Abstract
Caveolin (Cav)-1 has been involved in the pathogenesis of ischemic injuries. For instance, modulations of Cav-1 expression have been reported in animal models of myocardial infarction and cerebral ischemia-reperfusion. Furthermore, ablation of the Cav-1 gene in mice has been shown to increase the extent of ischemic injury in models of cerebral and hindlimb ischemia. Cav-1 has also been suggested to play a role in myocardial ischemic preconditioning. However, the role of Cav-1 in myocardial ischemia (MI)-induced cardiac dysfunction still remains to be determined. We determined the outcome of a permanent left anterior descending coronary artery (LAD) ligation in Cav-1 knockout (KO) mice. Wild-type (WT) and Cav-1 KO mice were subjected to permanent LAD ligation for 24 h. The progression of ischemic injury was monitored by echocardiography, hemodynamic measurements, 2,3,5-triphenyltetrazolium chloride staining, β-binding analysis, cAMP level measurements, and Western blot analyses. Cav-1 KO mice subjected to LAD ligation display reduced survival compared with WT mice. Despite similar infarct sizes, Cav-1 KO mice subjected to MI showed reduced left ventricular (LV) ejection fraction and fractional shortening as well as increased LV end-diastolic pressures compared with their WT counterparts. Mechanistically, Cav-1 KO mice subjected to MI exhibit reduced β-adrenergic receptor density at the plasma membrane as well as decreased cAMP levels and PKA phosphorylation. In conclusion, ablation of the Cav-1 gene exacerbates cardiac dysfunction and reduces survival in mice subjected to MI. Mechanistically, Cav-1 KO mice subjected to LAD ligation display abnormalities in β-adrenergic signaling.
Keywords: contractility, signal transduction, β-adrenergic receptors
ischemic heart disease is a leading cause of morbidity and mortality in Western countries. Numerous signaling pathways have been implicated in the cardiac dysfunction associated with ischemic heart disease. However, it still remains unclear how these different signaling pathways spatially and temporally interact with each other. Caveolae are 50- to 100-nm invaginations of the plasma membrane that compartmentalize numerous signaling molecules such as endothelial nitric oxide (NO) synthase (eNOS), ERK1/2, and tyrosine kinases as well as different heterotrimeric G protein subunits (8, 13, 20, 27, 35, 36). Caveolin (Cav) proteins represent the principal structural components of the caveolar domains (9, 26, 30). The Cav gene family consists of three distinct genes: namely, Cav-1, Cav-2, and Cav-3 (9, 30, 33, 39). Cav-1 and Cav-2 are usually coexpressed and are particularly abundant in endothelial cells, fibroblasts, smooth muscle cells, adipocytes, and epithelial cells, whereas Cav-3 appears to be muscle specific and is expressed in cardiac, skeletal, and smooth muscle cells (32, 33, 37, 39). Although Cav-3 was originally believed to be the only isoform expressed in cardiomyocytes, Cav-1 and Cav-2 expressions have been recently reported in mouse, rat, and human cardiomyocytes (2, 3, 11, 22, 29).
Interestingly, Cav-1 has recently been involved in the pathogenesis of ischemic injuries (12, 15, 25). For instance, modulations of endogenous Cav-1 expression have been reported in rat models of ischemic acute renal failure, myocardial infarction, and cerebral ischemia-reperfusion (12, 15, 25). Importantly, the generation of Cav knockout (KO) mice strongly supports the role of Cav-1 in the pathogenesis of ischemic injuries (12, 38). Indeed, we and others (12, 38) have previously reported that ablation of the Cav-1 gene in mice increased the extent of ischemic injury in models of cerebral and hindlimb ischemia. Recent evidence has also suggested that Cav-1 and caveolar domains might be involved in the pathogenesis of myocardial ischemic injury (21, 22). Indeed, disruption of caveolar domains was shown to attenuate the cardioprotective effects of ischemic preconditioning in adult rat cardiomyocytes subjected to simulated ischemia-reperfusion (21). The phosphorylation of Cav-1 was further suggested to contribute to cardiac protection after ischemic preconditioning (22). Accordingly, ablation of the Cav-1 gene has been reported to abolish isoflurane-induced cardiac protection in mice (22). However, the role of Cav-1 in myocardial ischemia (MI)-induced cardiac dysfunction still remains elusive. Here, we determined the outcome of a permanent left anterior descending coronary artery (LAD) ligation in Cav-1 KO mice.
METHODS
Animals.
This study was conducted according to guidelines of the National Institutes of Health (NIH) and Thomas Jefferson University Institute for Animal Studies. Cav-1 KO mice were generated as we have previously described (26). All mice used in this study were on the FVB/N genetic background and were housed in a barrier facility at the Thomas Jefferson University.
Materials.
Cav-1 and Cav-3 mouse monoclonal antibodies were the generous gifts of Dr. Roberto Campos-Gonzalez (BD-Pharmingen, San Diego, CA). Rabbit polyclonal antibodies to PKA-C and phospho-PKA-C (Thr197) were purchased from Cell Signaling Technology (Danvers, MA). A mouse monoclonal antibody to GAPDH was purchased from Fitzgerald Industries (Acton, MA). Rabbit and mouse horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from BD-Pharmingen.
Surgical procedures.
