Abstract
Podocytes are considered terminally differentiated cells in the mature kidney under normal conditions. In the face of injury, podocytes may proceed along several possible pathways, including dedifferentiation and proliferation, persistent cell cycle arrest, hypertrophy, apoptosis, or necrosis. There is mounting evidence that transdifferentiation into a dysregulated phenotype may also be a potential cell fate. We have previously reported that the transcript of SM22α, an actin-binding protein considered one of the earliest markers of smooth muscle differentiation, is upregulated nearly 70-fold in glomeruli of rats with passive Heymann nephritis (PHN). In contrast, the SM22α transcript is absent in normal adult rat glomeruli. The purpose of this study was to define SM22α's expression during kidney development and its role in glomerular diseases characterized by podocyte injury and proteinuria. During glomerulogenesis and podocyte differentiation, SM22α was expressed in glomeruli. This expression disappeared with glomerular maturation. Along with SM22α induction in PHN, confirmed at both mRNA and protein levels, SM22α was also induced across a broad range of proteinuric diseases, including experimental animal models (puromycin aminonucleoside nephropathy, adriamycin nephropathy, passive nephrotoxic nephritis, and diet-induced obesity) and human diseases (collapsing glomerulopathy, diabetic nephropathy, classic focal segmental glomerulosclerosis, IgA nephropathy, minimal-change disease, membranous nephropathy, and membranoproliferative glomerulonephritis). Crescentic glomerulonephritis was induced in SM22α +/+ and SM22α −/− mice by intraperitoneal injection of sheep anti-rabbit glomeruli antibody 12.5 mg/20 g body wt × 2 doses (n = 12–15/group), with mice euthanized at 7 and 14 days. Compared with SM22α −/− mice, SM22α +/+ mice demonstrated worse disease by histopathological parameters. In addition, there was greater apoptosis (cleaved caspase-3 immunostaining), fewer podocytes (Wilms' tumor-1 immunostaining), and less proliferation (Ki-67 immunostaining) in diseased SM22α +/+ mice. Furthermore, there was decreased activation of Erk1/2 in diseased SM22α +/+ mice. We conclude that the de novo expression of SM22α in glomerular epithelial cells affects the course of crescentic glomerulonephritis.
Keywords: podocyte, transgelin, crescentic glomerulonephritis, apoptosis, proliferation, transdifferentiation
diseases of the glomerulus are leading causes of chronic and end-stage kidney disease. Glomerular diseases can be categorized based on the principal cell type injured, considered a result of injury primarily to four resident glomerular cell types: mesangial cell, visceral epithelial cell (podocyte), endothelial cell, and/or parietal epithelial cell (PEC). Besides the cell type, the form of injury determines the response to injury, ultimately governing the histological and clinical manifestations of disease.
The highly specialized and terminally differentiated podocyte, a critical component of the glomerular filtration barrier, is a major target of injury in both diabetic and nondiabetic glomerular disease. It functions to prevent urinary protein leakage and to provide structural support to the capillary wall, thereby counteracting the hydrostatic pressure within the glomerulus (48). Common to many human kidney diseases and experimental animal models is a strong link between podocyte injury and progressive kidney disease. In fact, there is compelling evidence that a decline in podocyte number strongly correlates with, and likely underlies, the development of glomerulosclerosis (39, 47, 63).
Over the last several years, an ever-expanding body of literature has emerged defining more clearly the structure of the podocyte as a polarized cell that consists of three primary compartments: 1) a cell body containing a thin layer of cortical actin and a microtubule organizing center, 2) the major or “primary” foot processes containing microtubules and vimentin-type intermediate filaments, and 3) the interdigitating foot processes containing an elaborate microfilament-based contractile apparatus composed of actin, myosin-II, α-actinin, talin, and vinculin (25, 41). The podocyte's complex actin cytoskeleton provides support to the glomerular tuft and facilitates the cell's ability to alter shape continually and dynamically as it responds to demands of the filtration process.
A large number of actin-binding proteins have evolved to facilitate and manipulate actin filament formation and turnover. Two recently identified actin-binding proteins in podocytes are α-actinin-4 and synaptopodin. Mutations of α-actinin-4 lead to podocyte foot process effacement and proteinuria in both humans and experimental animal models (27, 29). Synaptopodin interacts directly with α-actinin-4 and modulates its expression by elongating α-actinin-4-induced actin filaments (3, 42). Indeed, proteinuria may occur in response to mutations in any one of a number of proteins that form the highly complex filtration slit diaphragm between adjacent interdigitating podocyte foot processes. Thus proteins regulating and stabilizing the actin cytoskeleton are critical in the podocyte's normal function.
Although our understanding of the mechanisms of glomerular injury, and more specifically podocyte injury, has been advanced significantly over the last several years, in many glomerular diseases, the exact molecular pathways leading to injury have not yet been fully defined. To devise newer and more specific therapeutic interventions, there is a need to understand more fully the mediators involved in the initiation and progression of glomerular injury, particularly at the cellular level. In a quest to identify novel mediators of glomerular injury, we performed a microarray study of mRNA extracted from isolated rat glomeruli at days 3 and 6 of passive Heymann nephritis (PHN), the experimental model of membranous nephropathy. In this study, we found an upregulation of SM22α mRNA, with a 69.77-fold increase at day 3 and a 38.66-fold increase at day 6 of disease (22).
SM22α, also known as transgelin, is a shape change-sensitive 22- to 25-kDa actin-binding protein of the calponin family, localized to the cytoskeleton apparatus. SM22α is considered a smooth muscle cell (SMC) lineage-restricted protein, expressed abundantly and exclusively in visceral and vascular SMCs postnatally and is one of the earliest markers of smooth muscle differentiation. For many years, its function was unknown. Although SM22α localizes to the cytoskeleton of SMCs (58), SM22α −/− mice are viable, fertile, and exhibit no obvious phenotypic abnormalities (66). However, more recently, several functions have been elucidated, including SM22α's role in 1) organization of actin distribution (21, 66); 2) inhibition of phenotypic modulation of SMCs from contractile to synthetic/proliferative cells (14); 3) regulation of calcium-independent SMC contraction (26); 4) proliferation (11); 5) cell migration (18); and 6) tumor suppression (4, 43, 65).
In this study, we sought to determine why SM22α, a protein considered to be a marker of SMC differentiation, would be expressed de novo in glomeruli in diseases characterized by podocyte injury and proteinuria, and what role SM22α may be playing in glomerular epithelial cell (GEC) injury.
MATERIALS AND METHODS
Rodent Disease Models Characterized by Podocyte Injury and Proteinuria
The animal care committee of the University of Washington School of Medicine reviewed and approved the experimental protocols of all proteinuric disease models detailed below. All animal procedures were conducted in accord with the Institutional Animal Care and Use Committee.
We have previously reported that the mRNA expression of smooth muscle protein SM22α was increased in isolated glomeruli of the PHN rat model of membranous nephropathy. In contrast, the transcript of SM22α is absent in glomeruli isolated from control rats (22). To determine SM22α expression during kidney development and in immune- and non-immune-mediated forms of podocyte injury, we examined mouse embryonic and early postnatal kidney tissues and kidney biopsies obtained from rodent disease models characterized by podocyte injury and proteinuria (49).
Embryonic and Early Postnatal Kidney Development
The kidney tissues from mice during embryonic and early postnatal development, at time points before full kidney development, were evaluated to determine baseline expression of SM22α during active glomerulogenesis and podocyte differentiation. Mice were euthanized at embryonic day 19 and postnatal days 1, 2, 5, and 8. Euthanasia was induced by cervical dislocation. SM22α immunostaining in kidneys was compared with that in normal adult mice.
Models of Podocyte Injury
PHN model of experimental membranous nephropathy.
In rats, the PHN model is a well-characterized analog of human membranous nephropathy. We have previously reported utilizing this model for transcriptional profiling to identify novel mediators in proteinuric diseases via a microarray study (22). PHN was induced in male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) by a single intraperitoneal injection of sheep anti-Fx1A antibody (5 ml/kg body wt) as previously described (49). Control animals were injected with preimmune sheep serum (5 ml/kg body wt). Animals were euthanized at days 3, 6, 10, and 30 following disease induction for renal biopsies and isolation of glomeruli (n = 5/group/time point). Euthanasia was induced by exsanguination following anesthetization with 100% ethyl ether (VWR, West Chester, PA).
Diet-induced obesity model of experimental obesity-related glomerulopathy.
Tissues were kindly provided by Dr. Francis Kim and Dr. Michael W. Schwartz of the University of Washington School of Medicine, Department of Medicine (Seattle, WA) in a tissue-sharing collaboration, during which the mice were euthanized for the purposes of their own study (28). Briefly, age-matched (6- to 12-wk-old) male C57BL6 mice were fed either low-fat (10% saturated fat) or high-fat (60% saturated fat) diets that were otherwise matched for calories and micronutrients for 8 wk. Before euthanasia, mice were anesthetized with isoflurane. Tissues were obtained for renal biopsies.
Puromycin aminonucleoside nephropathy model of experimental focal segmental glomerulosclerosis.
With hallmarks of podocyte foot process effacement and heavy proteinuria, puromycin aminonucleoside nephropathy (PAN) is considered analogous to the spectrum of minimal-change disease and focal segmental glomerulosclerosis (FSGS) in humans. The PAN model was induced in male Sprague-Dawley rats (Charles River Laboratories) as previously described (36). Rats aged 60 days, weighing 200–300 g, received a single injection of PA dissolved in 0.9% NaCl, at 6 mg/100 g body wt via the tail vein (n = 6/group/time point). The control animals were injected with an equal volume of 0.9% NaCl. Each animal was anesthetized with 100% ethyl ether in an inhalation chamber before tail vein injections. Following disease induction, rats were euthanized at days 7 and 14 by exsanguination following anesthetization with 100% ethyl ether. Tissues were obtained for renal biopsies.
Adriamycin nephropathy model of experimental FSGS.
