Abstract
Renal dopamine receptor function and ion transport inhibition are impaired in essential hypertension. We recently reported that caveolin-1 (CAV1) and lipid rafts are necessary for normal D1-like receptor-dependent internalization of Na-K-ATPase in human proximal tubule cells. We now hypothesize that CAV1 is necessary for the regulation of urine sodium (Na+) excretion (UNaV) and mean arterial blood pressure (MAP) in vivo. Acute renal interstitial (RI) infusion into Sprague-Dawley rats of 1 μg·kg−1·min−1 fenoldopam (FEN; D1-like receptor agonist) caused a 0.46 ± 0.15-μmol/min increase in UNaV (over baseline of 0.29 ± 0.04 μmol/min; P < 0.01). This increase was seen in Na+-loaded rats, but not in those under a normal-sodium load. Coinfusion with β-methyl cyclodextrin (βMCD; lipid raft disrupter, 200 μg·kg−1·min−1) completely blocked this FEN-induced natriuresis (P < 0.001). Long-term (3 day) lipid raft disruption via continuous RI infusion of 80 μg·kg−1·min−1 βMCD decreased renal cortical CAV1 expression (47.3 ± 6.4%; P < 0.01) and increased MAP (32.4 ± 6.6 mmHg; P < 0.001) compared with vehicle-infused animals. To determine whether the MAP rise was due to a CAV1-dependent lipid raft-mediated disruption, Na+-loaded rats were given a bolus RI infusion of CAV1 siRNA. Two days postinfusion, cortical CAV1 expression was decreased by 73.6 ± 8.2% (P < 0.001) and the animals showed an increase in MAP by 17.4 ± 2.9 mmHg (P < 0.01) compared with animals receiving scrambled control siRNA. In summary, acute kidney-specific lipid raft disruption decreases CAV1 expression and blocks D1-like receptor-induced natriuresis. Furthermore, chronic disruption of lipid rafts or CAV1 protein expression in the kidney induces hypertension.
Keywords: dopamine receptors, renal proximal tubule, GRK4, lipid rafts
the principal characteristic of essential hypertension is failure of the kidneys to eliminate sodium (Na+) at normal blood pressure (BP) levels. When Na+ intake is increased, there is accumulation of extracellular fluid volume resulting in an increase in BP. In turn, the increased BP leads to elimination of the excess Na+ and water through a process termed pressure-natriuresis (P-N). In several forms of hypertension, the P-N curve is shifted to the right so that a higher BP level is required to excrete the same amount of Na+ (10, 18). The cause of this shift in the P-N curve in essential hypertension is not well-understood. Dopamine secreted by renal proximal tubule cells (RPTC) has been shown to be an important mechanism contributing up to 60% of the renal natriuretic response. However, natriuresis is increased by dopamine receptor stimulation primarily in conditions of a mild Na+ load (33, 37). In humans under conditions of Na+ deficit (8) and in normally hydrated rats (24), no increase in urine Na+ excretion (UNaV) was seen following dopamine receptor stimulation.
Dopamine receptors belong to two receptor subfamilies: D1-like (D1 and D5) and D2-like (D2, D3, and D4). We proposed that a defect in the renal dopaminergic response to Na+ loading is an important mechanism in hypertension and salt sensitivity (13, 47) due to activating variants of G protein-coupled receptor kinase-4 (GRK4) that hyperphosphorylate, internalize, and desensitize the dopamine D1 receptor (D1R). In a preliminary communication, we reported that the P-N curve in salt-sensitive human GRK4γ 486V transgenic mice fed a high-Na+ diet is shifted to the right (40).
Caveolins, localized in lipid rafts of plasma membranes, tether and regulate signaling complexes into functional units (e.g., G protein-coupled receptors and kinases, GPCRs and GRKs, respectively) (27, 32). We previously reported that fenoldopam (FEN; D1-like receptor agonist) stimulates caveolin-2 (CAV2) and D1R association in human embryonic kidney 293 cells (44). Furthermore, FEN-stimulated membrane D1R recruitment (38) and cAMP accumulation were greater in membranes with CAV2-containing lipid rafts than in membranes depleted of CAV2 with antisense oligonucleotides or in nonlipid rafts without CAV2 (44). Studies recently demonstrated that rat RPTCs express CAV1 under certain physiologic conditions (6), but the specific physiologic function of CAV1 has yet to be determined. CAV1 has been shown to inhibit GRK4 (16) while CAV2 does not (44). Therefore, we decided to investigate the role of CAV1 in rat BP regulation.
The dopamine receptor defect that has been implicated in human essential hypertension has been studied extensively in our laboratory in human subjects as well as in immortalized human RPTCs (5, 13, 47). In numerous cell lines that were derived from kidney tissue from hypertensive subjects, the D1-like receptors have diminished agonist-induced recruitment to the plasma membrane and coupling to adenylyl cyclase. We compared these uncoupled cell lines to their normally coupled counterparts and recently reported that CAV1 and lipid rafts are necessary for the D1-like receptor-dependent reduction in Na+ transport in normally coupled RPTCs (16). Blocking CAV1 expression in D1-like receptor-uncoupled human RPTCs had no effect on Na-K-ATPase or Na+ transport.
In vivo studies demonstrated that FEN increased the fractional excretion of Na+ (FENa) by a proximal tubule mechanism in rats (48) and in humans (20). Our previous studies implicated the proximal tubule as the site where FEN induces natriuresis in a Na+-loaded animal model (model used because the normal-Na+ rats did not show an increase in natriuresis following FEN) (34). We repeated the comparison of animals fed a normal- vs. a high-Na+ diet in our current study to determine whether lipid rafts and CAV1 are necessary for D1-like receptor-induced natriuresis and maintenance of normal BP. Specifically, we first performed acute renal interstitial (RI) infusion of the lipid raft-disrupting agent β-methyl cyclodextrin (βMCD) concurrently with D1-like receptor stimulation and measured UNaV and mean arterial pressure (MAP). Second, the effect of 3-day chronic RI infusion of βMCD on MAP was examined. Third, we blocked renal CAV1 expression by infusing CAV1 siRNA into the RI space in vivo and measured MAP 2 days later. We hypothesized that chronic RI infusion of either βMCD or CAV1 siRNA might inactivate the dopamine D1-like receptor and increase MAP.
