Abstract
Cholecystokinin (CCK) is produced by discrete endocrine cells in the proximal small intestine and is released following the ingestion of food. CCK is the primary hormone responsible for gallbladder contraction and has potent effects on pancreatic secretion, gastric emptying, and satiety. In addition to fats, digested proteins and aromatic amino acids are major stimulants of CCK release. However, the cellular mechanism by which amino acids affect CCK secretion is unknown. The Ca2+-sensing receptor (CaSR) that was originally identified on parathyroid cells is not only sensitive to extracellular Ca2+ but is activated by extracellular aromatic amino acids. It has been postulated that this receptor may be involved in gastrointestinal hormone secretion. Using transgenic mice expressing a CCK promoter driven/enhanced green fluorescent protein (GFP) transgene, we have been able to identify and purify viable intestinal CCK cells. Intestinal mucosal CCK cells were enriched >200-fold by fluorescence-activated cell sorting. These cells were then used for real-time PCR identification of CaSR. Immunohistochemical staining with an antibody specific for CaSR confirmed colocalization of CaSR to CCK cells. In isolated CCK cells loaded with a Ca2+-sensitive dye, the amino acids phenylalanine and tryptophan, but not nonaromatic amino acids, caused an increase in intracellular Ca2+ ([Ca2+]i). The increase in [Ca2+]i was blocked by the CaSR inhibitor Calhex 231. Phenylalanine and tryptophan stimulated CCK release from intestinal CCK cells, and this stimulation was also blocked by CaSR inhibition. Electrophysiological recordings from isolated CCK-GFP cells revealed these cells to possess a predominant outwardly rectifying potassium current. Administration of phenylalanine inhibited basal K+ channel activity and caused CCK cell depolarization, consistent with changes necessary for hormone secretion. These findings indicate that amino acids have a direct effect on CCK cells to stimulate CCK release by activating CaSR and suggest that CaSR is the physiological mechanism through which amino acids regulate CCK secretion.
Keywords: hormone, secretion
cholecystokinin (CCK) is the major hormonal regulator of gallbladder contraction (29). However, it also has important actions to stimulate pancreatic enzyme secretion, delay gastric emptying, and induce satiety and reduce food intake (27). CCK is produced by discrete enteroendocrine cells of the upper small intestine, also called I cells, and is released upon ingestion of a meal (41). The major nutrients that stimulate CCK release are fats and ingested proteins. Of these, the specific meal components that cause CCK release include fatty acids and amino acids.
In some species, proteins appear to stimulate CCK secretion by virtue of their ability to inhibit intralumenal trypsin activity (20, 31). It has been proposed that this occurs through the interaction of protein and trypsin allowing for an endogenous CCK-releasing factor to go undigested, and thus interact with CCK cells to stimulate CCK secretion (22, 28). However, this trypsin interaction pathway may not be active in all species. Amino acids, the structural component of proteins, have a direct effect on CCK release in most species studied, including humans, monkeys, dogs, and rodents (1, 17, 26, 27, 37, 39).
Of the amino acids that stimulate CCK secretion, the aromatic amino acids, l-phenylalanine and l-tryptophan, are the most effective (27). Nonaromatic amino acids have much less effect on CCK secretion. In the CCK-expressing cell line, STC-1 derived from a mouse, phenylalanine was shown to stimulate CCK release and increase intracellular Ca2+ concentrations ([Ca2+]i) through a mechanism that was dependent on an L-type Ca2+ channel (33). However, the mechanism by which phenylalanine interacts with the native CCK cell is unknown. Moreover, a growing body of evidence indicates that mouse STC-1 cells are very different from native mouse intestinal CCK cells. Currently, there are few data indicating how nutrients of any type stimulate CCK release from native CCK cells. It is not even known whether nutrients have a direct or an indirect effect on native CCK cells.
Two possible mechanisms by which phenylalanine may stimulate CCK cells have been proposed: 1) interaction with a cell membrane cotransporter (25, 43) or 2) binding to a low-affinity plasma membrane receptor (32, 33). The extracellular Ca2+ ([Ca2+]o)-sensing receptor (CaSR) recognizes and responds to (i.e., “senses”) [Ca2+]o as its principal physiological ligand. However, it was recently demonstrated that CaSR is activated not only by [Ca2+]o but also by aromatic amino acids, establishing its capacity to sense nutrients (12). Several investigators have speculated on the role of CaSR in gut endocrine cell function (7, 10, 12), but little has been offered as evidence for this role. CaSR has been shown to play a role in amino acid-stimulated secretion in STC-1 cells (24), but it is unknown whether CaSR is important in differentiated intestinal I cells. We postulate that amino acids stimulate CCK cells through activation of the CaSR.
Study of CCK secretion has been limited by the lack of suitable experimental models. Isolation of native CCK cells has been difficult because neuroendocrine cells comprise <0.5% of the mucosal cell population, and it has proven tedious to enrich these cells (30). In the current study, we have used a transgenic mouse line in which enhanced green fluorescent protein (eGFP) is expressed downstream from the mouse CCK promoter. These mice (hereafter known as CCK-GFP mice) express eGFP in intestinal CCK cells (9). Isolated mucosal eGFP-expressing CCK cells were then prepared and purified using fluorescence-activated cell sorting (FACS). From these cells, we discovered using quantitative PCR that CaSR was highly expressed in CCK cells. This finding was the basis for subsequent studies indicating that aromatic amino acids stimulate CCK release through activation of CaSR.
METHODS
CCK-GFP transgenic mice.
CCK-GFP BAC transgenic mice were obtained from the Mutant Mouse Regional Resource Center located in Columbia, MO. The transgenic mice had been developed by GENSAT (Gene Expression Nervous System Atlas) to express eGFP in the CCK-producing cells of the brain of embryonic and adult mice (19). These mice were bred to Swiss-Webster (Taconic) wild-type mice to establish a colony in our laboratory (9). All studies were approved by the Institutional Animal Care and Use Committee at Duke University.
Immunochemistry of CCK-GFP cells.
