Abstract
The balance between angiotensin II (ANG II) and nitric oxide plays an important role in renal function and is thought to contribute to the progression of renal injury in experimental hypertension. In the present study, we investigated the extent of blood pressure (BP)-dependent and BP-independent pathways of renal injury following 2 wk of hypertension produced by intravenous infusion of ANG II (5 ng·kg−1·min−1)+Nω-nitro-l-arginine methyl ester (l-NAME; 1.4 μg·kg−1·min−1) in male Sprague-Dawley rats. An aortic balloon occluder was positioned between the renal arteries to maintain (24 h/day) BP to the left kidney (servo-controlled) at baseline levels, whereas the right kidney (uncontrolled) was chronically exposed to elevated BP. Over the 14-day experimental protocol, the average BP to uncontrolled kidneys (152.7 ± 1.8 mmHg) was significantly elevated compared with servo-controlled (113.0 ± 0.2 mmHg) kidneys and kidneys from sham rats (108.3 ± 0.1 mmHg). ANG II+l-NAME infusion led to renal injury that was focal in nature and mainly confined to the outer medulla. Despite the differences in BP between servo-controlled and uncontrolled kidneys, there was a similar ∼3.5-fold increase in renal outer medullary tubular injury, ∼2-fold increase in outer medullary interstitial fibrosis, ∼2-fold increase in outer medullary macrophage infiltration, and a significant increase in renal oxidative stress, all of which are indicative of BP-independent mediated pathways. The results of this study have important implications regarding the pathogenesis of renal injury in various experimental models of hypertension and provide novel insights regarding the variable association observed between hypertension and renal injury in some human populations.
Keywords: hypertension, angiotensin II, nitric oxide, inflammation, oxidative stress
the progression of renal damage varies widely among hypertensive populations (10, 17, 36, 43) as well as in experimental models of hypertension (2, 29). While elevated blood pressure (BP), per se, clearly plays a dominant role in the progression of renal injury in many forms of hypertension (2, 25, 29), it is also understood that renal injury continues to worsen in some individuals despite optimal BP control (10, 17, 45). Several such BP-independent mechanisms have been proposed and are thought to directly contribute to renal injury and/or alter the susceptibility to BP-induced renal injury and broadly include genetic-, dietary-, and neurohumoral-related mechanisms. We have recently reported that elevated circulating levels of ANG II increase the susceptibility to both BP-dependent and BP-independent pathways of tubulointerstitial injury and fibrosis compared with equivalent pressor doses of norepinephrine (NE) (29). A better understanding of the mechanisms that alter the susceptibility to BP-dependent vs. BP-independent renal injury will provide important insights regarding the variable association between hypertension and renal injury observed among human populations as well as in different experimental models of hypertension.
Several BP-independent mechanisms by which ANG II can result in renal injury have been proposed (i.e., oxidative stress, renal ischemia, inflammation, etc.), and it has become well established that nitric oxide (NO) plays an important role to counterbalance these deleterious pathways (1). Elevated ANG II and decreased NO levels have been reported in models of diabetes, heart failure, atherosclerosis, and hypertension (1, 12, 19, 33, 38, 48). It is possible that the extent of BP-independent injury induced by ANG II would be exacerbated in situations of reduced NO availability. It is also possible that a severe imbalance in the renal ANG II-NO axis could make the kidney more susceptible to elevated BP, resulting in severe BP-dependent renal injury. However, the extent to which renal injury is due to elevated BP vs. BP-independent factors has not been directly established in a model of hypertension associated with elevated ANG II and decreased NO in rats.
Until recently, it has not been possible to precisely quantify the extent to which elevated BP, per se, contributes to renal injury in various forms of hypertension. To do so requires precise and continuous measurement of BP (18) as well as the ability to continuously maintain BP to one kidney within the normotensive range while the contralateral kidney is exposed to elevated BP. Many previous studies which have claimed BP-independent mechanisms of renal injury have utilized the tail-cuff technique to measure BP in conjunction with the administration of various hypertensive and/or antihypertensive treatment regimens to different groups of rats. Such approaches have made it difficult to delineate the BP-dependent vs. BP-independent pathways of renal injury due to the spontaneous fluctuations of BP and the difficulty in precisely matching BP in various groups of rats with different pressor and/or antihypertensive treatments. In light of these factors, our laboratory recently developed a technique in rats that allows BP to one kidney to be precisely servo-controlled to baseline levels during the intravenous administration of various hypertensive agents while the contralateral kidney of the same rat is exposed to elevated BP for periods of up to several weeks (24). A servo-controlled aortic occluder cuff is positioned between the renal arteries so that BP to the left kidney can be maintained at baseline levels via the inflation of the cuff following the onset of hypertension while the right kidney is chronically exposed to elevated BP. BP is continuously measured 24 h/day from the implanted intra-arterial catheters above (carotid) and below (femoral) the aortic occluder. Thus this technique is adequately designed to separate BP-dependent vs. BP-independent patterns of renal injury in experimental models of hypertension in rodents. The goal of the present study was to assess the extent of BP-dependent and BP-independent pathways of renal injury in hypertension produced by the simultaneous administration of ANG II and the nonspecific NO synthase inhibitor Nω-nitro-l-arginine methyl ester (l-NAME) for 2 wk in rats.
METHODS
Experimental animals.
All studies were performed in 10- to 11-wk-old male Sprague-Dawley rats (Harlan) that were provided water ad libitum and maintained on a 0.4% NaCl AIN-76 rodent diet (Dyets, Bethlehem, PA) throughout the study. All protocols were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Surgical preparation.
