Abstract
NHE8, the newest member of the sodium/hydrogen exchanger family, is expressed in the epithelial cells of the intestine and the kidney. Intestinal expression of NHE8 is significantly higher than that of NHE2 and NHE3 at a young age, suggesting that NHE8 is an important player for intestinal sodium absorption during early development. The current study was designed to explore if NHE8 plays a compensatory role for the loss of NHE2 and NHE3 function in NHE2X3 double-knockout (NHE2X3 DKO) mice. We further explored the regulatory mechanism(s) responsible for the change in NHE8 expression in NHE2X3 DKO mice. We found that >95% of NHE2X3 DKO mice survived through weanling. However, only 60% of male NHE2X3 DKO mice and 88% of female NHE2X3 DKO mice survived to 6 wk of life. We also found that the expression of NHE8 in wild-type female mice was higher compared with wild-type male mice after puberty. In NHE2X3 KDO mice, NHE8 expression was increased in females but not in males. Using Caco-2 cells as a model of the small intestine, we showed that testosterone inhibited endogenous NHE8 expression by reducing NHE8 mRNA synthesis, whereas estrogen had no effect on NHE8 expression. Thus our data show for the first time that intestinal NHE8 has a compensatory role in NHE2X3 DKO mice and this regulation is gender-dependent.
Keywords: intestine, sodium/hydrogen exchanger 8, sex hormone, gender
sodium absorption occurs in the small intestine, and this process is mediated by several transporter families, including the sodium/hydrogen exchangers (NHEs). The NHEs are plasma membrane-bound antiporters that mediate the movement of extracellular sodium ions in cells in exchange for intracellular hydrogen ions. To date, nine mammalian NHEs have been discovered. These proteins have broad physiological functions, including intracellular pH homeostasis, cell volume regulation, acid-base regulation, and electroneutral NaCl transport. Three out of the nine NHEs (NHE2, -3, and -8) are expressed at the apical membrane of enterocytes (29, 31). These apically expressed NHEs have distinct transporter kinetic characteristics and expression patterns during development. NHE2 is highly sensitive to the inhibitor HOE-694, whereas NHE3 is highly sensitive to the inhibitor S-3226, but NHE8 is sensitive to both inhibitors (8, 28). The expression of NHE8 in the small intestine is higher at a young age and declines in adults; in contrast, the expression of NHE2 and NHE3 is higher after weanling but is lower at the young age (6, 7, 28, 29).
NHE2 is expressed at the apical membrane of the intestinal epithelia, and, at first, it was thought to play a role in intestinal sodium absorption. However, interruption of NHE2 function in mice displays no obvious small intestinal phenotype such as diarrhea and sodium malabsorption (14, 24). Therefore, NHE2 does not participate in intestinal Na+ absorption. In contrast, NHE3 knockout (KO) mice display diarrhea and impaired acid-base balance, suggesting an important role for NHE3 in intestinal and renal Na+ absorption (25). Furthermore, NHE3 does not play a compensatory role in the loss of NHE2 KO mice and vice versa NHE2 cannot compensate for the loss of NHE3 (14). Because NHE3 KO mice survive to adulthood regardless of diarrhea and impaired acid-base balance, another mechanism must compensate for the loss of NHE3. In fact, an amiloride-sensitive Na+ absorption was detected in NHE3KO mice that contributed to ∼30% of Na+ absorption (11). Because this observation was reported before the discovery of NHE8, we hypothesized that NHE8 might be the unidentified amiloride-sensitive NHE activity observed in NHE3 KO mice, and NHE8 could partially compensate for the loss of NHE3.
In the present study, we created NHE2X3 double-knockout (DKO) mice to study the possible compensatory role of NHE8 in the small intestine. Our results indicated that NHE8 expression was decreased by ∼54% in male mice and by ∼30% in female mice from 4 to 6 wk of age. In NHE2X3 DKO mice, NHE8 expression was increased in both male and female mice at 4 wk of age, but this increase was abolished in 6-wk-old male mice. Furthermore, testosterone treatment inhibited NHE8 expression at RNA and protein levels while estradiol treatment had no effect on NHE8 expression in Caco-2 cells. These observations suggested that NHE8 would partially compensate for the loss of NHE2 and NHE3, but this compensatory mechanism was inhibited by testosterone.
MATERIALS AND METHODS
Animals.
NHE2X3 DKO were produced by breeding NHE2+/−/ NHE3+/− mice together. To increase the number of NHE2X3 DKO mice, NHE2−/−/NHE3+/− breeding pairs were also used. Body weight was recorded at 3, 4, and 6 wk of age. Survival rate was calculated by recoding the percentage of surviving mice at each age group. Serum was collected for electrolyte analysis. Jejunal mucosa scrapings were collected and used for mRNA purification and brush-border membrane vesicle (BBMV) isolation. Electrolyte measurement was performed by the university animal care pathology services laboratory (the University of Arizona, Tucson, AZ). All animal work was approved by the University of Arizona Institutional Animal Care and Use Committee.
Cell culture.
