Abstract
Increasing evidence suggests that chronic kidney disease may develop following acute kidney injury and that this may be due, in part, to hypoxia-related phenomena. Hypoxia-inducible factor (HIF) is stabilized in hypoxic conditions and regulates multiple signaling pathways that could contribute to renal fibrosis. As transforming growth factor (TGF)-β is known to mediate renal fibrosis, we proposed a profibrotic role for cross talk between the TGF-β1 and HIF-1α signaling pathways in kidney cells. Hypoxic incubation increased HIF-1α protein expression in cultured human renal tubular epithelial cells and mouse embryonic fibroblasts. TGF-β1 treatment further increased HIF-1α expression in cells treated with hypoxia and also increased HIF-1α in normoxic conditions. TGF-β1 did not increase HIF-1α mRNA levels nor decrease the rate of protein degradation, suggesting that it enhances normoxic HIF-1α translation. TGF-β receptor (ALK5) kinase activity was required for increased HIF-1α expression in response to TGF-β1, but not to hypoxia. A dominant negative Smad3 decreased the TGF-β-stimulated reporter activity of a HIF-1α-sensitive hypoxia response element. Conversely, a dominant negative HIF-1α construct decreased Smad-binding element promoter activity in response to TGF-β. Finally, blocking HIF-1α transcription with a biochemical inhibitor, a dominant negative construct, or gene-specific knockdown decreased basal and TGF-β1-stimulated type I collagen expression, while HIF-1α overexpression increased both. Taken together, our data demonstrate cooperation in signaling between Smad3 and HIF-1α and suggest a new paradigm in which HIF-1α is necessary for normoxic, TGF-β1-stimulated renal cell fibrogenesis.
Keywords: acute kidney injury, fibrosis
acute kidney injury increases morbidity and mortality in the pediatric and adult population (1, 3, 14, 37). Disruption of renal blood flow in low-perfusion shock states can lead to ischemic injury of the glomeruli and especially renal tubules. Additionally, the normally low oxygen tension in the renal medulla renders the kidney vulnerable to hypoperfusion states, and possible induction of hypoxic/ischemic cell injury (41). The hypoxia-inducible factors (HIF) mediate adaptation to local hypoxia by regulating gene expression in response to low oxygen tension (9). HIF-1 is a heterodimeric transcription factor consisting of an oxygen-sensitive α-subunit that binds to a β-subunit, leading to nuclear translocation and subsequent activation of HIF response elements (34). In normoxia, HIF-1α is rapidly eliminated by oxygen-dependent prolyl hydroxylase (PHD) modification of HIF-1α, leading to von Hippel Lindau tumor suppressor-mediated ubiquitin tagging and proteosomal degradation. Under hypoxic conditions, PHD activity is attenuated, thereby stabilizing the expression and activity of HIF-1α (10). The HIF-1 complex that is formed promotes the expression of proangiogenic factors such as vascular endothelial growth factor, endoglin, and erythropoietin and of profibrotic factors such as plasminogen activator inhibitor-1 and tissue inhibitor of metalloproteinase 1 (5, 13, 15). Cellular targeting to inactivate HIF-1α and chemical HIF-1α inhibition protect against in vivo tubulointerstitial fibrosis in mice subjected to unilateral ureteral obstruction (UUO) (12, 32). Furthermore, mice subjected to UUO demonstrated increased HIF-1α nuclear staining (13). However, the signaling mechanism by which HIF-1α stimulates acute fibrogenic changes is not well understood.
A major contributor to collagen accumulation in progressive kidney disease is the activation of the Smad family of signaling proteins by transforming growth factor-β (TGF-β) (6, 23, 33). TGF-β is a pleiotropic cytokine involved in multiple events regulating cell growth, including differentiation, proliferation, epithelial-mesenchymal transition, and cell migration. TGF-β has been shown to mediate fibrogenesis in models of diabetic nephropathy and glomerulosclerosis (35). It transmits intracellular signals via type I (TβRI) and type II (TβRII) receptors, a transmembrane serine/threonine kinase complex that ultimately phosphorylates the receptor-activated Smads (R-Smads) (22). The R-Smads then heteromultimerize with Smad4 and translocate to the nucleus to regulate TGF-β target genes, including collagen I, by direct and indirect action (38). The phosphorylation of R-Smads (Smad2 and Smad3) is critical for fibrogenic signal transduction and is influenced by cross talk with other signaling pathways such as protein kinase C-δ and extracellular signal-regulated kinase (11, 28).
Potential cross talk between HIF-1α and TGF-β/Smad is not well understood. Genes that play primary roles in renal fibrogenesis are targets of both TGF-β and HIF-1α (10, 16, 31). Tumor microenvironments, milieu that are both fibrogenic and angiogenic, are known to have increased levels of HIF-1α and TGF-β. Some evidence suggests synergistic and possibly cooperative binding of Smad3 and HIF-1α at the promoter regions for vascular endothelial growth factor (VEGF), endoglin, and Sp1 (20, 29, 31). However, while hypoxia has been shown to increase fibrosis, TGF-β production, and Smad expression/activity (2, 8, 17, 43), the synergy or interaction of Smad activity with simultaneous HIF-1α upregulation is not well-characterized. Nonetheless, animal studies developed strong evidentiary support for the hypothesis that, during progressive kidney disease, renal ischemia promotes fibrosis through synergy between HIF-1α and Smads.
Here, we present data suggesting that HIF-1α may be an important contributor to extracellular matrix accumulation under both hypoxic and normoxic conditions. We found that, in human kidney epithelial cells, normoxic TGF-β1 stimulation increases HIF-1α expression by a mechanism that is distinct from, and additive to, hypoxic stabilization. Blocking HIF-1α activity decreases Smad3 signaling, and, conversely, blocking Smad3 activity inhibits certain HIF-1α-mediated responses. Activity of both molecules is required for TGF-β1-stimuated COL1A2 promoter activation. Taken together, these data describe cross talk between Smad and HIF signaling and suggest a paradigm by which these pathways cooperate in TGF-β1-mediated fibrogenesis even in the absence of hypoxia.
MATERIALS AND METHODS
Materials.
