Abstract
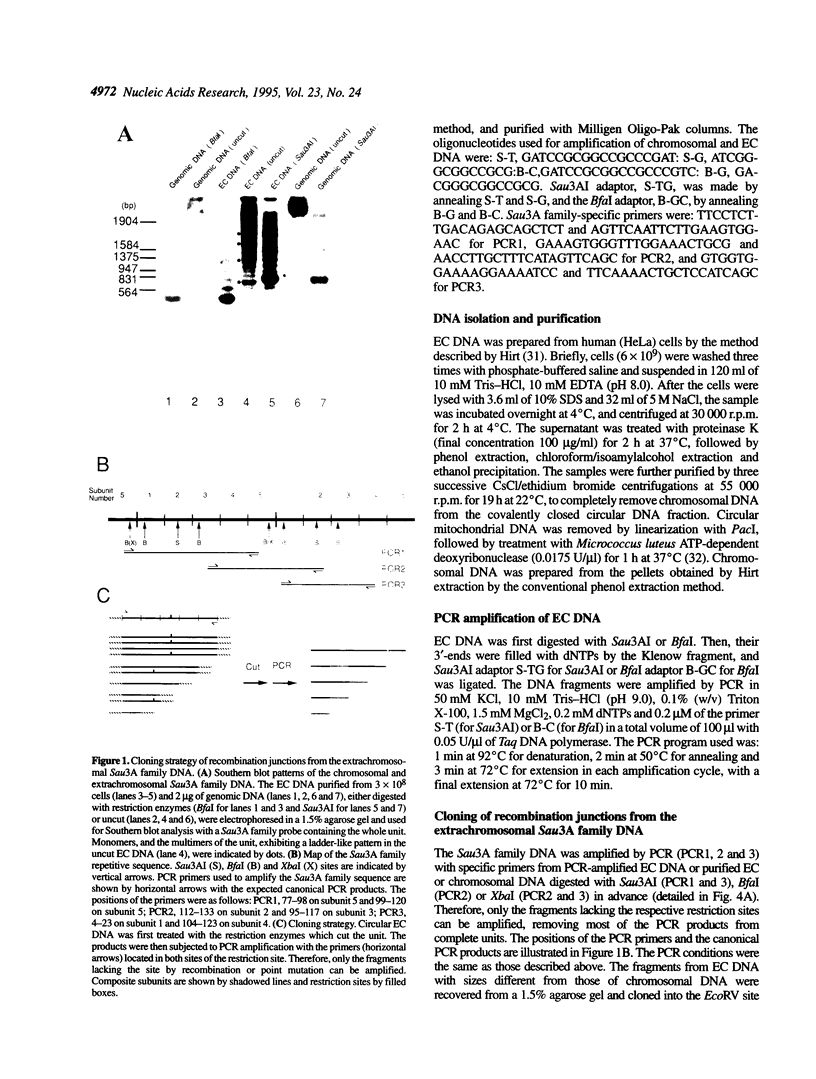
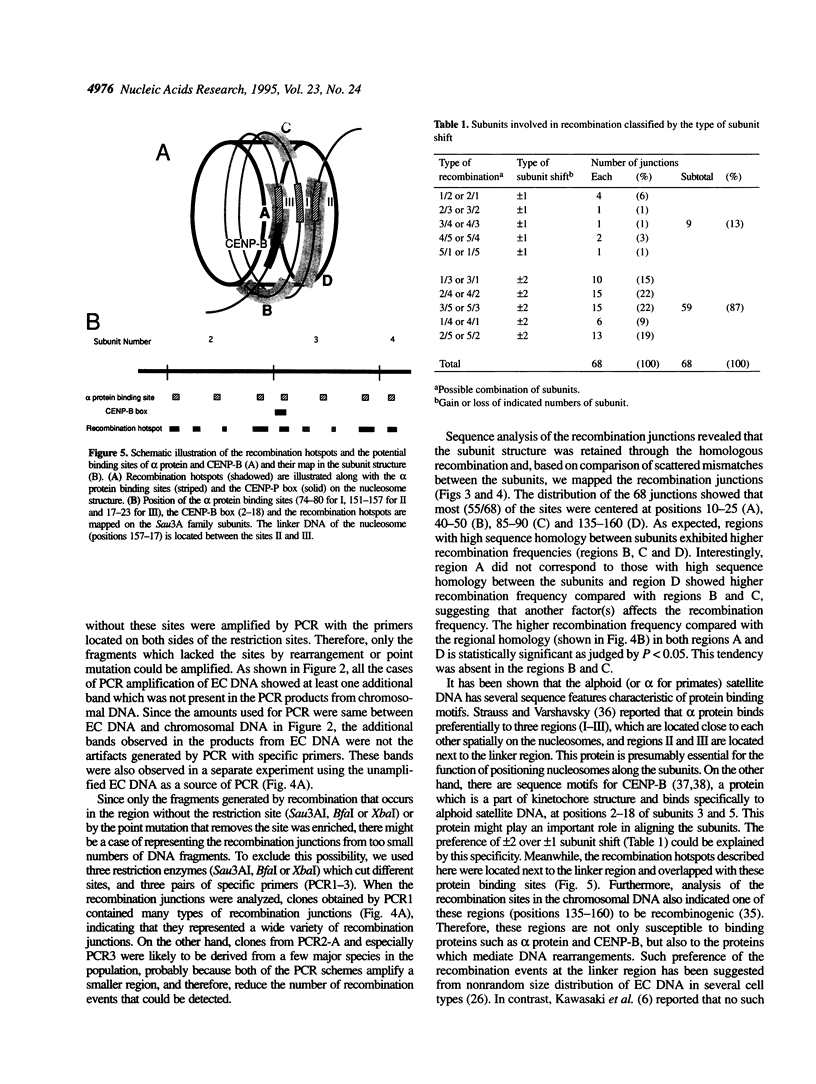
The human alphoid Sau3A repetitive family DNA is one of the DNA species that are actively amplified to form extrachromosomal circular DNA in several cell lines. The circularization takes place between two of the five approximately 170 bp subunits with an average of 73.1% homology as well as between identical subunits. To investigate the nature of the recombination reaction, we cloned and analyzed the subunits containing recombination junctions. Analysis of a total of 68 junctions revealed that recombination had occurred preferentially at four positions 10-25 (A), 40-50 (B), 85-90 (C) and 135-160 (D) in the 170bp subunit structure. Two regions (B and C) were overlapped with the regions with higher homology between subunits, while other two regions (A and D) cannot be explained solely by the regional homology between the subunits. These regions were located at both junctions of the nucleosomal and the linker region, and overlapped with the binding motifs for alpha protein and CENP-B. Approximately 90% of the recombination occurred between the subunits located next but one (+/- 2 shift), although the frequency of recombination between the adjoining subunits (+/- 1 shift) was approximately 10%.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Gene conversion: some implications for immunoglobulin genes. Cell. 1981 Jun;24(3):592–594. doi: 10.1016/0092-8674(81)90082-9. [DOI] [PubMed] [Google Scholar]
- Cooke C. A., Bernat R. L., Earnshaw W. C. CENP-B: a major human centromere protein located beneath the kinetochore. J Cell Biol. 1990 May;110(5):1475–1488. doi: 10.1083/jcb.110.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagrelius T. J., Livingston D. M. Location of DNAase I sensitive cleavage sites in the yeast 2 micron plasmid DNA chromosome. J Mol Biol. 1984 Feb 15;173(1):1–13. doi: 10.1016/0022-2836(84)90400-5. [DOI] [PubMed] [Google Scholar]
- Flavell A. J., Ish-Horowicz D. The origin of extrachromosomal circular copia elements. Cell. 1983 Sep;34(2):415–419. doi: 10.1016/0092-8674(83)90375-6. [DOI] [PubMed] [Google Scholar]
- Fujimoto S., Yamagishi H. Isolation of an excision product of T-cell receptor alpha-chain gene rearrangements. Nature. 1987 May 21;327(6119):242–243. doi: 10.1038/327242a0. [DOI] [PubMed] [Google Scholar]
- Gaubatz J. W. Extrachromosomal circular DNAs and genomic sequence plasticity in eukaryotic cells. Mutat Res. 1990 Sep-Nov;237(5-6):271–292. doi: 10.1016/0921-8734(90)90009-g. [DOI] [PubMed] [Google Scholar]