Human intestinal epithelial cells (Caco-2) were purchased from American Type Culture Collection (ATCC, Manassas, VA) and cultured according to ATCC guidelines. Cells were cultured at 37°C in a 95% air-5% CO2 atmosphere and passaged every 48–72 h. For hormone treatment experiments, cells were incubated with 100 nM estradiol (Sigma-Aldrich, St. Louis, MO) or testosterone (Sigma-Aldrich) for 18 h. For gene transcription study, cells were pretreated with actinomycin D (100 nM) for 2 h before the addition of hormones.
Protein purification and western blot analysis.
BBMVs were prepared from mouse jejunal mucosa as previously described (29). Total protein was prepared from cultured Caco-2 cells with RIPA buffer (7). BBMV protein (30 μg) and total protein (40 μg) were used for Western blot. NHE8 antibody was used as a 1:3,000 dilution in these experiments (29). A 1:5,000 dilution of the β-actin antiserum (Sigma-Aldrich) was used to determine β-actin protein abundance. Western detection was performed with the BM Chemiluminescence Western Blotting Kit (Roche Diagnostics, Indianapolis, IN). A ratio of NHE8 protein intensity over β-actin protein intensity was used for protein expression quantitation.
RNA purification and PCR analysis.
RNA was purified from mouse jejunal mucosa and Caco-2 cells using Trizol reagent (Invitrogen, Carlsbad, CA). Total RNA (500 ng) was reverse-transcribed using the qScript kit (Quanta Biosciences, Gaithersburg, MD), and 10% of the reverse transcription reaction was used for real-time PCR. TaqMan technology was used to determine the expression levels of NHE8 using mouse and human NHE8 and TATA-binding protein (TBP) primers from Applied Biosystems (Foster City, CA). Resulting data were analyzed using the comparative cycle threshold (Ct) method. The target gene Ct values are adjusted relative to a calibrator (normalized Ct value obtained from control groups) and expressed as 2−ΔΔCt (Applied Biosystems User Bulletin no. 2: Rev B “Relative Quantitation of Gene Expression”). TBP data were used as an endogenous reference to normalize expression levels.
Transient transfection and functional promoter analysis.
Caco-2 cells were cultured in 24-well plates. When cell density reached 60–70%, Caco-2 cells were transfected with human NHE8 gene promoter constructs (pGL3b/−89 and pGL3b/−671) (27) using Effectene (Qiagen, Valencia, CA) according to the manufacturer's instruction. Cells were harvested for promoter reporter assays 40 h after transfection. Promoter reporter assay was performed using a dual luciferase assay kit according to the manufacturer's instruction (Promega). Luciferase activities were measured with a luminometer (Femtomaster FB 12; Berthold Detection System, Pforzheim, Germany). Renilla luciferase activity driven by pRL-CMV (Promega) was used as an internal control to calculate the relative luciferase activity. To test the effect of testosterone on human NHE8 promoter activity, transfected cells were treated with 100 nM testosterone for 18 h before promoter reporter assay.
Statistical analysis.
Student's t-test was used to compare values of the experimental data. P values <0.05 were considered significant.
RESULTS
Growth and survival rate in NHE2X3 DKO mice.
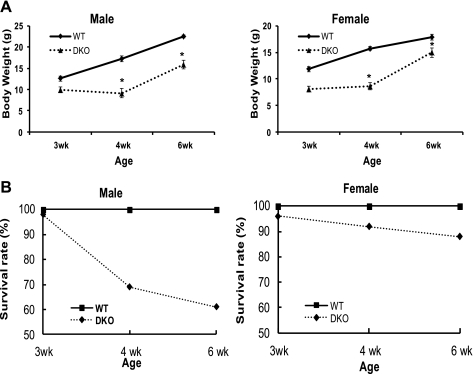
NHE2X3 DKO mice were produced by breeding NHE2+/− × NHE3+/− and NHE2−/− × NHE3+/− mice. About two hundred NHE2X3 DKO mice were collected in this study. Body weight and survival rate were recorded at the ages of 3, 4, and 6 wk. Serum sodium level was also measured at the age of 4 and 6 wk. As shown in Fig. 1A, NHE2X3 DKO mice have lower body weight compared with their wild-type littermates. The body weight of male NHE2X3 DKO mice was reduced by ∼22%, ∼47%, and ∼30% at 3, 4, and 6 wk of age, respectively, compared with male wild-type littermates. The body weight of female NHE2X3 DKO mice was reduced by ∼32%, ∼45%, and ∼16% at 3, 4, and 6 wk of age, respectively, compared with female wild-type littermates. The greatest reduction on gaining body weight occurred between the age of 3 and 4 wk in NHE2X3 DKO mice. At the same time, the survival rate is higher in the female compared with the male double-knockout mice. In male NHE2X3 DKO mice, the survival rate was reduced to 70% at the age of 4 wk and 60% at the age of 6 wk. In female NHE2X3 DKO mice, the survival rate was reduced to 92% at the age of 4 wk and 88% at the age of 6 wk (Fig. 1B). Furthermore, the serum sodium level in NHE2X3 DKO mice was lower than their wild-type littermates at 4 and 6 wk of age (Table 1).
