Abstract
Intestinal absorption of biotin is mediated via the sodium-dependent multivitamin transporter (SMVT). Studies from our laboratory and others have characterized different aspects of the human SMVT (hSMVT), but nothing is currently known about protein(s) that may interact with hSMVT and affect its physiology/biology. In this study, a PDZ-containing protein PDZD11 was identified as an interacting partner with hSMVT using yeast two-hybrid screen of a human intestinal cDNA library. The interaction between hSMVT and PDZD11 was confirmed by in vitro GST-pull-down assay and in vivo in a mammalian cell environment by a two-hybrid luciferase and coimmunoprecipitation assays. Furthermore, confocal imaging of live human intestinal epithelial HuTu-80 cells expressing hSMVT-GFP and DsRed-PDZD11 demonstrated colocalization of these two proteins. We also examined the functional consequence of the interaction between hSMVT and PDZD11 in HuTu-80 cells and observed significant induction in [3H]biotin uptake upon coexpression of hSMVT and PDZD11. In contrast, knocking down of PDZD11 with gene-specific small interfering RNA led to a significant decrease in biotin uptake; biotinylation assay showed this to be associated with a marked decrease in level of expression of hSMVT at the cell membrane. By truncation approach, we also demonstrated that the PDZ binding domain that is located in the COOH-terminal tail of hSMVT polypeptide is involved in the interaction with PDZD11. These results demonstrate for the first time that PDZD11 is an interacting partner with hSMVT in intestinal epithelial cells and that this interaction affects hSMVT function and cell biology.
Keywords: biotin transport, protein-protein interactions, yeast two-hybrid analysis
the water-soluble micronutrient biotin is essential for many cellular functions. It operates as a coenzyme for five carboxylases involved in the intermediate metabolism of gluconeogenesis, fatty acid synthesis, and catabolism of branched chains amino acids and odd-carbon fatty acids (10, 11, 20). Biotin deficiency leads to serious clinical abnormalities including neurological disorders, growth retardation including congenital malformation and death, and dermal abnormalities (10, 11, 26). Furthermore, a role for biotin in normal immune functions and cell proliferation has been suggested (2, 9).
It is well known that humans and other mammals cannot synthesize biotin and, thus, must obtain the vitamin from exogenous sources via intestinal absorption. Absorption of biotin in the intestine occurs via a Na+-dependent, carrier-mediated mechanism and is mediated through the sodium-dependent multivitamin transporter (SMVT; for humans it is referred as hSMVT) (3, 14, 15). The hSMVT system is expressed exclusively at the apical membrane domain of the polarized enterocytes and is capable of transporting the vitamin against a concentration gradient (19, 22). Studies from our laboratory (3) have determined the contribution of the hSMVT system toward overall carrier-mediated biotin uptake by human intestinal epithelial cells with the results suggesting a predominant role for this system in uptake of the vitamin (3). We also characterized certain aspects of the hSMVT cell biology in intestinal epithelial cells with regards to membrane targeting and intracellular trafficking (22). Other studies from our laboratory (4, 17, 18) have characterized transcriptional regulation of the SLC5A6 gene (which encodes hSMVT) and cloned the involved promoters.
The existence of proteins that interact with membrane transporters and affect their physiology (function) and cell biology has been well documented in recent years for a variety of membrane carriers (12, 13, 23). Modulation of the function of an interacting protein of a given membrane transporter could impact the overall transport event as has recently been shown in the case of the monocarboxylate transporters (25) and in our laboratory with the reduced folate carrier (1). Thus, for detailed understanding of the intestinal biotin absorption process, knowledge about identity and function of protein(s) that interacts with hSMVT is essential. There is nothing currently known about protein(s) that interacts with the hSMVT system in human intestinal epithelial cells and the physiological and cell biological consequences of such interactions. To fill this gap in our knowledge, we used a yeast two-hybrid (Y2H) approach and identified a PDZ-containing protein PDZD11, which interacts with hSMVT. PDZD11, which is also known as plasma membrane calcium ATPase-interacting single-PDZ protein (PISP; Ref. 7) or as ATPase-interacting PDZ protein (AIPP1; Ref. 21), is a ubiquitously expressed small protein that has been recently shown to interact with other membrane transporters and influence their activity [e.g., Ca-ATPase and Menkes copper ATPase (ATP7A); Refs. 7, 21]. We confirmed the interaction between hSMVT and PDZD11 both in vitro and in vivo and demonstrated the contribution of PDZD11 to functionality of the hSMVT system in intestinal epithelial cells.
EXPERIMENTAL PROCEDURES
Materials.
[3H]Biotin (specific activity: >30 Ci/mmol, radiochemical purity: >98%) was purchased from American Radiolabeled Chemical (St. Louis, MO). All chemicals and reagents used in this study were of analytical/molecular biology grade and were purchased from commercial sources. Human-derived intestinal epithelial Caco-2 and HuTu-80 cells and Chinese hamster ovarian CHO-K1 cells were purchased from American Type Culture Collection (Manassas, VA) and maintained in DMEM or F-12K (CHO-K1 cells) growth media supplemented with 10% (vol/vol) FBS, penicillin (100,000 U/l), and streptomycin (10 mg/l) in 75-cm2 plastic flasks at 37°C in a 5% CO2-95% air atmosphere with media changes every 2–3 days.
Y2H assay.