Following an overnight fast, rats were anesthetized with a mixture of ketamine (30 mg/kg im) and acepromazine (2 mg/kg im). Surgery was performed under sterile conditions while rats were maintained on a 37°C warming board. As described previously (24), indwelling catheters were implanted in the right carotid and left femoral artery and vein and an inflatable silastic vascular occluder (1.5-mm lumen diameter, 2.5-mm outer diameter; In Vivo Metric, Healdsburg, CA) was positioned around the aorta between the left and right renal arteries through a midsagittal abdominal incision. The vascular occluder cuff was attached to flexible Tygon tubing (0.76-mm lumen diameter, 2.29-mm outer diameter), which was tunneled subcutaneously and exteriorized at the back of the neck, along with the arterial and venous catheters. The vascular occluder cuff was connected to a custom-built infusion pump driven by a stepper motor (MDrive 23, Intelligent Motion Systems, Marlborough, CT) to allow for chronic servo-control of BP to the left kidney (29). Sham rats underwent identical surgical and implantation procedures; however, the vascular occluder cuff was never inflated. Rats recovered for at least 10 days following surgery, during which 3 days of 24-h baseline control BP measurements were obtained.
Chronic servo-control of renal perfusion pressure.
Rats were placed in a bidirectional turntable cage system (Rodent Workstation with Raturn system; Bioanalytical Systems, West Lafayette, IN) for several days before surgery. Following surgery, rats were returned to their cages, and arterial pressure above (carotid artery) and below (femoral artery) the vascular occluder cuff was monitored 24 h/day throughout the study. A continuous infusion of saline or drug was administered intravenously at a rate of 6.9 μl/min throughout the study. For chronic servo-control of BP to the left kidney, a computerized servo-control pump that adjusted the inflation of the aortic vascular occluder cuff in response to changes in femoral arterial BP was connected to the vascular occluder cuff. Chronic servo-control of BP to the left kidney began immediately following the increase in BP and was maintained within ±10 mmHg of the average BP obtained over the 3 days of baseline measurements.
Experimental design.
After obtaining 3 days of stable baseline BP, the intravenous solution was switched to ANG II (5 ng·kg−1·min−1) + l-NAME (1.4 μg·kg−1·min−1) for servo-control rats while sham rats continued to receive saline. Both of these doses are known to be subpressor when administered alone to rats fed a 0.4% NaCl diet (41, 46), whereas our pilot data indicated a sustained increase in mean arterial pressure (MAP) to ∼150 mmHg when administered together. These doses of ANG II and l-NAME were selected in the present study to produce a sustained increase in MAP over 14 days similar in magnitude to the increase elicited by ANG II (25 ng·kg−1·min−1) in our previous servo-control studies (24, 29).
Two groups of rats were studied following the 14-day experimental protocol: 1) ANG II+l-NAME-infused servo-control rats (n = 7) and sham rats (n = 7) used for renal histological and immunohistochemistry analyses; and 2) ANG II+l-NAME-infused servo-control rats (n = 6) and sham rats (n = 6) used for renal superoxide measurements. Three servo-control rats and four sham rats were used for both protocols. Following the 14-day experimental protocol, all rats were anesthetized with pentobarbital sodium (100 mg/kg) and the kidneys were quickly removed and bisected in the transverse plane. For each kidney, one-half was immediately immersion fixed in 10% neutral buffered formalin for histological and immunohistochemical analysis while the other half of the kidney was dissected to separate the cortex and outer medulla, which were immediately snap frozen for tissue superoxide analysis.
Histological and immunohistochemical analysis.
Kidney sections (3 μm) were mounted on slides and stained with hematoxylin and eosin to evaluate vascular, glomerular, and tubular pathology. The arterial smooth muscle wall:lumen cross-sectional area (CSA) ratio was used to assess vascular remodeling in 20 randomly selected interlobular arterioles spanning the entire cortex. The CSA of the vessel wall and lumen was examined using a ×20 objective lens and calculated using Metamorph image-analysis software (Molecular Devices, Downington, PA). As described previously (29), glomerular injury was determined in 30 randomly selected superficial cortical and 20 randomly selected juxtamedullary glomeruli spanning the entire cortex per kidney section. Using a ×40 objective lens, glomeruli were evaluated for the presence of glomerulosclerosis and results are presented as the percentage of glomeruli exhibiting sclerosis in >50% of the glomerular tuft area. Outer medullary tubular injury was assessed via the quantification of the area percentage of outer medullary tubular protein casts using Metamorph image-analysis software. All immunostaining protocols were performed using a robotic DAKO autostainer (S3400; Dako Cytomation, Carpinteria, CA) to ensure that all sections were stained together under identical conditions. As described previously (29), outer medullary interstitial fibrosis was determined by immunostaining with an antibody for α-smooth muscle actin (α-SMA; Dako Cytomation) and detected with an Envision/HRP detection kit (Dako Cytomation). The percent α-SMA-positive region in the outer medulla was quantified (Metamorph) in 20 randomly chosen frames spanning the entire outer medulla using a ×20 objective lens. Adjoining serial sections were immunostained for the presence of macrophages using an antibody for ED-1, as described previously (22). The number of ED-1-positive cells within the outer medulla was quantified (Metamorph) in 20 randomly chosen frames spanning the entire outer medulla using a ×20 objective lens. These data are expressed as the average number of ED-1-positive cells per ×20 frame. All images were captured with a Nikon E400 (Nikon Instruments, Melville, NY) microscope equipped with a Spot Insight color CCD camera (Diagnostic Instruments, Sterling Heights, MI). All analyses were blinded to treatment.
Renal superoxide production.
As described previously (30, 42), renal cortical and outer medullary tissues were homogenized in a potassium phosphate buffer, pH 7.4, containing 250 mM sucrose, 1 mM EDTA, 1 mM dithiothreitol, 2 mM pepstatin, 1 mM leupeptin, and 0.1 mM phenylmethylsulfonyl. The homogenate was centrifuged at 1,000 g for 5 min, and the protein concentration of the supernatant was determined using a Coomassie blue protein assay (Pierce, Rockford, IL) with bovine serum albumin used as a standard. The detection of superoxide was determined using a 2-hydroxyethidium fluorescence microtiter plate assay. Briefly, 20 μg of renal cortical or outer medullary protein was incubated with dihydroethidium (DHE; 10 μM), salmon testes DNA (0.5 mg/ml), and PBS. Following a 35-min incubation, the increase in 2-hydroxyethidium fluorescence was measured at an excitation of 485 nm and an emission of 570 nm. Background fluorescence was accounted for by subtracting the fluorescence of wells containing only PBS, DHE, and DNA.