Except where otherwise indicated, reagents were purchased from commercial sources. Active recombinant TGF-β1 (R&D Systems, Minneapolis, MN) was reconstituted as a 4-μg/ml stock solution in 4 mM HCl with 1 mg/ml bovine serum albumin. Antibodies were purchased from the following vendors: mouse anti-HIF-1α from BD Lifesciences; rabbit polyclonal anti-HIF-1α from Cayman Chemical (Ann Arbor, MI); goat anti-mouse IgG-horseradish peroxidase (HRP), mouse anti-goat IgG-HRP antibodies from Santa Cruz Biotechnology (Santa Cruz, CA); mouse anti-β-actin from Sigma (St. Louis, MO); rabbit polyclonal anti-PHD2 from Novus Biologicals (Littleton, CO); and goat anti-rabbit IgG-HRP from Promega (Madison, WI). The TβRI inhibitor, SB431542, was purchased from Calbiochem/EMD (La Jolla, CA). A chemical inhibitor of HIF-1α, CAY10585, was purchased from Cayman Chemical and used as directed. Puromycin was purchased from Sigma and used at 3 μg/ml.
Plasmid constructs.
The -378COL1A2-Luc construct containing the sequence 378 bp of the α2 (I) collagen promoter and 58 bp of the transcribed sequence fused to the luciferase reporter gene was constructed as previously described (23). The HRE-Luc construct (trimeric units of HRE sequences cloned into the pGL2 basic vector) was a gift from J. M. Leiden (7). The Smad-binding element-luciferase construct (SBE-Luc) was kindly provided by B. Vogelstein (42) and used as previously described (28). Dominant negative (DN) HIF-1α, constructed as described previously (18), was a generous gift from M. L. Kuo. DN Smad3A in pEXL vector was obtained from H. F. Lodish and X. Liu (19). A nondegradable mutant (39) (Addgene plasmid 18955) where proline residues 402 and 564 are substituted with alanines and pEXL control plasmid was obtained through Addgene (http://www.addgene.org). Control plasmid pCEP4 was purchased from Sigma and the pcDNA3 vector was purchased from Invitrogen (Carlsbad, CA).
Cell culture.
Human renal tubular epithelial cells (HKC) were the generous gift of L. Racusen (26). Mouse embryonic fibroblasts (MEF) were a kind gift from E. Bottinger (40). Both cell types were grown in Dulbecco's modified eagle medium/F12 supplemented with 10% heat-inactivated FBS, glutamine, penicillin-streptomycin, amphotericin B, and HEPES buffer.
Stable HIF-1α knockdown cell line.
To generate stable knockdown cell lines, pGIPZ clones (RHS4349, empty vector; RHS4430–98486415, human and mouse HIF-1α) were obtained from Open Biosystems Products (Huntsville, AL). Bacterial cultures in Escherichia coli were first subjected to CaPO4 transfection for lentiviral packaging in HEK293 FT cells (Invitrogen) using psPAX2 and pMD2.G according to the Open Biosystems protocol. HKC cells were incubated with viral supernatants, and after 48 h, puromycin was added in a concentration previously determined to result in 100% cell death of noninfected HKC cells to select infected cells. Once cells reached confluence, they were frozen or used for experiments up to passages 4–6 in the continual presence of puromycin.
Cell treatments.
Cells were changed to medium containing 1% (HKC) or 0.5% (MEF) FBS 24 h before treatment. In experiments involving hypoxia, cells were exposed to normoxia (21% O2, 5% CO2) or hypoxia (1.5% O2, 5% CO2, 93.5% N2), carried out in a glove-box hypoxia chamber (Coy Laboratory Products, Grass Lake, MI) before treatment with TGF-β1 (1 ng/ml) or vehicle (BSA); in experiments involving inhibitors, cells were treated with 10 μM SB431542 or 30 μM CAY10585 for 30 min before addition of TGF-β1 or vehicle.
Preparation of cell lysates and Western blot analysis.
Cells were washed twice with ice-cold PBS and lysed on ice in RIPA buffer (50 mM Tris·HCL, pH 7.5; 150 mM NaCl; 1% Nonidet P-40; 0.5% deoxycholate; 0.1% SDS) containing serum protease and phosphatase inhibitors obtained from Sigma. Cleared cell lysates were subjected to SDS-PAGE (8–10% polyacrylamide gels), transferred onto PVDF membranes (Millipore, Bedford, MA), and probed with antibodies as indicated in the figure legends. Densitometric analysis was performed using the NIH Image 1.61 program for Macintosh.
Transfection.
HKC were plated in triplicate in six-well plates, at 0.7–1.4 × 105 cells per well. Eighteen to 24 h later, cells were switched to 1% FBS medium and transfected with 0.5 μg of the indicated constructs along with CMV-SPORT-β-galactosidase (Invitrogen/GIBCO BRL) as a control for transfection efficiency. Transfection was performed with the Fugene6 transfection reagent (Roche Applied Science, Indianapolis, IN) as previously described (24). After 3 h of transfection, 1 ng/ml of TGF-β1 or vehicle was added to the culture medium. Twenty-four hours after incubation, cells were harvested with 300 μl of reporter lysis buffer and luciferase and β-galactosidase activities were measured with a commercial kit (Promega), as directed.
RNA isolation and analysis.
Total RNA was isolated from cell cultures by using the TRIzol method as previously described (28) and treating samples with amplification grade DNase (Invitrogen), or by using commercial kits for RNA isolation from Qiagen (RNeasy) or USB/Affymetrix (PrepEase). qPCR was performed on samples using the following template-specific primers human HIF-1α: forward 5′-AGCCGAGGAAGAACTATGAAC-3′ and reverse 5′-ATTTGA TGGGTGAGGAATGGG-3′. A 1-μg aliquot of RNA was analyzed using SYBR green and TaqMan PCR master mix. All genes were normalized to 18S levels using the 18S TaqMan set from Applied Biosystems or the template-specific primer for β2-microglobulin, forward 5′-TGTCTGGGTTTCATCCATCCGACA-3′ and reverse 5′-TCACACGGCAGGCATACTCTT-3′. Samples were analyzed in triplicate and three replicated experiments were performed.
Statistics.
All data are expressed as means ± SE. Two-way ANOVA, with Bonferroni post hoc tests (Prism 4.0, www.graphpad.com), was used to evaluate differences between groups. Values of P < 0.05 were considered significant.
RESULTS
TGF-β1 increases HIF-1α expression in normoxia and hypoxia.
