Abstract
The mechanisms controlling the activity of NADPH oxidase 5 (Nox5) are unique in that they are independent of the protein: protein interactions that coordinate the activation of other Nox isoforms. Instead, the primary driving force for Nox5 activity is calcium. However, in a previous study we reported that the protein kinase C (PKC)-agonist PMA could induce a sustained activation of Nox5 that was independent of calcium changes. This apparent calcium-independent activation was found to be mediated by the PKC-dependent phosphorylation of specific serine and threonine residues on Nox5 which increased the calcium sensitivity of the enzyme and enabled activation at resting levels of calcium. However, the specific kinase(s) mediating the phosphorylation and activation of Nox5 are not known. As PKC can activate the MEK/ERK1/2 signaling pathway, we hypothesized that Nox5 is activated by the coordinated phosphorylation of both MAPK and PKC pathways. The inhibition of MEK1 using PD-98059 and U-0126 significantly reduced the phosphorylation and activity of Nox5 in response to PMA but not to the calcium-mobilizing stimulus ionomycin. Dominant negative MEK1 and knockdown of endogenous MEK1/2 using a specific small interfering RNA also inhibited Nox5 activity in response to PMA. The mutation of S498 to a nonphosphorylatable residue and to a lesser degree T494 blocked the ability of ERK to stimulate Nox5 activity. However, a constitutively active form of MEK1 failed to increase Nox5 activity in the absence of PMA stimulation. These results suggest that the MEK/ERK1/2 pathway is necessary but not sufficient to regulate the PMA-dependent activation of Nox5.
Keywords: reduced nicotinamide adenine dinucleotide phosphate oxidase 5, reactive oxygen species, protein kinase C, mitogen-activated protein kinase
reactive oxygen species (ROS) are important contributors to cellular physiology and participate in intracellular signaling, proliferation, migration, and vascular reactivity (1, 27, 39, 45). The aberrant production of ROS, including superoxide and hydrogen peroxide, is commonly observed in diseases such as cancer, inflammation, hypertension, and diabetes and occurs alongside disturbances in cell and organ function (32). Thus the signaling mechanisms that coordinate the appropriate production of ROS at the right time and location are very important in our understanding of the mechanisms underlying the control of ROS production in physiological and pathophysiological states.
The NADPH oxidases (Nox) comprise a family of proteins that are a major source of ROS production in mammalian cells (7). There are seven related Nox enzymes that have been designated: Nox1–5 and dual oxidase 1 and 2. All are membrane-bound proteins that span the membrane six times. They contain two centrally coordinated nonidentical heme residues and COOH-terminal regions with FAD- and NADPH-binding domains. The catalysis originates with the transfer of electrons from NADPH via a flavin domain to the heme residues and, ultimately, to molecular oxygen to produce superoxide (28). The control of electron flow and thus ROS production are achieved by interactions of Nox subunits with other proteins, phosphorylation, and the elevation of intracellular calcium (7).
Nox2 is the best characterized of the Nox family. It is predominantly expressed in phagocytes such as macrophages and neutrophils (40), and its activity can be regulated by the interactions between the membrane-bound subunit p22phox and several cytoplasmic subunits including p47phox, p40phox, p67phox, and the small G proteins Rac and Rap1a. The production of ROS is initiated by the phosphorylation of p47phox and the subsequent translocation of cytosolic subunits (7, 48). The mechanism for the activation of Nox1 and -3 are analogous and involve both phosphorylation-dependent events and changes in protein: protein interactions (4, 5, 11, 12). However, unlike Nox1–3, both Nox4 and Nox5 do not require cytosolic subunits for their activation. Nox4 has been shown to be a constitutively active (CA) enzyme and is found bound to the integral membrane protein p22phox (35), whereas Nox5 is primarily regulated by calcium (6, 20).
Nox5 was originally described as a gene expressed in testis, spleen, and lymph nodes (6). However, it has been subsequently found to be expressed in other tissues including blood vessels and vascular smooth muscle (23, 25, 36), and the expression of Nox5 is increased in diseased blood vessels (23). When Nox5 is compared with the other Nox isoforms, considerably less is known about the functional significance of Nox5 as it present in the genomes of human and other species but has been lost from rodent genomes (mice and rats), which have become our primary models for experimentation (28). The basic transmembrane structure of Nox5 is similar to that described for the other Nox isoforms, but what distinguishes Nox5 is a unique amino terminus that encodes four calcium-binding EF-hands (6, 7). The elevation of intracellular calcium is sensed by the EF-hands, which then trigger an intramolecular conformational change that facilitates electron flow and superoxide production. While calcium is absolutely required for Nox5 activity, discrepancies between the amount of calcium needed to initiate ROS production versus that measured inside cells led to the discovery by our laboratory and others that the calcium sensitivity of Nox5 can be modified by the specific phosphorylation of serine/threonine residues (24, 44). The activator of protein kinase C (PKC), phorbol 12-myristate 13-acetate (PMA), elicits a robust slow and sustained production of superoxide from Nox5 without modifying cellular calcium levels. This is achieved via increased calcium sensitivity through the phosphorylation of T494 and Ser498 (24). However, the kinase(s) that regulate Nox5 activity are unknown and may not necessarily be PKC isoforms.
While PMA is widely considered to selectively activate PKC isoforms, an important but underappreciated ability of PKC is to further activate members of the mitogen-activated protein kinase (MAPK) family such as extracellular signal-regulated kinase 1 and 2 (ERK1/2) (29, 42). MAPKs are Ser/Thr kinases that are comprised of three major groups: the ERK1/2, the c-Jun NH2-terminal kinase, and p38 MAPK (9). The prototypical MAPK pathway is the ERK1/2 pathway, which is classically activated by the GTPase Ras via a series of MAPKs Raf-MEK1/2-ERK1/2 (9). MAPK pathways have been shown to play important roles in intracellular signaling in response to Nox-derived ROS. For example, in vascular smooth muscle cells (VSMCs) and cardiac myocytes, ROS derived from the angiotensin II stimulation of Nox isoforms causes increased contraction (17, 47) and endothelial dysfunction via MAPK activation (33). Interestingly, the activation of both the ERK1/2 signaling pathway and Nox5 (25) have been reported to promote cellular proliferation. Despite this close relationship between ROS and MAPK, the ability of MAPK pathways to regulate ROS output via a direct effect on Nox enzymes or influence Nox5 activity has not yet been reported. In the present study, we show that MAPK mediates the direct activation and phosphorylation of Nox5 via the MEK1-ERK1/2 pathway.
