Abstract
Hyperglycemia in diabetes mellitus promotes oxidative stress in endothelial cells, which contributes to development of cardiovascular diseases. Nuclear factor erythroid 2-related factor-2 (Nrf2) is a transcription factor activated by oxidative stress that regulates expression of numerous reactive oxygen species (ROS) detoxifying and antioxidant genes. This study was designed to elucidate the homeostatic role of adaptive induction of Nrf2-driven free radical detoxification mechanisms in endothelial protection under diabetic conditions. Using a Nrf2/antioxidant response element (ARE)-driven luciferase reporter gene assay we found that in a cultured coronary arterial endothelial cell model hyperglycemia (10–30 mmol/l glucose) significantly increases transcriptional activity of Nrf2 and upregulates the expression of the Nrf2 target genes NQO1, GCLC, and HMOX1. These effects of high glucose were significantly attenuated by small interfering RNA (siRNA) downregulation of Nrf2 or overexpression of Keap-1, which inactivates Nrf2. High-glucose-induced upregulation of NQO1, GCLC, and HMOX1 was also prevented by pretreatment with polyethylene glycol (PEG)-catalase or N-acetylcysteine, whereas administration of H2O2 mimicked the effect of high glucose. To test the effects of metabolic stress in vivo, Nrf2+/+ and Nrf2−/− mice were fed a high-fat diet (HFD). HFD elicited significant increases in mRNA expression of Gclc and Hmox1 in aortas of Nrf2+/+ mice, but not Nrf2−/− mice, compared with respective standard diet-fed control mice. Additionally, HFD-induced increases in vascular ROS levels were significantly greater in Nrf2−/− than Nrf2+/+ mice. HFD-induced endothelial dysfunction was more severe in Nrf2−/− mice, as shown by the significantly diminished acetylcholine-induced relaxation of aorta of these animals compared with HFD-fed Nrf2+/+ mice. Our results suggest that adaptive activation of the Nrf2/ARE pathway confers endothelial protection under diabetic conditions.
Keywords: diabetes, oxidative stress, endothelial dysfunction
in diabetic patients poorly controlled hyperglycemia leads to multiple vascular complications, including diabetic microvascular disease (retinopathy, neuropathy, and nephropathy) and macrovascular disease (ischemic heart disease and stroke), which are a major cause of patient mortality. There is strong evidence that the mechanisms by which hyperglycemia promotes the development of the aforementioned diseases include impairment of endothelial cell function. A common feature of endothelial cell dysfunction in hyperglycemia is increased production of reactive oxygen species (ROS) by mitochondria (27, 32) and/or NAD(P)H oxidases (6) and accumulation of oxidatively damaged macromolecules. Recent studies indicate that in cultured endothelial cells under oxidative stress conditions adaptive mechanisms can manifest themselves that involve responses mediated by nuclear factor erythroid 2-related factor-2 (Nrf2). Nrf2 is a transcription factor that regulates expression of numerous ROS detoxifying and antioxidant genes and is known to have an important role in redox homeostasis in endothelial cells (4, 17, 18, 22). However, the role of Nrf2-driven free radical detoxification mechanisms in endothelial protection under diabetic conditions is not well understood.
The present study was designed to determine whether hyperglycemia activates Nrf2 in endothelial cells and whether induction of Nrf2-regulated ROS detoxification systems protects endothelial function in type 2 diabetes. To test our hypotheses, we assessed Nrf2 activation in response to high-glucose treatment in cultured primary human coronary arterial endothelial cells (CAECs) and characterized the effect of disruption of the Nrf2/antioxidant response element (ARE) pathway on high-glucose-induced antioxidant gene expression. The relevance of Nrf2-mediated effects in vivo was tested by assessing high-fat diet-induced endothelial dysfunction and vascular oxidative stress in both wild-type and Nrf2−/− mice.
METHODS
Cell cultures, knockdown of Nrf2, and Keap-1 overexpression.
Primary human CAECs (purchased from Cell Applications) were cultured in MesoEndo Cell Growth Medium (Cell Applications) supplemented with 10% serum in the presence of varying glucose concentrations (5–30 mmol/l). Mannitol was used as osmotic control. To disrupt Nrf2 signaling, Nrf2 was downregulated by RNA interference using proprietary small interfering RNA (siRNA) sequences (Origen) and the electroporation-based Amaxa Nucleofector technology (Amaxa, Gaithersburg, MD), as we have previously reported (7, 8, 38). Cell density at transfection was 30%. Experiments were performed on day 2 after the transfection, when gene silencing was optimal. Keap-1 overexpression was achieved in CAECs by transfection with a Keap-1 full-length cDNA encoding plasmid (Origen) as described previously (10). In separate experiments, CAECs were pretreated with N-acetylcysteine (25 mmol/l) or polyethylene glycol (PEG)-catalase (1,000 U/ml) and then exposed to high glucose (30 mmol/l). In other studies, CAECs were treated with H2O2 (10−5 mol/l) or the prototypical Nrf2 activator sulforaphane (2.5 μmol/l) to induce Nrf2-mediated gene transcription.
Measurement of high-glucose-induced changes in mitochondrial ROS production in CAECs.
The effect of high-glucose treatment (30 mmol/l, for 24 h) on mitochondrial O2·− production in CAECs was assessed by flow cytometry (Guava Easycyte, Millipore, Billerica, MA) using MitoSOX Red (Invitrogen, Carlsbad CA), a mitochondrion-specific hydroethidine-derivative fluorescent dye, as previously reported (38). Cell debris (low forward and side scatter) and apoptotic cells [terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) positive] were gated out for analysis (38). The data are presented as fold change in the mean intensity of MitoSOX fluorescence compared with the respective controls. Fluorescent images of CAECs stained with MitoSOX were captured with a Nikon Eclipse Ti-U inverted fluorescent microscope equipped with a CoolSnap HQ camera. Hoechst 33258 was used for nuclear counterstaining.
Transient transfection and Nrf2 and NF-κB reporter gene assays.
The effect of increasing glucose concentrations (from 5 to 30 mmol/l) on Nrf2 activity in CAECs was assessed with a reporter gene assay. Upon activation Nrf2 translocates to the nucleus, where it binds to the ARE to activate transcription of phase II and antioxidant defense enzymes. We used an ARE reporter comprised of tandem repeats of the ARE transcriptional response element upstream of firefly luciferase (SA Biosciences, Frederick, MD) and a Renilla luciferase plasmid under the control of the cytomegalovirus (CMV) promoter (as an internal control).