Fig. 1.
Body weight and survival curve of NHE2X3 DKO mice. Body weight and survival rate were recorded at the age of 3, 4, and 6 wk. A total of 83 NHE2X3 DKO mice and 128 wild-type mice were used. A: body weight curve of male and female NHE2X3 DKO mice. Data are presented as means ± SE for each age group. WT, wild-type mice; DKO, NHE2X3 DKO mice. B: survival curve of male and female NHE2X3 DKO mice. Data are presented as percentage for each age group. *P < 0.05 for NHE2X3 DKO mice vs. wild type.
Table 1.
Serum sodium ion concentration*
| Age |
||
|---|---|---|
| Mouse | 4wk | 6wk |
| Wild type | 153 ± 2 (n = 11) | 161 ± 1 (n = 6) |
| NHE2X3 DKO | 133 ± 5 (n = 12) | 138 ± 6 (n = 6) |
| P Value | 0.003 | 0.009 |
Values are means ± SE; n, no. of mice. Units are mM. NHE2X3 DKO, sodium/hydrogen exchanger double knockout.
The normal range of serum sodium ion concentration is 140–160 mM.
Intestinal NHE8 mRNA expression in wild-type mice.
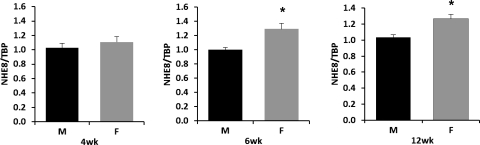
Four-, 6-, and 12-wk-old male and female mice were killed. RNA was purified from mouse jejunal mucosa, and real-time PCR was performed to determine the abundance of NHE8 mRNA. As indicated in Fig. 2, NHE8 mRNA expression was similar between 4-wk-old males and females (1.02 ± 0.07 in male mice and 1.10 ± 0.07 in female mice; n = 28 mice). By 6 wk of age, NHE8 expression was higher in female mice compared with male mice (1.01 ± 0.04 in male mice and 1.30 ± 0.08 in female mice; P < 0.01; n = 26 mice). At 12 wk of age, NHE8 expression was higher in female mice compared with male mice (1.05 ± 0.07 in male mice and 1.28 ± 0.02 in female mice; P < 0.01; n = 18 mice).
Fig. 2.
NHE8 mRNA expression in wild-type male and female mice. RNAs were isolated from the intestinal mucosa of male and female mice at the age of 4, 6, and 12 wk. Real-time PCR was performed using mouse-specific NHE8 and TATA-binding protein (TBP) primers. Data are means ± SE for each age group. *P ≤ 0.01 for female mice vs. male mice. Twenty-eight 4-wk-old mice (12 males, 16 females), 26 6-wk-old mice (13 for each gender), and 18 12-wk-old mice (9 for each gender) were used. M, male mice; F, female mice.
Intestinal NHE8 protein expression in wild-type mice.
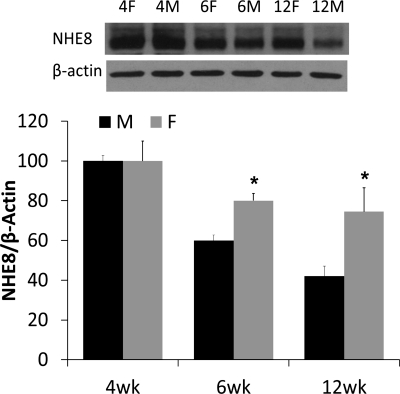
Male and female mice at the age of 4, 6, and 12 wk were killed, and BBMV protein was isolated from mouse jejunal mucosa. BBMV protein was then used for Western blot to determine NHE8 protein abundance. As shown in Fig. 3, NHE8 protein level was the same between male and female mice at 4 wk of age. The abundance of NHE8 at the age of 6 wk was reduced by ∼40% in male mice and by ∼20% in female mice compared with values at 4 wk of age. At 12 wk of age, the abundance of NHE8 was further reduced by ∼60% in male mice and by ∼25% in female mice compared with values at 4 wk of age. The overall protein expression level of NHE8 is reduced more dramatically in male mice than that in female mice at 6 wk of age (P < 0.01, n = 4 groups, 4–5 mice in each group).
Fig. 3.
NHE8 protein expression in wild-type male and female mice. Brush-border membrane vesicles (BBMVs) were isolated from the intestinal mucosa of male and female mice at the age of 4, 6, and 12 wk. BBMV protein (30 μg) was loaded on SDS-PAGE, and immunoblots were performed. The expression of NHE8 protein is calculated by the optical density of NHE8 band over that of the β-actin band. Bar chart shows the NHE8 protein expression indicated as means ± SE in the sum of four independent experiments. *P ≤ 0.01 for female mice vs. male mice. Inset: corresponding Western blot images.
Intestinal NHE8 expression in 4-wk-old NHE2X3 DKO mice.
