Abstract
A polycyclic collapse: Use of a carefully designed acyclic intermediate provided the means to execute a cascade-based construction which formed the entire core of the polyketide-derived dalesconols in a single flask. A number of additional and carefully controlled synthetic operations completed an expeditious synthesis of both of these highly bioactive natural products as well as structural congenors.
Keywords: dalesconol, polyphenol, cascade reaction, total synthesis
As part of a program seeking to identify new classes of potent immunosuppressants, Tan and co-workers recently isolated and characterized dalesconol A and B (1 and 2, Scheme 1) from a culture of Daldinia eschsholzii IFB-TL01 residing inside the gut of the mantis species Tenodora aridifolia.[1] Apart from possessing an unprecedented carbon-based skeleton containing seven fused rings of various sizes, these isolates indeed possessed immunosuppressive activity (IC50 values of 0.16 µg/mL and 0.25 µg/mL, respectively), levels comparable to that of the clinically utilized cyclosporin A (IC50 = 0.06 µg/mL), but with significantly reduced background cytotoxicity.[2] Intriguingly, racemic mixtures of either 1 or 2 were found to be more potent than their separated enantiomers.[3] Subsequently, She, Lin and colleagues obtained the same natural products (1 and 2) from a marine-based endophytic fungus (Sporothrix sp. #4335) that grew on the inshore mangrove tree Kandelia candel, naming them as sporothrin A and B;[4] they also isolated and characterized a related metabolite (sporothrin C, 3). Their activity screens revealed that 1 was a potent acetylcholinesterase inhibitor and that both 1 and 2 possessed modest antitumor activity. As such, members of this structurally novel natural product family could serve as valuable leads for future pharmaceutical development. In this communication, we describe the first total syntheses of dalesconol A and B (1 and 2) through an expedient and scalable route capable of providing the material supplies needed for more thorough biochemical applications.
Scheme 1.
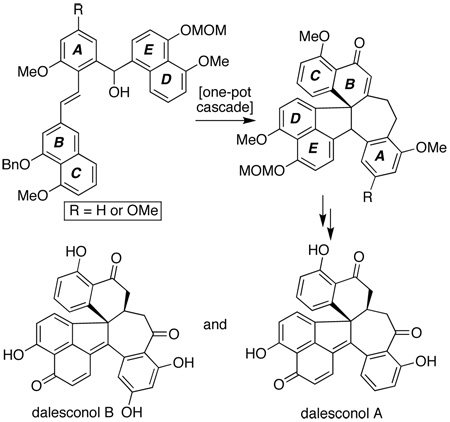
Retrosynthetic analysis of the dalesconols (1 and 2) based on an attempt to utilize key intermediate 5, a variant of 6 which has already led to a variety of resveratrol-derived polycyclic natural products (7–9).
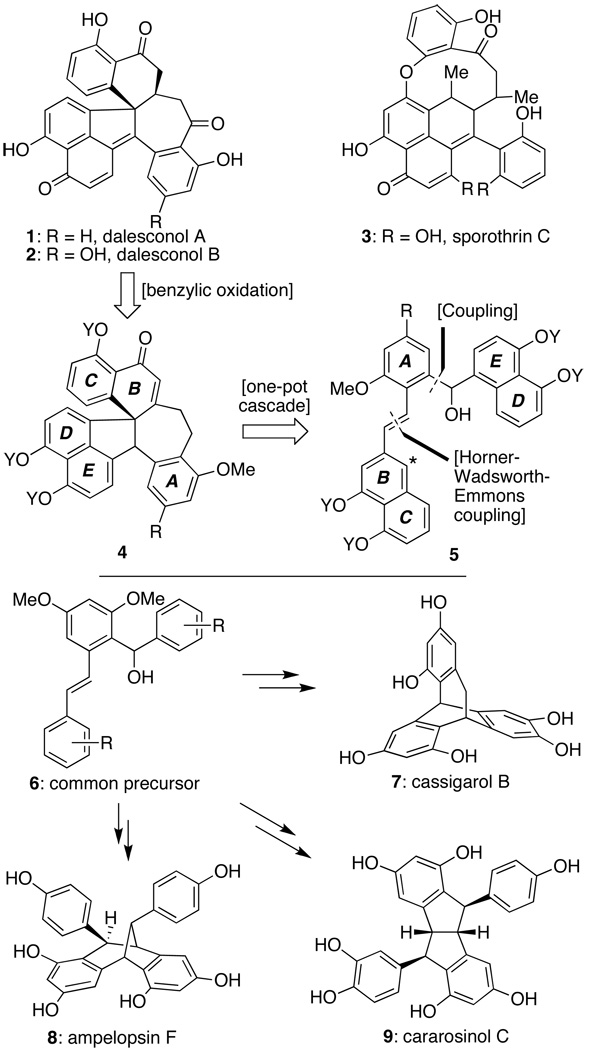