The adriamycin (ADR) nephropathy model, proven to be a robust experimental analog of human FSGS, was chosen to examine more closely the expression of SM22α in the initiation and propagation of FSGS in mice. ADR nephropathy was induced in male BALB/c mice, a strain carrying the recessive ADR susceptibility gene, aged 12 wk, by tail vein injection of ADR (12 mg/kg body wt × 2 doses; n = 8/group) as previously described (35). Control animals were injected with an equal volume of vehicle only (0.9% NaCl). Euthanasia was induced by cervical dislocation at weeks 1, 2, 8, and 11. Tissues were obtained for renal biopsies.
Passive nephrotoxic nephritis model of experimental crescentic glomerulonephritis.
It has recently been reported that SM22α mRNA is upregulated in kidneys in anti-glomerular basement membrane (GBM) nephritis in rats (45). Therefore, we chose experimental crescentic glomerulonephritis (GN) in mice as the principal model in our efforts to delineate the role of SM22α in proteinuric diseases.
The SM-CreERT2(ki) transgenic mice were generated by “knocking in” a tamoxifen-activated Cre recombinase-encoding sequence into the endogenous SM22α locus as previously described (30). Genotypes of mice were determined by PCR analysis of tail DNA. The wild-type SM22α allele was detected using primers RF67 (5′-CTCAGAGTGGAAGGCCTGCTT-3′) and RF90 (5′-CACACCATTCTTCAGCCACA-3′), which amplify a 276-bp fragment spanning the 3′ side of intron 1 and the 3′ side of exon 2 of the SM22α gene. The SM-CreERT2(ki) allele was detected using primers RF67 (see above) and SC135 (5′-GGCGATCCCTGAACATGTCC-3′), which amplify a 22-bp product. Primer SC135 is located on the 5′ side of the cre gene (30). Genotyping was confirmed by immunohistochemistry (IHC) for SM22α in renal biopsy specimens. Homozygous SM-CreERT2(ki) transgenic mice do not express endogenous SM22α protein (14) and thus will be referred to in shorthand as “SM22α −/− mice” from this point forward. As previously reported, SM22α −/− mice are viable, fertile, and exhibit no obvious phenotypic abnormalities (14, 66).
After a preliminary dose-finding study, the passive nephrotoxic nephritis model, characterized by crescentic GN and progressive glomerulosclerosis, with podocyte injury as a prominent feature, was induced in male SM22α +/+ and SM22α −/− mice, aged 12 wk, by intraperitoneal injection of sheep anti-rabbit glomeruli antibody [12.5 mg/20 g body wt × 2 doses (at days 0 and 3; n = 12–15 animals/group)]. The sheep anti-rabbit glomeruli antibody was produced by immunizing sheep with whole rabbit glomeruli, as previously described (49). Anti-serum was heat inactivated, and IgG was isolated using caprylic acid precipitation of serum proteins. Control animals did not receive the sheep anti-rabbit glomeruli antibody. Weekly and before death, a weight was recorded and urine was collected from each mouse in metabolic cages for 24-h protein and creatinine excretion. Urine protein was measured by the sulfosalicylic acid method (standards from Dade Diagnostics, Brisbane, Australia). Urine creatinine levels were determined via a colorimetric microplate assay (Cayman Chemical, Ann Arbor, MI), and a protein-to-creatinine ratio (PCR) was calculated.
Euthanasia was induced by cervical dislocation. Blood samples obtained at time of euthanasia were centrifuged (12,000 g for 5 min), and plasma was collected for measurement of blood urea nitrogen (BUN) via a QuantiChrom Urea Assay kit (Bioassay Systems, Hayward, CA). Tissues were obtained for renal biopsies.
Glomerular Protein Extraction and Western Blot Analysis
Protein was extracted from isolated glomeruli at days 3 and 6 of PHN rats and their respective negative controls (n = 5/time point/group). Preparation of isolated glomeruli was performed at 4°C, with kidney tissue from one rat used per preparation. After discarding of medulla and papilla, finely minced cortices were passed through three differential sieves (200, 140, 80 μm) using 2,000 ml of normal saline. Glomeruli were washed and resuspended in 50 ml of saline. Quantitation of glomeruli was performed in aliquots of glomerular suspension by phase microscopy, with purity >95% for all preparations. Following centrifugation (1,000 rpm at 4°C for 10 min), the supernatant was decanted, and protein isolation and purification were performed from the pelleted glomeruli. Briefly, the purified glomeruli were lysed in buffer A [20 mM Tris·HCl, pH 7.2, 2 mM MgCl2, 0.5% NP-40, 150 mM NaCl, 1 mM DTT containing 1× complete protease inhibitor cocktail (Roche, Indianapolis, IN) and the phosphatase inhibitors sodium orthovanadate (0.1 mmol/l) and sodium fluoride (50 mmol/l; Sigma-Aldrich, St. Louis, MO)]. Protein concentrations were determined by the BCA protein assay (Pierce, Rockford, IL).
We next performed Western blotting to ensure that SM22α protein expression was also upregulated in isolated glomeruli of PHN rats. Thirty micrograms of protein extracts from glomeruli taken from experimental and control rats, and 15 μg of protein extract from rat aorta as the positive control, were separated under reduced conditions on 12% SDS-PAGE and transferred to polyvinyldifluoride membranes (PerkinElmer, Boston, MA). Membranes were incubated with a polyclonal rabbit anti-SM22α antibody, kindly provided by Dr. Julian Solway (University of Chicago, Chicago, IL). To ensure equal protein loading, an antibody to housekeeping protein GAPDH (Abcam, Cambridge, MA) was used. Bands were detected with chromagen 5-bromo-4-chloro-3-inodyl phosphate/nitro blue tetrazolium (Sigma-Aldrich).
IHC
Indirect immunoperoxidase immunostaining, for SM22α, cleaved caspase-3, Wilms' tumor protein-1 (WT-1), phosphorylated p44/p42 MAPK (Erk1/2), Ki-67, nephrin, podocin, synaptopodin, desmin, collagen type I, and E-cadherin, was performed on formalin-fixed paraffin-embedded kidney specimens. Briefly, 4-μm tissue sections were deparaffinized in Histo-Clear (National Diagnostics, Atlanta, GA) and rehydrated in graded ethanol. Antigen retrieval was achieved with heat-induced epitope unmasking with EDTA, pH 6 (SM22α, nephrin, podocin); EDTA, pH 8 (cleaved caspase-3, synaptopodin); citrate, pH 6 (WT-1, Ki-67); and citrate, pH 7 (pErk1/2, E-cadherin). Endogenous peroxidases were blocked with 3% H2O2, followed by overnight incubation with primary antibody diluted in 1% BSA in PBS. The following primary antibodies were utilized: SM22α, diluted 1:100 (Abcam); cleaved-caspase-3, diluted 1:200 (Cell Signaling Technology, Danvers, MA); WT-1, diluted 1:1,000 (Santa Cruz Biotechnology, Santa Cruz, CA); pErk1/2, diluted 1:250 (Cell Signaling); Ki-67, diluted 1:100 (Thermo-Scientific, Fremont, CA); nephrin, diluted 1:1,500 (Fitzgerald, Concord, MA); podocin, diluted 1:2,000 (Abcam); synaptopodin, diluted 1:10 (Fitzgerald); desmin, diluted 1:100 (Abcam); collagen type I, diluted 1:100 (Millipore, Temecula, CA); and E-cadherin, diluted 1:100 (Cell Signaling). Omission of a primary antibody was used as a negative control. After washing in PBS, sections were incubated with biotinylated secondary antibody, diluted in 1% BSA in PBS, for 1 h at room temperature. For synaptopodin, Ki-67, desmin, and collagen type I, either the rabbit on rodent horseradish peroxidase-polymer (Biocare Medical, Concord, CA) or the ImmPRESS reagent (Vector Laboratories, Burlingame, CA) was used. ABC-Elite reagent (Vector Laboratories) was utilized for signal amplification, and 3,3′-diaminobenzidine (DAB) (Sigma-Aldrich) was utilized as a chromagen. Slides were counterstained with hematoxylin, dehydrated, and coverslipped. In addition, histopathology was examined after periodic acid-Schiff staining, and a disease severity score was calculated.
Human Diseases Characterized by Proteinuria
To validate the experimental data, renal pathology archives at the University of Washington Medical Center (Seattle, WA) were reviewed for representative biopsies from adult patients with diseases characterized by proteinuria of such severity that a renal biopsy was obtained (n = 2/disease category), including examples of collapsing glomerulopathy, advanced diabetic nephropathy, FSGS, IgA nephropathy, minimal-change disease, membranous nephropathy, and membranoproliferative glomerulonephritis (MPGN). A transplant protocol biopsy was utilized as the “normal” control. Standard procedures were used to process formalin-fixed tissue for light microscopic evaluation. The tissue sections were 3 μm in thickness. As a negative control, the primary antibody was omitted. The patient demographics and clinical data were blinded to the authors. The use of the tissues for this purpose was approved by the University of Washington Institutional Review Board.
Statistical Analysis
For comparison of mean values between two groups, the unpaired t-test was used. The quantitative analyses involve an assessment of all glomeruli within each tissue specimen, with the averages calculated across all animals within the group. All values are means ± SD or SE, except where otherwise indicated. Statistical significance was evaluated using GraphPad Prism version 5.02 (GraphPad Software, La Jolla, CA). The experimental findings were considered statistically significant if P < 0.05. All photomicrographs were made at similar intensity and background. During data analysis, the observer was blinded to the treatment categories.
RESULTS
SM22α is Present During Active Glomerulogenesis and Podocyte Differentiation
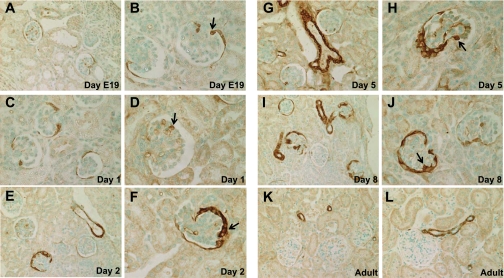
We performed IHC for SM22α in kidneys obtained at embryonic day 19 and postnatal days 1, 2, 5, and 8 in mice (Fig. 1). The newborn mouse kidney displays glomeruli at different stages of development, from the S-shaped body stage through the capillary loop stage to mature glomeruli. At the earliest time point, there was SM22α staining in presumptive podocytes and PECs (Fig. 1, A and B). The staining intensity progressively increased during the early postnatal development period (Fig. 1, C–J). In the mature normal kidney, there was no longer SM22α staining apparent in glomeruli. However, there remained expression in the vasculature, as would be expected for a smooth muscle lineage-restricted protein (Fig. 1, K and L). There results demonstrate that SM22α is transiently present in glomeruli during glomerulogenesis and podocyte differentiation and absent in normal adult glomeruli.
