Abstract
Pregnancy is a time of greatly increased uterine blood flow to meet the needs of the growing fetus. Increased uterine blood flow is also observed in the follicular phase of the ovarian cycle. Simultaneous fura-2 and 4,5-diaminofluoresceine (DAF-2) imaging reveals that cells of the uterine artery endothelium (UA Endo) from follicular phase ewes produce marginally more nitric oxide (NO) in response to ATP than those from luteal phase. However, this is paralleled by changes in NO in response to ionomycin, suggesting this is solely due to higher levels of endothelial nitric oxide synthase (eNOS) protein in the follicular phase. In contrast, UA Endo from pregnant ewes (P-UA Endo) produces substantially more NO (4.62-fold initial maximum rate, 2.56-fold overall NO production) in response to ATP, beyond that attributed to eNOS levels alone (2.07-fold initial maximum rate, 1.93-fold overall with ionomycin). The ATP-stimulated intracellular free calcium concentration ([Ca2+]i) response in individual cells of P-UA Endo comprises an initial peak followed by transient [Ca2+]i bursts that are limited in the luteal phase, not altered in the follicular phase, but are sustained in pregnancy and observed in more cells. Thus pregnancy adaptation of UA Endo NO output occurs beyond the level of eNOS expression and likely through associated [Ca2+]i cell signaling changes. Preeclampsia is a condition of a lack of UA Endo adaptation and poor NO production/vasodilation and is associated with elevated placental VEGF165. While treatment of luteal NP-UA Endo and P-UA Endo with VEGF165 acutely stimulates a very modest [Ca2+]i and NO response, subsequent stimulation of the same vessel with ATP results in a blunted [Ca2+]i and an associated NO response, with P-UA Endo reverting to the response of luteal NP-UA Endo. This demonstrates the importance of adaptation of cell signaling over eNOS expression in pregnancy adaptation of uterine endothelial function and further implicates VEGF in the pathophysiology of preeclampsia.
Keywords: connexin 43, preeclampsia, capacitative entry, calcium burst, transient receptor potential channel 3, endothelial nitric oxide synthase
pregnancy is associated with a need to expand maternal blood volume, increase cardiac output, and redistribute blood to the uterus to meet the needs of the growing fetus. Normal maternal vascular adaptation during pregnancy includes enhanced vasodilation, with the greatest effect seen in the uterus. Increased production of the potent vasodilator nitric oxide (NO) is of central importance to this adaptation, which is otherwise characterized by a well-described resistance to pressors during pregnancy (5). Failure of this adaptation in humans is an early hallmark of preeclampsia, a common condition that is characterized by poor uterine perfusion and often leads to intrauterine growth restriction (16). Over the past 15 years, studies of these maternal adaptation events at a cellular and molecular level in ovine uterine artery endothelium (UA Endo, i.e., endothelial cells directly on or freshly recovered from the uterine artery itself), as well as the corresponding endothelial cells expanded in primary culture (UAEC), have suggested that enhanced endothelial nitric oxide synthase (eNOS) activity in UA Endo ex vivo is associated with remapping of postreceptor signaling pathways as well as increased expression of eNOS itself (2, 21). With regard to post receptor signaling, UAEC isolated from pregnant ewes and maintained in primary culture still respond to ATP with a more prolonged capacitative intracellular free calcium concentration ([Ca2+]i) response than in cells from NP ewes (4, 8). More recently, we (22) have used video imaging to show that in individual cells this sustained capacitative phase of elevated [Ca2+]i, which is enhanced by pregnancy, occurs in the form of an extended initial [Ca2+]i peak followed by a series of repeated [Ca2+]i bursts. Furthermore, through a pregnancy-specific increase in Cx43 gap junction function, a greater number of endothelial cells are recruited to respond (22). Evidence that pregnancy-specific increases in [Ca2+]i elevation are absolutely necessary to achieve maximal eNOS activity are indicated by significant declines in eNOS activity when the [Ca2+]i response is eliminated (20). While these in vitro findings potentially explain the basis of pregnancy-adapted NO production in vivo, the question is whether it truly occurs in vivo? We (23) have previously performed imaging of freshly isolated UA Endo sheets en mass and shown that the more sustained nature of the [Ca2+]i response during pregnancy occurs and is directly associated with the more sustained NO production seen during pregnancy. We have not, however, established if [Ca2+]i bursts occur in the individual cells of intact vascular endothelium or if the number of cells responding in pregnancy in vivo changes, as otherwise suggested by recent UAEC studies in vitro (22). Also, it remains unclear to what degree these changes in enhanced Ca2+ signaling contribute to pregnancy-adapted NO output in vivo beyond that due to a simple increase in eNOS expression. We herein hypothesize that in vivo it is indeed pregnancy adaptation of Ca2+ signaling that accounts for the pregnancy adaptation of NO output above and beyond that accounted for by a simple increase in eNOS expression. We would further hypothesize that processes that interfere with the pregnancy adaptation of Ca2+ signaling in vivo will in turn reverse otherwise enhanced NO production back down to the level seen in the nonpregnant state. To that end, we report for the first time these studies of changes in [Ca2+]i and NO in real time in individual endothelial cells of the intact UA vessel ex vivo in response to ATP. Further, we compare the responses in UA Endo from the luteal phase (luteal NP-UA Endo), follicular phase (follicular NP-UA Endo), and pregnant ewes (P-UA Endo) to a physiologic agonist ATP and pharmacologic agent ionomycin, and in so doing we observe that changes in eNOS expression and changes in [Ca2+]i-mediated eNOS activation by ATP play different relative roles in these unique physiologic states. Finally, in further exploring the effect of growth factors (VEGF165) known to be locally elevated in diseases such as preeclampsia (11) on intact UA Endo initially alone, and then on subsequent G-protein coupled receptor agonist (ATP)-stimulated [Ca2+]i and NO responses, we find surprising inhibitory actions on pregnancy-adapted Ca2+ signaling that are paralleled by corresponding changes in NO production. These inhibitory changes not only support the causal relationship between Ca2+ bursts and NO production but are also consistent with an inhibitory role for VEGF165 in the pathophysiology of preeclampsia.
MATERIALS AND METHODS
Materials.
ATP (disodium salt) and ionomycin were purchased from Sigma (St. Louis, MO). PGF2 (for sheep synchronization) was purchased from Dinoprost Tromethamine-Lutalyse (Upjohn, Kalamazoo, MI). DAF-2 DA and all other general reagents were from Sigma, and for cell imaging fura-2 AM was from Molecular Probes (Carlsbad, CA).
Synchronization of follicular and luteal phase ewes.
Procedures for animal handling and protocols for experimental procedures were approved by the University of Wisconsin-Madison Research and Animal Care and Use Committees of both the Medical School and the College of Agriculture and Life Sciences and follow the recommendations of the “Report of the American Veterinary Medical Association Panel on Euthanasia.” Mixed Western breed ewes (50–60 kg) exhibiting normal estrous cycles were implanted with a vaginal progesterone controlled internal drug-releasing device (CIDR; 0.3 g; Latinagro de Mexico, Monterrey, Mexico) for 12–14 days. To ensure removal of any endogenous progesterone, on the day before CIDR removal, sheep were given two PGF2α injections (7.5 mg im, 4 h apart). At CIDR removal, animals were given an intramuscular injection of 500 IU pregnant mare serum gonadotrophin (Sioux Biochemical, Sioux Center, IA). UA samples were obtained from follicular phase sheep (n = 6) 48 h after CIDR removal around the peak of the estrous-induced rise in uterine blood flow during the periovulatory period; day 0 of the ovarian cycle (6). Uterine arteries were obtained from luteal phase sheep (n = 7) on day 10 of the ovarian cycle, i.e., 12 days after CIDR removal and pregnant mare serum gonadotrophin injection (6). This synchronization protocol results in ewes showing behavioral estrus within ∼40–44 h and ovulation ∼56 h after CIDR removal (6, 13). We visually confirmed that the ovaries of the follicular phase ewes had preovulatory follicles (6 mm) and fully regressed avascular corpora lutea (∼4–7 mm), whereas the luteal phase sheep had one or more large, functional vascular corpora lutea (10–12 mm). We (6, 13) have previously reported the estrogen and progesterone levels using this method. Moreover, the follicular phase sheep had elevated uterine blood flow compared with the luteal phase animals (data not shown), demonstrating the physiologic functional differences in the animal preparations and confirming our previous observations.
