Abstract
In response to iron loading, hepcidin synthesis is homeostatically increased to limit further absorption of dietary iron and its release from stores. Mutations in HFE, transferrin receptor 2 (Tfr2), hemojuvelin (HJV) or bone morphogenetic protein 6 (BMP6) prevent appropriate hepcidin response to iron, allowing increased absorption of dietary iron, and eventually iron overload. To understand the role each of these proteins plays in hepcidin regulation by iron, we analyzed hepcidin mRNA responsiveness to short and long-term iron challenge in iron-depleted Hfe, Tfr2, Hjv and Bmp6 mutant mice. After 1-day (acute) iron challenge, Hfe−/− showed a smaller hepcidin increase than their wild-type strain-matched controls, Bmp6−/− nearly no increase, and Tfr2 and Hjv mutants no increase in hepcidin expression, indicating that all four proteins participate in hepcidin regulation by acute iron changes. After a 21-day (chronic) iron challenge, Hfe and Tfr2 mutants increased hepcidin expression to nearly wild-type levels but a blunted increase of hepcidin was seen in Bmp6−/− and Hjv−/− mice. BMP6, whose expression is also regulated by iron, may mediate hepcidin regulation by iron stores. None of the mutant strains (excepting Bmp6−/− mice) had impaired BMP6 mRNA response to chronic iron loading. Conclusion: TfR2, HJV and BMP6 and, to a lesser extent, HFE, are required for the hepcidin response to acute iron loading, but are partially redundant for hepcidin regulation during chronic iron loading, and are not involved in the regulation of BMP6 expression. Our findings support a model in which acute increases in holotransferrin concentrations transmitted through HFE, TfR2 and HJV augment BMP receptor sensitivity to BMPs. A distinct regulatory mechanism that senses hepatic iron may modulate hepcidin response to chronic iron loading.
Keywords: Hereditary hemochromatosis, bone morphogenetic protein 6, hemojuvelin, HFE, transferrin receptor 2
Systemic iron homeostasis is dependent on the hepatic peptide hormone hepcidin, its receptor/iron channel ferroportin, and the feedback regulation of the two molecules by iron. Hepcidin controls the delivery of dietary and recycled iron to plasma by binding to the iron exporter ferroportin and inducing its endocytosis and degradation (1). Increases in body iron levels stimulate hepcidin production in the liver, limiting further absorption of iron. The mechanism of hepcidin regulation by iron is incompletely understood. Human studies have shown that blood hepcidin concentrations rapidly increase in response to oral iron challenge, are proportional to increases in diferric transferrin (holo-Tf) concentrations (2, 3), and strongly correlate with iron stores as reflected by serum ferritin (3). In mice, hepcidin mRNA increase within 24h after the switch from low-iron to standard diet (4), and in vivo imaging of hepcidin promoter-reporter constructs in mice (5) confirmed high responsiveness of hepcidin promoter activity to iron loading. Mouse primary hepatocytes treated with holo-Tf increase their hepcidin mRNA expression twofold or more (2, 6). The aggregate evidence indicates that hepcidin regulation both in vivo and in cultured hepatocytes is predominantly transcriptional.
As indicated by mutations that cause hereditary hemochromatosis and inappropriately low hepcidin levels relative to the iron load, the genes encoding the proteins HFE, transferrin receptor 2 (TfR2), hemojuvelin (HJV) and bone morphogenetic protein 6 (BMP6) play fundamental roles in modulating hepcidin responses to iron (7–10). The response of hepcidin to iron loading in the various mouse mutants has not been systemically compared. We hypothesized that the mutant mouse models would show distinct defects in hepcidin and BMP6 responses to short or long-term iron challenges.
EXPERIMENTAL PROCEDURES
Animals
Hfe−/− (The Jackson Laboratory, Bar Harbor, ME), Tfr2Y245X/Y245X (from Dr. Robert Fleming, St. Louis University, MO) and Hjv −/− (from Dr. Paul Schmidt, Children’s Hospital Boston, MA) mutant mice were maintained at UCLA, and Bmp6−/− mice at the University of Toulouse. All animal studies were approved by the Animal Research Committees at the respective institutions. The wild-type mice used as controls were matched for the background strain of each mutant: C57BL/6 for Hfe−/−, FVB for Tfr2Y245X, 129S6/SvEvTac for Hjv −/−, and CD1 for Bmp6 −/−. Both genders were used for the experiments. The strain names will be abbreviated as C57BL/6, FVB, 129S6, CD1, Hfe, Tfr2, Hjv and Bmp6.
Dietary iron challenge
All mice were fed a standard diet (NIH 31 rodent diet, 336 ppm iron, Harlan Teklad, Indianapolis, IN) until 4 weeks of age, and were then iron-depleted. Iron-depletion in WT mice (C57BL/6, FVB, 129S6 and CD1) was achieved by placing animals on a diet containing less than 4 ppm iron for 14 days. The iron restriction did not cause a decrease in hemoglobin levels. Mutant mice (Hfe, Tfr2, Hjv and Bmp6) and an additional group of C57BL/6 were iron-depleted by a combination of phlebotomy and low iron diet. After iron depletion, the mice were re-fed with standard diet for 1 or 21 days. The number of mice that were used in each group of non-phlebotomized and phlebotomized mice is listed in Supplemental Table IS.
