Abstract
The second window of ischemic preconditioning (SWOP) provides maximal protection against ischemia through regulation of the inducible nitric oxide synthase (iNOS), yet its application is limited by the inconvenience of the preliminary ischemic stimulus required for prophylaxis. Overexpression of H11 kinase/Hsp22 (Hsp22) in a transgenic mouse model provides cardioprotection against ischemia that is equivalent to that conferred by SWOP. We hypothesized that short-term, prophylactic overexpression of Hsp22 would offer an alternative to SWOP in reducing ischemic damage through a nitric oxide (NO)-dependent mechanism. Adeno-mediated overexpression of Hsp22 was achieved in the area at risk of the left circumflex (Cx) coronary artery in chronically instrumented swine and compared with LacZ controls (n = 5/group). Hsp22-injected myocardium showed an average fourfold increase in Hsp22 protein expression compared with controls and a doubling in iNOS expression (both P < 0.05). Four days after ischemia-reperfusion, regional wall thickening was reduced by 58 ± 2% in the Hsp22 group vs. 82 ± 7% in the LacZ group, and Hsp22 reduced infarct size by 40% (both P < 0.05 vs. LacZ). Treatment with the NOS inhibitor NG-nitro-l-arginine (l-NNA) before ischemia suppressed the protection induced by Hsp22. In isolated cardiomyocytes, Hsp22 increased iNOS expression through the transcription factors NF-κB and STAT, the same effectors activated by SWOP, and reduced by 60% H2O2-mediated apoptosis, which was also abolished by NOS inhibitors. Therefore, short-term, prophylactic conditioning by Hsp22 provides NO-dependent cardioprotection that reproduces the signaling of SWOP, placing Hsp22 as a potential alternative for preemptive treatment of myocardial ischemia.
Keywords: gene delivery, myocardial ischemia, preconditioning
although angioplasty and thrombolytic therapy have reduced the mortality of acute ischemic heart disease, they do not prevent the deterioration of cardiac function after myocardial infarction (MI) that eventually leads to heart failure. Despite the progress in stem cell research, the only alternative approach to restore impaired cardiac function due to MI to date is cardiac transplantation, which is severely limited by donor availability. A promising concept is preemptive conditioning of the heart (32) [or prophylactic cardioprotection (6)], in which the activation of prosurvival pathways in patients at risk of future heart attack might prevent cell death if an episode of potentially lethal ischemia occurs. In this study, we tested the hypothesis that H11 kinase/Hsp22 (Hsp22) represents a candidate for such an approach.
Hsp22, which belongs to the crystallin family of small heat shock proteins (24), shows an expression pattern that is restricted to a limited number of tissues, including heart and skeletal muscle (8, 24, 28). We found that Hsp22 mRNA and protein expression are upregulated in a swine model of myocardial stunning (11) and in a swine model of repetitive ischemia reproducing myocardial hibernation (9). Using tissue samples obtained at the time of bypass surgery, we found that a similar adaptation occurs in human hibernating myocardium (9). These data support the hypothesis that Hsp22 may promote cell survival in a context of acute, repetitive, and chronic ischemia. A transgenic (TG) mouse with cardiac-specific overexpression of Hsp22 showed a reduction in infarct size (IS) that was quantitatively equivalent to the cardioprotection conferred by ischemic preconditioning (12), the “gold standard” method of IS reduction (34), together with an upregulation of the inducible isoform of nitric oxide synthase (iNOS) (11), the mediator of the second window of preconditioning (SWOP) (20, 30, 33). Despite its powerful prophylactic effect, the clinical relevance of SWOP is limited by the requirement of short and repetitive episodes of ischemia-reperfusion needed to provide the preconditioning stimulus. For this reason, Hsp22 might represent a potential biological alternative. Although the TG model is useful to study the molecular mechanisms involved, it would be more clinically relevant if short-term, preemptive conditioning of the heart by Hsp22 could provide cardioprotection. Accordingly, the goal of the present investigation was to test in a swine model the hypothesis that short-term overexpression of Hsp22 reduces myocardial damage and improves regional contractile function after coronary artery occlusion and reperfusion through signaling mechanisms that reproduce those activated by SWOP.
MATERIALS AND METHODS
Swine model.
Domestic swine (22–25 kg) were sedated with ketamine (10–20 mg/kg im) and xylazine (2.2 mg/kg im), and general anesthesia was maintained with isoflurane (0.5–2.0 vol%). Left thoracotomy was performed in the fifth intercostal space. A hydraulic occluder was implanted around the left circumflex (Cx) coronary artery (11). Two catheters were implanted in the aorta and left atrium for pressure measurements and for microsphere injection (11). A miniature pressure transducer (Konigsberg) was implanted in the left ventricle (LV) via the apex to measure LV pressure and LV change in pressure with time (dP/dt) (11). Two pairs of ultrasound crystals were placed in the posterior (potentially ischemic area) and anterior (remote area) wall to measure regional myocardial function. The adenovirus harboring the sequence encoding Hsp22 (8) or the LacZ control was diluted in 1 ml of PBS at a concentration of 1010 CFU/ml. Aliquots of 1 ml were administered directly in the area at risk (AAR) by multiple injections (20 sites) with a 30-gauge needle. Animals used in this study were maintained in accordance with the Guide for the Care and Use of Laboratory Animals and protocols approved by the Institutional Animal Care and Use Committee of the University of Medicine and Dentistry of New Jersey.