The Y2H screens were performed [by Hybrigenics (Paris, France) as previously described (6)] by using the COOH-terminal tail (amino acids 553–635) of the hSMVT as a bait to screen a human intestinal random-primed cDNA library. Briefly, the bait was cloned as a COOH-terminal fusion to the LexA DNA-binding domain into plasmid pB27. A random primed human intestinal cDNA library was constructed into the pP6 plasmid. Colonies were screened by mating Y187 (mat α) and L40ΔGal4 (mat a) yeast strains. The prey fragments of the positive clones were amplified by PCR and sequenced at their 5′- and 3′-junctions, and the resulting sequences were used to identify the corresponding interacting proteins in the GenBank database (NCBI). Each protein interaction was assigned with a statistical confidence score (5).
GST pull-down assay.
To generate pGEX-4T-1-PDZD11, the open reading frame of human PDZD11 (OriGene, Rockville, MD) was inserted in frame into BamHI/ XhoI sites of pGEX-4T-1 vector (Invitrogen, Carlsbad, CA). GST-PDZD11 fusion protein and GST were produced in BL-21 Escherichia coli cells and used in GST pull-down assay as described before (1). Briefly, cleared (14,000 g) postnuclear extract of Caco-2 cells (1 mg of total soluble protein) was incubated with either GST-PDZD11 (100 μg) or GST (100 μg) bound to glutathione-Sepharose4B beads (GE Healthcare, Piscataway, NJ) in CelLytic M cell lysis reagent (Sigma, St. Louis, MO) for 2 h at 4°C. Bound proteins were eluted with 10 mM glutathione and processed for Western blotting using the rabbit polyclonal anti-SMVT (Santa Cruz Biotechnology, Santa Cruz, CA).
Mammalian two-hybrid assay.
CheckMate mammalian two-hybrid system (Promega, Madison, WI) was used according to manufacturer's recommendations. Briefly, the open reading frame for hSMVT and PDZD11 were cloned in frame into pBIND and pACT vector, respectively, using SalI/XbaI sites (hSMVT) and BamHI/XbaI sites (PDZD11). pBIND-hSMVT and pACT-PDZD11 (2 μg each) were cotransfected into CHO-K1 cells to generate hSMVT fused with the DNA-binding domain of GAL4 and PDZD11 fused with the activation domain of VP16, respectively. The pG5luc vector, containing the Firefly luciferase gene under the control of a transcriptional unit comprising five GAL4-binding elements upstream of a minimal TATA box, served as a reporter plasmid. Transfection of CHO-K1 cells (80–90% confluency) was performed using Lipofectamine 2000 (Invitrogen). Renilla-normalized Firefly Luciferase activity was determined after 72 h of transfection using a dual luciferase assay system (Promega).
Coimmunoprecipitation.
Coimmunoprecipitation was performed as described by us previously with minor modifications (1). Briefly, Caco-2 cells (80–90% confluency) were transfected with the expression plasmid pFLAG-CMV-2-PDZD11 or pFLAG-CMV-2-hSMVT (wild type or lacking TSL peptide from 633–635 amino acid sequence) to generate FLAG-tagged PDZD11 or FLAG-tagged hSMVT (wild type or truncated), respectively. Forty-eight hours after transfection, cells were lysed in CelLytic M cell lysis reagent. Cleared cell lysates were incubated with anti-FLAG M2 antibody-conjugated beads (Sigma), following manufacturer's instructions. Bound proteins were separated in NuPAGE 4–12% Bis-Tris gradient minigels (Invitrogen) and analyzed by Western blotting. hSMVT and PDZD11 were detected with rabbit polyclonal anti-SMVT (Santa Cruz Biotechnology) or goat polyclonal anti-human PDZD11 antibodies (R&D Systems, Minneapolis, MN), respectively, and anti-rabbit or anti-goat antibodies conjugated to horseradish peroxidase (Sigma). FLAG-tagged PDZD11 was detected with monoclonal anti-FLAG M2-peroxidase antibody (Sigma). FLAG-tagged hSMVT was detected with monoclonal anti-FLAG M2 antibody (Sigma) and anti-mouse antibodies conjugated to horseradish peroxidase.
Confocal imaging.
The human derived intestinal epithelial HuTu-80 cells grown on glass-bottomed petri dishes (MatTek, Ashland, MA) were transiently cotransfected either with hSMVT-GFP and pDsRed-C1-PDZD11 plasmids (3 μg each) at 90% confluency using Lipofectamine 2000. Live cell confocal imaging was performed 48 h after transfection as described by us previously (22). Briefly, fluorophores were excited by using a 488-nm line from an argon ion laser, and a 543-nm line from a HeNe ion laser-emitted fluorescence was monitored with a 515 ± 30-nm band-pass (GFP) or a 570 ± 50+-nm long-pass (DsRed) filter.
RNA interference assays.
MISSION Predesigned small interfering (si)RNAs (Sigma) targeted the PDZD11 sequence were 5′-CUGAAAGGAGGAUGAUGAU[dT][dT], 5′-CUACAAUUAUCAUCGCCAA[dT][dT] and 5′-CUGCAUUACCCAAACCAUA[dT][dT]. MISSION siRNA Universal Negative Control #1 (Sigma) was used as nontargeting control for siRNA experiments. HuTu-80 cells (90% confluent) were transiently transfected with the combination of three siRNA duplexes (1 μg of each; totally 3 μg of siRNAs/well of a 12-well plate) using Lipofectamine 2000. Assays for silencing were performed on confluent monolayers 24 h after transfection. siRNA assays were performed to determine PDZD11/hSMVT mRNA and protein expression level (by real-time PCR and Western blotting, respectively) and the effect of PDZD11 silencing on biotin uptake.