Phlebotomy
Mutant mice were phlebotomized to reduce the baseline iron load to levels similar to those of WT mice. Before phlebotomy, iron-overloaded mutant mice had increased Hb and MCV. Blood was removed from the retroorbital sinus (300–500 μL per week) until MCV was lowered to the WT range. Depending on the mutant strain, repeat phlebotomies were carried out for 3–7 weeks. To control for the possible confounding effect of phlebotomy on hepcidin response to iron, we also phlebotomized WT controls. We only used the C57BL/6 strain because our earlier experiments indicated that FVB and 129S6 had a very similar hepcidin response to iron as C57BL/6 mice. Bondi et al. showed in two WT strains that removal of 500 μL of blood resulted in transient suppression of hepcidin, with hepcidin mRNA returning to normal levels 2–3 days after phlebotomy (11). Our mice were therefore analyzed 1–2 weeks after the last phlebotomy.
Holotransferrin and apotransferrin administration
Six-week old male mice (4–6 per treatment) were placed on low iron diet for two weeks and then received 10 mg human holotransferrin or apotransferrin (Sanquin, Amsterdam, Netherlands)(12) in 200 μl saline intraperitoneally, or saline alone, and were analyzed after 6 hours.
Analysis of iron parameters and mRNA expression
Hematological parameters, serum iron, and tissue iron were measured as previously described (4, 13). Gene expression of Hamp1, Bmp6 and the reference gene β-actin were assessed by qRT-PCR (4, 13). Primer sequences are listed in the supplemental data. In every qRT-PCR run, an internal control sample was included to allow comparisons between different PCR plates. Results in figures are expressed as - ΔCt, i.e. the Ct differences between reference and target genes within each group of mice. The approximate fold induction between two conditions was calculated as 2−ΔΔCt.PCR efficiencies ranged between 90 and 100% for all transcripts.
Statistics
Statistics were calculated using SigmaStat (SyStat Software, Richmond, CA). Because some measurements were not normally distributed, the null hypotheses (no hepcidin increase after acute or chronic iron challenge, or no differences in hepcidin −ΔCt and iron loading between strains or between mutant vs wild-type) were tested by the non-parametric Mann-Whitney rank sum test. Data are reported as median (25%,75%), and also referred to as median and interquartile range. Comparisons between all mouse strains were tested by One Way ANOVA and Dunn’s method.
Mice of both genders were used in the study. Each treatment group had a similar number of male (m) and female (f) mice except for three acute iron challenge groups [129S6 (4m, 2f), Hjv (4m, 2f), and Bmp6 (2m, 5f)], and two chronic iron loading groups [Hfe (6m, 4f), and Hjv (6m, 3f)]. Because female mice in general have higher hepcidin levels than male mice, for these particular groups we performed statistical analysis using gender as a co-variable (two-way ANOVA). Gender had no significant effect on the outcome in any of the comparisons.
RESULTS
Hepcidin response to acute iron challenge is blunted in mouse models of hereditary hemochromatosis
Iron depletion
In wild-type mice (C57BL/6, FVB, 129S6 and CD1), iron depletion was achieved by low-iron diet for 2 weeks, and liver iron content was lowered to less than 1 μmole/g wet weight (Supplemental Table IS). However, Hfe, Tfr2, Hjv and Bmp6 mice had elevated liver iron concentrations and could not be iron-depleted by 2 weeks of low iron diet alone (data not shown). In order to make their iron stores comparable to those of WT mice, mutant mice were iron-depleted by weekly phlebotomies and low-iron (4 ppm) diet until their elevated mean corpuscular volume (MCV), a convenient index of erythropoietic iron availability, was lowered to the WT range of ~50 fL. A total of 1.5–3.5 ml blood (300–500 μL weekly) was removed from mutant mice, with Hfe requiring fewer, and Hjv and Bmp6 mice greater number of phlebotomies to reach the target MCV. Iron and erythropoietic activity have opposing effects on hepcidin expression, and to control for the potentially confounding effects of anemia, a group of WT mice (C57BL/6) was also subjected to the same phlebotomy protocol, with ~1 ml of blood removed. At the last phlebotomy, the average hemoglobin concentration in C57BL/6 mice was 7.1 g/dL, and in Hfe, Tfr2, Hjv and Bmp6 mutants 9.7, 8.9, 6.8 and 9.9 g/dL. At the time of iron challenge (1–2 weeks after the last phlebotomy in case of the phlebotomized mice, or after 2 weeks of low iron diet for non-phlebotomized mice), all WT and mutant mice had decreased liver iron content to near 1 μmol/g wet weight (Figure 1 and Supplemental Table IS), with medians (25–75%) ranging from 0.2 (0.1,0.2) μmole/g wet weight in 129S6 to 1.5 (1.2, 1.7) μmol/g wet weight in Hjv mutant mice showing effective depletion of liver iron stores.
Figure 1. Serum iron and nonheme liver iron increase after acute dietary iron challenge.