MATERIALS AND METHODS
Cell culture and transfection.
COS-7 cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) containing l-glutamine, penicillin, streptomycin, and 10% (vol/vol) fetal bovine serum (FBS). COS-7 cells were transfected with Lipofectamine 2000 (Invitrogen). Negative control, MEK1 and MEK2 small interfering RNA (siRNA) were obtained from Qiagen, and HEK293 cells were transfected using Effectene. The siRNA used were experimentally verified sequences obtained from Qiagen (31) and checked for homology to all other sequences of the genome using a nonredundant database and designed with HP OnGuard that provides asymmetry (43), 3′-UTR/seed region analysis (21), single nucleotide polymorphism avoidance, and interferon motif avoidance (26). Human aortic VSMCs (HAVSMCs) were obtained and cultured in SmBM media from Lonza.
DNA constructs.
Hemagglutinin (HA)-Nox5, T494A and S498A Nox5 mutants, and aequorin have been previously described (13, 24). HA-tagged wild-type (WT) MEK1, dominant negative (DN) MEK1 (K97R) CA-MEK1 (S218D), HA-tagged WT and DN-ERK2 (K52R) were generated by PCR. All constructs were verified by bidirectional sequencing.
Immunoprecipitation and immunoblotting.
COS-7 cells were lysed in lysis buffer (4°C) containing 50 mM Tris·HCl (pH 7.4), 100 mM NaF, 15 mM Na4P2O7, 1 mM Na3VO4, 1% vol/vol Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml pepstatin A, and 5 μg/ml aprotinin. Lysates were centrifuged at 10,000 g to concentrate insoluble material. Nox5 was extracted from detergent-resistant microdomains by the addition of 1% SDS and subsequently diluted 1:10 in lysis buffer. Protein extracts were precleared by incubation with protein A/G-agarose for 2 h at 4°C with rocking. Agarose beads were then pelleted by centrifugation at 1,000 g. HA-Nox5 in precleared lysates was immunoprecipitated by incubation with preconjugated agarose: anti-HA antibody overnight at 4°C with rocking. Immunoprecipitated proteins were eluted from the beads by boiling for 5 min in 2× sample buffer. Immunoprecipitates or cell lysates were immunoblotted with various antibodies as detailed within the experimental protocols.
Measurement of ROS.
COS-7 cells were transfected with cDNAs-encoding Nox5 or control plasmids (green fluorescent protein, red fluorescent protein, or lacZ), and 24 h later cells were replated into white tissue culture-treated 96-well plates (ThermoLabsystems) at a density of ∼5 × 104 cells per well. The cells were incubated at 37°C in phenol-free DMEM (Sigma) containing 400 μM of the luminol analog L-012 (Wako) for a minimum of 20 min before the addition of agonists (10, 24). Luminescence was quantified over time using a POLARstar OPTIMA (BMG Labtech). The specificity of L-012 for ROS was confirmed by transfecting cells with a control plasmid such as green fluorescent protein or lacZ or by coincubation of a superoxide scavenger such as Tiron (5 mM). Both of these interventions yielded virtually undetectable levels of luminescence under control-, PMA-, or ionomycin-stimulated conditions (10, 24). Superoxide was also measured using the cytochrome-c assay. COS-7 cells expressing Nox5 were incubated in phenol-free DMEM (Sigma-Aldrich) in the presence and absence of superoxide dismutase (Sigma-Aldrich). Acetylated cytochrome-c was added at a concentration of 1 μg/ml, and 20 min later the supernatants were transferred to a POLARstar OPTIMA (BMG Labtech). Superoxide was quantified as the superoxide dismutase-sensitive increase in absorbance at λ (550–540 nM).
Isolated Nox5 activity assay.
COS-7 cells coexpressing Nox5 and HA-ERK2 were lysed in MOPS (30 mM, pH 7.2, 4°C)-based buffer containing KCl (100 mM), Triton (0.3%), and protease inhibitors (Sigma). Adherent cells were gently rocked, the lysis buffer was aspirated, and the cells were then washed three times with PBS (4°C). The remaining cytoskeletal fractions were resuspended in the above MOPS buffer, sonicated at low power (setting 2, 5 × 1-s bursts, Fisher Scientific Sonic Dismembrator), and spun down at 10,000 g at 4°C. The supernatant was then aspirated and the pellet was resuspended in MOPS buffer with mild sonication (setting 2, 5 × 1-s bursts). The cell-free extract was aliquoted into buffers containing L-012 (400 μM), 1 mM MgCl2, 100 μM FAD (Sigma), and 100 nM of free calcium. The concentration of free calcium was obtained using the molecular probes calcium-calibration kit (K2EGTA, CaEGTA, 100 mM KCl, and 30 mM MOPS, pH 7.2) as previously described (24). After a brief period of equilibration, reduced NADPH (Sigma) was injected to a final concentration of 100 μM and the production of ROS was monitored over time.
Calcium measurements.
Changes in the intracellular calcium concentration in response to either ionomycin or PMA were measured using aequorin in COS-7 cells in response to either ionomycin or PMA. COS-7 cells were transfected with cytosolic aequorin, and 48 h later the aequorin was activated by incubating the cells in Ca2+-free DMEM (Biosource) containing 5 μM coelenterazine (Sigma) for 1 h. The loading media was then replaced with calcium-replete media before cell stimulation (13).
Statistics.