Detection of renal apoptosis.
The presence of apoptotic cells was determined in formalin-fixed tissues using a DeadEnd Fluorometric deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) System assay (Promega). A Nikon Eclipse 80i (Nikon Instruments, Melville, NY) equipped with a High Performance CCD camera (Cohu, San Diego, CA) and an X-cite 120 Mercury Halide lamp (Lumen Dynamics, Mississauga, ON) was used to capture images. A DAPI (UV-2E/C) and FITC (HQ:F) filter were used to assess normal and apoptotic nuclei, respectively. Using a ×20 objective lens, 10 randomly selected images spanning the entire corticomedullary region of the kidney were obtained. The percentage of apoptotic cells per total ×20 frame was quantified in a blinded fashion using standard thresholding techniques (Metamorph). The percentage of apoptotic cells per ×20 view was averaged over the 10 frames for each kidney.
Statistical methods.
Data are presented as means ± SE. A two-way repeated-measures ANOVA followed by a Tukey post hoc test was used to determine daily differences in BP across groups over the 3 days of baseline measurements as well as over the 14-day experimental protocol. A paired t-test was used to assess differences between servo-controlled and uncontrolled kidneys while an unpaired t-test was used to compare servo-controlled and sham kidneys. P < 0.05 was considered significant.
RESULTS
BP to servo-controlled and uncontrolled kidneys and kidneys of sham rats.
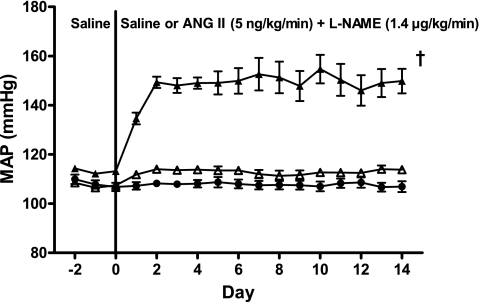
The MAP of the servo-controlled (n = 10) and sham (n = 9) rats used for both renal histology and analysis of tissue superoxide metabolism are combined and presented in Fig. 1. MAP to the uncontrolled kidneys averaged 152.7 ± 1.8 mmHg over the 14 days of ANG II+l-NAME infusion and was significantly (P < 0.05) elevated compared with the average 14-day MAP (113.0 ± 0.2 mmHg) of servo-controlled kidneys and the MAP (108.3 ± 0.1 mmHg) to kidneys of sham rats. No significant differences in MAP existed between servo-controlled kidneys and kidneys from sham rats across all time points.
Fig. 1.
Daily 24-h averages of mean arterial pressure (MAP) to servo-controlled (▵, n = 10) and uncontrolled (▴, n = 10) kidneys from ANG II (5 ng·kg−1·min−1)+Nω-nitro-l-arginine methyl ester (l-NAME; 1.4 μg·kg−1·min−1)-infused rats and kidneys from saline-infused sham rats (●, n = 9). Values are means ± SE. †P < 0.05 vs. servo-controlled and sham MAP over days 1–14.
Interlobular arterial remodeling and glomerular injury.
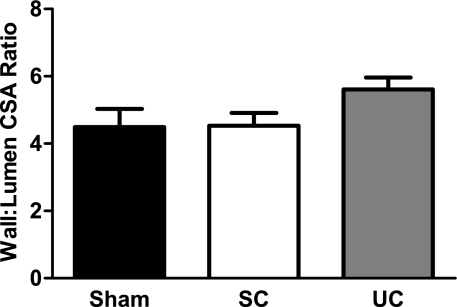
The average wall:lumen CSA ratio of interlobular arterioles from uncontrolled (5.6 ± 0.4) and servo-controlled (4.5 ± 0.4) kidneys from ANG II+l-NAME-infused rats and kidneys from sham rats (4.5 ± 0.5) is presented in Fig. 2. The wall:lumen CSA ratio was elevated (P = 0.09) in interlobular arteries from uncontrolled vs. servo-controlled kidneys, suggesting a direct role of elevated BP as a cause of the observed remodeling, although this did not reach statistical significance. The role of elevated BP in these changes is reinforced by the evidence that the interlobular wall:lumen CSA ratios in servo-controlled kidneys was similar to kidneys from sham rats.
Fig. 2.
Vascular smooth muscle wall:lumen cross sectional area (CSA) ratio in 20 randomly selected interlobular arteries from servo-controlled (SC; n = 7) and uncontrolled (UC; n = 7) kidneys from ANG II (5 ng·kg−1·min−1)+l-NAME (1.4 μg·kg−1·min−1)-infused rats and kidneys from saline-infused sham rats (n = 7). Chronically elevated arterial pressure tended (P = 0.09) to alter interlobular arterial remodeling. Values are means ± SE.
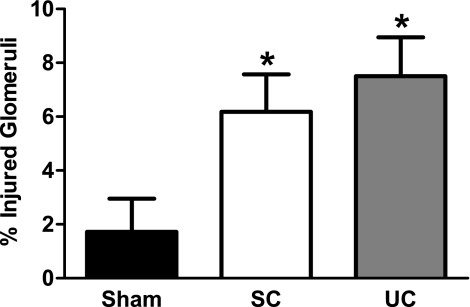
No differences were observed between the average superficial cortical and juxtamedullary glomerulosclerosis within each kidney; therefore, these values have been averaged and presented as a single glomerular injury score. As shown in Fig. 3, glomerulosclerosis was only modest, but significantly elevated to nearly the same extent in both uncontrolled (7.5 ± 1.5%) and servo-controlled (6.2 ± 1.4%) kidneys compared with kidneys from sham rats (1.7 ± 1.2%). These data demonstrate that the glomerular injury in this form of hypertension is modest, but significantly elevated by the direct effects of elevated ANG II+decreased NO, independently of elevated BP.
Fig. 3.
Summary of percentage of glomerular injury in servo-controlled (n = 7) and uncontrolled (n = 7) kidneys from ANG II (5 ng·kg−1·min−1)+l-NAME (1.4 μg·kg−1·min−1)-infused rats and saline-infused sham rats (n = 7). Glomerular injury was modestly, but significantly elevated by BP-independent factors. Values are mean ± SE. *P < 0.05 vs. kidneys from sham rats.