Fig. 1.
SM22α expression during active glomerulogenesis and podocyte differentiation. Representative micrographs of immunohistochemistry (IHC) for SM22α at embryonic day 19 (A and B), postnatal day 1 (C and D), day 2 (E and F), day 5 (G and H), day 8 (I and J), and in an adult mouse (K and L). During glomerular development, there was a progressive increase in the expression of SM22α, as determined by IHC, prenatally and during the early postnatal period, in a distribution that included developing podocytes, parietal epithelial cells, and endothelial cells (arrows indicate examples of positive cells). In adult mice, following kidney maturation, SM22α staining was absent in glomeruli, but remained positive in adjacent vessels.
SM22α De Novo Expression in PHN Model of Membranous Nephropathy
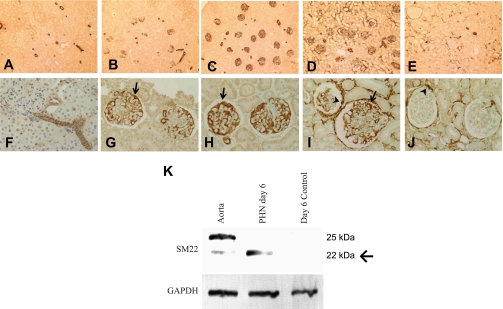
Given the microarray data that showed upregulation of SM22α in the PHN rat model of membranous nephropathy (22), we next examined SM22α expression by IHC at various stages of PHN. Correlating with data from normal adult mice, in tissue from control rats that received vehicle only, there was no glomerular staining for SM22α (Fig. 2, A and F). The positively stained vessels served as the internal positive control. By day 3 of disease, SM22α staining appeared in glomeruli in a podocyte distribution (Fig. 2, B and G). At day 6, the point of peak proteinuria, there was marked SM22α staining in glomeruli, most notably in the glomerular tuft (Fig. 2, C and H), which extended to PECs by day 10 (Fig. 2, D and I). Later in the disease course, the immunostaining had decreased in the glomerular tuft, but remains prominent in PECs and the interstitium at day 30 (Fig. 2, E and J). Thus in PHN there was de novo expression of SM22α in the glomerular tuft that later extended to PECs and the interstitium.
Fig. 2.
SM22α levels in passive Heymann nephritis (PHN) model of membranous nephropathy. A–J: representative micrographs of IHC for SM22α. Shown are control tissue (A and F) and tissue following disease induction at day 3 (B and G), day 6 (C and H), day 10 (D and I), and day 30 (E and J). In control tissue, only arterioles stained positive for SM22α. Following disease induction, there was a progressive increase in SM22α staining in glomeruli from days 3–10. By day 30, the immunostaining had decreased in the glomerular tuft, but remained prominent in parietal epithelial cells (PECs) and in the interstitium. Original magnification ×10 (A–E) and ×20 (F–J). Arrows indicate positive podocytes and arrowheads indicate positive PECs. K: Western blot for SM22α. In protein extracted from isolated normal glomeruli, no SM22α was detected. Protein extracted from isolated glomeruli of PHN rats showed abundantly expressed SM22α by day 6 of disease. Protein extracted from the aorta served as the positive control. GAPDH was used as a housekeeping protein to ensure equal protein loading. Arrow indicates band of interest at 22 kDa.
To validate the IHC results and to confirm that an increase in SM22α mRNA corresponded to an increase in protein expression, Western blot analysis was next performed on protein extracts from isolated glomeruli of control and PHN rats. Protein extracted from the aorta served as the positive control. At day 6, the protein extracted from glomeruli of control rats showed no expression of SM22α by Western blotting. In contrast, protein extracted from glomeruli of PHN rats at day 6 of disease showed the presence of a band at 22 kDa corresponding to SM22α. The Western blotting of protein extracted from the aorta showed bands at 22 and 25 kDa (Fig. 2K). Multiple bands on Western blotting for SM22α have been described in the literature and have been attributed to C-terminal proteolysis (16, 52). GAPDH was used as the housekeeping protein to ensure equal protein loading.
Together, these results show that SM22α transcript and protein levels are dramatically increased in PHN. Further studies delineated below were undertaken to determine whether SM22α was increased in other models of glomerular disease.
Upregulation of SM22α in Rodent Disease Models Characterized by Podocyte Injury and Proteinuria
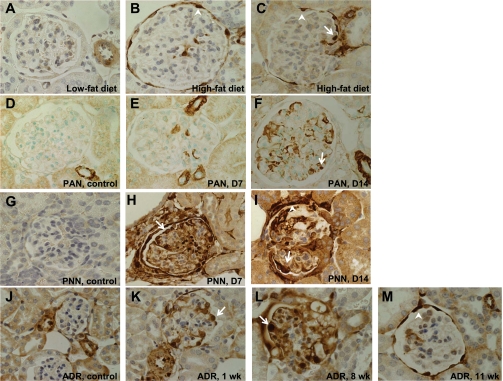
We next sought to determine whether the de novo expression of SM22α was a phenomenon that could be applied more generally to other models of podocyte injury and proteinuria, including the diet-induced obesity (DIO) mouse model of obesity-related glomerulopathy, the PAN rat model of experimental FSGS, the passive nephrotoxic nephritis mouse model of crescentic GN, and the ADR nephropathy mouse model of FSGS (Fig. 3). In DIO, tissue from mice fed a low-fat diet (10% saturated fat) for 8 wk did not show significant SM22α staining (Fig. 3A). In contrast, tissue from mice fed a high-fat diet (60% saturated fat) for 8 wk exhibited SM22α staining in both podocytes and PECs (Fig. 3, B and C). In PAN, there was a progressive increase in SM22α staining, with none seen in glomeruli from control rats and de novo appearance of intraglomerular staining by day 7 that increased at day 14 (Fig. 3, D–F). In experimental crescentic GN, tissue from mice that received vehicle alone did not show positive intraglomerular staining for SM22α. Following disease induction, there was a marked increase in SM22α staining in glomeruli at days 7 and 14 (Fig. 3, G–I). There was absent SM22α staining in tissue from ADR nephropathy mice that received vehicle only. By week 1 of disease, SM22α staining was present in a predominantly podocyte distribution. By week 8, staining involved both podocytes and PECs. At 11 wk, the cells positive for SM22α are mostly PECs. Thus we observed a pattern akin to that seen in PHN, with strong podocyte expression of SM22α during the early phase of disease that evolved into primarily PEC expression later in disease (Fig. 3, J–M). These results provide supporting data that the de novo expression of SM22α may have general applicability to kidney diseases characterized by podocyte injury and proteinuria.
Fig. 3.
SM22α IHC in rodent disease models characterized by podocyte injury and proteinuria. Representative micrographs of IHC for SM22α. Original magnification ×40. Arrows indicate examples of positive podocytes; arrowheads indicate examples of positive PECs. A–C: diet-induced obesity (DIO) in mice. A: in mice fed a low-fat diet, there was no significant glomerular staining for SM22α. An adjacent vessel served as the internal positive control. B and C: in mice fed a high-fat diet, there was positive SM22α staining in both podocytes and PECs. D–F: puromycin aminonucleoside nephropathy (PAN) in rats. D: in rats that received vehicle alone, there was positive staining for SM22α in vessels only. E and F: by day 7 of disease, and increasing by day 14, there was de novo expression of SM22α in podocytes. G–I: passive nephrotoxic nephritis (PNN) in mice. G: in mice that received vehicle alone, SM22α staining was negative in glomeruli. H and I: by day 7, and continuing at day 14, there was de novo expression of SM22α, with positive staining in podocytes and PECs. J–M: adriamycin (ADR) nephropathy in mice. J: in mice receiving vehicle only, there was no significant staining in glomeruli. K: by 1 wk after administration of ADR, there was de novo expression of SM22α in podocytes and PECs. L: staining of SM22α increased by week 8 following ADR administration. M: by week 11 of disease, the SM22α staining of podocytes had diminished, but the staining in PECs remained strong.
Upregulation of SM22α Expression in Human Diseases Characterized by Proteinuria
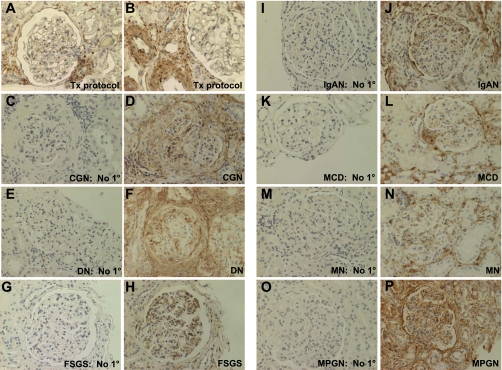
To determine whether the observations in rodent models were relevant to adult human disease, we next examined SM22α expression in human glomerular diseases characterized by proteinuria, including collapsing glomerulopathy, diabetic nephropathy, classic FSGS, IgA nephropathy, minimal-change disease, membranous nephropathy, and MPGN (Fig. 4). There was no significant staining for SM22α in the transplant protocol biopsy, considered the “normal” control (Fig. 4, A and B). In addition, there was no staining for SM22α when the primary antibody was omitted (Fig. 4, C, E, G, I, K, M, O). In contrast, in all diseases examined, there was marked intraglomerular staining for SM22α in a distribution that included podocytes, PECs, and mesangial cells. In some instances, there was also marked periglomerular tubulointerstitial staining associated with positively stained inflammatory cells (polymorphonuclear leukocytes) within the interstitium (Fig. 4, D, F, H, J, L, N, and P).
Fig. 4.
