Abstract
Serotonin transporter (SERT) regulates extracellular availability of serotonin and is a potential pharmacological target for gastrointestinal disorders. A decrease in SERT has been implicated in intestinal inflammatory and diarrheal disorders. However, little is known regarding regulation of SERT in the intestine. Epidermal growth factor (EGF) is known to influence intestinal electrolyte and nutrient transport processes and has protective effects on intestinal mucosa. Whether EGF regulates SERT in the human intestine is not known. The present studies examined the regulation of SERT by EGF, utilizing Caco-2 cells grown on Transwell inserts as an in vitro model. Treatment with EGF from the basolateral side (10 ng/ml, 24 h) significantly stimulated SERT activity (∼2-fold, P < 0.01) and mRNA levels compared with control. EGF increased the activities of the two alternate promoter constructs for human SERT gene: SERT promoter 1 (hSERTp1, upstream of exon 1a) and SERT promoter 2 (hSERTp2, upstream of exon 2). Inhibition of EGF receptor (EGFR) tyrosine kinase activity by PD168393 (1 nM) blocked the stimulatory effects of EGF on SERT promoters. Progressive deletions of the SERT promoter indicated that the putative EGF-responsive elements are present in the −672/−472 region of the hSERTp1 and regions spanning −1195/−738 and −152/+123 of hSERTp2. EGF markedly increased the binding of Caco-2 nuclear proteins to the potential AP-1 cis-elements present in EGF-responsive regions of hSERTp1 and p2. Overexpression of c-jun but not c-fos specifically transactivated hSERTp2, with no effects on hSERTp1. Our findings define novel mechanisms of transcriptional regulation of SERT by EGF via EGFR at the promoter level that may contribute to the beneficial effects of EGF in gut disorders.
Keywords: serotonin transport, SERT, c-jun, AP1, EGF
the gastrointestinal tract is a major source of endogenous serotonin (5-HT), where it plays critical roles in regulation of motility as well as fluid and electrolyte transport (3, 21, 37). High levels of this amine have been implicated in several inflammatory and diarrheal conditions including carcinoid syndrome and enterotoxin-induced diarrhea (42). The extracellular availability of 5-HT is regulated by serotonin transporter (SERT), which functions to rapidly internalize 5-HT via a Na+- and Cl−-dependent process. This mechanism of deactivation of 5-HT is necessary to terminate 5-HT signaling and prevent the 5-HT receptors from desensitization (27).
Previous studies from our laboratory and others have demonstrated that SERT is functionally present in human intestinal epithelial cells (20, 27, 28) and is localized predominantly to the apical membranes (20). Downregulation of SERT is implicated in the pathophysiology of various functional gut disorders, although the mechanisms underlying are not fully understood. For example, SERT expression is reduced in experimentally induced colitis (9) and in the gut of patients with ulcerative colitis and irritable bowel syndrome (IBS) (10, 15). Accordingly, transgenic mice with targeted deletion of SERT frequently exhibit diarrhea interspersed with transient constipation (8). Moreover, deletion of SERT increases susceptibility to colitis in IL-10-deficient mice (23). In addition, we previously demonstrated (13) that a decrease in SERT function and expression may underlie diarrhea associated with infection by an important foodborne pathogen, enteropathogenic Escherichia coli. Furthermore, proinflammatory cytokines such as TNF-α and IFN-γ either alone or in combination have been shown to decrease SERT expression in Caco-2 cells (17). Given that SERT downregulation contributes to pathophysiology of gut disorders, agents that increase SERT function and consequently decrease 5-HT levels offer potential for therapeutic modalities in the gut. However, such strategies harnessing the regulatory mechanisms that increase SERT function and expression in intestinal epithelial cells have not been investigated. Interestingly, transcription of human SERT gene is under the control of two alternate promoters, i.e., the previously identified upstream promoter (hSERTp1) and the recently identified intestine-specific promoter (hSERTp2), both of which are active in intestinal epithelial cells (25). However, no studies are currently available on the transcriptional regulation of SERT gene at the promoter level. The present studies were undertaken to examine the modulation of SERT gene expression by an important growth factor, epidermal growth factor (EGF), and to delineate the mechanisms involved.
EGF is an important 53-amino acid polypeptide and growth hormone secreted by salivary glands, liver, pancreas, kidney, and intestine (7). EGF mediates its effects via binding to its receptor, EGFR, expressed on the basolateral surface in human intestinal epithelial cells (7, 30, 31). EGF exhibits a wide range of physiological effects including stimulation of electrolyte and nutrient absorption and protective effects on intestinal mucosa in colitis such as the necrotizing colitis model and in human subjects (2, 14, 18, 33, 35, 36, 43, 44, 46, 48). Our studies demonstrate for the first time that EGF upregulates SERT function and expression via EGFR and through mechanisms involving potential AP-1 cis-elements of the SERT promoters. Although EGF appears to induce the activity of both the SERT promoters, stimulation of hSERTp2 appears to partly involve c-jun, whereas the transcription factor mediating effects on hSERTp1 remains to be identified. Therefore, our findings suggest that EGF effects on the alternate SERT promoters occur via distinct mechanisms that may be exploited to upregulate SERT function and expression in the treatment of intestinal disorders where SERT is downregulated.
METHODS
Cell culture.
Caco-2 cells were grown in T-75 (75 cm2) plastic flasks at 37°C in a 5% CO2 environment. The culture medium consisted of high-glucose MEM, 20% fetal bovine serum, 20 mM HEPES, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Cells used for these studies were between passages 25 and 45 and were plated on 12-well Transwell filters at a density of 2 × 104 cells/well. Cells were used for experiments at days 10–14 after plating. Fully differentiated Caco-2 monolayers were treated with EGF (1–10 ng/ml) from the basolateral side for time periods ranging from 1 to 24 h in the serum-free cell culture medium supplemented with 0.1% bovine serum albumin (BSA). In experiments involving promoter studies, Caco-2 cells were transiently cotransfected with SERT promoter constructs or c-fos and c-jun expression vectors by electroporation utilizing Amaxa technology (Amaxa) and plated in Transwell plates.
[3H]serotonin uptake.
Uptake of [3H]5-HT from the apical compartment of Caco-2 cells in response to EGF was measured by assessing Na+-dependent and Cl−-dependent [3H]5-HT uptake as previously described by us (13). [3H]5-HT uptake was initiated by the addition of 300 μl of uptake buffer containing 25–50 nM [3H]5-HT for a time period of 5 min (linear range of uptake). The uptake was stopped by washing the cells twice with 1 ml of ice-chilled 1× PBS. The cells were then solubilized in 0.5 ml of 0.5 N NaOH overnight at 4°C. The incorporated radioactivity was measured with a liquid scintillation counter (Packard), and protein was estimated by the method of Bradford (6).
Real-time RT-PCR studies.
The relative abundance of SERT mRNA in control and EGF-treated Caco-2 cells was quantitated by real-time RT-PCR. Histone was used as an internal control. Briefly, RNA was extracted from Caco-2 cells with a total RNA kit according to the manufacturer's instructions (Stratagene). cDNA formation and PCR amplification were carried out with the SYBR Green one-step real-time PCR master mix and the Mx3000p machine (Stratagene). The primers for hSERT and β-actin or histone were as described previously (12, 20).