Eight-week-old wild-type (WT) and Cav-1 KO FVB/N male mice were anesthetized with a mixture of xylazine (5 mg/kg) and ketamine (50 mg/kg) (Pfizer Animal Health, Monmouth, NJ), intubated, and ventilated with a small rodent ventilator (Harvard Apparatus, Holliston, MA) at a rate of 110 cycles/min with a tidal volume of 2 ml/min and a positive end-expiratory pressure of 2 cmH2O. A left side thoracotomy was performed, and the pericardium was incised. MI was then induced through permanent ligation of the LAD with a 8-0 silk suture (Fine Science Tools, Foster City, CA) proximal to its bifurcation from the main stem (n = 24 and 31 for WT and Cav-1 KO mice, respectively). The incision was subsequently closed with a 5-0 silk suture (Harvard Apparatus). Sham-operated mice were subjected to the same experimental procedure except for the ligation of the LAD (n = 20 mice/group). WT and Cav-1 KO mice were then allowed to recover in a temperature-controlled environment.
Echocardiography.
Transthoracic echocardiography was performed in anesthetized mice [2% (vol/vol) isoflurane] at 24 h post-LAD ligation using the VisualSONICS VeVo 770 imaging system with a 12-MHz scanhead (n = 6–10 mice/group). Two-dimensional targeted M-mode imaging was obtained from the short-axis view at the level of the greatest left ventricular (LV) dimension. We performed three measurements for each animal, and the average was taken in consideration for analysis. All three measurements were highly reproducible. End diastole was determined at the maximal LV diastolic dimension, and end systole was taken at the peak of posterior wall motion. VisualSONICS analysis software was used to calculate LV fractional shortening (FS) and ejection fraction (EF) using previously described formulas (23, 24). The percentage of LV FS was calculated as follows: FS (in %) = (LVEDD − LVESD)/LVEDD × 100, where LVEDD and LVESD are LV end-diastolic and end-systolic diameters, respectively. The percentage of LV EF was calculated as follows: EF (in %) = (LVEDV − LVESV)/LVEDV × 100, where LVEDV and LVESV are LV end-diastolic and end-systolic volumes, respectively.
Hemodynamic experiments.
Twenty-four hours after LAD ligation, mice were anesthetized with xylazine (5 mg/kg) and ketamine (50 mg/kg) followed by 1,000 units of heparin (Sigma-Aldrich). After stable anesthesia was obtained, the right carotid artery was isolated and incised, and a Millar catheter (SPR-671, Millar Instruments, Houston, TX) was advanced into the LV for hemodynamic measurements (n = 7–12 mice/group). LV pressures were measured and recorded with the Ponemah P3 data-acquisition system (LDS Test and Measurement, Middleton, WI).
Determination of the area at risk and infarct size.
Twenty-four hours after LAD ligation, mice were anesthetized with xylazine (5 mg/kg) and ketamine (50 mg/kg). Two milliliters of 2% Evans blue dye (Sigma-Aldrich) was then retrogradely injected into the aorta to delineate the in vivo area at risk (AAR; n = 21 and 18 for WT and Cav-1 KO mice, respectively). The heart was then excised, placed in an acrylic mouse heart matrix (Harvard Apparatus), and sectioned perpendicularly to the long axis in 2-mm sections. The 2-mm sections were weighed, placed in individual wells of a 12-well cell culture plate, and incubated with 1% 2,3,5-triphenyltetrazolium chloride (TTC) solution for 15 min at 37°C. Each section was visualized with a Z2 Zoom trinocular microscope and photographed with a digital camera (Powershot S31S, Canon). The AAR and infarct area were quantified using NIH ImageJ software. Infarct size was determined using the following previously described equation (17): weight of infarction = (A1 × W1) + (A2 × W2) + (A3 × W3), where A is the percent area of infarction obtained by planimetry of sections 1–3 and W is the weight of the same numbered sections.
Determination of cardiac NO metabolites.
Measurements of the total nitrate/nitrite concentration in LVs of sham-WT, MI-WT, sham-Cav-1 KO, and MI-Cav-1 KO were performed using a Nitrate/Nitrite Colorimetric Assay Kit (Cayman Chemical, Ann Harbor, MI), which is based on the enzymatic conversion of nitrate to nitrite by nitrate reductase, followed by the spectrophotometric quantitation of nitrite levels using Griess reagent (n = 7–8 mice/group).
Cardiac β-adrenergic receptor density and cAMP measurements.
β-Adrenergic receptor (β -AR) density was measured in isolated cardiac plasma membranes from the viable, healthy portion of the LV free wall by saturation ligand binding with [125I]iodocyanopindolol, as previously described (n = 4–6 mice/group) (14, 28). Nonspecific binding was determined in the presence of 20 μM alprenolol. Data were analyzed by nonlinear regression analysis using GraphPad Prism (GraphPad software). cAMP levels in cardiac lysates of the viable, healthy portion of the LV free wall were measured with a Cyclic AMP PLUS EIA kit (BioMol, Plymouth Meeting, PA), as previously described (n = 4–6 mice/group) (14, 28).
Immunoblot analyses.
Whole LVs from sham-WT, MI-WT, sham-Cav-1 KO, and MI-Cav-1 KO mice were homogenized in RIPA lysis buffer containing protease and phosphatase inhibitors (n = 6 mice/group). Lysates were centrifuged at 12,000 g for 10 min to remove the insoluble debris. Bicinchoninic acid reagent was subsequently used to determine the protein concentration of each sample as well as the volume required for 50 μg of protein. Proteins were then separated by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were placed in blocking solution for 30 min and subsequently washed with 10 mM Tris, 150 mM NaCl, and 0.05% Tween 20 (1× Tris-buffered saline-Tween 20). Membranes were incubated with a given primary antibody for 3 h. Finally, HRP-conjugated secondary antibodies were used to detect bound primary antibody using SuperSignal chemiluminescence substrate (Pierce Biotechnology, Rockford, IL). Western blots were quantitated using NIH ImageJ software (using the mean gray value for each band).