Real-time PCR analysis.
Total RNA (1 μg) was isolated from HuTu-80 cells, DNase I treated, and subjected to reverse transcription using SuperscriptII (Invitrogen) and oligo-dT primers. Then, the reverse transcription products were subjected to real-time PCR in a CFX96 real-time system (Bio-Rad, Hercules, CA) using iQ SYBR Green Supermix (Bio-Rad) and primers specific for hSMVT (forward: 5′-TGTCTACCTTCTCCATCATGGA-3′ and reverse: 5′-TAGAGCCCAATGGCAAGAGA-3′), PDZD11 (forward: 5′-CCCTCGAGCTATGGACAGCCGGATTCCTTATGATGACTAC-3′ and reverse: 5′-GGGGATCCGTGCACAGTCCTCTCTTTTTGGCGATG-3′), and 18S rRNA (forward: 5′-GGGAGGTAGTGACGAAAAATAACAAT-3′ and reverse: 5′-TTGCCCTCCAATGGATCCTC-3′). Real-time PCR conditions were as previously described (18), and data were normalized to 18S rRNA and then quantified using a relative relationship method supplied by the iCycler manufacture (Bio-Rad) described previously (16).
Uptake assays.
Biotin uptake experiments were performed on confluent monolayers of the human intestinal epithelial HuTu-80 cells 24 h posttransfection. Uptake was measured at 37°C in Krebs-Ringer buffer [containing (in mM) 133 NaCl, 4.93 KCl, 1.23 MgSO4, 0.85 CaCl2, 5 glucose, 5 glutamine, 10 HEPES, and 10 MES; pH 7.4]. Labeled and unlabeled biotin was added to the incubation medium at the onset of incubation, and uptake was examined during the initial linear period. The reaction was terminated by the addition of 2 ml of ice-cold buffer followed by immediate aspiration. Cells were then rinsed twice with ice-cold buffer, lysed with 1 ml of 1 N NaOH, neutralized with HCl, and then measured for radioactive content using a scintillation counter. The protein content of cell digests was measured in parallel wells using a Bio-Rad DC protein assay kit (Bio-Rad).
Cell surface protein biotinylation.
For biotinylation of cell surface proteins, the Pierce cell surface protein isolation kit (Pierce Biotechnology, Rockford, IL) was used according to manufacturer's recommendations. Briefly, HuTu-80 cells (90% confluent) were transiently transfected with the PDZD11 siRNA or control siRNA using Lipofectamine 2000. Twenty-four hours after transfection cells were labeled with EZ-Link Sulfo-NHS-SS-Biotin and lysed, and the equal amount of cell lysates (0.5 mg of total soluble protein) was used for the isolation of the biotinylated proteins with NeutrAvidin agarose. The bound proteins were released with sample loading buffer for SDS-PAGE and analyzed by Western blotting using anti-SMVT antibodies. Data were normalized to the total amount of cellular hSMVT.
Statistical analysis.
Transport data presented in this paper are the results of three separate experiments and are expressed as means ± SE (in fmol·mg protein−1 per unit time). Uptake of biotin by the carrier-mediated system was determined by subtracting the uptake by passive diffusion (determined from the slope of the line between uptake at a high pharmacological concentration of 1 mM and the point of origin) from the total uptake. Statistical analysis was performed by the Student's t-test or one-way ANOVA with statistical significance being set at P < 0.05. Western blot analysis, real-time PCR, uptake studies, and imaging studies were all performed on at least three separate occasions utilizing different samples. Data presented are from a representative set of experiments.
RESULTS
Identification of PDZD11 as the interacting partner for hSMVT by Y2H screening.
To identify interacting partner(s) for the human sodium-dependent multivitamin transporter hSMVT, the COOH-terminal cytosolic domain of transporter (amino acids 553–635) was used as a bait fragment in a LexA fusion construct for a Y2H screen against preys in a random-primed human intestinal cDNA library. We used the COOH terminus as the bait because recent studies from our laboratory (22) have shown that this sequence is important for the function of the hSMVT and its targeting to cell membrane. A total of 1.14 × 108 colonies were screened using a mating approach with Y187 (mat α) and L40ΔGal4 (mat a) yeast strains (6). Ninty-one His+ colonies were selected on a medium lacking tryptophan, leucine, and histidine. The prey fragments of the positive clones were amplified by PCR and sequenced at their 5′- and 3- junctions. The resulting sequences were used to identify the corresponding interacting proteins in the GenBank database (NCBI) using a fully automated procedure and were found to represent a total of 28 different proteins. With the use of bioinformatics analysis on each putative interacting protein (prey identification and sequencing, Selected Interacting Domain determination, functional annotation, and Predicted Biological Score calculation) (5), a positive clone, PDZD11 (also known as PISP/AIPP1; Refs. 7, 21), with high confidence of interaction was identified. In addition, a “one-by-one” Y2H interaction assay was performed between the bait N-LexA-hSMVT-C and N-GAL4 activation domain-PDZD11-C prey clone. The results of “one-by-one” Y2H interaction assay confirmed the solid colony growth for hSMVT-PDZD11 interaction pair (as well as for positive control) under restrictive growth conditions (selective medium lacking tryptophan, leucine, and histidine but containing 3-aminotriazole) compared with negative controls. Altogether, Y2H screening data clearly indicate that the PDZ-containing protein PDZD11 might be a promising interacting partner for hSMVT.