To deplete iron stores (“iron-depleted” condition), mutant mice (Hfe, Tfr2, Hjv and Bmp6) were placed on a low-iron diet and weekly phlebotomies, and strain-matched WT mice (C57BL/6, 129S6, FVB and CD1) were placed on low iron diet for two weeks. An additional control group of C57BL/6 mice (“Ph”) were subjected to low iron diet and phlebotomy. Acute iron challenge consisted of placing the mice on standard chow for 1 day. The values in the graphs represent median and interquartile range. For comparison to chronic liver iron loading (Figure 4, bottom panel), note that the scale for acute iron loading is 5-fold smaller..
Acute iron loading
After 1 day of standard iron diet (336 ppm iron), both WT and mutants significantly increased their serum iron concentration as well as liver iron stores (Figure 1, Supplemental Table IS). The increase in liver iron content after only 1 day of standard iron diet could be expected if mice absorbed as little as 10% of the iron from their food. The average daily consumption of food in mice is about 4 grams (14). Thus, with standard chow containing 336 ppm iron, mice ingest up to 1.3 mg (~25 μmoles) per day, and would plausibly load the liver (approximate mass 1 g) with 1–3 μmoles of iron, as was observed.
Acute hepcidin response to dietary iron
After iron depletion, all mice had similarly low hepcidin mRNA, ranging from −ΔCt median (25–75% range) of −12.0(−12.7,−11.9) in Bmp6 mutants to −7.3(−12.1,−4.8) in FVB mice, with statistically insignificant differences (except for the difference between Hfe and Bmp6 mutants, P<0.05, one way ANOVA on ranks). After acute iron challenge, phlebotomized WT C57BL/6 increased their hepcidin mRNA −ΔCt by 12.9 or nearly 8,000-fold (p<0.001, Figure 2). This response was similar to the hepcidin increase observed in diet-depleted C57BL/6 mice switched from low-iron diet to standard diet (Figure 2) demonstrating that phlebotomy and anemia did not impair hepcidin regulation by acute iron loading. A similar magnitude of hepcidin mRNA increase was also observed in diet-depleted FVB, CD1, and 129S6 mice switched from low-iron to standard diet (Figure 2). In contrast, Tfr2 and Hjv mutants failed to increase hepcidin in response to 1-day iron feeding, and Bmp6 mice had a negligible response (Figure 2) indicating that these proteins play essential roles in sensing acute changes in body iron levels. Among mutant mice, Hfe showed a partial increase in hepcidin expression (11-fold, p=0.027), a much smaller increase than in the matching WT strain C57BL/6 (p<0.001), indicating that HFE plays a somewhat redundant role in sensing acute iron changes. Other proteins, possibly TfR2 or HJV, may partially compensate for the lack of HFE.
Figure 2. Hepcidin response to acute dietary iron challenge is impaired in hemochromatotic mice.
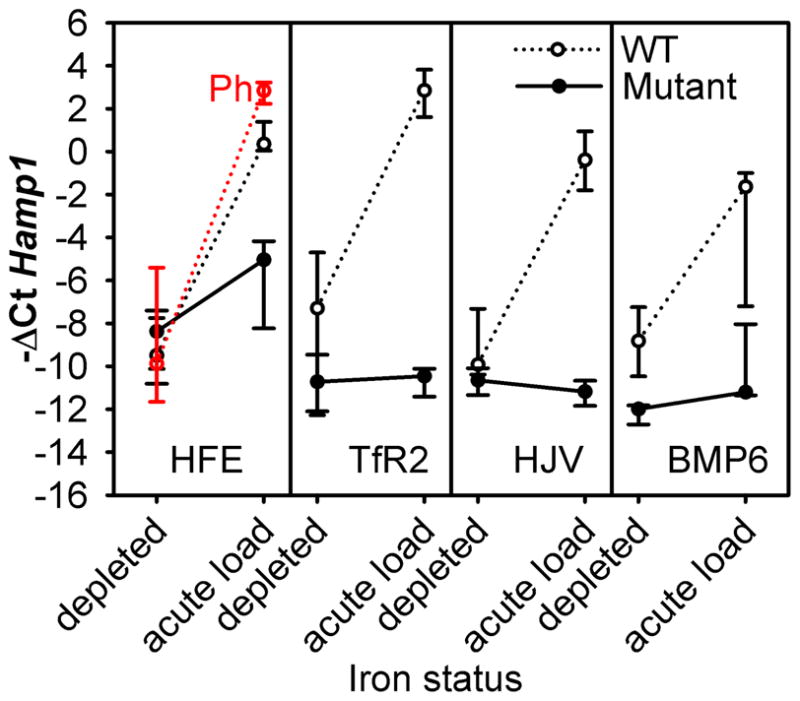
Animals were iron-depleted as described in Figure 1. Hamp1 mRNA levels were measured either after iron depletion alone or after an additional 1-day dietary iron challenge (standard diet, 336 ppm iron). The values in the graphs represent median and interquartile range.