SM22α IHC in human diseases characterized by proteinuria. Representative micrographs of IHC for SM22α. A and B: protocol biopsy of transplant kidney tissue, considered “normal” control. There was no significant staining for SM22α within the glomeruli. The staining in vessels served as the internal positive control. C–P: in all cases, with omission of the primary antibody, there was no significant staining. In diseased tissues, there was positive intraglomerular staining for SM22α in a podocyte, PEC, and mesangial cell distribution. In some instances, there was marked periglomerular and tubulointerstitial staining associated with positively stained polymorphonuclear leukocytes within the interstitium. In addition, peritubular capillaries intensely stained positively for SM22α. C and D: collapsing glomerulopathy (CGN). E and F: diabetic nephropathy (DN). G and H: focal segmental glomerulosclerosis (FSGS). I and J: IgA nephropathy (IgAN). K and L: minimal change disease (MCD). M and N: membranous nephropathy (MN). O and P: membranoproliferative glomerulonephritis (MPGN).
Renal Function and Proteinuria in Diseased Mice with Crescentic GN
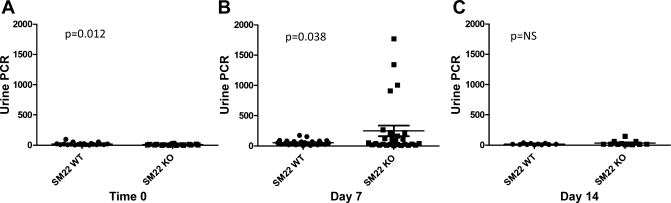
To determine the role of SM22α in experimental glomerular disease, crescentic GN was induced in SM22α +/+ and SM22α −/− mice. Biochemical parameters were assessed to determine the severity of renal dysfunction in SM22α +/+ and SM22α −/− mice. There was no statistically significant difference in plasma BUN between SM22α +/+ and SM22α −/− mice (data not shown). At baseline, before disease induction, the SM22α +/+ mice exhibited a statistically significantly higher urinary PCR (means ± SE; +/+: 21.28 ± 4.947; −/−: 8.835 ± 1.529; P = 0.0120) (Fig. 5A). At day 7 following disease induction, there was a statistically significant increase in the urinary PCR in SM22α −/− mice compared with SM22α +/+ mice (means ± SE; +/+: 54.83 ± 8.271; −/−: 248.9 ± 87.35; P = 0.038) (Fig. 5B). By 14 days following disease induction, there was no longer a statistically significant difference in the urinary PCR between SM22α +/+ and SM22α −/− mice (+/+: 16.09 ± 3.244; −/−: 34.22 ± 11.76; P = 0.168) (Fig. 5C).
Fig. 5.
Urinary protein-to-creatinine ratio (PCR) in SM22α +/+ and SM22α −/− mice at baseline and following induction of experimental crescentic GN. A: at time 0, SM22α +/+ mice had a statistically significantly greater PCR at baseline compared with SM22α −/− mice (P < 0.05). B: at day 7 of disease, SM22α −/− mice had a statistically significantly greater PCR compared with SM22α +/+ mice (P < 0.05). C: at day 14 following disease induction, there was no statistically significant difference in PCR between SM22α +/+ and SM22α −/− mice.
Less Disease in SM22α −/− Mice with Experimental Crescentic GN
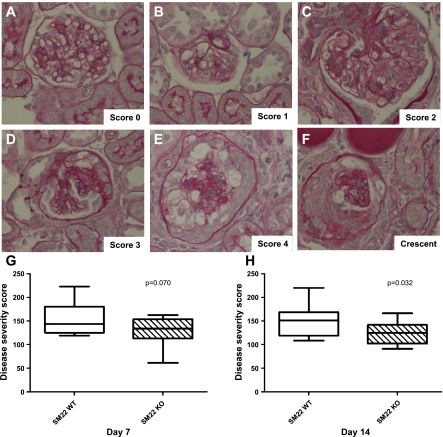
The histopathology of mice that received an anti-glomerular antibody was typified in the acute phase by capillary loop dilatation, mesangial matrix expansion and proliferative changes, cellular crescent formation, and infiltrating inflammatory cells as previously described (49) (Fig. 6). To assess semiquantitatively the severity of glomerular injury, a glomerular disease severity score (GDS score) was calculated by examining histopathological sections stained with periodic acid-Schiff, as a modification of what has been described previously (35), using the following scale: 0 = normal glomerulus (Fig. 6A), 1 = minimal disease with an affected area <25% of glomerular area (Fig. 6B), 2 = disease-affected area 25–50% of glomerular area (Fig. 6C), 3 = disease-affected area 50–75% of glomerular area (Fig. 6D), and 4 = disease-affected area >75% of glomerular area or presence of crescents (Fig. 6, E and F). The GDS score was calculated by multiplying the number of glomeruli with a severity score of 1 by one, 2 by two, and so on. These values were summed to obtain the final GDS score. By day 7 of disease, there was a not-yet-significant trend toward a higher GDS score in SM22α +/+ mice compared with SM22α −/− mice (means ± SE; +/+: 152.9 ± 9.507; −/−: 130.9 ± 7.131; P = 0.0704) (Fig. 6G). By day 14 following disease induction, there was a statistically significant difference in GDS score, with SM22α −/− mice showing less disease (+/+: 141.8 ± 9.763; −/−: 124.5 ± 6.853; P = 0.032) (Fig. 6H).
Fig. 6.
Histopathological changes by periodic acid-Schiff (PAS) staining in SM22α +/+ and SM22α −/− mice with experimental crescentic GN. A–F: histopathology (original magnification ×40). Representative micrographs of PAS staining of tissues from diseased mice are shown to illustrate the scoring system used to obtain the disease severity score. A: score 0 = normal glomerulus. B: score 1 = minimal disease, characterized by capillary loop dilatation. C: score 2 = mesangial matrix expansion and proliferative changes, affecting <50% of glomerular tuft. D: score 3 = proliferative changes affecting 50–75% of glomerular tuft. E: score 4 = proliferative changes affecting >75% of glomerular tuft. F: crescent, extracapillary proliferation of cells within the urinary space. G and H: disease severity score. G: at day 7 of disease, there was no significant difference in disease severity score between SM22α +/+ and SM22α −/− mice. H: at day 14 following disease induction, SM22α +/+ mice exhibited statistically significantly greater disease severity compared with SM22α −/− mice, as semiquantitatively assessed by disease severity score (P < 0.05).
Podocyte Apoptosis Decreased in SM22α −/− Mice in Experimental Crescentic GN
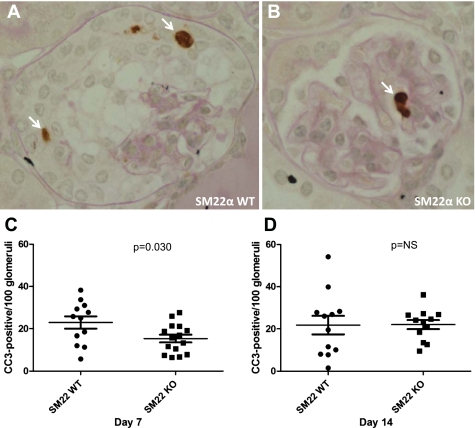
Given worse disease, by histopathological parameters, in SM22α +/+ mice with experimental crescentic GN, IHC for cleaved caspase-3, the activated form of the main effector caspase, was next performed as a surrogate marker of apoptosis as previously described (7). Activation, or cleavage, of caspase-3 occurs in response to a variety of proapoptotic stimuli and constitutes a final common pathway in the cell death process (Fig. 7). At day 7 of disease, there was a statistically significant difference in the number of positively stained glomerular cells for cleaved caspase-3 (per 100 glomeruli counted) between SM22α +/+ mice and SM22α −/− mice, with increased caspase-3 cleavage in SM22α +/+ mice (means ± SE; +/+: 22.91 ± 2.888; −/−: 15.39 ± 1.79; P = 0.030) (Fig. 7C). By day 14 following disease induction, the difference in caspase-3 cleavage was no longer statistically significant between the groups (+/+: 21.80 ± 4.385; −/−: 22.02 ± 2.157; P = 0.964) (Fig. 7D). Thus early in the course of experimental crescentic GN there is less apoptosis in SM22α −/− mice.
Fig. 7.
Apoptosis detection by IHC for cleaved caspase-3 (CC3) in SM22α +/+ and SM22α −/− mice following induction of crescentic GN. A and B: representative micrographs of CC3 staining in diseased glomerular tissue from SM22α +/+ mice (A) and SM22α −/− mice (B). Arrows indicate positively stained cells. C and D: quantification of positively stained cells for CC3 in tissues from diseased SM22α +/+ and SM22α −/− mice. C: at day 7 following disease induction, there were statistically significantly more cells staining positively for CC3 in SM22α +/+ tissue compared with SM22α −/− tissue (P < 0.05). D: by day 14, this difference was no longer statistically significant.
Podocyte Number Increased in SM22α −/− Mice with Experimental Crescentic GN
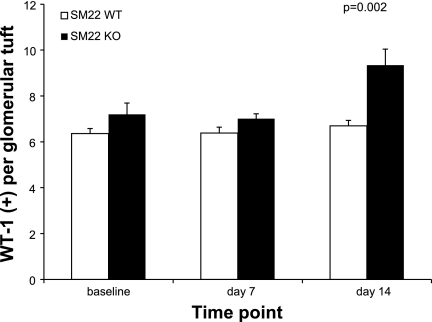
Because of increased apoptosis in SM22α +/+ mice at day 7 following disease induction, IHC for WT-1, a surrogate marker for podocyte number, was next performed to determine whether podocyte number changed in crescentic GN. By WT-1 staining, there was no significant difference in the baseline complement of podocytes between SM22α +/+ and SM22α −/− mice before disease induction (means ± SE; +/+: mean 6.359 ± 0.218; −/−: 7.20 ± 0.490; P = 0.166). By day 7 following disease induction, there was a not-yet-significant trend toward fewer WT-1-positive cells per glomerular tuft in SM22α +/+ vs. SM22α −/− mice (means ± SE; +/+: 6.386 ± 0.252; −/−: 7.007 ± 0.213; P = 0.071). By day 14 of disease, there was a statistically significant difference in the number of WT-1-positive cells between SM22α +/+ and SM22α −/− mice, with a greater number of positive cells seen in SM22α −/− mice (means ± SE; +/+: 6.695 ± 0.240; −/−: 9.340 ± 0.700; P = 0.002) (Fig. 8). Thus, correlating with the cleaved caspase-3 data, less apoptosis in SM22α −/− mice at the early time point in crescentic GN was associated with increased podocyte number at the later time point.