GST-PDZD11 fusion protein binds with hSMVT.
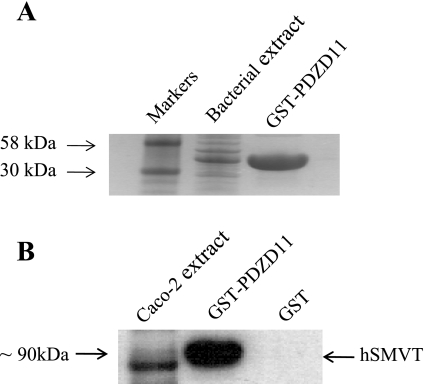
GST pull-down assay was used to further confirm the interaction between hSMVT and PDZD11 in vitro. We performed pull-down experiments with GST-PDZD11 affinity purified from BL-21 Escherichia coli (Fig. 1A) using extracts from human intestinal epithelial Caco-2 cells. The results showed that the GST-PDZD11 fusion protein but not GST alone (negative control) pulled down the hSMVT from the cell extract (Fig. 1B), suggesting specific interaction between PDZD11 and hSMVT in Caco-2 cells.
Fig. 1.
Pull-down of human sodium-dependent multivitamin transporter (hSMVT) from Caco-2 cells by GST-tagged PDZD11. A: generation and purification of GST-PDZD11. GST-PDZD11 fusion protein was produced in BL-21 Escherichia coli cells and purified as described in experimental procedures. Protein samples were run on NuPAGE 4–12% Bis-Tris gradient gels and stained with Coomassie blue. B: pull-down of hSMVT from Caco-2 cells by GST-PDZD11 but not by GST alone. Postnuclear extract of Caco-2 cells (1 mg of total soluble protein) was incubated with either GST-PDZD11 (100 μg) or GST (100 μg) bound to glutathione-Sepharose4B beads. Bound proteins were eluted with 10 mM glutathione and analyzed by Western blotting using the anti-hSMVT antibodies.
hSMVT and PDZD11 interact in vivo in mammalian two-hybrid luciferase assay.
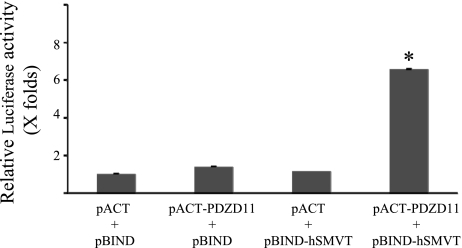
hSMVT and PDZD11, expressed as GAL4 and VP16 fusion constructs, respectively, were checked for possible interaction in CHO-K1 cells by using a mammalian two-hybrid system. CHO-K1 cells were cotransfected with pBIND-hSMVT and pACT-PDZD11 plasmids along with the pG5luc reporter vector, and Renilla-normalized Firefly Luciferase activity was determined after 72 h of transfection. The results (Fig. 2) showed that the luciferase activity in cells expressing GAL4-hSMVT and VP16-PDZD11 fusion constructs is significantly (P < 0.01) higher compared with negative controls (>6-fold increase over the negative controls). These data further suggest that the hSMVT interacts with PDZD11 in mammalian cells.
Fig. 2.
Interaction of hSMVT and PDZD11 in mammalian 2-hybrid luciferase assay. pBIND-hSMVT and pACT-PDZD11 were cotransfected into CHO-K1 cells along with pG5luc vector to generate fusion proteins. Renilla-normalized Firefly Luciferase activity was determined after 72 h of transfection using a dual luciferase assay system. Data are presented as means ± SE of at least 3 independent experiments and Firefly luciferase expression given in folds over the background (pACT/pBIND empty vectors), which was set at 1. Note that statistical SEs of the mean bars are not visible on the scale of the graph presented. *P < 0.01.
hSMVT and PDZD11 interact in vivo in human intestinal epithelial cells.
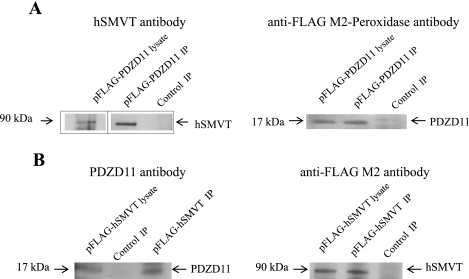
We also tried to confirm the interaction between hSMVT and PDZD11 in human intestinal epithelial cells by coimmunoprecipitation assay. hSMVT and PDZD11 were expressed as the FLAG-tagged fusion proteins in Caco-2 cells, and we examined the ability of these fusions to bind the endogenous PDZD11 and hSMVT, respectively. Nontransfected Caco-2 cells served as a negative control in our immunoprecipitation assay. Anti-FLAG M2 antibody-conjugated beads were incubated with extracts prepared from transfected and nontransfected cells, and then the immunoprecipitates were subjected to immunoblot analysis. The results (Fig. 3A) showed that the FLAG-PDZD11 immunoprecipitate contains the endogenous hSMVT. In a reciprocal coimmunoprecipitation assay (Fig. 3B), the FLAG-hSMVT immunoprecipitate contains the endogenous PDZD11. In contrast, neither hSMVT nor PDZD11 from nontransfected Caco-2 cells was found among the proteins bound to anti-FLAG M2 antibody-conjugated beads. These data suggest that hSMVT interacts with PDZD11 in vivo in human intestinal epithelial cells.