As revealed in Scheme 1, our synthetic approach to the dalesconols (1 and 2) was based primarily on the idea that an appropriately protected form of 5 could be converted into their complete cores (such as that represented by 4) in a single, cascade-based operation. That key step would employ among its operations a Friedel–Crafts cyclization initiated by ionization of its hydroxyl function followed by an oxidative C–C bond forming event; these processes would utilize the starred carbon within 5 as both nucleophile and electrophile to transform it into the lone quaternary center of the natural products. Subsequent adjustments in oxidation state would then complete the target molecules. This overall analysis was inspired by our earlier studies towards members of the resveratrol class of oligomeric polyphenols,[5] where cascade-based operations using the structurally similar precursor 6 enabled the preparation of a number of architectures, including the [3.2.2]-, [3.2.1]-, and [3.3.0]-bicyclic frameworks of natural products 7–9.[6] Thus, if the envisioned cascade could be achieved (i.e. 5→4), then the power of the general structural subtype represented by 5 and 6 as precursors to controllably access structurally and biosynthetically diverse architectures would be enhanced.
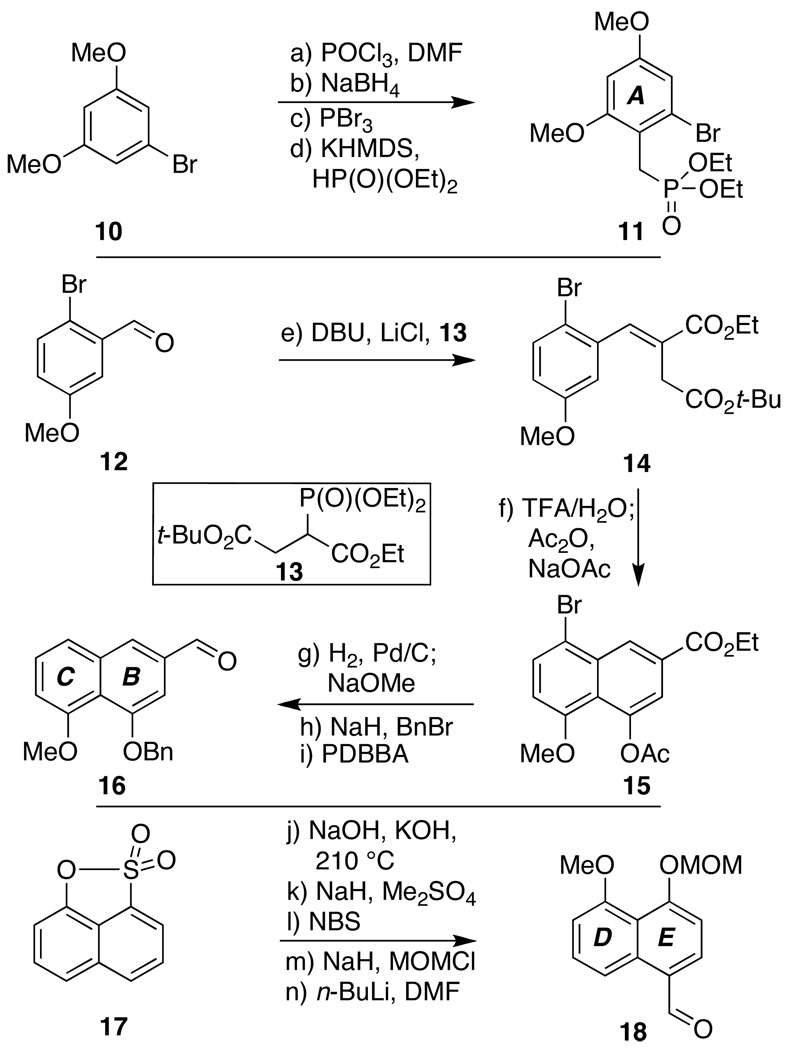
Our explorations to test this overall hypothesis began with the preparation of three phenolic precursors, hoping to unite them smoothly into a defined form of 5 via the retrosynthetic disconnections indicated in Scheme 1, targeting dalesconol B (2) specifically. Following several rounds of protecting group selections to achieve proper reactivity in later steps (vide infra), fragments 11, 16, and 18 were smoothly prepared from commercial materials in 4, 5, and 5 linear steps, respectively, as shown in Scheme 2. Given the conventional nature of many of these operations, detailed discussion of the entire sequence is not warranted. However, we do wish to note the following: 1) each fragment was readily synthesized on multigram scale; 2) extensive efforts to form a variant of 14 via Stobbe condensations (with a free carboxylic acid instead of the t-butyl ester) proved low yielding and capricious, particularly on scale;[7] 3) attempted DIBAL-H reduction of the ester within 15 to the aldehyde failed, with only PDBBA (formed by admixing DIBAL-H with KOt-Bu)[8] giving the desired chemoselectivity;[9] and 4) commercial sultone 17 had to be recrystallized prior to use to achieve a high yielding alkali fusion reaction en route to 18.[10]
Scheme 2.