Statistical analysis.
All data are expressed as means ± SE. Survival was compared using the log-rank test. Differences between other variables were evaluated by either an unpaired Student's t-test or one-way ANOVA followed by Tukey's multiple-group comparisons test, where appropriate. Statistical significance was assumed at P < 0.05.
RESULTS
Cav-1 KO mice subjected to MI display reduced survival.
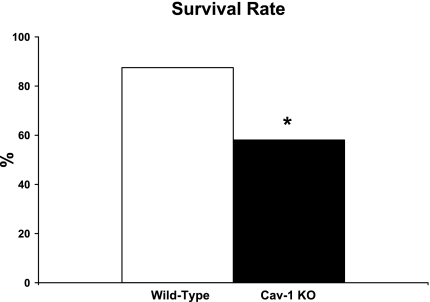
To gain insight on the role of Cav-1 in the pathogenesis of myocardial ischemic injury, male WT and Cav-1 KO mice were subjected to permanent LAD ligation for 24 h. The mortality rate was then determined as the percentage of mice that initially recovered from surgery but subsequently died within the next 24 h. Interestingly, Cav-1 KO mice show a marked reduction in survival at 24 h postischemia (58.1 ± 2.6%) compared with WT mice subjected to the same procedure (87.5 ± 1.7%, P < 0.05; Fig. 1). Note that most Cav-1 KO mice died at later time points rather than early after surgery and that dead animals were not used for any of the analyses presented below.
Fig. 1.
Caveolin (Cav)-1 knockout (KO) mice subjected to permanent left anterior descending coronary artery (LAD) ligation for 24 h showed reduced survival compared with wild-type (WT) mice subjected to the same procedure. n = 24–31 mice/group. *P < 0.05 vs. WT mice.
WT and Cav-1 KO mice subjected to MI display similar infarct size.
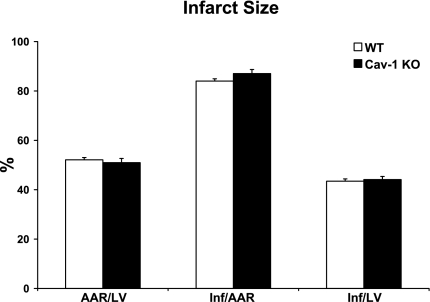
As shown in Fig. 2, Cav-1 KO mice subjected to permanent LAD ligation for 24 h showed similar infarct size/AAR (87.1 ± 1.6%) and infarct size/LV (44.1 ± 1.2%) as WT mice subjected to the same procedure [84.0 ± 1.6% and 43.5 ± 0.9%, respectively, P = not significant (NS)]. Importantly, both WT (52.1 ± 1.6%) and Cav-1 KO (51.0 ± 1.7%) mice subjected to MI displayed comparable AARs (AAR/LV, P = NS; Fig. 2).
Fig. 2.
Cav-1 KO mice subjected to permanent LAD ligation for 24 h showed similar area at risk (AAR)/left ventricle (LV), infarct size (Inf)/AAR, and Inf/LV as WT mice subjected to the same procedure. n = 18–21 mice/group.
Cav-1 KO mice subjected to MI display impaired LV function.
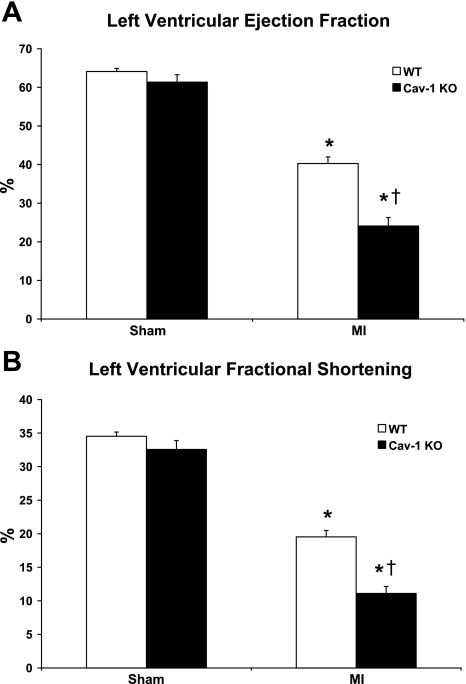
As expected, both WT and Cav-1 KO mice subjected to permanent LAD ligation for 24 h displayed reduced LV function as assessed by transthoracic echocardiography (P < 0.05; Fig. 3 and Table 1). However, Cav-1 KO mice subjected to MI show reduced LV EF (24.1 ± 2.2%) and LV FS (11.1 ± 1.1%) compared with WT mice subjected to the same procedure (40.3 ± 1.7% and 19.6 ± 1.0%, respectively, P < 0.05; Fig. 3). Accordingly, Cav-1 KO mice subjected to MI also displayed increased LV end-diastolic pressures (LVEDP) compared with their WT counterparts (P < 0.05; Table 2). Importantly, heart rates were similar in all groups (P = NS; Table 1).
Fig. 3.
Cav-1 KO mice subjected to permanent LAD ligation for 24 h showed reduced LV ejection fraction (A) and fractional shortening (B) compared with WT mice subjected to the same procedure. n = 6–10 mice/group. *P < 0.05 vs. sham-operated mice (sham); †P < 0.05 vs. WT mice subjected to myocardial ischemia (MI).
Table 1.