The experiments were initiated 3–4 days after surgery. Hemodynamics and LV function recordings were made with a Notocord acquisition system and a multiple-channel oscillograph. Aortic and left atrial pressures were measured with strain gauge manometers. LV dP/dt was obtained by electronically differentiating the LV pressure signal (Triton). Regional myocardial wall thickness was measured by ultrasonic transit-time dimension gauge (Triton). After baseline recording, occlusion of the Cx artery was performed by inflating the hydraulic occluder for 60 min, followed by complete deflation. In an additional group of animals, NG-nitro-l-arginine (l-NNA, 35 mg/kg; Sigma-Aldrich, St. Louis, MO) was administered as a bolus 1 h before coronary occlusion (27).
Completion of the Cx coronary artery occlusion was confirmed by measuring regional myocardial blood flow with the color microsphere technique. Microspheres (15 ± 1 μm; BioPAL, Worcester, MA) were suspended by ultrasound for 30 min. Each injection of microsphere suspension, containing ∼3–5 million microspheres, was administered through the left atrial catheter and flushed with saline. An arterial blood reference sample was withdrawn at a rate of 7.75 ml/min for 120 s. Tissue samples were collected at the end of the study. All samples were weighed, transferred into sodium-free polypropylene vials, and dried overnight at a temperature of 70°C in an oven. The samples were analyzed by a spectroscopic method (BioPAL) and expressed as milliliters per minute per gram of tissue.
Four days after coronary artery reperfusion, animals were euthanized with pentobarbital. The heart was removed and perfused with Alcian blue (0.05% in saline), after which the heart was sliced into six to eight rings. The individual rings were weighed and photographed from both sides. IS was measured on each slide by digital area calculation software. IS and AAR were corrected by the weight of each ring and expressed as AAR-to-LV and IS-to-AAR ratios.
Mouse model.
Surgery was performed on 3- to 4-mo-old TG mice (8) with cardiac-specific overexpression of Hsp22 and their wild-type (WT) littermates. The left coronary artery was occluded for a period of 45 min (12), followed by reperfusion for 24 h. At the end of each experiment, the coronary artery was religated and IS was measured after incubation in 1% triphenyltetrazolium chloride (TTC) at 37°C for 15 min, after delineation of the AAR by Alcian blue (12). Measurement of the infarct area and the AAR from both sides of each section was performed with Image-Pro software.
Virus preparation.
The adenovirus harboring the Hsp22 sequence was prepared by the COS-TPC (Terminal Protein Complex) method with the Adeno-X system (Clontech) as described previously (8, 22). The recombinant adenovirus was propagated in HEK293 cells. Titers were determined on 293 cells overlaid with Dulbecco's modified Eagle's medium (DMEM) plus 5% equine serum and 0.5% agarose. An adenovirus harboring LacZ was prepared similarly and used as a negative control (29).
Culture of cardiac myocytes.
Cultures of ventricular cardiac myocytes were prepared from Sprague-Dawley rat pups (29). Myocytes were dispersed from the ventricles by digestion with 0.1% collagenase type IV (Worthington), 0.1% trypsin (GIBCO), and 15 μg/ml DNase I (Sigma-Aldrich). Cell suspensions were applied on a discontinuous Percoll gradient (1.060/1.082 g/ml) with DMEM-F-12 (1:1; Invitrogen), 17 mmol/l NaHCO3, 2 mmol/l glutamine, and 50 μg/ml gentamicin. Myocytes were plated at a density of 106 cells per well. The culture medium was changed to a serum-free medium after 24 h. Adeno-mediated overexpression of Hsp22 or the LacZ control was performed for 36 h as described previously (29). Myocytes were treated for the last 24 h of incubation with 25 μg/ml SN50 (Calbiochem), 25 μmol/l AG490 (Sigma-Aldrich), 10 μmol/l l-NNA. or 1 mmol/l aminoguanidine (Sigma-Aldrich). Apoptosis was induced by addition of 100 μmol/l H2O2 (Sigma-Aldrich) to the cardiac myocytes for 3 h.
Immunoblotting.
Tissues were homogenized at 4°C in a lysis buffer (in mmol/l: 25 Tris·HCl at pH 8.0, 150 NaCl, 15 KCl, 1 EDTA, and 1 DTT, with 0.5% Triton X-100 and 5% glycerol) supplemented with protease, kinase, and phosphatase inhibitors and then centrifuged at 12,000 g for 20 min at 4°C. Extracts were denatured by boiling, resolved on SDS-PAGE gels, and transferred to nitrocellulose membranes. The antibodies against Hsp22 (8), Hsp25, Hsp70, iNOS, and glyceraldehyde-3-phosphate dehydrogenase (Cell Signaling) were added at a 1/1,000 dilution for Hsp22 and a 1/500 dilution for the other antibodies and incubated overnight. After washing and incubation with the secondary antibody, signal detection was performed by chemiluminescence (NEN/Dupont).
Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling analysis.