- Gaubatz J. W., Flores S. C. Tissue-specific and age-related variations in repetitive sequences of mouse extrachromosomal circular DNAs. Mutat Res. 1990 Jan;237(1):29–36. doi: 10.1016/0921-8734(90)90029-q. [DOI] [PubMed] [Google Scholar]
- Hansen B. M., Marcker K. A. DNA sequence and transcription of a DNA minicircle isolated from male-fertile sugar beet mitochondria. Nucleic Acids Res. 1984 Jun 11;12(11):4747–4756. doi: 10.1093/nar/12.11.4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Iwasaki T., Ohki R., Kiyama R., Oishi M. Analysis of recombination junctions in extrachromosomal circular DNA obtained by in-gel competitive reassociation. FEBS Lett. 1995 Apr 24;363(3):239–245. doi: 10.1016/0014-5793(95)00325-4. [DOI] [PubMed] [Google Scholar]
- Iwasato T., Shimizu A., Honjo T., Yamagishi H. Circular DNA is excised by immunoglobulin class switch recombination. Cell. 1990 Jul 13;62(1):143–149. doi: 10.1016/0092-8674(90)90248-d. [DOI] [PubMed] [Google Scholar]
- Jones R. S., Potter S. S. Characterization of cloned human alphoid satellite with an unusual monomeric construction: evidence for enrichment in HeLa small polydisperse circular DNA. Nucleic Acids Res. 1985 Feb 11;13(3):1027–1042. doi: 10.1093/nar/13.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. S., Potter S. S. L1 sequences in HeLa extrachromosomal circular DNA: evidence for circularization by homologous recombination. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1989–1993. doi: 10.1073/pnas.82.7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki I., Bae Y. S., Eki T., Kim Y., Ikeda H. Homologous recombination of monkey alpha-satellite repeats in an in vitro simian virus 40 replication system: possible association of recombination with DNA replication. Mol Cell Biol. 1994 Jun;14(6):4173–4182. doi: 10.1128/mcb.14.6.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyama R., Matsui H., Oishi M. A repetitive DNA family (Sau3A family) in human chromosomes: extrachromosomal DNA and DNA polymorphism. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4665–4669. doi: 10.1073/pnas.83.13.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyama R., Matsui H., Okumura K., Oishi M. A group of repetitive human DNA families that is characterized by extrachromosomal oligomers and restriction-fragment length polymorphism. J Mol Biol. 1987 Feb 20;193(4):591–597. doi: 10.1016/0022-2836(87)90342-1. [DOI] [PubMed] [Google Scholar]
- Kiyama R., Oishi M., Kanda N. Chromosomal localization of Sau3A repetitive DNA revealed by in situ hybridization. Chromosoma. 1988;96(5):372–375. doi: 10.1007/BF00330704. [DOI] [PubMed] [Google Scholar]