Echocardiographic data
| Sham-WT | MI-WT | Sham-Cav-1 KO | MI-Cav-1 KO | |
|---|---|---|---|---|
| Heart rate, beats/min | 367 ± 6 | 374 ± 9 | 375 ± 13 | 374.2 ± 6 |
| Anterior wall diameter | ||||
| Diastolic, mm | 0.80 ± 0.04 | 0.83 ± 0.06 | 0.79 ± 0.03 | 0.67 ± 0.02*†‡ |
| Systolic, mm | 1.29 ± 0.05 | 1.10 ± 0.11 | 1.23 ± 0.04 | 0.89 ± 0.05*† |
| Posterior wall diameter, mm | ||||
| Diastolic, mm | 0.77 ± 0.03 | 0.79 ± 0.05 | 0.82 ± 0.04 | 0.76 ± 0.05 |
| Systolic, mm | 1.23 ± 0.03 | 1.08 ± 0.08 | 1.20 ± 0.05 | 0.89 ± 0.07*† |
| LV diameter, mm | ||||
| End diastolic, mm | 4.01 ± 0.09 | 4.44 ± 0.06* | 3.80 ± 0.15 | 4.41 ± 0.12*† |
| End systolic, mm | 2.59 ± 0.06 | 3.57 ± 0.08* | 2.51 ± 0.11 | 3.92 ± 0.11*†‡ |
Values are means ± SE; n = 6–10 mice/group. Mice were divided into the following groups: wild-type mice subjected to sham operation (sham-WT), WT mice subjected to myocardial ischemia (MI-WT), caveolin-1 knockout mice subjected to sham operation (sham-Cav-1 KO), and caveolin-1 knockout mice subjected to myocardial ischemia (MI-Cav-1 KO). LV, left ventricular.
P < 0.05 vs. sham-WT;
P < 0.05 vs. sham-Cav-1 KO;
P < 0.05 vs. MI-WT.
Table 2.
Morphometric and hemodynamic variables
| Sham-WT | MI-WT | Sham-Cav-1 KO | MI-Cav-1 KO | |
|---|---|---|---|---|
| BW, g | 24.8 ± 0.5 | 25.0 ± 0.3 | 24.6 ± 0.4 | 23.0 ± 0.3*†‡ |
| LV/BW, % | 0.314 ± 0.005 | 0.351 ± 0.007* | 0.359 ± 0.004* | 0.352 ± 0.004* |
| Wet lung weight/BW, % | 0.136 ± 0.003 | 0.203 ± 0.007* | 0.187 ± 0.003* | 0.266 ± 0.008*†‡ |
| Dry lung weight/BW, % | 0.031 ± 0.001 | 0.038 ± 0.001* | 0.044 ± 0.001* | 0.055 ± 0.001*†‡ |
| Dry/wet lung weight, % | 23.2 ± 0.3 | 19.4 ± 0.5* | 23.8 ± 0.3 | 20.9 ± 0.4*†‡ |
| Mean arterial pressure, mmHg | 85.7 ± 3.2 | 73.1 ± 5.0* | 76.7 ± 3.2 | 67.2 ± 4.5* |
| LV end-diastolic pressure, mmHg | 2.8 ± 0.2 | 5.7 ± 0.4* | 2.6 ± 0.2 | 8.3 ± 0.7*†‡ |
| LV +dP/dt, mmHg/s | 5,670 ± 330 | 5,166 ± 316 | 5,407 ± 281 | 4,692 ± 317* |
| LV −dP/dt, mmHg/s | 3,935 ± 229 | 3,362 ± 314 | 3,977 ± 194 | 2,842 ± 259*† |
Values are means ± SE; n = 7–12 mice/group. BW, body weight.
P < 0.05 vs. sham-WT;
P < 0.05 vs. sham-Cav-1 KO;
P < 0.05 vs. MI-WT.
WT and Cav-1 KO mice subjected to MI display similar cardiac NO levels.
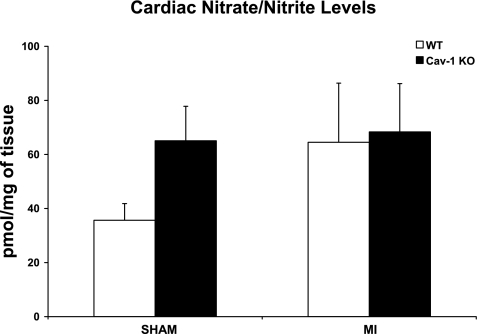
Although there was a trend toward increased cardiac NO metabolites in Cav-1 KO mice subjected to sham surgery compared with their WT counterparts (65.1 ± 12.7 vs. 35.7 ± 6.2 pmol/mg tissue, P = 0.069; Fig. 4), both WT and Cav-1 KO mice subjected to MI displayed similar cardiac NO metabolites (64.5 ± 21.9 vs. 68.3 ± 17.9 pmol/mg, P = NS; Fig. 4).
Fig. 4.
Although there was a trend toward increased cardiac nitric oxide (NO) metabolites in Cav-1 KO mice subjected to sham surgery compared with their WT counterparts, both WT and Cav-1 KO mice subjected to permanent LAD ligation for 24 h showed similar cardiac NO metabolites. n = 7–8 mice/group.