MATERIALS AND METHODS
General Animal Preparation
The experimental protocols were approved by the University of Virginia Animal Care and Use Committee and were conducted on 12-wk-old female Sprague-Dawley rats (Harlan, Teklad) that were housed in a vivarium under controlled conditions (temperature: 21 ± 1°C; humidity: 60 ± 10%; and light: 8 AM to 8 PM). Rats received either a normal- or a high-Na+ diet (0.28 and 4.0% Na+, respectively). The rats that were Na+-loaded received the high-Na+ diet for 1 wk before and during the experiments. On days 6–7, the rats were placed in metabolic cages and 24-h urine samples were collected to measure UNaV to ensure that they were Na+-loaded. Representative UNaV was 0.69 and 6.34 μmol/min for the normal- and high-Na+ diets, respectively. Experiments were conducted at similar times each day to prevent any diurnal variations in BP.
Experimental Protocols
1) Effects of short-term RI infusion of FEN on UNaV and MAP in normal and Na+-loaded rats.
On the day of experimentation, the rats were placed under general anesthesia with pentobarbital sodium via an intraperitoneal (IP) injection and a tracheostomy was performed to assist respiration. Direct cannulation of the right internal jugular vein using PE-10 tubing provided intravenous (IV) access through which vehicle (VEH) 5% dextrose in water (D5W) was infused at 20 μl/min to prevent dehydration. Cannulation of the right carotid artery using PE-50 tubing provided arterial access for MAP measurements with a digital BP analyzer (Micromed). Pressures were recorded every 5 min and averaged for all periods of the study. Following a midline laparotomy, the right kidney was removed, and the remaining ureter was cannulated (PE-10) to collect urine for the quantification of UNaV. An open-bore microinfusion catheter (PE-10) was inserted under the renal capsule into the cortex of the remaining (left) kidney for the RI infusion of pharmacologic agents or VEH at 2.5 μl/min with a syringe pump (Harvard, model 55-222). Vetbond tissue adhesive (3M Animal Care Products) was used to secure the catheter and prevent interstitial pressure loss. Four groups were investigated (n = 6 per group): 1) control normal Na+: rats received RI infusion of VEH during both the 60-min control and 60-min treatment periods; 2) FEN normal Na+: rats received RI infusion of VEH during the 60-min control period, followed by a 60-min treatment period with RI infusion of FEN (1 μg·kg−1·min−1); 3) control high Na+: rats received RI infusion of VEH during both the 60-min control and 60-min treatment periods; and 4) FEN high Na+: rats received RI infusion of VEH during the 60-min control period, followed by a 60-min treatment period with RI infusion of FEN (1 μg·kg−1·min−1). UNaV and MAP were measured for each period and the remaining (left) kidneys were removed for use in the coimmunoprecipitation dot-blot assay for Na-K-ATPase-adaptor protein 2 (AP2) association.
2) Effects of short-term RI infusion of FEN+βMCD and βMCD alone on UNaV and MAP in Na+-loaded rats.
On the day of experimentation, the same procedures in protocol 1 were performed. Two groups were investigated (n = 6 per group): 1) FEN + βMCD high Na+: rats received RI coinfusion of FEN (1 μg·kg−1·min−1) + βMCD (200 μg·kg−1·min−1) during the 60-min treatment period following a 60-min control period with RI infusion of VEH and 2) βMCD high Na+: rats received RI infusion of βMCD alone during the 60-min treatment period following a 60-min control period with RI infusion of VEH. UNaV and MAP were measured for each period and the kidneys were collected for the use in the coimmunoprecipitation dot-blot assay for Na-K-ATPase-AP2 association.
3) Effects of 3-day RI infusion of βMCD on MAP.
DAY 0.
Rats (n = 6) were placed under short-term anesthesia with ketamine and xylazine via an IP injection. Using a sterile technique, a midline laparotomy was performed and the right kidney was removed. A catheter (PE-10) connected to a 3-day osmotic mini-pump (Alzet, model 1003D) infusing βMCD (80 μg·kg−1·min−1) at 1 μl/h was inserted into the renal cortex of the remaining (left) kidney and secured with mesh and Vetbond tissue adhesive. Rats received intramuscular injections (IM) of Buprenex following the surgery and then twice daily for 2 days. The antibiotic Baytril was added to the drinking water to prevent infection.
DAY 3.
The rats were placed under general anesthesia with pentobarbital sodium via an IP injection and a tracheostomy was performed to assist respiration. Cannulation of the right carotid artery using PE-50 tubing provided arterial access for MAP measurements. The left kidney was removed and snap-frozen in liquid nitrogen for use in Western blotting to determine the effect of βMCD on CAV1 protein levels.
4) Effects of CAV1 siRNA on MAP.
DAY 0.
Rats (n = 6) were placed under short-term anesthesia with ketamine and xylazine via an IP injection. Using a sterile technique, a midline laparotomy was performed and the right kidney was removed. Twenty microliters of either scrambled control siRNA (SCR) or CAV1 siRNA (15 μg) were mixed with 20 μl of transfer polymer and 64 μl of delivery buffer (Mirus TransIT In Vivo Gene Delivery System) and infused interstitially into the renal cortex of the remaining (left) kidney at a rate of 2.5 μl/min. Previous studies demonstrated that antisense selectively targets the kidney (and not surrounding organs) and preferentially appears in the proximal tubule using this method (35, 41). Rats received IM injections of Buprenex following the surgery and then twice daily for the next 2 days. The antibiotic Baytril was added to the drinking water to prevent infection.