Fig. 3.
PDZD11 coimmunoprecipitates with hSMVT from Caco-2 cells. Immunoprecipitation of FLAG-PDZD11 (A) or FLAG-hSMVT (B) from Caco-2 cell lysates was performed on anti-FLAG M2 affinity gel, and immunoprecipitates (IP) were analyzed by immunoblotting using the antibodies indicated. Control IP, immunoprecipitate obtained from nontransfected cells. Note: The boxed lanes of A (left) are from the exact same gel with exact same exposure and are grouped together for clarity of data presentation.
hSMVT and PDZD11 colocalize in human intestinal epithelial cells.
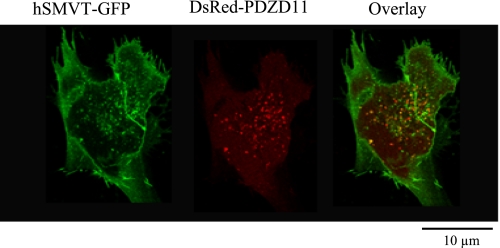
We next examined whether hSMVT and PDZD11 colocalized in human intestinal epithelial cells by means of live cell confocal microscopy. Human intestinal epithelial HuTu-80 cells were transiently cotransfected with hSMVT-GFP and pDsRed- C1-PDZD11 plasmids, and confocal imaging was performed 48 h posttransfection. hSMVT showed its expected pattern of localization being at the plasma membrane and in intracellular vesicular compartment (Fig. 4). As for PDZD11, the expression was found in the cytoplasm. Merged images of the cells showed significant colocalization of the two proteins (Fig. 4).
Fig. 4.
Subcellular colocalization of hSMVT and PDZD11 in HuTu-80 cells. HuTu-80 cells were transiently cotransfected with hSMVT-GFP and pDsRed-C1-PDZD11 plasmids, and 48 h after transfection, imaging was performed by confocal microscopy as described in experimental procedures.
PDZD11 affects biotin uptake by human intestinal epithelial cells.
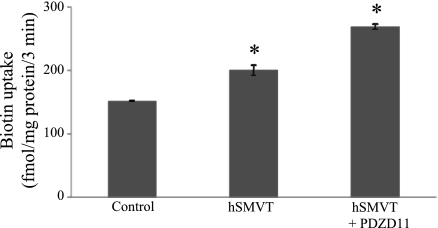
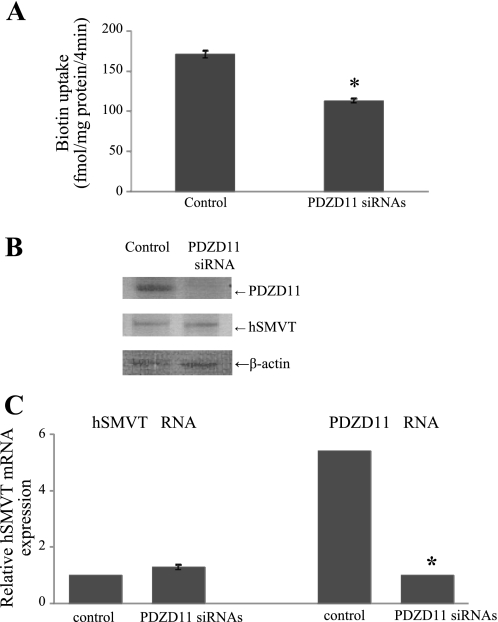
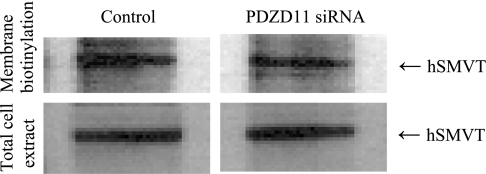
To study the consequences of the hSMVT/PDZD11 protein-protein interaction on the functionality of the hSMVT system, we first measured the carrier-mediated [3H]biotin uptake (9 nM; pH 7.4) in HuTu-80 cells overexpressing hSMVT and PDZD11. HuTu-80 cells were transiently cotransfected with the pcDNA3.1-hSMVT and pFLAG-CMV-2-PDZD11 expression plasmids followed by uptake measurement in 24 h posttransfection. Uptake results (Fig. 5) showed that coexpression of both proteins to lead to a significant (P < 0.01) stimulation in biotin uptake compared with cells transfected with hSMVT alone. To further confirm the contribution of PDZD11 to carrier-mediated biotin uptake by HuTu-80 cells, we examined the effect of silencing the PDZD11 gene by means of siRNA on the ability of the cells to transport the vitamin. The results (Fig. 6A) show that silencing of PDZD11 significantly (P < 0.01) inhibited carrier-mediated [3H]biotin (9 nM) uptake by HuTu-80 cells. The effectiveness and specificity of PDZD11 gene knockdown were verified by determining protein and mRNA expression level for PDZD11 as well as for hSMVT. Western blotting data (Fig. 6B) and real-time PCR data (Fig. 6C) showed the marked reduction of PDZD11 protein and mRNA expression level, respectively, in PDZD11 siRNA-pretreated cells compared with cells pretreated with the nontargeting negative siRNA control (control cells). Importantly, no reduction in hSMVT protein and mRNA expression level was detected in the PDZD11 siRNA-pretreated cells compared with control cells, indicating the specificity of the observed effect. To further understand the reasons for the observed biotin uptake inhibition, the cell surface expression level of hSMVT was examined in PDZD11 siRNA-pretreated HuTu-80 cells. To achieve this, we performed the biotinylation of cell surface proteins in PDZD11 siRNA-pretreated as well as in control cells and compared the cell surface level of hSMVT in those cells (data were normalizied to the total cellular level of hSMVT). The results (Fig. 7) showed that cell surface expression of hSMVT significantly (P < 0.05) decreased in PDZD11 siRNA-pretreated cells compared with cells pretreated with the nontargeting negative siRNA control. No significant difference, however, was found in the total cellular hSMVT level of expression between control and PDZD11 siRNA-pretreated cells. Altogether, these data suggest that PDZD11 appears to influence the biotin uptake through affecting the hSMVT protein functionality via a mechanism that may involve protein delivery and/or retention at the plasma membrane.