Promoter cloning and plasmid construction.
SERT gene is under the control of two alternate promoters 1) upstream of exon 1a (referred to here as hSERTp1) and 2) upstream of exon 2 and extending upstream of exon 1c (25) (referred to here as hSERTp2). The 5′-flanking regions of hSERT gene were cloned [−872/+2 for hSERTp1 relative to the transcription initiation site and −1195/+123 for hSERTp2 (where +1 is the start of exon 2)] by PCR utilizing human genomic DNA, gene-specific primers, and Elongase enzyme mix (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. For both hSERTp1 and hSERTp2, the forward primers were designed to contain an internal site for the KpnI restriction enzyme (underlined) and the reverse primers contained a site for the HindIII enzyme (underlined). The sequences for cloning full-length promoters and progressive deletions are given in Table 1.
Table 1.
Primers used for cloning of SERT promoter constructs
| Primer Sequence | |
|---|---|
| hSERTp1 | |
| hSERTp1 (−872/+2) | 5′-CGGGGTACCACTCCCGGGCTCAGCTGATCCTCCAC-3′ |
| hSERTp1A (−672/+2) | 5′-CGGGGTACCAGCTTTGAACTGTAGCTGGTTAACAA-3′ |
| hSERTp1B (−472/+2) | 5′-CGGGGTACCCGGGATGGGGACGATGGGGAGGTGTC-3′ |
| hSERTp1C (−272/+2) | 5′-CGGGGTACCGCTCCTCCCTGCGAGCGTGTGTGTGT-3′ |
| Reverse primer | 5′-CCCAAGCTTTTGTGCGGAGGGGCGCCGG-3′ |
| hSERTp2 | |
| hSERTp2 (1995/+123) | 5′-CGGGGTACCATAGGCCGGAGGGAAGTACAGAGG-3′ |
| hSERTp2A (−738/+123) | 5′-CGGGGTACCGGTGAGCAGCAGTGCCGTTTAAGG-3′ |
| hSERTp2B (−152/+123) | 5′-CGGGGTACCGGACTGCCATGTAGCAAATAGG-3′ |
| Reverse primer | 5′-CCCAAGCTTTCCTGCTGGTTAGTAAATGACA-3′ |
The two alternate promoters of serotonin transporter (SERT) gene, upstream of exon 1a-(referred here as hSERTp1) and upstream of exon 2 and extending upstream of exon 1c (referred here as hSERTp2) were cloned by PCR utilizing gene-specific primers. For both hSERTp1 and hSERTp2, the forward primers were designed to contain an internal site for the KpnI restriction enzyme (underlined) and the reverse primers contained a site for the HindIII enzyme (underlined).
The PCR amplified products were excised from 1% agarose gel and purified with the Sephaglas BandPrep Kit (Amersham Pharmacia Biotech, Piscataway, NJ). The amplified 5′-flanking regions (hSERTp1 and hSERTp2) were then digested with KpnI and HindIII enzymes and cloned into luciferase reporter gene vector pGL-2 basic (Promega, Madison, WI). The orientation and the sequence of the insert were confirmed by sequencing.
Transient transfection and luciferase assay.
For transfection studies, Caco-2 cells were transfected with FuGENE as previously described (1, 38). In some sets of experiments the Amaxa Nucleofector System was utilized for transfection according to the manufacturer's instructions (1, 38). Briefly, for Amaxa electroporation 1 × 106 cells were trypsinized and then electroporated in 100 μl of solution T (supplied by Amaxa for Caco-2 cells) with hSERTp-luciferase constructs (30 μg) and 2.0 μg of pCMVβ (β-galactosidase mammalian expression vector; Clontech, Mountain View, CA) for 24 wells. At 24 h after transfection, cells were treated basolaterally with EGF (10 ng/ml) for 24 h in serum-free medium containing 0.1% BSA. In some experiments, along with 10 μg of SERT promoter construct and 1.0 μg of pCMVβ (β-galactosidase mammalian expression vector), 20 μg of mammalian expression vectors for c-fos and c-jun were cotransfected per 24 well. The c-jun and c-fos vectors were a generous gift from Dr. Nancy Colburn, National Cancer Institute (Frederick, MD). Control cells without c-jun or c-fos expression were transfected with equal amounts of empty vector pcDNA3.1. Cells were then plated on Transwell inserts in 20% FBS containing Caco-2 medium. At 24–48 h after transfection, cells were lysed in reporter lysis buffer and the activities of both firefly luciferase (Promega) and β-galactosidase (Clontech) were measured by luminometer according to the manufacturer's instructions with commercially available kits. The promoter activity was expressed as a ratio of luciferase to β-galactosidase activity (relative luciferase activity) in each sample.
Nuclear extracts and electrophoretic mobility shift assay.
Nuclear extracts were prepared from control or EGF-treated Caco-2 cells grown on Transwells with a commercially available kit (Thermo Scientific, Rockford, IL). The sequences of the potential AP-1 binding sites utilized for electrophoretic mobility shift assay (EMSA) are as follows: hSERTp1: −545 to −525: CAGCCGGTCAGTCAGATAAAC; hSERTp2: proximal AP-1 (−978 to −951): AGAGCTGAGCTGACTTCCCTGCGCCCAG, distal AP-1 (−1479 to −1437): AAACCCTAGTGACTGACATTGCCTGGTG.
AP-1 consensus double-stranded oligonucleotide (Santa Cruz Biotechnology) and duplex oligonucleotides of potential AP-1 binding sites of both promoters (custom synthesized from IDT, San Diego, CA) were end labeled with T4-polynucleotide kinase and [γ-32P]ATP (Amersham, Arlington Heights, IL). DNA/protein binding reactions were performed by EMSA as previously described (1, 26, 38). For some experiments, EMSA was performed by a nonradioactive digoxigenin (DIG) labeling method utilizing a commercially available kit from Roche Diagnostics (Mannheim, Germany).
Statistical analysis.
Results are expressed as means ± SE. Student's t-test or one-way ANOVA was utilized for statistical analysis. A P value ≤ 0.05 was considered statistically significant.
RESULTS
Effects of EGF on SERT function and mRNA levels.