Synthesis of key phenolic building blocks 11, 16, and 18: a) POCl3 (3.0 equiv), DMF (6.0 equiv), 90 °C, 6 h; aq. KOH, 0→25 °C, 12 h, 99%; b) NaBH4 (2.0 equiv), MeOH, 0 °C, 30 min, 96%; c) PBr3 (1.0 equiv), pyridine (cat.), Et2O, 25 °C, 4 h, 96%; d) KHMDS (0.5 M in toluene, 1.8 equiv), HP(O)(OEt)2 (2.0 equiv), 0 °C, 15 min; then add s.m., THF, 0→25 °C, 12 h, 94%; e) LiCl (1.3 equiv), DBU (1.0 equiv), 13 (1 equiv), CH3CN, 25 °C, 12 h, 99%; f) TFA/H2O (9/1), 25 °C, 90 min; NaOAc (1.4 equiv), Ac2O, 140 °C, 1 h, 83%; g) H2 (1 atm), Pd/C (10%), MeOH/CH2Cl2 (2/1), 25 °C, 24 h; filter, NaOMe (3.0 equiv), 0→25 °C, 2 h, 99%; h) NaH (2.0 equiv), BnBr (2.0 equiv), DMF, 0→25 °C, 1 h, 77%; i) PDBBA (0.9 equiv), THF, −20→0 °C, 1.5 h, 67%; j) NaOH/KOH/17 (1/5/1 by weight), 210 °C, 40 min, 53%; k) NaH (1.0 equiv), THF, 0 °C, 10 min; Me2SO4 (1.0 equiv), 0→25 °C, 14h, 99%; l) NBS (1.0 equiv), CH3CN, 25 °C, 1 h, 98%; m) NaH (1.2 equiv), MOMCl (1.5 equiv), DMF, 0 °C, 1.5 h, 99%; n) n-BuLi (1.6 M in hexanes, 1.2 equiv), THF, −78 °C, 20 min; DMF (4.0 equiv), THF, −78 °C, 1.5 h, 96%. DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene, DMF = N,N-dimethylformamide, PDBBA = potassium diisobutyl-tert-butoxyaluminum hydride, KHMDS = potassium bis(trimethylsilyl)amide, MOM = methoxymethyl.
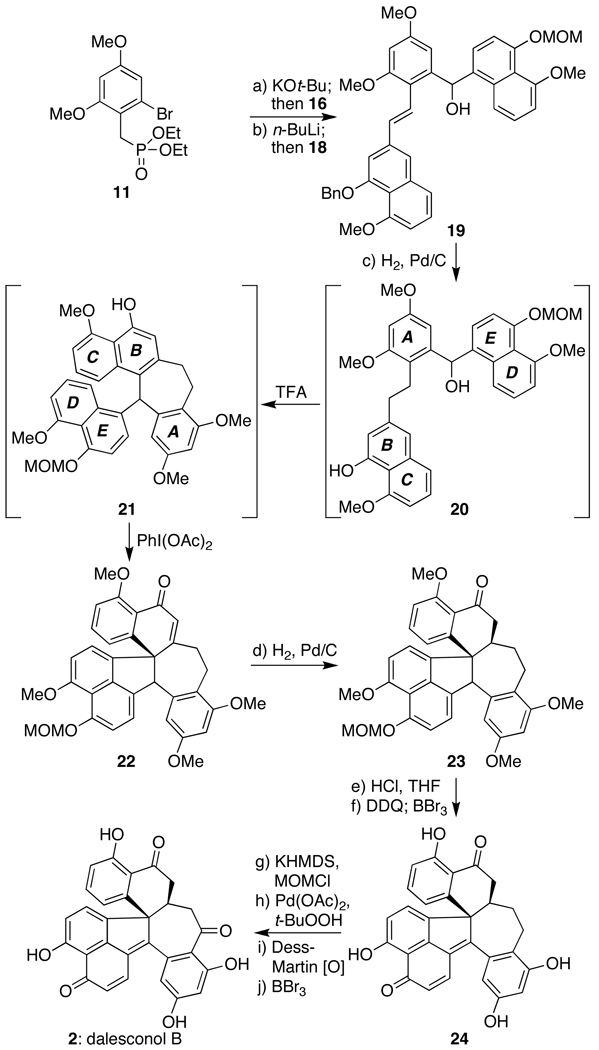
With these fragments in hand, they were then united into key intermediate 19 (a fully defined form of retron 5, cf. Scheme 1) in 58% overall yield via an initial Horner–Wadsworth–Emmons olefination between the anion derived from 11 and aldehyde 16, followed by halogen-lithium exchange and nucleophilic attack onto the aldehyde function of 18 (Scheme 3). As such, we could now test our ability to convert this material into the entire dalesconol framework. Following extensive studies, this goal was indeed realized; Scheme 3 presents the sequence in its current level of optimization.