Sections from both swine tissue and isolated cardiac myocytes were treated with proteinase K for permeabilization (9) and labeled with 2 nmol/l of biotin-conjugated dUTP and 0.1 U/μl of deoxynucleotidyltransferase for 1 h at 37°C (15). Incorporation of biotin-16-dUTP was measured with FITC-ExtrAvidin (Sigma-Aldrich). Nuclear counterstaining was performed with DAPI. Slides were read under fluorescence in a ×40 objective field.
Statistical analysis.
Results are presented as means ± SE. Student's t-test was used for two-group comparison. Two-way analysis of variance (ANOVA) with post hoc Bonferroni/Dunn correction was used for multigroup comparison. A value of P < 0.05 was considered significant.
RESULTS
Gene delivery of Hsp22 in the swine heart.
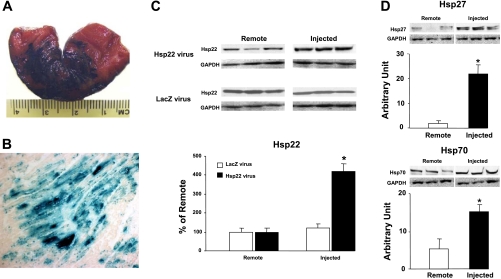
We first injected the adenovirus harboring the LacZ sequence in the AAR of the Cx artery. LacZ staining of the AAR 48 h after injection showed the diffusion of the dye (Fig. 1A). Furthermore, tissue samples were harvested, fixed in formalin, and processed for LacZ staining. As shown in Fig. 1B, a homogeneous pattern of LacZ expression was found in the injected area.
Fig. 1.
Gene delivery of Hsp22 in the swine heart. A: macroscopic aspect of the injected area at risk (AAR) after LacZ staining. B: LacZ staining in the circumflex (Cx) territory injected with the adenovirus harboring the LacZ sequence (magnification ×10). C: expression of Hsp22 in different samples taken from the Cx territory injected with the adenovirus harboring Hsp22 or LacZ and compared with samples from the remote area (n = 5/group). D: abundance of Hsp27 and Hsp70 in myocardium with Hsp22 overexpression compared with remote, noninjected area (n = 4/group). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. *P < 0.05 vs. corresponding remote area.
The adenovirus harboring the Hsp22 sequence was injected in the AAR of the left Cx artery. Immunoblotting showed an average fourfold increase in abundance of Hsp22 protein in the injected territory compared with the remote area (Fig. 1C), which reproduces the increase found in the TG mouse (8). Injection of the adenovirus harboring LacZ did not affect Hsp22 expression (Fig. 1C). There was no overexpression of Hsp22 in the liver of the same animals (not shown), demonstrating the absence of systemic release of the adenovirus. We showed previously (12) that the TG mouse overexpressing Hsp22 also displays an increased expression of other Hsp proteins, such as Hsp27 and Hsp70. In accordance with that finding, both proteins were also significantly increased in swine myocardium injected with the Hsp22 virus (Fig. 1D), but not with the LacZ virus (not shown).
Hsp22 improves postischemic myocardial function.
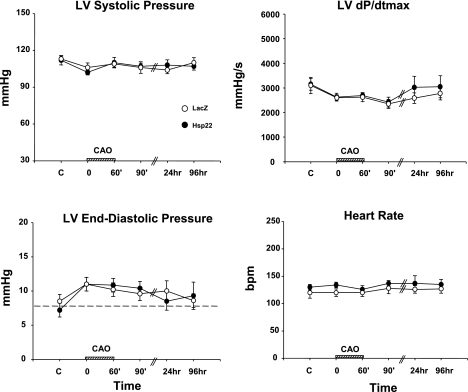
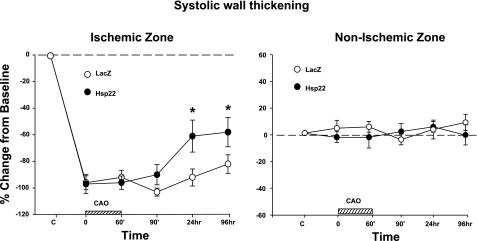
Three days after injection of the adenovirus, the Cx territory was submitted to 60-min coronary artery occlusion followed by 4 days of reperfusion. LV systolic and end-diastolic pressure, LV dP/dt, and heart rate were measured at different time points and did not show any significant difference between the Hsp22-injected myocardium compared with the LacZ control at any time of the protocol (Fig. 2). The systolic wall thickening in the nonischemic zone showed no difference between groups at any time point, whereas in the ischemic area systolic wall thickening was reduced by 100% in both groups at the onset of ischemia, and such reduction was maintained during the entire ischemic episode (Fig. 3). Subendocardial and subepicardial blood flow measured by microspheres were comparable between the groups, both in the normal and ischemic areas (Table 1). Four days after reperfusion, wall thickening in the ischemic region was reduced by 82 ± 7% in the LacZ group but only by 58 ± 2% in the Hsp22 group (P < 0.05), which demonstrates a significant improvement in recovery of contractility in myocardium pretreated with Hsp22 (Fig. 3).
Fig. 2.