Fig. 5.
Overexpression of PDZD11 increases biotin uptake in HuTu-80 cells. HuTu-80 cells were transiently cotransfected with the pcDNA3.1-hSMVT and pFLAG-CMV-2-PDZD11 expression plasmids followed by carrier-mediated [3H]biotin uptake (9 nM; pH 7.4; 3 min) measurement in 24 h posttransfection. Control, nontransfected cells. Values are means ± SE of at least 3 independent experiments. hSMVT alone induced significant (*P < 0.01) induction in biotin uptake compared with control. PDZD11 when coexpressed with hSMVT caused significant (*P < 0.01) increase in biotin uptake compared with hSMVT alone.
Fig. 6.
Silencing of PDZD11 with small interfering (si)RNAs decreases carrier-mediated biotin uptake in HuTu-80 cells. HuTu-80 cells were treated with the PDZD11-specific siRNAs or with the nontargeting negative control siRNA (control) as described in experimental procedures. A: initial rate of carrier-mediated biotin uptake by HuTu-80 cells. Cells were incubated at 37°C in Krebs-Ringer buffer (pH 7.4). [3H]biotin (9 nM) was added to the incubation medium with or without unlabeled biotin at the onset of incubation. Uptake was measured after 4 min of incubation. Carrier-mediated component was calculated as described in experimental procedures. Data are means ± SE of at least 3 separate uptake determinations. B: Western blot analysis was performed using 30 μg protein from the cell lysates. Blot was probed with the polyclonal antibodies directed against PDZD11 or hSMVT, and data were normalized to the amount of β-actin. Data presented are from the representative set of experiments. C: total RNA was isolated and real-time PCR was performed using gene-specific primers. Data are from 3 different experiments and expressed relative to 18S rRNA as means ± SE. Note that some statistical SEs of the mean bars are not visible on the scale of the graph presented. *P < 0.01.
Fig. 7.
Effect of PDZD11 siRNA on the cell surface expression level of hSMVT. HuTu-80 cells were transiently transfected with the PDZD11 siRNA or control siRNA (control). Twenty four hours after transfection cells were labeled with EZ-Link Sulfo-NHS-SS-Biotin and lysed, and the equal amount of cell lysates (0.5 mg of total soluble protein) was used for the isolation of the biotinylated proteins with NeutrAvidin agarose. The bound proteins were released with sample loading buffer for SDS-PAGE and analyzed by Western blotting using anti-SMVT antibodies (top). Data were normalized to the total amount of cellular hSMVT (bottom). Image shown represents 3 separate sets of experiments.
PDZ binding domain of hSMVT is involved in hSMVT/PDZD11 interaction in human intestinal epithelial cells.
The hSMVT polypeptide contains a PDZ binding domain in its COOH terminus (amino acids 632–635) that comprise E-T-S-L amino acids. To determine the requirement of this domain for the interaction between hSMVT and PDZD11, we performed coimmunoprecipitation experiments on extracts from Caco-2 cells expressing FLAG-wild-type hSMVT or FLAG-hSMVT lacking three amino acids (T-S-L) from the PDZ binding domain. The results (Fig. 8) showed that the wild-type hSMVT immunoprecipitate, but not the truncated hSMVT immunoprecipitate, contains PDZD11. These data indicate that the interaction between hSMVT and PDZD11 in human intestinal epithelial cells occurs through this PDZ binding domain of hSMVT.
Fig. 8.
PDZ binding domain of hSMVT is involved in interaction between hSMVT and PDZD11 in HuTu-80 cells. Immunoprecipitation of FLAG-hSMVT (wild type or lacking T-S-L peptide corresponding to 633–635 amino acid sequence of hSMVT) from Caco-2 cell lysates was performed on anti-FLAG M2 affinity gel, and immunoprecipitates were analyzed by immunoblotting using the antibodies indicated.