CCK-GFP transgenic mice were fasted overnight and perfused with ice-cold 3.5% paraformaldehyde in PBS (10 mM sodium phosphate, pH 7.4, containing 0.9% sodium chloride). The proximal 10 cm of the small intestine were harvested, flushed to remove debris, and fixed in 3.5% paraformaldehyde for 3 h at 4°C. The tissue was then washed in PBS for 30 min at 4°C. It was sequentially equilibrated in 10% sucrose in 0.1 M sodium phosphate, pH 7.4, 20% sucrose in PBS, and 30% sucrose in PBS and frozen in optimum cutting temperature compound. Five-micrometer transverse sections of the mold were cut using a cryostat set at −19°C. Immunohistochemical staining was performed with CCK-specific antisera as previously described (9). CaSR was identified in slides prepared from intestinal sections. Slides were washed one time in PBST (PBS with 0.05% Tween 20), blocked in 5% normal donkey serum (diluted in PBST) for 45 min, rinsed one time with PBST, and incubated with 1:800 dilution of rabbit CaSR antibody (BIOMOL International, Plymouth Meeting, PA) for 30 min at room temperature. The slides were then washed two times in PBST (1 min) and incubated in 1:1,000 dilution of donkey anti-rabbit antibody coupled to Cy3 (Jackson ImmunoResearch Laboratories, West Grove, PA) for 30 min in the dark. Slides were washed two times with PBST (1 min), counterstained with mounting medium containing DAPI, and mounted with cover slips. Images were taken using a Zeiss 510 inverted confocal microscope as described previously (9).
Image acquisition.
Images were collected using Zeiss LSM software. Samples were imaged on a Zeiss inverted confocal microscope using ×40/1.3 oil (Zeiss Plan NeoFluar) or ×63/1.4 oil (Zeiss Plan Apochromat) objectives. Single optical sections were taken using sequential multitrack acquisition with excitation at 405 nm (DAPI), 488 nm (endogenous GFP), and 561 nm (Cy3), emission filters of BP 420–480, BP505–550, and LP575, with pinholes set to 1 Airy unit for each channel, line averaging of 8 or 16 at 1,024 or 2,048 pixel resolution. Maximal intensity projection of the Z-stack collected using the confocal microscope was constructed using Imaris 7.1.1 software.
Preparation of intestinal cells.
Intestinal cell isolation was performed as previously described with the following modifications (45). CCK-GFP transgenic mice were fasted overnight after which animals were killed, and ∼10 cm of the proximal small intestine were harvested. The intestine was flushed with PBS, everted, and threaded on a wire, and the ends were tied with suture. For flow cytometry, the intestine was incubated in oxygenated buffer [96 mM NaCl, 1.5 mM KCl, 8 mM KH2PO4, 5.6 mM Na2HPO4, 27 mM sodium citrate, pH 7.4, 2% Dextran 580,000, 2.5 mg/ml BSA, and 1 mg/ml hyaluronidase (Sigma Chemical)] for 10 min at 37°C in a water bath. The wires were then transferred to a new tube containing fresh buffer and incubated with shaking at ∼150–200 rpm for 20 min at 37°C. The dissociated cells were harvested by centrifugation at 300 g for 4 min, washed with incubation buffer, resuspended in RPMI medium containing gentamicin, and filtered through a sterile 40-μm nylon mesh cell strainer. For live cell imaging, secretion, and electrophysiology, intestine was incubated in Hank's balanced salt solution (HBSS) (GIBCO, Carlsbad, CA) for 10 min at 37°C and transferred to a cell dispersion solution consisting of HBSS containing 0.6 mg/ml collagenase CLSPA (Worthington, Lakewood, NJ), 0.02 μg/ml dispase I (Boehringer Mannhein, Indianapolis, IN), and 2% BSA. Cells were incubated at 37°C for 10 min with gentle shaking every 3 min. The mucosa was gently scraped with the back of a scalpel blade, and cells were aspirated into HBSS and centrifuged at 120 g for 3 min. Cells were transferred to HBSS containing 5 mM glucose, 10 mM HEPES, 0.1% BSA, and 2% FBS (Biowhittaker, Walkersville, MD). Immediately before imaging and secretion studies, cells were rinsed three times and incubated in HBSS with 5 mM glucose and 10 mM HEPES, pH 7.4.
Flow cytometry.
Cells were sorted at the Duke Comprehensive Cancer Center's Flow Cytometry Shared Resource. Propidium iodide (PI) was added to the cell suspension at a concentration of 1 μg/ml. Sorting and analyses were carried out in a Becton-Dickinson DiVa cell sorter. Dead cells were excluded by gating on forward and side scatter and by eliminating PI-positive events. Viable cells were sorted into RPMI medium containing 10 mg/ml BSA. Toward the end of sorting, when eGFP-positive cells had been collected, an equal number of non-GFP-expressing cells was collected as a control for quantitative PCR analysis.
Real-time PCR.
RNA was isolated using the RNeasy Micro kit (Qiagen, Valencia, CA) following the manufacturer's recommendations. RNA was eluted in 14 μl of pure water and analyzed on a Picochip (Agilent 2100 Bioanalyzer) for determination of concentration and integrity. RNA from three preparations was pooled to generate a sample for PCR analysis. RNA (50 ng) was reverse transcribed using the Quantiscript reverse transcription kit (Qiagen). The cDNA was preamplified using TaqMan Preamp Master mix (95°C for 10 min, 1 cycle; 95°C for 15 s, 60°C for 4 min, 14 cycles). Quantitative real-time PCR was performed with Taqman gene expression assays (Applied Biosystems, Foster City, CA) on Stratagene's Mx3000P QPCR System. The identifications of TaqMan gene expression assays used were as follows: β-actin (Mm02619580), CaSR (Mm00443375), CCK (Mm00446170), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Mm99999915). The cDNA expression values were compared using the delta delta cycle threshold calculations. The expression of target genes in GFP-positive CCK-producing cells was normalized to the non-GFP-positive cells (calibrator), and GAPDH was used as the normalizer.
Ca2+ imaging.