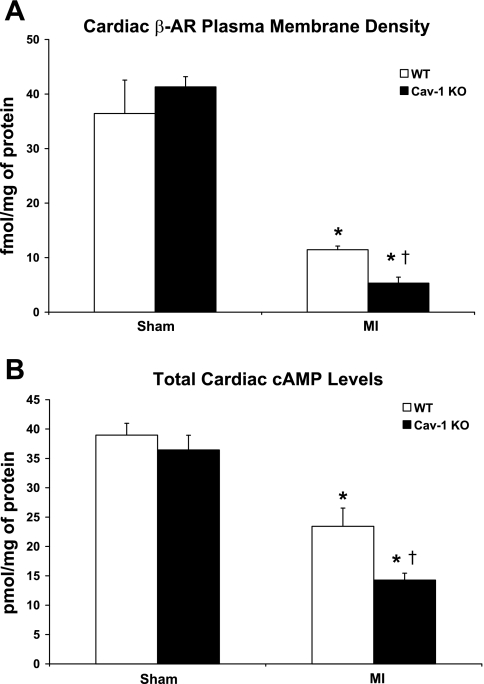
Cav-1 KO mice subjected to MI display reduced plasma membrane β-AR density.
Both WT and Cav-1 KO mice subjected to permanent LAD ligation for 24 h displayed reduced β-AR density at the plasma membrane compared with WT and Cav-1 KO mice subjected to sham surgery (P < 0.05; Fig. 5A). Importantly, Cav-1 KO mice subjected to MI exhibited a marked reduction in plasma membrane β-AR density compared with WT mice subjected to the same procedure (5.3 ± 1.1 vs. 11.5 ± 0.7 fmol/mg, respectively, P < 0.05; Fig. 5A).
Fig. 5.
Cav-1 KO mice subjected to permanent LAD ligation for 24 h showed reduced β-adrenergic receptor (β-AR) plasma membrane density (A) and cardiac cAMP levels (B) compared with WT mice subjected to the same procedure. n = 4–6 mice/group. *P < 0.05 vs. sham mice; †P < 0.05 vs. WT mice subjected to MI.
Cav-1 KO mice subjected to MI display reduced cardiac cAMP levels and PKA activity.
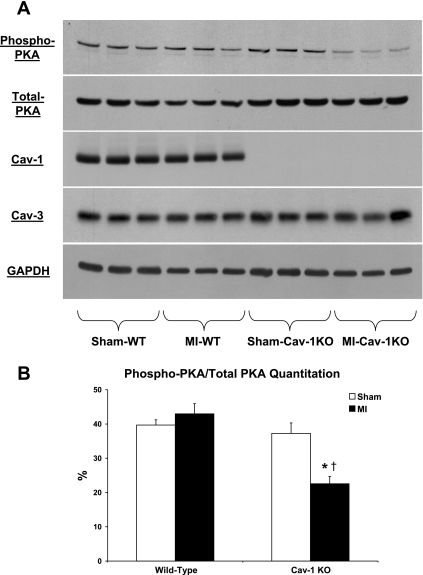
Both WT and Cav-1 KO mice subjected to permanent LAD ligation for 24 h displayed reduced cardiac cAMP levels compared with WT and Cav-1 KO mice subjected to sham surgery (P < 0.05; Fig. 5B). Importantly, Cav-1 KO mice subjected to MI exhibited a greater reduction of cardiac cAMP levels compared with WT mice subjected to the same procedure (14.3 ± 1.2 vs. 23.4 ± 3.1 pmol/mg, respectively, P < 0.05; Fig. 5B). Our Western blot analyses further demonstrated that hearts of Cav-1 KO mice subjected to MI displayed reduced phosphorylation of PKA on the Thr197 residue compared with those of WT mice subjected to the same procedure (P < 0.05; Fig. 6).
Fig. 6.
A: immunoblot analyses showing reduced phosphorylation of PKA in hearts of Cav-1 KO mice subjected to permanent LAD ligation for 24 h compared with those of WT mice subjected to the same procedure. As expected, hearts of Cav-1 KO mice lack Cav-1 expression. Moreover, hearts of WT and Cav-1 KO mice subjected to either sham surgery or LAD ligation displayed similar Cav-3 protein levels (3 hearts are shown for each group). Immunoblot analyis with total PKA and GAPDH are shown as controls for equal protein loading. B: quantitation of phospho-PKA levels. n = 6 mice/group. *P < 0.05 vs. sham mice; †P < 0.05 vs. WT mice subjected to MI.
DISCUSSION
Caveolar microdomains are particularly abundant in cells of the cardiovascular system such as endothelial cells, smooth muscle cells, cardiac fibroblasts, and cardiomyocytes (32, 33, 37, 39). Interestingly, caveolar microdomains have previously been involved in cardioprotection (21, 22). For instance, a reduction of caveolae number has been reported in the rabbit myocardium after severe anoxia/reoxygenation (6). Moreover, exposure of adult mouse cardiomyocytes to isoflurane before simulated ischemia-reperfusion has been shown to increase the number of caveolae and reduce cell death (22). Disruption of caveolae with methyl-β-cyclodextrin (MβCD) has further been shown to abolish ischemic preconditioning- and opioid-induced cardioprotection in adult rat cardiomyocytes subjected to simulated ischemia-reperfusion (21).