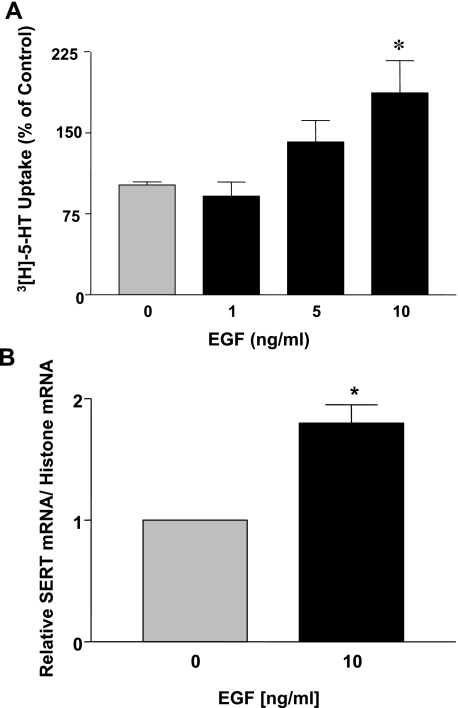
Previous studies have demonstrated that EGF influences intestinal epithelial transport processes by both rapid posttranslational events involving phosphorylation and/or transcriptional mechanisms via alteration in gene expression (2, 18, 33, 35, 36, 43, 44, 46, 48). Therefore, we first examined short-term effects of EGF on SERT function. Caco-2 monolayers were treated with EGF (5–10 ng/ml) from the basolateral side for a time period of 1 h, and SERT function was assessed by [3H]5-HT uptake from the apical side. There was no significant difference in SERT activity in response to EGF compared with control (Table 2). These data indicate that SERT function is not altered by acute treatment with EGF in human intestinal epithelial cells. To examine the long-term effects, Caco-2 monolayers were treated basolaterally with EGF (1–10 ng/ml) for a time period of 24 h. Treatment with both 5 and 10 ng/ml EGF increased SERT function, with no alteration observed at 1 ng/ml (Fig. 1A). For all subsequent experiments the 10 ng/ml EGF concentration was utilized. To examine whether long-term EGF treatment affects SERT mRNA expression, real-time RT-PCR using hSERT and histone (internal control) gene-specific primers was utilized. Corresponding with the function, SERT mRNA levels were significantly increased by approximately twofold in response to 24-h incubation of Caco-2 monolayers with EGF (Fig. 1B). These results indicate that EGF upregulates SERT gene expression.
Table 2.
SERT function is not altered by acute treatment with EGF
| Treatment, 10 ng/ml | SERT function, % of control |
|---|---|
| 0 | 100 ± 3.5 |
| 5 | 95 ± 2.9 |
| 10 | 110 ± 13.7 |
Data are shown as mean ± SE % of control values obtained from at least 3 different experiments performed in triplicate. Caco-2 cells grown on Transwell inserts were treated with epidermal growth factor for a time period of 1 h from the basolateral side in serum-free Caco-2 medium supplemented with 0.1% bovine serum albumin (BSA). SERT function was measured as [3H]serotonin uptake from the apical side.
Fig. 1.
Epidermal growth factor (EGF) treatment of Caco-2 cells increases serotonin transporter (SERT) function and expression. Caco-2 cells grown on Transwell inserts were treated with EGF (1–10 ng/ml, 24 h) from the basolateral side in serum-free Caco-2 medium supplemented with 0.1% bovine serum albumin (BSA). A: SERT function was measured as [3H]serotonin ([3H]5-HT) uptake from the apical side. Data are shown as % of control (means ± SE of values obtained from at least 3 different experiments performed in triplicate). Absolute mean control value: 0.57 pmol·mg protein−1·5 min−1. *P < 0.01 compared with control. B: total RNA was extracted, and quantitative real-time RT-PCR was performed with SYBR Green fluorescent dye. Human SERT (hSERT) mRNA levels were normalized to the levels of histone mRNA. Data are shown as means ± SE of values obtained from at least 4 different experiments performed in triplicate. *P < 0.01 compared with control.
Effect of EGF on promoter activity of hSERT.
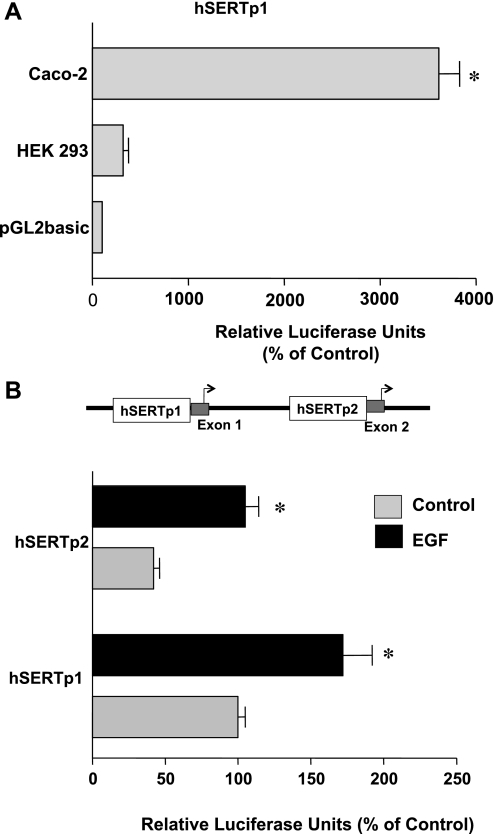
Given that SERT mRNA expression was increased in response to EGF, we next examined whether EGF influences SERT promoter activity. SERT gene is under the control of two different alternate promoters (25). For these experiments, we cloned the two previously identified hSERT alternate promoters: hSERTp1 (flanking a region between −872 and +2, upstream of exon 1a) and a −1995 bp fragment of the recently identified intestine-specific promoter that we named hSERTp2 (upstream of exon 2 and extending upstream of exon 1c). These promoter constructs were transiently cotransfected into Caco-2 cells along with the β-galactosidase mammalian expression vector as an internal control to adjust for transfection efficiency. Results demonstrated that the hSERTp1 was highly active in Caco-2 cells (∼36-fold increase) compared with cells transfected with the pGL2 empty vector alone (Fig. 2A). The hSERTp1 promoter construct demonstrated a significantly lower activity in nonintestinal HEK-293 cells (Fig. 2A).
Fig. 2.
Effects of EGF on hSERT promoter activities in Caco-2 cells. A: hSERTp1 is active in Caco-2 cells. Caco-2 and HEK-293 cells were transiently cotransfected with the hSERTp1 promoter construct along with the β-galactosidase mammalian expression vector. Promoter activity was measured by firefly luciferase assay and represented as relative luciferase units normalized to β-galactosidase activity to adjust for transfection efficiency. The activity of the pGL2 empty vector alone in Caco-2 cells or HEK-293 cells is represented as 100%. hSERTp1 was highly active in Caco-2 cells. Results were obtained from 3 separate experiments and are expressed as means ± SE. *P < 0.0001 compared with empty vector. B: effect of EGF on activity of hSERTp1 and hSERTp2. Caco-2 cells were transiently cotransfected with hSERTp1 or hSERTp2 fragments along with β-galactosidase vector to adjust for transfection efficiency. Twenty-four hours after transfection, cells were incubated with 10 ng/ml EGF for another 24 h. hSERTp1 exhibited higher activity than hSERTp2 in Caco-2 cells. EGF increased the activity of both hSERTp1 and hSERTp2. Data are represented as % of control and represent means ± SE of 5 or 6 different experiments performed in triplicate. *P < 0.05 compared with control.
Similar to the findings of Linden et al. (25), hSERTp2 was also active in Caco-2 cells, although its activity was significantly less compared with hSERTp1 (Fig. 2B). Interestingly, EGF treatment (10 ng/ml, 24 h) of cells transiently transfected with hSERTp1 or hSERTp2 significantly increased the promoter activities of both the promoters by ∼2- to 2.5-fold (Fig. 2B). Similar results were obtained by 8-h treatment with EGF (data not shown). These data demonstrate that EGF stimulates SERT via transcriptional mechanisms.
EGF effects are tyrosine kinase dependent.