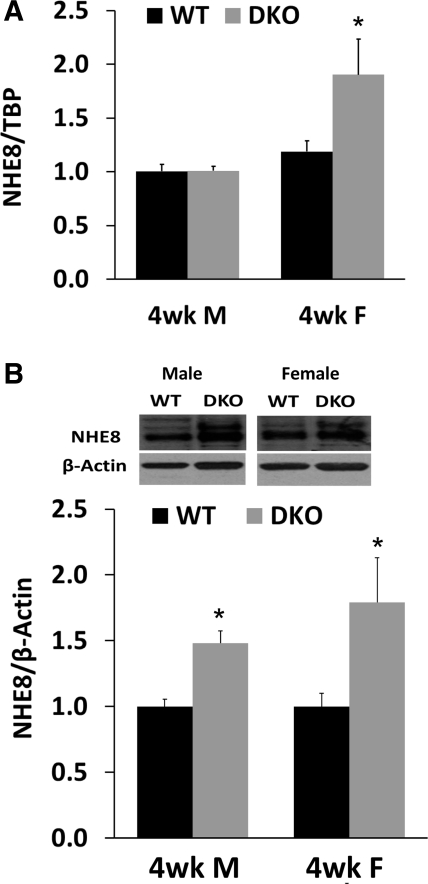
Jejunal mucosa was collected from 4-wk-old wild-type and NHE2X3 DKO mice. RNA was prepared and used for real-time PCR. BBMV was isolated for Western blot. As shown in Fig. 4, the expression of NHE8 mRNA in male mice was similar between wild-type and the NHE2X3 DKO mice (1.00 ± 0.06 in wild-type male mice, 1.10 ± 0.03 in NHE2x3 DKO male mice; n = 29 mice). In female mice, NHE8 mRNA abundance was higher in NHE2X3 DKO mice compared with wild-type mice (1.01 ± 0.04 in wild-type female mice, 1.90 ± 0.33 in NHE2X3 DKO female mice; P < 0.01; n = 30 mice) (Fig. 4A). The expression of BBM NHE8 protein was also significantly increased in both male and female NHE2X3 DKO mice compared with their wild-type littermates (1.0 ± 0.05 and 1.0 ± 0.10 in wild-type males and females, 1.5 ± 0.10 and 1.75 ± 0.45 in NHE2X3 DKO males and females; P < 0.01; n = 4 groups, 5 mice in each group) (Fig. 4B).
Fig. 4.
NHE8 expression in 4-wk-old NHE2X3 DKO mice. A: NHE8 mRNA expression in 4-wk-old male and female mice. RNA was isolated from the intestinal mucosa of 4-wk-old male and female wild-type mice and NHE2X3 DKO mice. Real-time PCR was performed using mouse-specific NHE8 and TBP primers. The changes in NHE8 gene expression are analyzed by the comparative cycle threshold (Ct) method. Data are means ± SE for each age group. *P ≤ 0.01 for NHE2X3 DKO mice vs. wild-type mice. Thirty wild-type mice (15 males, 15 females) and 29 NHE2X3 DKO mice (14 males, 15 females) were used. B: NHE8 protein expression in 4-wk-old male and female mice. BBMV was isolated from the intestinal mucosa of 4-wk-old male and female wild-type mice and NHE2X3 DKO mice. Western blot was used to detect NHE8 and β-actin protein abundances in BBMV preparations. The expression of NHE8 protein is calculated by the optical density of the NHE8 band over that of the β-actin band. Data are means ± SE for each gender group. *P ≤ 0.01 for NHE2X3 DKO mice vs. wild-type mice. Three independent experiments were performed. Inset: corresponding Western blot images.
Intestinal NHE8 expression in 6-wk-old NHE2X3 DKO mice.
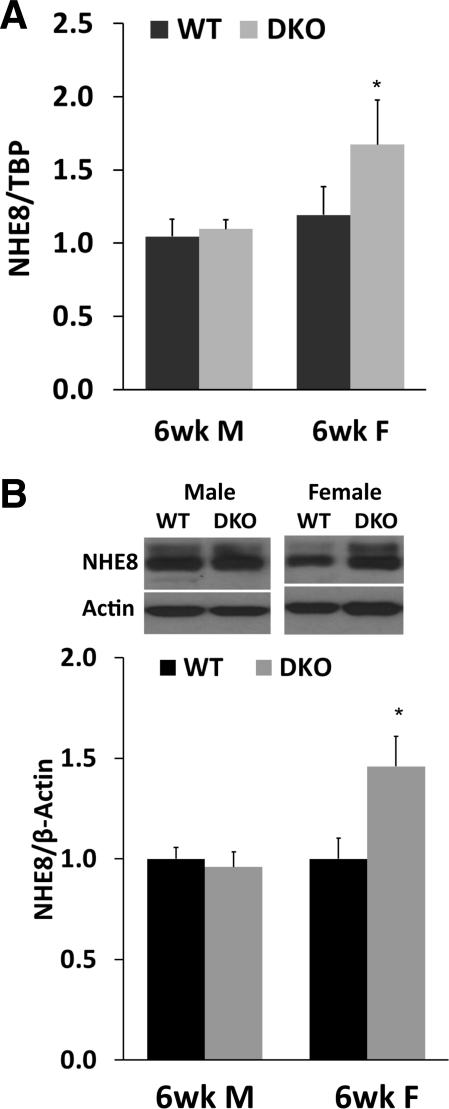
Jejunal mucosa was collected from 6-wk-old wild-type and NHE2X3 DKO mice. RNA was prepared and used for real-time PCR. BBMV was isolated for Western blot. As shown in Fig. 5A, NHE8 expression in male mice was similar between wild-type mice and NHE2X3 DKO mice (1.015 ± 0.12 in wild-type male mice, 1.19 ± 0.09 in NHE2x3 DKO male mice; n = 18). The expression of NHE8 mRNA in NHE2X3 DKO female mice was significantly higher compared with their wild-type female mice (1.10 ± 0.06 in wild-type mice, 1.67 ± 0.303 in NHE2X3 DKO; P < 0.01; n = 12). NHE8 protein expression in female NHE2X3 DKO mice was also increased compared with their female wild-type mice (1.00 ± 0.10 in wild-type mice, 1.50 ± 0.25 in NHE2X3 DKO mice; P < 0.01; n = 4 groups, 4 mice in each group). The expression of NHE8 protein in male NHE2X3 DKO mice remained unchanged compared with their wild-type mice (1.0 ± 0.10 in wild-type mice, 0.97 ± 0.15 in NHE2X3 DKO mice; n = 3 groups, 4 mice in each group) (Fig. 5B).