The effect of high-glucose treatment on NF-κB activity in CAECs was tested by a reporter gene assay as described previously (13). We used a NF-κB reporter comprised of an NF-κB response element upstream of firefly luciferase (NF-κB-Luc, Stratagene) and a Renilla luciferase plasmid under the control of the CMV promoter.
Transfections in CAECs were performed with the Amaxa Nucleofector technology, as we have previously reported (7, 14, 16). Firefly and Renilla luciferase activities were assessed after 24 h with the Dual Luciferase Reporter Assay Kit (Promega) and a Tecan Infinite M200 plate reader.
Quantitative real-time RT-PCR.
A quantitative real-time RT-PCR technique was used to analyze mRNA expression of the Nrf2/ARE target genes NAD(P)H:quinine oxidoreductase 1 (NQO1), heme oxygenase-1 (Hmox1), and γ-glutamylcysteine synthetase (GCLC) and mRNA expression of the NF-κB target gene ICAM1 and the proinflammatory cytokine TNF in high-glucose-treated CAECs and in aortic segments, as previously reported (11, 15, 40, 42). In brief, total RNA was isolated with a Mini RNA Isolation Kit (Zymo Research, Orange, CA) and was reverse transcribed with SuperScript III reverse transcriptase (Invitrogen) as described previously (13, 15). A real-time RT-PCR technique was used to analyze mRNA expression with the Stratagen MX3000, as reported previously (13). Amplification efficiencies were determined with a dilution series of a standard vascular sample. Quantification was performed with the efficiency-corrected ΔΔCq method (where Cq is quantification cycle). The relative quantities of the human reference genes GAPDH, HPRT, and ACTB (β-actin) and the mouse reference genes Hprt, Ywhaz, and Actb were determined, and a normalization factor was calculated based on their geometric mean for internal normalization. Oligonucleotides used for quantitative real-time RT-PCR are listed in Table 1. Fidelity of the PCR reaction was determined by melting temperature analysis and visualization of product on a 2% agarose gel.
Table 1.
Oligonucleotides for real-time RT-PCR
| mRNA Targets | Descriptions | Species | Sense | Antisense |
|---|---|---|---|---|
| NQO1 | NAD(P)H:quinine oxidoreductase 1 (NAD(P)H dehydrogenase, quinine 1) | Homo sapiens | AGACCTTGTGATATTCCAGTTC | GGCAGCGTAAGTGTAAGC |
| GCLC | γ-Glutamylcysteine synthetase (glutamate-cysteine ligase, catalytic subunit) | H. sapiens | CAGTGGTGGATGGTTGTG | ATTGATGATGGTGTCTATGC |
| HMOX1 | Heme oxygenase-1 | H. sapiens | AAGTATCCTTGTTGACACG | TGAGCCAGGAACAGAGTG |
| ICAM1 | Intercellular adhesion molecule 1 | H. sapiens | CTCTCGCTCTGTCACC | GGAAGTCTGGGCAATGT |
| HPRT | Hypoxanthine phosphoribosyltransferase 1 | H. sapiens | CCGTGTGTTAGAAAAGTAAGAAGC | AACTGCTGACAAAGATTCACTGG |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | H. sapiens | AACGAATTTGGCTACAGC | AGGGTACTTTATTGATGGTACAT |
| ACTB | β-Actin | H. sapiens | CGGTGAAGGTGACAGCAG | TGTGTGGACTTGGGAGAGG |
| Nqo1 | NAD(P)H:quinone oxidoreductase 1 | Mus musculus | ATGAAGGAGGCTGCTGTAG | AGATGACTCGGAAGGATACTG |
| Gclc | γ-Glutamylcysteine synthetase | M. musculus | AGCATCTGGAGAACTAATGACTG | CAAGTAACTCTGGACATTCACAC |
| Hmox1 | Heme oxygenase-1 | M. musculus | CTGTGAACTCTGTCCAATG | AACTGTGTCAGGTATCTCC |
| Icam1 | Intercellular adhesion molecule 1 | M. musculus | TTCACCAGTCACATAAACACT | CTTGCACGACCCTTCTA |
| Tnf | Tumor necrosis factor (TNF superfamily, member 2) | M. musculus | GCACCACCATCAAGGACTC | AGGTCTGAAGGTAGGAAGGC |
| Hprt | Hypoxanthine phosphoribosyltransferase 1 | M. musculus | TGCTGCGTCCCCAGACTTTTG | AGATAAGCGACAATCTACCAGAGG |
| Ywhaz | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, ζ polypeptide | M. musculus | ACTGTCTTGTCACCAACCATTC | GGGCTGTAGAGAGGATGAGG |
| Actb | β-Actin | M. musculus | AATAAGTGGTTACAGGAAGTC | ATGAAGTATTAAGGCGGAAG |
Western blotting.
To analyze protein expression of the Nrf2 targets NQO1, glutathione peroxidase (GPX), and GCLC, Western blotting was performed as described previously (12) with the following primary antibodies: rabbit anti-GCLC (Abcam, ab41463; 1 μg/ml in 5% milk), rabbit anti-NQO1 (Abcam; ab34173, 1:2,000 in 5% milk), rabbit anti-GPX (Abcam, ab22604; 1:6,000 in 5% milk). All polyvinylidene difluoride (PVDF) membranes were incubated in primary antibodies overnight at 4°C. A donkey anti-rabbit secondary antibody was used (Abcam, ab16284; 1:2,000 in 5% milk). Mouse anti-β-actin (Abcam, ab6276; 1:10,000 in 5% milk) and Coomassie staining were used for normalization purposes.
TUNEL assay.
Cellular stress resistance to high-glucose-induced apoptosis was assessed by growing cells in 96-well plates and treating them with high glucose (30 mmol/l, for 24 h). After the treatment period the ratio of TUNEL-positive cells, a marker of apoptosis, was determined by flow cytometry (Guava Easycyte) using the Guava TUNEL Assay (Millipore) according to the manufacturer's guidelines.
Animal studies.