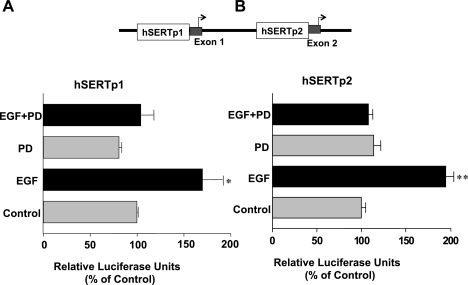
EGFR is a 170-kDa transmembrane protein containing an intrinsic cytoplasmic tyrosine kinase domain and docking sites for various signaling effector molecules (39, 49). To examine whether the effects of EGF are dependent on EGFR, we measured the effects of EGF on SERT promoter activity in the presence of the EGFR-specific tyrosine kinase inhibitor PD168393 (1 nM). Treatment of cells with EGF resulted in an increase in hSERTp1 (Fig. 3A) and hSERTp2 (Fig. 3B) activities. However, inhibition of EGFR tyrosine kinase activity abrogated the stimulatory effects of EGF on hSERTp1 and hSERTp2 (Fig. 3). These data indicate that the effects of EGF on SERT are EGFR signaling dependent.
Fig. 3.
Stimulation of hSERTp1 and hSERTp2 is EGF receptor (EGFR) dependent. Caco-2 cells were transfected with hSERTp1 (A) or hSERTp2 (B) fragments along with β-galactosidase vector. Twenty-four hours after transfection, cells were pretreated from the basolateral side with the cell-permeant EGFR tyrosine kinase activity inhibitor PD168393 (PD, 1 nM) for 1 h, followed by coincubation with EGF (10 ng/ml) for another 24 h. SERT promoter activities were assessed by luciferase assays normalized to β-galactosidase activity. Values represent % of control derived from means ± SE of 3 different experiments. *P < 0.05, **P < 0.01 compared with respective controls.
EGF-responsive region in hSERT promoters.
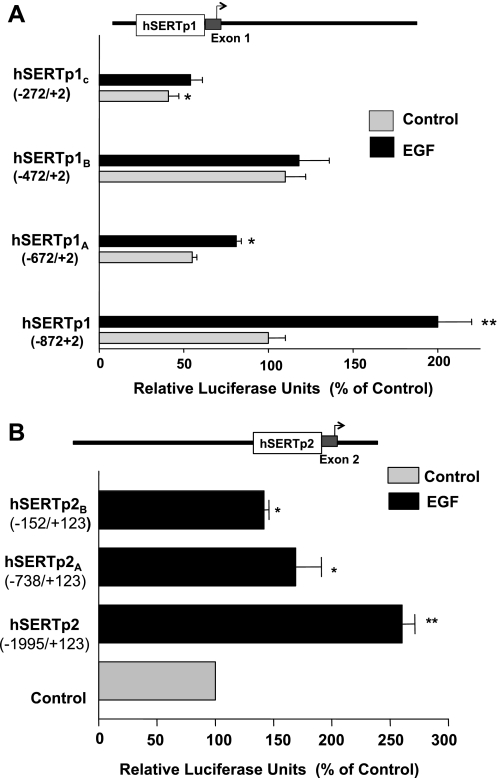
To determine which specific regions in hSERTp are responsive to EGF stimulation, we cloned different fragments of hSERTp1 and hSERTp2 representing 5′ progressive deletions in the promoters. The basal activity of the deletion constructs for hSERTp1 is shown in Fig. 4A. Deletion to −272 bp significantly attenuated the promoter activity of hSERTp1, indicating that this region harbors critical cis-elements required for basal activity of the promoter. The effect of EGF on the deletion constructs was next examined. EGF treatment stimulated hSERTp1 activity (−872/+2) by approximately twofold compared with control. However, the hSERTp1A deletion construct (−672/+2) showed significantly lower stimulation (∼1.4-fold), and further deletion to −472/+2 (hSERTp1B) or −272/+2 (hSERTp1C) fragment abolished the EGF-induced stimulation compared with the respective controls (taken as 100%). These data show that the EGF response elements are located in the region spanning the area of −872/−472 of the hSERTp1 (Fig. 4A).
Fig. 4.
EGF-responsive regions of hSERTp1 and hSERTp2. A: hSERTp1: progressive 5′ deletions of hSERTp1 treated with EGF. Different promoter constructs of hSERTp1 were treated with EGF (10 ng/ml, 24 h). Twenty-four hours after treatment, cells were harvested for measurement of promoter activity by luciferase assay. Values were normalized to β-galactosidase to adjust for transfection efficiency. The stimulatory effect of EGF was abolished on deletion to −472/+2 bp. Data were obtained from at least 5 different experiments performed in triplicate and are shown as means ± SE. Results are expressed as percentages of control. *P < 0.05, **P < 0.001 compared with control. B: hSERTp2: progressive 5′ deletion constructs of hSERTp2 were cotransfected in Caco-2 cells with β-galactosidase vector, and effect of EGF on promoter activity was measured. EGF-responsive region predominantly spans −1995/−738 region of hSERTp2. Data were obtained from at least 3 different experiments performed in triplicate and are shown as means ± SE. Results are expressed as % of control. *P < 0.01, **P < 0.001 compared with control.
Previous studies of Linden et al. (25) have demonstrated that hSERTp2 and its different truncated constructs (upstream of exon 2 and extending to exon 1c) are active in Caco-2 cells, with no significant activity in human choriocarcinoma JAR cells. Similar to these previous studies, the present studies showed that the basal activity of the full-length hSERTp2 (−1995/+123) was increased upon deletion to smaller fragments, i.e., −738/+123 bp (hSERTp2A) and −152/+123 bp (hSERTp2C), indicating the presence of suppressor elements in the truncated regions [promoter activity in relative luciferase units under basal conditions: hSERTp2 100 ± 11; hSERTp2A 916 ± 102 (P < 0.001); hSERTp2B 317 ± 72.8 (P < 0.001)]. To narrow the EGF-responsive region of hSERTp2, the effect of EGF was assessed with these deletion constructs (Fig. 4B). EGF treatment of Caco-2 cells resulted in ∼2.5-fold stimulation of the full-length promoter. This stimulation in promoter activity by EGF was attenuated with the use of hSERTp2A and hSERTp2B constructs flanking region −738/+123 and region −152/+123 compared with their respective controls (taken as 100%). These data indicate that the EGF-responsive region lies predominantly in the −1995/−738 bp region as well as in the region spanning −152/+123 of the hSERTp2.
Involvement of AP-1 in EGF-mediated effects on SERT promoters.