Single cells were loaded with 2.5 μM X-Rhod-1 with 0.1% Pluronic F147 (Molecular Probes, Eugene, OR) in HBSS. X-Rhod-1, a rhodamine derivative that shows a large shift in fluorescent intensity upon Ca2+ binding, was selected as a Ca2+ indicator because of its relatively low dissociation constant and empirically sufficient loading in CCK-GFP cells. Cells were washed in HBSS buffer containing 5 mM glucose and 10 mM HEPES, pH 7.4, plated on poly-d-lysine-coated glass cover slips, and placed in a perfusion chamber to which secretagogues were added.
Cells were imaged on an inverted Axio Imager confocal microscope equipped with GFP and RFP filters using a ×10 dry objective and LSM software provided by Zeiss. GFP signal was used to identify CCK-positive cells and the RFP filter to detect changes in X-Rhod-1 intensity corresponding to Ca2+ changes. A simple perfusion chamber was secured on the microscope.
Using Metamorph (Meta Imaging Series) software, GFP-positive cells were selected as a region of analysis. Regions containing non-GFP cells were selected as background. Green and red channel integrated intensities were recorded across time for these two regions. Intensity changes were expressed as percent change from time 0 for each condition.
Measurements of CCK secretion.
For measurements of CCK release in vitro, ∼3 × 106 dispersed cells were separated into aliquots in 24-well plates and incubated in a water bath at 37°C in room air. CCK was measured by a sensitive and specific radioimmunoassay as we have previously described using antisera no. 9322, which recognizes biologically active forms of CCK (33). The assay utilizes 125I-labeled CCK-8 as tracer and is sensitive to CCK concentrations in the low picomolar range. Cells were incubated with alanine or the aromatic amino acids phenylalanine or tryptophan. Control conditions included incubation media only (negative control) or a hyperpolarizing concentration of KCl (50 mM). The specificity of amino acid-stimulated effects was confirmed using the CaSR antagonist Calhex 231 (Santa Cruz Biotechnology, Santa Cruz, CA). CCK was measured in the incubation media after 15 min exposure to the appropriate reagent. Total protein content of wells was measured to ensure that equal numbers of cells were present in each well. The variation between wells was <5%. Cell viability was assessed before and after the secretion experiments by measuring lactate dehydrogenase (LDH) in the incubation media (14). None of the stimulatory conditions caused an increase in LDH release.
Electrophysiology studies.
Whole cell currents were recorded with the patch-clamp technique. CCK-GFP cells were plated on poly-d-lysine-coated glass cover slips. Each cover slip was placed in a 1-ml recording chamber and perfused with control or test solutions. The bath was grounded with an Ag-AgCl electrode separated from the bathing solution by a short Ringer-agar bridge. Recording pipettes were made from Corning 7052 borosilicate glass. When filled with the internal solution, they had resistances of 2–5 MΩ. The standard internal solution contained (in mM): 10 NaCl, 140 KCl, 10 HEPES, 2 EGTA, 5 MgATP, and 0.1 Na2-GTP (pH 7.4 with CsOH); the external solution contained (in mM): 140 NaCl, 4 KCl, 2 CaCl2, 2 MgCl2, 5 glucose, and 5 HEPES (pH 7.4 with NaOH). Experiments were performed at room temperature.
Data were filtered at 1 kHz and sampled at 5 kHz (8-pole Bessel filter, 3 dB down). The potential inside the pipette was clamped at the required level. Voltage commands and current measurements were performed using an Axonpatch-1D amplifier with a Digidata 1200 interface and pCLAMP 8.2 software (Axon Instruments) (16, 21, 23). The peak conductance of whole cell Ca2+ currents at each potential was calculated from the corresponding peak current (36).
Statistical analysis.
Experimental results were expressed as means ± SE. Statistical determinations of significance among groups were examined by one-way ANOVA with the Tukey posttest, using GraphPad Prism version 5.02 (GraphPad Software, San Diego, CA) or Student's t-test where appropriate.
RESULTS
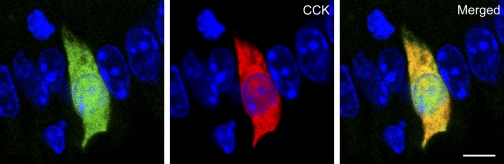
We used CCK-GFP mice to develop a model for studying native small intestinal CCK cells in vitro. To confirm that GFP was appropriately targeted to CCK cells, we immunostained cells for CCK and identified cells by dual immunofluorescence. GFP and CCK colocalized in I cells of transgenic mice with little aberrant GFP expression (Fig. 1). By virtue of their endogenous green fluorescence, we were able to isolate and study CCK cells in vitro.
Fig. 1.
Immunohistochemical characterization of cholecystokinin (CCK) cells from the proximal small intestine of a mouse in which enhanced green fluorescent protein (GFP) is expressed downstream from the mouse CCK promoter (CCK-GFP) mouse. Intestinal CCK cell expressing endogenous GFP (left), stained with CCK-specific antisera (center), and merged image showing colocalization of GFP and CCK (right). Microscopic images are from a section of the proximal small intestine of a CCK-GFP mouse. Sections were stained with DAPI to identify cell nuclei (blue). Images were captured with a ×63 objective and ×1.4 Zeiss LSM software zoom. Scale bar = 5 μm.
Identification of CaSR in CCK-GFP cells.
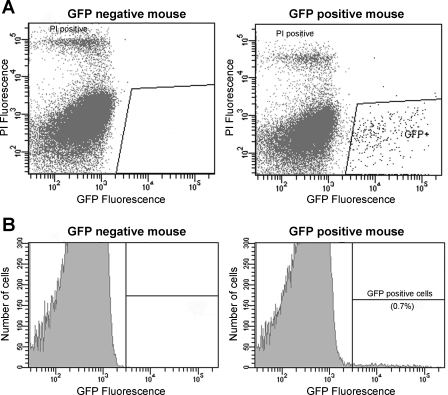
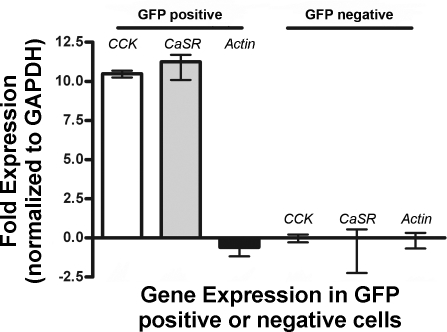
We and others have previously proposed that phenylalanine stimulates CCK secretion through a membrane receptor (32, 33) and CaSR appeared to be a likely candidate (10, 12). To investigate this possibility, we performed FACS to highly enrich CCK-GFP cells. Approximately 0.3–0.7% of mucosal cells were gated as GFP positive and isolated by FACS (Fig. 2). These cells were used for real-time PCR analysis. GFP-positive cells prepared from dispersed intestinal mucosa were compared with GFP-negative cells. As shown in Fig. 3, CCK-GFP cells, as expected, expressed CCK mRNA. Importantly, this same population of cells also expressed CaSR mRNA. β-Actin mRNA expression was similar in GFP-positive and GFP-negative cells.