Isolated uterine artery preparation.
Nonpregnant (follicular, n = 6; luteal, n = 7) and late pregnant (n = 12; 125 ± 4 days of gestation) ewes were euthanized with pentobarbital sodium (50–70 mg/kg). Microdissection of uterine artery was performed as described previously (23). Briefly, the third (from nonpregnant sheep) and fourth (from pregnant sheep) branches of the main uterine arteries with a similar external diameter were separated from the surrounding tissue and were rapidly dissected and immersed in ice-cold Krebs buffer (in mM: 125 NaCl, 5 KCl, 1 MgSO4, 1 KH2PO4, 6 glucose, 25 HEPES, and 2 CaCl2 pH 7.4). Uterine arteries were further carefully dissected in a dissection station and placed in cold Krebs buffer and then transferred to a 0.9-mm microdissecting dish filled with Sylgard (World Precision Instruments) immersed in ice-cold Krebs buffer. The arterial segment was cut open along its longitudinal axis and pinned onto the Sylgard with the lumen side upward. Care was taken not to disrupt the endothelium. Four additional supportive pins were placed around the lumen-opened artery with the top ends 1.2 mm over the arterial endothelium. After the artery was fixed, the 0.9-mm dish was put into a recording chamber with the vessel lumen side downward facing the objective of an inverted microscope. A 1.2-mm space between the arterial endothelium and the glass bottom of the recording chamber was created by the support pins. This kept the artery endothelium from direct contact with glass bottom and allowed the solution free passage. The recording chamber was filled with Krebs buffer, and after a 30-min equilibration, the endothelium was ready for dye loading.
In situ simultaneous measurement of [Ca2+]i and NO in intact UA Endo.
Simultaneous [Ca2+]i and NO fluorescent imaging analysis was performed using a high-speed excitation and emission wavelength switching systems as described by Yi et al. (24). After loading with both DAF-2 DA (20 μM; Molecular Probes) and fura-2 AM (10 μM) for 90 min, the chamber with the isolated UA was mounted on an inverted microscope (Diaphot 150; Nikon) so that a ×20 phase/fluor objective was focused on the lumenal endothelium and individual endothelial cells are visualized (Fig. 1; only well-focused endothelial cells had a strong signal, and these cells were randomly selected). The excitation light from a xenon lamp was filtered to provide wavelengths of 340 ± 10 and 380 ± 10 (for fura-2) and 480 ± 20 nm (for DAF-2) with a high-speed wavelength switcher (Lambda 10–2; Sutter, Novato, CA). Emission light from endothelial cells was passed through a dichroic mirror (505 nm) and through an emission filter of 520 ± 20 nm for both fura-2 and DAF-2 with a high-speed rotating filter wheel (Lambda 10–2; Sutter). The fluorescence images were recorded by a digital camera (PixelFly; Cooke). InCyt Im3 imaging and analysis software (Intracellular Imaging) was used to acquire, digitize, and store the images and data for off-line processing and statistical analysis. The relative fluorescence intensities for fura-2 and DAF-2 were quantitatively comparable. When the endothelium was only loaded with fura-2 AM, no signal at 480/535 nm (for NO imaging) was detected. In contrast, when the endothelium was only loaded with DAF-2 DA, no detectable signal could be recorded at 340/510- and 380/510-nm wavelengths (for [Ca2+]i imaging; data not shown). To reduce photobleaching of these fluorescent dyes, images were acquired at 5-s intervals. Background and autofluorescence were obtained from control-unloaded endothelium. F340/F380, a fluorescence ratio of excitation at 340 nm to that at 380 nm, was determined after background and autofluorescence subtraction, and [Ca2+]i was calculated in real time by comparison to a standard curve established for the same settings using buffers of known free [Ca2+]. Because there is no significant change in baseline fluorescence during a 60-min experiment, we expressed the intracellular NO production as relative fluorescence (f), which is the net increment of DAF-2 fluorescence relative to its basal value (f = ΔF/F0), where F is DAF-2 fluorescence intensity obtained during experiments and F0 is its basal fluorescence intensity.
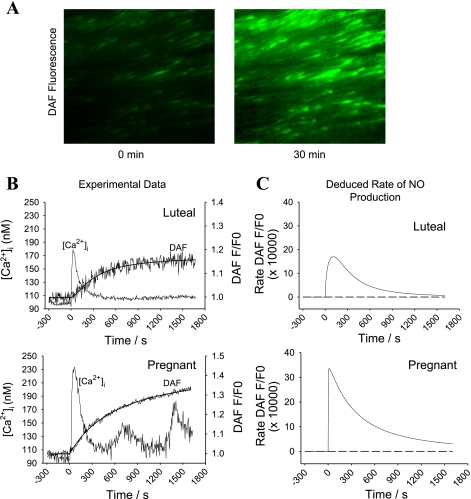
Fig. 1.
Simultaneous in situ measurements of intracellular free Ca2+ concentration ([Ca2+]i) and nitric oxide (NO) levels in individual cells of intact uterine artery endothelium (UA Endo). A: representative 4,5-diaminofluorescein (DAF-2) fluorescence image taken at an excitation wavelength (λ) of 485 nm and an emission λ of 535 nm after incubation under control conditions (left) or with ATP (100 uM; right) for 25 min. B: representative simultaneous recordings of ATP-induced increase in [Ca2+]i and DAF-2 fluorescence from a single intact endothelial cell of UA Endo from luteal (top) and pregnant ewes (bottom). Note the upper response shows a single [Ca2+]i peak (burst), while the lower shows 3 clear successive [Ca2+]i bursts. C: conversion of relative DAF-2 fluorescence (F/F0) to rate increase of DAF-2 fluorescence, thus providing a measure of endothelial NO synthase (eNOS) activity with time.
Conversion of DAF-2 fluorescence intensity to rate increase of NO production.
Unlike the reversible reaction between fura-2 and [Ca2+]i, NO does not dissociate from DAF-2 since this dye covalently reacts with NO, so the detected NO-sensitive fluorescence with DAF-2 primarily represents a cumulative amount of NO produced within the cells as described above. An obvious disadvantage of this method for continuous NO measurements is that the plateau phase of the NO-DAF fluorescence curve actually represents the termination of NO production within the cells. To more accurately present the time-dependent relationship between eNOS activity and [Ca2+]i in the cells, we established a line of best fit to the data and then performed a differential conversion of the NO-DAF fluorescence curve to calculate rate increases in DAF-2 fluorescence (i.e., changes in eNOS activity). More specifically, regression analysis of the NO-DAF fluorescence curve recordings for each given stimuli was performed using SigmaPlot 2000. From this curve, rate increases in DAF-2 fluorescence per 5 s (data collection interval) were derived.
Statistical analysis.
In all statistical comparisons of groups, bar graph data are presented as means ± SE and were analyzed by Student's t-test or ANOVA, as appropriate. A value of P < 0.05 was considered statistically significant.
RESULTS
Simultaneous measurement of [Ca2+]i and NO production in intact endothelium of uterine arteries.