Physiological parameters of the swine model. Left ventricular (LV) systolic pressure, LV maximum change in pressure with time (dP/dtmax), LV end-diastolic pressure, and heart rate [beats/min (bpm)] in LacZ-injected vs. Hsp22-injected hearts under control conditions (C), during coronary artery occlusion (CAO), and during the 4 days of reperfusion (n = 5/group). The hatched bar on x-axis represents time of CAO.
Fig. 3.
Effect of Hsp22 conditioning on postischemic functional recovery: wall thickening [calculated as % of baseline (horizontal line)] in both ischemic and nonischemic zones of hearts injected with the adenovirus harboring LacZ or Hsp22 at the different time points of the protocol defined in Fig. 2 (n = 5/group). *P < 0.05 vs. corresponding LacZ. The hatched bar on x-axis represents time of CAO.
Table 1.
Myocardial blood flow during coronary artery occlusion
| Normal Zone |
Ischemic Zone |
|||||||
|---|---|---|---|---|---|---|---|---|
| Endocardium |
Epicardium |
Endocardium |
Epicardium |
|||||
| CAO5′ | CAO50′ | CAO5′ | CAO50′ | CAO5′ | CAO50′ | CAO5′ | CAO50′ | |
| LacZ (n = 5) | 1.11 ± 0.10 | 1.20 ± 0.10 | 1.02 ± 0.11 | 1.15 ± 0.13 | 0.04 ± 0.02 | 0.02 ± 0.01 | 0.04 ± 0.02 | 0.03 ± 0.02 |
| H11 (n = 4) | 1.19 ± 0.11 | 1.13 ± 0.10 | 0.84 ± 0.07 | 0.77 ± 0.04 | 0.05 ± 0.03 | 0.03 ± 0.02 | 0.08 ± 0.03 | 0.01 ± 0.03 |
Values (in ml·min−1·g−1) are mean ± SE absolute flow in both subendocardium and subepicardium of the remote and ischemic areas after the onset (5 min CAO) and before the end (50 min CAO) of coronary artery occlusion. H11, H11 kinase/Hsp22. No significant difference between groups was detected.
Hsp22 reduces infarct size after ischemia-reperfusion.
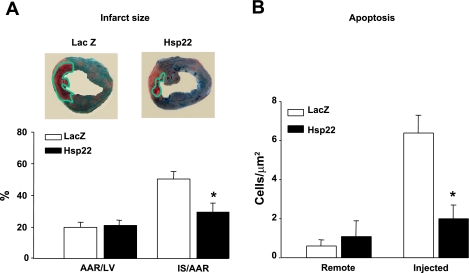
Upon completion of the physiological measurements, animals were euthanized 4 days after Cx reperfusion for measurement of IS. Hearts were perfused with Alcian blue, after which both the AAR and the IS were quantitated by planimetry. A representative example of staining is shown for both groups in Fig. 4A. The AAR, measured as a percentage of LV weight, did not show significant difference between groups (Fig. 4A). In contrast, the injection of Hsp22 significantly (P < 0.05) reduced the IS-to-AAR ratio by ∼40% compared with the LacZ group (Fig. 4A).
Fig. 4.
Reduction of infarct size (IS) by Hsp22. A: delineation of AAR and IS performed 4 days after reperfusion in hearts injected with LacZ vs. Hsp22. Photographs show a representative example from both groups, where the green line delimits the IS. Graph shows AAR as % of LV weight and IS/AAR in both groups (n = 5/group). B: terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) measurement in both remote and ischemic areas (n = 4/group). At least 10,000 DAPI-positive nuclei were measured in each sample. *P < 0.05 vs. corresponding LacZ.
An improvement of cardiac cell survival in Hsp22-injected myocardium was further confirmed by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) analysis, which measures apoptosis and autolytic cell death. In nonischemic myocardium, the number of TUNEL-positive cells did not significantly differ between control and Hsp22-injected myocardium (Fig. 4B). After ischemia, apoptosis increased significantly by ∼10-fold in LacZ controls and by a nonsignificant 2-fold in Hsp22-treated hearts (Fig. 4B). Therefore, the functional improvement after ischemia-reperfusion in hearts treated with Hsp22 compared with LacZ corresponds to a reduction of irreversible ischemic damage by both necrosis and apoptosis.
Cardioprotection by Hsp22 is NO dependent.
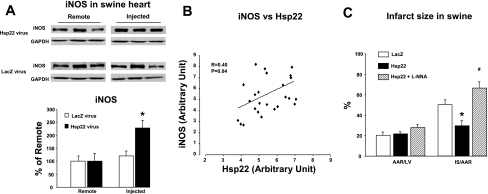
Chronic overexpression of Hsp22 in a cardiac-specific TG mouse model is accompanied by an upregulation of the expression of iNOS (12). Because iNOS is the mediator of the SWOP (29) and because the cardioprotection conferred by Hsp22 overexpression in the TG mouse is quantitatively similar to that provided by the SWOP (7), we determined whether short-term overexpression of Hsp22 in the swine is sufficient to upregulate iNOS. As shown in Fig. 5A, iNOS expression increased by an average of 2.5-fold in the myocardium injected with the Hsp22 adenovirus compared with the remote area, whereas it was unaffected by the LacZ adenovirus. Importantly, such increase of iNOS upon overexpression of Hsp22, albeit of limited amplitude, is quantitatively comparable to the upregulation found in different models of SWOP (6). There was a significant correlation between the overexpression level of Hsp22 and the increased expression of iNOS when individual data collected from four different hearts were plotted (Fig. 5B).