Fig. 2.
Fluorescence-activated cell sorting (FACS) of GFP-positive cells. A: dispersed cells from GFP-negative or GFP-positive mouse proximal small intestine were subjected to FACS after addition of propidium iodide (PI). GFP signal was detected with a 530/30-nm bandpass filter and PI signal with a 575/26-nm bandpass filter. The PI-positive cells (top left) were considered nonviable and excluded. Fluorescent cells selected for sorting are shown in the area outlined. B: histogram showing the percent of GFP-positive cells (0.7% of total cells) that could only be identified when cells were isolated from a GFP-positive mouse duodenum. An equal number of positive and negative cells was collected and used for real-time PCR experiments.
Fig. 3.
Quantitative real-time PCR analysis of GFP-positive and GFP-negative cells isolated by FACS. Fold expressions of CCK, Ca2+-sensing receptor (CaSR), and actin RNAs [relative to the abundance of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) RNA] are shown. Data were obtained from 3 independent experiments.
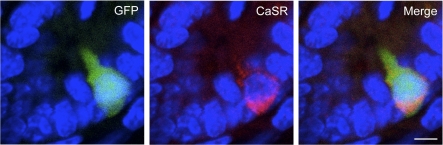
To further confirm expression of the CaSR protein and to possibly localize CaSR on CCK cells, we immunostained CCK-GFP cells with CaSR-specific antisera (Fig. 4). CaSR appeared on both the apical and basolateral regions of CCK cells. This localization of CaSR does not discriminate the site at which amino acids act to stimulate CCK cells (luminal or basolateral). During the course of these studies, we noticed that most but not all CCK cells expressed CaSR (Fig. 5). From 137 cells that stained positively for either GFP or CaSR, 29% expressed only GFP, 6% expressed only CaSR, and 65% expressed both CaSR and GFP.
Fig. 4.
CaSR immunostaining in CCK-GFP cells. Sections of small intestine from CCK-GFP mice expressing endogenous GFP (left) or stained with antisera against CaSR (center). A merged overlay of these images showing colocalization of CaSR in CCK-GFP cells is shown on the right. Images were obtained at ×40 magnification. Scale bar = 5 μm.
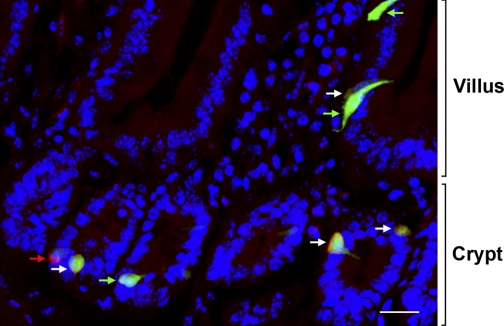
Fig. 5.
Distribution of GFP- and CaSR-immunostained cells in the CCK-GFP mouse duodenum. Maximum intensity projection of a Z-stack showing GFP-only positive cells (green arrows), CaSR-only positive cell (red arrow), and double-positive cells (white arrows). The crypt-villus axis is shown on the right. Scale bar = 20 μm.
Aromatic amino acids increase Ca2+ fluorescence in CCK-GFP cells.
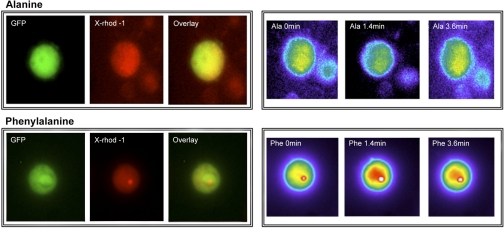
Small intestinal cells isolated from CCK-GFP transgenic mice were loaded with 2.5 μM X-Rhod-1, a rhodamine derivative that shows a large shift in fluorescence intensity upon Ca2+ binding. GFP-positive cells were selected as a region of analysis, and regions containing non-GFP cells were selected as background. As shown in Fig. 6, GFP-expressing cells could be easily identified among dispersed intestinal mucosal cells, whereas X-Rhod-1 was taken up in all cells. Cells of interest were selected, and changes in X-Rhod-1 intensity were captured over time. Phenylalanine (10 mM) produced significant elevations in Ca2+ fluorescence that lasted >5 min. Phenylalanine did not influence X-Rhod-1 fluorescence in non-GFP cells. In contrast to phenylalanine, the nonaromatic amino acid alanine did not produce an increase in [Ca2+]i in GFP-expressing cells.
Fig. 6.
Phenylalanine increases intracellular Ca2+ ([Ca2+]i) in intestinal CCK-GFP cells. Isolated CCK-expressing GFP cells were prepared from CCK-GFP transgenic mice and identified by endogenous GFP fluorescence (left, GFP). All cells were loaded with 2.5 μM X-Rhod-1 (X-Rhod-1) and identified by fluorescence using an RFP filter. Overlays of GFP and X-Rhod-1 images are shown (overlay). The same GFP-positive cells were imaged following addition of 30 mM l-alanine (row on top) or 10 mM l-phenylalanine (row on bottom). Increases in Ca2+ fluorescence were indicated by increased brightness (greater red intensity). As shown in the bottom right, an elevation in [Ca2+]i was seen within 1.4 min and lasted for >3.6 min following phenylalanine administration. In contrast, alanine did not produce an elevation in [Ca2+]i. Results are representative of >20 cells studied.
[Ca2+]i stores contribute to amino acid-stimulated [Ca2+]i.