We (23) have previously described changes in [Ca2+]i and NO in freshly isolated endothelium cell sheets removed en mass from the vessel surface. In this former study, imaging was undertaken using a single 340-nm excitation of fura-2 and 485 nm for DAF-2 and entire cell group responses recorded. Herein (Fig. 1) we now use ratiometric imaging for fura-2 and record for the first time simultaneously changes in [Ca2+]i and NO production in individual endothelial cells while they are still present and intact on the luminal surface of the uterine artery ex vivo. Thus our data represent a snapshot of the physiologic in vivo conditions from a per cell basis. Figure 1A shows sample images in which the 100 uM ATP-induced rise of DAF-2 green fluorescence in intact UA Endo from pregnant ewes is clearly apparent, even by eye. In Fig. 1B, a digitized online recording of [Ca2+]i and DAF-2 fluorescence in this arterial preparation is also shown for single representative cells. ATP induced substantially more NO production in P-UA Endo than luteal NP-UA Endo, with a progressive increase in DAF-2 signal for pregnancy-derived vessels. In comparison, DAF-2 fluorescence in the signal from luteal NP-UA Endo reaches an earlier plateau. Since the detected change in DAF-2 fluorescence is due to covalent bonding to the DAF-2 dye, and so is irreversible, a progressive increase indicates the continued NO production while a plateau indicates NO production has ceased. A differential calculation as presented in Fig. 1C shows the actual rate of increase of DAF-2 fluorescence/NO production, thereby revealing these time-dependent changes in eNOS activity in these individual cells.
ATP induces more [Ca2+]i bursts in P-UA Endo than follicular and luteal NP-UA Endo.
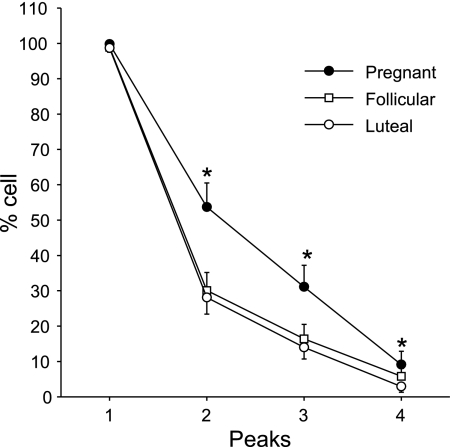
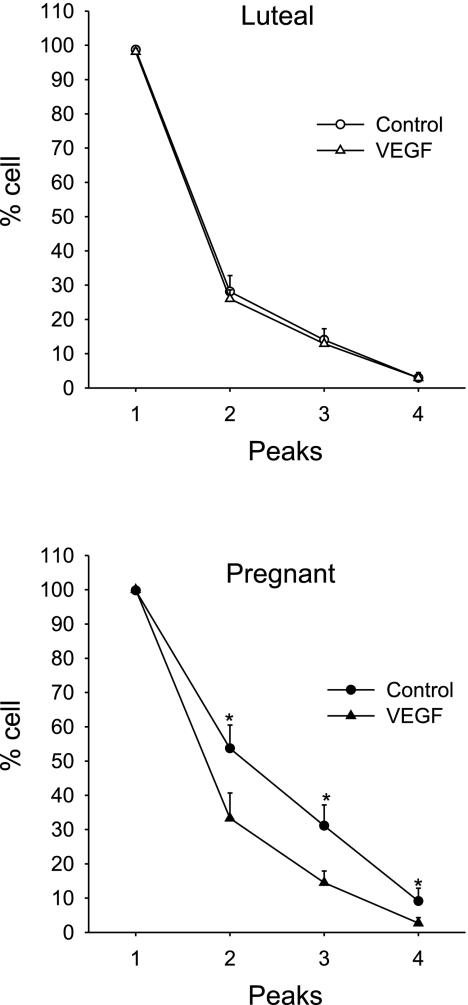
Over a 25-min period, we found that the sustained phase [Ca2+]i response in individual cells of the P-UA Endo ex vivo, just as in cultured passage 4–5 P-UAEC in vitro (22), does not take the form of an extended plateau. Rather, following the initial ATP-stimulated [Ca2+]i peak, the sustained Ca2+ entry was maintained in the form of a series of [Ca2+]i bursts (Fig. 1B). Furthermore, the number of bursts was clearly greater in P-UA Endo (Fig. 1B, bottom) vs. luteal NP-UA Endo (Fig. 1B, top). While the data in Fig. 1B are an illustration of typical results for single endothelial cells, Fig. 2 summarizes the overall likelihood of seeing each successive burst in a repeated burst pattern in P-UA Endo, as well as follicular and luteal NP-UA Endo. The number of cells showing burst behavior was significantly greater (almost double for burst 2 and 3) in P-UA Endo compared with both luteal and follicular NP-UA Endo, with no apparent difference in percent number of cells showing [Ca2+]i burst activity in the follicular vs. luteal phase.
Fig. 2.
Summary of the effects of ATP (100 uM) on [Ca2+]i bursts in endothelial cells of UA Endo from luteal, follicular, and pregnant sheep. Incidence of [Ca2+]i bursts was determined as in Fig. 1 for 40 or more individual cells per image field. Percentage of cells responding with each successive burst are shown. Data are means ± SE of UA Endo from 6 to 8 ewes per group. *P < 0.05 vs. luteal.
ATP induces greater [Ca2+]i synchronization in P-UA Endo than NP-UA Endo.
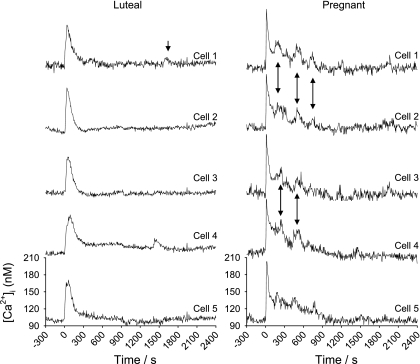
Since digital video imaging was used to image many endothelial cells simultaneously, it was possible to analyze individual cell responses in neighboring cells to evaluate the level of cell synchronization. Imaging using fura-2 is ratiometric, so a peak (indicated by arrows) can be verified as opposing changes in signal recorded at 340- and 380-nm excitation wavelengths. We have defined a peak as that which shows appropriate changes at 340- vs. 380-nm excitation, has a clear maximum and exceeds twice basal. Such analysis revealed that, as in passage 4–5 cultured P-UAEC (22), the rapid [Ca2+]i peak and subsequent bursts in P-UA Endo ex vivo were synchronous among cells (Fig. 3). While this synchronization of [Ca2+]i burst responses was apparent in groups of cells in P-UA Endo, it was not seen at all in luteal or follicular NP-UA Endo.
Fig. 3.
Synchronous [Ca2+]i bursts in neighboring cells in response to 100 μM ATP over 40 min. Multiple individual cell tracings from endothelium of a single intact UA from luteal NP (left) and pregnant (right) ewes are shown. After the initial peak, continued synchronous bursts by cells are as indicated by arrows.
Effect of ATP on [Ca2+]i and NO production in luteal NP-, follicular NP-, and P-UA Endo.