Scheme 3.
Total synthesis of dalesconol B (2): a) KOt-Bu (1.0 M in THF, 1.1 equiv), THF, −78 °C, 20 min; 16 (1.0 equiv), −78→25 °C, 2 h, 87%; b) n-BuLi (1.6 M in hexanes, 1.5 equiv), THF, −78 °C; 18 (2.0 equiv), −78→25 °C, 4 h, 67%; c) H2 (1 atm), Pd/C (10%, 1 equiv), EtOAc/EtOH (2/3), 25 °C, 45 min; filter, solvent removal, TFA (1.0 equiv), 2,2,2-trifluoroethanol, −45 °C, 15 min; PhI(OAc)2 (1.1 equiv), −45 °C, 20 min, 32% overall; d) H2 (1 atm), Pd/C (10%, 1.0 equiv), EtOH/EtOAc (3/1), 25 °C, 3–10 h, 84%; e) conc. HCl (40 equiv), THF, 0→25 °C, 3 h, 99%; f) DDQ (0.97 equiv), CH2Cl2, 25 °C, 1 h; −78 °C, BBr3 (1.0 M in CH2Cl2, 25 equiv), −78→0 °C, 12 h, 73%; g) KHMDS (0.5 M in THF, 5.0 equiv), MOMCl (20 equiv), THF, 0 °C, 20 min, 91%; h) Pd(OAc)2 (1.0 equiv), t-BuOOH (25 equiv), K2CO3 (10 equiv), CH2Cl2, 72 h, 25 °C, 42%; i) Dess–Martin periodinane (5.0 equiv), NaHCO3 (10 equiv), CH2Cl2, 25 °C, 2 h, 99%; j) BBr3 (1.0 M in CH2Cl2, 25 equiv), CH2Cl2, −78 °C, 15 min, 73%. DDQ = 2,3-dichloro-5,6-dicyano-1,4-benzoquinone.
In the event, compound 19 was taken up in a mixture of EtOAc and EtOH (2/3) and subjected to 1 atmosphere of H2 gas in the presence of a full equivalent of Pd/C (10%); under these specific conditions, the benzyl protecting group was excised and the double bond uniting the A and B rings was reduced in quantitative yield. Use of any other solvent combinations or ratios, as well as catalytic loadings of palladium, led to significant amounts of material in which the alcohol group on the carbon bridging the A and E rings was reduced as well. Next, following filtration and solvent removal, the crude residue was resuspended in 2,2,2-trifluoroethanol and treated with a full equivalent of TFA at −45 °C for 15 min. During this time, the alcohol function was ionized, thereby initiating a Friedel–Crafts reaction which generated the 7-membered ring within 21.[11] Subsequent addition of 1.1 equivalents of PhI(OAc)2 to the same pot at −45 °C, followed by 20 min of additional reaction time, then converted the strategically deprotected B-ring phenol into an oxidized material with a p-disposed carbocation that was engaged by the D-ring to fashion the complete dalesconol core as expressed in 22.[12] Globally, these operations provided 22 in 32% isolated yield, thereby accounting for an overall efficiency level of 75% per step based on its 4 distinct operations.