All statistical analyses were performed using Instat software and were made using a two-tailed Student's t-test or ANOVA with a post hoc test where appropriate. Differences are considered significant at P < 0.05.
RESULTS
PMA induces ERK1/2 phosphorylation.
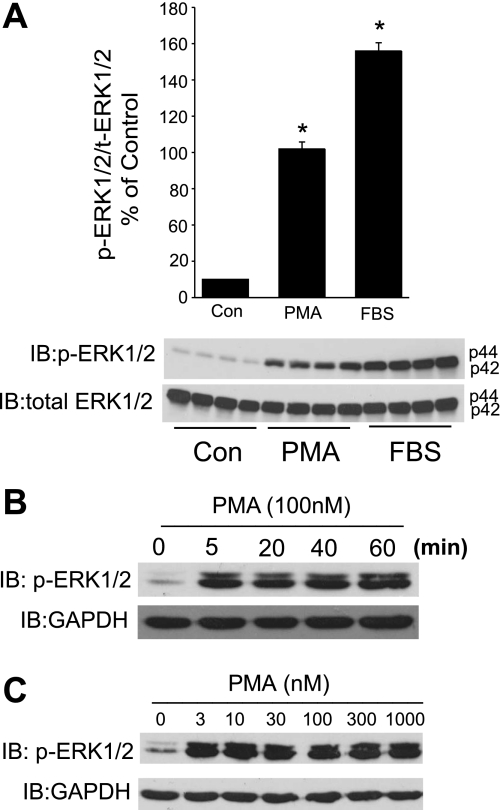
We first investigated whether the activator of PKC, PMA, could influence the phosphorylation of ERK1/2. As shown in Fig. 1A, PMA (100 nM) induced a robust and significant increase in the phosphorylation of ERK1/2 in serum-starved COS-7 cells. FBS (20%) was used as a positive control and also robustly activated ERK1/2. To assess the time course of ERK1/2 activation, COS-7 cells were exposed to PMA for 0, 5, 10, 20, 40, and 60 min. As shown in Fig. 1B, the activation of ERK1/2 occurred rapidly (5 min) and phosphorylation was sustained over 60 min. The concentration of PMA required to activate ERK1/2 was low with 3 nM eliciting near maximal activation (Fig. 1C).
Fig. 1.
PMA activates ERK1/2. A: serum-starved COS-7 cells were stimulated with PMA (100 nM) or FBS (20%) for 20 min, and cell lysates were immunoblotted (IB) for phosphorylated (p) ERK1/2 vs. total (t) ERK1/2 (bottom). A, top: relative densitometry of p-ERK1/2 vs. t-ERK1/2. Results are presented as means ± SE; n = 4 experiments. *P < 0.05. Con, control. B: COS-7 cells were stimulated with PMA (100 nM) for the times indicated. C: COS-7 cells were stimulated with increasing concentrations of PMA (0–1,000 nM). Blots are representative of 3 separate experiments.
Activation of MEK1 and ERK1/2 is necessary for the PMA-dependent activation of Nox5.
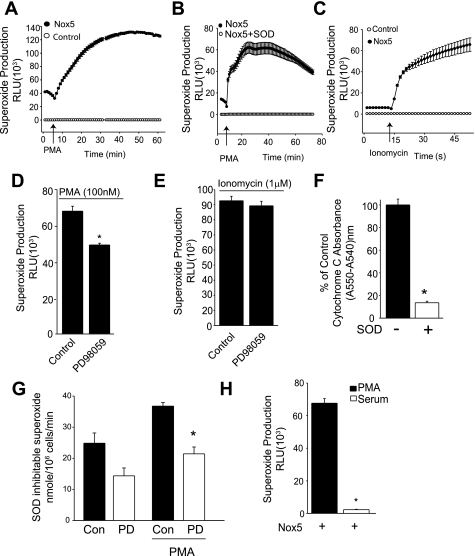
To determine whether ERK1/2 participates in the activation of Nox5 by PMA, we next measured superoxide production in COS-7 cells expressing Nox5 using L-012. As shown in Fig. 2A, PMA induced a large and sustained increase in superoxide production that was not present in cells expressing a control plasmid (lacZ) instead of Nox5. The PMA-dependent increase in superoxide was also completely reversed with supplemental superoxide dismutase (Fig. 2B). Increased superoxide production in response to the calcium ionophore, ionomycin, was also observed only in cells expressing Nox5 (Fig. 2C) and absent in control-transfected cells. To assess the role of MEK-ERK pathway in PMA-dependent activation of Nox5, COS-7 cells expressing Nox5 were stimulated with PMA (100 nM) in the presence and absence of a pharmacological inhibitor of MEK1 (intermediary kinase in MAPK pathway, 20 μM PD-98059). As shown in Fig. 2D, the MEK1 inhibitor significantly reduced superoxide production from Nox5, suggesting that ERK1/2 participates in Nox5 phosphorylation and superoxide production. To determine the specificity of PD-98059 for PMA-dependent activation, we next assessed whether it influences the response to ionomycin, which activates Nox5 exclusively via the elevation of calcium and does not induce a change in its phosphorylation state (24). As shown in Fig. 2E, PD-98059 did not affect ionomycin-stimulated superoxide production. Equivalent results were obtained with the structurally dissimilar MEK1/2 inhibitor U-0126 (10 μM, data not shown). To confirm these results using a different method to measure superoxide levels, we next employed the cytochrome-c assay. The specificity of this assay is shown in Fig. 2F, where the increased absorbance of cytochrome-c in COS-7 cells expressing Nox5 is reversed by the presence of supplemental superoxide dismutase. The pretreatment of cells with PD-98059 significantly attenuated the PMA-dependent increase in superoxide production as determined using cytochrome-c reduction (Fig. 2G). In Fig. 1A, we showed that FBS was more efficacious than PMA in stimulating the phosphorylation of ERK1/2. To assess whether FBS can activate Nox5, COS-7 cells were transfected with Nox5 and then serum starved (0.1% FBS) for 12 h. Cells were then exposed to 20% FBS, and superoxide was measured using L-012. As shown in Fig. 2H, PMA, but not FBS, was able to activate Nox5.