Male ICR wild-type mice (Nrf2+/+) were purchased from Taconic, and male Nrf2 knockout mice on an ICR background (Nrf2−/−) were obtained from a well-characterized colony (30) at the National Institute on Aging. The mice were housed in an environmentally controlled vivarium with unlimited access to water and a controlled photoperiod (12 h light, 12 h dark). Body weight was recorded biweekly. All mice were maintained according to National Institutes of Health guidelines, and all animal use protocols were approved by the Institutional Animal Care and Use Committees of the participating institutions. At 20 wk of age, mice were assigned to two groups and were fed a standard AIN-93G diet (SD) or AIN-93G modified to provide 60% of calories from fat [high-fat diet (HFD)], as described previously (29). Nrf2+/+ (n = 10) and Nrf2−/− (n = 16) mice fed HFD in these cohorts gained 43.0 ± 3.0% and 42.0 ± 4.6% of their starting weight, respectively (35).
Serum biomarkers.
Blood glucose was determined with an Ascensia Elite glucose meter (Bayer, Mishawaka, IN). Serum insulin levels were determined with an Ultra Sensitive Mouse Insulin ELISA Kit (Crystal Chem, Downers Grove, IL). Total cholesterol, nonesterified fatty acid, and triglyceride levels were determined with the Cholesterol E kit, the NEFA-HR kit, and the L-Type Triglyceride M kit (each manufactured by Wako Chemicals, Richmond, VA), respectively. Adiponectin levels were determined with a Mouse Adiponectin ELISA kit (GenWay Biotech, San Diego, CA) according to the manufacturer's instructions.
Assessment of effect of HFD on vascular endothelial function.
Animals were euthanized at 36 wk of age, aortas were isolated, and endothelial function was assessed by measuring relaxation of aortic ring preparations to acetylcholine (ACh) as previously described (29). In brief, an aorta ring segment 2 mm in length was isolated from each animal and mounted on 40-μm stainless steel wires in myograph chambers (Danish Myo Technology) for measurement of isometric tension. The vessels were superfused with Krebs buffer solution (in mM: 118 NaCl, 4.7 KCl, 1.5 CaCl2, 25 NaHCO3, 1.1 MgSO4, 1.2 KH2PO4, and 5.6 glucose; at 37°C, gassed with 95% air and 5% CO2). After an equilibration period of 1 h during which an optimal passive tension was applied to the rings (as determined from the vascular length-tension relationship), relaxation of precontracted (by 10−6 mol/l phenylephrine) vessels to ACh (from 10−9 to 10−6 mol/l) was obtained.
Measurement of vascular ROS production.
The oxidative fluorescent dyes dihydroethidium (DHE) and 5-(and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (H2DCFDA) were used to assess vascular O2·− production and cytoplasmic peroxide levels in segments of en face preparations of the aortas as we have previously reported (10, 11, 29). In brief, vessels were incubated with DHE (3 × 10−6 mol/l; at 37°C) or H2DCFDA and the time course for the increase of ethidium fluorescence or H2DCFDA fluorescence was recorded for 30 min with a Tecan Infinite M200 plate reader (8, 12). The slope factor was calculated and normalized to tissue mass. Unstained aortas and vessels preincubated with PEG-SOD or PEG-catalase were used for background correction and negative control, respectively.
Data analysis.
Gene expression data were normalized to the respective control mean values. Statistical analyses of data were performed by Student's t-test or by two-way ANOVA followed by the Tukey post hoc test, as appropriate. P < 0.05 was considered statistically significant. Data are expressed as means ± SE.
RESULTS
High glucose increases transcriptional activity of Nrf2 in CAECs.
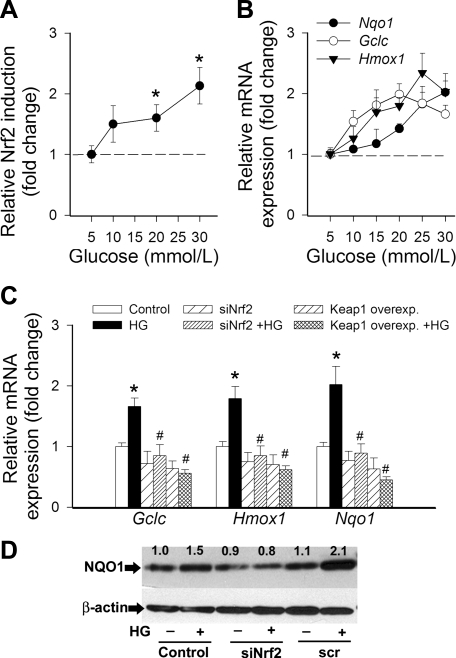
To determine the effect of hyperglycemia on Nrf2 activation, we transiently transfected CAECs with a Nrf2/ARE-driven reporter gene construct and then treated the cells with high glucose. A significant, concentration-dependent increase in luciferase activity compared with the vector control was noted upon stimulation with glucose (Fig. 1A).
Fig. 1.
A: reporter gene assay showing the effects of increasing concentrations of glucose on nuclear factor erythroid 2-related factor-2 (Nrf2)/antioxidant response element (ARE) reporter activity in cultured primary human coronary arterial endothelial cells (CAECs). Cells were transiently cotransfected with ARE-driven firefly luciferase and cytomegalovirus (CMV)-driven Renilla luciferase constructs followed by glucose treatment. Cells were then lysed and subjected to luciferase activity assay. After normalization relative luciferase activity was obtained from 4–6 independent transfections. Data are means ± SE. *P < 0.05. B: effect of glucose on mRNA expression of Nqo1, Gclc, and Hmox1 in cultured primary CAECs. Data are means ± SE (n = 5 in each group). The effects of glucose were significant (P < 0.05) for each target. C: effects of small interfering RNA (siRNA) downregulation of Nrf2 (siNrf2) or overexpression of Keap-1 on high glucose (HG, 30 mmol/l)-induced mRNA expression of Nqo1, Gclc, and Hmox1 in cultured primary CAECs. Data are means ± SE (n = 5 in each group). *P < 0.05 vs. control; #P < 0.05 vs. HG only. D: representative Western blots showing protein expression of NQO1 in CAECs treated with HG, with or without siNrf2 treatment (scr, scrambled siRNA control). β-Actin was used as a loading control. Numbers are normalized densitometric values (see methods). Experiments were repeated 3 times with similar results.
High glucose upregulates Nrf2/ARE-driven genes in CAECs: role for increased ROS production.