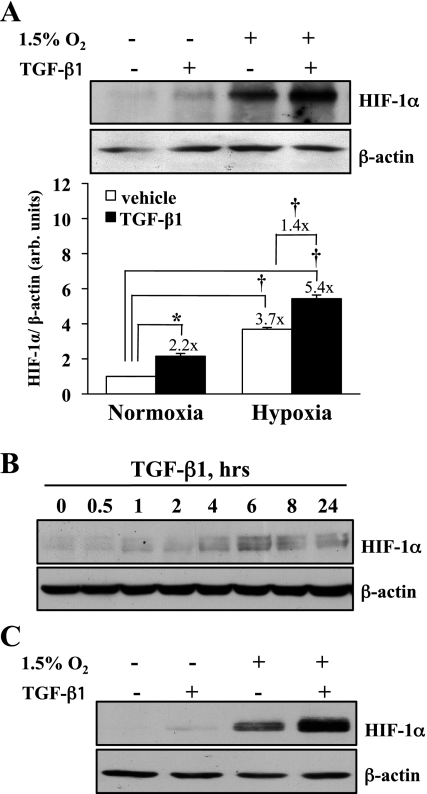
HIF-1α protein and mRNA expression were measured in response to TGF-β1 and hypoxia. HKC were exposed to normoxic (21% O2, 5% CO2) or hypoxic (1.5% O2, 5% CO2) conditions in the presence of 1 ng/ml TGF-β1 or vehicle for 6 h. As anticipated, HIF-1α expression was significantly increased (3.7-fold, P < 0.0001) under hypoxic conditions (Fig. 1A). However, HIF-1α expression was also significantly increased by TGF-β1 alone under normoxic conditions (2.2-fold, P < 0.0002) and TGF-β1 significantly enhanced hypoxia-stimulated HIF-1α expression by 43% (1.4-fold, P < 0.0001) over that seen with hypoxia alone (P < 0.0001). A time course of treatment indicated that TGF-β-stimulated HIF-1α expression is maximal at 6 h (Fig. 1B). Similar increases in HIF-1α expression were observed 6 h after TGF-β1 treatment of MEF in normoxia or hypoxia (Fig. 1C).
Fig. 1.
Transforming growth factor (TGF)-β1 stimulation increases normoxic hypoxia-inducible factor (HIF)-1α protein expression. A: human epithelial kidney cells (HKC) were stimulated with TGF-β1 (1 ng/ml) in normoxia or hypoxia (1.5% O2, 5% CO2) for 6 h. Whole cell lysates were assessed for HIF-1α expression by Western blot. A representative figure (top) and densitometric analyses normalized to β-actin controls (bottom) from 5 separate experiments are shown in the graph. TGF-β1 significantly increased HIF-1α protein expression in normoxia (*P < 0.0002). In vehicle-treated cells, hypoxia significantly increased HIF-1α expression 3.7-fold over normoxia (†P < 0.0001), with TGF-β1 and hypoxia increasing expression 5.4-fold over normoxic controls (†P < 0.0001). The relative increase in expression seen by TGF-β1 stimulation was 2.2-fold in normoxia and 1.4-fold in hypoxia. B: time course of HIF-1α protein expression after TGF-β1 treatment in HKC. Representative blot of 3 separate experiments is shown. C: mouse embryonic fibroblasts (MEF) treated as in A and assessed for HIF-1α protein expression. Representative blot of 3 separate experiments is shown.
TGF-β does not increase HIF-1α mRNA expression or decrease HIF-1α degradation.
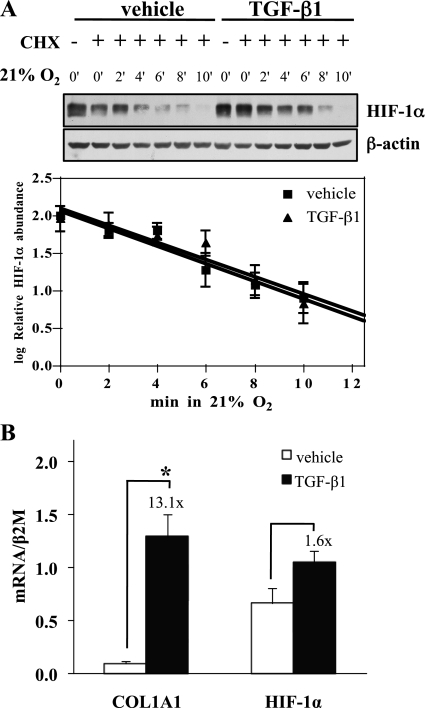
To begin to investigate the mechanism by which TGF-β increases HIF-1α expression, we examined the effect of TGF-β1 on HIF-1α protein degradation. Cells were subjected to hypoxia to increase the amount of HIF-1α protein to easily detectable levels, treated with cycloheximide to prevent new protein synthesis, and then switched to normoxia. Protein disappearance curves generated in the presence or absence of TGF-β1 (Fig. 2A) were virtually identical, indicating that, unlike hypoxia, TGF-β does not increase HIF-1α abundance by stabilizing the protein. Next, we meaured HIF-1α mRNA expression. TGF-β1 did not increase the specific HIF-1α mRNA levels (Fig. 2B). PHD2 levels were not altered by TGF-β1 treatment (data not shown). Although they are not definitive, these results are consistent with previous results, suggesting that other growth factors may increase HIF-1α levels via an effect on translation of the message into protein (4, 25).
Fig. 2.
TGF-β1 does not increase normoxic HIF-1α protein expression through protein degradation or mRNA expression. A: HKC were treated with vehicle or 1 ng/ml TGF-β1 for 6 h in 1.5% O2 before 15-min pretreatment with 10 μg/ml cycloheximide (CHX). Zero time point cells were harvested in 1.5% O2 and remaining cultures were transferred to 21% O2 and harvested at the indicated times. Whole cell lysates were assessed for HIF-1α expression by Western blot. Representative figure (top) and densitometric analyses normalized to β-actin controls (bottom) from 4 separate experiments are shown in the graph. Although TGF-β1 increased the amount of HIF-1α protein detected, it did not change the rate of HIF-1α degradation in normoxia. B: HKC were stimulated with vehicle or TGF-β1 for 6 h. Lysates were assessed for COL1A1 and HIF-1α mRNA expression by qPCR and a representative result is shown. TGF-β1 significantly increased COL1A1 expression (*P < 0.001) without significantly affecting HIF-1α expression.
TGF-β1-induced increase in HIF-1α expression requires TβRI kinase activity.