Although Cav-3 was originally believed to be the only caveolin isoform present in cardiomyocytes, it is now well recognized that Cav-1 is expressed in all cardiac cell types including cardiomyocytes (2, 3, 11, 22, 29). For instance, coimmunoprecipitation of Cav-1 with Cav-3 was observed in lysates of adult mouse and rat cardiomyocytes (11). Western blot and immunofluorescence analyses also revealed the expression of Cav-1 in adult mouse cardiomyocytes (2, 3, 22). Moreover, freeze-fracture electron microscopy combined with immunogold labeling recently demonstrated the expression of Cav-1 in human cardiomyocytes (29). Importantly, Cav-1 was also recently involved in the pathogenesis of myocardial ischemic injuries (22, 25). For instance, although its total protein expression remained unchanged, a dissociation of Cav-1 from caveolae to the cytosol was reported in hearts of rats subjected to MI (25). This dissociation was suggested to increase cytosolic Cav-1/eNOS complexes and to, consequently, reduce NO production (25). Furthermore, the phosphorylation of Cav-1 has been suggested to contribute to ischemic preconditioning-induced cardioprotection (22). Indeed, Patel et al. (22) reported an increased phosphorylation of Cav-1 in hearts of WT mice after isoflurane exposure and ischemic preconditioning. Mechanistically, the phosphorylation of Cav-1 appears necessary for the recruitment of COOH-terminal Src kinase and the subsequent deactivation of Src (1, 22). Accordingly, Cav-1 KO mice have been shown to be resistant to isoflurane-induced cardiac protection (22). However, although it appears to be involved in ischemic preconditioning, the role of Cav-1 in MI-induced cardiac dysfunction remains unclear. Our present results demonstrate that Cav-1 KO mice subjected to permanent LAD ligation for 24 h displayed reduced survival compared with their WT counterparts. Interestingly, despite similar AAR and infarct sizes, hearts of Cav-1 KO mice subjected to MI displayed impaired cardiac function, as indicated by reduced LV FS and EF and increased LVEDP compared with their WT counterparts.
Mechanistically, Cav-1 is particularly well known for its negative regulation of eNOS activity (7, 8). Indeed, direct interaction of eNOS with the Cav-1 scaffolding domain (residues 82–101) has previously been shown to inhibit eNOS activity (7, 8). Accordingly, Cav-1 KO mice display decreased vascular tone and microvascular hyperpermeability secondary to eNOS hyperactivation (26, 34). Therefore, to evaluate if increased NO production could be involved in the impaired cardiac function observed in Cav-1 KO mice subjected to MI, we subsequently determined the cardiac NO metabolites in hearts of WT and Cav-1 KO mice subjected to either sham surgery or LAD ligation. Interestingly, although there was a trend toward increased cardiac NO metabolites in Cav-1 KO mice subjected to sham surgery compared with WT mice subjected to the same procedure, both WT and Cav-1 KO mice subjected to LAD ligation displayed similar cardiac nitrate/nitrite levels. Thus, the impaired cardiac function observed in Cav-1 KO mice subjected to LAD ligation is unlikely to be ascribed to increased NO production. However, further studies with NO inhibitors are warranted to confirm such a conclusion.
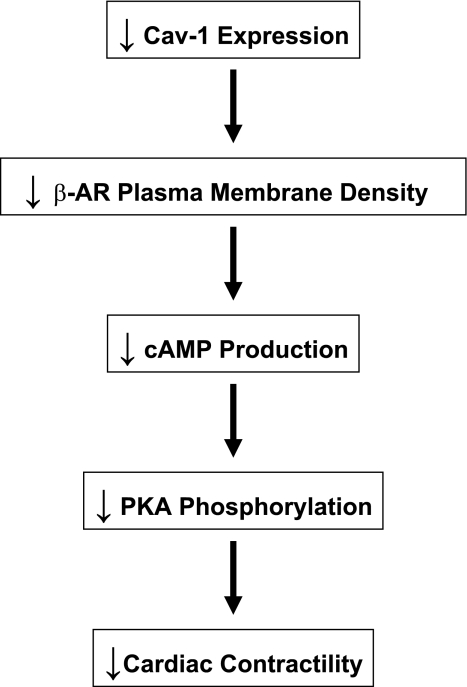
Activation of the β-adrenergic signaling pathway also plays an important role in the regulation of cardiac function. The β-adrenergic signaling pathway consists of β-ARs activating the G protein subunit, Gαs, which, in turn, stimulates the effector, adenylate cyclase (AC). AC is then responsible for the production of the second messenger cAMP, which activates PKA. PKA subsequently phosphorylates different proteins involved in cardiac inotropy, chronotropy, and lusitropy, such as L-type Ca2+ channels, phospholamban, and troponin I. Importantly, caveolar domains have previously been shown to compartmentalize several molecules involved in the β-adrenergic signaling pathway, such as β-AR, Gαs, AC, cAMP, and PKA (20, 27, 35, 36). Caveolar domains may thus serve as platforms to optimize cardiac β-adrenergic signaling. Accordingly, disruption of caveolar domains with MβCD has previously been shown to inhibit forskolin- and isoproterenol-stimulated cAMP production in cultured rat neonatal cardiomyocytes (18). On the other hand, Cav proteins may also promote signaling via enhanced receptor-effector coupling. In fact, Cav-3 has been shown to directly interact with β-AR, Gαs, and AC in rat cardiomyocytes (10, 19, 31). However, although previous reports have investigated the function of caveolae and Cav-3 in β-adrenergic signaling, the role of Cav-1 in the modulation of cardiac β-adrenergic signaling still remains elusive. Interestingly, our present results demonstrate that hearts of Cav-1 KO mice subjected to MI exhibit reduced plasma membrane β-AR density. In addition, hearts of Cav-1 KO mice subjected to permanent LAD ligation displayed reduced cAMP levels as well as reduced PKA activity, as assessed by the phosphorylation of PKA on the Thr197 residue, compared with their WT counterparts. The impaired LV function observed in Cav-1 KO mice subjected to MI could thus be attributed, at least in part, to reduced β-adrenergic signaling (Fig. 7). Importantly, as Cav-1 KO cardiomyocytes retain caveolae formation (4), the defects in β-adrenergic signaling observed here are likely to be ascribed to a lack of Cav-1 protein expression. Cav-1 might thus act as a chaperone or scaffolding protein that spatially and temporally facilitates the interactions of the different members of the β-adrenergic signaling pathway. Accordingly, decreased responses to the AC agonist forskolin as well as reduced cAMP levels in response to the β3-AR agonist CL-316,243 were recently reported in primary adipocytes derived from Cav-1 KO mice (16). Furthermore, ablation of the Cav-1 gene has been shown to alter β-AR function and reduce PKA activity in the mouse small intestine (5).