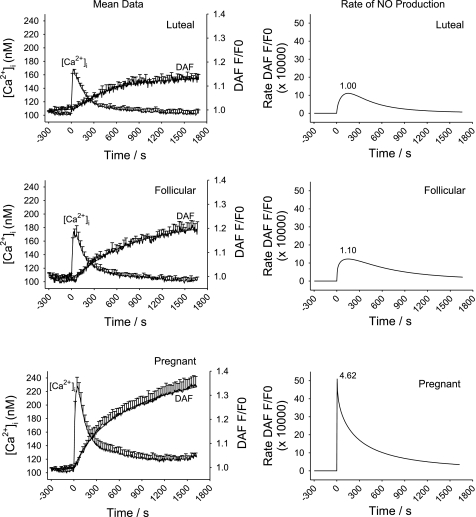
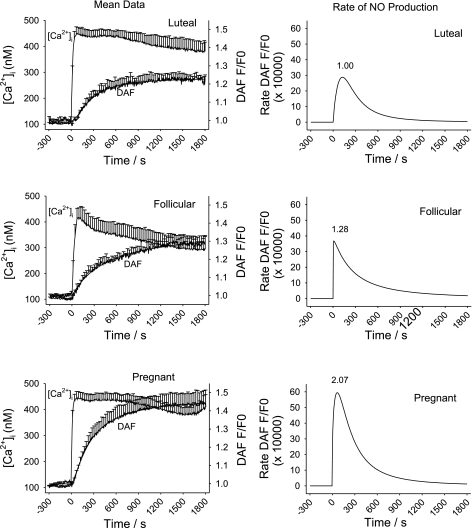
In Fig. 4, we show the combined average response traces of ATP (100 uM)-induced increase in [Ca2+]i and DAF-2 fluorescence (total NO production; Fig. 4, left) and rate increase of DAF-2 fluorescence (NO production rate, i.e., eNOS activity; Fig. 4, right) from n = 6–8 ewes. Note that this average is inclusive of all cells in the view field, regardless of responder or nonresponder. This representation alone demonstrates that ATP induced a greater initial [Ca2+]i peak and a more sustained average [Ca2+]i response (consisting in part of more [Ca2+]i bursts) compared with follicular or luteal NP-UA Endo. More detailed analysis of this average data, as depicted in Table 1, confirms the initial [Ca2+]i peak was almost twice the magnitude in vessels from pregnant ewes compared with those from nonpregnant ewes (P < 0.05). In previous studies, we have also reported the sustained phase of the [Ca2+]i response to ATP in freshly isolated UA Endo shows a clearly different magnitude of average response at 500 s compared with that in NP-UAEC prepared from luteal phases ewes. The temporal differences in the [Ca2+]i response in UA Endo while still intact on the vessel surface are such that the point where this difference is greatest between luteal phase and pregnant ewes, i.e., at 900 s when [Ca2+]i elevation has returned to a near basal for luteal NP-UA Endo, yet in P-UA Endo the [Ca2+]i elevation has only fallen to an elevated plateau. Measures of average [Ca2+]i levels at 900 s do reveal the mean sustained [Ca2+]i elevation in pregnancy is 3.85-fold higher than in luteal NP-UA Endo (Fig. 4; Table 1) while luteal and follicular phase ewes are not significantly different. Overall NO production per cell, i.e., the cumulative DAF-2 signal at 1,500 s is also summarized in Table 1. ATP (100 uM) stimulated an overall 2.56-fold increase in overall NO production in P-UA Endo and a 1.48-fold increase in follicular NP-UA Endo compared with luteal NP-UA Endo. ATP-stimulated NO production in P-UA Endo is 1.73-fold over that in follicular NP-UA Endo. For maximum rate of NO production, this was clearly greatest in P-UA over follicular NP-UA Endo, which was in turn greater than luteal NP-UA Endo (Fig. 4), with a pregnancy associated peak rate of NO production 4.62-fold of that observed in luteal nonpregnant phase ewes, while follicular phase ewes were only 1.10-fold of luteal phase controls.
Fig. 4.
Averaged ATP (100 uM)-induced [Ca2+]i increase and NO production in intact UA Endo from luteal, follicular, and pregnant sheep. UA Endo from nonpregnant luteal (n = 7 ewes; top) and follicular (n = 6 ewes; middle) and pregnant (n = 8 ewes; bottom) ewes were dual loaded with fura-2 AM and DAF-2 DA and multiple cells simultaneously imaged as described. Overall responses of all individual cells in each field, regardless of response, were generated to match the equivalent of that previously reported for whole field imaging (by photometry) by Yi et al. (20). Means ± SE of combined animal responses in each group are shown. Data for DAF-2 imaging were also subjected to a curve-fit process and time-dependent changes in eNOS activity were derived (right).
Table 1.
Comparison of numeric data for initial peak Ca2+, sustained phase Ca2+, and overall NO output
| Ca2+ Peak, nM |
Ca2+ 900 s, nM |
NO 1,500 s (Relative to Basal-No Units) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Means | SE | Fold luteal | Means | SE | Fold luteal | Means | SE | Fold luteal | |
| Luteal | ||||||||||
| Iono | 9 | 335.2 | 35.7 | 1.00 | 0.229 | 0.029 | 1.00 | |||
| ATP | 11 | 65.7 | 6.0 | 1.00 | 5.0 | 3.1 | 1.00 | 0.128 | 0.016 | 1.00 |
| %Max | 56.07% | |||||||||
| Follicular | ||||||||||
| Iono | 8 | 314.8 | 41.2 | 0.94 | 0.292 | 0.023 | 1.28* | |||
| ATP | 10 | 71.9 | 14.2 | 1.09 | 6.2 | 2.5 | 1.24 | 0.190 | 0.025 | 1.48* |
| %Max | 64.89% | |||||||||
| Pregnant | ||||||||||
| Iono | 12 | 333.8 | 27.4 | 1.00 | 0.442 | 0.067 | 1.93*† | |||
| ATP | 12 | 125.2 | 18.9 | 1.90*† | 19.3 | 5.6 | 3.85* | 0.328 | 0.041 | 2.56*† |
| %Max | 74.13% | |||||||||
All values are from data shown in Figs. 4 and 5 and are means ± SE; n = group sizes. Data are also reexpressed as fold mean luteal values. Overall nitric oxide (NO) output is as indicated by 4,5-diaminofluoresceine signal at 1,500 s. %Max is percentage of the NO produced in response to ATP relative to maximal NO output [in response to ionomycin (Iono)]. Relative to the luteal uterine artery endothelium (UA Endo) efficiency of 56.07%, follicular UA Endo improved by a further 8.82% and pregnancy UA Endo increased by 18.06% overall. Of note, relative changes in maximal endothelial NO synthase activity in pregnant UA Endo was even greater in response to ATP (as shown in Figs. 4 and 5).
P < 0.05, significance from luteal UA Endo response.
P < 0.05, significance from follicular UA Endo response.
Ionomycin increases [Ca2+]i and NO production in luteal NP-, follicular NP-, and P-UA Endo.
To directly address the question of how much NO production was increased in these three physiologic states due to changes in eNOS expression alone (14, 15), challenge with ionomycin was used as a normalization control (23). Ionomycin is a Ca2+ ionophore that can rapidly induce Ca2+ influx independently of P2Y2 receptor activation and that has been previously used as a means to achieve maximal [Ca2+]i-dependent eNOS activation and so an indirect functional measure of eNOS expression (23). We thus compared maximal ionomycin-induced [Ca2+]i or NO production among luteal NP-, follicular NP-, and P-UA Endo (Fig. 5; summarized in Table 1). Of note, [Ca2+]i at 900 s was not calculated since [Ca2+]i does not drop substantially from maximum in that time (Fig. 5). There was no difference in the maximal [Ca2+]i response among luteal NP-, follicular NP-, and P-UA Endo (Table 1). However, the initial maximum NO production rate (Fig. 5) for P-UA Endo was 2.07-fold of luteal control and for follicular phase ewes was 1.28-fold of luteal control. The alternative measure of overall NO production in response to ionomycin (DAF-2 signal at 1,500-s stimulation) revealed a 1.93-fold increase in P-UA Endo and 1.28-fold in follicular NP-UA Endo compared with luteal NP-UA Endo. Reexpression of the ATP-stimulated overall NO responses as a percentage of maximum (i.e., percentage of the ionomycin response) showed that while luteal phase ewes show a 56.07% response to ATP, follicular phase ewes show a 64.89% response, and pregnant ewes show a 74.13% response, confirming that factors beyond eNOS expression were at play in pregnancy adaptation. When the maximum rates of NO production were examined, the vessels from follicular phase ewes showed a 1.10-fold increase relative to luteal phase ewes, while those from pregnant ewes showed a 4.62-fold increase. These findings again indicate that while the small but significant difference in NO production during the follicular vs. luteal phase was mostly due to changes in eNOS expression, changes in NO production occurring in P-UA Endo had to also be due to additional factors beyond eNOS expression.