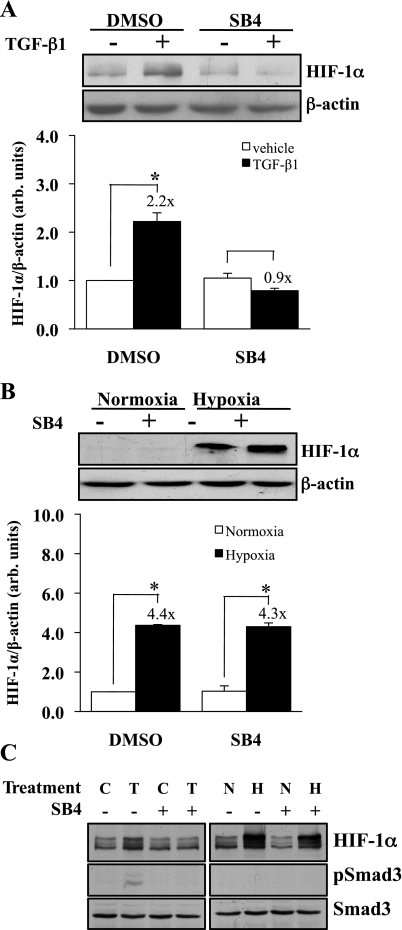
We examined whether TβRI kinase (ALK5) activity mediated the normoxic increase in HIF-1α expression induced by TGF-β1. HKC were pretreated with 5 μM TβRI kinase inhibitor SB-431542 or DMSO 30 min before stimulation with TGF-β1 (1 ng/ml) for 6 h (Fig. 3A). The twofold increase in TGF-β1-stimulated HIF-1α protein expression (P < 0.0001) was eliminated by the addition of the ALK5 kinase inhibitor, indicating that TGF-β1-enhanced HIF-1α expression requires TβRI kinase activity. Since we observed a role for TGF-β1 in HIF-1α expression under normoxic conditions, a similar experiment was performed to determine whether TβRI also plays a role in hypoxic stabilization of HIF-1α. HKC were incubated with SB431542 or vehicle for 30 min and then exposed to normoxic (21% O2, 5% CO2) or hypoxic (1.5% O2, 5% CO2) conditions for 6 h (Fig. 3B). There was no difference in hypoxia-induced HIF-1α protein expression comparing the presence with the absence of the TβRI inhibitor (×4.3 vs. ×4.4). Thus, TβRI/ALK5 activity is essential for TGF-β1-stimulated, but not hypoxia-induced, increased HIF-1α expression. Control experiments confirmed the efficacy of the ALK5 kinase inhibitor, and hypoxia did not stimulate Smad3 phosphorylation (Fig. 3C).
Fig. 3.
Normoxic, but not hypoxic, increased HIF-1α expression is TβRI dependent. A: HKC were treated with the TβRI receptor kinase inhibitor (SB431542, 5 mM) or DMSO for 30 min and then with TGF-β1 (1 ng/ml) or vehicle for 6 h. Whole cell lysate was analyzed by Western blot for HIF-1α. Representative blots (top) and densitometric analyses (bottom) corrected for β-actin-loading controls from 6 separate experiments are shown. TGF-β1 significantly increased HIF-1α expression (*P < 0.0001). Addition of the receptor kinase inhibitor eliminated the enhanced expression. B: HKC were incubated under hypoxia (1.5% O2, 5% CO2) or normoxia in the presence of the TβRI receptor antagonist (SB431542, 5 μM) or DMSO for 6 h. Whole cell lysate was analyzed by Western blot for HIF-1α protein. Representative blots (top) and densitometric analyses (bottom) corrected for β-actin-loading controls from 6 separate experiments are shown. Hypoxia significantly increased the expression of HIF-1α over normoxic controls (*P < 0.0001), with addition of the receptor kinase inhibitor having no effect. C: blots from cells as in A and B were probed for phospho-Smad3 and Smad3 (C, vehicle; T, 1 ng/ml TGF-β1; N, normoxia; H, hypoxia). SB4 completely blocked Smad3 phosphorylation (left middle). Hypoxia did not stimulate Smad3 phosphorylation.
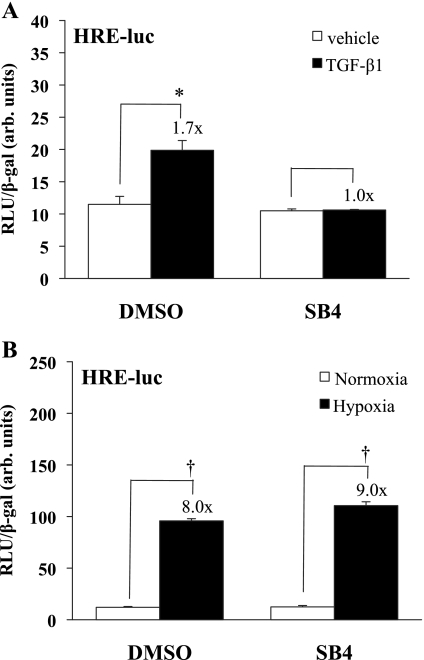
We next asked whether TGF-β1-increased HIF-1α expression results in increased HIF-dependent transcriptional activity. HKC were transfected with a reporter plasmid containing a hypoxia response element featuring multiple sequential HIF-1α binding sites, fused with a luciferase construct (HRE-Luc), and stimulated with 1 ng/ml TGF-β1 in the presence or absence of 5 μM SB431542. As shown in Fig. 4A, TGF-β1 significantly increased HRE-luc reporter activity 1.7-fold (P < 0.001) over vehicle treatment under normoxic conditions and this increase was eliminated with the addition of the TβRI kinase inhibitor. In contrast to the results with TGF-β stimulation, although HRE-luc reporter activity increased significantly under hypoxic conditions (8- to 9-fold, P < 0.0001), there was no effect of the TβRI kinase inhibitor on this activity seen under hypoxic conditions (Fig. 4B). These data suggest that HIF-1α expression and activity stimulated by TGF-β1, but not by hypoxia, are dependent on TβRI receptor kinase activity in kidney epithelial cells.
Fig. 4.