Having completed this critical operation, the completion of dalesconol B (2) required several adjustments in oxidation state prior to removal of the phenolic protecting groups. The first of these events, hydrogenation of the double bond within 22, occurred chemoselectively when performed in a 3/1 mixture of EtOH and EtOAc at 25 °C. This step provided 23 as a single diastereomer of unknown configuration in 84% yield;[13] other solvents and/or prolonged reaction times led to unwanted conversion of the benzylic ketone into the corresponding alcohol as well. Following removal of the lone MOM protecting group (HCl, THF) and DDQ-mediated oxidation[14] to the corresponding p-quinone methide, an X-ray crystal structure of the resultant intermediate (not shown, see Supporting Information section) confirmed the stereochemistry as that desired for the target structure and as drawn in compound 23. In practice, however, the DDQ-oxidation step was followed directly by exposure to BBr3 in the same pot to unveil all the free phenols, thereby providing a material (i.e. 24) needing only a benzylic oxidation adjacent to the A-ring to reach the target structure.
Though simply stated, this final operation proved challenging to effect as no oxidation protocol with the free phenols of 24 led to the desired product; either the starting material was recovered unchanged or complete decomposition was observed. Following reprotection of all the phenols as MOM ethers, however, exposure of the resultant material to Pd(OAc)2, K2CO3, and t-butylhydrogen peroxide in CH2Cl2 in an open flask at 25 °C over 3 days[15] uniquely effected conversion of the desired methylene into a benzylic alcohol.[16] Interestingly, no ketone or hydroperoxide products were observed for this transformation, a result counter to previous literature reports; this result, we believe, is indicative of the truly unique nature of the 7-membered ring within these molecules and perhaps explains the numerous failed attempts in achieving its oxidation. In any event, with an alcohol finally installed, further oxidation with Dess–Martin periodinane, followed by MOM-ether cleavage with BBr3 in CH2Cl2, completed the target molecule (2) in 73% overall yield.[17] Thus, a total of 15 linear operations, with only half of these occurring after the preparation of key intermediate 19, were needed to achieve the total synthesis. To date, over 20 mg of dalesconol B (2) have been prepared.
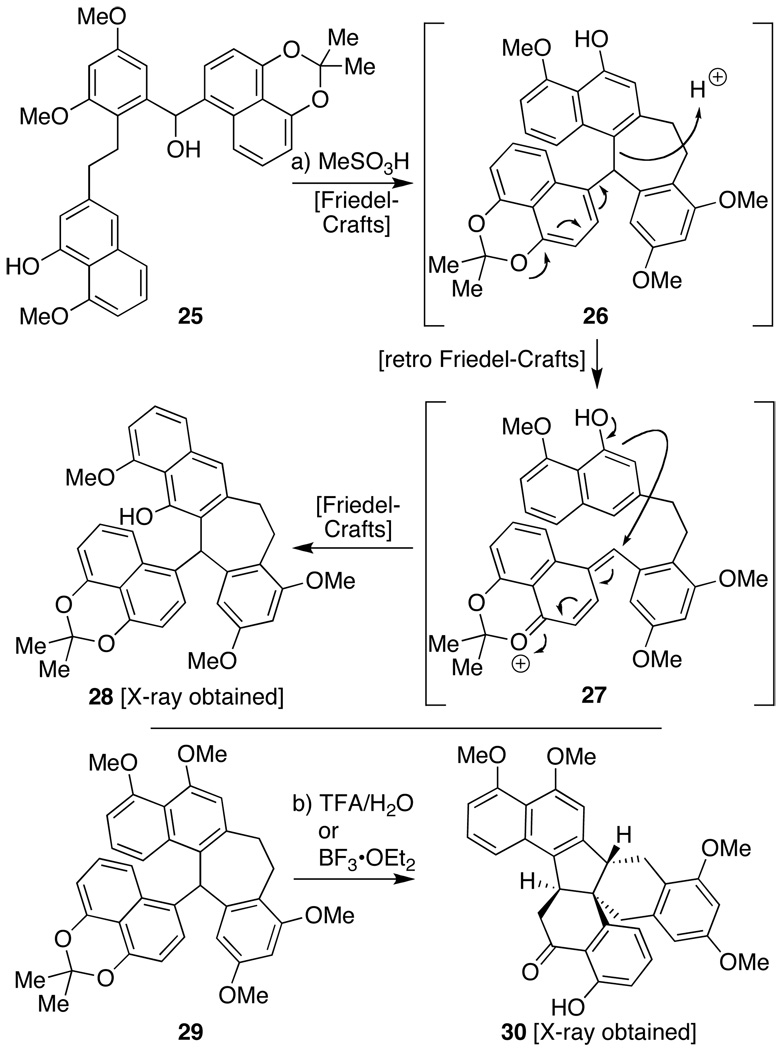
It is important to stress, however, that the sequence delineated above, particularly the cascade-based sequence converting 19 into 22, required several generations of approaches to achieve. The main challenge, as we observed on numerous occasions, was that subtle alteration of reaction conditions and/or the mere alteration or absence of a single protecting group afforded a number of unanticipated skeletal rearrangements. For instance, exposure of a molecule with a free B-ring phenol (i.e. 25, Scheme 4) to a stronger acid than used above (MeSO3H in CH2Cl2) led to an alternate 7-membered ring adduct (i.e. 28). As indicated, we believe this structure, one whose connectivities were confirmed by X-ray crystallographic analysis, is the product of a retro-Friedel–Crafts/Friedel–Crafts sequence as 26 was observed during the course of the reaction, though it could not be isolated in any significant quantity. Based on the evaluation of a number of crystal structures of related intermediates, materials of general architecture 28 appear to have significantly less steric strain than those like 26.