- Kiyama R., Okumura K., Matsui H., Bruns G. A., Kanda N., Oishi M. Nature of recombination involved in excision and rearrangement of human repetitive DNA. J Mol Biol. 1987 Dec 20;198(4):589–598. doi: 10.1016/0022-2836(87)90202-6. [DOI] [PubMed] [Google Scholar]
- Koenig M., Hoffman E. P., Bertelson C. J., Monaco A. P., Feener C., Kunkel L. M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987 Jul 31;50(3):509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Liskay R. M., Letsou A., Stachelek J. L. Homology requirement for efficient gene conversion between duplicated chromosomal sequences in mammalian cells. Genetics. 1987 Jan;115(1):161–167. doi: 10.1093/genetics/115.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto H., Masukata H., Muro Y., Nozaki N., Okazaki T. A human centromere antigen (CENP-B) interacts with a short specific sequence in alphoid DNA, a human centromeric satellite. J Cell Biol. 1989 Nov;109(5):1963–1973. doi: 10.1083/jcb.109.5.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura K., Kiyama R., Oishi M. Sequence analyses of extrachromosomal Sau3A and related family DNA: analysis of recombination in the excision event. Nucleic Acids Res. 1987 Sep 25;15(18):7477–7489. doi: 10.1093/nar/15.18.7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson K. E., Deka N., Schmid C. W., Misra R., Schindler C. W., Rush M. G., Kadyk L., Leinwand L. A transposon-like element in human DNA. Nature. 1985 Jul 25;316(6026):359–361. doi: 10.1038/316359a0. [DOI] [PubMed] [Google Scholar]
- Riabowol K., Shmookler Reis R. J., Goldstein S. Interspersed repetitive and tandemly repetitive sequences are differentially represented in extrachromosomal covalently closed circular DNA of human diploid fibroblasts. Nucleic Acids Res. 1985 Aug 12;13(15):5563–5584. doi: 10.1093/nar/13.15.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard M., Belmaaza A., Gusew N., Wallenburg J. C., Chartrand P. Integration of a vector containing a repetitive LINE-1 element in the human genome. Mol Cell Biol. 1994 Oct;14(10):6689–6695. doi: 10.1128/mcb.14.10.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showe L. C., Ballantine M., Nishikura K., Erikson J., Kaji H., Croce C. M. Cloning and sequencing of a c-myc oncogene in a Burkitt's lymphoma cell line that is translocated to a germ line alpha switch region. Mol Cell Biol. 1985 Mar;5(3):501–509. doi: 10.1128/mcb.5.