Glucose, in a concentration-dependent manner, significantly increased mRNA expression of the known Nrf2 targets NQO1, GCLC, and HMOX1 (Fig. 1B). Overexpression of Keap-1 or siRNA knockdown of Nrf2 prevented high-glucose-induced upregulation of NQO1, GCLC, and HMOX1 (Fig. 1C). Western blotting showed that protein expression of NQO1 was upregulated by high-glucose treatment, and this effect was prevented by siRNA downregulation of NRF2 (Fig. 1D).
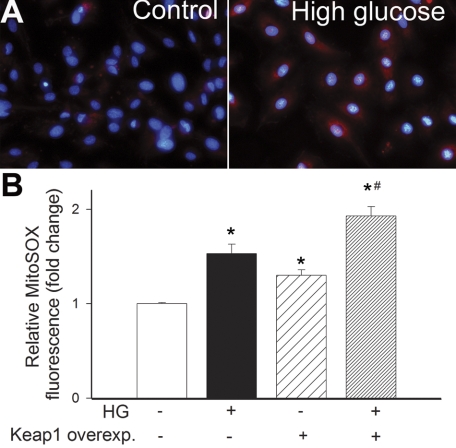
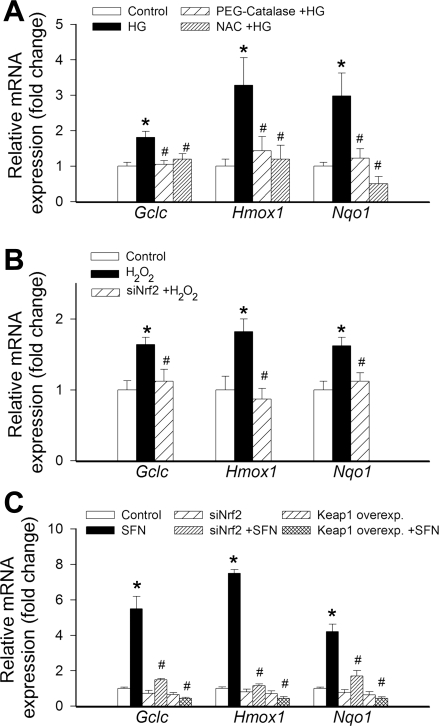
Hyperglycemia significantly increases mitochondrial ROS production, as shown by the intense MitoSOX staining in high-glucose-treated CAECs (Fig. 2). Overexpression of Keap-1 significantly increased MitoSOX staining in high-glucose-treated CAECs (Fig. 2B). To determine the role of increased ROS in high–glucose-mediated induction of Nrf2 targets, we treated the CAECs with scavengers of H2O2. We found that pretreatment with both PEG-catalase and N-acetylcysteine prevented high-glucose-mediated upregulation of NQO1, GCLC, and HMOX1 (Fig. 3A). Treatment of CAECs with H2O2 also resulted in upregulation of NQO1, GCLC, and HMOX1, and these effects were prevented by knockdown of Nrf2 (Fig. 3B). The prototypical Nrf2 activator sulforaphane also induced NQO1, GCLC, and HMOX1, and these effects were prevented by both siRNA knockdown of Nrf2 and overexpression of Keap-1 (Fig. 3C).
Fig. 2.
A: representative fluorescent images showing stronger MitoSOX staining (red fluorescence) in high glucose-treated cultured primary human CAECs compared with untreated controls. Original magnification ×10. Blue fluorescence: nuclear counterstaining with Hoechst 33258. B: in CAECs high glucose (HG, 30 mmol/l) induces mitochondrial oxidative stress, as shown by the significant increases in the mean fluorescence intensity of oxidized MitoSOX (flow cytometry data). Overexpression of Keap-1 significantly increases HG-induced mitochondrial O2·− production. Data are means ± SE (n = 6 in each group). *P < 0.05 vs. baseline; #P < 0.05 vs. HG only.
Fig. 3.
A: effect of pretreatment with polyethylene glycol (PEG)-catalase (1,000 U/ml) and N-acetylcysteine (NAC, 25 mmol/l) on high glucose (HG, 30 mmol/l, for 24 h)-induced changes in mRNA expression of Nqo1, Gclc, and Hmox1 in cultured primary human CAECs. Data are means ± SE (n = 5 in each group). *P < 0.05 vs. control; #P < 0.05 vs. HG only. B: effect of siRNA knockdown of Nrf2 (siNrf2) on H2O2 (10−5 mol/l, for 24 h)-induced changes in mRNA expression of Nqo1, Gclc, and Hmox1 in cultured primary human CAECs. Data are means ± SE (n = 5 in each group). *P < 0.05 vs. control; #P < 0.05 vs. H2O2 only. C: effects of siNrf2 and overexpression of Keap-1 on sulforaphane (SFN, 2.5 μmol/l, for 24 h)-induced changes in mRNA expression of Nqo1, Gclc, and Hmox1 in cultured primary human CAECs. Data are means ± SE (n = 5 in each group). *P < 0.05 vs. control; #P < 0.05 vs. SFN only.
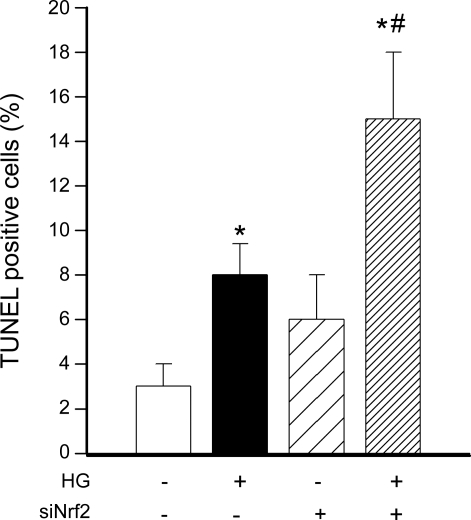
Nrf2-dependent attenuation of high-glucose-induced endothelial apoptosis.
To assess the role of adaptive Nrf2 activation in regulation of endothelial apoptosis, CAECs were treated with high glucose and TUNEL assay was performed. The level of TUNEL-positive apoptotic cells was low in untreated samples. Treatment with high glucose significantly increased the rate of apoptosis in CAECs (Fig. 4). siRNA knockdown of Nrf2 significantly augmented the proapoptotic effect of high-glucose treatment (Fig. 4).
Fig. 4.