[18] Indeed, even 21 (cf. Scheme 3) can rearrange into such products if appropriate care is not taken (in terms of total reaction stir time, temperature, or equivalents of acid used). Similarly, when efforts were made to deprotect the ketal within compound 29 and similar materials with B-ring phenol protection (which prevented their initial rearrangement into materials like 28), both protic and Lewis acidic conditions led to deprotection and concomitant rearrangement to unique polycycle 30. The exact mechanism for this event is the subject of current investigations.
Scheme 4.
Selected challenges encountered in executing the cascade-based construction of the dalesconol core: skeletal rearrangements deriving from differential protection of the phenols: a) MeSO3H (10 equiv), CH2Cl2, −78→0 °C, 1 h, 77%; b) TFA/H2O (9/1), 25 °C, 24 h or BF3•OEt2 (10 equiv), CH2Cl2, −78→25 °C, 7 h, 59%.
Finally, we wished to determine if the developed sequence could be applied to prepare dalesconol A (1) as well. As shown in Scheme 5, that goal was achieved starting with phosphonate 31,[19] obtained from o-anisaldehyde, through the same general sequence of events as described above for dalesconol B (2). Interestingly, though there is one fewer phenol in the A-ring within all of these intermediates, no fundamental change in reactivity was observed, though some differences in reaction time and temperature were required for individual steps to reach completion. As a testament to the strength of the developed chemistry, our first attempt to execute this sequence, in which we started with just 100 mg of o-anisaldehyde, led to the preparation of a characterizable amount of dalesconol A (1). The yields and experimental description for this synthesis in the Supporting Information section represent additional, larger pushes of material.
Scheme 5.
Total synthesis of dalesconol A (1): a) KOt-Bu (1.0 M in THF, 1.1 equiv), THF, −78 °C, 20 min; 14 (1.0 equiv), −78→25 °C, 3 h, 79%; b) n-BuLi (1.6 M in hexanes, 1.5 equiv), THF, −78 °C; 18 (2.0 equiv), −78→25 °C, 4 h, 51%; c) H2 (1 atm), Pd/C (10%, 1 equiv), EtOAc/EtOH (2/3), 25 °C, 1 h; filter, solvent removal, TFA (1.0 equiv), 2,2,2-trifluoroethanol, −45 °C, 15 min; PhI(OAc) 2 (1.1 equiv), −45 °C, 20 min, 27% overall; d) H2 (1 atm), Pd/C (10%, 1.0 equiv), EtOH/EtOAc (3/1), 25 °C, 3–10 h, 65%; e) conc. HCl (30 equiv), THF, 0→25 °C, 2 h, 99%; f) DDQ (1.1 equiv), benzene, 25 °C, 30 min, 77%; g) Pd(OAc)2 (1.0 equiv), t-BuOOH (25 equiv), K2CO3 (10 equiv), CH2Cl2, 72 h, 25 °C, 41%; i) Dess–Martin periodinane (5.0 equiv), NaHCO3 (10 equiv), CH2Cl2, 25 °C, 2 h, 99%; j) BBr3 (1.0 M in CH2Cl2, 15 equiv), CH2Cl2, −78→25 °C, 5 h, 66%.