Stimulation of HIF-1α activity by TGF-β1, but not by hypoxia, is TβRI kinase dependent. A: HKC were transfected with the HIF response element (HRE) construct and treated in triplicate with SB431542 or DMSO and then TGF-β1 (1 ng/ml) or vehicle. Luciferase assay results, corrected for transfection efficiency using β-gal controls, are shown from a study representative of 3 separate experiments. The addition of TGF-β1 increased the HRE response 1.7-fold (*P < 0.001), but the response was eliminated in the presence of the receptor kinase inhibitor. B: HKC were transfected with the HRE construct and treated in triplicate with SB431542 or DMSO under hypoxia (1.5% O2) or normoxia. Luciferase assay results, corrected for transfection efficiency using β-gal controls, are shown from a study representative of 3 separate experiments. Hypoxia increased reporter activity 8- to 9-fold (†P < 0.0001) in the presence or absence of the receptor kinase inhibitor.
TGF-β1 stimulation of the HRE is Smad3 dependent.
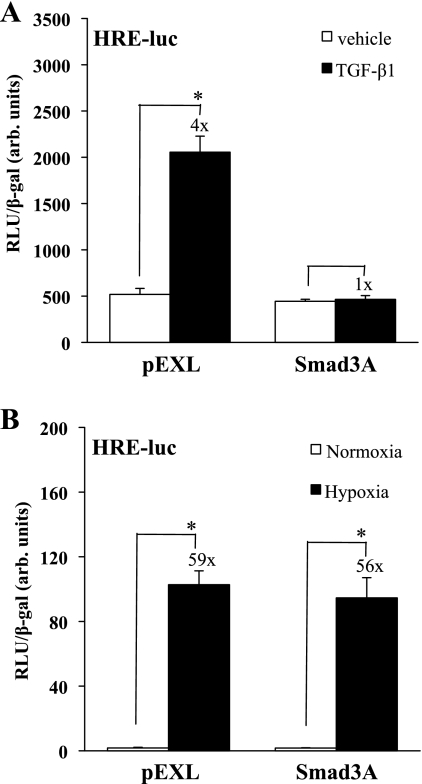
We next sought to determine whether there was a reciprocal effect of inhibiting the TGF-β1 pathway on HIF-1α-dependent signaling. HKC were cotransfected with the HRE-Luc construct and either an empty vector (pEXL) or a DN Smad3 construct (Smad3A) that contains a point mutation that prevents COOH-terminal phosphorylation, interfering with TβR activation of endogenous Smad3. The transfected cells were stimulated with TGF-β1 (1 ng/ml) or vehicle and then assayed for relative luciferase activity (Fig. 5A). TGF-β1 induced a fourfold increase in HRE-Luc reporter activity (P < 0.0001) in pEXL-transfected cells that was eliminated in the presence of Smad3A. In similarly transfected cells, Smad3A had no significant effect on hypoxic stimulation of HRE-Luc reporter activity (Fig. 5B). These results indicate that Smad3 COOH-terminal phosphorylation is required for TGF-β1-stimulated HRE-Luc reporter activity.
Fig. 5.
COOH-terminal Smad3 mutation decreases stimulation of an HRE in epithelial cells treated with TGF-β. A: HKC were cotransfected with an HRE-luc construct and a mutated Smad3 construct impairing COOH-terminal phosphorylation (Sd3A) or pEXL vector (EV). Cells were treated in triplicate with TGF-β1 (1 ng/ml) or vehicle. Luciferase assay results, corrected for transfection efficiency using β-gal controls, are shown from a study representative of 3 separate experiments. TGF-β1 significantly increased HRE reporter activity in pEXL-transfected cells (*P < 0.0001), and the Smad3A construct blocked TGF-β1-stimulated reporter activity. B: experiments were repeated using cotransfections of HKC with HRE-Luc and Sd3A or EV under normoxic or hypoxic conditions. A study representative of 3 separate experiments is shown. Hypoxia equally stimulated reporter activity in EV or Smad3A-transfected cells (*P < 0.0001).
HIF-1α contributes to Smad-binding element activity.
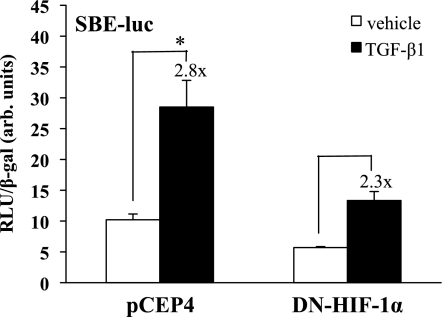
Because ligand binding to TβRI affects HIF-1α expression, we examined whether HIF-1α plays a role in TGF-β1 signal transduction. To determine whether HIF-1α is required for Smad3 activation of its canonical promoter sequence, a SBE-luciferase reporter construct was cotransfected into HKC together with a DN HIF-1α construct that effectively competes with endogenous HIF-1α for dimerization with HIF-1β, blocking the binding of HIF1α·HIF-1β complex with the target HRE-responsive element sequence and its subsequent transcriptional activation (18). Cells were treated with TGF-β1 (1 ng/ml) or vehicle and assayed for luciferase activity (Fig. 6). TGF-β1 induced a significant 2.8-fold increase in SBE-Luc activity in empty vector-transfected cells (P < 0.01). The presence of DN HIF-1α resulted in a 44% reduction in basal SBE-Luc reporter activity (×1.0 to ×0.56) and also reduced TGF-β1-stimulated activity (×1.0 to ×1.3), so that TGF-β-stimulated activity in the presence of DN HIF was only slightly higher than control, basal activity, although the fold TGF-β1 inductions were similar (×2.8 vs. ×2.3). These results indicate that Smad3 transcriptional activity through the SBE is, in part, mediated by functional HIF-1α under normoxic conditions. These results suggest that functional normoxic HIF-1α is required for full SBE-Luc activity.
Fig. 6.
Dominant negative (DN) HIF decreases the normoxic activity of the Smad-binding element (SBE). HKC were cotransfected with a DN HIF construct or empty vector (pCEP4) and a SBE-luc reporter construct and treated in triplicate with TGF-β1 (1 ng/ml) or vehicle. Luciferase assay results, corrected for transfection efficiency using β-gal controls, are shown from an experiment representative of 3 separate studies. Fold increase with pCEP4 was ×2.8 (*P < 0.01) while in DN HIF it was ×2.3. The basal level of SBE-luciferase expression was also decreased with the DN HIF by 33%.
HIF-1α is required for TGF-β1-stimulated collagen production.