Fig. 5.
Overexpression of Hsp22 increases inducible nitric oxide synthase (iNOS) expression. A: increased expression of iNOS in swine myocardium injected with the adenovirus harboring Hsp22 compared with the remote area. Injection with the adenovirus harboring LacZ does not affect iNOS expression. Immunoblotting shows 3 representative examples per group. Bar graph shows means ± SE of n = 6 per group. GAPDH was used as a loading control. *P < 0.05 vs. corresponding remote area. B: correlation between Hsp22 overexpression and iNOS abundance in myocardial samples from 4 different hearts. C: measurement of AAR/LV and IS/AAR in swine myocardium injected with adenovirus harboring LacZ or Hsp22 in the presence or absence of NG-nitro-l-arginine (l-NNA). *P < 0.05 vs. corresponding LacZ; #P < 0.05 vs. Hsp22 without l-NNA.
We showed previously (10) in the same model that nitric oxide (NO) blockade by the NOS inhibitor l-NNA totally prevents the cardioprotection conferred by SWOP. To determine whether a similar mechanism mediates the cardioprotection conferred by Hsp22, additional pigs pretreated with the Hsp22 adenovirus received a bolus injection of l-NNA 1 h before Cx artery occlusion. The AAR and IS were measured in these animals after 4 days of reperfusion. Although the AAR was not affected, l-NNA totally abolished the reduction in IS observed in animals treated with Hsp22 compared with LacZ control animals (Fig. 5C).
Hsp22-induced iNOS expression is mediated by NF-κB and STAT.
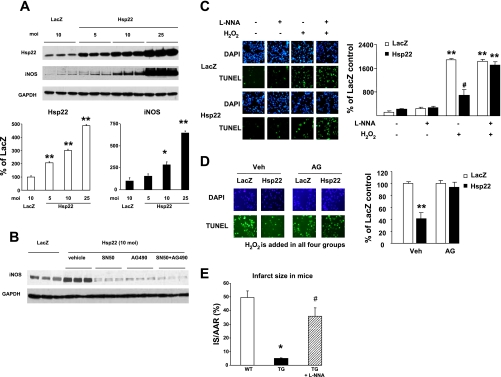
The transcriptional pathways involved in Hsp22-mediated increase in iNOS were investigated more mechanistically in isolated cardiac myocytes in order to test whether it reproduces those activated by SWOP. First, we determined in vitro whether the increased expression of iNOS can be directly related to the overexpression of Hsp22. For that purpose, a dose-response experiment was performed in isolated neonatal rat cardiac myocytes infected with the Hsp22 adenovirus in a range from 5 to 25 multiplicity of infection (moi), and iNOS expression was measured by immunoblotting 48 h after infection. The expression of iNOS increased proportionately to the amount of Hsp22 adenovirus added to the cardiac myocytes (Fig. 6A), further confirming the correlation observed in the swine heart. The two main transcription factors activated by SWOP and regulating the expression of iNOS are STAT (3) and NF-κB (21). The increased expression of iNOS by Hsp22 overexpression was totally abolished in presence of AG490, a STAT inhibitor, or in the presence of SN50, an NF-κB inhibitor (Fig. 6B). These data show that Hsp22 is responsible for iNOS induction through a transcriptional pathway similar to that for SWOP. It is known that SWOP has a window of efficacy of ∼24–96 h following the preconditioning stimulus (5). Upon virus injection, it takes at least 24 h for the construct to be translated into effective protein. Therefore, the time windows of protection conferred by SWOP versus gene delivery seem comparable.
Fig. 6.
Hsp22-mediated iNOS expression provides cardioprotection. A: dose-dependent increase in iNOS expression in response to different amounts [multiplicity of infection (moi)] of Hsp22 adenovirus in isolated cardiac myocytes (n = 3/group). *P < 0.05, **P < 0.01 vs. LacZ control. B: Hsp22-mediated increase in iNOS expression is suppressed by both the NF-κB inhibitor SN50 and the STAT inhibitor AG490. C: TUNEL/DAPI staining and quantitation in cardiac myocytes infected with the Hsp22 adenovirus vs. LacZ control and pretreated or not with l-NNA, in the presence or absence of H2O2. **P < 0.01 vs. corresponding group without H2O2; #P < 0.01 vs. corresponding LacZ. At least 500 DAPI-positive nuclei were measured in each sample. D: TUNEL/DAPI staining and quantitation in cardiac myocytes infected with the Hsp22 adenovirus vs. LacZ control in the presence of H2O2 and pretreated with vehicle (Veh) or with 1 mmol/l aminoguanidine (AG). **P < 0.01 vs. corresponding LacZ. At least 500 DAPI-positive nuclei were measured in each sample. E: IS/AAR in transgenic (TG) mice overexpressing Hsp22 in the presence or absence of l-NNA compared with wild-type (WT) littermates (n = 4/group). *P < 0.05 vs. WT; #P < 0.05 vs. TG without l-NNA.