Hepcidin response to holo-Tf
Because one day feeding with standard diet increased both serum and liver iron, we asked whether an increase in serum iron induced by a small dose of parenteral iron is sufficient for hepcidin induction. We injected C57BL/6 mice with saline, iron-free apotransferrin (apo-Tf) or holo-Tf (10 mg/mouse which for holo-Tf equals 14 μg or 0.25 μmoles of iron), a dose estimated to be hundred-times smaller than the amount of iron ingested daily on standard diet, and ten-times smaller than the amount absorbed assuming 10% absorption rate. Injection of holo-Tf raised serum iron concentrations from 30 to 130 μM within 6h, and hepcidin mRNA increased to the maximal levels seen in this strain. Hepcidin mRNA levels in holo-Tf-treated mice were more than 100-fold or 16-fold higher than in saline or apo-Tf-treated mice (p=0.015 and 0.026 respectively, Supplemental Figure 1S). Although −ΔCt in apo-Tf-injected animals was somewhat higher than in saline-injected, the difference was not statistically significant. Apo- or holo-Tf injections had no significant effect on the expression of the inflammatory marker C-reactive protein (not shown). Thus, an acute increase in holo-Tf concentrations is sufficient to induce maximal hepcidin expression even if the amount of iron delivered is quite small.
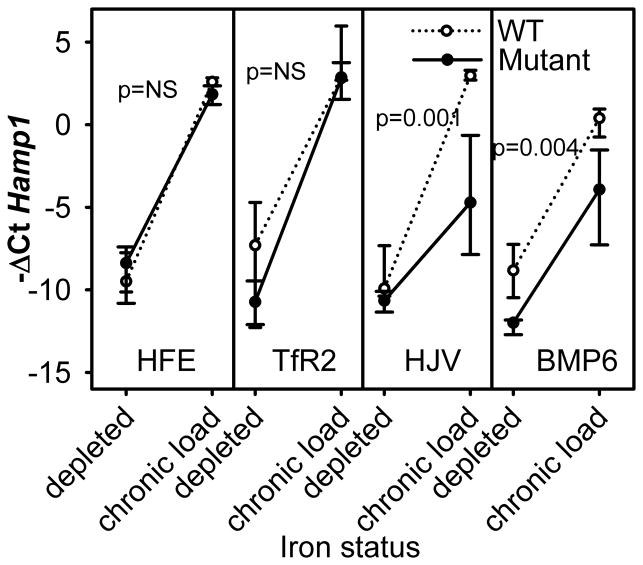
Hepcidin response to chronic iron challenge is blunted in HJV and BMP6 knockout mice, but not in HFE and Tfr2 mutants
Chronic iron loading
For all four strains of WT mice, the iron-depleted condition was achieved by feeding mice 4 ppm iron-restricted diet for 14 days, and the chronic iron challenge consisted of 21 days of standard iron diet. For all hemochromatosis mutants, we phlebotomized the mice to iron-deplete them. The chronically iron-loaded condition was achieved by maintaining mice on standard iron diet until they reached the same age as chronically loaded WT mice (9 weeks of age).
In WT mice, 21 days of standard iron diet increased liver iron to 2–5 μmoles Fe/g wet weight (Supplemental Table IS and Supplemental Figure 2S). Iron loading in the mutant strains was more severe: Hfe had double, Tfr2 mutant quadruple, and Hjv and Bmp6 5–6 times the amount of liver iron of the WT mice (all differences statistically significant). As expected, the median spleen iron content was lower in all mutants than in the corresponding WT mice, despite more severe systemic iron overload in mutants (Supplemental Figure 2S, Supplemental Table IS, all differences statistically significant except 129S6 vs Hjv).
Chronic hepcidin responses
At baseline, hepcidin levels were low in both WT and mutants (Figure 3, Supplemental Table IS), but significantly lower only in Bmp6 mutants compared to their WT counterparts (p=0.002). In the chronically iron-loaded group, C57BL/6, FVB and 129S6 WT mice had a very similar increase in hepcidin mRNA (Figure 3, Supplemental Table IS), reaching at least 1000-fold higher hepcidin mRNA expression than the iron-depleted group, while the less iron-sensitive CD1 mice increased hepcidin ~600-fold.
Figure 3. Hepcidin response to chronic dietary iron challenge.
Medians and interquartile range are shown. Hepcidin mRNA expression was measured by qRT-PCR in iron-depleted and iron-loaded mutant mice and their respective WT strains.
Chronically-loaded Hfe and Tfr2 mice also had at least 1000-fold higher hepcidin mRNA than iron-depleted mice, reaching the level of hepcidin expression detected in chronically-loaded WT mice (Figure 3). Hjv and Bmp6 had impaired hepcidin response to chronic iron loading (significantly different from all other mice, p<0.001 by one way ANOVA), but notably a partial response was still preserved in both types of mutants. In iron-loaded Hjv and Bmp6 mice, hepcidin mRNA was ~64-fold and 270-fold higher than in iron-depleted mice. Importantly, both Hjv and Bmp6 chronically-loaded mice failed to reach hepcidin mRNA levels of the matching WT strain (p=0.001 and p=0.004 respectively). Although C57BL/6 mice had a similar hepcidin response to iron loading whether they were depleted by diet alone or diet and phlebotomy (Figure 2), we reconsidered the possibility that hepcidin induction in Hjv and Bmp6 mice may not be related to iron loading but to the difference in erythropoietic activity between the iron-depleted state when mice were anemic and iron-loaded state when mice were not anemic. To assess the potential effect of anemia apart from iron loading, we analyzed the correlation between hepcidin mRNA expression and Hb levels in iron-depleted Hjv and Bmp6 mice which did not undergo any iron challenge (Supplemental Figure 3S). Hepcidin expression changed very little with increasing Hb values suggesting that iron load rather than the severity of anemia caused hepcidin increase in iron-fed Hjv and Bmp6 mice.