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield S. W., Helinski D. R. Multiple mechanisms generate extrachromosomal circular DNA in Chinese hamster ovary cells. Nucleic Acids Res. 1986 Apr 25;14(8):3527–3538. doi: 10.1093/nar/14.8.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G. R., Debatisse M., Giulotto E., Wahl G. M. Recent progress in understanding mechanisms of mammalian DNA amplification. Cell. 1989 Jun 16;57(6):901–908. doi: 10.1016/0092-8674(89)90328-0. [DOI] [PubMed] [Google Scholar]
- Stary A., Sarasin A. Molecular analysis of DNA junctions produced by illegitimate recombination in human cells. Nucleic Acids Res. 1992 Aug 25;20(16):4269–4274. doi: 10.1093/nar/20.16.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss F., Varshavsky A. A protein binds to a satellite DNA repeat at three specific sites that would be brought into mutual proximity by DNA folding in the nucleosome. Cell. 1984 Jul;37(3):889–901. doi: 10.1016/0092-8674(84)90424-0. [DOI] [PubMed] [Google Scholar]
- Sunnerhagen P., Sjöberg R. M., Bjursell G. Increase of extrachromosomal circular DNA in mouse 3T6 cells on perturbation of DNA synthesis: implications for gene amplification. Somat Cell Mol Genet. 1989 Jan;15(1):61–70. doi: 10.1007/BF01534670. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Gorham J., Cossman J., Jaffe E., Croce C. M. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985 Sep 27;229(4720):1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. On the possibility of metabolic control of replicon "misfiring": relationship to emergence of malignant phenotypes in mammalian cell lineages. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3673–3677. doi: 10.1073/pnas.78.6.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton P. E., Waye J. S., Willard H. F. Nonrandom localization of recombination events in human alpha satellite repeat unit variants: implications for higher-order structural characteristics within centromeric heterochromatin. Mol Cell Biol. 1993 Oct;13(10):6520–6529. doi: 10.1128/mcb.13.10.6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard H. F., Waye J. S. Chromosome-specific subsets of human alpha satellite DNA: analysis of sequence divergence within and between chromosomal subsets and evidence for an ancestral pentameric repeat. J Mol Evol. 1987;25(3):207–214. doi: 10.1007/BF02100014. [DOI] [PubMed] [Google Scholar]
- Yamagishi H., Tsuda T., Fujimoto S., Toda M., Kato K., Maekawa Y., Umeno M., Anai M. Purification of small polydisperse circular DNA of eukaryotic cells by use of ATP-dependent deoxyribonuclease. Gene. 1983 Dec;26(2-3):317–321. doi: 10.1016/0378-1119(83)90205-6. [DOI] [PubMed] [Google Scholar]