Fig. 8.
Wilms' tumor (WT)-1 IHC in SM22α +/+ and SM22α −/− mice in experimental crescentic GN. Quantification of WT-1-positive cells per glomerular tuft is shown. Before disease induction and at day 7 of disease, there was not a statistically significant difference in the number of WT-1-positive cells between groups. By day 14, there were significantly more WT-1-positive cells in glomeruli of SM22α −/− mice compared with SM22α +/+ mice (P < 0.05).
Increased Proliferation in SM22α −/− Mice with Experimental Crescentic GN
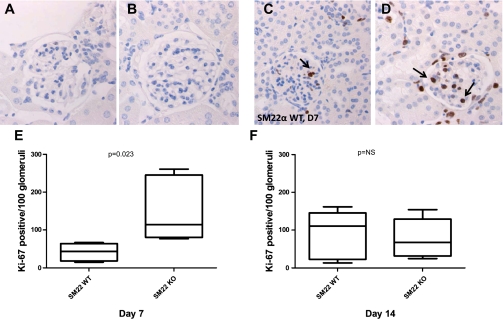
To determine whether the increase in podocyte number in SM22α −/− mice was due to increased proliferation, immunostaining for proliferation marker Ki-67 was next performed (Fig. 9). At baseline, there was no significant evidence of proliferation, by immunostaining for Ki-67, in both SM22α +/+ and SM22α −/− mice, as would be expected given very little turnover in the mature adult kidney under normal physiological conditions (Fig. 9, A and B). By day 7 following disease induction, there was greater proliferation in the SM22α −/− mice compared with that seen in the SM22α +/+ mice (means ± SE; +/+: 41.76 ± 10.37 positive cells in glomerular tuft/100 glomeruli; −/−: 153.2 ± 38.45; P = 0.023) (Fig. 9, C–E). By day 14, the difference between the groups was no longer statistically significant (+/+: 89.44 ± 28.52; −/−: 77.75 ± 23.41; P = 0.760) (Fig. 9F). Therefore, increased proliferation early in the course of disease, in association with decreased apoptosis, contributed to increased podocyte number in the SM22α −/− mice.
Fig. 9.
IHC for Ki-67 in SM22α +/+ and SM22α −/− mice in experimental crescentic GN. Representative micrographs of IHC for Ki-67 are shown. A and C: SM22α +/+ mice at baseline (A) and day 7 of disease (C). B and D: SM22α −/− mice at baseline (B) and day 7 of disease (D). Original magnification ×40. Arrows indicate examples of positively stained cells in a podocyte distribution. E and F: quantification of Ki-67-positive cells per 100 glomeruli counted. E: at day 7 following disease induction, there was a statistically significantly greater number of Ki-67-positive cells in tissue from SM22α −/− mice compared with SM22α +/+ mice (P < 0.05). F: this difference was no longer significant by day 14 of disease.
Reduced pErk1/2 in SM22α +/+ Mice in Experimental Crescentic GN
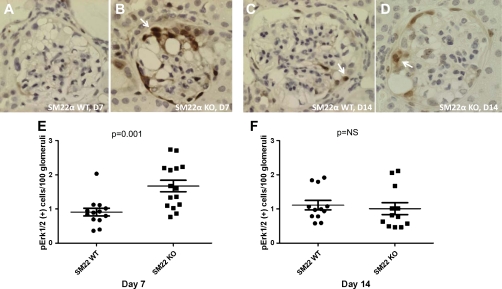
Erk1/2 are members of the MAPK superfamily mediating cell proliferation, cell survival, and apoptosis (37). We and others have previously identified pErk1/2 as an important mediator in podocyte survival and apoptosis in glomerular injury models (7). Therefore, we next performed IHC for pErk1/2 to determine its role in apoptosis observed in experimental crescentic GN (Fig. 10, A–D). At day 7 of disease, there were more cells per 100 glomeruli counted that stained positive for pErk1/2 in SM22α −/− mice compared with SM22α +/+ mice (means ± SE; +/+: 0.908 ± 0.114; −/−: 1.672 ± 0.169; P = 0.001) (Fig. 10E). By day 14 following disease induction, this difference was no longer statistically significant (+/+: 1.111 ± 0.137; −/−: 1.009 ± 0.175; P = 0.653) (Fig. 10F). These results provide supporting data that increased levels of pErk1/2 in SM22α −/− mice may confer a survival advantage.
Fig. 10.
IHC for pErk1/2 in SM22α +/+ and SM22α −/− mice in experimental crescentic GN. Representative micrographs of IHC for pErk1/2 are shown. A and C: SM22α +/+ mice at day 7 (A) and day 14 of disease (C). B and D: SM22α −/− mice at day 7 (B) and day 14 of disease (D). Original magnification ×40. Arrows indicate examples of positively stained cells in a podocyte and PEC distribution. E and F: quantification of pErk1/2-positive cells per 100 glomeruli counted. E: at day 7 following disease induction, there was a statistically significantly greater number of pErk1/2-positive cells in tissue from SM22α −/− mice compared with SM22α +/+ mice (P < 0.05). F: this difference was no longer significant by day 14 of disease.
Expression of Other Markers Associated with Phenotypic Transition
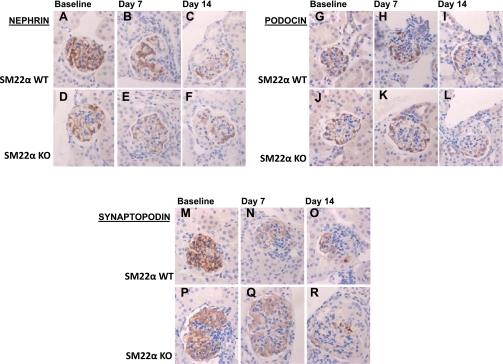
To determine whether the de novo expression of SM22α in glomeruli following injury is accompanied by other alterations in protein expression that might suggest a phenotypic transition, immunostaining to detect loss of podocyte-specific proteins (nephrin, podocin, and synaptopodin), loss of epithelial markers (E-cadherin), and acquisition of mesenchymal markers (desmin and collagen type I) was next performed (33). The staining for podocyte-specific proteins was compared by calculating a score, based on the percentage of the glomerular tuft area that had retained positive staining, using the following scale: 1 = area of preserved staining <25%, 2 = area of preserved staining 25–50%, 3 = area of preserved staining 50–75%, and 4 = area of preserved staining >75%. The total score was calculated by multiplying the number of glomeruli with a score of 1 by one, 2 by two, and so on. These values were summed to obtain the final score (Table 1). In general, immunostaining for all podocyte-specific proteins was decreased following disease induction. However, there was no significant difference in nephrin staining between SM22α +/+ and SM22α −/− mice (Table 1, Fig. 11, A–F). At baseline, podocin staining was significantly greater in SM22α +/+ mice compared with SM22α −/− mice, and this difference remained significant at day 14 following disease induction (Table 1, Fig. 11, G–L). There was no difference in synaptopodin staining between the two groups (Table 1, Fig. 11, M–R). These results suggest that the injury-induced glomerular expression of SM22α is not associated with a greater reduction in the expression of the podocyte-specific proteins nephrin, podocin, and synaptopodin.
Table 1.
Immunohistochemistry for podocyte-specific markers, SM22α WT vs. KO
| <25% |
25–50% |
50–75% |
>75% |
Total Score |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Podocyte-Specific Proteins | WT | KO | WT | KO | WT | KO | WT | KO | WT | KO | P Value |
| Nephrin | |||||||||||
| Baseline | 14.61 ± 14.08 | 27.49 ± 14.62 | 6.995 ± 3.25 | 47.86 ± 3.13 | 23.17 ± 6.78 | 21.10 ± 8.60 | 56.11 ± 10.20 | 3.88 ± 2.56 | 322.5 ± 40.57 | 202.0 ± 27.68 | 0.134 |
| Day 7 | 37.79 ± 7.68 | 39.63 ± 3.04 | 28.29 ± 2.10 | 28.08 ± 4.27 | 27.64 ± 5.84 | 25.96 ± 1.53 | 6.08 ± 2.39 | 6.13 ± 2.74 | 201.6 ± 18.41 | 198.2 ± 7.44 | 0.867 |
| Day 14 | 39.46 ± 7.51 | 36.74 ± 10.47 | 22.51 ± 3.06 | 26.29 ± 3.43 | 27.25 ± 3.91 | 29.89 ± 7.03 | 11.01 ± 4.46 | 7.83 ± 3.96 | 210.3 ± 16.56 | 210.3 ± 21.58 | 0.999 |
| Podocin | |||||||||||
| Baseline | 0.91 ± 0.02 | 17.51 ± 0.14 | 9.36 ± 1.80 | 54.89 ± 1.00 | 57.57 ± 2.90 | 23.88 ± 1.12 | 32.84 ± 4.94 | 3.73 ± 2.26 | 323.7 ± 7.47 | 213.8 ± 3.54 | 0.006* |
| Day 7 | 19.98 ± 11.94 | 15.72 ± 4.15 | 32.44 ± 2.90 | 42.93 ± 6.23 | 42.80 ± 12.47 | 37.48 ± 8.76 | 4.79 ± 2.81 | 3.87 ± 1.62 | 232.4 ± 27.70 | 229.5 ± 15.94 | 0.932 |
| Day 14 | 20.56 ± 2.14 | 32.99 ± 6.71 | 39.18 ± 3.48 | 52.11 ± 2.58 | 35.20 ± 3.24 | 14.92 ± 4.916 | 4.96 ± 1.85 | 0 | 224.3 ± 1.95 | 182 ± 11.77 | 0.024* |
| Synaptopodin | |||||||||||
| Baseline | 0 | 0.78 ± 0.13 | 1.26 ± 1.26 | 5.92 ± 2.72 | 10.44 ± 4.13 | 20.35 ± 0.80 | 88.53 ± 5.62 | 72.03 ± 0.24 | 387.9 ± 7.55 | 361.8 ± 4.11 | 0.093 |
| Day 7 | 20.35 ± 6.50 | 9.79 ± 3.74 | 27.43 ± 9.04 | 14.88 ± 5.68 | 27.85 ± 3.15 | 33.97 ± 4.95 | 24.72 ± 15.24 | 42.24 ± 3.73 | 257.6 ± 39.45 | 310.4 ± 15.50 | 0.281 |
| Day 14 | 17.11 ± 2.35 | 14.87 ± 4.33 | 14.99 ± 0.73 | 13.32 ± 3.56 | 22.11 ± 0.68 | 32.33 ± 3.75 | 46.05 ± 2.44 | 39.74 ± 10.53 | 297.6 ± 7.05 | 297.2 ± 21.13 | 0.985 |
Values are means ± SΕ expressed as the percentage of glomerular tuft area with positive staining. WT, wild-type; KO, knockout.