Serum and liver iron in chronic vs acute iron loading
Serum iron levels in WT mice and mutants after chronic iron loading were not significantly higher (and mostly were lower) than those measured after acute iron challenge (Figure 4). In contrast, liver iron content in all mutants was higher after chronic loading when compared to acute iron challenge (Figure 4, Supplemental Table IS). The amount of liver iron in chronically- as compared to acutely-loaded animals was: in Hfe 5 and 2 μmol Fe/g wet liver (p=0.004), in Tfr2 16 and 4 (p=0.001), in Hjv 26 and 4 (p<0.001), and in Bmp6 26 and 1 μmol/g wet liver (p=0.001). Therefore, any additional increase in hepcidin expression after chronic as compared to acute iron loading in chronically-loaded mutant mice cannot be accounted for by changes in serum iron levels, but is related to increased iron stores.
Figure 4. Serum and liver iron after acute vs chronic dietary iron loading.
Serum iron concentrations after chronic iron challenge were similar or lower than those reached after acute iron challenge, in both WT and mutant mice. Liver non-heme iron content in mutants increased after chronic iron loading when compared to acute challenge (p values for the difference of medians shown above the line connecting the acute and chronic loading values). The order of hepatic iron loading was Hjv = Bmp6 > Tfr2 > Hfe. The p values for the comparison of mutant and WT iron loading are shown near or between the corresponding points on the graph.
Hepcidin response relative to iron load
Even though hepcidin levels in all mutant mice increased with chronic iron loading, the severity of iron overload relative to the WT strain of the same genetic background depended on the mutation. To assess the appropriateness of hepcidin response to iron loading, we correlated hepcidin expression with liver iron content of mutant mice and matching WT strains (Figure 5). Hfe mice had the mildest impairment in hepcidin response, followed by Tfr2 mice, whereas Bmp6 and Hjv mice showed a more severe impairment. Although Hjv and Bmp6 mice accumulated the most iron during chronic loading, their hepcidin expression did not reach the levels observed in the WT strains.
Figure 5. HFE, Tfr2, HJV and BMP6 mutations differentially alter the sensitivity of hepcidin response to iron.
In the absence of HFE or TfR2, modestly higher liver concentrations were needed to reach the increase in hepcidin mRNA comparable to the WT strain. BMP6 and HJV loss dramatically decreased hepcidin responses to iron loading, and hepcidin mRNA remained low even at 10-fold higher liver iron concentrations. The values in the graphs represent median and interquartile range.
The dependence of hepcidin on serum iron and hepatic iron
To determine how serum iron and liver iron contribute to hepcidin regulation, we performed best sets linear regression analysis of the dependence of hepcidin mRNA on serum and liver iron in each group of mice (Supplemental Table IIS). In wild-type strains, hepcidin showed mixed dependence of serum and liver iron, ranging from predominant dependence on serum iron in C57BL/6 mice to predominant dependence on liver iron in CD1 mice. In all mutant strains, hepcidin mRNA was dependent on liver iron only.
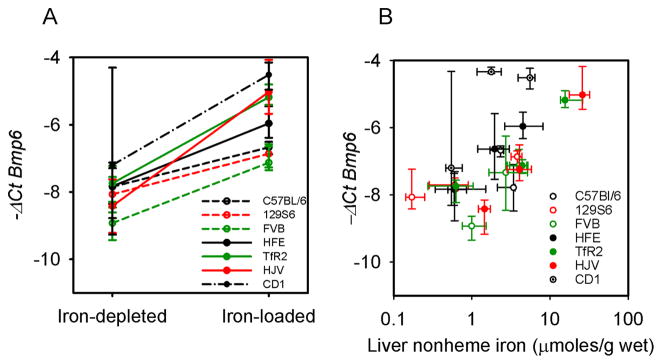
Bmp6 mRNA increase in response to iron loading is not impaired in mouse models of hereditary hemochromatosis
BMP6 plays an important role in the regulation of hepcidin by iron (9, 15). To understand whether BMP6 regulation by iron was altered in mutant mice, we measured Bmp6 mRNA in our chronic iron loading model. In all cases, Bmp6 mRNA increased with iron loading (Figure 6A), roughly proportionally to the liver iron content (Figure 6B). There was a 4-fold increase in Bmp6 mRNA in WT and Hfe mice which loaded the least iron, a 6-fold increase in Tfr2 mutants, and an 8-fold upregulation in Hjv which loaded the most iron. These results indicate that Bmp6 expression is regulated independently of Hfe, Tfr2 and Hjv. Similar results were reported in other studies using Hfe or Hjv knockout mice (16, 17). In the acute iron-loading model, Bmp6 mRNA levels tended to increase but did not reach statistical significance (not shown).
Figure 6. BMP6 expression reflects iron load in wt and mutant strains.