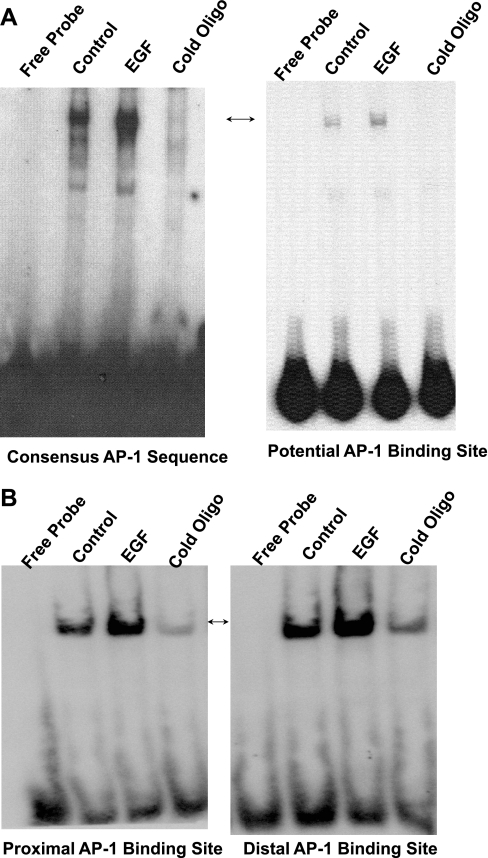
EGF response has been shown to be mediated through various elements including serum-response elements, c-myb, sp1, and AP-1 binding sequences in promoter regions of various genes (16, 22, 32, 34, 46–48). Analysis of the potential EGF-responsive region in hSERTp1 indicated the presence of potential cis-elements for AP-1, c-myb1, c-myb2, ETS, and MZF1. We examined the involvement of AP-1 in EGF-mediated effects. Results in Fig. 5A demonstrate that EGF increased the binding of 32P-labeled consensus AP-1 oligonucleotide (Santa Cruz) to nuclear extracts (Fig. 5A, left). Similarly, the binding of the nuclear proteins to the probe corresponding to the potential AP-1 site of hSERTp1 promoter was increased in response to EGF treatment (Fig. 5A, right). The observed DNA-protein complex was eliminated in the presence of an excess of unlabeled probe, indicating the binding specificity of the complexes.
Fig. 5.
Effect of EGF on nuclear protein binding to potential AP-1 cis-elements of hSERTp1 (A) and hSERTp2 (B). A: 8 μg of nuclear extracts from control (untreated) or EGF-treated (10 ng/ml, 24 h) Caco-2 cells was incubated with the 32P-labeled AP-1 cis-element of hSERTp1 (right) or with 32P-labeled consensus AP-1 sequence (left). Protein-DNA complexes competed in the presence of excess of cold unlabeled oligonucleotide probe. B: nuclear extracts from control (untreated) or EGF-treated (10 ng/ml, 24 h) Caco-2 cells were incubated with digoxigenin (DIG)-labeled AP-1 potential cis-element of hSERTp2. Binding of both the potential AP-1 sites located proximal to the transcription site (left) and the distal AP-1 site (right) is shown. Protein-DNA complexes competed in the presence of excess of cold unlabeled oligonucleotide probe, showing specificity of binding. Representative gels of 3 or 4 separate experiments with similar results are shown.
Regarding hSERTp2, two AP-1 sites have been predicted in the region that our studies narrowed as the predominant EGF response element (−1995/−738 bp). We named these sites the proximal AP-1 [proximal to the transcription initiation site (−978/−951 bp)] and distal AP-1 (−1479 to −1437 bp) sites. EMSA was performed with DIG-labeled double-stranded oligonucleotide probes corresponding to the potential AP-1 cis-elements of hSERTp2 and nuclear extracts isolated from control and EGF-treated cell monolayers. Under control (untreated) conditions, both the proximal and distal potential AP-1 binding sites of hSERTp1 and -2 displayed binding to Caco-2 nuclear extract proteins, which was eliminated in the presence of 125-fold excess of cold unlabeled probe, indicating the specificity of binding (Fig. 5B). Interestingly, EGF treatment resulted in a remarkable increase in the DNA-protein binding for both the proximal and distal potential AP-1 cis-elements of hSERTp2 (Fig. 5B). These data suggest that AP-1 may contribute to stimulatory effects of EGF on hSERT promoters.
c-Jun transactivates hSERTp2 activity.
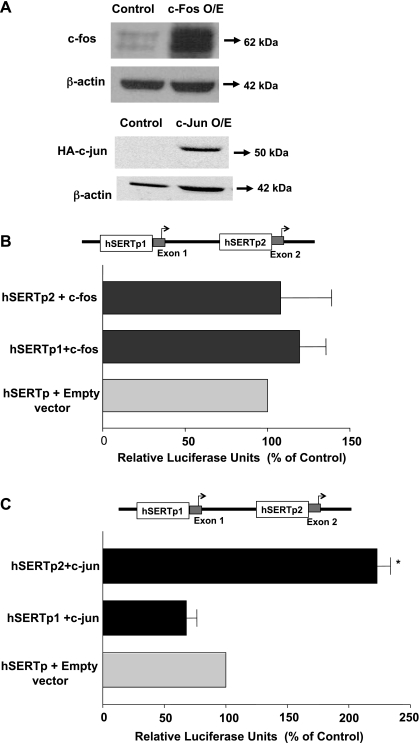
The AP-1 transcription complex is a family of dimeric transcription factors composed of c-fos and c-jun proteins (40). To examine whether c-jun or c-fos directly influences the activity of SERT promoters, Caco-2 cells were cotransfected with the hSERTp1 or hSERTp2 promoter along with mammalian expression vectors for c-fos or c-jun-hemagglutinin (HA)-tagged protein. The overexpression of c-fos and c-jun-HA fusion protein in Caco-2 cells was assessed by Western blotting utilizing anti-c-fos and anti-HA antibodies, respectively. Increased expression of c-fos and c-jun-HA-tagged fusion protein was evident in cells transfected with the expression vectors for these proteins compared with transfection with pcDNA3 vector alone (Fig. 6A). β-Actin was utilized as an internal control.
Fig. 6.
Effect of c-fos or c-jun overexpression on hSERTp1 and hSERTp2 activities. A: expression of c-jun or c-fos. Cells were cotransfected with hSERT promoter constructs and mammalian expression vectors for c-fos or hemagglutinin (HA)-tagged c-jun along with the β-galactosidase vector. Cells were harvested after 24 h, and protein lysates were run on SDS-PAGE, followed by immunoblotting with anti-c-fos or anti-HA antibodies to examine the protein expression of c-fos and c-jun, respectively. β-Actin was used as an internal control. O/E, overexpressing. Data were obtained from 3 separate experiments. B: effect of c-fos overexpression on hSERTp1 and hSERTp2 activities. Caco-2 cells were transiently transfected with c-fos mammalian expression vector for 24 h along with promoter construct hSERTp1 or hSERTp2. Control cells were transfected with empty vector for c-fos (PCDN3.1) and hSERTp1 or hSERTp2. Promoter activity was measured by luciferase assay normalized to β-galactosidase to adjust for transfection efficiency. c-fos overexpression failed to alter the activity of hSERTp1 or hSERTp2 compared with respective controls. Data are % of respective controls represented as means ± SE of 5 individual experiments performed in triplicate. C: c-jun overexpression transactivates hSERTp2 activity. Caco-2 cells were transiently transfected with c-jun mammalian expression vector for 24 h along with promoter construct hSERTp1 or hSERTp2. Control cells were cotransfected with empty vector and hSERTp1 or hSERTp2. c-jun overexpression transactivated hSERTp2, with no effect on hSERTp1 compared with their respective controls. Data are represented as % of control represented as means ± SE of 3 individual experiments performed in triplicate. *P < 0.001 compared with control.