To further confirm a link between NO and cell survival by Hsp22, apoptosis induced by H2O2 was measured in cardiac myocytes overexpressing LacZ or Hsp22, in the presence or absence of l-NNA. TUNEL staining showed a significant increase in apoptosis after addition of H2O2 in presence of the LacZ control, and such increase was reduced by ∼60% upon overexpression of Hsp22 (Fig. 6C). Addition of l-NNA in the absence of H2O2 did not affect apoptosis in either group (Fig. 6C). Addition of l-NNA in the presence of H2O2 did not affect the rate of apoptosis in the LacZ group; however, it totally abolished the cytoprotective effect of Hsp22 (Fig. 6C). These experiments were repeated upon incubation of cardiac myocytes with H2O2 in the presence or absence of aminoguanidine, which is considered to be a more specific inhibitor of iNOS than other NOS isoforms, and again the cardioprotection provided by Hsp22 was abolished (Fig. 6D). These data were further corroborated in the TG mouse model of Hsp22 overexpression that we characterized before (8, 12, 23, 29). Both WT and TG mice were submitted to 45-min ischemia followed by 24-h reperfusion. The AAR was measured after staining with Alcian blue, and IS was determined by TTC staining. In agreement with our previous observation (12), the Hsp22 TG mouse was characterized by a 80% reduction in IS/AAR compared with the WT mice (Fig. 6E). However, upon pretreatment of TG mice with l-NNA, IS increased markedly compared with nontreated TG mice and became statistically comparable to the IS/AAR observed in WT mice (Fig. 6E).
DISCUSSION
This study demonstrates the potential of Hsp22 as a mechanism of preemptive conditioning of the ischemic heart, providing at least a 40% reduction of IS for an average fourfold increase in Hsp22 expression. Our study draws two main conclusions. First, we show that short-term overexpression of Hsp22 is sufficient to activate two important signaling pathways mediating SWOP, i.e., iNOS and Hsps (including Hsp70 and Hsp27) (34). Second, we also demonstrate, using different models both in vivo (short-term overexpression in the swine and cardiac-specific overexpression in the TG mouse) and in vitro (isolated cardiac myocytes), that the cardioprotection provided by Hsp22 is NO dependent, consistent with the observations made in the SWOP (4). As such, manipulation of Hsp22 expression represents an attractive candidate for prophylactic cardioprotection (6), in order to reproduce the effects of the SWOP without the inconvenience of the preliminary episodes of ischemia-reperfusion required to obtain the protection by ischemic preconditioning.
The design of the present study was based on our previous observations (12) in a TG mouse model of cardiac-specific overexpression of Hsp22 showing major cardioprotection against lethal ischemia. However, the TG model presents two limitations. First, there is a possibility of nonspecific effects resulting from the chronic overexpression of the transgenic protein. Second, from a therapeutic point of view, it would be much more powerful if the heart could be protected against irreversible damage by short-term overexpression of the protein. The present study addresses both limitations. Therefore, biological manipulation of Hsp22 activity could be of therapeutic relevance, as an alternative to pharmacological approaches such as adenosine or opioid agonists (19, 32, 34).
The molecular mechanisms by which Hsp22 promotes cardioprotection are progressively unraveling. We recently showed (29) both in vivo and in vitro that Hsp22 activates a serial signaling pathway of cell survival that includes the bone morphogenetic protein (BMP) receptor, phosphatidylinositol 3-kinase (PI3K), and its major effector, Akt. We also know from the TG model (12) and from the in vitro data presented here that Hsp22 overexpression is sufficient to increase iNOS abundance. A possible mechanism relies on the Akt-mediated activation of NF-κB, the transcription factor responsible for induction of iNOS during the SWOP (35). This mechanism is supported by the observation reported here that NF-κB inhibition prevents Hsp22-mediated iNOS expression. We also show here the potential role of STAT, a stress-responsive transcription factor activated by Hsp22 and also by SWOP (3). Therefore, short-term overexpression of Hsp22 reproduces the main characteristics of the signaling pathways activated by SWOP. It was beyond the scope of the present investigation to test whether all the signaling mechanisms of Hsp22 activated both in vivo (8, 12) and in vitro (29) in rodent models also apply to the swine. Instead, we focused on iNOS expression as the potential mediator of cardioprotection. Using three different models, we confirmed that the cardioprotection conferred by Hsp22 is NO dependent. Our experiments with aminoguanidine further imply a role specifically for iNOS compared with other NOS isoforms. Although cardioprotection by NO donors has been limited so far (32), it is possible that stimulating the formation of NO from iNOS inside the cardiac myocytes might have better biological efficiency than that provided by NO donors diffusing from the vasculature. It is also possible that endogenous control of iNOS expression (2- to 3-fold increase in expression) may help avoid the excessive formation of NO, which would be toxic through generation of superoxides (13). However, despite an increased expression of iNOS, it remains undetermined whether the final effector is increased NO production or another antioxidative mechanism. Although previous studies in vitro have shown that increased expression of iNOS in cardiac myocytes is followed by increased production of NO (1, 25), it was shown recently that delayed preconditioning in vivo in the rat heart, although increasing iNOS expression, does not necessarily result in increased NO production but rather affects peroxynitrite production (2).