Fig. 2.
Inhibition of MEK1/2 reduces PMA, but not ionomycin stimulated superoxide release from NADPH oxidase 5 (Nox5). A: superoxide release from COS-7 cells transfected with hemagglutinin (HA)-Nox5 or a control plasmid (lacZ) in response to PMA (100 nM). RLU, relative light units. B: superoxide release from COS-7 cells expressing Nox5 in the presence and absence of SOD (100 U/ml). C: superoxide from COS-7 cells expressing Nox5 or a control plasmid (lacZ) in response to ionomycin (1 μM). COS-7 cells expressing HA-Nox5 were stimulated with PMA (100 nM; D) or ionomycin (1 μM; E) in the presence and absence of the MEK inhibitor PD-98059 (20 μM). Superoxide in A–E was measured with L-012. F: superoxide release from COS-7 cells transfected with Nox5 was measured using the reduction of cytochrome-c in the presence and absence of SOD (100 U/ml). G: superoxide release was measured from control and PMA-stimulated COS-7 cells expressing Nox5 using cytochrome-c. Cells were preincubated with vehicle (Con) or PD-98059 (PD, 20 μM). H: detection of superoxide using L-012 in COS-7 cells expressing HA-Nox5 in response to PMA (100 nM) or FBS (20%). Results are presented as means ± SE; n = 4–8 experiments. *P < 0.05 vs. control.
Active MEK1 is necessary but not sufficient for the PMA-dependent phosphorylation and activation of Nox5.
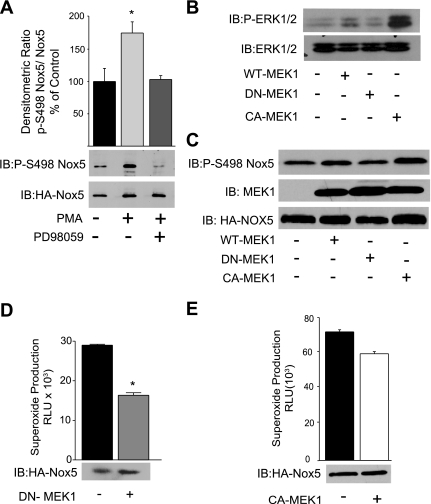
We next assessed whether the pharmacological inhibition of MEK1/2 with PD-98059 modulates the phosphorylation of Nox5 at the activating phosphorylation site S498. As shown in Fig. 3A, PMA robustly phosphorylated Nox5, and this was significantly attenuated in cells pretreated with the MEK1 inhibitor PD-980589.
Fig. 3.
MEK1 is necessary, but not sufficient, for Nox5 phosphorylation and activation. A: COS-7 cells expressing HA-Nox5 were stimulated with PMA (100 nM) in the presence and absence of the MEK inhibitor PD-98059 (20 μM), and Nox5 was immunoprecipitated and immunoblotted for p-S498 vs. t-Nox5 (bottom). A, top: densitometric analysis of p-Nox5 (S498) vs. t-Nox5. B: COS-7 cells were cotransfected with HA-Nox5 and either control (LacZ), wild-type (WT)-MEK1, dominant negative (DN)-MEK1 (K97R), or constitutively active (CA)-MEK1 (S218D). Cell lysates were immunoblotted for p-ERK1/2 and t-ERK1/2 or p-S498 Nox5, MEK1, and t-Nox5 (C). D: superoxide release was measured from cells expressing HA-Nox5 and DN-MEK1 using L-012 in the presence of PMA (100 nM). E: superoxide release from cells expressing HA-Nox5 and CA-MEK1. Results are presented as means ± SE; n = 4–6 experiments. *P < 0.05 vs. control.
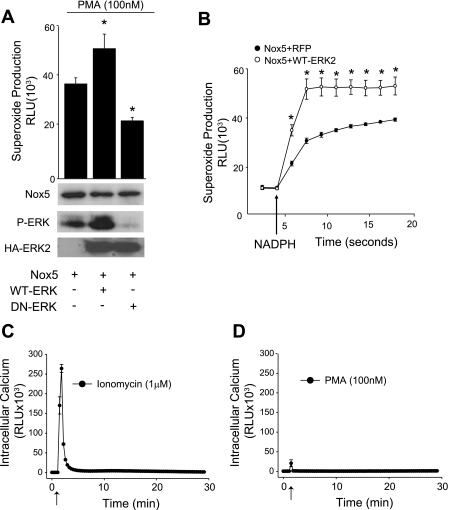
As pharmacological inhibitors may have unintended nonspecific effects, we next generated WT, DN (K97R), and CA-MEK1 (S218D). These mutants were cotransfected together with Nox5 in COS-7 cells. We first confirmed the effectiveness of DN and CA mutations of MEK1 by Western blot analysis for phosphorylated ERK1/2. As shown in Fig. 3B, the expression of CA-MEK1 greatly increased ERK1/2 phosphorylation, WT-MEK1 slightly increased ERK1/2 phosphorylation, and DN-MEK1 did not modify basal ERK1/2 phosphorylation. Similar results were found with regard to the ability of MEK1 mutants to alter the PMA-dependent phosphorylation of Nox5 on S498 with the CA-MEK1 increasing the phosphorylation of Nox5 to the greatest extent, and no change was observed from cells cotransfected with the DN-MEK1 (Fig. 3C). To assess the effects of these mutants on Nox5 activity, we measured superoxide in COS-7 cells coexpressing DN-MEK1 or CA-MEK1 with Nox5. As shown in Fig. 3D, DN-MEK1 significantly reduced PMA-stimulated superoxide production. In contrast, the coexpression of CA-MEK1 did not increase basal or unstimulated Nox5 activity (Fig. 3E), suggesting that active MEK1 alone is not sufficient to activate Nox5.
Endogenous MEK is necessary for PMA-stimulated Nox5 activity.