DAY 2.
The rats were placed under general anesthesia with pentobarbital sodium via an IP injection and a tracheostomy was performed to assist respiration. Cannulation of the right carotid artery using PE-50 tubing provided arterial access for MAP measurements. The left kidney was removed and snap-frozen in liquid nitrogen for use in Western blotting to confirm that the siRNA caused a reduction in CAV1 protein levels.
Immunofluorescent Staining of Endogenous Proximal Tubular CAV1
Kidneys were removed from normal- or high-Na+ rats (1 wk on diet) and were fixed with 4% paraformaldehyde. Frozen 8-μm cortical sections were made, unmasked with 1% sodium dodecyl sulfate (SDS) for 5 min, blocked, and probed with rabbit anti-CAV1 primary antibody (1:200; BD Transduction Laboratories) for 1 h at room temperature. Alexa-647 donkey anti-rabbit secondary antibody (Invitrogen) was added for 1 h, washed, and slides were mounted in Fluoromount G. Images were taken on Olympus IX81 spinning disk confocal microscope with a ×60 1.2 NA water immersion lens using Slidebook 5.0 software collected using identical exposure and filter settings. Background levels of staining were gated using an equal amount of nonspecific rabbit IgG antibody (Sigma; 0.8 μg/ml) as a primary antibody. Images were quantified using ImageJ imaging software.
Renal Cortical CAV1 Protein Expression
Superficial surface shavings were taken from the frozen renal cortex to obtain only proximal tubules and not deeper nephron segments. One hundred fifty milligrams of this tissue were processed by lysis in 1 ml M-PER (Thermo Scientific) containing Halt protease and phosphatase inhibitor cocktail with EDTA. Protein concentration was determined by BCA assay (Bio-Rad) and 20 μg of protein were loaded per lane for electrophoresis and Western blot analysis (17). The proteins of interest were detected using rabbit polyclonal antibodies to CAV1 (1:500 dilution; BD Transduction Laboratories) and mouse monoclonal antibody to β-actin (1:2,000; Sigma) followed by goat anti-rabbit infrared dye (IR Dye 800, Li-Cor) and goat anti-mouse infrared dye (IR Dye 680, Li-Cor) secondary antibodies (1:15,000). Imaging was performed on an Odyssey infrared imaging system (Li-Cor).
Coimmunoprecipitation Dot-Blot Assay for AP2-Na-K-ATPase Association
Measurement of Na-K-ATPase internalization by association with AP2 was performed as previously reported (16). Immediately following the acute experimental conditions in protocols 1 and 2, the kidneys were excised, decapsulated, and snap-frozen in liquid nitrogen. One hundred fifty milligrams of superficial (shaved) cortex were lysed in ice-cold lysis buffer (M-PER with Halt protease and phosphatase inhibitor cocktail) using a tissue homogenizer. Two micrograms of rabbit anti-AP2 antibody (α-adaptin, Santa Cruz Biotechnology, sc-10761) or a nonspecific rabbit IgG (Sigma) were added to 500 μg lysate in 1 ml lysis buffer for 2 h. Protein A sepharose was incubated for 30 min with the lysate, washed three times in lysis buffer, and eluted with LDS loading buffer with reducing agent (Invitrogen). The eluate was immuno-dot-blotted for Na-K-ATPase using a 1:1,000 dilution of anti-Na-K-ATPase alpha antibody (Millipore clone C464.6). The Na-K-ATPase signal was normalized to the amount of AP2 pulled down using a replicate well that was probed with a mouse anti-AP2 monoclonal antibody (α-adaptin, Santa Cruz Biotechnology, sc-17771). Following the addition of both Na-K-ATPase and AP2 mouse monoclonal antibodies, goat anti-mouse IR Dye 800 secondary antibody was added, and the cells were imaged using an Odyssey infrared imaging system. The specificity of this immuno dot-blot method was previously verified by Western blot analysis (16).
siRNA to CAV1
CAV1 siRNA (target sequence 5′-AACCAGAAGGGACACACAGTT-3′ and scrambled control 5′-AAAGAGCGACTTTACACACTT-3′) (9, 39) were designed and ordered prehybridized (Sigma-Genosys).
Statistical Analysis
Data are expressed as means ± 1 SE. Comparisons within and among groups were made by repeated measures or factorial ANOVA, respectively, followed by Holm-Sidak or Duncan's test. T-test was used for two-group comparisons. A P value of <0.05 was considered significant.
RESULTS
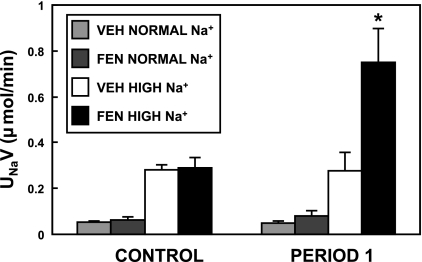
Initially, we performed acute studies on rats that had received either a normal- or high-Na+ diet, with all animals (n = 6/group) receiving 1-h RI infusion of VEH for baseline and subsequently 1 h of the drug(s) to be tested. We reconfirmed our previous finding that the normal-diet rats show no change in natriuresis following RI FEN (D1-like receptor agonist, 1 μg·kg−1·min−1) infusion (unpublished observations, Carey RM). In the high-Na+ rats, however, FEN caused a 0.47 ± 0.13-μmol/min increase (P < 0.01) in UNaV from baseline (Fig. 1).
Fig. 1.
Effect of acute renal interstitial infusion of fenoldopam (FEN) on natriuresis in normal- and Na+-loaded rats. Rats fed a diet of normal or high sodium (Na+) for 7 days received a 1-h control period with D5W vehicle (VEH), which was followed by a 1-h infusion (period 1) of VEH or FEN (1 μg·kg−1·min−1). The Na+-loaded rats showed a 0.46 ± 0.13-μmol/min increase in urine Na+ excretion (UNaV) from baseline after FEN infusion (*P < 0.01, n = 6); the normal-Na+ rats showed no natriuretic response to FEN.