In primary human CAECs high glucose (HG) significantly increased apoptotic cell death as shown by increased terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) positivity (assessed by flow cytometry, see methods; P < 0.05 vs. control). siRNA knockdown of Nrf2 (siNrf2) significantly augmented HG-induced endothelial apoptosis. Data are mean ± SE (n = 6 for each group). *P < 0.05 vs. untreated; #P < 0.05 vs. HG only.
Nrf2-dependent attenuation of high-glucose-induced NF-κB activation and proinflammatory gene expression in CAECs.
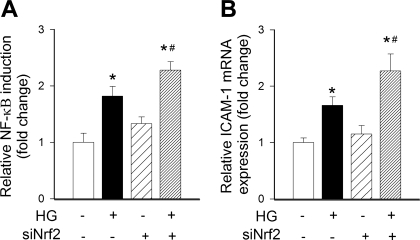
To assess the role of adaptive Nrf2 activation in regulation of proinflammatory gene expression, CAECs were treated with high glucose and NF-κB activity and NF-κB-driven gene expression were assayed. Treatment with high glucose significantly increased transcriptional activity of NF-κB (Fig. 5A) and upregulated ICAM-1 (Fig. 5B). siRNA knockdown of Nrf2 significantly augmented both high-glucose-induced NF-κB activation (Fig. 5A) and high-glucose-induced ICAM-1 induction (Fig. 5B).
Fig. 5.
A: reporter gene assay showing that in primary human CAECs high glucose (HG) significantly increased NF-κB activity. Cells were transiently cotransfected with NF-κB-driven firefly luciferase and CMV-driven Renilla luciferase constructs followed by HG treatment. Cells were then lysed and subjected to luciferase activity assay. After normalization relative luciferase activity was assessed from 4–6 independent transfections. siRNA knockdown of Nrf2 (siNrf2) significantly augmented HG-induced endothelial NF-κB activation. Data are means ± SE (n = 5 in each group). *P < 0.05 vs. untreated; #P < 0.05 vs. HG only. B: in cultured endothelial cells HG significantly increased mRNA expression of ICAM-1. Knockdown of Nrf2 significantly augmented HG-induced ICAM-1 mRNA expression. Data are means ± SE (n = 5 in each group). *P < 0.05 vs. untreated; #P < 0.05 vs. HG only.
Effect of high-fat diet on various biomarkers in mouse sera.
We compared male Nrf2+/+ and Nrf2−/− mice after 16 wk of HFD. Fasting blood glucose levels differed significantly between the two strains on the control diet, yet both strains developed comparable relative hyperglycemia on HFD (Nrf2+/+: control diet 5.5 ± 0.6 mmol/l, HFD 8.6 ± 0.4 mmol/l; Nrf2−/−: control diet 2.8 ± 0.2 mmol/l, HFD 5.7 ± 0.8 mmol/l). HFD tended to increase serum levels of free fatty acids both in wild type [Nrf2+/+: 0.88 ± 0.06 meq/l, Nrf2+/+ (HFD): 1.15 ± 0.08 meq/l; P < 0.05] and Nrf2−/− mice [Nrf2−/−: 0.88 ± 0.12 meq/l, Nrf2−/− (HFD): 0.92 ± 0.07 meq/l]. Serum cholesterol levels were also elevated by feeding HFD in both strains [Nrf2+/+: 245 ± 5 mg/dl, Nrf2+/+ (HFD): 337 ± 20 mg/dl (P < 0.05); Nrf2−/−: 123 ± 8 mg/dl, Nrf2−/− (HFD): 267 ± 9 mg/dl (P < 0.05)]. Serum triglyceride and insulin levels (assessed in fed animals) were not significantly affected by HFD feeding in either strain (data not shown). Serum adiponectin levels were decreased in HFD-fed wild-type mice [Nrf2+/+: 23.2 ± 2.6 μg/ml, Nrf2+/+ (HFD): 13.6 ± 0.4 μg/ml; P < 0.05], whereas they were unchanged in HFD-fed Nrf2−/− mice [Nrf2−/−: 15.6 ± 0.8 μg/ml, Nrf2−/− (HFD): 15.3 ± 0.4 μg/ml; not significant].
Upregulation of Nrf2/ARE-driven genes in aortas of HFD-fed mice.
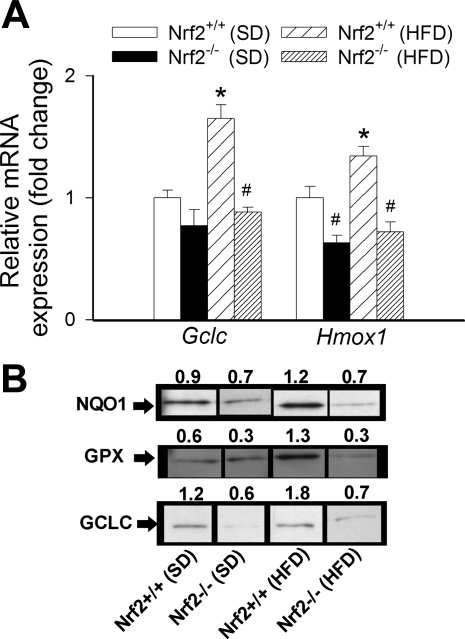
The HFD elicited significant increases in mRNA expression of Gclc and Hmox1 in aortas of Nrf2+/+ mice (Fig. 6A). By contrast, HFD did not result in any significant change in mRNA expression of Gclc and Hmox1 in aortas of Nrf2−/− mice, compared with the respective SD-fed control mice (Fig. 6A). Western blot experiments confirmed that genetic depletion of Nrf2 prevents HFD-induced upregulation of protein expression of Nrf2 targets (NQO1, GPX, and GCLC) in mouse aortas (Fig. 6B).
Fig. 6.
A: expression of Gclc and Hmox1 mRNA in aortas isolated from Nrf2+/+ mice and Nrf2−/− mice fed a standard diet (SD) or a high-fat diet (HFD). Data are means ± SE (n = 5 or 6 in each group). *P < 0.05 vs. SD; #P < 0.05 vs. Nrf2+/+. B: analysis of protein expression of NQO1, GPX, and GCLC in aortic segments from Nrf2+/+ mice and Nrf2−/− mice fed SD or HFD. Shown are spliced bands from Western blot experiments, organized according to the experimental groups. Numbers are normalized densitometric values for the respective bands (see methods). The molecular masses of the bands recognized by the antibodies directed against NQO1, GPX, and GCLC are approximately 31, 22, and 73 kDa, respectively.