Outer medullary tubular injury, interstitial fibrosis, and macrophage infiltration.
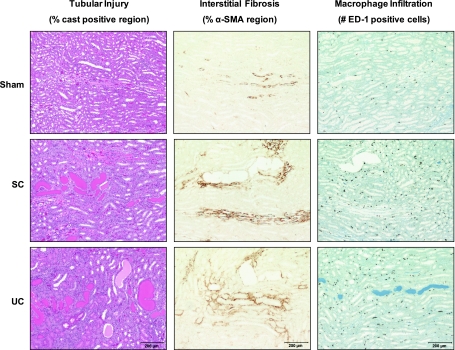
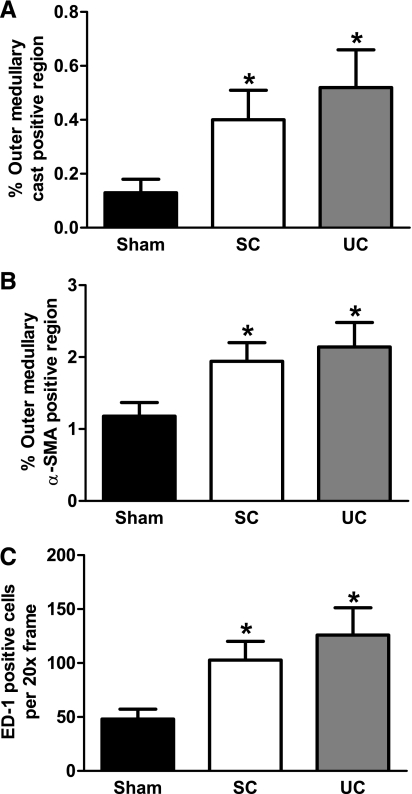
Representative images from servo-controlled and uncontrolled kidneys and kidneys from sham rats are shown in Fig. 4 for 1) outer medullary tubular necrosis, 2) outer medullary interstitial fibrosis, and 3) outer medullary macrophage density. Overall, outer medullary injury and infiltration of macrophages were mainly due to the direct effects of elevated ANG II and decreased NO, independently of elevated BP. The results of the semiquantitative analysis of outer medullary tubular necrosis are presented in Fig. 5A. Outer medullary tubular necrosis was significantly (P < 0.05) elevated in both servo-controlled (3-fold) and uncontrolled (4-fold) kidneys compared with kidneys from sham rats. No significant effects of elevated BP on outer medullary tubular injury were observed as the level of injury was similar between servo-controlled and uncontrolled kidneys. Similarly, outer medullary interstitial fibrosis was significantly (P < 0.05) increased in both servo-controlled (64%) and uncontrolled (81%) kidneys compared with kidneys from sham rats (Fig. 5B). These data demonstrate that the significant increase in outer medullary α-SMA staining following 14 days of ANG II+l-NAME infusion was primarily due to the direct effects of elevated ANG II and decreased NO, independently of elevated BP. Figure 5C summarizes the average number of ED-1-positive cells per ×20 frame in kidneys of ANG II+l-NAME-treated rats and kidneys from sham rats. Macrophage infiltration was significantly (P < 0.05) elevated in both servo-controlled (2.1-fold) and uncontrolled (2.6-fold) kidneys compared with kidneys from sham rats. Similar to the previous indices of outer medullary injury, these data implicate a direct role of elevated ANG II+decreased NO in the macrophage infiltration within the renal outer medulla of ANG II+l-NAME-induced hypertensive rats.
Fig. 4.
Representative images (×10 objective lens) of outer medullary tubular injury (hematoxylin and eosin), fibrosis [α-smooth muscle actin (SMA)], and macrophage infiltration (ED-1) in SC and UC kidneys from ANG II (5 ng·kg−1·min−1)+l-NAME (1.4 μg·kg−1·min−1)-infused rats and kidneys from saline-infused sham rats.
Fig. 5.
Semiquantitative injury score for outer medullary tubular injury (% tubular cast-positive region; A), fibrosis (% α-SMA-positive region; B), and macrophage infiltration (no. of ED-1-positive cells/×20 frame; C) in SC (n = 7) and UC (n = 7) kidneys from ANG II (5 ng·kg−1·min−1)+l-NAME (1.4 μg·kg−1·min−1)-infused rats and kidneys from saline-infused sham rats (n = 7). Values are means ± SE. *P < 0.05 vs. kidneys from sham rats.
Superoxide production in renal tissue homogenates.
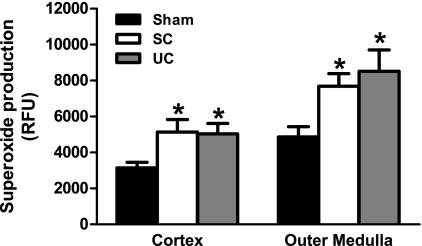
Renal cortical and outer medullary superoxide production is summarized in Fig. 6. Renal cortical superoxide production was significantly (P < 0.05) elevated in both servo-controlled [5,138 ± 699 relative fluorescent units (RFU)] and uncontrolled (5,034 ± 584 RFU) kidneys compared with kidneys from sham rats (3,146 ± 309 RFU). Similarly, outer medullary superoxide production was significantly (P < 0.05) elevated in both servo-controlled (7,689 ± 693) and uncontrolled (8,517 ± 1,192) kidneys compared with kidneys from sham rats (4,861 ± 579). These data demonstrate the direct effects of elevated ANG II+decreased NO, independently of elevated BP, on the elevated superoxide production observed in both the renal cortex and outer medulla of ANG II+l-NAME-induced hypertensive rats.
Fig. 6.
Renal cortical and outer medullary superoxide production, expressed as 2-hydroxyethidium relative fluorescent units (RFU), in tissue homogenates from SC (n = 6) and UC (n = 6) kidneys from ANG II (5 ng·kg−1·min−1)+l-NAME (1.4 μg·kg−1·min−1)-infused rats and kidneys from saline-infused sham rats (n = 6). Superoxide production was increased in ANG II+l-NAME-infused rats via the direct effects of BP-independent factors. Values are means ± SE. *P < 0.05 vs. kidneys from sham rats.