In conclusion, we have developed a short, direct route to dalesconol A and B (1 and 2) that is capable of providing both natural products, as well as several analogs, to enable a fuller evaluation of their biochemical potential. Key elements of the sequence include a one-pot cascade which sequentially forged two rings and the lone quaternary carbon to complete the entire polycyclic core of the targets from an acyclic material, a unique benzylic oxidation to fashion the final oxygen atom of the targets, and the demonstration that alteration in phenol protection and/or reaction conditions could afford a number of unique structures in addition to the target molecules. Indeed, the ability to obtain not only 22, but also 28 and 30 from intermediates of general structure 5 and 6 reaffirms their power as privileged starting materials for the controlled generation of a variety of distinct architectures.[20]
Supplementary Material
Footnotes
We thank the NSF (CHE-0619638) for an X-ray diffractometer and Prof. Gerard Parkin, Mr. Wesley Sattler, and Mr. Aaron Sattler for performing all of the crystallographic analyses. We also thank Dr. George Sukenick for NMR assistance. Financial support was provided by Columbia University, the National Institutes of Health (R01GM84994), the NSF (Predoctoral Fellowships to T.C.S. and A.G.R.), Eli Lilly (Grantee award to S.A.S), the Camille and Henry Dreyfus Foundation (New Faculty Award to S.A.S.), and the Research Corporation for Science Advancement (Cottrell Scholar Award to S.A.S.).
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Zhang YL, Ge HM, Zhao W, Dong H, Xu Q, Li SH, Li J, Zhang J, Song YC, Tan RX. Angew. Chem. 2008;120:5907–5910. doi: 10.1002/anie.200801284. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2008;47:5823–5826. doi: 10.1002/anie.200801284. [DOI] [PubMed] [Google Scholar]
- 2.For a recent total synthesis of cyclosporin A, see: Wu X, Stockdill JL, Wang P, Danishefsky SJ. J. Am. Chem. Soc. 2010;132:4098–4100. doi: 10.1021/ja100517v.
- 3.This unique biological activity can perhaps be explained if both enantiomers can enter the binding pocket, a phenomenon that was only just recently observed: Mentel M, Blankenfeldt W, Breinbauer R. Angew. Chem. 2009;121:9248–9251. doi: 10.1002/anie.200902997. Angew. Chem. Int. Ed. 2009;48:9084–9087. doi: 10.1002/anie.200902997.
- 4.Wen L, Cai X, Xu F, She Z, Chan WL, Vrijmoed LLP, Jones EBG, Lin Y. J. Org. Chem. 2009;74:1093–1098. doi: 10.1021/jo802096q. [DOI] [PubMed] [Google Scholar]
- 5.a) Snyder SA, Zografos AL, Lin Y. Angew Chem. 2007;119:8334–8339. doi: 10.1002/anie.200703333. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:8186–8191. doi: 10.1002/anie.200703333. [DOI] [PubMed] [Google Scholar]; b) Snyder SA, Breazzano SP, Ross AG, Lin Y, Zografos AL. J. Am. Chem. Soc. 2009;131:1753–1765. doi: 10.1021/ja806183r. [DOI] [PubMed] [Google Scholar]
- 6.For the isolation and characterization of these 3 particular natural products, see: Baba K, Maeda K, Tabata Y, Doi M, Kozawa M. Chem. Pharm. Bull. 1988;36:2977–2983. Guo-Xun Y. Planta Medica. 2005;71:569. doi: 10.1055/s-2005-864162. Ohshima Y, Ueno Y, Hisamachi K, Takeshita M. Tetrahedron. 1993;49:5801–5804.
- 7.For a related Stobbe condensation, see: Bloomer JL, Stagliano KW, Gazzillo JA. J. Org. Chem. 1993;58:7906–7912. The preparation and use of phosphonate 13 was inspired by the following article: Tamiya M, Ohmori K, Kitamura M, Kato H, Arai T, Oorui M, Suzuki K. Chem. Eur. J. 2007;13:9791–9823. doi: 10.1002/chem.200700863.
- 8. Chae M, Song J, An D. Bull. Kor. Chem. Soc. 2007;28:2517–2518. The sodium and lithium analogs have been made as well: Song JI, An DK. Chem. Lett. 2007;36:886–887. Kim MS, Choi YM, An DK. Tetrahedron Lett. 2007;48:5061–5064.
- 9.A sequence using LiAlH4 to completely reduce the ester of 15, followed by Dess–Martin oxidation, afforded a commensurate yield of product, albeit at the cost of an additional synthetic step.
- 10.For recent uses of the alkali fusion reaction, see: Poirier M, Simard M, Wuest JD. Organometallics. 1996;15:1296–1300. Snyder SA, Tang Z-Y, Gupta R. J. Am. Chem. Soc. 2009;131:5744–5745. doi: 10.1021/ja9014716.