Increased oxidative stress in aortas of HFD-fed Nrf2−/− mice.
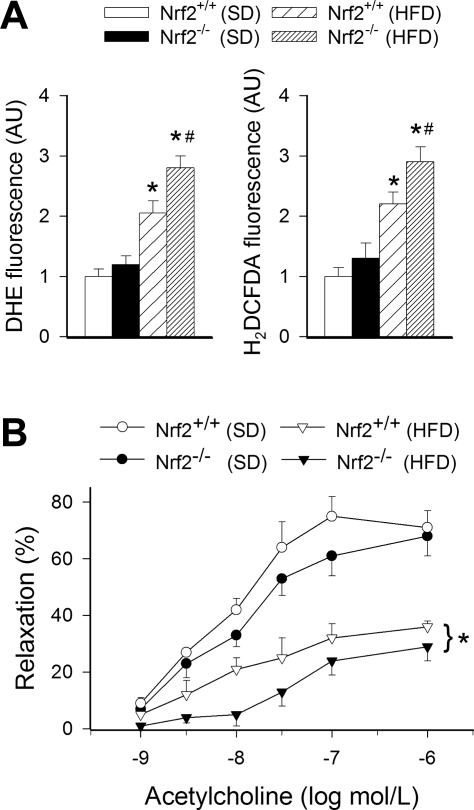
The HFD elicited significant increases in DHE and H2DCFDA fluorescent signals (measures of vascular O2·− and H2O2 production, respectively) in aortas of Nrf2+/+ mice (Fig. 7A). HFD-induced vascular oxidative stress was more severe in Nrf2−/− mice, as shown by the significantly greater DHE and H2DCFDA fluorescent signals observed in aortas of HFD-fed Nrf2−/− mice compared with responses obtained in vessels from HFD-fed Nrf2+/+ mice (Fig. 7A).
Fig. 7.
A: superoxide and peroxide production in segments of aortas isolated from Nrf2+/+ mice and Nrf2−/− mice fed SD or HFD, as measured by the dihydroethidium (DHE, left) and dichlorofluorescein [5-(and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (H2DCFDA), right] fluorescence methods. Data are means ± SE (n = 5 or 6 in each group). AU, arbitrary units. *P < 0.05 vs. SD; #P < 0.05 vs. Nrf2+/+. B: relaxation to acetylcholine in aortic segments isolated from Nrf2+/+ mice and Nrf2−/− mice fed SD or HFD. Data are means ± SE (n = 5 or 6 for each data point). *P < 0.05 vs. Nrf2+/+.
Diminished endothelial function in aortas of HFD-fed Nrf2−/− mice.
The HFD elicited significant endothelial dysfunction in aortas of Nrf2+/+ mice, as shown by the impaired relaxation responses to ACh (Fig. 7B). HFD-induced endothelial dysfunction was more severe in Nrf2−/− mice, as shown by the significantly diminished ACh-induced relaxations of aortas of these animals, compared with responses obtained in vessels from HFD-fed Nrf2+/+ mice (Fig. 7B).
Increased proinflammatory gene expression in aortas of HFD-fed Nrf2−/− mice.
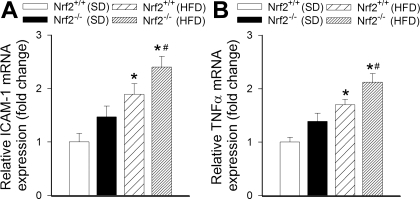
The HFD elicited significant increases in mRNA expression of Icam1 and Tnf in aortas of Nrf2+/+ mice (Fig. 8, A and B, respectively). In aortas of HFD-fed Nrf2−/− mice mRNA expression of Icam1 and Tnf was significantly greater than in vessels of HFD-fed wild-type mice (Fig. 8, A and B, respectively).
Fig. 8.
Expression of ICAM-1 (A) and TNFα (B) mRNA in aortas isolated from Nrf2+/+ mice and Nrf2−/− mice fed SD or HFD. Data are means ± SE (n = 5 or 6 in each group). *P < 0.05 vs. SD; #P < 0.05 vs. Nrf2+/+.
DISCUSSION
Here we show for the first time that adaptive activation of the Nrf2/ARE pathway has a critical role in endothelial protection in response to diabetic conditions both in vitro and in vivo. We base this conclusion on the following lines of evidence. We demonstrate that in human CAECs hyperglycemia significantly increases the transcriptional activity of Nrf2 (Fig. 1A) and upregulates several ARE-regulated genes involved in free radical metabolism (Fig. 1, B–D), including NQO1 (a key component of the plasma membrane redox system), heme oxygenase-1, and γ-glutamylcysteine synthetase (the rate-limiting enzyme for glutathione synthesis). The aforementioned effects of high glucose are mediated predominantly by Nrf2, as siRNA knockdown of Nrf2 inhibits induction of antioxidant genes by high glucose (Fig. 1, C and D). Furthermore, overexpression of Keap-1 also abolished the adaptive antioxidant response in response to hyperglycemia (Fig. 1C). Keap-1 is a cytosolic repressor protein that interacts with Nrf2, preventing its nuclear translocation.
Our findings suggest that increased mitochondrial ROS generation is a major mechanism by which hyperglycemia promotes oxidative stress in human CAECs (Fig. 2). Similar conclusions have been reached previously in studies from other laboratories as well (27, 31, 32, 38). Disruption of Nrf2 signaling significantly increases hyperglycemia-induced mitochondrial oxidative stress, indicating that adaptive upregulation of Nrf2-driven antioxidant systems effectively attenuates cellular oxidative stress under diabetic conditions (Fig. 2). Because administration of catalase prevented high-glucose-induced upregulation of Nrf2 targets (Fig. 3A), it is likely that increased H2O2 levels have a central role in activation of Nrf2 in metabolically stressed endothelial cells. This concept is further supported by the findings that exogenous administration of H2O2 significantly increased expression of Nrf2-driven genes, an effect that was abolished by knockdown of Nrf2 (Fig. 3B). The induction of GCLC, HMOX1, and NQO1 was attenuated by treatment with the thiol antioxidant N-acetylcysteine, suggesting that thiol oxidation is largely mediating the effects of H2O2 on Nrf2-responsive genes (Fig. 3A).