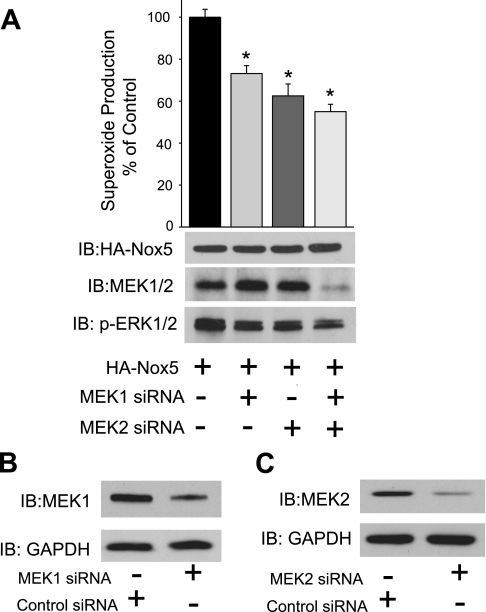
After observing a decrease in Nox5 activity due to the inhibition of MEK1 activity using a DN construct, we next wanted to determine whether the knockdown of endogenous MEK1 and MEK2 would support this finding. To achieve this, we cotransfected HEK293 cells with siRNA targeting MEK1 or MEK2 or a combination of MEK1 and MEK2-specific siRNA together with Nox5. The inhibition of MEK1 and MEK2, both separately and together, reduced the PMA-dependent increase in superoxide production from Nox5 and the phosphorylation of ERK1/2 (Fig. 4A). Interestingly, the combination of MEK1 and MEK2 siRNA was necessary to show a reduction in the total MEK expression using an antibody that recognizes both MEK1 and MEK2 isoforms. To show the effectiveness of MEK1 and MEK2 siRNA, we reprobed cell lysates using antibodies that selectively recognize either MEK1 or MEK2. As shown in Fig. 4, B and C, siRNA to MEK1 and MEK2 reduced the expression of the respective isoforms.
Fig. 4.
Silencing of MEK1 and MEK2 antagonizes PMA-dependent phosphorylation of ERK1/2 and the activation of Nox5. HEK293 cells were cotransfected with HA-Nox5 and either a control (nontargeting) small interfering RNA (siRNA) or siRNA selective for MEK1 or MEK2 and a combination of both MEK1 and MEK2 siRNA, and 48 h later cells were stimulated with PMA (100 nM). A: PMA-stimulated superoxide release was monitored using L-012 and cell lysates probed for t-Nox5, combined MEK1/MEK2, p-ERK1/2, and t-ERK1/2 (bottom). B: HEK293 cells were transfected with siRNA selective for MEK1, and lysates were immunoblotted for MEK1 vs. GAPDH. C: HEK293 cells were transfected with siRNA selective for MEK2, and lysates were immunoblotted for MEK2 vs. GAPDH. Results are presented as means ± SE; n = 6 experiments. *P < 0.05 vs. siRNA control.
ERK2 directly influences Nox5 activity.
Thus far, our results support a role for MEK1 in regulating Nox5 activity. Therefore, our next goal was to establish whether ERK, the terminal MAPK in the MAPK signaling cascade, could directly influence Nox5 activity. To achieve this we first cotransfected COS-7 cells with Nox5 and either WT-ERK2 or a DN-ERK2 and measured Nox5-dependent superoxide production. As shown in Fig. 5A, we observed a significant increase in Nox5 activity in cells expressing the WT-ERK2, whereas the DN-ERK2 significantly reduced Nox5-dependent superoxide production in response to PMA. We also observed increased ERK1/2 phosphorylation in cells expressing the WT-ERK2, whereas the DN-ERK2 significantly reduced ERK1/2 phosphorylation (Fig. 5A, bottom). To determine whether ERK can directly modify Nox5 activity, we next conducted an isolated Nox5 activity assay in which Nox5 was extracted from COS-7 cells expressing either Nox5 and a control cDNA (lacZ) or Nox5 and ERK2. Nox5 was then activated in the presence of a low concentration of calcium, NADPH, and FAD to produce superoxide (10, 24). As shown in Fig. 5B, ERK2 significantly increased Nox5 activity in a cell-free system, indicating that the ability of ERK to alter Nox5 activity is likely to be a result of a direct modification to Nox5 rather than the indirect alteration of other cytoplasmic proteins or secondary changes in the levels of Nox substrates or cofactors. To determine whether the level of intracellular calcium has any role in the PMA-dependent activation of Nox5, we expressed the calcium-sensitive photoprotein aequorin in COS-7 cells and measured intracellular calcium in response to PMA or ionomycin. As shown in Fig. 5, C and D, ionomycin triggered a robust mobilization of intracellular calcium, whereas PMA produced a very small calcium transient that was not distinguishable from the injection artifact (data not shown). This suggests that PMA- and ERK1/2-mediated activation of Nox5 occurs independently of changes in the intracellular calcium concentration.
Fig. 5.
ERK potentiates PMA-dependent Nox5 activity in both intact cells and isolated activity assays but does not modify intracellular calcium levels. A: COS-7 cells were cotransfected with HA-Nox5 and either control (lacZ), WT-ERK2, or DN-ERK2 (K52R), and superoxide release was measured in response to PMA (100 nM). B: superoxide release from Nox5 in an isolated activity assay. Nox5 was extracted from detergent resistant microdomains of cells cotransfected with HA-Nox5 and a control (LacZ) or WT-ERK2 and exposed to PMA. RFP, red fluorescent protein. Nox5 was incubated in a buffer containing 100 nM CaCl2, 100 μM FAD, and superoxide, initiated by injection of 100 μM NADPH (indicated by arrow). C and D: measurement of intracellular calcium in aequorin-transfected COS-7 cells in response to ionomycin (1 μM; C, initiation indicated by arrow) and PMA (100 nM; D, initiation indicated by arrow) over the times indicated. Results are presented as means ± SE; n = 4–6 experiments. *P < 0.05 vs. lacZ control.