Measurement of the promoter activity demonstrated that c-fos overexpression did not alter either hSERTp1 or hSERTp2 (Fig. 6B). c-jun overexpression exhibited differential effects on the two promoters (Fig. 6C). Data showed that hSERTp1 activity was not significantly altered by c-jun overexpression. In contrast, a remarkable stimulation was observed in the activity of hSERTp2, indicating that c-jun is the major regulator of the hSERTp2 and may be involved in EGF-mediated stimulation of hSERTp2 (Fig. 6C). These data indicate that although both promoters exhibited increased DNA-protein binding of putative AP-1 cis-elements in response to EGF, upregulation of hSERTp2 may involve increased binding to c-jun, whereas activation of hSERTp1 by EGF may occur via binding of another putative protein.
c-Jun binds to potential AP-1 cis-elements of hSERTp2.
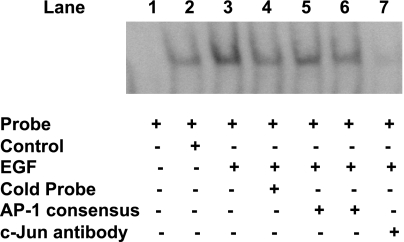
Since the above studies indicated that c-jun transactivates hSERTp2, we next investigated the ability of c-Jun to bind potential AP-1 cis-elements in response to EGF. EMSA was performed utilizing the distal AP-1 cis-element as a DIG-end-labeled probe. As shown in Fig. 7, the binding of labeled potential AP-1 cis-element to Caco-2 nuclear proteins was significantly increased in the presence of EGF (lane 3) compared with control (lane 2), which was competed out in the presence of excess of unlabeled probe (lane 4) or unlabeled consensus AP-1 sequence (lanes 5 and 6). To confirm the identity of the binding protein in this complex, a specific c-jun antibody was added. The presence of the anti-c-jun antibody blocked the formation of the DNA-protein complex (lane 7). These results suggest that the c-jun antibody binds to a site on the transcription factor that is essential for DNA binding and further indicate the role of c-jun in EGF-mediated stimulation of hSERTp2 activity.
Fig. 7.
c-jun binds to potential AP-1 cis-elements of hSERTp2. Nuclear extracts from control (untreated) or EGF-treated (10 ng/ml, 24 h) Caco-2 cells were incubated with DIG-labeled distal AP-1 cis-element of the hSERTp2. DNA-protein complexes in response to EGF treatment were competed by excess of unlabeled probe (lane 4). Excess of cold oligonucleotides representing the AP-1 consensus sequence (125-fold excess, lane 5; 150-fold excess, lane 6) competed out the increase in binding observed in EGF-treated nuclear extracts (lane 3). Addition of c-jun antibody abolished the increase in binding in response to EGF treatment (lane 7). These data indicate that c-jun binds to the potential AP-1 cis-elements of hSERTp2. A representative blot is shown.
DISCUSSION
EGF is a key regulator of a number of epithelial functions such as restitution, tight junction permeability, electrolyte transport, as well as nutrient and phosphate absorption (2, 18, 33, 35, 36, 43, 44, 46, 48). Also, EGFR-mediated signaling has been shown previously to be protective in cases of inflammation or infection by Clostridium difficile, indicating the emerging role of growth factors in treatment of intestinal disorders (14, 29, 41). In the present study, we for the first time demonstrate the upregulation of SERT by EGF in intestinal epithelial cells at the promoter level via transcriptional mechanisms. These findings are of clinical importance, as induction of SERT expression may be beneficial in pathophysiological cases in which SERT expression is downregulated, such as diarrheal and inflammatory bowel diseases.
The present studies utilized the well-established human intestinal epithelial cell line Caco-2 as an in vitro model to understand mechanistic insights underlying EGF-mediated effects on SERT. Given that SERT is highly expressed in the ileum, Caco-2 cells represent an excellent in vitro model for our studies, as on differentiation these cells manifest many anatomic and functional similarities to absorptive ileal enterocytes. Several previous studies have utilized Caco-2 cells to study regulation of various human intestinal ion transport processes including SERT (1, 5, 17, 19, 20, 37, 46, 47). Also, Caco-2 cells have been shown to express EGFRs on the basolateral surface (4) and thus have been widely used to examine regulation of various ion transporters in response to EGF (45, 47, 48).
With respect to mechanisms underlying EGF-mediated effects, previous studies have shown that EGF influences epithelial ion transporters acutely as well as via modulation of gene expression. For example, EGF-mediated upregulation of Na+/H+ exchanger (NHE)3 occurred rapidly via activation of a phosphatidylinositol 3-kinase (PI3K)- and AKT-dependent process (11). Similarly, activation of the EGFR has been shown to reduce carbachol-stimulated ion transport via a signaling pathway involving PI3K and the extracellular signal-regulated kinase (ERK) isoforms of the mitogen-activated protein kinase (MAPK) family (24, 44). On the other hand, EGF is known to upregulate NHE2 and NKCC1 gene expression (33, 46) and has been shown to decrease NHE8 and NaPi-II transporter by transcriptional mechanisms (45, 48). Interestingly, our studies demonstrated that EGF did not exert acute effects on SERT but stimulated SERT function over a 24-h time period by increasing SERT mRNA via transcriptional mechanisms.
Recent studies have established that SERT gene has multiple transcription start sites under the control of two different promoters (25). An alternative promoter and transcription start site that is 12 kb downstream of the previously recognized site has been identified (25). This is consistent with our previous Northern blot studies showing the presence of multiple SERT transcripts in the human ileum (20). However, the physiological relevance of the existence of different variants and alternate promoters is not very clear at present. It is believed that the downstream alternate promoter (hSERTp2) represents the intestine-specific promoter, which is not active in other nonintestinal tissues where SERT is expressed, such as brain (25). Although not experimentally proven yet, it can be speculated that the activity of hSERTp2 in the intestine may be driven by intestine-specific transcription factors that are not expressed in other nonintestinal tissues. Since intestinal SERT has characteristics similar to neuronal SERT (which include sensitivity to the serotonin selective reuptake inhibitor fluoxetine and similarity in kinetic parameters) (20, 27, 28), understanding the regulation of the expression of SERT via two alternate promoters may create new opportunities for targeting SERT specifically in the intestine with no adverse effects in other regions. Interestingly, similar to the studies of Linden et al. (25), both promoters (hSERTp1 and hSERTp2) were found to be highly active in Caco-2 cells, with hSERTp1 exhibiting higher activity. However, no studies are currently available that address the regulation of the SERT promoters in the intestine. Our studies provide novel findings that EGF stimulated the activities of both hSERTp1 and hSERTp2 via activation of EGFR.
EGFR consists of a transmembrane receptor domain and a cytoplasmic protein tyrosine kinase domain, which gets autophosphorylated after ligand binding (39). Our studies show that inhibition of EGFR tyrosine kinase activity blocked the stimulatory effects of EGF on the two promoters, indicating that EGF-mediated transcriptional effects on SERT are specific. With respect to the EGF-responsive region, previous studies have shown that EGF decreases NHE8 expression via reduced SP3 binding to NHE8 promoter in Caco-2 cells (48). Similarly, c-myb has been implicated in decreasing NaPi-IIb cotransporter in response to EGF treatment of Caco-2 cells (45). Other EGF response elements include AP-1 binding sequences from the c-fos gene and Sp1 binding sequences (16, 22, 32, 34). Our studies demonstrated that EGF induced the stimulation of the two SERT gene promoters, albeit by distinct mechanisms, via AP-1. AP-1 is a multimeric transcription factor complex generally associated with growth factor response, cellular proliferation, differentiation, and stress. The AP-1 complex consists of homodimers of the jun family or heterodimers of jun family members and the related fos proteins (40).