Hyperglycemia-induced endothelial oxidative stress has been implicated in the development of diabetic complications, in part by inducing endothelial apoptosis and by promoting endothelial activation and vascular inflammation. Our present findings (Fig. 4) and results from previous investigations (28) suggest that induction of Nrf2-driven free radical detoxification pathways confers significant antiapoptotic effects in endothelial cells exposed to hyperglycemia. Furthermore, our results (Fig. 5) suggest that adaptive Nrf2 activation also effectively attenuates hyperglycemia-induced NF-κB activation and NF-κB-driven proinflammatory gene expression in endothelial cells, extending previous findings (3).
Furthermore, results from the present study show that in wild-type mice metabolic stress associated with HFD can upregulate Nrf2 target genes in the vasculature, whereas genetic depletion of Nrf2 prevents induction of free radical detoxification mechanisms in vessels of HFD-fed Nrf2−/− mice (Fig. 6). We found that the HFD elicits significant oxidative stress in blood vessels of wild-type mice, which is associated with endothelial dysfunction (Fig. 7), extending earlier findings (29, 35). Genetic lack of a functional Nrf2/ARE pathway results in significant increases in vascular ROS levels and a more severe endothelial functional impairment in aortas of HFD-fed Nrf2−/− mice compared with vessels of HFD-fed wild-type control mice (Fig. 7). These findings provide evidence that Nrf2-driven free radical detoxification pathways are physiologically important endogenous homeostatic mechanisms that play an important role in vasoprotection in metabolic diseases. The HFD-fed mouse is an accepted model of early type 2 diabetes, which recapitulates many aspects of diabetes mellitus, including hyperglycemia, oxidative stress, and endothelial dysfunction. Because in this model hyperglycemia is not the only factor contributing to vascular impairment, we propose that activation of Nrf2 represents a common pathway by which oxidative stress induced by diverse stimuli associated with diabetes mellitus and consumption of a HFD (including hyperglycemia, hyperlipidemia, increased levels of oxidized lipids, and advanced glycation end products) activate homeostatic mechanisms by which the deleterious effects of metabolic stress are attenuated in the vasculature. This concept is supported by the observation that hyperglycemia, oxidized lipoproteins (1), and advanced glycation end products (19) can separately elicit an Nrf2-driven adaptive response in endothelial cells. It is likely that adaptive Nrf2 activation also protects other organs, including the liver (34), the brain (26), and the heart (20), from the deleterious effects of oxidative stress associated with a HFD. Further studies are warranted to test the vasoprotective role of adaptive Nrf2 activation in other models of hyperglycemia/type 2 diabetes.
We posit that pathological conditions that impair the ability of cells to mount an effective Nrf2/ARE-mediated antioxidant response render the vascular system vulnerable to the deleterious effects of metabolic diseases. Importantly, there is a significant age-related dysregulation of Nrf2-dependent pathways in vascular cells. As a result, the same level of oxidative stress that elicits significant induction of Nrf2-dependent genes in arteries of young rats fails to upregulate Nrf2-dependent free radical detoxification pathways in vessels of aged rats (Csiszar and Ungvari, unpublished observations). Similarly, aortic expression of Nrf2-driven antioxidant enzymes markedly increases in young mice fed a HFD but tends to decrease or only modestly increase in middle-aged mice fed a HFD, despite the fact that vascular oxidative stress is greater in HFD-fed middle-aged mice than in young mice (5). The intimate link between aging and vascular Nrf2 activation is also underscored by the observations that arteries of extremely long-lived muroid rodents (Peromyscus leucopus, maximal life span: ∼8 yr) exhibit an increased expression of Nrf2-driven antioxidant enzymes, decreased cellular and mitochondrial levels of ROS, and increased resistance to the proinflammatory and proapoptotic effects of hyperglycemia compared with vessels of shorter-lived Mus musculus (12, 24, 36, 37). Moreover, many of the effects of caloric restriction, which decreases cellular and mitochondrial levels of ROS and exerts anti-inflammatory and antiapoptotic vascular effects in aging (8), also depend on the presence of functional Nrf2 (30).
Because the metabolic stress-induced Nrf2-dependent adaptive response is relatively weak and cannot compensate completely for the increased cellular oxidative stress in diabetes (especially in aging), there is a clear opportunity for pharmacological intervention to facilitate the efficiency of Nrf2-driven homeostatic mechanisms. In that regard, it is significant that in endothelial cells and other cell types Nrf2 can be activated pharmacologically by the polyphenol resveratrol (2, 21, 25, 33, 35) or by sulforaphane (43), which results in significant induction of cellular antioxidant systems (23, 33, 39) (Fig. 3C), increases in GSH levels (23, 38), and consequential reduction of oxidative stress (23, 33, 39, 43). Importantly, both resveratrol and sulforaphane can effectively attenuate vascular ROS production and improve endothelial function in animal models of diabetes mellitus (29, 45) and/or attenuate hyperglycemia-induced endothelial oxidative stress (38, 43). Thus we posit that facilitation of the induction of Nrf2-driven homeostatic pathways by pharmacological treatments can contribute importantly to an intervention strategy for the prevention of vascular diseases in diabetic patients.