Fig. 5.
Averaged ionomycin (2 uM)-induced [Ca2+]i increase and NO production in intact UA Endo from luteal, follicular, and pregnant sheep. UA Endo from nonpregnant luteal (n = 7 ewes; top) and follicular (n = 6 ewes; middle), and pregnant (n = 8 ewes; bottom) ewes was dual loaded with fura-2 AM and DAF-2 DA and multiple individual cells simultaneously imaged as described. Average values were then calculated for all recorded cells (as in Fig. 4), and values shown are means ± SE of all these average responses combined. Data for DAF-2 imaging was also subjected to a curve-fit process and time-dependent changes in eNOS activity rate were derived (right).
Effects of VEGF on ATP-induced [Ca2+]i and NO production in luteal NP- and P-UA Endo.
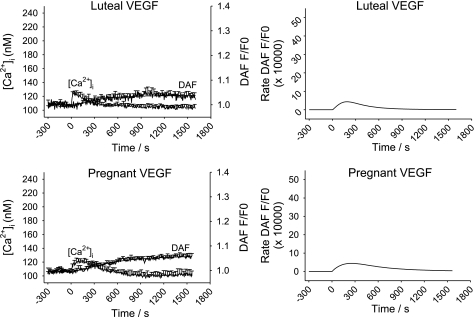
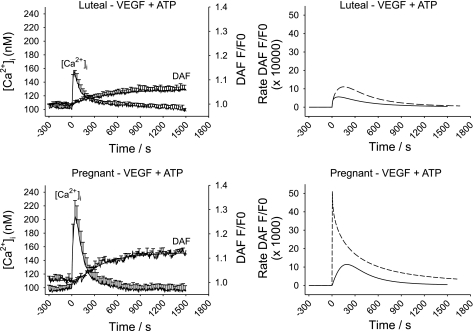
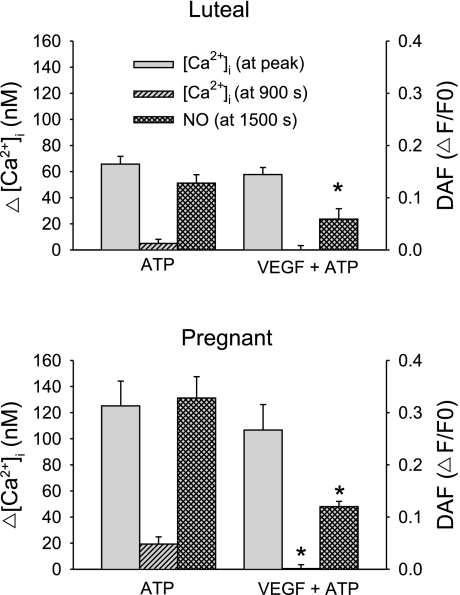
While our data showing changes in sustained phase [Ca2+]i signaling suggest a causal mechanism for pregnancy-enhanced NO production, was that indeed the case? The answer to that question was revealed by examining the effect of VEGF pretreatment. Our choice of VEGF dose (10 ng/ml) was based on that previously shown in both NP- and P-UAEC to be both maximally mitogenic (1) and stimulating maximal eNOS activation and ERK-1/2 phosphorylation (10). Of note, this is the equivalent of 518 pM, which is at the higher end of levels reported in pregnancy and within the range commonly reported in preeclamptic subjects (12). As shown in Fig. 6, VEGF165 alone stimulates a [Ca2+]i response in 70–90% of cells with changes in [Ca2+]i and NO production that were much smaller than that caused by ATP (Fig. 4). Notably, the [Ca2+]i response was of limited magnitude but also more prolonged and lacked evidence of [Ca2+]i bursts otherwise seen in response to ATP. Such a response is consistent with the slower activation of PLCγ observed in many other cell types in response to VEGF (25). In the majority of cases, rises in [Ca2+]i in individual UA Endo cells were accompanied by rises in NO. Of particular interest, however, was our finding that treatment with VEGF (10 ng/ml) for 30 min also altered the effect of subsequent ATP treatment on UA Endo response at the level of [Ca2+]i and NO. The initial ATP-induced [Ca2+]i peak (Figs. 7 and 8) in luteal NP-UA Endo or P-UA Endo remained essentially unchanged, but the subsequent probability of ATP-stimulated sustained [Ca2+]i bursts (Fig. 9) and correspondingly the subsequent average ATP-induced sustained phase [Ca2+]i response (Fig. 8) were both substantially reduced in P-UA Endo, and of particular relevance they were inhibited down to the level coincident with that in luteal NP-UA Endo. Furthermore overall NO production (both overall NO production and maximum NO production rate) in P-UA Endo (Figs. 7 and 8) was considerably blunted (63% overall; Fig. 8) and more comparable after VEGF pretreatment to that of luteal NP-UA Endo before VEGF treatment. Thus simple VEGF165 pretreatment had not only fully reversed the pregnancy-adapted [Ca2+]i burst function of UA Endo down to a nonpregnancy level but in so doing had reduced the associated NO production to nonpregnancy levels.
Fig. 6.
Averaged VEGF (10 ng/ml)-induced [Ca2+]i increase and NO production in intact UA Endo from luteal phase and pregnant sheep. UA Endo from nonpregnant luteal (n = 7 ewes; top) and pregnant (n = 8 ewes; bottom) ewes were dual loaded with fura-2 AM and DAF-2 DA, and multiple individual cells were simultaneously imaged. Average responses of all cells in each field, regardless of response, were generated as in Fig. 4. Values are combined means ± SE of UA Endo responses from 7 to 8 ewes.
Fig. 7.
Effect of VEGF (10 ng/ml) pre exposure on average ATP-induced [Ca2+]i and NO production in intact UA Endo from luteal phase and pregnant sheep. After pretreatment with VEGF (10 ng/ml), UA Endo from nonpregnant luteal (top) and pregnant ewes (bottom) were further stimulated with ATP (100 uM) for 25 min and responses of multiple cells further were recorded. Average responses of all cells recorded in each sample were derived, and data shown are means ± SE of these combined averages of UA Endo from 6 to 8 ewes. Data for DAF-2 imaging were also subjected to a curve-fit process, and time-dependent changes in eNOS activity/NO production were derived (right) as shown with the solid lines. Dashed lines represent the NO production from Fig. 4 (ATP alone without VEGF pretreatment) for comparison.
Fig. 8.
Summary of effect of VEGF (10 ng/ml) pretreatment on average ATP-induced [Ca2+]i and NO production in intact UA Endo from luteal phase and pregnant sheep. After pretreatment with VEGF (10 ng/ml) or vehicle for 30 min, UA Endo from nonpregnant luteal (top) and pregnant ewes (bottom) were then stimulated with ATP (100 uM) for a further 25 min. Responses from Figs. 4 and 7 were used to determine [Ca2+]i at both the initial peak and at 900 s and DAF-2 signals at 1,500 s. All data are means ± SE of that from 6 to 8 ewes. *P < 0.05 vs. ATP, no VEGF pretreatment.
Fig. 9.
Summary of effects of VEGF pretreatment on subsequent ATP (100 uM)-induced [Ca2+]i bursts in intact UA Endo from luteal phase and pregnant sheep. The incidence of [Ca2+]i bursts was determined for 40 or more individual cells per field in replicate data sets from n = 6–8 ewes. Average percentages of cells responding with each successive burst in vessels without or with VEGF165 pretreatment are shown for nonpregnant luteal (top) and pregnant (bottom) UA Endo. Data are means ± SE of UA Endo from 6 to 8 ewes. *P < 0.05, compared with no VEGF pretreatment.