A. BMP6 mRNA levels were measured by qRT-PCR in mutants and in their respective WT strains before and after chronic iron loading. Expression increased significantly in all the groups tested (p values: C57BL/6=0.018, 129S6=0.026, FVB<0.001, Hfe p=0.037, Tfr2 =0.001, and Hjv =0.003). Medians and interquartile range are shown. BMP6 was also measured in mice before and after 1 day iron feeding, but the increase did not reach statistical significance. B. BMP6 mRNA expression is proportional to the liver iron load (non-heme iron) in WT and mutant mice.
DISCUSSION
Mouse models
We used four mouse models of hereditary hemochromatosis, Hfe−/−, Tfr2Y245X/Y245X, Hjv−/− and Bmp6−/−, in order to examine the role of these proteins in hepcidin regulation by acute and chronic iron loading in vivo. Although the Tfr2Y245X/Y245X is not a null mutant, truncated TfR2 is not detectable on the cell membrane both because of nonsense-mediated mRNA decay and defective subcellular transport of the truncated protein (18). All four models were reported to have reduced hepcidin mRNA (7–10, 15, 19). Importantly, to accurately assess hepcidin response to iron loading in mutant mice, we iron-depleted the mutant mice by phlebotomy so that the liver iron content in iron-depleted state was similar to that observed in WT mice.
Acute and chronic iron loading
We found that chronic iron loading of WT and mutant mice did not raise serum iron more than 1 day iron-loading (Figure 4). The iron stores in the liver of the mutant mice, however, substantially increased with chronic loading compared to 1 day challenge (Figure 4). The chronic increase in iron stores was greatest in Hjv and Bmp6 mice, intermediate in Tfr2 mutant mice, and least in Hfe mice. The order of severity of iron loading is the same as reported in the human mutations involving HJV, TfR2 and HFE. On standard iron diet, WT mice modestly increased their liver iron content compared to 1 day iron loading (Figure 4 and Supplemental Table IS).
Hepcidin response to acute iron loading
After 1 day iron challenge, Tfr2 and Hjv mice did not increase their hepcidin expression, Bmp6 mice increased it minimally, whereas Hfe showed a significant but still blunted response. The result indicates that all four proteins participate in hepcidin regulation by acute iron changes (presumably by holo-Tf concentrations), but that TfR2, HJV and BMP6 are required for this effect. Interestingly, not only serum iron, but also the liver iron stores of WT and mutant mice increased significantly within one day of iron challenge, due to the large food consumption by mice relative to their body mass. To confirm that serum iron is a sufficient signal regulating hepcidin expression, we tested hepcidin responsiveness to a holo-Tf injection that contained much smaller amount of iron (14 μg) than was loaded into the liver by 1 day’s diet (more than 100 μg). Hepcidin increased by ~100-fold within 6 hours compared to solvent controls, and reached maximal hepcidin expression seen in the C57BL/6 mice which indicates that holo-Tf alone can modulate hepcidin mRNA levels.
Hepcidin response to chronic iron loading
Hepcidin expression after chronic iron loading of Hfe and Tfr2 mice reached much higher levels than after acute loading and became similar to those of iron-loaded WT mice (Figures 3 and 5). Although Hjv and Bmp6 mutant mice accumulated the most iron after chronic loading with standard chow, their hepcidin expression rose only modestly and remained severely depressed when assessed relative to their total liver iron load (Figure 5). The BMP6-deficient iron phenotype may be partially ameliorated if other BMPs associate with HJV to mediate signaling and increase hepcidin expression in response to iron loading. Current models of hepcidin regulation have focused on holo-Tf concentration as the key regulator of hepcidin, through the incompletely defined pathway centered on TfR1 and TfR2 as the likely holo-Tf sensors, interacting with HFE, HJV and BMP6/BMPR. Increased hepcidin expression after chronic as compared to acute iron loading suggests that hepcidin is regulated not only by plasma iron, which is similar after acute and chronic iron loading, but also by intracellular iron stores, which are much higher after chronic than acute iron loading. Based on our findings, the pathway involved in hepcidin regulation by intracellular iron is mainly dependent on HJV and BMP6, with a small, if any, nonredundant contribution from TfR2 or HFE. The greater severity of iron overload in the combined TfR2/HFE deficiency as compared to the deficiency of either alone indicates that the two molecules may partially compensate for each other, both in mice and in humans (20, 21). Notably, both Hjv and Bmp6 mice significantly increased hepcidin with chronic iron loading, albeit less than WT strains. Although the loss of either molecule may preserve sufficient BMP receptor activity to regulate hepcidin inefficiently, there is also evidence that alternative pathways are involved in iron regulation. Holo-Tf was reported to activate the ERK/MAPK pathway (6), and it is possible that chronic stimulation of this pathway in Hjv and Bmp6 mice results in hepcidin increase, unrelated to the rise in hepatic iron stores. Alternatively, another as yet unidentified pathway may play a role in sensing intracellular iron and signaling to hepcidin. We did not detect additional hepcidin suppression in diet iron-depleted C57BL/6 mice when they were subjected to phlebotomy (Figure 2), or any dependence of hepcidin on the severity of post-phlebotomy anemia in Hjv and Bmp6 mice (Supplemental Figure 3S). Nevertheless, our studies cannot exclude that anemia-related erythropoietic factors contribute to the relative hepcidin suppression after phlebotomy and conversely, that the reversal of anemia contributes to the rise of hepcidin after iron loading.