Percentage of apoptotic cells in formalin-fixed renal tissue.
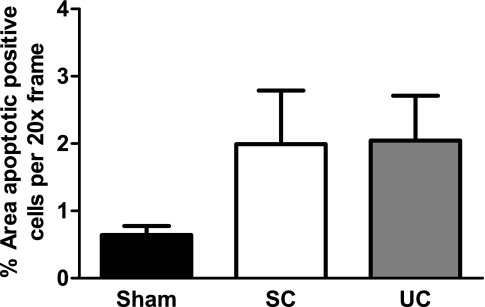
The average percentage of apoptotic cells in servo-controlled (n = 6) and uncontrolled (n = 6) kidneys from ANG II+l-NAME-induced hypertensive rats and kidneys (n = 5) from saline-infused sham rats is presented in Fig. 7. The overall level of apoptotic cells was modest among all kidneys. While there was an elevated level of apoptosis in both the servo-controlled and uncontrolled kidneys compared with kidneys from sham rats, indicative of a BP-independent mechanism, these differences did not reach significance. These data suggest that renal apoptosis is not likely a major contributor to the observed BP-independent injury in this model of hypertension.
Fig. 7.
Average percentage of renal apoptotic cells/×20 frame in paraffin-embedded tissue sections from SC (n = 6) and UC (n = 6) kidneys from ANG II (5 ng·kg−1·min−1)+l-NAME (1.4 μg·kg−1·min−1)-infused rats and kidneys from saline-infused sham rats (n = 5). While the percentage of apoptotic cells was similarly elevated in both SC and UC kidneys compared with kidneys from sham rats, the level of apoptosis was modest and the difference did not reach statistical significance. Values are means ± SE.
DISCUSSION
The novel finding from this study is that the renal injury that occurs in ANG II-induced hypertension in the presence of reduced nitric oxide synthase (NOS) activity (2 wk of ANG II+l-NAME administration) is mediated via BP-independent pathways. These results are in contrast to those observed when hypertension is produced by 2 wk of ANG II administration to rats with normal levels of NOS activity, in which nearly 85% of the renal injury could be accounted for by the direct effects of elevated BP per se (24, 29). The results of the present study, therefore, have important implications regarding the pathogenesis of renal injury in different forms of hypertension, particularly those involving alterations in the ANG II-NO axis.
For hypertension to result in renal injury, elevated levels of systemic BP must be transmitted to the renal microvasculature (2). The lack of a significant BP-induced component of renal injury as well as the similar pattern and magnitude of injury in both the servo-controlled (normotensive) and uncontrolled (hypertensive) kidneys suggest a role for BP-independent-mediated injury in ANG II+l-NAME-induced hypertensive rats. Bidani and colleagues (2) have suggested that models of hypertension associated with significant preglomerular vasoconstriction would be more prone to ischemia-mediated as opposed to BP-induced renal injury. Although renal blood flow (RBF) was not directly measured in the present study, it is not unreasonable to infer that the combination of increased ANG II and decreased NO resulted in significant preglomerular vasoconstriction and renal ischemia. Nishiyama et al. (27) has demonstrated that the maintenance of RBF in ANG II-induced hypertensive rats (MAP ∼153 mmHg) is dependent upon renal NO production. In their study, chronic ANG II administration for 2 wk did not alter RBF, as assessed by radioactive microspheres in conscious rats. However, when l-NAME was coadministered with ANG II they observed a significant reduction in RBF. Although future research is required to elucidate the direct role of renal ischemia in the subsequent renal injury in this model of hypertension, the general observation that renal injury originated via BP-independent pathways has important implications regarding the pathogenesis of renal injury in various models of hypertension.
In ANG II+l-NAME-infused rats, the extent of BP-independent renal injury was modest and primarily consisted of outer medullary tubular necrosis and interstitial fibrosis with a small, but significant, degree of BP-independent glomerular injury. The disproportionate level of outer medullary injury compared with the small degree of superficial glomerular injury is similar to that observed in models of ANG II-induced hypertension (15, 24, 29). Examining the pattern of renal injury observed in the present study within the context of the pattern observed in our previous ANG II studies provides additional insights regarding the role of NO in altering the magnitude of BP-dependent and BP-independent pathways of injury in ANG II-induced hypertension. It should be recognized that a similar level and duration of hypertension were produced in both the present study (average MAP ∼153 mmHg over 14 days) and our previous servo-control studies (24, 29) in ANG II-induced hypertensive rats (average MAP ∼157 mmHg over 14 days), although it was necessary to alter the levels of dietary salt intake (0.4 vs. 4.0% NaCl, respectively) and doses of ANG II (5 vs. 25 ng·kg−1·min−1, respectively) to achieve similar levels of hypertension in the presence of l-NAME. In both models of hypertension, superficial glomerular injury was modest and independent of elevated BP. The protection from elevated BP in this region of the kidney can be explained by the highly efficient autoregulation of RBF, in which the preglomerular vasculature responds with proportionate vasoconstriction in response to elevations in BP, thus largely preventing excessive BP transmission to the glomeruli (3). In addition, the superimposed ANG II- or ANG II+l-NAME-induced vasoconstriction would further attenuate the transmission of elevated systemic BP to the superficial glomeruli (2). The major difference between these two models is the lack of a predominant BP-induced component of juxtamedullary glomerular and outer medullary injury in ANG II+l-NAME-infused rats because we have previously demonstrated a large BP-dependent component of injury in this region of the kidney in ANG II-infused rats (24, 29). These differences may relate to the regulation of medullary blood flow (23). We (6, 24) and others (16) have suggested that the diminished autoregulation of blood flow in the renal outer medulla may increase the susceptibility of this region of the kidney to BP-induced injury. Indeed, prominent outer medullary injury is observed in many models of hypertension, including those associated with elevated levels of ANG II (15, 24, 29). However, in the present study when NO was reduced in the presence of elevated ANG II, there was not a significant BP-dependent component of juxtamedullary glomerular or outer medullary injury. Previous studies have demonstrated that ANG II-induced NO production within the kidney attenuates the vasoconstrictor effects of ANG II (9, 14, 40, 47, 49). Specifically, our laboratory has demonstrated that ANG II-induced NO production plays a key role in preserving outer medullary blood flow in response to modest doses of ANG II (5 ng·kg−1·min−1) (49). These studies suggest that increased outer medullary vascular resistance induced by ANG II+l-NAME would significantly attenuate the transmission of elevated systemic BP to the outer medulla and protect it from barotrauma-induced injury.