- 11.With the double bond present, or with any oxidized form of the double bond, the desired Friedel-Crafts cyclization could not be achieved. Thus, though a decrease in oxidation state was required before a penultimate oxidation event to complete the targets, that change proved absolutely necessary in this case.
- 12.The use of PhI(OAc)2 to forge such types of systems is, of course, well known. For selected examples, see: Kita Y, Takada T, Gyoten M, Tohma H, Zenk MH, Eichhorn J. J. Org. Chem. 1996;61:5857–5864. Kita Y, Arisawa M, Gyoten M, Nakajima M, Hamada R, Tohma H, Takada T. J. Org. Chem. 1998;63:6625–6633. Tohma H, Morioka H, Takizawa S, Arisawa M, Kita Y. Tetrahedron. 2001;57:345–352. Baxendale IR, Ley SV, Piutti C. Angew. Chem. 2002;114:2298–2301. doi: 10.1002/1521-3773(20020617)41:12<2194::aid-anie2194>3.0.co;2-4. Angew. Chem. Int. Ed. 2002;41:2194–2197. doi: 10.1002/1521-3773(20020617)41:12<2194::aid-anie2194>3.0.co;2-4. Lion CJ, Vasselin DA, Schwalbe CH, Matthews CS, Stevens MFG, Westwell AD. Org. Biomol. Chem. 2005;3:3996–4001. doi: 10.1039/b510240e. Baxendale IR, Deeley J, Griffiths-Jones CM, Ley SV, Saaby S, Tranmer GK. Chem. Comm. 2006:2566–2568. doi: 10.1039/b600382f. Kelly DR, Baker SC, King DS, de Silva DS, Lord G, Taylor JP. Org. Biomol. Chem. 2008;6:787–796. doi: 10.1039/b716915a. However, to the best of our knowledge, the transformation has never been used in such a cascade-based event as described here. For excellent reviews on such reactions in synthesis, see: Pouységu L, Deffieux D, Quideau S. Tetrahedron. 2010;66:2235–2261. Quideau S, Pouységu L, Deffieux D. Curr. Org. Chem. 2004;8:113–148.
- 13.Molecular modeling suggested that there would be a preference for the desired approach of hydrogen in this reaction, though the overall bias was not profound. For precedent that the desired hydrogenation could be achieved without loss of the benzylic ketone, see: Kametani T, Kondoh H, Tsubuki M, Honda T. J. Chem. Soc. Perkin Trans. 1. 1990:5–10. Miles DH, Lho D-S, Chittawong V, Payne AM. J. Org. Chem. 1990;55:4034–4036. Nevertheless, this reaction could be capricious, with over-reduction easily achieved if the reaction was not carefully monitored, particularly on scale-up.
- 14.For a general reference on DDQ, see: Buckle DR. In: Encyclopedia of Reagents for Organic Synthesis. Paquette LA, editor. Vol. 3. Chichester: John Wiley and Sons; 1995. pp. 1299–1704.
- 15.a) Yu J-Q, Corey EJ. Org. Lett. 2002;4:2727–2730. doi: 10.1021/ol0262340. [DOI] [PubMed] [Google Scholar]; b) Yu J-Q, Corey EJ. J. Am. Chem. Soc. 2003;125:3232–3233. doi: 10.1021/ja0340735. [DOI] [PubMed] [Google Scholar]
- 16.Only one other oxidant, Mn(OAc)3, delivered similar material without the formation of significant amounts of by-products: Shing TKM, Yeung Y-Y, Su PL. Org. Lett. 2006;8:3149–3151. doi: 10.1021/ol0612298. Other oxidants which failed to effect this transformation include DDQ (in the presence and absence of water), Jones reagent, Ag2O, N-bromosuccinimide, and ceric ammonium nitrate (CAN).
- 17.The permethylated form of 24 could also be oxidized to a ketone over 2 steps under the reported conditions; however, despite numerous attempts, it could never be fully deprotected to dalesconol B (2); the non-hydrogen bound phenol within ring A proved resistant to cleavage over several attempts.
- 18.See the Supporting Information section for these structures. Based on MMFF94 calculations, rearranged compound 28 is approximately 6.8 kcal/mol more stable than 26.
- 19.See the Supporting Information section for the synthetic route.
- 20.For a recent review on the concept of privileged architectures in drug discovery and development, see: Welsch M, Snyder SA, Stockwell BS. Curr. Opin. Drug Discovery. 2010 doi: 10.1016/j.cbpa.2010.02.018. in press.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.