Fig. 5.
NHE8 expression in 6-wk-old NHE2X3 DKO mice. A: NHE8 mRNA expression in 6-wk-old NHE2X3 DKO mice. RNA was isolated from the intestinal mucosa of 6-wk-old mice. Real-time PCR was performed using mouse-specific NHE8 and TBP primers. The changes in NHE8 gene expression are analyzed by the comparative Ct method. Data are means ± SE for each age group. *P ≤ 0.01 for NHE2X3 DKO mice vs. wild-type mice. Eighteen wild-type mice (9 males, 9 females) and 12 NHE2X3 DKO mice (6 males, 6 females) were used. B: NHE8 protein expression in 6-wk-old NHE2X3 DKO mice. BBMV was isolated from the intestinal mucosa of 6-wk-old mice. Western blot was used to detect NHE8 and β-actin protein abundances in BBMV preparations. The expression of NHE8 protein is calculated by the optical density of NHE8 band over that of the β-actin band. Data are means ± SE for each gender group. *P ≤ 0.01 for NHE2X3 DKO mice vs. wild-type mice. Three independent experiments were performed. Inset: corresponding Western blot images.
Effect of sex hormones on NHE8 expression in Caco-2 cells.
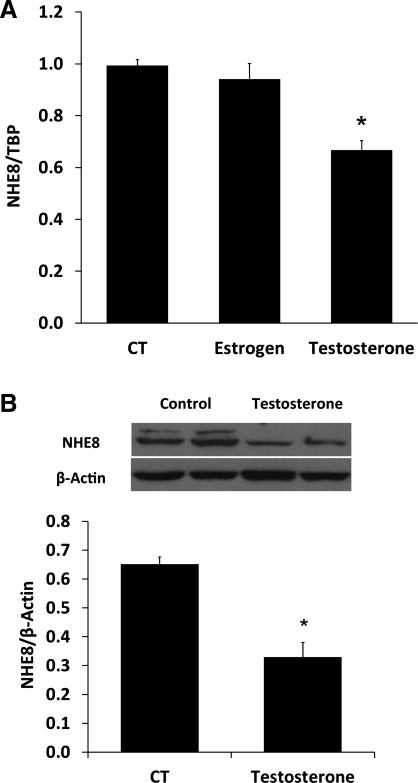
Real-time PCR and Western blotting were used to assess the expression of endogenous NHE8 in Caco-2 cells. Testosterone treatment (100 nM, 18 h) reduced NHE8 gene expression by ∼40% in Caco-2 cells compared with untreated cells (n = 4; p < 0.01) while estradiol treatment (100 nM, 18 h) had no effect on NHE8 expression (Fig. 6A). At NHE8 protein levels, testosterone treatment also reduced NHE8 protein expression by ∼50% in Caco-2 cells (1.0 ± 0.05 in control cells; 0.35 ± 0.10 in testosterone cells; n = 3; P < 0.05) (Fig. 6B).
Fig. 6.
Effect of sex hormone on NHE8 expression in human intestinal epithelial cells. Caco-2 cells were cultured in standard medium or sex hormone-containing medium (100 nM) for 18 h before harvest. RNAs were isolated from cells and were used for RT-PCR. Real-time PCR was performed with human NHE8 or TBP primers in separate reactions. Total protein was prepared from cells and used for Western blot. Results are means ± SE from 3 separate experiments. *P < 0.01 for control (CT) vs. testosterone treatment. A: effect of sex hormones on NHE8 mRNA expression in Caco-2 cells. B: effect of testosterone on NHE8 protein expression in Caco-2 cells.
Effect of testosterone on human NHE8 gene transcription in Caco-2 cells.