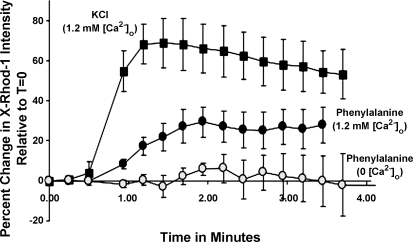
To determine whether the increases in [Ca2+]i required [Ca2+]o, we examined the effects of phenylalanine on Ca2+ fluorescence in CCK-GFP cells in Ca2+-free buffer or physiological buffer containing 1.2 mM Ca2+. As shown in Fig. 7, Ca2+ fluorescence was substantially reduced in Ca2+-free buffer, suggesting that phenylalanine-stimulated increases in [Ca2+]i result in large part from influx of [Ca2+]o. Of note, the L-type Ca2+ channel blocker diltiazem did not significantly reduce phenylalanine-stimulated Ca2+ fluorescence (data not shown).
Fig. 7.
Extracellular Ca2+ ([Ca2+]o)-dependent effects of phenylalanine on [Ca2+]i in intestinal CCK-GFP cells. Cells were treated with 30 mM phenylalanine (circles) or a depolarizing concentration of 50 mM KCl (squares), and [Ca2+]i fluorescence was measured. Experiments were performed in the presence (filled symbols) or absence (open symbols) of 1.2 mM [Ca2+]o. Results are from 5 cells/condition and are representative of 3 experiments.
Effects of aromatic amino acids on CCK secretion.
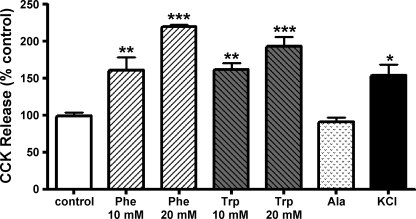
Mucosal cells were isolated from the proximal 10 cm of small intestine of CCK-GFP transgenic mice, and total CCK was measured by radioimmunoassay. Cells were incubated with l-stereoisomers of alanine, the aromatic amino acids phenylalanine or tryptophan, or hyperpolarizing concentrations of KCl. CCK was measured in the incubation media (Fig. 8). Both phenylalanine and tryptophan produced significant, dose-dependent increases in CCK secretion. In contrast, alanine did not stimulate CCK release. A concentration of KCl (50 mM) that is known to depolarize the plasma membrane of electrically excitable cells also caused CCK release. The d-stereoisomers of phenylalanine and tryptophan did not stimulate CCK release (data not shown).
Fig. 8.
Effects of amino acids on CCK secretion. Dispersed intestinal cells were incubated with 10 or 20 mM l-phenylalanine (Phe), l-tryptophan (Trp), l-alanine (Ala), or 50 mM KCl. CCK released in the media was measured by RIA. These data are representative of 3 experiments (n = 3). *P < 0.05, **P < 0.01, and ***P < 0.005.
Effects of CaSR blockade.
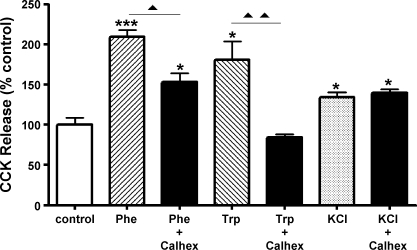
If CaSR is responsible for the effects of aromatic amino acids on CCK cells that have been described above, we would expect that blockade of CaSR would inhibit those effects. To this end, we tested the CaSR antagonist Calhex 231 on phenylalanine and tryptophan-stimulated CCK release. As shown in Fig. 9, CaSR inhibitor treatment significantly inhibited the effects of both phenylalanine and tryptophan but had no effect on KCl-stimulated CCK secretion.
Fig. 9.
CaSR antagonism with Calhex 231 blocks amino acid-induced CCK secretion. Cells were incubated in the presence or absence of Calhex 231 (20 μM) administered 5 min before addition of 20 mM l-phenylalanine, l-tryptophan, or 50 mM KCl. CCK released in the media was measured 15 min following the addition of test substances. *P < 0.05 and ***P < 0.005, amino acid vs. control; ▴P < 0.05 and ▴▴P < 0.01, amino acid vs. amino acid + Calhex 231. Results are from 1 of 3 experiments.
Electrophysiological properties of CCK cells.
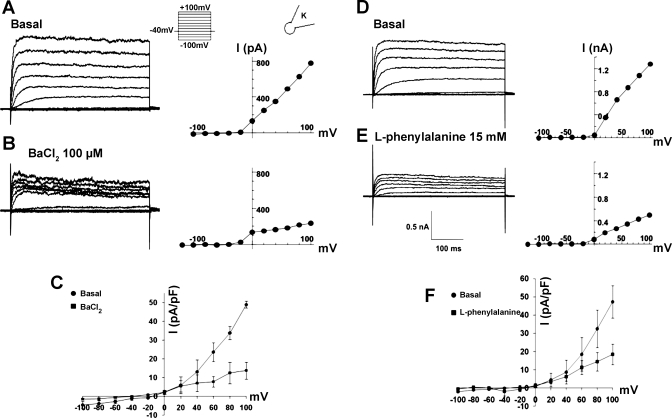
We have examined the membrane currents expressed in the CCK cells using the whole cell patch-clamp technique. We observed a strongly outwardly rectifying current in response to membrane depolarization (Fig. 10). The channel carries very little inward current at hyperpolarized potentials. Further analysis indicates that the current is carried by a delayed-rectifier K+ channel. The current was blocked by 100 μM Ba2+. Elevation of extracellular K+ ([K+]o) reduced the outward driving force for K+ and decreases the current (data not shown) . The current is blocked in a dose-dependent manner by the secretagogue phenylalanine.
Fig. 10.
Whole cell recordings from CCK-GFP cells showing inhibition of K+ current activity by barium chloride and l-phenylalanine. A and B: membrane currents (pulse protocol in inset, holding potential −40 mV, step size 20 mV) and current-voltage (I-V) plots during control and exposure to 100 μM barium. C: average data from the I-V relationship from 5 cells. D and E: currents and I-V plot from another cell during control and exposure to 15 mM l-phenylalanine. F: average data from 5 cells.