Significant difference (P < 0.05).
Fig. 11.
IHC for podocyte-specific proteins nephrin, podocin, and synaptopodin in SM22α +/+ and SM22α −/− mice in experimental crescentic GN. Representative micrographs of IHC for nephrin (A–F), podocin (G–L), and synaptopodin (M–R) are shown (original magnification ×40). At baseline, there is no significant difference in nephrin staining between SM22α +/+ and SM22α −/− tissues, with strong linear staining seen along the glomerular capillary loops (A and D). Following disease induction, there is diminished nephrin staining, with no significant difference between the two groups at the early (B and E) and late (C and F) time points. At baseline, there is more podocin staining in SM22α +/+ tissue compared with SM22α −/− tissue (G and J). At day 7 following disease induction, there is no significant difference in podocin staining for SM22α +/+ and SM22α −/− mice (H and K). By day 14 following injury, the SM22α +/+ tissue demonstrates greater podocin staining compared with that seen in SM22α −/− mice (I and L). At baseline, there is no significant difference in staining for synaptopodin in SM22α +/+ and SM22α −/− mice (M and P). Following disease induction, there is diminished synaptopodin staining, with no significant difference between the 2 groups at the early (N and Q) and late (O and R) time points.
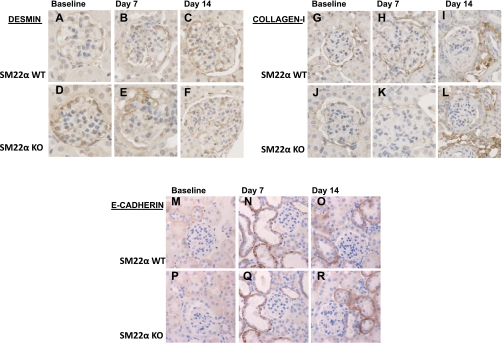
Upon examining other markers that have been reported to be lost or gained during phenotypic transition in epithelial cells in other disease models, we found that there was not a significant difference in the expression of desmin (Fig. 12, A–F), collagen type I (Fig. 12, G–L), and E-cadherin (Fig. 12, M–R) between SM22α +/+ and SM22α −/− mice, at baseline or following disease induction. Thus there was not a greater downregulation in podocyte-specific markers or an epithelial marker in the SM22α +/+ mice following disease induction; and there was not a greater upregulation in mesenchymal markers in the SM22α +/+ mice following disease induction.
Fig. 12.
IHC for desmin, collagen type I, and E-cadherin in SM22α +/+ and SM22α −/− mice in experimental crescentic GN. Representative micrographs of IHC for intermediate filament protein desmin (A–F), interstitial matrix component collagen type I (G–L), and epithelial marker E-cadherin (M–R) are shown (original magnification ×40). At baseline, there is no significant difference in desmin staining between SM22α +/+ and SM22α −/− tissues (A and D). Following disease induction, there is increased desmin staining within the glomerular tuft area, with no significant difference between the two groups at the early (B and E) and late (C and F) time points. At baseline, there is no significant staining for collagen type I within the glomerular tuft area (G and J). Following disease induction, there is no significant increase in staining within the glomerular tuft area, but there is an increase in tubulointerstitial staining in both SM22α +/+ and SM22α −/− mice (H and K, I and L). At baseline, there is no significant staining in the glomerular tuft area for E-cadherin in SM22α +/+ and SM22α −/− mice (M and P). Following disease induction, there is increased tubular epithelial staining for E-cadherin, with no significant difference between the 2 groups at the early (N and Q) and late (O and R) time points.
DISCUSSION
The highly specialized and terminally differentiated podocyte is a critical component of the glomerular filtration barrier. The podocyte's cytoarchitecture is due, in part, to an intricate actin cytoskeleton that scaffolds the glomerular tuft and facilitates the cell's ability to alter shape continually and dynamically in response to microenvironment changes and to demands of the filtration process. Proteins regulating and stabilizing the actin cytoskeleton are critical in the podocyte's normal function.
Underscoring the actin cytoskeleton's role in maintaining podocyte structure and function was the de novo expression of the actin-binding protein SM22α, traditionally considered a SMC lineage-restricted protein, in injured glomeruli across a diverse array of glomerular diseases. Although SM22α was transiently present in glomeruli during development, its expression was absent in normal adult glomeruli. A major finding in our study was that the later reappearance of SM22α within injured glomeruli may be a generalizable phenomenon during the initiation and/or progression of diseases characterized by podocyte injury and proteinuria. We found marked SM22α upregulation in experimental animal models of membranous nephropathy (22), FSGS, crescentic GN, and obesity-related glomerulopathy. Indeed, other investigators have shown independently that SM22α is upregulated in PAN (40) and anti-GBM nephritis (45). In addition, we found abundant expression of SM22α in human proteinuric diseases, including collapsing glomerulopathy, diabetic nephropathy, classic FSGS, IgA nephropathy, minimal-change disease, membranous nephropathy, and MPGN. Similarly, Miao et al. (40) have shown that SM22α is present in biopsies from children with minimal-change disease, FSGS, and membranous nephropathy. Taken together, the findings suggest that de novo expression of SM22α may occur broadly across many diseases affecting the glomerulus, both in humans and animals.
In addition to SM22α, upregulation of other SMC proteins in podocytes has been described. Saleem et al. (55) recently reported an upregulation of genes associated with SMC differentiation in cultured podocytes, including smoothelin and calponin, and demonstrated that podocytes have angiotensin II-modulated contractile properties and express proteins associated with a SMC phenotype, including smooth muscle (SM) myosin heavy chain, α-smooth muscle actin (α-SMA), and myocardin (55). Furthermore, Ransom et al. (51) detected gelsolin and capping protein by proteomic analysis in cultured podocytes, suggesting that the podocyte's actin cytoskeleton can undergo rapid reorganization. Thus there is a developing paradigm that podocytes may upregulate a repertoire of proteins traditionally thought to be restricted to SMCs.
To determine the role of glomerular injury-induced SM22α, we next focused on experimental crescentic GN. Crescentic GN has been a useful model for examining the potential for GEC transdifferentiation, characterized by loss of some phenotypes accompanied by acquisition of new phenotypes in differentiated cells (5). One of the best illustrations of cell transdifferentiation is epithelial-to-mesenchymal transdifferentiation (EMT). Ng et al. (17) and others (44) propose that EMT occurs in the anti-GBM model of crescentic GN, with PECs and, to a lesser extent, podocytes demonstrating de novo expression of α-SMA early in disease, accompanied by loss of the epithelial marker E-cadherin. During the course of disease, the normal cuboidal epithelium of PECs transforms into a morphology featuring an elongated cell body and loss of polarity, microvilli, and tight junctions (17, 44). Li et al. (32) report that injured podocytes are capable of EMT, with loss of epithelial P-cadherin, zonula occludens-1, and nephrin, and acquisition of mesenchymal Fsp1, desmin, collagen I, and fibronectin. This group proposes that EMT is a potential pathway leading to podocyte dysfunction and proteinuria (32). In the current study, following disease induction, there was decreased expression by immunostaining of podocyte proteins that play critical roles in maintaining the normal filtration barrier, namely, nephrin, podocin, and synaptopodin. However, the absence of SM22α did not lessen the degree of diminished staining seen following injury. Furthermore, we did not find a significant difference between groups in desmin, E-cadherin, or collagen type I immunostaining in our disease model. Therefore, whether the de novo expression of SM22α in GECs can be classified as an example of EMT is as yet unclear. We hypothesize, however, that the injured GEC may be transdifferentiating into a more SMC-like phenotype.
The pathways that lead to GEC transdifferentiation are poorly understood and are likely varied. The literature implicates growth factors such as transforming growth factor (TGF)-β as one mechanism potentially leading to GEC transdifferentiation (17, 32, 44). TGF-β receptors are increased in PECs by day 3 of crescentic GN. In addition, many cells within cellular and fibrocellular crescents express TGF-β1 (9), suggesting a dominant role for TGF-β1 in regulating the transdifferentiation process during the evolution of crescent formation (17, 44).
Over the years, multiple studies have highlighted the central role that TGF-β and its downstream mediators have in activating cellular processes that underlie kidney disease progression. The TGF-β isoforms are widely expressed and act on many mammalian cell types (6). Of special interest to our current study, TGF-β has been implicated in the regulation of SMC differentiation, inducing SM22α expression via a TGF-β control element in the 5′ regulatory region (1). In addition, TGF-β induces expression of SMC differentiation marker genes (including α-SMA, SM myosin heavy chain, and SM22α) in SMCs that have undergone partial dedifferentiation in culture (23) and in a variety of non-smooth muscle precursor cell types in culture, including multipotent embryonic 10T1/2 cells and neural crest cells (10, 24, 57, 62). These observations indicate an important role for TGF-β in promoting the differentiation of mesenchymal cells toward a contractile SM phenotype (1).