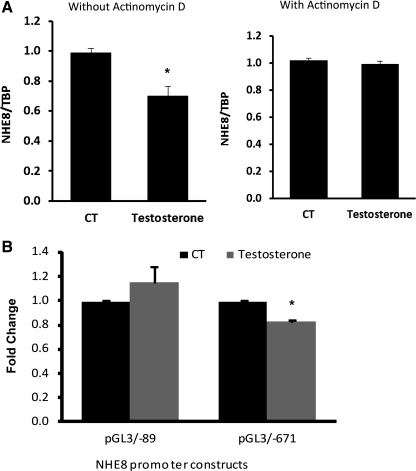
To explore if testosterone-mediated NHE8 expression downregulation is due to reduced gene transcription, Caco-2 cells were treated with 100 nM testosterone for 18 h in the presence or absence of actinomycin D (100 nM). In the absence of actinomycin D treatment, testosterone inhibited NHE8 gene expression by ∼40%. In the presence of actinomycin D, the inhibitory effect of testosterone on NHE8 mRNA expression was completely abolished (Fig. 7A). Furthermore, transfection with NHE8 gene promoter constructs (pGL3b/−89 and pGL3b/−671) showed that testosterone inhibited NHE8 gene promoter activity by ∼20% in transfected Caco-2 cells, and this inhibition occurred only in promoter construct pGL3/−671 but not pGL3/−89 (Fig. 7B).
Fig. 7.
The mechanism of testosterone effect on human NHE8 gene expression. A: actinomycin D's effect on testosterone-mediated NHE8 gene expression inhibition. Caco-2 cells were cultured in standard medium or testosterone-containing medium (100 nM) in the presence or absence of actinomycin D for 18 h before harvest. RNAs were isolated from these cells. Real-time PCR was performed with human NHE8 or TBP primers in separate reactions. Results are means ± SE from 3 separate experiments. *P < 0.01 for control vs. testosterone treatment. B: cells were cotransfected with pRL-CMV and pGL3 basic (pGL3b) or human NHE8 promoter constructs (pGL3b/−89 and pGL3b/−671). Testosterone was applied 18 h before measuring promoter activities. Promoter activity is shown as a relative activity, which is a ratio of firefly luciferase activity driven by NHE8 promoter over Renilla luciferase activity driven by CMV promoter. The degree of inhibition is shown as the ratio of luciferase activity in testosterone-treated cells over luciferase activity in vehicle-treated cells. Results are means ± SE from 4 separate experiments.
DISCUSSION
The nine identified mammalian NHE isoforms have different functions, tissue distribution, and membrane localization (31). Of these nine NHEs, only four of them are expressed in the small intestine. NHE1 is expressed at the basolateral membrane of the intestinal epithelial cells, and NHE2, NHE3, and NHE8 are expressed at the apical membrane of the intestinal epithelial cells (30, 31). Knockout animal models revealed that NHE2 plays little or no role in intestinal sodium absorption while NHE3 is the main contributor of the intestinal and renal sodium absorption (11, 14, 24, 25). NHE8 is highly expressed at early life, and it has been suggested to have a role in intestinal sodium absorption during early development when NHE2 and NHE3 are expressed at very low levels (28, 29).
Disruption of NHE3 function results in diarrhea and impaired acid-base balance in mice (25). Studies in NHE3 KO mice suggested that an unidentified NHE is expressed in the gut, and this NHE contributes to ∼30% of sodium absorption (11, 14). In our initial work, we found that NHE8 expression was increased in young NHE3 KO mice. To further study the role of NHE8 in the intestine, we created a NHE2X3 DKO mouse model. Body weight and serum sodium level of NHE2X3 DKO mice were lower than their wild-type littermates. Almost no body weight was gained in NHE2X3 DKO mice between 3 and 4 wk of age. This period in development coincides with a dramatic increase in NHE3 expression (7). These observations suggested that NHE3 is important for the development after weanling. After 4 wk of age, the NHE2X3 DKO mice started to gain body weight again with the rate similar to their wild-type littermates. By 6 wk of age, the body weights of male and female NHE2X3 DKO mice were ∼70% and ∼84% of the wild-type mice, respectively. Interestingly, only 60% of male NHE2X3 DKO mice survived after 6 wk of age while 88% of female NHE2X3 DKO mice survived after 6 wk of age, which indicated that NHE8 expression may be regulated differently in male and female mice. A previous study by another group reported ∼79% survival in NHE2X3 DKO mice (14). However, this study used a very small number of animals (24 NHE2X3 DKO mice), and there was no gender description for these mice. If the male and the female mice in our study were combined, the total survival rate would be 74%, which is very close to the survival rate reported by the earlier group.