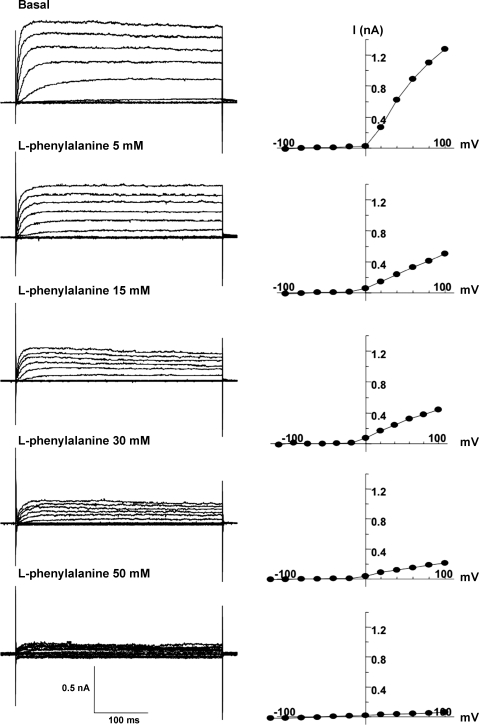
In the basal state, CCK cells are polarized by the K+ channel at their normal resting potential. The channel was activated at −20 mV, and the amplitude of the current increased progressively with further depolarization. The channel carried little current at hyperpolarized potentials. Elevation of [K+]o (5–25 mM) reduced the outward driving force of K+ and decreased the current. We could not determine an effect on the reversal potential of the current because of the strong outward rectification. The current was blocked by 100 μM barium (Fig. 10A). These characteristics identified the channel as an outwardly rectifying K+ channel. The current was blocked by l-phenylalanine in a dose-dependent manner (Fig. 10, D–F). Figure 11 shows the effects of 5–50 mM l-phenylalanine on the K+ current during continuous recording from a single cell. Supporting data from two to four cells during exposure to 5, 15, 30, or 50 mM concentrations of l-phenylalanine were obtained.
Fig. 11.
Dose-response effect of l-phenylalanine (5–50 mM) on membrane current and the resulting I-V relationships during continuous recording from a single cell. The voltage-clamp protocol was the same as that in Fig. 10A. l-Phenylalanine reduced the current in a dose-dependent manner.
Thus blockade of the channel or an increase of [K+]o depolarized the membrane into a potential range that activates an inward Ca2+ current. These data suggest that the delayed-rectifier channel plays a role in CCK secretion.
DISCUSSION
In the present study, we demonstrated that the aromatic amino acids l-phenylalanine and l-tryptophan stimulated the release of CCK from native intestinal CCK cells. Several lines of evidence indicated that [Ca2+]i was important in stimulating CCK release and that amino acids regulate this messenger through CaSR. First, phenylalanine and tryptophan stimulated an increase in [Ca2+]i as measured by Ca2+ fluorescence. Second, this increase was reduced by the removal of Ca2+ from the extracellular medium. Third, phenylalanine and tryptophan stimulated CCK secretion under the same conditions that were associated with increasing [Ca2+]i. Fourth, CCK-GFP cells express the CaSR. Finally, the effects of phenylalanine on [Ca2+]i and CCK release were blocked by the CaSR antagonist Calhex 231.
The role of Ca2+ signaling in CCK secretion has been examined previously in different experimental models. In rat mucosal cells, the CCK-releasing factor, monitor peptide, was shown to stimulate CCK secretion in Ca2+-containing media but not Ca2+-free media (3). In addition, the Ca2+ chelator EGTA and Ca2+ channel blocker manganese inhibited monitor peptide-stimulated CCK secretion. In STC-1 cells, bombesin stimulated CCK release in a Ca2+-dependent manner, and this effect was blocked by treatment with L-type Ca2+ channel blockers nicardipine or diltiazem, indicating that the CCK release required influx of [Ca2+]o into the cell through L-type Ca2+ channels to stimulate CCK secretion (42).
Native CCK cells are neuroendocrine in origin and are electrically excitable. Depolarization of the cell membrane induces CCK secretion, typical of other endocrine cell types (32). In both native intestinal CCK cells and in STC-1 cells, it appears that [Ca2+]o is necessary for CCK secretion. In isolated, vascularly perfused intestinal preparations, CCK release was observed following administration of the Ca2+ ionophore A-23187, peptone, or forskolin (13). These increases were blocked by removal of [Ca2+]o. The current study suggests that Ca2+ signaling pathways are different in native CCK cells and STC-1 cells. We have previously demonstrated that phenylalanine-stimulated increases in [Ca2+]i in STC-1 cells involved an L-type Ca2+ channel influx pathway, since secretion was blocked by the L-type Ca2+ channel blocker diltiazem (33). In addition, stimulation of CCK secretion was accompanied by an increase in cytosolic Ca2+ levels, an effect that was also blocked by diltiazem. Finally, patch-clamp recordings showed stimulation of Ca2+ channel activity after the addition of phenylalanine, indicating that phenylalanine stimulated release of CCK via a Ca2+-dependent process. In contrast, our findings in native CCK cells indicate that, although stimulation of CCK release is Ca2+ dependent, it does not involve L-type Ca2+ channel activation since the L-type Ca2+ channel blocker diltiazem had no effect in CCK-GFP cells.
CCK-GFP cells expressed a robust inward-rectifying potassium current. The modulation of this current is consistent with a significant role in the regulation of CCK secretion, since it is blocked by the l-aromatic acids phenylalanine and tryptophan but not by alanine. We propose that blockade of the inward rectifier depolarizes the cell into a range of potential at which Ca2+ entry occurs. We were not able to identify the Ca2+ entry pathway in our preliminary experiments; however, the results suggest that the coupling of Ca2+ entry to secretion is different in CCK-GFP and STC-1 cells.