Underscoring the significance of TGF-β in the regulation of SM22α expression is the presence of several Smad-binding sites in the SM22α promoter region. As the primary signaling intermediates downstream of TGF-β receptors, Smad proteins are essential for full activation of the SM22α gene following TGF-β stimulation (8, 50). Thus it is likely that expression of SM22α in non-SMCs is driven by TGF-β. Indeed, TGF-β is known to increase SM22α in mesenchymal cells, fibroblasts (31, 61), and epithelial cells of the intestine (59), breast (59), and prostate (4, 65). Hence, we speculate that SM22α gene transcription is activated by TGF-β, known to be upregulated in crescentic GN, potentially leading to GEC transdifferentiation.
To determine the significance of disease-induced SM22α, we examined SM22α +/+ and SM22α −/− mice with crescentic GN. There was less proteinuria in SM22α +/+ mice at day 7, suggesting that the potential role of SM22α in stabilizing the podocyte actin cytoskeleton may be adaptive early in disease. At day 7 following disease induction, there was no significant difference in the podocyte-specific proteins nephrin, podocin, and synaptopodin, suggesting that a differential expression of these specific podocyte proteins could not account for the significant difference in proteinuria. At day 14 following disease induction, there was worse histopathological disease in SM22α +/+ mice, suggesting that the more stable actin cytoskeleton may be detrimental later in disease. The podocyte, analogous in many ways to a microvascular pericyte, with an actin cytoskeleton composed of a rich network of coordinated stress fibers, requires highly dynamic foot processes that can reorganize in minutes to respond to changes in intraglomerular pressure and distention of capillary loops (13, 56). Decreasing actin turnover and remodeling, and thereby hindering the dynamic actin cytoskeletal rearrangements necessary to respond to a changing microenvironment, may be particularly devastating to the podocyte.
Recent evidence illustrates that changes in the dynamic state of actin may change cell fate (15). Increased turnover of filamentous actin promotes cell longevity, whereas decreased actin turnover triggers cell death (20). Moreover, SM22α is the mammalian homolog of yeast actin-bundling protein Scp1. Just as studies in yeast have shown that elevated Scp1 levels cause inappropriate actin clumping and cell death (64), studies in mammalian cells have shown that senescent cells have higher levels of SM22α (12, 19, 60). Indeed, a cell that loses its ability to signal to, and rearrange, its actin cytoskeleton elicits a signal for cell death (20). We found that GEC apoptosis was greater in SM22α +/+ mice with crescentic GN, supporting the supposition that SM22α-induced actin stabilization may trigger cell death.
Podocyte apoptosis has been considered detrimental, due to the cell's relative inability to proliferate, and podocyte depletion is key in the development of glomerulosclerosis. Accordingly, we next focused on changes in podocyte number in diseased SM22α +/+ and SM22α −/− mice. Although there was increased apoptosis in SM22α +/+ mice, the number of WT-1-positive cells was not significantly different between baseline and day 7 time points, suggesting a balance between expected proliferation and apoptosis in crescentic GN. In contrast, by day 14, diseased SM22α −/− mice exhibited an increased number of WT-1-positive cells, suggesting the balance had tipped toward proliferation in these mice. As confirmation that increased proliferation, together with decreased apoptosis, led to a greater number of WT-1-positive cells in SM22α −/− mice, we found that the number of Ki-67-positive cells was significantly greater at day 7 following disease induction in SM22α −/− mice compared with that seen in SM22α +/+ mice.
A cell's decision to replicate its genetic material and divide is influenced by extracellular signals, including nutrients, mitogens, and extracellular matrix. A key signal transduction pathway responsible for integrating environmental signals and relaying the information to the cell cycle is the Ras-dependent Erk1/2 MAPK pathway (38). Erk1/2 functions in regulating cell proliferation, differentiation, and survival, and it is necessary for the downregulation of cyclin-dependent kinase (CDK) inhibitors, including p21 and p27 (34).
Dong et al. (11) report that SM22α overexpression inhibits Raf-1-MEK1/2-Erk1/2 signaling through segregation of Ras with Raf-1, blocking Erk1/2 activation and resulting in cell cycle arrest. This cell cycle arrest is associated with increased p21 and p27 and decreased cyclin D1 in vascular SMCs stimulated by PDGF-BB. Overexpression of a truncated SM22α missing the actin-binding domain also decreased the activity of Raf-1-MEK1/2-Erk1/2 signaling and inhibited cell proliferation, suggesting that SM22α's antiproliferative effect is independent of its known function in actin cytoskeleton remodeling (11).
Given Erk1/2's role in mediating cell survival and the evidence that SM22α's antiproliferative effect occurs via blocking Erk1/2 activation, we next examined the expression of activated Erk1/2 (pErk1/2) in SM22α +/+ and SM22α −/− mice with crescentic GN. We found that pErk1/2 levels were greater in SM22α −/− mice, suggesting that increased proliferation in SM22α −/− mice may be secondary to pErk1/2. Podocytes are specialized cells with a limited potential to proliferate under exceptional circumstances. In contrast, PECs maintain the ability readily to proliferate in diseased states and to respond secondarily to injury affecting neighboring glomerular cells (46). There is mounting evidence that PECs may serve as local progenitor cells for podocytes following injury (2, 53, 54). We propose that by blocking proliferation via suppression of Erk1/2, SM22α may be blocking the potential for PECs to transform into podocytes and repopulate the denuded basement membrane. Indeed, our immunostaining findings demonstrate that the primary site of Erk1/2 activation in crescentic GN was the parietal epithelium.
In summary, we hypothesize that upregulation of TGF-β in crescentic GN leads to GEC transdifferentiation toward a contractile SMC phenotype through the induction of SM22α expression. The de novo expression of SM22α has several possible roles in injured GECs: 1) its actin-bundling effect may lead to a cytoskeleton that is too stable, triggering apoptosis; 2) its repression of Erk1/2 activation may lead to cell cycle arrest by upregulating CDK-inhibitors p21 and p27; and 3) its block of Erk1/2 activation may limit proliferation and prevent PECs from serving as progenitor cells for podocytes.
Our findings add to the evolving body of literature illuminating the structural and functional similarities between podocytes and SMCs, and the expression by podocytes of proteins thought to be specific to SMCs (40, 45, 55). In addition, our work strengthens the developing paradigm that there is plasticity in the podocyte's phenotype in response to injurious stimuli. It is unclear whether the de novo expression of SM proteins in podocytes is an adaptive or maladaptive response to injurious stimuli. In our study, the SM22α +/+ mice displayed worse disease in crescentic GN, suggesting that transdifferentiation of podocytes into a SM22α-expressing phenotype in crescentic GN may be maladaptive. Possible mechanisms for this effect include increased apoptosis, decreased proliferation, and cell cycle arrest.
A major question that arises from this study appears to be whether proliferation in crescentic GN is a good or bad occurrence. In the mature glomerulus, podocytes do not readily proliferate under normal conditions or in response to a wide variety of injuries. Hence, traditionally, podocyte proliferation has been considered a “bad” thing, with nonreparative proliferation characterized by the podocyte's escape from cell cycle blockade. However, with our recent understanding of the role of PECs in the response to podocyte injury, it is now appreciated that PECs proliferate in many forms of injury, including those not associated with crescent formation, and demonstrate a role in repair following podocyte injury. Several studies have reported that PECs may serve as local progenitor cells, being recruited into the glomerular tuft with an ability to transform into podocytes (2, 53, 54). Thus we are increasingly becoming more aware that not all GEC proliferation is the same. Ongoing studies are further defining the role that SM22α may play in GEC differentiation, proliferation, and survival.