DISCUSSION
The intestine plays a central role in regulating body biotin homeostasis since it is the normal route through which the vitamin is obtained from exogenous sources. This regulation appears to utilize an efficient uptake mechanism mediated by the SMVT system. While significant information has been generated in recent years dealing with the physiology and cell biology of SMVT and its transcriptional regulation (3, 4, 15, 17, 18, 22), much less is known about possible involvement of accessory protein(s) that may interact with this membrane carrier and influence its physiology/biology at the posttranslational level. We addressed this issue in this study by using the Y2H approach to screen a human intestinal cDNA library and have identified a PDZ-containing protein, PDZD11, as an interacting partner for hSMVT. Recent studies (7, 21) have shown that PDZD11 interacts with other membrane proteins, namely the Ca2+-ATPase (PMCA) and the Menkes copper ATPase (ATP7A) and influence their biology. PDZD11 is a ubiquitously expressed small protein (140 amino acids) that is composed mainly of a single PDZ domain. Proteins containing PDZ domains have been reported to be involved in protein transport/targeting events and in the processes of formation/stabilization of functional protein complexes (reviewed in Refs. 8, 24). PDZ domains appear to recognize specific COOH-terminal motifs on partner proteins. The hSMVT polypeptide has a class I PDZ binding domain (with the sequence ETSL) located at its COOH-terminal tail. It is important to mention here that we used the full COOH-terminal tail (amino acids 553–635) of the hSMVT protein (i.e., with its PDZ binding domain) as a bait in our library screening.
The interaction between hSMVT and PDZD11 initially identified in our Y2H screening was further confirmed by multiple experimental approaches, namely in vitro GST-pull-down assay and in vivo in a mammalian cell environment by two-hybrid luciferase and coimmunoprecipitation assays. Furthermore, confocal imaging of living human intestinal epithelial monolayer expressing hSMVT-GFP and DsRed-PDZD11 demonstrated colocalization of the two proteins, thus further supporting existence of direct interaction between them.
Following demonstration that direct interaction between hSMVT and PDZD11 exists both in vitro and in vivo, we examined the effect of this protein-protein interaction on functionality ([3H]biotin uptake) of the hSMVT system in human intestinal epithelial HuTu-80 cells. Thus we transfected these cells with the recombinant PDZD11 along with hSMVT and then examined the initial rate of biotin uptake. The results showed a significant induction in carrier-mediated biotin uptake in cells expressing both the PDZD11 and hSMVT compared with those expressing the hSMVT alone, clearly suggesting a role for PDZD11 in hSMVT function in human intestinal epithelial cells. We also tested the effect of knocking down the endogenous PDZD11 by means of gene-specific siRNA approach on initial carrier-mediated biotin uptake by HuTu-80 cells. We validated the effectiveness of our siRNA approach and its specificity by demonstrating a significant reduction of PDZD11 protein/RNA level, but not hSMVT, in cells treated with the gene-specific siRNA. The results showed a significant decrease in biotin uptake by HuTu-80 cells in siRNA-treated cells confirming the importance of PDZD11 in hSMVT transport physiology. Since PDZD11 colocalized with hSMVT in the intracellular vesicular compartment in HuTu-80 cells, it is possible that the effect of PDZD11 is mediated via influencing cell biology of the hSMVT protein as suggested in the case of two other membrane transporters shown to interact with PDZD11, namely the Ca2+-ATPase and the Menkes copper ATPase (7, 21). To further understand the role of PDZD11 in hSMVT cell biology, we performed cell surface biotinylation assay with PDZD11 siRNA-treated HuTu-80 cells and evaluated plasma membrane expression of hSMVT. We found a significant decrease in the hSMVT plasma membrane level under conditions of PDZD11 knockdown, indicating that PDZD11 affects the biotin uptake process through the mechanism that may involve hSMVT protein delivery and/or retention at the plasma membrane.
Since the hSMVT polypeptide contains PDZ binding domain (with the sequence ETSL) located at its COOH-terminal tail, we next examined the involvement of this domain in hSMVT/PDZD11 protein-protein interaction. Our coimmunoprecipitation data obtained from Caco-2 cells expressing wild-type hSMVT, or truncated hSMVT lacking PDZ binding domain, clearly indicate that the interaction between hSMVT and PDZD11 in human intestinal epithelial cells occurs through the PDZ binding domain of hSMVT.
In summary, our data report for the first time the identification of PDZD11 as an interacting protein partner with the hSMVT in human intestinal epithelial cells, which appear to play a role in hSMVT transport physiology and cell biology.