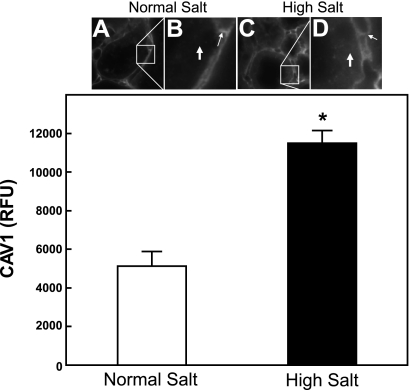
We also carried out immunofluorescent staining (Fig. 2) on normal- and Na+-loaded rats. In the normal-salt rats, proximal tubule staining was found to be very low when identically exposed confocal images were properly gated, using identical amounts of nonspecific rabbit IgG as a negative control antibody. Using the same confocal settings as above, Na+-loaded rats showed a 224 ± 12% (P < 0.001) increase in proximal tubule-specific CAV1 staining. Quantitative peritubular capillary-specific CAV1 staining did not change between the normal- and high-Na+ conditions (Fig. 2, B and D, small arrows). Additionally, the distal tubule-specific CAV1 staining did not change between the normal- and high-Na+ rats (data not shown).
Fig. 2.
Immunofluorescent staining of endogenous proximal tubular caveolin-1 (CAV1) in fixed frozen renal cortical sections from normal- and Na+-loaded rats. Kidneys were removed from normal- or Na+-loaded rats. Fixed frozen 8-μm cortical sections were stained with rabbit anti-CAV1 primary antibody and Alexa-647 donkey anti-rabbit secondary antibody. Tissue from normal-Na+ animals is shown in A and B and that of high-Na+ animals in C and D. The boxes in A and C (×600 magnification) are enlarged in B and D. Proximal tubular cellular localization is indicated by the large white arrows, which show over 2-fold increased CAV1 immunofluorescent staining in the high-Na+ conditions (D; *P < 0.001, n = 6). The unchanged peritubular capillary CAV1 staining is indicated by the smaller white arrows in B and D.
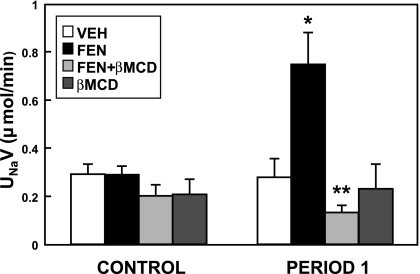
All subsequent experiments were performed using rats fed a high-Na+ diet. When the cholesterol and lipid raft disruptor βMCD (200 μg·kg−1·min−1) was infused into the RI space, there was no significant change in UNaV (Fig. 3). However, coinfusion of βMCD with FEN abolished the FEN-induced natriuresis (P < 0.001). In this acute study, MAP did not change significantly (data not shown).
Fig. 3.
Effect of acute renal interstitial coinfusion of β-methyl cyclodextrin (βMCD) on D1-like receptor-mediated urine sodium excretion. Left (CONTROL period): D5W vehicle (VEH) was infused into the renal cortex of all the animals for 1 h to record baseline UNaV measurements. Right (period 1): the second hour where the same animals received VEH, FEN (1 μg·kg−1·min−1), βMCD alone (200 μg·kg−1·min−1), or a combination of FEN+βMCD. The FEN-induced natriuresis (*P < 0.01, FEN vs. VEH, n = 6) was completely blocked by the coinfusion of βMCD (**P < 0.001, FEN vs. FEN+βMCD, n = 6).
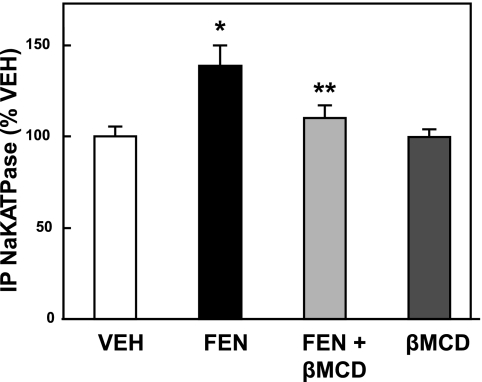
In an attempt to correlate the observed natriuresis with an inhibition of Na-K-ATPase, we measured the association of AP2 with Na-K-ATPase by immunoprecipitation and dot-blot as previously reported for cells in culture (16). Figure 4 shows a 38.56 ± 11.29% (P < 0.01) increase in AP2-Na-K-ATPase association from the kidney lysates of rats infused with FEN as described above for the acute study. The addition of βMCD blocked this FEN-dependent increase by 73.35 ± 4.55% (P < 0.05 vs. FEN alone). βMCD alone had no effect compared with VEH.
Fig. 4.
Effect of short-term renal interstitial coinfusion of βMCD on D1-like receptor-induced internalization of Na-K-ATPase. At the end of the acute study shown in Fig. 3, kidneys from the rats were removed and cortical tissue lysates were made. Adaptor protein 2 (AP2) was immunoprecipitated and the filter-trapped precipitate was then immuno-dot-blotted for Na-K-ATPase. Values are expressed as a percentage of VEH control (set at 100%). FEN (1 μg·kg−1·min−1) stimulated the AP2-mediated Na-K-ATPase internalization (*P < 0.01 FEN vs. VEH, n = 6) and this effect was blocked by the coinfusion of βMCD (200 μg·kg−1·min−1; **P < 0.05 FEN vs. FEN+βMCD, n = 6).