To understand if the low survival rate in male NHE2X3 DKO mice was due to low NHE8 expression, we first compared the expression pattern of NHE8 in wild-type male and female mice. Our data indicated that NHE8 expression indeed differs between males and females. At 4 wk of age, NHE8 expression was the same between male and female mice. However, at 6 wk of age, NHE8 expression in male mice was 30% lower than in female mice. By 12 wk of age, NHE8 expression in male mice was 50% lower than in female mice. These observations suggested that NHE8 expression is regulated by a gender-specific mechanism and female mice have higher NHE8 expression levels. We therefore compared NHE8 regulation in NHE2X3 DKO male and female mice. Our results showed that NHE8 was upregulated in female NHE2X3 DKO mice, but this compensatory mechanism was absent in male NHE2X3 DKO mice. In female NHE2X3 DKO mice, NHE8 mRNA and protein expression increased >50% compared with wild-type female mice. In male NHE2X3 DKO mice, NHE8 mRNA expression level remained unchanged, but the protein level increased by ∼50% only in 4-wk-old mice. The levels of NHE8 expression correlated with the survival rate in NHE2X3 DKO mice. These observations indicated that NHE8 was upregulated in NHE2X3 DKO mice, and this compensatory mechanism was gender-specific. Interestingly, NHE8 expression was also increased in young NHE3 KO mice but not in adult NHE3 KO male mice (data not shown). It is possible that other sodium transporters instead of NHE8 compensate for the loss of NHE3 in adult NHE3 KO mice, such as the epithelial sodium channel protein (25).
Gender-dependent gene expression has been observed in mammals, and this difference is linked to sex hormones. Testosterone is the principal male sex hormone and is an anabolic steroid. It is found in mammals, reptiles, birds, and other vertebrates (9, 22). In mammals, testosterone is primarily secreted in the testes of males and the ovaries of females. In men, testosterone plays a key role in the development of male reproductive tissues such as the testis and prostate as well as promoting secondary sexual characteristics such as increased muscle, bone mass, and hair growth (17). Although testosterone is a male-specific steroid hormone, it has been known to regulate certain transporter expression via a gender-specific manner. In male rodents, testosterone inhibits sodium-glucose cotransporter expression by reducing SGLT-1 mRNA abundance (23). Testosterone also stimulates epithelial sodium channel protein (10, 13, 18–21), organic anion transporters (2–5, 12, 15, 16), and organic cation transporters (1, 26). All of this testosterone-mediated transporter gene regulation occurred in the kidney but not in the small intestine.
In the current study, we observed the gender-specific expression of NHE8 in the small intestine in mice. To understand the mechanism of gender-specific NHE8 expression, we tested the effect of sex hormones on NHE8 expression in Caco-2 cells. Estradiol had no effect on NHE8 expression in Caco-2 cells, but testosterone significantly reduced NHE8 expression at both mRNA and protein expression levels. These observations indicated that testosterone was indeed an important regulator of NHE8 expression in the intestine. To further understand if this inhibition is mediated by transcriptional regulation, we treated cells with actinomycin D. Actinomycin D treatment completely blocked the inhibitory effect of testosterone on NHE8 expression in Caco-2 cells, indicating that the inhibitory effect of testosterone on NHE8 occurred at the gene transcription level. Transfection with human NHE8 gene promoter constructs in Caco-2 cells showed a similar response of NHE8 promoter activity in the promoter construct pGL3/−671 but not pGL3/−89, suggesting that the testosterone-responsive region is located between −671 and −89 bp of the human NHE8 gene. Interestingly, no androgen receptor (AR) binding motif was found in this human NHE8 gene promoter region, which suggested an AR-independent mechanism for testosterone's regulation of NHE8 gene expression. Future study is forthcoming to identify the mechanism involved.
In summary, we have shown that the intestinal NHE8 expression exhibits a gender difference after puberty, and this difference was mediated by the inhibitory effect of testosterone. Furthermore, NHE8 could partially compensate for the loss of NHE3 in NHE2X3 DKO female mice. These studies, for the first time, directly demonstrated that the intestinal NHE8 expression regulation was gender-specific, and testosterone is an important regulator of NHE8 gene in the intestine.