DISCUSSION
Previous studies (2) have shown increased vascular flow in uterine artery in the follicular phase and particularly the late pregnant state is associated with increased eNOS expression, but direct studies have also suggested that NO metabolites are produced in pregnancy and particularly in hypoxic pregnancy at a level over and above that accounted for by increased eNOS expression. Isolation of uterine artery endothelial cells from luteal vs. late pregnant ewes and maintenance in culture for up to 2 wk removes differences in eNOS expression, and yet enhanced eNOS activation is still observed in cells from late pregnancy in a manner most closely associated with more sustained [Ca2+]i signaling in response to ATP (1, 4). In turn, detailed studies in recent years of [Ca2+]i signaling in UAEC infer that sustained phase [Ca2+]i signaling in UAEC occurs through transient receptor potential channel 3 (TRPC3)/inositol triphosphate receptor 2 (IP3R2) interaction (9) and that the pregnancy-specific enhancement of this response in fact occurs as a series of [Ca2+]i bursts in individual cells (22) that are mediated by TRPC3 (9) but are also potentiated by a pregnancy-specific increases in Cx43-mediated gap junction function in a cell density-dependent manner (21, 22). The finding that cells responded with such a burst pattern dependent on cell-cell communication via Cx43 gap junctions raised the possibility that pregnancy could not only synchronize cell responses with each other but also perhaps potentiate and so recruit neighboring cells to respond. Our most recent studies (22) of UAEC do indeed show that while 25% of cells are synchronous when derived from the NP luteal ewes, >50% of cells are synchronous in cultures derived from pregnant ewes, and this difference together with enhanced eNOS activation through repeated [Ca2+]i bursts is all lost when inhibitors of Cx43 gap junctions are applied. An important conclusion of these recent studies in cultured cells (22) in addition to prior in vivo studies (14, 15) now leaves us with three possible causes of enhanced NO production in pregnancy: 1) increased eNOS expression, 2) increased cell signaling “gain” per cell through more sustained [Ca2+]i bursts, and 3) increased recruitment of cells to also respond via improved Cx43 communication. In this study, we have evaluated the extent to which these three phenomena are important in defining changes in NO production in intact vessels ex vivo when taken from animals in three physiologic states, namely the luteal phase, follicular phase, and late pregnant states. The use of ex vivo imaging leaves the vessel endothelial cells in their natural state of cell-cell contact in vivo and leaves physiologic changes of eNOS expression in vivo undisturbed, and the observation ex vivo allows us to control the exposure to single or combined agonists as needed.
While we have recently reported the occurrence of [Ca2+]i bursts in UAEC in primary culture and the synchronization of such bursts in P-UAEC in particular, no such studies have been undertaken to date in intact vessels. Herein we report for the first time that [Ca2+]i bursts are indeed observed in the endothelium of intact vessels ex vivo, and such burst behavior is clearly enhanced both per cell and in number of cells responding in pregnancy compared with the luteal and follicular phase nonpregnant states (which were otherwise indistinguishable). Compared with UAEC in culture, the burst pattern in vessels from pregnant ewes tends to exhibit a continuous series of broader peaks with less time near basal in the “valleys,” but the response is otherwise very similar, and synchronization of cell responses is also clearly observed in pregnancy. The broadening of the [Ca2+]i bursts when observed relative to cells in culture is possibly a product of the cells being in contact with the basal lamina and so is potentially influenced by underlying vascular smooth muscle (VSM). This in turn raises the possibility that in the uterine vasculature gap junction coupling between VSM and endothelium could allow electric field effects or even passage of ions and/or small molecules between the two layers. While we have not performed any experiments to investigate this directly, and proof of this would require further dedicated study, we are, however, confident that the nature of the response observed in individual cells herein is qualitatively similar and quantitatively only moderately different due to the underlying VSM since the overall average [Ca2+]i and NO data shown for intact vessels in Figs. 5 and 6 relate closely to the data obtained previously using isolated plaques of endothelial cells that were imaged after removal from the vessel and plating to glass (23). Also, with regard to our focus herein, [Ca2+]i bursts in individual cells still on the vessel wall are clearly occurring ex vivo and in a more sustained and synchronous manner during pregnancy in particular as we have recently described in UAEC in vitro (22).
Having established the effects of physiologic state on [Ca2+]i bursts in response to agonists such as ATP, we were also able to further isolate the relative effect of changes in eNOS expression alone on NO production using receptor-independent stimulation of vessels with ionomycin, as performed previously (23). In this instance, average NO production (both maximal rate and overall NO production) in response to ionomycin treatment followed closely the previously reported ranking of eNOS expression by Western blot (14, 15), with the rank order being late pregnant >> follicular > luteal phase ewes. When we further consider the effect of the physiologic agonist ATP on [Ca2+]i and NO in these vessels, we found that pregnancy enhances both average sustained [Ca2+]i response per cell and the number of cells responding with bursts, which is clearly matched by an enhancement of both maximum rate and overall NO response beyond that due to increased eNOS expression levels. In contrast, there is little change in the initial or sustained [Ca2+]i responses in the follicular phase nonpregnant state in response to ATP compared with that in the luteal phase and little change in the number of cells showing sustained bursts. The maximum rate and overall NO responses to ATP in UA Endo of follicular phase ewes are increased only to an extent that corresponds closely with the previously reported eNOS expression level as assessed by Western blot (15) or in response to ionomycin (Ref. 23 and herein). A direct comparison of the overall NO production per cell in response to ATP relative to that of ionomycin for each physiologic state further confirms these findings. Follicular phase UA Endo cells show only a ∼9% higher overall NO production per cell relative to the ionomycin response compared with that seen in the luteal phase, and given that there is also no increase in the number of cells showing sustained bursts in the follicular phase compared with luteal phase UA Endo, it is not surprising changes in overall NO output match changes in eNOS expression. In contrast, during pregnancy there is not only a greater 18% rise in NO output per cell relative to the ionomycin response, i.e.,18% per cell over and above that already due to increased eNOS expression, but also a doubling of the number of cells showing sustained bursts. Thus, while pregnancy-related changes in eNOS expression can improve NO output per cell, it is the additional adaptive programming of Ca2+ signaling both through more sustained [Ca2+]i bursts per cell and increased recruitment of cells to burst in this way that brings about the remarkable further increase in NO output by UA Endo in pregnancy.