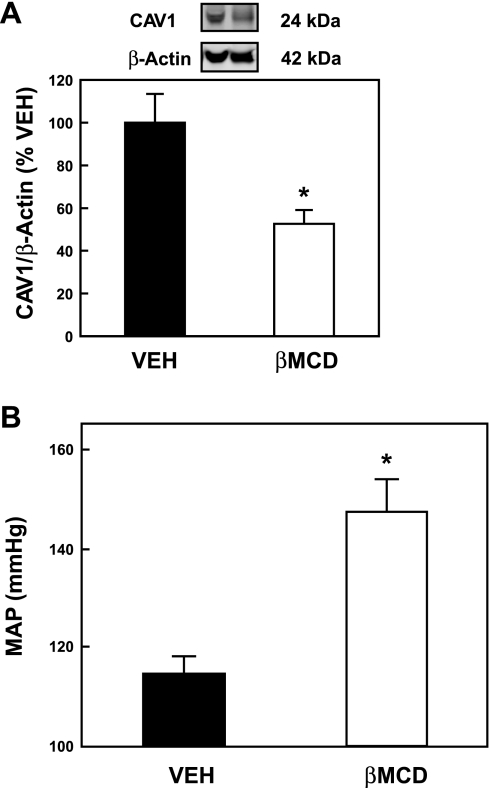
A longer-term (3 day) RI infusion of βMCD (80 μg·kg−1·min−1) was subsequently administered using an osmotic minipump. βMCD caused a 47.3 ± 6.4% reduction in cortical CAV1 expression as measured by Western blot analysis (Fig. 5A; P < 0.01 vs. VEH-infused animals). β-Actin was used as a loading control and quantification of CAV1 was calculated as a ratio to β-actin. Figure 5B shows that the 3-day RI infusion of βMCD (80 μg·kg−1·min−1) produced a 32.4 ± 6.6-mmHg rise in MAP (P < 0.001 vs. VEH-infused animals), indicating that chronic reduction in lipid rafts increases MAP.
Fig. 5.
Effect of long-term renal interstitial infusion of βMCD on CAV1 protein levels and mean arterial pressure (MAP). A: Western blot analysis of cortical kidney tissue lysates from animals receiving a 3-day infusion of D5W vehicle (VEH) or βMCD (80 μg·kg−1·min−1). The CAV1 protein was quantified as a ratio of the β-actin protein measured concurrently in the Western blot (*P < 0.001, n = 6). B: same animals as in A had MAP measurements performed at the end of the 3-day infusion with VEH or βMCD. The rats that received βMCD showed a 32.4 ± 6.6-mmHg rise in MAP (*P < 0.001, n = 6).
Since βMCD is not a specific disrupting agent for CAV1, we employed siRNA knockdown techniques to determine whether a CAV1-dependent lipid raft-mediated disruption would also cause an increase in MAP. We knocked down CAV1 using a bolus RI infusion of CAV1 siRNA into the kidney and then measured MAP and kidney proteins 2 days later. We confirmed that this subcapsular method of infusion to produce knockdown was specific to the kidney by comparing CAV expression in other nearby organs. We examined heart, aorta, and liver tissue and saw no decrease in CAV1. We also measured the expression of CAV2 and discovered that it did not change significantly in any of the organs, including the kidney (data not shown). Previous use of this method showed preferential targeting of the proximal tubule (41).
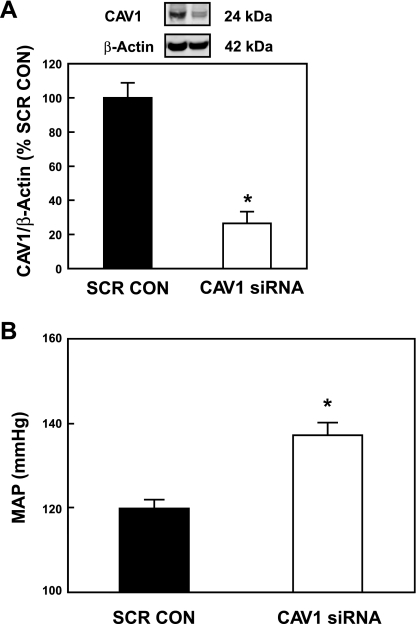
Figure 6A shows that the RI infusion of CAV1 siRNA knocked down CAV1 protein expression in the renal cortex of the kidney. Calculated as a ratio of β-actin levels and compared with SCR controls, CAV1 was significantly reduced by 73.6 ± 8.2% (P < 0.001) as determined by Western blot. When CAV1 was measured in these animals 2 days post-RI infusion of CAV1 siRNA, MAP was increased by 17.4 ± 2.9 mmHg (P < 0.01, n = 5) compared with control animals receiving SCR (Fig. 6B). This increase in MAP was less than that seen in the continuously infused βMCD animals, but it was still a significant change for that 2-day time period.
Fig. 6.
Long-term (2-day) effect of a bolus renal interstitial infusion of CAV1 siRNA on CAV1 protein levels and MAP. A: Western blot analysis of cortical kidney tissue lysates from animals receiving a bolus infusion of CAV1 siRNA or scrambled control (SCR CON). The CAV1 protein was quantified as a ratio of the β-actin protein, measured concurrently in the Western blot (*P < 0.001, n = 6). B: MAP was measured in the same animals as in A. The rats who received CAV1 siRNA showed an increase in MAP of 17.4 ± 2.9 mmHg, compared with the SCR CON animals (*P < 0.01, n = 5).