ERK2 activates Nox5 via S498 phosphorylation.
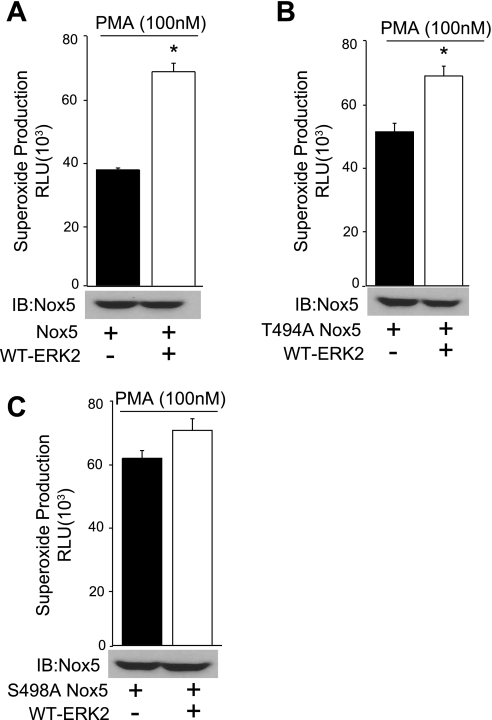
Previously, we have shown that PMA-dependent activation of Nox5 occurs via the increased phosphorylation of S498 and T494. To determine whether these residues are important in the activation of Nox5 by ERK, we expressed Nox5 S498A and T494A, which contain a mutation of the individual phosphorylation sites to the nonphosphorylatable residue alanine. We then cotransfected COS-7 cells with either WT Nox5 or the Nox5 mutants (S498A or T494A) and ERK2. As shown in Fig. 6, A–C, the mutation of S498 to alanine virtually abolished ERK2-mediated increases in Nox5 activity, whereas the mutation of T494 to alanine had a minimal effect. The data suggest that ERK2 promotes the phosphorylation of Nox5 on S498 and increases its activity.
Fig. 6.
Mutation of S498A prevents ERK-dependent increases in Nox5 activity. COS-7 cells were cotransfected with either WT HA-Nox5 (A) or S498A (B) or T494A (C) mutants together with WT-ERK2 or control DNA (lacZ), and superoxide was measured in response to PMA (100 nM). Results are presented as means ± SE; n = 4 to 5 experiments. *P < 0.05 vs. lacZ control.
The MEK-ERK pathway contributes to PMA-dependent superoxide production in human VSMCs.
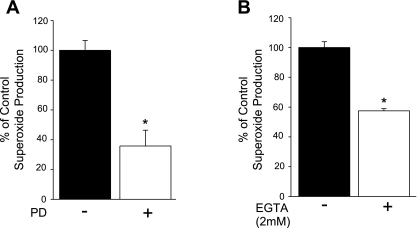
To determine the physiological significance of the MEK-ERK in a cell type that natively expresses Nox5, we next measured superoxide production in response to PMA in HAVSMCs in the presence and absence of the MEK inhibitor PD-98059. We found that the inhibition of MEK1/2 reduced the PMA-dependent superoxide production (Fig. 7A). To determine the contribution of Nox5 to superoxide production in human aortic VSMCs, we exposed cells to the calcium chelator EGTA. As shown in Fig. 7B, EGTA significantly reduced superoxide release from VSMCs.
Fig. 7.
PMA-stimulated superoxide release from human vascular smooth muscle cells (VSMCs) is inhibited by MEK1/2 inhibitors and chelation of calcium. A: VSMCs were treated with PMA (100 nM) in the presence or absence of PD-98059 (20 μM), and superoxide was measured using L-012. B: VSMCs were treated with PMA (100 nM) in the presence or absence of EGTA (2 mM), and superoxide levels were measured using L-012. Results are presented as means ± SE; n = 5 experiments. *P < 0.05 vs. control.
DISCUSSION
The major findings of this study are that the ability of PMA to stimulate increased Nox5 activity does not exclusively involve the participation of PKC isoforms. We found that the inhibition of ERK1/2 signaling using a number of complementary approaches including a pharmacological inhibitor, DN-MEK1, siRNA to MEK1, and a DN-ERK2 all reduced the ability of Nox5 to produce superoxide in response to PMA. The inhibition of ERK1/2 signaling prevented the phosphorylation of Nox5 on S498, and the mutation of this residue prevented ERK2 from stimulating Nox5 activity. Collectively, these results suggest that PKC can activate Nox5, at least in part, via the ERK2-dependent phosphorylation of S498.
The molecular regulation of Nox5 was originally thought to be unique in its simplicity and unitary dependence on intracellular calcium. This was largely based on a comparison to the more complex regulation of the other Nox isoforms that depend on the interactions of numerous subunits and phosphorylation-dependent events for superoxide generation. However, we have since found that the regulation of Nox5 is not quite as simple as first thought. The ability of PMA to increase Nox5 activity without elevating intracellular calcium led to the discovery of PMA-dependent phosphorylation and calcium sensitization. Because PMA is an established agonist for PKC activation, it was reasonable to assume that PKC might be involved in the direct phosphorylation of Nox5. Evidence to support this is derived from the ability of the pharmacological inhibitors of PKC to significantly attenuate PMA-dependent increases in the phosphorylation of Nox5 and reduce superoxide production (24, 44). However, these inhibitors may not be entirely specific for PKC and cannot discriminate between its multiple isoforms or determine whether PKC operates through a secondary kinase cascade such as MEK/ERK to directly modulate Nox5 activity.