Fig. 7.
Diagram summarizing the effect of a genetic ablation of Cav-1 on cardiac contractility in mice subjected to permanent LAD ligation for 24 h. Mechanistically, we show that ablation of the Cav-1 gene reduces β-AR plasma membrane density, which, in turn, reduces cAMP generation and PKA phosphorylation.
Study limitations.
In the present study, we used a mouse model of permanent LAD ligation. However, permanent coronary occlusion is relatively rare clinically compared with occlusion/reperfusion. Importantly, Patel et al. (22) previously reported similar infarct sizes in WT and Cav-1 KO mice subjected to LAD ligation for 30 min followed by 2 h of reperfusion. Nevertheless, future studies should determine the effect of a genetic ablation of Cav-1 on cardiac function and activation of the β-adrenergic signaling pathway in models of coronary occlusion/reperfusion.
The exclusion of dead animals from the AAR and infarct size measurements could have created a potential selection bias as Cav-1 KO animals that died could have had larger infarcts. However, as mentioned above, similar infarct sizes have been reported in WT and Cav-1 KO mice subjected to coronary occlusion/reperfusion (22). Nonetheless, our results demonstrate the importance of exacerbated contractile dysfunction in the absence of infarct alterations. Indeed, despite similar AAR and infarct sizes, hearts of Cav-1 KO mice subjected to MI displayed impaired cardiac function, as indicated by reduced LV FS and EF and increased LVEDP compared with their WT counterparts. Exacerbated contractile dysfunction could thus contribute to the reduced survival observed in Cav-1 KO mice subjected to MI.
Conclusions.
Ablation of the Cav-1 gene exacerbates cardiac dysfunction and reduces survival in mice subjected to myocardial ischemic injury. Mechanistically, Cav-1 KO mice subjected to permanent LAD ligation display abnormalities in β-adrenergic signaling.
GRANTS
This work was supported by grants from the National Institutes of Health (NIH) and the American Heart Association (to M. P. Lisanti) as well as by NIH Grants R01-HL-085503 and P01-HL-075443 (to W. J. Koch). J.-F. Jasmin is the recipient of an American Lung Association Biomedical Research Grant. A. Lymperopoulos is the recipient of a Scientist Development Grant Award from the American Heart Association (National Center).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Cao H, Sanguinetti AR, Mastick CC. Oxidative stress activates both Src-kinases and their negative regulator Csk and induces phosphorylation of two targeting proteins for Csk: caveolin-1 and paxillin. Exp Cell Res 294: 159– 171, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Cho WJ, Chow AK, Schulz R, Daniel EE. Matrix metalloproteinase-2, caveolins, focal adhesion kinase and c-Kit in cells of the mouse myocardium. J Cell Mol Med 11: 1069– 1086, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chow AK, Cena J, El Yazbi AF, Crawford BD, Holt A, Cho WJ, Daniel EE, Schulz R. Caveolin-1 inhibits matrix metalloproteinase-2 activity in the heart. J Mol Cell Cardiol 42: 896– 901, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Cohen AW, Park DS, Woodman SE, Williams TM, Chandra M, Shirani J, Pereira dS, Kitsis RN, Russell RG, Weiss LM, Tang B, Jelicks LA, Factor SM, Shtutin V, Tanowitz HB, Lisanti MP. Caveolin-1 null mice develop cardiac hypertrophy with hyperactivation of p42/44 MAP kinase in cardiac fibroblasts. Am J Physiol Cell Physiol 284: C457– C474, 2003 [DOI] [PubMed] [Google Scholar]
- 5. El Yazbi AF, Cho WJ, Schulz R, Daniel EE. Caveolin-1 knockout alters beta-adrenoceptors function in mouse small intestine. Am J Physiol Gastrointest Liver Physiol 291: G1020– G1030, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Frank JS, Beydler S, Kreman M, Rau EE. Structure of the freeze-fractured sarcolemma in the normal and anoxic rabbit myocardium. Circ Res 47: 131– 143, 1980 [DOI] [PubMed] [Google Scholar]
- 7. Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the NOS caveolin binding domain in vivo. J Biol Chem 272: 25437– 25440, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Garcia-Cardena G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci USA 93: 6448– 6453, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glenney JR, Jr, Soppet D. Sequence and expression of caveolin, a protein component of caveolae plasma membrane domains phosphorylated on tyrosine in Rous sarcoma virus-transformed fibroblasts. Proc Natl Acad Sci USA 89: 10517– 10521, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Head BP, Patel HH, Roth DM, Lai NC, Niesman IR, Farquhar MG, Insel PA. G-protein-coupled receptor signaling components localize in both sarcolemmal and intracellular caveolin-3-associated microdomains in adult cardiac myocytes. J Biol Chem 280: 31036– 31044, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem 281: 26391– 26399, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Jasmin JF, Malhotra S, Singh DM, Mercier I, Rosenbaum DM, Lisanti MP. Caveolin-1 deficiency increases cerebral ischemic injury. Circ Res 100: 721– 729, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Lisanti MP, Scherer PE, Tang ZL, Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol 4: 231– 235, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Lymperopoulos A, Rengo G, Funakoshi H, Eckhart AD, Koch WJ. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nat Med 13: 315– 323, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Mahmoudi M, Willgoss D, Cuttle L, Yang T, Pat B, Winterford C, Endre Z, Johnson DW, Gobe GC. In vivo and in vitro models demonstrate a role for caveolin-1 in the pathogenesis of ischaemic acute renal failure. J Pathol 200: 396– 405, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Mattsson CL, Andersson ER, Nedergaard J. Differential involvement of caveolin-1 in brown adipocyte signaling: impaired β3-adrenergic, but unaffected LPA, PDGF and EGF receptor signaling. Biochim Biophys Acta 1803: 983– 989, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Michael LH, Entman ML, Hartley CJ, Youker KA, Zhu J, Hall SR, Hawkins HK, Berens K, Ballantyne CM. Myocardial ischemia and reperfusion: a murine model. Am J Physiol Heart Circ Physiol 269: H2147– H2154, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Ostrom RS, Bundey RA, Insel PA. Nitric oxide inhibition of adenylyl cyclase type 6 activity is dependent upon lipid rafts and caveolin signaling complexes. J Biol Chem 279: 19846– 19853, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Ostrom RS, Gregorian C, Drenan RM, Xiang Y, Regan JW, Insel PA. Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. J Biol Chem 276: 42063– 42069, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Ostrom RS, Violin JD, Coleman S, Insel PA. Selective enhancement of beta-adrenergic receptor signaling by overexpression of adenylyl cyclase type 6: colocalization of receptor and adenylyl cyclase in caveolae of cardiac myocytes. Mol Pharmacol 57: 1075– 1079, 2000 [PubMed] [Google Scholar]
- 21. Patel HH, Head BP, Petersen HN, Niesman IR, Huang D, Gross GJ, Insel PA, Roth DM. Protection of adult rat cardiac myocytes from ischemic cell death: role of caveolar microdomains and delta opioid receptors. Am J Physiol Heart Circ Physiol 291: H344– H350, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Patel HH, Tsutsumi YM, Head BP, Niesman IR, Jennings M, Horikawa Y, Huang D, Moreno AL, Patel PM, Insel PA, Roth DM. Mechanisms of cardiac protection from ischemia-reperfusion injury: a role for caveolae and caveolin-1. FASEB J 21: 1565– 1574, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Pleger ST, Most P, Boucher M, Soltys S, Chuprun JK, Pleger W, Gao E, Dasgupta A, Rengo G, Remppis A, Katus HA, Eckhart AD, Rabinowitz JE, Koch WJ. Stable myocardial-specific AAV6-S100A1 gene therapy results in chronic functional heart failure rescue. Circulation 115: 2506– 2515, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Pleger ST, Remppis A, Heidt B, Volkers M, Chuprun JK, Kuhn M, Zhou RH, Gao E, Szabo G, Weichenhan D, Muller OJ, Eckhart AD, Katus HA, Koch WJ, Most P. S100A1 gene therapy preserves in vivo cardiac function after myocardial infarction. Mol Ther 12: 1120– 1129, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Ratajczak P, Damy T, Heymes C, Oliviero P, Marotte F, Robidel E, Sercombe R, Boczkowski J, Rappaport L, Samuel JL. Caveolin-1 and -3 dissociations from caveolae to cytosol in the heart during aging and after myocardial infarction in rat. Cardiovasc Res 57: 358– 369, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem 276: 38121– 38138, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Razani B, Rubin CS, Lisanti MP. Regulation of cAMP-mediated signal transduction via interaction of caveolins with the catalytic subunit of protein kinase A. J Biol Chem 274: 26353– 26360, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, Koch WJ. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation 119: 89– 98, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robenek H, Weissen-Plenz G, Severs NJ. Freeze-fracture replica immunolabelling reveals caveolin-1 in the human cardiomyocyte plasma membrane. J Cell Mol Med 12: 2519– 2521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell 68: 673– 682, 1992 [DOI] [PubMed] [Google Scholar]
- 31. Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of β-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J Biol Chem 275: 41447– 41457, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, Couet J, Kohtz DS, van Donselaar E, Peters P, Lisanti MP. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem 272: 29337– 29346, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci USA 93: 131– 135, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schubert W, Frank PG, Woodman SE, Hyogo H, Cohen DE, Chow CW, Lisanti MP. Microvascular hyperpermeability in caveolin-1−/− knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, l-NAME, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem 277: 40091– 40098, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Schwencke C, Okumura S, Yamamoto M, Geng YJ, Ishikawa Y. Colocalization of β-adrenergic receptors and caveolin within the plasma membrane. J Cell Biochem 75: 64– 72, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Schwencke C, Yamamoto M, Okumura S, Toya Y, Kim SJ, Ishikawa Y. Compartmentation of cyclic adenosine 3′,5′-monophosphate signaling in caveolae. Mol Endocrinol 13: 1061– 1070, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J Biol Chem 271: 9690– 9697, 1996 [DOI] [PubMed] [Google Scholar]
- 38. Sonveaux P, Martinive P, DeWever J, Batova Z, Daneau G, Pelat M, Ghisdal P, Gregoire V, Dessy C, Balligand JL, Feron O. Caveolin-1 expression is critical for vascular endothelial growth factor-induced ischemic hindlimb collateralization and nitric oxide-mediated angiogenesis. Circ Res 95: 154– 161, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Tang Z, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem 271: 2255– 2261, 1996 [DOI] [PubMed] [Google Scholar]