DISCUSSION
Little is known about the role of lipid rafts and specifically CAV1 (a major structural protein within lipid rafts) in health and disease. The loss of CAV1 expression in the kidney is associated with hypertension and diabetes in the rat (3, 22, 26). CAV1 expression is lower in the proximal tubules of spontaneously hypertensive rats (SHR) compared with their Wistar-Kyoto (WKY) counterparts (6). Furthermore, a decrease of BP in SHR was correlated with an increase in CAV1 expression in the proximal tubule (28). This reciprocal relationship may be related to our recent findings that anti-natriuretic angiotensin II (ANG II) stimulation decreases, while natriuretic D1-like receptor activation increases, the expression of CAV1 in RPTCs (16). This previous study demonstrated that the reduction in CAV1 expression is associated with uncoupling of the D1R from adenylyl cyclase activation and from Na-K-ATPase internalization and inhibition of Na+ transport. However, the physiologic effect of CAV1 reduction on BP has not been studied. Since the natriuretic effect of D1-like receptor stimulation only occurs during high-Na+ intake in RI-infused rats, we tested the hypothesis that CAV1 is necessary for D1-like receptor-mediated natriuresis in vivo using Sprague-Dawley rats fed a high-Na+ diet.
In our previous in vitro study, we determined that lipid rafts and CAV1 expression were necessary for FEN-dependent Na-K-ATPase internalization and inhibition of Na+ transport of RPTCs. Similarly, in this current physiologic study, the consequence of reducing CAV1 expression by βMCD or CAV1 siRNA was a reduction in D1-like receptor-mediated natriuresis (short-term) and a significant rise in MAP (long-term). We also demonstrated a reduced association of Na-K-ATPase with AP2, a prerequisite for proper downregulation of Na-K-ATPase. The specific mediators of these effects could be GRK4 (14) or GRK2 (2, 42) activity. We are currently investigating these possibilities by coinfusion with either GRK2 or GRK4 siRNA and testing whether the acute natriuretic and chronic hypertensive effects can be reversed.
Previous reports have been equivocal about the presence of CAV1 in rat renal proximal tubules in situ. Zhuang et al. (49) recently reported that under normal physiologic conditions, CAV1 is undetectable in rat renal proximal tubules using numerous techniques, confirming their previous report using immunofluorescence. Others also failed to find immunoreactive CAV1 in proximal tubules in situ (7, 15). The demonstration of CAV1 in the rat and mouse proximal tubule may be inconsistent from various laboratories due to the difficulty in unmasking CAV1 using different immunohistochemical embedding and staining methods. Using Western blot analysis and immunofluorescence on microdissected rat renal proximal tubules, Bocanegra et al. (6) detected lower CAV1 expression in SHR compared with control (WKY) rats. CAV1 was also found to be expressed in mouse despite the lack of immunohistochemical staining when proximal tubule segments were isolated using collagenase and then examined by Western blot (45). Furthermore, certain physiologic stresses have been shown to cause transient expression of CAV1 in the renal proximal tubule (45). CAV1 and CAV2 expression increases dramatically from very low baseline expression levels in rat renal proximal tubules in response to gentamicin injection (15). In isolated mouse RPTCs, radiographic contrast media caused CAV1 shedding into the culture media (46). CAV1 and rBAT1 coimmunoprecipitate in primary cultured rat RPTCs (23), with CAV1 mRNA expression and CAV1 protein detected by RT-PCR and Western blotting, respectively. Our studies suggest that CAV1 is expressed at low concentrations in the normal rat kidney in situ as revealed by careful antigen rescue. Since we show here that CAV1 expression is upregulated after Na+ loading, we speculate that CAV1 may play only a minor role in unstressed renal function, but it can be upregulated to play its role as a scaffold protein to assist with events that help restore normal-Na+ balance.
The role of CAV1 on BP has been controversial. CAV1-null animals have been reported to have no increase in BP (1, 11), decreased BP (29), a decrease in BP variability (11), pulmonary hypertension (4, 12), and a 50% decrease in life span (31). Furthermore, losartan treatment lowered BP in SHR while raising CAV1 expression. We speculated that a decrease in the activity of the kidney cortical dopaminergic system by kidney-specific CAV1 knockdown would likely increase the activity of the intrarenal renin-angiotensin system (RAS) (16) since the dopaminergic system has been shown to be responsible for keeping angiotensin type 1 receptor (AT1R) activation in check (17, 25). It is possible that the decrease in CAV1 expression in the βMCD-treated rats could be associated with an activation of the RAS; however, this hypothesis remains to be tested. Interestingly, AT1R binding was shown to be lower in kidneys of the CAV1 knockout mice by receptor binding assays (43). This may be due to downregulation secondary to increased ANG II peptide production, but this remains to be examined.
The use of a nonselective cholesterol inhibitor (βMCD) to disrupt lipid rafts and thus CAV1 function can be problematic. βMCD alone has been shown to inhibit sodium-hydrogen exchanger-3 (30), which would be expected to result in natriuresis and hypotension (36). However, we observed the opposite effects. In most published studies, CAV1 expression is directly linked to cell membrane cholesterol levels (19, 21); therefore, we used this approach in our in vivo studies. However, we also performed parallel experiments in which we used the selective CAV1 mRNA knockdown strategy with siRNA, which demonstrated similar physiologic results as induced by βMCD. Future studies will focus on the chronic studies, investigating FEN-induced natriuresis in animals with a sustained increased BP following CAV1 siRNA treatment.
In summary, depletion of membrane CAV1 by reducing membrane cholesterol by βMCD resulted in a short-term reduction in D1-like receptor-stimulated natriuresis in Na+-loaded rats without a change in BP. Longer-term administration of βMCD or CAV1 siRNA into the kidney cortex resulted in a reduction in CAV1 and an increase in BP in Na+-loaded rats.
Perspectives
There is a growing appreciation that structural membrane proteins play a critical role in cell surface receptor-mediating effects within the cell. CAV1 is a component of lipid rafts, and it has been found to be associated with dopamine and ANG II activities. Previous work demonstrated that the D1-like receptor-mediated internalization of the Na+ pump (Na-K-ATPase) was CAV1 dependent. We now demonstrate that CAV1 depletion in Na+-loaded rats plays an integral role in the reduction of UNaV in the short term and an elevation of BP in the long term. Thus, we show that CAV1 plays a key role in maintaining appropriate UNaV and BP under conditions of Na+ loading.