Indeed, in our study, we found that PMA robustly stimulates the phosphorylation of ERK1/2 and that the MEK1/2 inhibitor PD-98059 and DN-MEK1 and MEK1 siRNA significantly reduce the PMA-dependent phosphorylation of S498 and the activity of Nox5. All of these findings suggest that the participation of ERK1/2 is necessary for Nox5 activation. However, we also found that a CA-MEK1 failed to activate Nox5, suggesting that this pathway, while necessary, is not sufficient to drive Nox5 activity in the absence of additional stimuli. In a previous study, we found that the ability of PMA to stimulate Nox5 activity could not be fully blocked by the mutation of a single PKC phosphorylation site and that the mutation of greater than two sites were required to fully suppress PMA-stimulated activity (24). Therefore, it is possible that the ERK1/2-dependent phosphorylation of S498 requires the cooperative phosphorylation of other sites on Nox5 to fully activate the enzyme, and this explains the inability of a CA-MEK1 to increase Nox5 activity in the absence of other stimuli. In agreement with this hypothesis, the single mutation of S498 to the nonphosphorylatable analog, alanine, nearly abolishes ERK2-dependent increases in Nox5 activity. In contrast, the mutation of T494 had a significantly reduced effect, and yet both sites are required for the PMA-dependent increase in Nox5 activity (24). The data suggest that S498 is the primary site regulated by ERK1/2 and that its phosphorylation is necessary, but not sufficient, to elicit changes in Nox5 activity.
Previous studies have shown that MAPKs can phosphorylate the regulatory subunits of other Nox enzymes and influence ROS output (3, 14, 18, 19, 46). The regulatory subunit p47phox, which can influence the activities of Nox1, 2, and 3, has been shown to be phosphorylated by ERK1/2 on S345, and this modification promotes increased Nox2 activity (16). Other Nox regulatory subunits that can be targeted by ERK include Nox organizer 1 and Nox activator 1, which regulate the activities of both Nox1 and Nox3 (30, 37). These effects are not limited to ERK, and other MAPKs such as p38 MAPK can also influence p47phox phosphorylation and Nox2 activity (16). However, the direct phosphorylation of Nox enzyme units by ERK has not yet been reported, and Nox5 may be the first example of a direct regulation. In addition to COS-7 cells, we found that the superoxide release from HAVSMCs, which express Nox5 (6, 23, 25), was also sensitive to the inhibition of MEK1/2. The ability of reduced calcium levels to suppress Nox5 activity also supports a role for Nox5 in the PMA-dependent release of superoxide and is consistent with findings obtained using angiotensin II and endothelin-1 stimulation of VSMCs (36).
A close relationship exists between ROS and MAPK signaling, and elevated ROS, particularly in the form of hydrogen peroxide, can promote increased ERK activity (2, 8, 22, 41, 49). Nox-generated ROS have been shown to activate the redox-sensitive transcription factor Nrf2, which is abolished by inhibitors of ERK1/2 signaling (38). Conversely, antioxidants have been shown to reverse ERK activation in response to growth factor signaling (15, 50). The mechanism of ERK activation by ROS is not fully understood, but it is likely to be an indirect target of altered upstream phosphatase and kinase activity (34).
In conclusion, the current work is, to the best of our knowledge, the first to demonstrate that MAPK signaling can directly influence the phosphorylation state of a transmembrane Nox enzyme and also that it contributes to the activation of Nox5 in response to PMA. The mechanism by which this occurs is via the increased phosphorylation of S498 and to a lesser extent the phosphorylation of T494, and these events promote greater activation of the enzyme at resting levels of calcium. The elevated production of ROS, which is known to occur in pathological states such as cardiovascular disease, may activate the MAPK/ERK1/2 pathway and thus promote Nox activation via the phosphorylation of p47phox or through the direct phosphorylation of Nox5, leading to greater ROS production, and thus may form an aberrant feed-forward cycle that contributes to the excessive production of ROS.
GRANTS
This work was supported in part by the Cardiovascular Discovery Institute at the Medical College of Georgia, by National Heart, Lung, and Blood Institute Grants HL-085827 and HL-092446, and by an American Heart Association established investigator award (to D. J. R. Fulton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Abid MR, Kachra Z, Spokes KC, Aird WC. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett 486: 252– 256, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Adderley SR, Fitzgerald DJ. Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J Biol Chem 274: 5038– 5046, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Avdi NJ, Winston BW, Russel M, Young SK, Johnson GL, Worthen GS. Activation of MEKK by formyl-methionyl-leucyl-phenylalanine in human neutrophils. Mapping pathways for mitogen-activated protein kinase activation. J Biol Chem 271: 33598– 33606, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem 278: 3510– 3513, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem 279: 46065– 46072, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, Krause KH. A Ca2+-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem 276: 37594– 37601, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245– 313, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Bhat NR, Zhang P. Hydrogen peroxide activation of multiple mitogen-activated protein kinases in an oligodendrocyte cell line: role of extracellular signal-regulated kinase in hydrogen peroxide-induced cell death. J Neurochem 72: 112– 119, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 410: 37– 40, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Chen F, Fulton DJ. An inhibitor of protein arginine methyltransferases, 7,7′-carbonylbis(azanediyl)bis-4-hydroxynaphthalene-2-sulfonic acid (AMI-1), is a potent scavenger of NADPH-oxidase-derived superoxide. Mol Pharmacol 77: 280– 287, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269: 131– 140, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Cheng G, Ritsick D, Lambeth JD. Nox3 regulation by NOXO1, p47phox, and p67phox. J Biol Chem 279: 34250– 34255, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Church JE, Fulton D. Differences in eNOS activity because of subcellular localization are dictated by phosphorylation state rather than the local calcium environment. J Biol Chem 281: 1477– 1488, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Clerk A, Michael A, Sugden PH. Stimulation of multiple mitogen-activated protein kinase sub-families by oxidative stress and phosphorylation of the small heat shock protein, HSP25/27, in neonatal ventricular myocytes. Biochem J 333: 581– 589, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Colavitti R, Pani G, Bedogni B, Anzevino R, Borrello S, Waltenberger J, Galeotti T. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J Biol Chem 277: 3101– 3108, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Dang PM, Stensballe A, Boussetta T, Raad H, Dewas C, Kroviarski Y, Hayem G, Jensen ON, Gougerot-Pocidalo MA, El-Benna J. A specific p47phox-serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J Clin Invest 116: 2033– 2043, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ding L, Chapman A, Boyd R, Wang HD. ERK activation contributes to regulation of spontaneous contractile tone via superoxide anion in isolated rat aorta of angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 292: H2997– H3005, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Downey GP, Butler JR, Tapper H, Fialkow L, Saltiel AR, Rubin BB, Grinstein S. Importance of MEK in neutrophil microbicidal responsiveness. J Immunol 160: 434– 443, 1998 [PubMed] [Google Scholar]