GRANTS
This work was supported by grants from the American Diabetes Association (to Z. Ungvari), the American Federation for Aging Research (to A. Csiszar), the University of Oklahoma College of Medicine Alumni Association (to A. Csiszar), the National Institutes of Health (NIH) (AG-031085 to A. Csiszar; AT-006526 and HL-077256 to Z. Ungvari; P01-AG-11370 to W. E. Sonntag), and the Intramural Research Program of NIH (to R. de Cabo).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Afonyushkin T, Oskolkova OV, Philippova M, Resink TJ, Erne P, Binder BR, Bochkov VN. Oxidized phospholipids regulate expression of ATF4 and VEGF in endothelial cells via NRF2-dependent mechanism: novel point of convergence between electrophilic and unfolded protein stress pathways. Arterioscler Thromb Vasc Biol 30: 1007– 1013, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Chen CY, Jang JH, Li MH, Surh YJ. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Commun 331: 993– 1000, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Chen XL, Dodd G, Thomas S, Zhang X, Wasserman MA, Rovin BH, Kunsch C. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol Heart Circ Physiol 290: H1862– H1870, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Chen XL, Varner SE, Rao AS, Grey JY, Thomas S, Cook CK, Wasserman MA, Medford RM, Jaiswal AK, Kunsch C. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J Biol Chem 278: 703– 711, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Collins AR, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, Boyadjian R, Bikineyeva A, Pratico D, Harrison DG, Hsueh WA. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res 104: e42– e54, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Cosentino F, Eto M, De Paolis P, van der Loo B, Bachschmid M, Ullrich V, Kouroedov A, Delli Gatti C, Joch H, Volpe M, Luscher TF. High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells: role of protein kinase C and reactive oxygen species. Circulation 107: 1017– 1023, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Csiszar A, Ahmad M, Smith KE, Labinskyy N, Gao Q, Kaley G, Edwards JG, Wolin MS, Ungvari Z. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol 168: 629– 638, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev 130: 518– 527, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol 294: H2721– H2735, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-TNF-alpha treatment in aging. Am J Pathol 170: 388– 698, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy RJ, Hintze TH, Wolin MS, Austad SN, Podlutsky A, Ungvari Z. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell 6: 783– 797, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: role of NF-kappaB inhibition. Am J Physiol Heart Circ Physiol 291: H1694– H1699, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Csiszar A, Smith KE, Koller A, Kaley G, Edwards JG, Ungvari Z. Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappaB activation by tumor necrosis factor-alpha, H2O2, and high intravascular pressure. Circulation 111: 2364– 2372, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159– 1166, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics 17: 21– 30, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Dai G, Vaughn S, Zhang Y, Wang ET, Garcia-Cardena G, Gimbrone MA., Jr Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ Res 101: 723– 733, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Fledderus JO, Boon RA, Volger OL, Hurttila H, Yla-Herttuala S, Pannekoek H, Levonen AL, Horrevoets AJ. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler Thromb Vasc Biol 28: 1339– 1346, 2008 [DOI] [PubMed] [Google Scholar]
- 19. He M, Siow RC, Sugden D, Gao L, Cheng X, Mann GE. Induction of HO-1 and redox signaling in endothelial cells by advanced glycation end products: a role for Nrf2 in vascular protection in diabetes. Nutr Metab Cardiovasc Dis ( March 12, 2010). doi: 10.1016/j.numecd.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 20. He X, Kan H, Cai L, Ma Q. Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. J Mol Cell Cardiol 46: 47– 58, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Hsieh TC, Lu X, Wang Z, Wu JM. Induction of quinone reductase NQO1 by resveratrol in human K562 cells involves the antioxidant response element ARE and is accompanied by nuclear translocation of transcription factor Nrf2. Med Chem 2: 275– 285, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Jones CI, Zhu H, 3rd, Martin SF, Han Z, Li Y, Alevriadou BR. Regulation of antioxidants and phase 2 enzymes by shear-induced reactive oxygen species in endothelial cells. Ann Biomed Eng 35: 683– 693, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 294: L478– L488, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Labinskyy N, Mukhopadhyay P, Toth J, Szalai G, Veres M, Losonczy G, Pinto JT, Pacher P, Ballabh P, Podlutsky A, Austad SN, Csiszar A, Ungvari Z. Longevity is associated with increased vascular resistance to high glucose-induced oxidative stress and inflammatory gene expression in Peromyscus leucopus. Am J Physiol Heart Circ Physiol 296: H946– H956, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Y, Cao Z, Zhu H. Upregulation of endogenous antioxidants and phase 2 enzymes by the red wine polyphenol resveratrol in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stress. Pharmacol Res 53: 6– 15, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, White CL, Purpera MN, Uranga RM, Bruce-Keller AJ, Keller JN. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem 114: 1581– 1589, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mukhopadhyay P, Rajesh M, Hasko G, Hawkins BJ, Madesh M, Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc 2: 2295– 2301, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Okouchi M, Okayama N, Alexander JS, Aw TY. NRF2-dependent glutamate-l-cysteine ligase catalytic subunit expression mediates insulin protection against hyperglycemia-induced brain endothelial cell apoptosis. Curr Neurovasc Res 3: 249– 261, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8: 157– 168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pearson KJ, Lewis KN, Price NL, Chang JW, Perez E, Cascajo MV, Tamashiro KL, Poosala S, Csiszar A, Ungvari Z, Kensler TW, Yamamoto M, Egan JM, Longo DL, Ingram DK, Navas P, de Cabo R. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci USA 105: 2325– 2330, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Quijano C, Castro L, Peluffo G, Valez V, Radi R. Enhanced mitochondrial superoxide in hyperglycemic endothelial cells: direct measurements and formation of hydrogen peroxide and peroxynitrite. Am J Physiol Heart Circ Physiol 293: H3404– H3414, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Drel VR, Obrosova IG, Pacher P. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am J Physiol Heart Circ Physiol 293: H610– H619, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rubiolo JA, Mithieux G, Vega FV. Resveratrol protects primary rat hepatocytes against oxidative stress damage: activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur J Pharmacol 591: 66– 72, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Tanaka Y, Aleksunes LM, Yeager RL, Gyamfi MA, Esterly N, Guo GL, Klaassen CD. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J Pharmacol Exp Ther 325: 655– 664, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol 299: H18– H24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ungvari Z, Buffenstein R, Austad SN, Podlutsky A, Kaley G, Csiszar A. Oxidative stress in vascular senescence: lessons from successfully aging species. Front Biosci 13: 5056– 5070, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Ungvari Z, Krasnikov BF, Csiszar A, Labinskyy N, Mukhopadhyay P, Pacher P, Cooper AJL, Podlutskaya N, Austad SN, Podlutsky A. Testing hypotheses of aging in long-lived mice of the genus Peromyscus: association between longevity and mitochondrial stress resistance, ROS detoxification pathways and DNA repair efficiency. Age (Dordr) 30: 121– 133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol 297: H1876– H1881, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol 292: H2417– H2424, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Ungvari ZI, Labinskyy N, Gupte SA, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol 294: H2121– H2128, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Ungvari ZI, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith KE, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293: H37– H47, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Xue M, Qian Q, Adaikalakoteswari A, Rabbani N, Babaei-Jadidi R, Thornalley PJ. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes 57: 2809– 2817, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNFalpha and vascular oxidative stress. Arterioscler Thromb Vasc Biol 29: 1164– 1171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]