GRANTS
This work was supported by Robert Wood Johnson Foundation Grant 58826 (to C. B. Marshall) and by National Institute of Diabetes and Digestive and Kidney Diseases Grants RO1 DK051096-06S1 (to C. B. Marshall) and DK60525, DK56799, and DK 51096 (to S. J. Shankland).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Adam PJ, Regan CP, Hautmann MB, Owens GK. Positive- and negative-acting Kruppel-like transcription factors bind a transforming growth factor beta control element required for expression of the smooth muscle cell differentiation marker SM22alpha in vivo. J Biol Chem 275: 37798–37806, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asanuma K, Kim K, Oh J, Giardino L, Chabanis S, Faul C, Reiser J, Mundel P. Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner. J Clin Invest 115: 1188–1198, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Assinder SJ, Stanton JA, Prasad PD. Transgelin: an actin-binding protein and tumour suppressor. Int J Biochem Cell Biol 41: 482–486, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Bariety J, Bruneval P, Hill GS, Mandet C, Jacquot C, Meyrier A. Transdifferentiation of epithelial glomerular cells. J Am Soc Nephrol 14, Suppl 1: S42–S47, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol 13: 2600–2610, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Brinkkoetter PT, Olivier P, Wu JS, Henderson S, Krofft RD, Pippin JW, Hockenbery D, Roberts JM, Shankland SJ. Cyclin I activates Cdk5 and regulates expression of Bcl-2 and Bcl-XL in postmitotic mouse cells. J Clin Invest 119: 3089–3101, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen S, Kulik M, Lechleider RJ. Smad proteins regulate transcriptional induction of the SM22alpha gene by TGF-beta. Nucleic Acids Res 31: 1302–1310, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coimbra T, Wiggins R, Noh JW, Merritt S, Phan SH. Transforming growth factor-beta production in anti-glomerular basement membrane disease in the rabbit. Am J Pathol 138: 223–234, 1991 [PMC free article] [PubMed] [Google Scholar]
- 10. Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 122: 103–111, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dong LH, Wen JK, Liu G, McNutt MA, Miao SB, Gao R, Zheng B, Zhang H, Han M. Blockade of the Ras-extracellular signal-regulated kinase 1/2 pathway is involved in smooth muscle 22 alpha-mediated suppression of vascular smooth muscle cell proliferation and neointima hyperplasia. Arterioscler Thromb Vasc Biol 30: 683–691, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Dumont P, Burton M, Chen QM, Gonos ES, Frippiat C, Mazarati JB, Eliaers F, Remacle J, Toussaint O. Induction of replicative senescence biomarkers by sublethal oxidative stresses in normal human fibroblast. Free Radic Biol Med 28: 361–373, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Endlich N, Kress KR, Reiser J, Uttenweiler D, Kriz W, Mundel P, Endlich K. Podocytes respond to mechanical stress in vitro. J Am Soc Nephrol 12: 413–422, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Feil S, Hofmann F, Feil R. SM22alpha modulates vascular smooth muscle cell phenotype during atherogenesis. Circ Res 94: 863–865, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Franklin-Tong VE, Gourlay CW. A role for actin in regulating apoptosis/programmed cell death: evidence spanning yeast, plants and animals. Biochem J 413: 389–404, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Fu Y, Liu HW, Forsythe SM, Kogut P, McConville JF, Halayko AJ, Camoretti-Mercado B, Solway J. Mutagenesis analysis of human SM22: characterization of actin binding. J Appl Physiol 89: 1985–1990, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Fujigaki Y, Sun DF, Fujimoto T, Suzuki T, Goto T, Yonemura K, Morioka T, Yaoita E, Hishida A. Mechanisms and kinetics of Bowman's epithelial-myofibroblast transdifferentiation in the formation of glomerular crescents. Nephron 92: 203–212, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Gimona M, Kaverina I, Resch GP, Vignal E, Burgstaller G. Calponin repeats regulate actin filament stability and formation of podosomes in smooth muscle cells. Mol Biol Cell 14: 2482–2491, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gonos ES, Derventzi A, Kveiborg M, Agiostratidou G, Kassem M, Clark BF, Jat PS, Rattan SI. Cloning and identification of genes that associate with mammalian replicative senescence. Exp Cell Res 240: 66–74, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Gourlay CW, Ayscough KR. The actin cytoskeleton: a key regulator of apoptosis and ageing? Nat Rev Mol Cell Biol 6: 583–589, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Han M, Dong LH, Zheng B, Shi JH, Wen JK, Cheng Y. Smooth muscle 22 alpha maintains the differentiated phenotype of vascular smooth muscle cells by inducing filamentous actin bundling. Life Sci 84: 394–401, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Hauser PV, Perco P, Muhlberger I, Pippin J, Blonski M, Mayer B, Alpers CE, Oberbauer R, Shankland SJ. Microarray and bioinformatics analysis of gene expression in experimental membranous nephropathy. Nephron Exp Nephrol 112: e43–e58, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hautmann MB, Madsen CS, Owens GK. A transforming growth factor beta (TGFbeta) control element drives TGFbeta-induced stimulation of smooth muscle alpha-actin gene expression in concert with two CArG elements. J Biol Chem 272: 10948–10956, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Hirschi KK, Rohovsky SA, D'Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol 141: 805–814, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ichimura K, Kurihara H, Sakai T. Actin filament organization of foot processes in rat podocytes. J Histochem Cytochem 51: 1589–1600, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Je HD, Sohn UD. SM22alpha is required for agonist-induced regulation of contractility: evidence from SM22alpha knockout mice. Mol Cells 23: 175–181, 2007 [PubMed] [Google Scholar]
- 27. Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, Pollak MR. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24: 251–256, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res 100: 1589–1596, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Kos CH, Le TC, Sinha S, Henderson JM, Kim SH, Sugimoto H, Kalluri R, Gerszten RE, Pollak MR. Mice deficient in alpha-actinin-4 have severe glomerular disease. J Clin Invest 111: 1683–1690, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuhbandner S, Brummer S, Metzger D, Chambon P, Hofmann F, Feil R. Temporally controlled somatic mutagenesis in smooth muscle. Genesis 28: 15–22, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Lawson D, Harrison M, Shapland C. Fibroblast transgelin and smooth muscle SM22alpha are the same protein, the expression of which is down-regulated in many cell lines. Cell Motil Cytoskeleton 38: 250–257, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Li Y, Kang YS, Dai C, Kiss LP, Wen X, Liu Y. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol 172: 299–308, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 21: 212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marra DE, Simoncini T, Liao JK. Inhibition of vascular smooth muscle cell proliferation by sodium salicylate mediated by upregulation of p21(Waf1) and p27(Kip1). Circulation 102: 2124–2130, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Marshall CB, Krofft RD, Pippin JW, Shankland SJ. The CDK-inhibitor p21 is prosurvival in adriamycin-induced podocyte injury, in vitro and in vivo. Am J Physiol Renal Physiol 298: F1140–F1151, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marshall CB, Pippin JW, Krofft RD, Shankland SJ. Puromycin aminonucleoside induces oxidant-dependent DNA damage in podocytes in vitro and in vivo. Kidney Int 70: 1962–1973, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: is subcellular localization the answer? Cell Cycle 8: 1168–1175, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene 26: 3227–3239, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with type II diabetes and microalbuminuria. Diabetologia 42: 1341–1344, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Miao J, Fan Q, Cui Q, Zhang H, Chen L, Wang S, Guan N, Guan Y, Ding J. Newly identified cytoskeletal components are associated with dynamic changes of podocyte foot processes. Nephrol Dial Transplant 24: 3297–3305, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Moeller MJ, Holzman LB. Imaging podocyte dynamics. Nephron Exp Nephrol 103: e69–e74, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Mundel P, Heid HW, Mundel TM, Kruger M, Reiser J, Kriz W. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol 139: 193–204, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nair RR, Solway J, Boyd DD. Expression cloning identifies transgelin (SM22) as a novel repressor of 92-kDa type IV collagenase (MMP-9) expression. J Biol Chem 281: 26424–26436, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Ng YY, Fan JM, Mu W, Nikolic-Paterson DJ, Yang WC, Huang TP, Atkins RC, Lan HY. Glomerular epithelial-myofibroblast transdifferentiation in the evolution of glomerular crescent formation. Nephrol Dial Transplant 14: 2860–2872, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Ogawa A, Sakatsume M, Wang X, Sakamaki Y, Tsubata Y, Alchi B, Kuroda T, Kawachi H, Narita I, Shimizu F, Gejyo F. SM22alpha: the novel phenotype marker of injured glomerular epithelial cells in anti-glomerular basement membrane nephritis. Nephron Exp Nephrol 106: e77–e87, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Ohse T, Pippin JW, Chang AM, Krofft RD, Miner JH, Vaughan MR, Shankland SJ. The enigmatic parietal epithelial cell is finally getting noticed: a review. Kidney Int 76: 1225–1238, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ. Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296: F213–F229, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Qiu P, Ritchie RP, Fu Z, Cao D, Cumming J, Miano JM, Wang DZ, Li HJ, Li L. Myocardin enhances Smad3-mediated transforming growth factor-beta1 signaling in a CArG box-independent manner: Smad-binding element is an important cis element for SM22alpha transcription in vivo. Circ Res 97: 983–991, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Ransom RF, Vega-Warner V, Smoyer WE, Klein J. Differential proteomic analysis of proteins induced by glucocorticoids in cultured murine podocytes. Kidney Int 67: 1275–1285, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Rho JH, Roehrl MH, Wang JY. Tissue proteomics reveals differential and compartment-specific expression of the homologs transgelin and transgelin-2 in lung adenocarcinoma and its stroma. J Proteome Res 8: 5610–5618, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20: 322–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J Am Soc Nephrol 17: 2443–2456, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Saleem MA, Zavadil J, Bailly M, McGee K, Witherden IR, Pavenstadt H, Hsu H, Sanday J, Satchell SC, Lennon R, Ni L, Bottinger EP, Mundel P, Mathieson PW. The molecular and functional phenotype of glomerular podocytes reveals key features of contractile smooth muscle cells. Am J Physiol Renal Physiol 295: F959–F970, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Seiler MW, Venkatachalam MA, Cotran RS. Glomerular epithelium: structural alterations induced by polycations. Science 189: 390–393, 1975 [DOI] [PubMed] [Google Scholar]
- 57. Shah NM, Groves AK, Anderson DJ. Alternative neural crest cell fates are instructively promoted by TGFbeta superfamily members. Cell 85: 331–343, 1996 [DOI] [PubMed] [Google Scholar]
- 58. Shapland C, Hsuan JJ, Totty NF, Lawson D. Purification and properties of transgelin: a transformation and shape change sensitive actin-gelling protein. J Cell Biol 121: 1065–1073, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shields JM, Rogers-Graham K, Der CJ. Loss of transgelin in breast and colon tumors and in RIE-1 cells by Ras deregulation of gene expression through Raf-independent pathways. J Biol Chem 277: 9790–9799, 2002 [DOI] [PubMed] [Google Scholar]
- 60. Thweatt R, Lumpkin CK, Jr, Goldstein S. A novel gene encoding a smooth muscle protein is overexpressed in senescent human fibroblasts. Biochem Biophys Res Commun 187: 1–7, 1992 [DOI] [PubMed] [Google Scholar]
- 61. Untergasser G, Gander R, Lilg C, Lepperdinger G, Plas E, Berger P. Profiling molecular targets of TGF-beta1 in prostate fibroblast-to-myofibroblast transdifferentiation. Mech Ageing Dev 126: 59–69, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Verbeek MM, Otte-Holler I, Wesseling P, Ruiter DJ, de Waal RM. Induction of alpha-smooth muscle actin expression in cultured human brain pericytes by transforming growth factor-beta 1. Am J Pathol 144: 372–382, 1994 [PMC free article] [PubMed] [Google Scholar]
- 63. Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 64. Winder SJ, Jess T, Ayscough KR. SCP1 encodes an actin-bundling protein in yeast. Biochem J 375: 287–295, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yang Z, Chang YJ, Miyamoto H, Ni J, Niu Y, Chen Z, Chen YL, Yao JL, di Sant'Agnese PA, Chang C. Transgelin functions as a suppressor via inhibition of ARA54-enhanced androgen receptor transactivation and prostate cancer cell growth. Mol Endocrinol 21: 343–358, 2007 [DOI] [PubMed] [Google Scholar]
- 66. Zhang JC, Kim S, Helmke BP, Yu WW, Du KL, Lu MM, Strobeck M, Yu Q, Parmacek MS. Analysis of SM22alpha-deficient mice reveals unanticipated insights into smooth muscle cell differentiation and function. Mol Cell Biol 21: 1336–1344, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]