- 19. El Benna J, Han J, Park JW, Schmid E, Ulevitch RJ, Babior BM. Activation of p38 in stimulated human neutrophils: phosphorylation of the oxidase component p47phox by p38 and ERK but not by JNK. Arch Biochem Biophys 334: 395– 400, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Fulton DJ. Nox5 and the regulation of cellular function. Antioxid Redox Signal 11: 2443– 2452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 27: 91– 105, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem 271: 4138– 4142, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, Harrison DG. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol 52: 1803– 1809, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jagnandan D, Church JE, Banfi B, Stuehr DJ, Marrero MB, Fulton DJ. Novel mechanism of activation of NADPH oxidase 5. calcium sensitization via phosphorylation. J Biol Chem 282: 6494– 6507, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Jay DB, Papaharalambus CA, Seidel-Rogol B, Dikalova AE, Lassegue B, Griendling KK. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic Biol Med 45: 329– 335, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol 23: 457– 462, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal 11: 1– 14, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Kawahara T, Quinn MT, Lambeth JD. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol 7: 109, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marme D, Rapp UR. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature 364: 249– 252, 1993 [DOI] [PubMed] [Google Scholar]
- 30. Kroviarski Y, Debbabi M, Bachoual R, Perianin A, Gougerot-Pocidalo MA, El-Benna J, Dang PM. Phosphorylation of NADPH oxidase activator 1 (NOXA1) on serine 282 by MAP kinases and on serine 172 by protein kinase C and protein kinase A prevents NOX1 hyperactivation. FASEB J 24: 2077– 2092, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Krueger U, Bergauer T, Kaufmann B, Wolter I, Pilk S, Heider-Fabian M, Kirch S, Artz-Oppitz C, Isselhorst M, Konrad J. Insights into effective RNAi gained from large-scale siRNA validation screening. Oligonucleotides 17: 237– 250, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med 43: 332– 347, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Laplante MA, Wu R, El Midaoui A, de Champlain J. NAD(P)H oxidase activation by angiotensin II is dependent on p42/44 ERK-MAPK pathway activation in rat's vascular smooth muscle cells. J Hypertens 21: 927– 936, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Lee MY, San Martin A, Mehta PK, Dikalova AE, Garrido AM, Datla SR, Lyons E, Krause KH, Banfi B, Lambeth JD, Lassegue B, Griendling KK. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol 29: 480– 487, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69– 82, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Montezano AC, Burger D, Paravicini TM, Chignalia AZ, Yusuf H, Almasri M, He Y, Callera GE, He G, Krause KH, Lambeth D, Quinn MT, Touyz RM. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ Res 106: 1363– 1373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oh H, Jung HY, Kim J, Bae YS. Phosphorylation of serine282 in NADPH oxidase activator 1 by Erk desensitizes EGF-induced ROS generation. Biochem Biophys Res Commun 394: 691– 696, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Papaiahgari S, Kleeberger SR, Cho HY, Kalvakolanu DV, Reddy SP. NADPH oxidase and ERK signaling regulates hyperoxia-induced Nrf2-ARE transcriptional response in pulmonary epithelial cells. J Biol Chem 279: 42302– 42312, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Ritsick DR, Edens WA, Finnerty V, Lambeth JD. Nox regulation of smooth muscle contraction. Free Radic Biol Med 43: 31– 38, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rotrosen D, Yeung CL, Leto TL, Malech HL, Kwong CH. Cytochrome b558: the flavin-binding component of the phagocyte NADPH oxidase. Science 256: 1459– 1462, 1992 [DOI] [PubMed] [Google Scholar]
- 41. Sano M, Fukuda K, Sato T, Kawaguchi H, Suematsu M, Matsuda S, Koyasu S, Matsui H, Yamauchi-Takihara K, Harada M, Saito Y, Ogawa S. ERK and p38 MAPK, but not NF-kappaB, are critically involved in reactive oxygen species-mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circ Res 89: 661– 669, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Schonwasser DC, Marais RM, Marshall CJ, Parker PJ. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol 18: 790– 798, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schwarz DS, Hutvagner G, Du T, Xu Z, Aronin N, Zamore PD. Asymmetry in the assembly of the RNAi enzyme complex. Cell 115: 199– 208, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Serrander L, Jaquet V, Bedard K, Plastre O, Hartley O, Arnaudeau S, Demaurex N, Schlegel W, Krause KH. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie 89: 1159– 1167, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature 401: 79– 82, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Tardif M, Rabiet MJ, Christophe T, Milcent MD, Boulay F. Isolation and characterization of a variant HL60 cell line defective in the activation of the NADPH oxidase by phorbol myristate acetate. J Immunol 161: 6885– 6895, 1998 [PubMed] [Google Scholar]
- 47. Touyz RM, He G, Deng LY, Schiffrin EL. Role of extracellular signal-regulated kinases in angiotensin II-stimulated contraction of smooth muscle cells from human resistance arteries. Circulation 99: 392– 399, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci 59: 1428– 1459, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J 333: 291– 300, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wung BS, Cheng JJ, Chao YJ, Hsieh HJ, Wang DL. Modulation of Ras/Raf/extracellular signal-regulated kinase pathway by reactive oxygen species is involved in cyclic strain-induced early growth response-1 gene expression in endothelial cells. Circ Res 84: 804– 812, 1999 [DOI] [PubMed] [Google Scholar]