CaSR recognizes and responds to (i.e., senses) [Ca2+]o as its principal physiological ligand (4). CaSR is expressed widely throughout the body, including parathyroid, kidney, and gastrointestinal tract, suggesting that this receptor has diverse functions in different tissues (6). It has been demonstrated that CaSR is activated not only by [Ca2+]o but also by amino acids, establishing its capacity to sense nutrients (12). In cells transfected with the cloned CaSR, l-amino acids, especially aromatic amino acids, including l-phenylalanine and l-tryptophan, stereoselectively mobilized Ca2+. In contrast, branched-chain amino acids were almost inactive, and charged amino acids, including arginine and lysine, were much less effective than aromatic amino acids. The profile of amino acids for activating CaSR is remarkably similar to that for stimulating CCK secretion.
CaSR has been noted on gastrinomas that, clinically, are sensitive to stimulation by elevated blood levels of Ca2+ (18). Moreover, gastrin-producing G cells and parietal cells of the stomach have been shown to express immunoreactive and functional CaSR (7, 40). Therefore, CaSR has been proposed to play a role in gastrin and gastric acid secretion (15). Subsequent studies indicated that CaSR participates in regulation of gastric acid secretion (8). Our data indicate that CaSR is highly expressed in CCK cells relative to other mucosal cells of the proximal small intestine.
Knowing that aromatic amino acids are agonist ligands of CaSR, it seemed reasonable that CaSR could mediate the ability of amino acids to stimulate CCK release from intestinal CCK cells (7) if, indeed, CCK cells possess CaSR. Our results demonstrate that CCK cells express CaSR mRNA as well as CaSR protein. Together these data suggest that CaSR mediates the ability of amino acids to stimulate CCK secretion. Immunostaining of CCK cells confirmed expression of the CaSR protein. However, staining did not localize CaSR exclusively to the apical or basolateral surface.
In addition to Ca2+, highly positively charged organic molecules, such as polyamines (e.g., spermine), polyarginine, and aminoglycoside antibiotics (e.g., neomycin) (5, 44), directly interact with and activate CaSR. These polycationic agonists are termed type 1 agonists and have the ability to activate the receptor even in the absence of [Ca2+]o. In contrast, type 2 agonists, which include aromatic l-amino acids, require [Ca2+]o to activate the CaSR (38). Our demonstration that phenylalanine's effects on [Ca2+]i require [Ca2+]o is consistent with its actions as a type 2 CaSR agonist. The physiological importance of circulating amino acids to regulate CaSR's activation outside the gastrointestinal tract is uncertain (11). However, our current findings suggest that amino acids may play a physiologically important role in nutrient regulation of gastrointestinal hormone secretion.
In a limited number of previous studies, the release of CCK has been examined in freshly isolated, partially enriched, intestinal I cells (26, 30). In canine jejunal cells, secretion of CCK was stimulated by depolarization of the plasma membrane by high potassium or by elevations in cAMP levels (2). We previously observed that stimulation of CCK secretion by potassium depolarization was inhibited by removal of [Ca2+]o (34, 35, 42). Here we have examined the membrane currents expressed in CCK-GFP cells using the whole cell patch-clamp technique. We observed a strongly outwardly rectifying current in response to membrane depolarization. Our preliminary analysis suggests a role of the delayed-rectifier K+ channel in the control of CCK secretion. We propose that, in the basal state, CCK cells are polarized by the K+ channel at their normal resting potential. Blockade of the channel or an increase of [K+]o depolarizes the membrane into a potential range that activates an inward Ca2+ current. Such a current would link membrane depolarization to CCK secretion. Future studies should examine the precise mechanism by which CaSR couples to K+ and Ca2+ channel activities.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-38626 and the Duke Comprehensive Cancer Center Core Facility.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Anika SM, Houpt TR, Houpt KA. Satiety elicited by cholecystokinin in intact and vagotomized rats. Physiol Behav 19: 761–766, 1977 [DOI] [PubMed] [Google Scholar]
- 2. Barber DL, Walsh JH, Soll AH. Release and characterization of cholecystokinin from isolated canine jejunal cells. Gastroenterology 91: 627–636, 1986 [DOI] [PubMed] [Google Scholar]
- 3. Bouras EP, Misukonis MA, Liddle RA. Role of calcium in monitor peptide-stimulated cholecystokinin release from perifused intestinal cells. Am J Physiol Gastrointest Liver Physiol 262: G791–G796, 1992 [DOI] [PubMed] [Google Scholar]
- 4. Brown EM. Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol Rev 71: 371–411, 1991 [DOI] [PubMed] [Google Scholar]
- 5. Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 366: 575–580, 1993 [DOI] [PubMed] [Google Scholar]
- 6. Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 81: 239–297, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Buchan AM, Squires PE, Ring M, Meloche RM. Mechanism of action of the calcium-sensing receptor in human antral gastrin cells. Gastroenterology 120: 1128–1139, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Ceglia L, Harris SS, Rasmussen HM, Dawson-Hughes B. Activation of the calcium sensing receptor stimulates gastrin and gastric acid secretion in healthy participants. Osteoporos Int 20: 71–78, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chandra R, Samsa LA, Vigna SR, Liddle RA. Pseudopod-like basal cell processes in intestinal cholecystokinin cells. Cell Tissue Res 341: 289–297, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Conigrave AD, Brown EM. Taste receptors in the gastrointestinal tract. II. L-amino acid sensing by calcium-sensing receptors: implications for GI physiology. Am J Physiol Gastrointest Liver Physiol 291: G753–G761, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Conigrave AD, Mun HC, Brennan SC. Physiological significance of L-amino acid sensing by extracellular Ca(2+)-sensing receptors. Biochem Soc Trans 35: 1195–1198, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Conigrave AD, Quinn SJ, Brown EM. L-amino acid sensing by the extracellular Ca2+-sensing receptor. Proc Natl Acad Sci USA 97: 4814–4819, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cuber JC, Bernard G, Fushiki T, Bernard C, Yamanishi R, Sugimoto E, Chayvialle JA. Luminal CCK-releasing factors in the isolated vascularly perfused rat duodenojejunum. Am J Physiol Gastrointest Liver Physiol 259: G191–G197, 1990 [DOI] [PubMed] [Google Scholar]