The involvement of AP-1 in hSERTp1 and hSERTp2 stimulation was evident from EMSA demonstrating increased binding of the potential AP-1 cis-element sites to nuclear proteins extracted from Caco-2 cells treated with EGF. Interestingly, overexpression of c-jun but not c-fos in Caco-2 cells specifically transactivated hSERTp2 activity, indicating an important role of c-jun in hSERTp2 stimulation. This was further evident from EMSA studies showing binding of c-jun to potential AP-1 cis-elements of hSERTp2 in response to EGF. It should be noted that deletion of hSERTp2 to −738/+123 and −152/+123 regions (which lack both the AP-1 sites present in full-length promoter) retained significant responsiveness to EGF. These data indicate that other potential cis-elements present in the −152/+123 region may also contribute to EGF-mediated stimulation of hSERTp2.
In contrast to hSERTp2, overexpression of both c-jun and c-fos failed to transactivate hSERTp1, indicating that EGF-mediated stimulation of hSERTp1 occurs indirectly via binding of other transcription factors to the potential AP-1 cis-elements identified in the EGF-responsive region of hSERTp1. More in-depth studies are needed to confirm the identity of the transcription factor(s) as well as the role of other potential cis-elements in basal as well as EGF-mediated stimulation of hSERTp1 and hSERTp2.
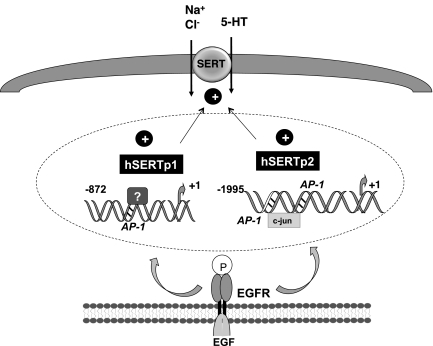
In conclusion, our findings provide novel information on the upregulation of SERT function and expression by EGF via transcriptional mechanisms. Our findings as depicted in Fig. 8 reveal that EGF-mediated stimulation of SERT function and expression occurred via tyrosine kinase activation of EGFR and involved stimulation of the activity of both of the SERT gene promoters by distinct mechanisms. Notably, hSERTp2 activation occurred partly via increased binding of c-jun to potential AP-1 cis-elements. However, the identity of the transcription factor(s) responsible for stimulation of hSERTp1 needs to be investigated. Our findings support the notion that the two alternate promoters of SERT gene can be regulated by growth factors via different mechanisms. Thus EGF signaling involving c-jun can be harnessed for specifically targeting hSERTp2 to induce SERT function specifically in the intestine and reducing the side effects of its upregulation in other organs where SERT is expressed, such as brain. Since clinical studies support the beneficial effects of EGF enemas in decreasing the inflammation and diarrhea associated with ulcerative colitis due to its wound-healing and intestinal epithelial restitution effects (14, 29, 35, 36), future studies are needed to investigate whether SERT upregulation can contribute to beneficial effects of EGF and can reverse the decreased SERT expression observed in cases of inflammation or diarrheal disorders. Also, since SERT−/− mice are more susceptible to colitis (23), it will be of interest to examine in the future whether SERT overexpression in intestinal epithelial cells will protect these mice from induced colitis.
Fig. 8.
Schematic of the proposed model of EGF-mediated effects on SERT.
GRANTS
These studies were supported by the Department of Veterans Affairs, National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-74458 (R. K. Gill), DK-71596 (W. A. Alrefai), DK-33349, and P01-DK-067887 (J. Malakooti) and Crohn's and Colitis Foundation of America (CCFA) Grant Ref. No. 1942 (S. Saksena).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Nancy Colburn, National Cancer Institute (Frederick, MD) for providing the mammalian expression vectors for c-Jun and c-Fos. We also thank Dr. Pradeep K. Dudeja and Dr. Krishnamurthy Ramaswamy for their input and critical review of the manuscript.
REFERENCES
- 1.Alrefai WA, Annaba F, Sarwar Z, Dwivedi A, Saksena S, Singla A, Dudeja PK, Gill RK. Modulation of human Niemann-Pick C1-like 1 gene expression by sterol: role of sterol regulatory element binding protein 2. Am J Physiol Gastrointest Liver Physiol 292: G369–G376, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Banan A, Zhang LJ, Shaikh M, Fields JZ, Farhadi A, Keshavarzian A. Key role of PLC-gamma in EGF protection of epithelial barrier against iNOS upregulation and F-actin nitration and disassembly. Am J Physiol Cell Physiol 285: C977–C993, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med 60: 355–366, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop WP, Wen JT. Regulation of Caco-2 cell proliferation by basolateral membrane epidermal growth factor receptors. Am J Physiol Gastrointest Liver Physiol 267: G892–G900, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Borthakur A, Gill RK, Tyagi S, Koutsouris A, Alrefai WA, Hecht GA, Ramaswamy K, Dudeja PK. The probiotic Lactobacillus acidophilus stimulates chloride/hydroxyl exchange activity in human intestinal epithelial cells. J Nutr 138: 1355–1359, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 7.Carpenter G, Cohen S. Epidermal growth factor. J Biol Chem 265: 7709–7712, 1990 [PubMed] [Google Scholar]
- 8.Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: abnormal intestinal motility and the expression of cation transporters. J Neurosci 21: 6348–6361, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coates MD, Johnson AC, Greenwood-Van Meerveld B, Mawe GM. Effects of serotonin transporter inhibition on gastrointestinal motility and colonic sensitivity in the mouse. Neurogastroenterol Motil 18: 464–471, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 126: 1657–1664, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Donowitz M, Janecki A, Akhter S, Cavet ME, Sanchez F, Lamprecht G, Zizak M, Kwon WL, Khurana S, Yun CH, Tse CM. Short-term regulation of NHE3 by EGF and protein kinase C but not protein kinase A involves vesicle trafficking in epithelial cells and fibroblasts. Ann NY Acad Sci 915: 30–42, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Esmaili A, Nazir S, Singla A, Dudeja A, Saksena S, Raheja G, Alrefai WA, Gill RK. Epidermal growth factor (EGF) stimulates serotonin transporter function and expression via transcriptional mechanisms in human intestinal epithelial cells (Abstract). Gastroenterology 136, Suppl 1: A-567, 2009 [Google Scholar]
- 13.Esmaili A, Nazir SF, Borthakur A, Yu D, Turner JR, Saksena S, Singla A, Hecht GA, Alrefai WA, Gill RK. Enteropathogenic Escherichia coli infection inhibits intestinal serotonin transporter function and expression. Gastroenterology 137: 2074–2083, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrell RJ. Epidermal growth factor for ulcerative colitis. N Engl J Med 349: 395–397, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Faure C, Patey N, Gauthier C, Brooks EM, Mawe GM. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology 139: 249–258, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisch TM, Prywes R, Roeder RG. An AP1-binding site in the c-fos gene can mediate induction by epidermal growth factor and 12-O-tetradecanoyl phorbol-13-acetate. Mol Cell Biol 9: 1327–1331, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foley KF, Pantano C, Ciolino A, Mawe GM. IFN-gamma and TNF-alpha decrease serotonin transporter function and expression in Caco2 cells. Am J Physiol Gastrointest Liver Physiol 292: G779–G784, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Ghishan FK, Kikuchi K, Riedel B. Epidermal growth factor up-regulates intestinal Na+/H+ exchange activity. Proc Soc Exp Biol Med 201: 289–295, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G, Dudeja PK. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J Clin Invest 117: 428–437, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill RK, Pant N, Saksena S, Singla A, Nazir TM, Vohwinkel L, Turner JR, Goldstein J, Alrefai WA, Dudeja PK. Function, expression, and characterization of the serotonin transporter in the native human intestine. Am J Physiol Gastrointest Liver Physiol 294: G254–G262, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gill RK, Saksena S, Tyagi S, Alrefai WA, Malakooti J, Sarwar Z, Turner JR, Ramaswamy K, Dudeja PK. Serotonin inhibits Na+/H+ exchange activity via 5-HT4 receptors and activation of PKC alpha in human intestinal epithelial cells. Gastroenterology 128: 962–974, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Godley JM, Brand SJ. Regulation of the gastrin promoter by epidermal growth factor and neuropeptides. Proc Natl Acad Sci USA 86: 3036–3040, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haub S, Ritze Y, Bergheim I, Pabst O, Gershon MD, Bischoff SC. Enhancement of intestinal inflammation in mice lacking interleukin 10 by deletion of the serotonin reuptake transporter. Neurogastroenterol Motil 22: 826–834, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keely SJ, Barrett KE. ErbB2 and ErbB3 receptors mediate inhibition of calcium-dependent chloride secretion in colonic epithelial cells. J Biol Chem 274: 33449–33454, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Linden DR, White SL, Brooks EM, Mawe GM. Novel promoter and alternate transcription start site of the human serotonin reuptake transporter in intestinal mucosa. Neurogastroenterol Motil 21: 534–541, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malakooti J, Memark VC, Dudeja PK, Ramaswamy K. Molecular cloning and functional analysis of the human Na+/H+ exchanger NHE3 promoter. Am J Physiol Gastrointest Liver Physiol 282: G491–G500, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Martel F. Recent advances on the importance of the serotonin transporter SERT in the rat intestine. Pharmacol Res 54: 73–76, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Martel F, Monteiro R, Lemos C. Uptake of serotonin at the apical and basolateral membranes of human intestinal epithelial (Caco-2) cells occurs through the neuronal serotonin transporter (SERT). J Pharmacol Exp Ther 306: 355–362, 2003 [DOI] [PubMed] [Google Scholar]
- 29.McCole DF, Rogler G, Varki N, Barrett KE. Epidermal growth factor partially restores colonic ion transport responses in mouse models of chronic colitis. Gastroenterology 129: 591–608, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Menard D, Corriveau L, Arsenault P. Differential effects of epidermal growth factor and hydrocortisone in human fetal colon. J Pediatr Gastroenterol Nutr 10: 13–20, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Menard D, Pothier P. Radioautographic localization of epidermal growth factor receptors in human fetal gut. Gastroenterology 101: 640–649, 1991 [DOI] [PubMed] [Google Scholar]
- 32.Merchant JL, Shiotani A, Mortensen ER, Shumaker DK, Abraczinskas DR. Epidermal growth factor stimulation of the human gastrin promoter requires Sp1. J Biol Chem 270: 6314–6319, 1995 [DOI] [PubMed] [Google Scholar]
- 33.O'Mahony F, Toumi F, Mroz MS, Ferguson G, Keely SJ. Induction of Na+/K+/2Cl− cotransporter expression mediates chronic potentiation of intestinal epithelial Cl− secretion by EGF. Am J Physiol Cell Physiol 294: C1362–C1370, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Ren Y, Satoh T, Yamada M, Hashimoto K, Konaka S, Iwasaki T, Mori M. Stimulation of the preprothyrotropin-releasing hormone gene by epidermal growth factor. Endocrinology 139: 195–203, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Riegler M, Sedivy R, Sogukoglu T, Castagliuolo I, Pothoulakis C, Cosentini E, Bischof G, Hamilton G, Teleky B, Feil W, Lamont JT, Wenzl E. Epidermal growth factor attenuates Clostridium difficile toxin A- and B-induced damage of human colonic mucosa. Am J Physiol Gastrointest Liver Physiol 273: G1014–G1022, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Riegler M, Sedivy R, Sogukoglu T, Cosentini E, Bischof G, Teleky B, Feil W, Schiessel R, Hamilton G, Wenzl E. Effect of growth factors on epithelial restitution of human colonic mucosa in vitro. Scand J Gastroenterol 32: 925–932, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Saksena S, Gill RK, Tyagi S, Alrefai WA, Sarwar Z, Ramaswamy K, Dudeja PK. Involvement of c-Src and protein kinase C delta in the inhibition of Cl−/OH− exchange activity in Caco-2 cells by serotonin. J Biol Chem 280: 11859–11868, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Saksena S, Singla A, Goyal S, Katyal S, Bansal N, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Mechanisms of transcriptional modulation of the human anion exchanger SLC26A3 gene expression by IFNgamma. Am J Physiol Gastrointest Liver Physiol 298: G159–G166, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 110: 669–672, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Shaulian E. AP-1—the Jun proteins: oncogenes or tumor suppressors in disguise? Cell Signal 22: 894–899 [DOI] [PubMed] [Google Scholar]
- 41.Sinha A, Nightingale J, West KP, Berlanga-Acosta J, Playford RJ. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med 349: 350–357, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Spiller R. Serotonin and GI clinical disorders. Neuropharmacology 55: 1072–1080, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Ulshen MH, Lyn-Cook LE, Raasch RH. Effects of intraluminal epidermal growth factor on mucosal proliferation in the small intestine of adult rats. Gastroenterology 91: 1134–1140, 1986 [DOI] [PubMed] [Google Scholar]
- 44.Uribe JM, Gelbmann CM, Traynor-Kaplan AE, Barrett KE. Epidermal growth factor inhibits Ca2+-dependent Cl− transport in T84 human colonic epithelial cells. Am J Physiol Cell Physiol 271: C914–C922, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Xu H, Collins JF, Bai L, Kiela PR, Ghishan FK. Regulation of the human sodium-phosphate cotransporter NaPi-IIb gene promoter by epidermal growth factor. Am J Physiol Cell Physiol 280: C628–C636, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Xu H, Collins JF, Bai L, Kiela PR, Lynch RM, Ghishan FK. Epidermal growth factor regulation of rat NHE2 gene expression. Am J Physiol Cell Physiol 281: C504–C513, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Xu H, Inouye M, Hines ER, Collins JF, Ghishan FK. Transcriptional regulation of the human NaPi-IIb cotransporter by EGF in Caco-2 cells involves c-myb. Am J Physiol Cell Physiol 284: C1262–C1271, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Xu H, Zhang B, Li J, Chen H, Tooley J, Ghishan FK. Epidermal growth factor inhibits intestinal NHE8 expression via reducing its basal transcription. Am J Physiol Cell Physiol 299: C51–C57, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheleznova NN, Wilson PD, Staruschenko A. Epidermal growth factor-mediated proliferation and sodium transport in normal and PKD epithelial cells. Biochim Biophys Acta (October 16, 2010). doi:10.1016/j.bbadis.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]