Comparing the magnitude of outer medullary tubular injury and interstitial fibrosis observed in the present study with those of our previous ANG II (29) servo-control study reinforces these concepts. In both studies, renal injury was analyzed and quantitated in a similar fashion. For example, outer medullary tubular injury (% cast-positive region) averaged a modest 0.4 and 0.5% in servo-controlled and uncontrolled kidneys, respectively, in ANG II+l-NAME rats. In ANG II rats (29), the outer medullary % cast-positive region was 0.3 and 1.3% in servo-controlled and uncontrolled kidneys, respectively, a striking and significant difference between the two kidneys. The same pattern is observed with the extent of outer medullary interstitial fibrosis. In ANG II+l-NAME rats, the % α-SMA-positive region, used as an index of outer medullary interstitial fibrosis, was 1.9 and 2.1% in servo-controlled and uncontrolled kidneys, respectively. In ANG II rats (29), the % α-SMA-positive region was 2.2 and 4.3% in servo-controlled and uncontrolled kidneys, respectively. Examination of both indices of outer medullary injury demonstrates the much higher degree of injury in uncontrolled kidneys from ANG II rats compared with uncontrolled kidneys of ANG II+l-NAME rats, in which the level of injury is similar in both servo-controlled kidneys of ANG II+l-NAME and ANG II rats. In summary, a comparison of these two models suggests that reduced NOS activity in ANG II-induced hypertensive rats greatly attenuates BP-induced outer medullary injury, but may enhance the susceptibility to renal ischemia, which is consistent with the modest levels of BP-independent renal injury observed in the present study.
Due to their potent vascular effects, the ability to regulate numerous cellular pathways associated with BP-independent injury (i.e., oxidative stress, inflammation, fibrosis, etc.), and their counterregulatory actions, a large amount of research has been devoted to the role of ANG II and NO in the pathogenesis of renal injury (1, 33, 38, 48). Although not an extensively studied model of hypertension, previous groups have investigated the chronic effects of ANG II+l-NAME administration on renal injury. Hu et al. (13) demonstrated that the administration of pressor doses of l-NAME (10 μg·kg−1·min−1) in combination with a small dose of ANG II (10 ng·kg−1·min−1) in rats exacerbated both the hypertension and renal injury compared with rats made hypertensive with l-NAME or ANG II alone; however, the magnitude of BP-dependent vs. BP-independent renal injury was not determined. Particularly relevant to the results of our study, Henke et al. (12) reported that suppression of endothelial NF-κB, a master regulatory transcription factor influencing several potential pathways of injury (i.e., oxidative stress, inflammation, etc.) attenuated renal injury in ANG II+l-NAME-induced hypertensive mice fed a 1% NaCl diet. They demonstrated that the albuminuria, tubular necrosis, interstitial fibrosis, and infiltration of immune cells were significantly attenuated in transgenic mice with endothelial cell-specific suppression of NF-κB, despite the lack of effect on the overall levels of BP compared with control mice. The authors concluded that suppression of endothelial NF-κB attenuated hypertension-induced renal damage; that is, NF-κB activation was part of a signaling cascade downstream of elevated BP. However, our study suggests that renal injury, as well as the associated inflammation and oxidative stress, developed independently of elevated BP, a conclusion that could only be made with the use of our chronic servo-control technique. Interestingly, both renal ischemia (4) and oxidative stress (31) have been reported to directly regulate the activation of NF-κB, which provides potential BP-independent mechanisms that could have contributed to renal injury observed in the present study.
Both renal oxidative stress and inflammation were increased in a BP-independent manner in the present study. Although not mutually exclusive, both are thought to contribute, in part, to the associated renal injury in different experimental models of hypertension. Excess production of reactive oxygen species and infiltration of inflammatory cells within the kidney have been reported in both ANG II (35)- and l-NAME (32)-induced models of hypertension. Previous studies have demonstrated that renal oxidative stress can be induced via BP-independent pathways. Our laboratory has shown that chronic administration of subpressor doses of ANG II for 7 days can lead to the expression of genes related to oxidative stress and interstitial fibrosis within the outer medulla (46). Using our chronic servo-control of the renal perfusion pressure technique, we recently reported that ANG II, independently of elevated BP, can directly increase superoxide production within the kidney (30), which could be contributing, in part, to the modest levels of BP-independent injury in models of hypertension associated with elevated levels of ANG II (24, 29). One of the well-known effects of increased production of reactive oxygen species (ROS) is a reduced level of NO bioavailability (5, 44), which can lead to further deteriorations of renal function and ultimately greater injury. The ANG II-induced increase in renal oxidative stress in conjunction with an already depressed level of NO availability could have led to the BP-independent pattern of renal injury either via direct deleterious actions or from subsequent alterations in renal function (i.e., ischemia and or hypoxia). It should be acknowledged that excess levels of ROS, in the absence of other stimuli, such as increased BP or renal ischemia, may not be sufficient to induce significant renal injury. For example, increased levels of ROS within the kidney have been observed at early time points in normotensive streptozotocin-induced diabetic rats (28) as well as in the hypertensive spontaneously hypertensive rat (37), neither of which exhibit significant renal injury until late in the progression of disease. Conversely, longer time periods of exposure to oxidative stress, in the absence of other injurious stimuli, may be required to stimulate significant renal injury.