GRANTS
This work was supported by Grants HL074940, DK039308, DK087998, HL081891, HL087998, and HL095796.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Helen E. McGrath for editorial assistance with this manuscript.
REFERENCES
- 1. Albinsson S, Shakirova Y, Rippe A, Baumgarten M, Rosengren BI, Rippe C, Hallmann R, Hellstrand P, Rippe B, Sward K. Arterial remodeling and plasma volume expansion in caveolin-1-deficient mice. Am J Physiol Regul Integr Comp Physiol 293: R1222–R1231, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Asghar M, Banday AA, Fardoun RZ, Lokhandwala MF. Hydrogen peroxide causes uncoupling of dopamine D1-like receptors from G proteins via a mechanism involving protein kinase C and G-protein-coupled receptor kinase 2. Free Radic Biol Med 40: 13–20, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bai Y, Ye S, Mortazavi R, Campese V, Vaziri ND. Effect of renal injury-induced neurogenic hypertension on NO synthase, caveolin-1, AKt, calmodulin and soluble guanylate cyclase expressions in the kidney. Am J Physiol Renal Physiol 292: F974–F980, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Benett RW, Persaud TV, Moore KL. Experimental studies on the effects of aluminum on pregnancy and fetal development. Anat Anz 138: 365–378, 1975 [PubMed] [Google Scholar]
- 5. Bengra C, Mifflin TE, Khripin Y, Manunta P, Williams SM, Jose PA, Felder RA. Genotyping of essential hypertension single-nucleotide polymorphisms by a homogeneous PCR method with universal energy transfer primers. Clin Chem 48: 2131–2140, 2002 [PubMed] [Google Scholar]
- 6. Bocanegra V, Manucha W, Pena MR, Cacciamani V, Valles PG. Caveolin-1 and Hsp70 interaction in microdissected proximal tubules from spontaneously hypertensive rats as an effect of Losartan. J Hypertens 28: 143–155, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Breton S, Lisanti MP, Tyszkowski R, McLaughlin M, Brown D. Basolateral distribution of caveolin-1 in the kidney. Absence from H+-atpase-coated endocytic vesicles in intercalated cells. J Histochem Cytochem 46: 205–214, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Carey RM, Siragy HM, Ragsdale NV, Howell NL, Felder RA, Peach MJ, Chevalier RL. Dopamine-1 and dopamine-2 mechanisms in the control of renal function. Am J Hypertens 3: 59S–63S, 1990 [DOI] [PubMed] [Google Scholar]
- 9. Cho KA, Ryu SJ, Park JS, Jang IS, Ahn JS, Kim KT, Park SC. Senescent phenotype can be reversed by reduction of caveolin status. J Biol Chem 278: 27789–27795, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Coffman TM, Crowley SD. Kidney in hypertension: guyton redux. Hypertension 51: 811–816, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Desjardins F, Lobysheva I, Pelat M, Gallez B, Feron O, Dessy C, Balligand JL. Control of blood pressure variability in caveolin-1-deficient mice: role of nitric oxide identified in vivo through spectral analysis. Cardiovasc Res 79: 527–536, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293: 2449–2452, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Felder RA, Jose PA. Mechanisms of disease: the role of GRK4 in the etiology of essential hypertension and salt sensitivity. Nat Clin Pract Nephrol 2: 637–650, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Felder RA, Sanada H, Xu J, Yu PY, Wang Z, Watanabe H, Asico LD, Wang W, Zheng S, Yamaguchi I, Williams SM, Gainer J, Brown NJ, Hazen-Martin D, Wong LJ, Robillard JE, Carey RM, Eisner GM, Jose PA. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci USA 99: 3872–3877, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujigaki Y, Sakakima M, Sun Y, Goto T, Ohashi N, Fukasawa H, Tsuji T, Yamamoto T, Hishida A. Immunohistochemical study on caveolin-1alpha in regenerating process of tubular cells in gentamicin-induced acute tubular injury in rats. Virchows Arch 450: 671–681, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Gildea JJ, Israel JA, Johnson AK, Zhang J, Jose PA, Felder RA. Caveolin-1 and dopamine-mediated internalization of Na-K-ATPase in human renal proximal tubule cells. Hypertension 54: 1070–1076, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gildea JJ, Wang X, Jose PA, Felder RA. Differential D1 and D5 receptor regulation and degradation of the angiotensin type 1 receptor. Hypertension 51: 360–366, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Guyton A, Hall J, Coleman T. The Dominant Role of the Kidneys in Long-Term Arterial Pressure Regulation in Normal and Hypertensive States. New York: Raven, 1995, p. 1311–1333 [Google Scholar]
- 19. Hailstones D, Sleer LS, Parton RG, Stanley KK. Regulation of caveolin and caveolae by cholesterol in MDCK cells. J Lipid Res 39: 369–379, 1998 [PubMed] [Google Scholar]
- 20. Hughes JM, Beck TR, Rose CE, Jr, Carey RM. The effect of selective dopamine-1 receptor stimulation on renal and adrenal function in man. J Clin Endocrinol Metab 66: 518–525, 1988 [DOI] [PubMed] [Google Scholar]
- 21. Ikonen E, Parton RG. Caveolins and cellular cholesterol balance. Traffic 1: 212–217, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Komers R, Schutzer WE, Reed JF, Lindsley JN, Oyama TT, Buck DC, Mader SL, Anderson S. Altered endothelial nitric oxide synthase targeting and conformation and caveolin-1 expression in the diabetic kidney. Diabetes 55: 1651–1659, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Kwak JO, Kim HW, Jung SM, Song JH, Hong SB, Oh KJ, Ko CB, Cha SH. Colocalization and interaction of b0,+-type amino acid transporter 1 (BAT1) with caveolin-1 in rat kidney. J Nephrol 18: 681–689, 2005 [PubMed] [Google Scholar]
- 24. Leander JD. Effects of specific D-1 and D-2 dopamine agonists (LY141865 and SKF-38393) and pergolide on urinary output of rats. Arch Int Pharmacodyn Ther 289: 290–295, 1987 [PubMed] [Google Scholar]