Regulation of hepcidin by serum iron vs liver iron
Linear multivariate analysis of the dependence of hepcidin mRNA on serum iron vs. liver iron revealed that in wild-type strains both serum and liver iron contributed to hepcidin regulation. In mutants, only liver iron contributed to hepcidin regulation, and the effect of serum iron was lost. Thus, liver and serum iron may independently regulate hepcidin expression. However, we cannot exclude the possibility that the effects of serum iron are mediated by hepatocyte loading through potential nonlinear effects. For example, at low liver iron concentrations, changes in holo-transferrin levels could cause small changes in liver iron content to which hepcidin mRNA transcription could be highly sensitive. Nevertheless, strong support for the independent regulation of hepcidin by the extracellular effects of serum iron (probably sensed as holotransferrin) is provided by the low hepcidin levels in hypotransferrinemic humans and mice (22, 23), despite hepatic iron overload. Although some of hepcidin decrease could be attributed to the suppressive effects of ineffective erythropoiesis in hypotransferrinemic mice, hepcidin remained low even when the potential effect of erythropoiesis was negated by erythrocyte transfusion or cytotoxic therapy.
BMP6 responses to iron loading
The expression of Bmp6 mRNA is regulated by iron (24) but the mechanism is not known. The continued rise of Bmp6 mRNA during chronic iron loading parallels the rise in hepatic iron content (Figure 6) suggesting that BMP6 production is predominantly responsive to intracellular iron stores. However, chronic iron loading of Bmp6 mice resulted in 270-fold increase in hepcidin mRNA indicating that BMP6 is not the sole mediator of the intracellular iron signal. Interestingly, when compared to the matching WT strain, hepcidin response to chronic iron loading in Hjv mice appears even more impaired than in Bmp6 mice, despite the significant increase in Bmp6 expression in chronically iron-loaded Hjv mice. This supports the concept that HJV acts as an essential potentiator or coreceptor for BMP6 signaling, but that BMP6 may not be the only factor regulating hepcidin via HJV. Intracellular iron could also signal by altering the cell surface localization of HJV, as indicated by the ability of ferric ammonium citrate or holo-Tf to decrease the release of soluble HJV in vitro (25). As soluble HJV suppressed hepcidin but membrane HJV stimulated it (25), this effect of iron would be expected to stimulate hepcidin production. How iron affects the release of soluble HJV is unknown, but the regulation may involve neogenin, a receptor for repulsive guidance molecules (26) or furin, a protease that cleaves hemojuvelin (27).
In summary, our comparative studies of four hereditary hemochromatosis mouse models point to at least two distinct pathways of hepcidin regulation by iron, one sensing extracellular holo-Tf concentrations and the other sensing intracellular iron, presumably in hepatocytes. It is possible that intracellular iron, partially signaling through HJV/BMP6 pathway, sets basal hepcidin expression, with membrane-bound HJV functioning to heighten the sensitivity of BMP receptors to BMP6 and possibly other ligands. Holo-Tf concentrations, signaling through TfR2, HFE and HJV, further increase the sensitivity of BMP receptors to the BMP ligands, through as yet unknown mechanisms. It is also possible that TfR2/HFE activate a parallel pathway to increase hepcidin expression. Although not directly addressed in this study, the involvement of non-hepatocyte cell types in the regulation of hepatocyte hepcidin synthesis remains to be explored and could contribute to iron homeostasis.
Supplementary Material
Acknowledgments
Financial Support:
This work was supported by National Institutes of Health grants R01 DK065029 (to T.G.), R01 DK082717 (to E.N.), the Will Rogers Fund (to T.G.), Ruth L. Kirschstein National Research Service Award GM007185 (to E.R.) and ANR-GENOPAT IRONREG (to M.P.R.).