Many systems of cardiac gene delivery have been reported, including direct myocardial injection, catheter-based end-myocardial injection, retroperfusion via a coronary sinus with concurrent occlusion of outflow from the sinus, intrapericardial injection via a specialized catheter, and aortic injection in the presence of either aortic clamping or cross-clamping of the aorta and pulmonary artery. An important consideration is that the present model, as accurate as it can be, does not allow a homogeneous overexpression of the protein, because of the delivery method of the adenovirus. We can therefore speculate that a more widespread delivery (i.e., more injection sites, higher volume of solvent, higher titer of the adenovirus) might achieve an even bigger reduction in IS. Despite this limitation, the reduction of IS provided by Hsp22 in the present study (∼40%) is close to the 60% reduction in IS provided by SWOP in the same model (10).
We chose the pig model, instead of more common models in rodents, for three specific reasons. First, this model closely reproduces the pathophysiology of ischemic heart disease found in patients. Second, this model includes the possibility of performing reproducible IS because of the lack of collaterals. Third, since the study was conducted in chronically instrumented animals, serial recording could be performed in a fully conscious state, which excludes the artifactual influence of anesthesia and recent surgery. As shown previously (9, 11), other major advantages are the possibility of performing a paired comparison, within the same heart, between AAR and remote myocardium and the possibility of separating subendocardium from subepicardium (11). This model has been used also as a preclinical model for gene therapy in the heart (16–18, 26). Unlike rodent species, the larger-animal model allows us to precisely obtain the tissue samples in different areas of myocardium, i.e., central ischemic area, adjacent and remote to ischemic areas. Also, the anatomy of the coronary circulation and the myocardial metabolic characteristics of pigs are similar to those of humans. In addition, preexisting collateral channels and anastomoses are almost nonexistent, as reflected by the transmural reduction of blood flow to almost zero levels during coronary artery occlusion.
In conclusion, a preemptive activation of Hsp22 and its downstream targets would confer a prophylactic cardioprotection during the following ischemic stress. However, like most of the cytoprotective signaling pathways already involved in preconditioning, the translation of these findings to the clinical area may prove difficult. In particular, the future of gene delivery for cardiovascular disease remains uncertain. However, alternative strategies for activating endogenous survival pathways are emerging, which get closer to potential clinical applications in conditions of ischemic heart disease. In particular, one approach already tested for other Hsps consists of the administration of cell-permeant recombinant peptides (14). It remains to be demonstrated whether such an approach may prove feasible for Hsp22.
GRANTS
This study was supported by National Institutes of Health Grants HL-072863, PO1-HL-069020, PO1-AG-027211, and RO1-HL-033107 and American Heart Association Grant 0230017N.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Balligand J, Ungureanu-Longrois D, Simmons W, Pimental D, Malinski T, Kapturczak M, Taha Z, Lowenstein C, Davidoff A, Kelly R. Cytokine-inducible nitric oxide synthase (iNOS) expression in cardiac myocytes. Characterization and regulation of iNOS expression and detection of iNOS activity in single cardiac myocytes in vitro. J Biol Chem 269: 27580– 27588, 1994 [PubMed] [Google Scholar]
- 2. Bencsik P, Kupai K, Giricz Z, Görbe A, Pipis J, Murlasits Z, Kocsis GF, Varga-Orvos Z, Puskás LG, Csonka C, Csont T, Ferdinandy P. Role of iNOS and peroxynitrite-matrix metalloproteinase-2 signaling in myocardial late preconditioning in rats. Am J Physiol Heart Circ Physiol 299: H512– H518, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther 120: 172– 185, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol 33: 1897– 1918, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Bolli R. The late phase of preconditioning. Circ Res 87: 972– 983, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Bolli R, Li QH, Tang XL, Guo Y, Xuan YT, Rokosh G, Dawn B. The late phase of preconditioning and its natural clinical application—gene therapy. Heart Fail Rev 12: 189– 199, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Danan IJ, Rashed ER, Depre C. Therapeutic potential of H11 kinase for the ischemic heart. Cardiovasc Drug Rev 25: 14– 29, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Depre C, Hase M, Gaussin V, Zajac A, Wang L, Hittinger L, Ghaleh B, Yu X, Kudej RK, Wagner T, Sadoshima J, Vatner SF. H11 kinase is a novel mediator of myocardial hypertrophy in vivo. Circ Res 91: 1007– 1014, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Depre C, Kim SJ, John AS, Huang Y, Rimoldi OE, Pepper JR, Dreyfus GD, Gaussin V, Pennell DJ, Vatner DE, Camici PG, Vatner SF. Program of cell survival underlying human and experimental hibernating myocardium. Circ Res 95: 433– 440, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Depre C, Park JY, Shen YT, Zhao X, Qiu H, Yan L, Tian B, Vatner SF, Vatner DE. Molecular mechanisms mediating preconditioning following chronic ischemia differ from those in classical second window. Am J Physiol Heart Circ Physiol 299: H752– H762, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Depre C, Tomlinson JE, Kudej