- 25. Li H, Armando I, Yu P, Escano C, Mueller SC, Asico L, Pascua A, Lu Q, Wang X, Villar VA, Jones JE, Wang Z, Periasamy A, Lau YS, Soares-da-Silva P, Creswell K, Guillemette G, Sibley DR, Eisner G, Gildea JJ, Felder RA, Jose PA. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J Clin Invest 118: 2180–2189, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Z, Rodriguez-Iturbe B, Ni Z, Shahkarami A, Sepassi L, Vaziri ND. Effect of hereditary obesity on renal expressions of NO synthase, caveolin-1, AKt, guanylate cyclase, and calmodulin. Kidney Int 68: 2766–2772, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu YH, Cook RF, Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol 126: 111–126, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mukai Y, Sato S. Polyphenol-containing azuki bean (Vigna angularis) extract attenuates blood pressure elevation and modulates nitric oxide synthase and caveolin-1 expressions in rats with hypertension. Nutr Metab Cardiovasc Dis 19: 491–497, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, Sessa WC. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med 204: 2373–2382, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murtazina R, Kovbasnjuk O, Donowitz M, Li X. Na+/H+ exchanger NHE3 activity and trafficking are lipid Raft-dependent. J Biol Chem 281: 17845–17855, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Park DS, Cohen AW, Frank PG, Razani B, Lee H, Williams TM, Chandra M, Shirani J, De Souza AP, Tang B, Jelicks LA, Factor SM, Weiss LM, Tanowitz HB, Lisanti MP. Caveolin-1 null (−/−) mice show dramatic reductions in life span. Biochemistry 42: 15124–15131, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol 48: 359–391, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pelayo JC, Fildes RD, Eisner GM, Jose PA. Effects of dopamine blockade on renal sodium excretion. Am J Physiol Renal Fluid Electrolyte Physiol 245: F247–F253, 1983 [DOI] [PubMed] [Google Scholar]
- 34. Salomone LJ, Howell NL, McGrath HE, Kemp BA, Keller SR, Gildea JJ, Felder RA, Carey RM. Intrarenal dopamine D1-like receptor stimulation induces natriuresis via an angiotensin type-2 receptor mechanism. Hypertension 49: 155–161, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Sanada H, Yatabe J, Midorikawa S, Katoh T, Hashimoto S, Watanabe T, Xu J, Luo Y, Wang X, Zeng C, Armando I, Felder RA, Jose PA. Amelioration of genetic hypertension by suppression of renal G protein-coupled receptor kinase type 4 expression. Hypertension 47: 1131–1139, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Siragy HM, Felder RA, Howell NL, Chevalier RL, Peach MJ, Carey RM. Evidence that intrarenal dopamine acts as a paracrine substance at the renal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 257: F469–F477, 1989 [DOI] [PubMed] [Google Scholar]
- 38. Trivedi M, Narkar VA, Hussain T, Lokhandwala MF. Dopamine recruits D1A receptors to Na-K-ATPase-rich caveolar plasma membranes in rat renal proximal tubules. Am J Physiol Renal Physiol 287: F921–F931, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Vijayasingam SM, Narendran K, Meers PD. Community-acquired legionellosis in Singapore. Ann Acad Med Singapore 20: 817–820, 1991 [PubMed] [Google Scholar]
- 40. Wang Z, Asico LD, Escano CS, Felder RA, Jose PA. Human G protein-coupled receptor kinase type 4 ξ (hGRK4ξ) wild-type prevents salt sensitivity while its variant, hGRK4g486V, promotes salt sensitivity in transgenic mice: role of genetic background. Hypertension 48: e27, 2006 [Google Scholar]
- 41. Wang ZQ, Felder RA, Carey RM. Selective inhibition of the renal dopamine subtype D1A receptor induces antinatriuresis in conscious rats. Hypertension 33: 504–510, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Watanabe H, Xu J, Bengra C, Jose PA, Felder RA. Desensitization of human renal D1 dopamine receptors by G protein-coupled receptor kinase 4. Kidney Int 62: 790–798, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Wyse BD, Prior IA, Qian H, Morrow IC, Nixon S, Muncke C, Kurzchalia TV, Thomas WG, Parton RG, Hancock JF. Caveolin interacts with the angiotensin II type 1 receptor during exocytic transport but not at the plasma membrane. J Biol Chem 278: 23738–23746, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Yu P, Yang Z, Jones JE, Wang Z, Owens SA, Mueller SC, Felder RA, Jose PA. D1 dopamine receptor signaling involves caveolin-2 in HEK-293 cells. Kidney Int 66: 2167–2180, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Zager RA, Johnson A, Hanson S, dela Rosa V. Altered cholesterol localization and caveolin expression during the evolution of acute renal failure. Kidney Int 61: 1674–1683, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Zager RA, Johnson AC, Hanson SY. Radiographic contrast media-induced tubular injury: evaluation of oxidant stress and plasma membrane integrity. Kidney Int 64: 128–139, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Zeng C, Villar VA, Eisner GM, Williams SM, Felder RA, Jose PA. G protein-coupled receptor kinase 4: role in blood pressure regulation. Hypertension 51: 1449–1455, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang Y, Yuan Z, Ge H, Ren Y. Effects of long-term ouabain treatment on blood pressure, sodium excretion, and renal dopamine D(1) receptor levels in rats. J Comp Physiol [B] 180: 117–124, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Zhuang Z, Marshansky V, Breton S, Brown D. Is caveolin involved in normal proximal tubule function? Presence in model PT systems but absence in situ. Am J Physiol Renal Physiol 300: F199–F206, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]