- 14. Decker T, Lohmann-Matthes ML. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods 115: 61–69, 1988 [DOI] [PubMed] [Google Scholar]
- 15. Dufner MM, Kirchhoff P, Remy C, Hafner P, Muller MK, Cheng SX, Tang LQ, Hebert SC, Geibel JP, Wagner CA. The calcium-sensing receptor acts as a modulator of gastric acid secretion in freshly isolated human gastric glands. Am J Physiol Gastrointest Liver Physiol 289: G1084–G1090, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Fisher RE, Gray R, Johnston D. Properties and distribution of single voltage-gated calcium channels in adult hippocampal neurons. J Neurophysiol 64: 91–104, 1990 [DOI] [PubMed] [Google Scholar]
- 17. Gibbs J, Falasco JD, McHugh PR. Cholecystokinin-decreased food intake in rhesus monkeys. Am J Physiol 230: 15–18, 1976 [DOI] [PubMed] [Google Scholar]
- 18. Goebel SU, Peghini PL, Goldsmith PK, Spiegel AM, Gibril F, Raffeld M, Jensen RT, Serrano J. Expression of the calcium-sensing receptor in gastrinomas. J Clin Endocrinol Metab 85: 4131–4137, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425: 917–925, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Green GM, Levan VH, Liddle RA. Interaction of dietary protein and trypsin inhibitor on plasma cholecystokinin and pancreatic growth in rats. Adv Exp Med Biol 199: 123–132, 1986 [DOI] [PubMed] [Google Scholar]
- 21. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- 22. Herzig KH, Schon I, Tatemoto K, Ohe Y, Li Y, Folsch UR, Owyang C. Diazepam binding inhibitor is a potent cholecystokinin-releasing peptide in the intestine. Proc Natl Acad Sci USA 93: 7927–7932, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hess P, Lansman JB, Tsien RW. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature 311: 538–544, 1984 [DOI] [PubMed] [Google Scholar]
- 24. Hira T, Nakajima S, Eto Y, Hara H. Calcium-sensing receptor mediates phenylalanine-induced cholecystokinin secretion in enteroendocrine STC-1 cells. FEBS J 275: 4620–4626, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab 296: E603–E613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koop I, Buchan AM. Cholecystokinin release from isolated canine epithelial cells in short-term culture. Gastroenterology 102: 28–34, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Liddle RA. Cholecystokinin . In: Gut Peptides, edited by Walsh JH, Dockray GJ. New York: Raven, 1994, p. 175–216 [Google Scholar]
- 28. Liddle RA. Regulation of cholecystokinin secretion by intraluminal releasing factors. Am J Physiol Gastrointest Liver Physiol 269: G319–G327, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest 75: 1144–1152, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liddle RA, Misukonis MA, Pacy L, Balber AE. Cholecystokinin cells purified by fluorescence-activated cell sorting respond to monitor peptide with an increase in intracellular calcium. Proc Natl Acad Sci USA 89: 5147–5151, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Louie DS, May D, Miller P, Owyang C. Cholecystokinin mediates feedback regulation of pancreatic enzyme secretion in rats. Am J Physiol Gastrointest Liver Physiol 250: G252–G259, 1986 [DOI] [PubMed] [Google Scholar]
- 32. Mangel AW. Electrophysiology of intestinal cholecystokinin secretion. Regul Pept 56: 121–129, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Mangel AW, Prpic V, Wong H, Basavappa S, Hurst LJ, Scott L, Garman RL, Hayes JS, Sharara AI, Snow ND, Walsh JH, Liddle RA. Phenylalanine-stimulated secretion of cholecystokinin is calcium dependent. Am J Physiol Gastrointest Liver Physiol 268: G90–G94, 1995 [DOI] [PubMed] [Google Scholar]
- 34. Mangel AW, Scott L, Liddle RA. Depolarization-stimulated cholecystokinin secretion is mediated by L-type calcium channels in STC-1 cells. Am J Physiol Gastrointest Liver Physiol 270: G287–G290, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Mangel AW, Snow ND, Misukonis MA, Basavappa S, Middleton JP, Fitz JG, Liddle RA. Calcium-dependent regulation of cholecystokinin secretion and potassium currents in STC-1 cells. Am J Physiol Gastrointest Liver Physiol 264: G1031–G1036, 1993 [DOI] [PubMed] [Google Scholar]
- 36. Marty A, Neher E. Tight-seal whole-cell recording. In: Single-Channel Recording, edited by Sakmann B, Neher E. New York, NY: Plenum, 1995, p. 31–52 [Google Scholar]
- 37. Meyer JH, Kelly GA. Canine pancreatic responses to intestinally perfused proteins and protein digests. Am J Physiol 231: 682–691, 1976 [DOI] [PubMed] [Google Scholar]
- 38. Nemeth EF, Steffey ME, Hammerland LG, Hung BC, Van Wagenen BC, DelMar EG, Balandrin MF. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci USA 95: 4040–4045, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Owyang C, Louie DS, Tatum D. Feedback regulation of pancreatic enzyme secretion. Suppression of cholecystokinin release by trypsin. J Clin Invest 77: 2042–2047, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ray JM, Squires PE, Curtis SB, Meloche MR, Buchan AM. Expression of the calcium-sensing receptor on human antral gastrin cells in culture. J Clin Invest 99: 2328–2333, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rehfeld JF. Cholecystokinin. Clin Gastroenterol 9: 593–607, 1980 [PubMed] [Google Scholar]
- 42. Snow ND, Prpic V, Mangel AW, Sharara AI, McVey DC, Hurst LJ, Vigna SR, Liddle RA. Regulation of cholecystokinin secretion by bombesin in STC-1 cells. Am J Physiol Gastrointest Liver Physiol 267: G859–G865, 1994 [DOI] [PubMed] [Google Scholar]
- 43. Taylor PM. Amino acid transporters: eminences grises of nutrient signalling mechanisms? Biochem Soc Trans 37: 237–241, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Tfelt-Hansen J, Brown EM. The calcium-sensing receptor in normal physiology and pathophysiology: a review. Crit Rev Clin Lab Sci 42: 35–70, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Ziomek CA, Schulman S, Edidin M. Redistribution of membrane proteins in isolated mouse intestinal epithelial cells. J Cell Biol 86: 849–857, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]