RK, Gaussin V, Thompson E, Kim SJ, Vatner DE, Topper JN, Vatner SF. Gene program for cardiac cell survival induced by transient ischemia in conscious pig. Proc Natl Acad Sci USA 98: 9336– 9341, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Depre C, Wang L, Sui X, Qiu H, Hong C, Hedhli N, Ginion A, Shah A, Pelat M, Bertrand L, Wagner T, Gaussin V, Vatner SF. H11 kinase prevents myocardial infarction by pre-emptive preconditioning of the heart. Circ Res 98: 280– 288, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br J Pharmacol 138: 532– 543, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flynn C, Smoke C, Furnish E, Komalavilas P, Thresher J, Yi Z, Mandarino L, Brophy C. Phosphorylation and activation of a transducible recombinant form of human HSP20 in Escherichia coli. Protein Expr Purif 52: 50– 58, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geng Y, Ishikawa Y, Vatner D, Wagner T, Bishop S, Vatner S, Homcy C. Apoptosis of cardiac myocytes in Gsα transgenic mice. Circ Res 84: 34– 42, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Grines C, Rubanyi G, Kleiman N, Marrott P, Watkins M. Angiogenic gene therapy with adenovirus 5 fibroblast growth factor-4 (Ad5FGF-4): a new option for the treatment of coronary artery disease. Am J Cardiol 92: 24N– 31N, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Grines C, Watkins M, Helmer G, Penny W, Brinker J, Marmur J, West A, Rade J, Marrott P, Hammond H, Engler R. Angiogenic gene therapy (AGENT) trial in patients with stable angina pectoris. Circulation 105: 1291– 1297, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Grines C, Watkins M, Mahmarian J, Iskandrian A, Rade J, Marrott P, Pratt C, Kleinman N. A randomized double blind placebo-controlled trial of Ad5FGF-4 gene therapy and its effect on myocardial perfusion in patients with stable angina. J Am Coll Cardiol 42: 1339– 1347, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ Res 94: 960– 966, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, Han H, Laubach VE, Ping P, Yang Z, Qiu Y, Bolli R. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci USA 96: 11507– 11512, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hall G, Hasday J, Rogers T. Regulating the regulator: NF-kappaB signaling in heart. J Mol Cell Cardiol 41: 580– 591, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Hase M, Depre C, Vatner S, Sadoshima J. H11 has dose-dependent and dual hypertrophic and proapoptotic functions in cardiac myocytes. Biochem J 388: 475– 483, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hedhli N, Wang L, Wang Q, Rashed E, Tian Y, Sui X, Madura K, Depre C. Proteasome activation during cardiac hypertrophy by the chaperone H11 kinase/Hsp22. Cardiovasc Res 77: 497– 505, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Kappe G, Verschuure P, Philipsen R, Staalduinen A, Van den Bogaart P, Boelens W, De Jong W. Characterization of two novel human small heat shock proteins: protein kinase-related HspB8 and testis-specific HspB9. Biochim Biophys Acta 1520: 1– 6, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Kurosaki K, Ikeda U, Maeda Y, Shimada K. Carvedilol stimulates nitric oxide synthesis in rat cardiac myocytes. J Mol Cell Cardiol 32: 333– 339, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Rutanen J, Rissanen TT, Markkanen JE, Gruchala M, Silvennoinen P, Kivela A, Hedman A, Hedman M, Heikura T, Orden MR, Stacker SA, Achen MG, Hartikainen J, Yla-Herttuala S. Adenoviral catheter-mediated intramyocardial gene transfer using the mature form of vascular endothelial growth factor-D induces transmural angiogenesis in porcine heart. Circulation 109: 1029– 1035, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Shen YT, Depre C, Yan L, Park JY, Tian B, Jain K, Chen L, Zhang Y, Kudej RK, Zhao X, Sadoshima J, Vatner DE, Vatner SF. Repetitive ischemia by coronary stenosis induces a novel window of ischemic preconditioning. Circulation 118: 1961– 1969, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith C, Yu Y, Kulka M, Aurelian L. A novel human gene similar to the protein kinase (PK) coding domain of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) codes for a serine-threonine PK and is expressed in melanoma cells. J Biol Chem 275: 25690– 25699, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Sui X, Li D, Qiu H, Gaussin V, Depre C. Activation of the bone morphogenetic protein receptor by H11 kinase/Hsp22 promotes cardiac cell growth and survival. Circ Res 104: 887– 895, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Takano H, Manchikalapudi S, Tang XL, Qiu Y, Rizvi A, Jadoon AK, Zhang Q, Bolli R. Nitric oxide synthase is the mediator of late preconditioning against myocardial infarction in conscious rabbits. Circulation 98: 441– 449, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Vitale J, Qiu H, Depre C. Pre-emptive conditioning of the ischemic heart. Cardiovasc Hematol Agents Med Chem 6: 92– 104, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Wang Y, Guo Y, Zhang SX, Wu WJ, Wang J, Bao W, Bolli R. Ischemic preconditioning upregulates inducible nitric oxide synthase in cardiac myocyte. J Mol Cell Cardiol 34: 5– 15, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Yellon D, Downey J. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev 83: 1113– 1151, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Zhang J, Ping P, Vondriska TM, Tang XL, Wang GW, Cardwell EM, Bolli R. Cardioprotection involves activation of NF-kappaB via PKC-dependent tyrosine and serine phosphorylation of IkappaB-alpha. Am J Physiol Heart Circ Physiol 285: H1753– H1758, 2003 [DOI] [PubMed] [Google Scholar]