We have previously implicated a causal relationship between pregnancy enhancement of sustained Ca2+ entry to UAEC, manifested as a series of [Ca2+]i bursts, and corresponding eNOS activation in UAEC (20) and in isolated endothelial sheets (23). Thus when the [Ca2+]i response is inhibited using 2-APB or U73122, there is a drop of ∼60% in the eNOS response (21). There are also diseased states, such as preeclampsia, in which UA Endo function is impaired at the level of NO production, and it has been suggested by at least some investigators (11) that VEGF165, a growth factor produced locally by placentas of preeclamptic subjects (17), may play a role in such vascular dysfunction. Coincidentally, VEGF165 is known from studies in other cell types to close Cx43 gap junctions, and this is considered a normal response to wounding situations (19), resulting in containment of blood flow through suppression of endothelial cell [Ca2+]i responses. We chose to further examine the effects of VEGF165 in intact vessel endothelium in two ways, first examining the action of VEGF165 alone and then if VEGF165 pretreatment could impair subsequent [Ca2+]i bursts in response to ATP. If this inhibition did indeed occur, we proposed the decline in [Ca2+]i bursts should also be accompanied by a drop in NO production in UA Endo preparations ex vivo if pregnancy-adapted [Ca2+]i bursts do indeed underlie the pregnancy-enhanced NO production beyond changes in eNOS expression. Treatment with VEGF165 alone stimulated a modest rise in [Ca2+]i in individual cells of luteal NP- and P-UA Endo, and the response was both a smaller maximum than that produced by ATP alone and did not include any observable repeated [Ca2+]i burst pattern otherwise seen for ATP in P-UA Endo in particular. Of note, this is apparently at odds with our previous report that while VEGF165 stimulates both ERK1/2 phosphorylation and eNOS activation in UAEC, there was no apparent effect of growth factors including VEGF on cellular [Ca2+]i in UAEC in culture. It should also be acknowledged, however, that at that time we imaged individual cells one at a time (4), whereas now we image ∼50–60 cells simultaneously using video imaging. Recent preliminary studies in UAEC using video imaging show that a minority of cells detectably respond to VEGF (<20%) compared with the much higher percentages (as many as 90% or more) to ATP (data not shown). This explains why we could not reliably detect responses when looking one cell at a time since our chances of success were less than 1 in 5 even though previous studies (7) of VEGF165 action using other cell function assays such as phospho-ERK nuclear translocation in UAEC shows a far higher percentage cells actually responding. Clearly, further studies are needed to understand the mechanism behind the effects of VEGF165 on cell [Ca2+]i compared with other signaling pathways. Regardless, the studies herein performed on ex vivo vessels show the pregnancy-enhanced NO production in response to VEGF165 is also observable in intact vessels and is associated with a modest [Ca2+]i response. The data presented in Fig. 6 are the average of the tracings from all cells. It is clear, as noted above, that by comparison with the responses to ATP (Fig. 4), the magnitude of both the [Ca2+]i and the NO response to VEGF165 is small for both luteal NP- and P-UA Endo. Nonetheless, a small magnitude of [Ca2+] response to VEGF165 compared with that for ATP may not explain the modest NO response alone. Close examination of individual cell recordings suggest that while the majority of cells showing an increase in [Ca2+]i also show an increase in NO, there are cells that show detectable NO production in the absence of detectable [Ca2+] elevation in response to VEGF while this is not the case for ATP. Likewise, there are also cells which show clearly detectable increases in [Ca2+]i and yet no correspondingly greater changes in NO. Further studies in UAEC are necessary to dissect out the relationship between VEGF165 effects on [Ca2+]i via VEGFR1 and VEGFR2 and the corresponding effects on NO that we (10) have recently shown in UAEC to be heavily dependent on VEGFR2 activation (10).
In view of the clear difference in the nature of the [Ca2+]i response to VEGF165 and ATP in UA vessels ex vivo, and the smaller magnitude of the NO produced in response to VEGF165, we were able to proceed to treat vessels with VEGF165 for 30 min and thereafter treat with additional ATP for a further 30 min to examine if VEGF165 could inhibit subsequent [Ca2+]i and/or NO responses to ATP. Of note, the initial [Ca2+]i peaks in UA Endo of ewes from both luteal phase and pregnant states were not markedly different from controls. The average sustained phase [Ca2+]i per cell and percentage of cells showing Ca2+ burst responses in UA Endo from luteal phase ewes were marginally effected and NO output was correspondingly reduced. Of greatest significance, however, was the finding that in UA Endo from pregnant ewes, both the average sustained phase [Ca2+]i per cell (Figs. 7 and 8) and percentage of cells showing sustained bursts (Fig. 9) in particular were dramatically impaired, with only half of the cells now showing both the second and third peak compared with control, and both the initial maximum and overall NO production correspondingly reduced all the way down to levels more similar to that of luteal phase ewes. This finding strongly supports the proposal that while the initial [Ca2+]i peak has little bearing on NO output, pregnancy enhancement of sustained phase [Ca2+]i bursts in UA Endo in vivo is indeed causally linked to pregnancy-enhanced NO production over and above any increase in eNOS expression. In addition, the small [Ca2+]i and NO responses attributed to VEGF alone, in terms of both number of cells responding and magnitude of responses, fail to compensate for the degree of inhibition it otherwise infers on the ATP responses by a wide margin. This means that VEGF165 pretreatment can essentially abolish pregnancy-related endothelial signaling adaptation, while providing only a minimal alternative response in return. Whether this VEGF inhibition of ATP-stimulated [Ca2+]i bursts and associated NO production during pregnancy is due to kinase mediated phosphorylation of Cx43, as the wound-healing literature would suggest, will require further study, and such studies are ongoing at this time. Of further and perhaps far greater significance with regard to a possible role for VEGF in preeclampsia, we can alternatively summarize our findings to state that given just 30 min of VEGF165 exposure, a uterine artery that had shown pregnancy-adapted/enhanced endothelial function is transformed in its response to that of a vessel from an unadapted or nonpregnant state, a hallmark of uterine artery dysfunction of preeclamptic subjects. As mentioned above, the dose of 10 ng/ml VEGF165 used herein corresponds to 518 pM and this relates well to the elevated levels reported in preeclamptic subjects but is above that commonly seen in normal pregnancy (12).
Conclusion
We noted above that there are three possibilities that could account for the changes in NO production in different stages of the ovarian cycle vs. pregnancy, namely increased eNOS expression, more sustained [Ca2+]i signaling per cell, and increased recruitment of cells to respond. It is now clear that while an increase in eNOS expression alone is largely sufficient to describe the difference between luteal and follicular phases, increases in all three processes are needed to fully account for the large increase in NO production exclusive to the pregnant state. Our additional finding that simple preexposure of UA Endo to VEGF165 for just 30 min can reverse pregnancy enhancement of [Ca2+]i bursts and brings corresponding NO production down to nonpregnant levels is important in itself in demonstrating a causal link between adapted [Ca2+]i signaling and pregnancy-enhanced NO production over and above that otherwise due to increased eNOS expression. This observation, however, is all the more significant given that long before this laboratory developed the uterine artery endothelial cell model and applied imaging techniques to cells and intact uterine vessels, some of the earliest descriptions of abnormal vascular function in pregnant subjects destined to become preeclamptic was that their vasculature continued to respond to infused pressors the same way as nonpregnant women whereas those subjects with healthy pregnancy showed enhanced vasodilation and resisted the effects of pressors (5). It is still not clear to this day if the preeclamptic women failed to adapt or if the adaptive response was suppressed. It is highly significant that the pretreatment of P-UA Endo ex vivo with VEGF165, a hormone reported to be locally produced at elevated levels by the preeclamptic placenta (17) and placental vasculature VSM (3), reproduces this phenomenon so clearly and resurrects the possibility that VEGF165 and indeed any factor which otherwise impairs pregnancy-enhanced [Ca2+]i signaling in UA Endo may play an active role in suppressing pregnancy adaptation of uterine artery endothelial function (11). Our recent work (22) suggests that Cx43 function may be a key mediator of both sustained [Ca2+]i signaling per cell and recruitment of additional P-UAEC to respond in normal pregnancy. Further mechanistic study of signaling via VEGF receptors expressed in UAEC and their relative roles in direct VEGF165 stimulation of [Ca2+]i and eNOS compared with the longer term blockade of [Ca2+]i bursts subsequently induced by ATP could certainly help identify a mechanistic basis for vascular endothelial dysfunction in preeclamptic subjects. However, is it also true that VEGF is an actual mediator of endothelial dysfunction in preeclampsia? For the field as a whole to move from “possible role” to an actual “proof of a role” for VEGF in the pathology of preeclampsia will require broader study by multiple groups. The work of others on understanding the origin of increased VEGF and sFlt needs to be continued, given that reported VEGF levels are clearly influenced by methods of blood collection, assay specificity and the potential impact of sFlt (12). It is particularly noteworthy that the suggested circulating VEGF levels overall still far exceeds circulating sFlt levels (12). The recent observation that placental vasculature VSM shows higher local levels of VEGF in preeclamptic subjects than in matched normotensive pregnant subjects (3) also implies that local endothelial VEGF exposure may be higher still than that assumed from the general circulation. For our part, areas still to be addressed are the way in which VEGF signals to the cell through VEGFR1 and VEGFR2 respectively both for VEGF acute action and subsequent inhibition of ATP responses, and if the nature of the VEGF inhibition changes with chronic VEGF treatment. If it turns out in UA Endo as in other cells (19) that the initial VEGF inhibition of ATP stimulated Ca2+ bursts is through ERK-1/2 mediated Cx43 phosphorylation, so blocking cell-cell communication, such inhibition of function could certainly be maintained long term. Nonetheless, what is unknown is if further changes in VEGFR1 or VEGFR2 stability at the plasma membrane (i.e., half-life on the cell surface) are also altered with chronic treatment, and if additional long term signaling cross talk could lead to altered coupling of the ERK pathway in a manner that impacts on Cx43 at multiple levels. At this time, it is hard to predict if the observed acute VEGF inhibition of vascular endothelial function would persist long term or if other mechanisms would work to restore Cx43 function.