GRANTS
This work was supported by a grant from the Department of Veterans Affairs and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-58057 and DK-56061.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1.Ashokkumar B, Nabokina SM, Ma TY, Said HM. Identification of dynein light chain road block-1 as a novel interaction partner with the human reduced folate carrier. Am J Physiol Gastrointest Liver Physiol 297: G480–G487, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baéz-Saldaña A, Díaz G, Espinoza B, Ortega E. Biotin deficiency induces changes in subpopulations of spleen lymphocytes in mice. Am J Clin Nutr 67: 431–437, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Balamurugan K, Ortiz A, Said HM. Biotin uptake by human intestinal and liver epithelial cells: role of the SMVT system. Am J Physiol Gastrointest Liver Physiol 285: G73–G77, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Dey S, Subramanian VS, Chatterjee NS, Rubin SA, Said HM. Characterization of the 5′ regulatory region of the human sodium-dependent multivitamin transporter, hSMVT. Biochim Biophys Acta 1574: 187–192, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Formstecher E, Aresta S, Collura V, Hamburger A, Meil A, Trehin A, Reverdy C, Betin V, Maire S, Brun C, Jacq B, Arpin M, Bellaiche Y, Bellusci S, Benaroch P, Bornens M, Chanet R, Chavrier P, Delattre O, Doye V, Fehon R, Faye G, Galli T, Girault JA, Goud B, de Gunzburg J, Johannes L, Junier MP, Mirouse V, Mukherjee A, Papadopoulo D, Perez F, Plessis A, Rossé C, Saule S, Stoppa-Lyonnet D, Vincent A, White M, Legrain P, Wojcik J, Camonis J, Daviet L. Protein interaction mapping: a Drosophila case study. Genome Res 15: 376–384, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fromont-Racine M, Rain JC, Legrain P. Toward a functional analysis of the yeast genome through exhaustive two-hybrid screens. Nat Genet 16: 277–282, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Goellner GM, DeMarco SJ, Strehler EE. Characterization of PISP, a novel single-PDZ protein that binds to all plasma membrane Ca2+-ATPase b-splice variants. Ann NY Acad Sci 986: 461–471, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Kiela PR, Ghishan FK. Ion transport in the intestine. Curr Opin Gastroenterol 25: 87–91, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matnhey KC, Griffin JB, Zempleni J. Biotin supply affects expression of biotin transporters, biotinylation of carboxylases and metabolism of interleukin-2 in Jurkat cells. J Nutr 132: 887–892, 2002 [DOI] [PubMed] [Google Scholar]
- 10.McMahon RJ. Biotin in metabolism and molecular biology. Annu Rev Nutr 22: 221–239, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Mock D. Biotin: Physiology, dietary sources and requirements. In: Encyclopedia of Human Nutrition, edited by Caballero B, Allen L, Prentice A. London: Elsevier, 2005 [Google Scholar]
- 12.Munehira Y, Ohnishi T, Kawamoto S, Furuya A, Shitara K, Imamura M, Yokota T, Takeda S, Amachi T, Matsuo M, Kioka N, Ueda K. Alpha1-syntrophin modulates turnover of ABCA1. J Biol Chem 279: 15091–15095, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Ortiz DF, Moseley J, Calderon G, Swift AL, Li S, Arias IM. Identification of HAX-1 as a protein that binds bile salt export protein and regulates its abundance in the apical membrane of Madin-Darby canine kidney cells. J Biol Chem 279: 32761–32770, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Prasad PD, Wang H, Kekuda R, Fujita T, Fei YJ, Devoe LD, Leibach FH, Ganapathy V. Cloning and functional expression of a cDNA encoding a mammalian sodium-dependent vitamin transporter mediating the uptake of pantothenate, biotin, and lipoate. J Biol Chem 273: 7501–7506, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Prasad PD, Wang H, Huang W, Fei YJ, Leibach FH, Devoe LD, Ganapathy V. Molecular and functional characterization of the intestinal Na+-dependent multivitamin transporter. Arch Biochem Biophys 366: 95–106, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Reidling JC, Nabokina SM, Balamurugan K, Said HM. Developmental maturation of intestinal and renal thiamin uptake: studies in wild-type and transgenic mice carrying human THTR-1 and -2 promoters. J Cell Physiol 206: 371–377, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Reidling JC, Said HM. Regulation of the human biotin transporter hSMVT promoter by KLF-4 and AP-2: confirmation of promoter activity in vivo. Am J Physiol Cell Physiol 292: C1305–C1312, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Reidling J, Nabokina SM, Said HM. Molecular mechanisms involved in the adaptive regulation of human intestinal biotin uptake: A study of the hSMVT system. Am J Physiol Gastrointest Liver Physiol 292: G275–G281, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Said HM, Redha R, Nylander W. Biotin transport in the human intestine: inhibition by anticonvulsant drugs. Am J Clin Nutr 49: 127–131, 1989 [DOI] [PubMed] [Google Scholar]
- 20.Said HM. Cell and molecular aspects of human intestinal biotin absorption. J Nutr 139: 158–162, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephenson SE, Dubach D, Lim CM, Mercer JF, La Fontaine S. A single PDZ domain protein interacts with the Menkes copper ATPase, ATP7A. A new protein implicated in copper homeostasis. J Biol Chem 280: 33270–33279, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Subramanian VS, Marchant JS, Boulware MJ, Ma TY, Said HM. Membrane targeting and intracellular trafficking of the human sodium-dependent multivitamin transporter in polarized epithelial cells. Am J Physiol Cell Physiol 296: C663–C671, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun AQ, Balasubramaniyan N, Liu CJ, Shahid M, Suchy FJ. Association of the 16-kDa subunit c of vacuolar proton pump with the ileal Na+-dependent bile acid transporter: protein-protein interaction and intracellular trafficking. J Biol Chem 279: 16295–16300, 2004 [DOI] [PubMed] [Google Scholar]
- 24.van Ham M, Hendriks W. PDZ domains-glue and guide. Mol Biol Rep 30: 69–82, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Wilson MC, Meredith D, Fox JE, Manoharan C, Davies AJ, Halestrap AP. Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: the ancillary protein for the insensitive MCT2 is EMBIGIN (gp70). J Biol Chem 280: 27213–27221, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Wolf B. Disorders of biotin metabolism. In: The Metabolic and Molecular Bases of Inherited Disease, edited by Scriver CR, Beaudet AL, Sly WS, Valle D. New York: McGraw-Hill Medical Publishing Division, 2001 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.