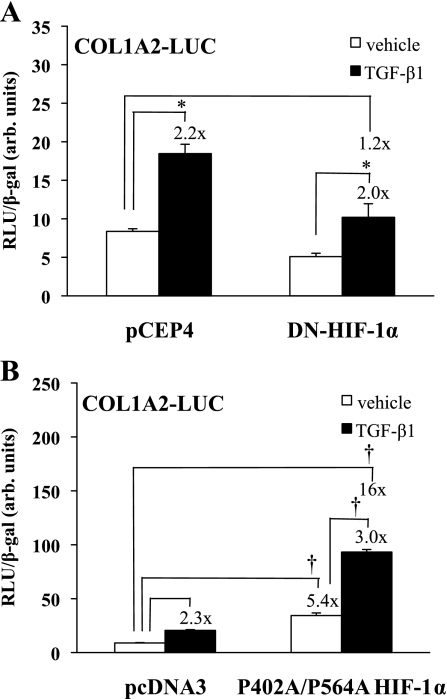
TGF-β1 stimulates α2(I) collagen gene transcription and protein accumulation in renal mesangial cells, and we previously showed that this is a Smad3-dependent event (23). We therefore sought to determine the influence of HIF-1α on TGF-β1-stimulated collagen expression. The DN HIF-1α construct or pCEP4 [empty vector (EV)] was cotransfected into HKC with a COL1A2 promoter-luciferase reporter construct, and the cells were treated with TGF-β1 (1 ng/ml) or vehicle (Fig. 7A). The DN HIF-1α construct reduced basal promoter activity by about half and, although it did not affect the relative degree of TGF-β1-dependent promoter stimulation (×2.2 vs. ×2.0), it reduced stimulated promoter activity to near that of basal levels (×1.0 compared with ×1.2). To examine the effect of increasing HIF-1α expression levels on α2(I) collagen gene activation, we cotransfected HKC with the COL1A2-luc construct and a construct, P402A/P564A-HIF-1α, that expresses nondegradable HIF-1α. This construct has prolines 402 and 564 mutated to alanines and cannot be hydroxylated by prolyl hydroxylases, and thus is not targeted for degradation by von Hippel-Lindau E3 ligase (39). Cells were treated with TGF-β1 (1 ng/ml) or vehicle and assayed for luciferase activity (Fig. 7B). TGF-β1 increased reporter activity 2.3-fold in pcDNA3-transfected cells. Transfection with P402A/P564A-HIF-1α increased basal reporter activity fivefold (P < 0.0001), and TGF-β1 treatment further increased activity another threefold (P < 0.0001).
Fig. 7.
Effect of HIF-1α on collagen promoter activity. A: HKC were cotransfected with a DN HIF construct or pCEP4 vector (EV) and a COL1A2 promoter-luciferase reporter construct and treated in triplicate with TGF-β1 (1 ng/ml) or vehicle. Luciferase assay results, corrected for transfection efficiency using β-gal controls, are shown from a study representative of 3 separate experiments. TGF-β1 increased reporter activity in pCEP4 and DN HIF-1α-transfected cells (*P < 0.05). Both basal and TGF-β1-stimulated reporter activities were decreased in the presence of a DN HIF, although the fold increase under TGF-β1 was unchanged. B: HKC were cotransfected with a nondegradable HIF-1α P402A/P564A mutant or pcDNA3 empty vector and a COL1A2 promoter-luciferase reporter construct and treated with TGF-β1 (1 ng/ml) or vehicle. Luciferase assay results, corrected for transfection efficiency using β-gal controls, are shown from a study representative of 3 separate experiments. The P402A/P564A mutant increased both basal and TGF-β1-stimulated reporter activity and increased the fold induction under TGF-β1 (†P < 0.0001).
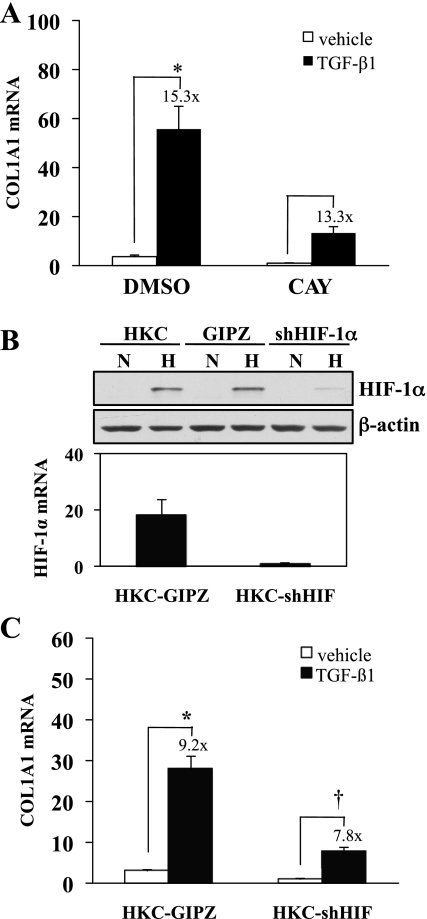
To further verify a role for HIF-1α in collagen gene activation, we inhibited HIF-1α expression using CAY10585, an inhibitor of HIF-1α transcription, and examined collagen mRNA expression as determined by quantitative PCR (Fig. 8A). This treatment repressed basal COL1A1 mRNA expression by 73% and reduced TGF-β1-stimulated mRNA expression by 76%, although TGF-β1-stimulated expression was similar (×15.3 compared with ×13.3). A role for HIF-1α in collagen expression was further supported by the development of a HKC line in which HIF-1α was stably knocked down (Fig. 8B). Similar to the results with the COL1A2 promoter, both basal and TGF-β1-stimulated type I collagen mRNA expression were decreased (Fig. 8C). Taken together, these results indicate that type I collagen expression is at least in part regulated by HIF-1α.
Fig. 8.