In closing, we have examined the effects of VEGF alone and on subsequent stimulation with ATP but the UA Endo in reality is under the control of many other endocrine factors and indeed physical forces. The question of whether VEGF can also inhibit responses to these other endocrine or paracrine factors arises, and it is indeed likely that those stimuli working though the same [Ca2+]i signaling pathway synchronized via Cx43 to illicit a prolonged capacitative Ca2+ entry response (as burst or otherwise) would also be inhibited by VEGF exposure. It is less clear if responses to physical forces such as altered sheer associated with pregnancy (18) would also be altered, simply because it is not yet known in UA Endo if shear mediates its otherwise known effects through Ca2+ signaling. One thing is clear, however, and that is that the question of a direct role for VEGF in endothelial dysfunction in preeclampsia subjects is back on the table and demands to be addressed. If correct, then a mechanistic understanding of this process could in turn potentially provide a basis for pharmacologic targeting of specific endothelial therapy for the first time in these subjects.
GRANTS
This work was funded by NIH Grants HL-079020, T32-HD-41921 [to I. M. Boedldt. (Principal Investigator) and D. S. Boeldt (Sponsored Trainee)], HL-49210, HL-87144, and HD-38843 (to R. R. Magness). This work was undertaken in partial fulfillment of the Ph.D. in Endocrinology and Reproductive Physiology of D. S. Boeldt.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Bird IM, Sullivan JA, Di T, Cale JM, Zhang L, Zheng J, Magness RR. Pregnancy-dependent changes in cell signaling underlie changes in differential control of vasodilator production in uterine artery endothelial cells. Endocrinology 141: 1107– 1117, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Bird IM, Zhang L, Magness RR. Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am J Physiol Regul Integr Comp Physiol 284: R245– R258, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Bosco C, Buffet C, Diaz E, Rodrigo R, Morales P, Barja P, Terra R, Parra-Cordero M. VEGF in the muscular layer of placental blood vessels: immuno-expression in preeclampsia and intrauterine growth restriction and its association with the antioxidant status. Cardiovasc Hematol Agents Med Chem 8: 87– 95, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Di T, Sullivan JA, Magness RR, Zhang L, Bird IM. Pregnancy-specific enhancement of agonist-stimulated ERK-1/2 signaling in uterine artery endothelial cells increases Ca(2+) sensitivity of endothelial nitric oxide synthase as well as cytosolic phospholipase A(2). Endocrinology 142: 3014– 3026, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Everett RB, Worley RJ, MacDonald PC, Gant NF. Effect of prostaglandin synthetase inhibitors on pressor response to angiotensin II in human pregnancy. J Clin Endocrinol Metab 46: 1007– 1010, 1978 [DOI] [PubMed] [Google Scholar]
- 6. Gibson TC, Phernetton TM, Wiltbank MC, Magness RR. Development and use of an ovarian synchronization model to study the effects of endogenous estrogen and nitric oxide on uterine blood flow during ovarian cycles in sheep. Biol Reprod 70: 1886– 1894, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Gifford SM, Cale JM, Tsoi S, Magness RR, Bird IM. Pregnancy-specific changes in uterine artery endothelial cell signaling in vivo are both programmed and retained in primary culture. Endocrinology 144: 3639– 3650, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Gifford SM, Yi FX, Bird IM. Pregnancy-enhanced Ca2+ responses to ATP in uterine artery endothelial cells is due to greater capacitative Ca2+ entry rather than altered receptor coupling. J Endocrinol 190: 373– 384, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Gifford SM, Yi FX, Bird IM. Pregnancy-enhanced store-operated Ca2+ channel function in uterine artery endothelial cells is associated with enhanced agonist-specific transient receptor potential channel 3-inositol 1,4,5-trisphosphate receptor 2 interaction. J Endocrinol 190: 385– 395, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Grummer MA, Sullivan JA, Magness RR, Bird IM. Vascular endothelial growth factor acts through novel, pregnancy-enhanced receptor signalling pathways to stimulate endothelial nitric oxide synthase activity in uterine artery endothelial cells. Biochem J 417: 501– 511, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hayman R, Brockelsby J, Kenny L, Baker P. Preeclampsia: the endothelium, circulating factor(s) and vascular endothelial growth factor. J Soc Gynecol Investig 6: 3– 10, 1999 [PubMed] [Google Scholar]
- 12. Hertig A, Liere P. New markers in preeclampsia. Clin Chim Acta 411: 1591– 1595, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Khan LH, Rosenfeld CR, Liu XT, Magness RR. Regulation of the cGMP-cPKG pathway and large-conductance Ca2+-activated K+ channels in uterine arteries during the ovine ovarian cycle. Am J Physiol Endocrinol Metab 298: E222– E228, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Magness RR, Shaw CE, Phernetton TM, Zheng J, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. II. Pregnancy effects on NO synthase expression. Am J Physiol Heart Circ Physiol 272: H1730– H1740, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Magness RR, Sullivan JA, Li Y, Phernetton TM, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. VI .Ovarian and pregnancy effects on eNOS and NO(x). Am J Physiol Heart Circ Physiol 280: H1692– H1698, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol Regul Integr Comp Physiol 272: R441– R463, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, Post M, Caniggia I. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab 90: 4299– 4308, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sprague B, Chesler NC, Magness RR. Shear stress regulation of nitric oxide production in uterine and placental artery endothelial cells: experimental studies and hemodynamic models of shear stresses on endothelial cells. Int J Dev Biol 54: 331– 339, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suarez S, Ballmer-Hofer K. VEGF transiently disrupts gap junctional communication in endothelial cells. J Cell Sci 114: 1229– 1235, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Sullivan JA, Grummer MA, Yi FX, Bird IM. Pregnancy-enhanced endothelial nitric oxide synthase (eNOS) activation in uterine artery endothelial cells shows altered sensitivity to Ca2+, U0126, and wortmannin but not LY294002–evidence that pregnancy adaptation of eNOS activation occurs at multiple levels of cell signaling. Endocrinology 147: 2442– 2457, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Yi FX, Boeldt DS, Bird IM. Pregnancy induced reprogramming of endothelial function in response to ATP: evidence for post receptor Ca2+ signaling plasticity. In: Extracellular ATP and Adensosine as the Regulators of Endothelial Cell Function, edited by Gerasimovskaya E, Kaczmarek E. New York: Springer, 2010, p. 197– 213 [Google Scholar]
- 22. Yi FX, Boeldt DS, Gifford SM, Sullivan JA, Grummer MA, Magness RR, Bird IM. Pregnancy enhances sustained Ca2+ bursts and endothelial nitric oxide synthase activation in ovine uterine artery endothelial cells through increased connexin 43 function. Biol Reprod 82: 66– 75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yi FX, Magness RR, Bird IM. Simultaneous imaging of [Ca2+]i and intracellular NO production in freshly isolated uterine artery endothelial cells: effects of ovarian cycle and pregnancy. Am J Physiol Regul Integr Comp Physiol 288: R140– R148, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Yi FX, Zhang AY, Campbell WB, Zou AP, VanBreemen C, Li PL. Simultaneous in situ monitoring of intracellular Ca2+ and NO in endothelium of coronary arteries. Am J Physiol Heart Circ Physiol 283: H2725– H2732, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Zachary I. VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans 31: 1171– 1177, 2003 [DOI] [PubMed] [Google Scholar]