GRANTS
This investigation was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-073638 and R01-DK-041274.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Alnouti Y, Petrick JS, Klaassen CD. Tissue distribution and ontogeny of organic cation transporters in mice. Drug Metab Dispos 34: 477– 482, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Buist SC, Cherrington NJ, Choudhuri S, Hartley DP, Klaassen CD. Gender-specific and developmental influences on the expression of rat organic anion transporters. J Pharmacol Exp Ther 301: 145– 151, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Buist SC, Cherrington NJ, Klaassen CD. Endocrine regulation of rat organic anion transporters. Drug Metab Dispos 31: 559– 564, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Buist SC, Klaassen CD. Rat and mouse differences in gender-predominant expression of organic anion transporter (Oat1-3; Slc22a6-8) mRNA levels. Drug Metab Dispos 32: 620– 625, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Cheng X, Klaassen CD. Tissue distribution, ontogeny, and hormonal regulation of xenobiotic transporters in mouse kidneys. Drug Metab Dispos 37: 2178– 2185, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collins JF, Kiela PR, Xu H, Zeng J, Ghishan FK. Increased NHE2 expression in rat intestinal epithelium during ontogeny is transcriptionally mediated. Am J Physiol Cell Physiol 275: C1143– C1150, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Collins JF, Xu H, Kiela PR, Zeng J, Ghishan FK. Functional and molecular characterization of NHE3 expression during ontogeny in rat jejunal epithelium. Am J Physiol Cell Physiol 273: C1937– C1946, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Counillon L, Scholz W, Lang HJ, Pouyssegur J. Pharmacological characterization of stably transfected Na+/H+ antiporter isoforms using amiloride analogs and a new inhibitor exhibiting anti-ischemic properties. Mol Pharmacol 44: 1041– 1045, 1993 [PubMed] [Google Scholar]
- 9. Cox RM, John-Alder HB. Testosterone has opposite effects on male growth in lizards (Sceloporus spp) with opposite patterns of sexual size dimorphism. J Exp Biol 208: 4679– 4687, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Gambling L, Dunford S, Wilson CA, McArdle HJ, Baines DL. Estrogen and progesterone regulate alpha, beta, and gammaENaC subunit mRNA levels in female rat kidney. Kidney Int 65: 1774– 1781, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Gawenis LR, Stien X, Shull GE, Schultheis PJ, Woo AL, Walker NM, Clarke LL. Intestinal NaCl transport in NHE2 and NHE3 knockout mice. Am J Physiol Gastrointest Liver Physiol 282: G776– G784, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Isern J, Hagenbuch B, Stieger B, Meier PJ, Meseguer A. Functional analysis and androgen-regulated expression of mouse organic anion transporting polypeptide 1 (Oatp1) in the kidney. Biochim Biophys Acta 1518: 73– 78, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Kienitz T, Allolio B, Strasburger CJ, Quinkler M. Sex-specific regulation of ENaC and androgen receptor in female rat kidney. Horm Metab Res 41: 356– 362, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Ledoussal C, Woo AL, Miller ML, Shull GE. Loss of the NHE2 Na+/H+ exchanger has no apparent effect on diarrheal state of NHE3-deficient mice. Am J Physiol Gastrointest Liver Physiol 281: G1385– G1396, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Ljubojevic M, Herak-Kramberger CM, Hagos Y, Bahn A, Endou H, Burckhardt G, Sabolic I. Rat renal cortical OAT1 and OAT3 exhibit gender differences determined by both androgen stimulation and estrogen inhibition. Am J Physiol Renal Physiol 287: F124– F138, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Lu R, Kanai N, Bao Y, Wolkoff AW, Schuster VL. Regulation of renal oatp mRNA expression by testosterone. Am J Physiol Renal Fluid Electrolyte Physiol 270: F332– F337, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens. Endocr Rev 8: 1– 28, 1987 [DOI] [PubMed] [Google Scholar]
- 18. Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension 37: 1199– 1208, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Reckelhoff JF, Granger JP. Role of androgens in mediating hypertension and renal injury. Clin Exp Pharmacol Physiol 26: 127– 131, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension 35: 480– 483, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Reckelhoff JF, Zhang H, Srivastava K, Granger JP. Gender differences in hypertension in spontaneously hypertensive rats: role of androgens and androgen receptor. Hypertension 34: 920– 923, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Reed WL, Clark ME, Parker PG, Raouf SA, Arguedas N, Monk DS, Snajdr E, Nolan V, Jr, Ketterson ED. Physiological effects on demography: a long-term experimental study of testosterone's effects on fitness. Am Nat 167: 667– 683, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Sabolic I, Skarica M, Gorboulev V, Ljubojevic M, Balen D, Herak-Kramberger CM, Koepsell H. Rat renal glucose transporter SGLT1 exhibits zonal distribution and androgen-dependent gender differences. Am J Physiol Renal Physiol 290: F913– F926, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Schultheis PJ, Clarke LL, Meneton P, Harline M, Boivin GP, Stemmermann G, Duffy JJ, Doetschman T, Miller ML, Shull GE. Targeted disruption of the murine Na+/H+ exchanger isoform 2 gene causes reduced viability of gastric parietal cells and loss of net acid secretion. J Clin Invest 101: 1243– 1253, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282– 285, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Urakami Y, Nakamura N, Takahashi K, Okuda M, Saito H, Hashimoto Y, Inui K. Gender differences in expression of organic cation transporter OCT2 in rat kidney. FEBS Lett 461: 339– 342, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Xu H, Chen H, Dong J, Li J, Chen R, Uno JK, Ghishan FK. Tumor necrosis factor-α downregulates intestinal NHE8 expression by reducing basal promoter activity. Am J Physiol Cell Physiol 296: C489– C497, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu H, Chen H, Dong J, Lynch R, Ghishan FK. Gastrointestinal distribution and kinetic characterization of the sodium-hydrogen exchanger isoform 8 (NHE8). Cell Physiol Biochem 21: 109– 116, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Xu H, Chen R, Ghishan FK. Subcloning, localization, and expression of the rat intestinal sodium-hydrogen exchanger isoform 8. Am J Physiol Gastrointest Liver Physiol 289: G36– G41, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Yun CH, Tse CM, Nath SK, Levine SA, Brant SR, Donowitz M. Mammalian Na+/H+ exchanger gene family: structure and function studies. Am J Physiol Gastrointest Liver Physiol 269: G1– G11, 1995 [DOI] [PubMed] [Google Scholar]
- 31. Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411– 443, 2005 [DOI] [PubMed] [Google Scholar]