HIF-1α is required for collagen expression. A: HKC were treated with HIF-1α inhibitor (CAY10585, 30 μM) or DMSO for 30 min and then treated with TGF-β1 (1 ng/ml) or vehicle for 24 h. RNA isolation and quantitative RT-PCR were performed on resulting samples. Results from a representative experiment corrected with 18S show TGF-β1-stimulated α1 collagen mRNA (*P < 0.05). The HIF-1α inhibitor decreased basal and TGF-β1-stimulated α1 collagen mRNA expression. B: uninfected HKC, HKC stably expressing empty vector (GIPZ) or short hairpin RNA directed against HIF-1α (shHIF-1α) were exposed to 21% O2 (N) or 1.5% O2 (H) for 6 h before lysates were harvested for Western analysis of HIF-1α protein expression (top). HIF-1α mRNA expression corrected with β2-microglobulin is shown for GIPZ and shHIF-1α cells. C: GIPZ and shHIF-1α were treated with vehicle or 1 ng/ml TGF-β1 for 24 h before harvest for mRNA. A representative graph from 3 separate experiments is shown. TGF-β1 treatment significantly increased COL1A1 expression over vehicle-treated GIPZ control (*P < 0.0001). Basal and TGF-β1-stimulated COL1A1 expression were reduced in shHIF-1α, but the fold induction (†P < 0.05) was similar to that of control (×9.2 vs. ×7.8).
DISCUSSION
TGF-β is an established mediator of kidney fibrosis. Because of recent clinical (13) and experimental (16, 17) evidence that hypoxic conditions promote fibrogenesis, we sought to determine the potential interaction between TGF-β- and hypoxia-stimulated signaling. TGF-β1 increased HIF-1α expression through a mechanism dependent on the kinase activity of TβRI. This TGF-β1-dependent HIF-1α expression is distinct from that of hypoxia-dependent stabilization, and the effects of the two stimuli are additive for HIF-responsive signaling. Inhibiting HIF-1α decreased TGF-β1-induced SBE-Luc activation, as well as collagen I mRNA expression and promoter activity. Conversely, interfering with Smad3 signaling decreased normoxic, TGF-β-stimulated, HIF-1α-mediated induction of HRE-Luc activity. Although it is possible that hypoxic stabilization of HIF-1α could amplify Smad3 activity in the absence of TGF-β, directly stimulating collagen expression, hypoxia alone did not activate Smad3 and we did not detect activation of the COL1A2 promoter in the presence of hypoxia alone (data not shown). Taken together, these data indicate the presence of two mechanisms of HIF-1α signaling: the classic, hypoxia-driven pathway, and a distinct, TGF-β1-stimulated mechanism in which Smad3 and HIF-1α cooperate. Our data further suggest that both Smad3 and HIF-1α are essential contributors to TGF-β1-stimulated collagen expression under normoxic conditions.
One potential mechanism of increased protein expression would be stabilization through a HIF-1α-protective effect due to its formation of a complex with Smad3, a finding that has been suggested by others (29–31). Although the mechanism by which TGF-β1 increases HIF-1α expression is not defined by the present set of experiments, the identical degradation slopes for HIF-1α in the presence or absence of TGF-β suggest that this enhanced HIF-1α activity does not reflect increased stability of the protein, as is the case with hypoxia-induced, HIF-1α-dependent signaling (34). Another potential mechanism of increased expression is increased transcription, but we did not observe increased HIF-1α mRNA expression after TGF-β1 treatment. A likely explanation for our findings is enhanced translation of HIF-1α mRNA into protein, as has been described for enhanced HIF activity in response to other growth factors (4, 25). In that regard, insulin has been reported to augment HIF-1α protein expression by enhancing translation in an mTOR-dependent manner (36), while angiotensin II enhances translation of HIF-1α in a reactive oxygen species-dependent manner (21, 27).
The Smad3-HIF-1α interdependence in our experimental system may have signaling implications beyond increased HIF-1α expression. Both Smads and HIF translocate to the nucleus to participate in the formation of transcription-regulatory complexes. In our studies, activation of a canonical Smad3-responsive promoter by TGF-β1 was inhibited by a DN HIF-1α construct, and activation of a canonical HIF-1α construct was blocked by DN Smad3. While these results could simply reflect decreased levels of expression and/or activity of HIF-1α and Smad3, cooperative binding of these two transcriptional regulators to promoter sequences has been reported for VEGF, endoglin, and Sp1 (29–31). Previous work from our laboratory and others found that Sp1 binding was necessary for TGF-β1-induced type I collagen mRNA expression (24, 44). Further studies are needed to define the interactions between native and mutant Smad3 and HIF-1α, and the implications of these interactions for gene transcription.
Hypoxic changes in the kidney may serve as an accelerant or progression factor for chronic kidney disease. Direct injury or reactive apoptosis of renal vascular capillary endothelial cells leads to ischemia and the activation of HIF-1α signaling (9). Haase and colleagues (13) reported that progression of kidney fibrosis is ameliorated in HIF-1α-deficient mice. The contribution of HIF-1α to progression has been felt to reflect ischemic changes in the kidney, supported by in vitro findings that hypoxia is a stimulus for extracellular matrix accumulation (20). However, our studies raise the possibility that HIF-1α plays a role before the development of chronic hypoxia/ischemia, at least for type I collagen expression. Thus, HIF-1α could participate in multiple aspects of fibrogenesis, not only those activated under hypoxic conditions. Alternatively, HIF could act as a potential contributor to the progression of fibrosis after acute kidney injury. Further studies will establish whether activation of HIF-1α under such transient ischemic conditions could synergize with basal levels of TGF-β1/Smad3 activity, initiating a fibrogenic program that outlasts the transient, hypoxic events of the acute injury.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK049362 and DK075663 (to H. W. Schnaper) and National Heart, Lung, and Blood Institute Grant HL35440 (to P. T. Schumacker).