List of Abbreviations
- Hfe
hereditary hemochromatosis gene
- TfR2
transferrin receptor 2
- TfR1
transferrin receptor 1
- HJV
hemojuvelin
- BMP6
bone morphogenetic protein 6
- HH
hereditary hemochromatosis
- HAMP
hepcidin antimicrobial peptide
- Holo-Tf
holo-transferrin
- Apo-Tf
apotransferrin
- RGM
repulsive guidance molecule
- SMAD
mothers against decapentaplegic homolog
Reference List
- 1.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 2.Lin L, Valore EV, Nemeth E, Goodnough JB, Gabayan V, Ganz T. Iron transferrin regulates hepcidin synthesis in primary hepatocyte culture through hemojuvelin and BMP2/4. Blood. 2007;110:2182–2189. doi: 10.1182/blood-2007-04-087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112:4292–4297. doi: 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 4.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flanagan JM, Truksa J, Peng H, Lee P, Beutler E. In vivo imaging of hepcidin promoter stimulation by iron and inflammation. Blood Cells Mol Dis. 2007;38:253–257. doi: 10.1016/j.bcmd.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramey G, Deschemin JC, Vaulont S. Cross-talk between the mitogen activated protein kinase and bone morphogenetic protein/hemojuvelin pathways is required for the induction of hepcidin by holotransferrin in primary mouse hepatocytes. Haematologica. 2009;94:765–772. doi: 10.3324/haematol.2008.003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawabata H, Fleming RE, Gui D, Moon SY, Saitoh T, O'Kelly J, et al. Expression of hepcidin is down-regulated in TfR2 mutant mice manifesting a phenotype of hereditary hemochromatosis. Blood. 2005;105:376–381. doi: 10.1182/blood-2004-04-1416. [DOI] [PubMed] [Google Scholar]
- 8.Niederkofler V, Salie R, Arber S. Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J Clin Invest. 2005;115:2180–2186. doi: 10.1172/JCI25683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41:478–481. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 10.Vujic SM, Kiss J, Herrmann T, Galy B, Martinache S, Stolte J, et al. Hfe acts in hepatocytes to prevent hemochromatosis. Cell Metab. 2008;7:173–178. doi: 10.1016/j.cmet.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Bondi A, Valentino P, Daraio F, Porporato P, Gramaglia E, Carturan S, et al. Hepatic expression of hemochromatosis genes in two mouse strains after phlebotomy and iron overload. Haematologica. 2005;90:1161–1167. [PubMed] [Google Scholar]
- 12.Li H, Rybicki AC, Suzuka SM, von BL, Breuer W, Hall CB, et al. Transferrin therapy ameliorates disease in beta-thalassemic mice. Nat Med. 2010;16:177–182. doi: 10.1038/nm.2073. [DOI] [PubMed] [Google Scholar]
- 13.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108:3730–3735. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32:435–443. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andriopoulos B, Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41:482–487. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corradini E, Garuti C, Montosi G, Ventura P, Andriopoulos B, Jr, Lin HY, et al. Bone morphogenetic protein signaling is impaired in an HFE knockout mouse model of hemochromatosis. Gastroenterology. 2009;137:1489–1497. doi: 10.1053/j.gastro.2009.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kautz L, Meynard D, Besson-Fournier C, Darnaud V, Al ST, Coppin H, et al. BMP/Smad signaling is not enhanced in Hfe-deficient mice despite increased Bmp6 expression. Blood. 2009;114:2515–2520. doi: 10.1182/blood-2009-02-206771. [DOI] [PubMed] [Google Scholar]
- 18.Fleming RE, Sly WS. Mechanisms of iron accumulation in hereditary hemochromatosis. Annu Rev Physiol. 2002;64:663–680. doi: 10.1146/annurev.physiol.64.081501.155838. [DOI] [PubMed] [Google Scholar]
- 19.Huang FW, Babitt JL, Wrighting DM, Samad TA, Xia Y, Sidis Y, et al. Hemojuvelin Acts as a Bone Morphogenetic Protein Co-Receptor To Regulate Hepcidin Expression. ASH Annual Meeting Abstracts. 2005;106:511. [Google Scholar]
- 20.Wallace DF, Summerville L, Crampton EM, Frazer DM, Anderson GJ, Subramaniam VN. Combined deletion of Hfe and transferrin receptor 2 in mice leads to marked dysregulation of hepcidin and iron overload. Hepatology. 2009;50:1992–2000. doi: 10.1002/hep.23198. [DOI] [PubMed] [Google Scholar]
- 21.Pietrangelo A, Caleffi A, Henrion J, Ferrara F, Corradini E, Kulaksiz H, et al. Juvenile hemochromatosis associated with pathogenic mutations of adult hemochromatosis genes. Gastroenterology. 2005;128:470–479. doi: 10.1053/j.gastro.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 22.Trombini P, Coliva T, Nemeth E, Mariani R, Ganz T, Biondi A, et al. Effects of plasma transfusion on hepcidin production in human congenital hypotransferrinemia. Haematologica. 2007;92:1407–1410. doi: 10.3324/haematol.11377. [DOI] [PubMed] [Google Scholar]
- 23.Bartnikas TB, Andrews NC, Fleming MD. Transferrin is a major determinant of hepcidin expression in hypotransferrinemic mice. Blood. 2010 doi: 10.1182/blood-2010-05-287359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kautz L, Meynard D, Monnier A, Darnaud V, Bouvet R, Wang RH, et al. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood. 2008;112:1503–1509. doi: 10.1182/blood-2008-03-143354. [DOI] [PubMed] [Google Scholar]
- 25.Lin L, Goldberg YP, Ganz T. Competitive regulation of hepcidin mRNA by soluble and cell-associated hemojuvelin. Blood. 2005;106:2884–2889. doi: 10.1182/blood-2005-05-1845. [DOI] [PubMed] [Google Scholar]
- 26.Zhang AS, Anderson SA, Meyers KR, Hernandez C, Eisenstein RS, Enns CA. Evidence that inhibition of hemojuvelin shedding in response to iron is mediated through neogenin. J Biol Chem. 2007;282:12547–12556. doi: 10.1074/jbc.M608788200. [DOI] [PubMed] [Google Scholar]
- 27.Silvestri L, Pagani A, Nai A, De DI, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8:502–511. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.