A novel finding of our study is that the inflammatory infiltrate within the kidney was not the result of an upstream BP-dependent stimulus, but rather originated from a BP-independent stimulus. The mechanisms by which renal inflammation can lead to renal injury are not completely understood, but it is thought that inflammatory cells can lead to increased ROS production, capillary rarefaction and/or ischemia, recruitment of various other cytokines and/or chemokines (i.e., IL-6), and stimulation of fibrotic pathways (7, 11, 21, 26, 34). Crowley et al. (7) demonstrated that inhibition of lymphocyte responses in ANG II-induced hypertensive mice attenuated renal injury in a BP-independent manner. Inhibition of lymphocyte production has also been reported to prevent salt-sensitive hypertension resulting from chronic ANG II administration (35) and chronic NOS inhibition (32) in rats. It is also understood that NO possesses anti-inflammatory actions (8) and thus likely plays a role in opposing the proinflammatory actions of ANG II. Nevertheless, it remains to be established whether the observed renal inflammation in the present study was a direct result of cellular inflammatory pathways invoked by the imbalance of ANG II and NO, or rather a result of other stimuli, such as renal ischemia.
Increased apoptotic cells have been reported in the kidneys of ANG II- and l-NAME-induced hypertensive rats and are thought to play a role in the subsequent renal injury in these models (31). Both oxidative stress and ischemia have been reported to increase the number of apoptotic cells within the kidney (20, 31, 39); therefore, we investigated the extent of renal cell apoptosis using the TUNEL assay on formalin-fixed kidneys. Levels of apoptosis tended to be elevated in both the servo-controlled and uncontrolled kidneys compared with kidneys from saline-infused sham rats, but did not reach levels of statistical significance. Possible explanations for the low levels of apoptosis include the modest degree of renal injury and the recognition that other pathways of cell death, such as necrosis, may play a more predominant role in the observed injury in this model of hypertension.
In conclusion, we have demonstrated that the renal injury observed in a model of hypertension associated with increased ANG II and decreased NO originates via BP-independent pathways. Both renal oxidative stress and inflammation were elevated in a BP-independent fashion in this model of hypertension, and it is likely that these pathways were enhanced by renal vasoconstriction and chronic ischemia, although additional studies would be required to confirm the involvement of these pathways in the pathogenesis of renal injury. Regardless of the specific pathways of injury, this study shows that one must be circumspect in interpreting mechanisms of renal injury when assessing models of hypertension associated with various combinations of elevated ANG II and reduced NOS activity. Most importantly, the results of this study have important implications regarding the variability observed in the association between hypertension and end-stage renal disease in various forms of human hypertension.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-081091 and HL-29587 and a predoctoral fellowship from American Heart Association (AHA-0615590Z).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank David Eick, Mike Kloehn, and Greg McQuestion for design and maintenance of the servo-control system; Glenn Slocum for microscopy assistance; and Carol Bobrowitz, Barb Fleming, Liz Berdan, and Mary Kaldunski for assistance with histological and immunohistochemistry protocols. We also thank R. P. Ryan for performing the TUNEL assay to evaluate the level of apoptosis in renal tissues and Dr. Carl Becker for expert input regarding the evaluation of renal histology.
REFERENCES
- 1. Adam A, Raij L. Nitric oxide-angiotensin II axis in renal and cardiovascular injury. J Nephrol 13: 211–220, 2000 [PubMed] [Google Scholar]
- 2. Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension 44: 595–601, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension 54: 393–398, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cao CC, Ding XQ, Ou ZL, Liu CF, Li P, Wang L, Zhu CF. In vivo transfection of NF-kappaB decoy oligodeoxynucleotides attenuate renal ischemia/reperfusion injury in rats. Kidney Int 65: 834–845, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Cowley AW., Jr Renal medullary oxidative stress, pressure-natriuresis, and hypertension. Hypertension 52: 777–786, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cowley AW., Jr Role of the renal medulla in volume and arterial pressure regulation. Am J Physiol Regul Integr Comp Physiol 273: R1–R15, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, Tharaux PL, Coffman TM. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol 295: F515–F524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest 96: 60–68, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deng X, Welch WJ, Wilcox CS. Role of nitric oxide in short-term and prolonged effects of angiotensin II on renal hemodynamics. Hypertension 27: 1173–1179, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Fogo AB. Hypertensive risk factors in kidney disease in African Americans. Kidney Int Suppl: S17–S21, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Franco M, Martinez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodriguez-Iturbe B. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 293: R251–R256, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Henke N, Schmidt-Ullrich R, Dechend R, Park JK, Qadri F, Wellner M, Obst M, Gross V, Dietz R, Luft FC, Scheidereit C, Muller DN. Vascular endothelial cell-specific NF-kappaB suppression attenuates hypertension-induced renal damage. Circ Res 101: 268–276, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Hu L, Sealey JE, Chen R, Zhou Y, Merali C, Shi Y, Laragh JH, Catanzaro DF. Nitric oxide synthase inhibition accelerates the pressor response to low-dose angiotensin II, exacerbates target organ damage, and induces renin escape. Am J Hypertens 17: 395–403, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Ikenaga H, Fallet RW, Carmines PK. Basal nitric oxide production curtails arteriolar vasoconstrictor responses to ANG II in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 271: F365–F373, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J, Schwartz SM. Renal injury from angiotensin II-mediated hypertension. Hypertension 19: 464–474, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Johnson RJ, Schreiner GF. Hypothesis: the role of acquired tubulointerstitial disease in the pathogenesis of salt-dependent hypertension. Kidney Int 52: 1169–1179, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, Coresh J, Patterson N, Tandon A, Powe NR, Fink NE, Sadler JH, Weir MR, Abboud HE, Adler SG, Divers J, Iyengar SK, Freedman BI, Kimmel PL, Knowler WC, Kohn OF, Kramp K, Leehey DJ, Nicholas SB, Pahl MV, Schelling JR, Sedor JR, Thornley-Brown D, Winkler CA, Smith MW, Parekh RS. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet 40: 1185–1192, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. Recommendations for blood pressure measurement in humans and experimental animals. Part 2: blood pressure measurement in experimental animals: a statement for professionals from the subcommittee of professional and public education of the American Heart Association council on high blood pressure research. Hypertension 45: 299–310, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Leon CA, Raij L. Interaction of haemodynamic and metabolic pathways in the genesis of diabetic nephropathy. J Hypertens 23: 1931–1937, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Liang HL, Hilton G, Mortensen J, Regner K, Johnson CP, Nilakantan V. MnTMPyP, a cell-permeant SOD mimetic, reduces oxidative stress and apoptosis following renal ischemia-reperfusion. Am J Physiol Renal Physiol 296: F266–F276, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liao TD, Yang XP, Liu YH, Shesely EG, Cavasin MA, Kuziel WA, Pagano PJ, Carretero OA. Role of inflammation in the development of renal damage and dysfunction in angiotensin II-induced hypertension. Hypertension 52: 256–263, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 48: 149–156, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Mattson DL, Lu S, Roman RJ, Cowley AW., Jr Relationship between renal perfusion pressure and blood flow in different regions of the kidney. Am J Physiol Regul Integr Comp Physiol 264: R578–R583, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Mori T, Cowley AW., Jr Role of pressure in angiotensin II-induced renal injury: chronic servo-control of renal perfusion pressure in rats. Hypertension 43: 752–759, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Mori T, Polichnowski A, Glocka P, Kaldunski M, Ohsaki Y, Liang M, Cowley AW., Jr High perfusion pressure accelerates renal injury in salt-sensitive hypertension. J Am Soc Nephrol 19: 1472–1482, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakagawa T, Kang DH, Ohashi R, Suga S, Herrera-Acosta J, Rodriguez-Iturbe B, Johnson RJ. Tubulointerstitial disease: role of ischemia and microvascular disease. Curr Opin Nephrol Hypertens 12: 233–241, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Nishiyama A, Fujisawa Y, Fukui T, Rahman M, Kondo N, Ogawa Y, Fanzhu L, Guoxing Z, Kimura S, Abe Y. Role of nitric oxide in regional blood flow in angiotensin II-induced hypertensive rats. Hypertens Res 24: 421–427, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia 46: 1153–1160, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Polichnowski AJ, Cowley AW., Jr Pressure-induced renal injury in angiotensin II versus norepinephrine-induced hypertensive rats. Hypertension 54: 1269–1277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Polichnowski AJ, Jin C, Yang C, Cowley AW., Jr Role of renal perfusion pressure versus angiotensin II on renal oxidative stress in angiotensin II-induced hypertensive rats. Hypertension 55: 1425–1430, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Quiroz Y, Bravo J, Herrera-Acosta J, Johnson RJ, Rodriguez-Iturbe B. Apoptosis and NFkappaB activation are simultaneously induced in renal tubulointerstitium in experimental hypertension. Kidney Int Suppl: S27–S32, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Quiroz Y, Pons H, Gordon KL, Rincon J, Chavez M, Parra G, Herrera-Acosta J, Gomez-Garre D, Largo R, Egido J, Johnson RJ, Rodriguez-Iturbe B. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from nitric oxide synthesis inhibition. Am J Physiol Renal Physiol 281: F38–F47, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Raij L. Workshop: hypertension and cardiovascular risk factors: role of the angiotensin II-nitric oxide interaction. Hypertension 37: 767–773, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Rodriguez-Iturbe B, Pons H, Herrera-Acosta J, Johnson RJ. Role of immunocompetent cells in nonimmune renal diseases. Kidney Int 59: 1626–1640, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Rodriguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincon J, Chavez M, Parra G, Herrera-Acosta J, Gomez-Garre D, Largo R, Egido J, Johnson RJ. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int 59: 2222–2232, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Ruggenenti P, Perna A, Zoccali C, Gherardi G, Benini R, Testa A, Remuzzi G. Chronic proteinuric nephropathies. II. Outcomes and response to treatment in a prospective cohort of 352 patients: differences between women and men in relation to the ACE gene polymorphism Gruppo Italiano di Studi Epidemologici in Nefrologia (Gisen). J Am Soc Nephrol 11: 88–96, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Schnackenberg CG, Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-iso prostaglandin f2alpha. Hypertension 33: 424–428, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Schulman IH, Zhou MS, Raij L. Interaction between nitric oxide and angiotensin II in the endothelium: role in atherosclerosis and hypertension. J Hypertens Suppl 24: S45–S50, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Singaravelu K, Devalaraja-Narashimha K, Lastovica B, Padanilam BJ. PERP, a p53 proapoptotic target, mediates apoptotic cell death in renal ischemia. Am J Physiol Renal Physiol 296: F847–F858, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest 100: 264–269, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Szentivanyi M, Jr, Maeda CY, Cowley AW., Jr Local renal medullary l-NAME infusion enhances the effect of long-term angiotensin II treatment. Hypertension 33: 440–445, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Taylor NE, Glocka P, Liang M, Cowley AW., Jr NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension 47: 692–698, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Walker WG, Neaton JD, Cutler JA, Neuwirth R, Cohen JD. Renal function change in hypertensive members of the Multiple Risk Factor Intervention Trial. Racial and treatment effects The MRFIT Research Group. JAMA 268: 3085–3091, 1992 [PubMed] [Google Scholar]
- 44. Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol 289: R913–R935, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 288: 2421–2431, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Yuan B, Liang M, Yang Z, Rute E, Taylor N, Olivier M, Cowley AW., Jr Gene expression reveals vulnerability to oxidative stress and interstitial fibrosis of renal outer medulla to nonhypertensive elevations of ANG II. Am J Physiol Regul Integr Comp Physiol 284: R1219–R1230, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Zhang Z, Rhinehart K, Solis G, Pittner J, Lee-Kwon W, Welch WJ, Wilcox CS, Pallone TL. Chronic ANG II infusion increases NO generation by rat descending vasa recta. Am J Physiol Heart Circ Physiol 288: H29–H36, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Zhou MS, Schulman IH, Raij L. Nitric oxide, angiotensin II, and hypertension. Semin Nephrol 24: 366–378, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Zou AP, Wu F, Cowley AW., Jr Protective effect of angiotensin II-induced increase in nitric oxide in the renal medullary circulation. Hypertension 31: 271–276, 1998 [DOI] [PubMed] [Google Scholar]