Present address of R. K. Basu: 3333 Burnet Ave., MLC 2005, Cincinnati, OH 45229.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71: 1028–1035, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Akman HO, Zhang H, Siddiqui MA, Solomon W, Smith EL, Batuman OA. Response to hypoxia involves transforming growth factor-β2 and Smad proteins in human endothelial cells. Blood 98: 3324–3331, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Bailey D, Phan V, Litalien C, Ducruet T, Merouani A, Lacroix J, Gauvin F. Risk factors of acute renal failure in critically ill children: a prospective descriptive epidemiological study. Pediatr Crit Care Med 8: 29–35, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor β-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem 275: 36803–36810, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Berra E, Pages G, Pouyssegur J. MAP kinases and hypoxia in the control of VEGF expression. Cancer Metastasis Rev 19: 139–145, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol 13: 2600–2610, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem 275: 25130–25138, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Falanga V, Qian SW, Danielpour D, Katz MH, Roberts AB, Sporn MB. Hypoxia upregulates the synthesis of TGF-β1 by human dermal fibroblasts. J Invest Dermatol 97: 634–637, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Haase VH. Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol 291: F271–F281, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haase VH. The VHL/HIF oxygen-sensing pathway and its relevance to kidney disease. Kidney Int 69: 1302–1307, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Hayashida T, Poncelet AC, Hubchak SC, Schnaper HW. TGF-β1 activates MAP kinase in human mesangial cells: a possible role in collagen expression. Kidney Int 56: 1710–1720, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Hewitson TD. Renal tubulointerstitial fibrosis: common but never simple. Am J Physiol Renal Physiol 296: F1239–F1244, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest 117: 3810–3820, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hui-Stickle S, Brewer ED, Goldstein SL. Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. Am J Kidney Dis 45: 96–101, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Kietzmann T, Roth U, Jungermann K. Induction of the plasminogen activator inhibitor-1 gene expression by mild hypoxia via a hypoxia response element binding the hypoxia-inducible factor-1 in rat hepatocytes. Blood 94: 4177–4185, 1999 [PubMed] [Google Scholar]
- 16. Kimura K, Iwano M, Higgins DF, Yamaguchi Y, Nakatani K, Harada K, Kubo A, Akai Y, Rankin EB, Neilson EG, Haase VH, Saito Y. Stable expression of HIF-1α in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol 295: F1023–F1029, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee K, Lee JH, Boovanahalli SK, Jin Y, Lee M, Jin X, Kim JH, Hong YS, Lee JJ. (Aryloxyacetylamino)benzoic acid analogues: a new class of hypoxia-inducible factor-1 inhibitors. J Med Chem 50: 1675–1684, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Lin MT, Kuo IH, Chang CC, Chu CY, Chen HY, Lin BR, Sureshbabu M, Shih HJ, Kuo ML. Involvement of hypoxia-inducing factor-1α-dependent plasminogen activator inhibitor-1 upregulation in Cyr61/CCN1-induced gastric cancer cell invasion. J Biol Chem 283: 15807–15815, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19. Liu X, Sun Y, Constantinescu SN, Karam E, Weinberg RA, Lodish HF. Transforming growth factor β-induced phosphorylation of Smad3 is required for growth inhibition and transcriptional induction in epithelial cells. Proc Natl Acad Sci USA 94: 10669–10674, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Norman JT, Clark IM, Garcia PL. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int 58: 2351–2366, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Page EL, Robitaille GA, Pouyssegur J, Richard DE. Induction of hypoxia-inducible factor-1α by transcriptional and translational mechanisms. J Biol Chem 277: 48403–48409, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Piek E, Moustakas A, Kurisaki A, Heldin CH, ten Dijke P. TGF-β type I receptor/ALK-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J Cell Sci 112: 4557–4568, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Poncelet AC, de Caestecker MP, Schnaper HW. The transforming growth factor-β/SMAD signaling pathway is present and functional in human mesangial cells. Kidney Int 56: 1354–1365, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Poncelet AC, Schnaper HW. Sp1 and Smad proteins cooperate to mediate transforming growth factor-β1-induced α2(I) collagen expression in human glomerular mesangial cells. J Biol Chem 276: 6983–6992, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 441: 437–443, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Racusen LC, Monteil C, Sgrignoli A, Lucskay M, Marouillat S, Rhim JG, Morin JP. Cell lines with extended in vitro growth potential from human renal proximal tubule: characterization, response to inducers, and comparison with established cell lines. J Lab Clin Med 129: 318–329, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Richard DE, Berra E, Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1α in vascular smooth muscle cells. J Biol Chem 275: 26765–26771, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Runyan CE, Schnaper HW, Poncelet AC. Smad3 and PKCδ mediate TGF-β1-induced collagen I expression in human mesangial cells. Am J Physiol Renal Physiol 285: F413–F422, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Sanchez-Elsner T, Botella LM, Velasco B, Corbi A, Attisano L, Bernabeu C. Synergistic cooperation between hypoxia and transforming growth factor-β pathways on human vascular endothelial growth factor gene expression. J Biol Chem 276: 38527–38535, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Sanchez-Elsner T, Botella LM, Velasco B, Langa C, Bernabeu C. Endoglin expression is regulated by transcriptional cooperation between the hypoxia and transforming growth factor-β pathways. J Biol Chem 277: 43799–43808, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Sanchez-Elsner T, Ramirez JR, Sanz-Rodriguez F, Varela E, Bernabeu C, Botella LM. A cross-talk between hypoxia and TGF-β orchestrates erythropoietin gene regulation through SP1 and Smads. J Mol Biol 336: 9–24, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-β1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest 112: 1486–1494, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schnaper HW, Jandeska S, Runyan CE, Hubchak SC, Basu RK, Curley JF, Smith RD, Hayashida T. TGF-β signal transduction in chronic kidney disease. Front Biosci 14: 2448–2465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol 15: 551–578, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Sharma K, Ziyadeh FN. Hyperglycemia and diabetic kidney disease. The case for transforming growth factor-β as a key mediator. Diabetes 44: 1139–1146, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem 277: 27975–27981, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Wrana JL, Attisano L. The Smad pathway. Cytokine Growth Factor Rev 11: 5–13, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Yan Q, Bartz S, Mao M, Li L, Kaelin WG., Jr The hypoxia-inducible factor 2α N-terminal and C-terminal transactivation domains cooperate to promote renal tumorigenesis in vivo. Mol Cell Biol 27: 2092–2102, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang YC, Piek E, Zavadil J, Liang D, Xie D, Heyer J, Pavlidis P, Kucherlapati R, Roberts AB, Bottinger EP. Hierarchical model of gene regulation by transforming growth factor β. Proc Natl Acad Sci USA 100: 10269–10274, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zappitelli M. Epidemiology and diagnosis of acute kidney injury. Semin Nephrol 28: 436–446, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, Kern SE. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell 1: 611–617, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Zhang H, Akman HO, Smith EL, Zhao J, Murphy-Ullrich JE, Batuman OA. Cellular response to hypoxia involves signaling via Smad proteins. Blood 101: 2253–2260, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Zhang W, Ou J, Inagaki Y, Greenwel P, Ramirez F. Synergistic cooperation between Sp1 and Smad3/Smad4 mediates transforming growth factor β1 stimulation of α2(I)-collagen (COL1A2) transcription. J Biol Chem 275: 39237–39245, 2000 [DOI] [PubMed] [Google Scholar]