Abstract
There is emerging evidence that aldosterone can promote diastolic dysfunction and cardiac fibrosis independent of blood pressure effects, perhaps through increased oxidative stress and inflammation. Accordingly, this investigation was designed to ascertain if mineralocorticoid receptor blockade improves diastolic dysfunction independently of changes in blood pressure through actions on myocardial oxidative stress and fibrosis. We used young transgenic (mRen2)27 [TG(mRen2)27] rats with increases in both tissue ANG II and circulating aldosterone, which manifests age-related increases in hypertension and cardiac dysfunction. Male TG(mRen2)27 and age-matched Sprague-Dawley rats were treated with either a low dose (∼1 mg·kg−1·day−1) or a vasodilatory, conventional dose (∼30 mg·kg−1·day−1) of spironolactone or placebo for 3 wk. TG(mRen2)27 rats displayed increases in systolic blood pressure and plasma aldosterone levels as well as impairments in left ventricular diastolic relaxation without changes in systolic function on cine MRI. TG(mRen2)27 hearts also displayed hypertrophy (left ventricular weight, cardiomyoctye hypertrophy, and septal wall thickness) as well as fibrosis (interstitial and perivascular). There were increases in oxidative stress in TG(mRen2)27 hearts, as evidenced by increases in NADPH oxidase activity and subunits as well as ROS formation. Low-dose spironolactone had no effect on systolic blood pressure but improved diastolic dysfunction comparable to a conventional dose. Both doses of spironolactone caused comparable reductions in ROS/3-nitrotryosine immunostaining and perivascular and interstitial fibrosis. These data support the notion mineralocorticoid receptor blockade improves diastolic dysfunction through improvements in oxidative stress and fibrosis independent of changes in systolic blood pressure.
Keywords: aldosterone, fibrosis, renin-angiotensin-aldosterone system
remodeling of cardiac tissue in hypertension is characterized by myocyte hypertrophy and interstitial fibrosis, which facilitate the development of left ventricular (LV) diastolic dysfunction (27, 41). In this context, increases in aldosterone levels are associated with increased heart failure mortality (9, 22), and blockade of mineralcorticoid receptors (MRs) improves morbidity and mortality (22, 25, 26), including those with preserved ejection fraction (EF), e.g., diastolic dysfunction (15, 18). Both cardiac myocytes and fibroblasts express MRs with a high affinity for aldosterone (17, 32, 38). However, the development of interstitial fibrosis and diastolic dysfunction by aldosterone actions on the MR occur when the renin-angiotensin-aldosterone system (RAAS) is inappropriately activated relative to salt intake, i.e., in the absence of salt deprivation (30, 32).
Inappropriate activation of the RAAS through aldosterone actions on the MR have been shown to promote the development of diastolic dysfunction through putative nongenomic actions including the generation of oxidative stress in models of chronic pressure overload (18, 22, 38). Oxidative stress is known to impair metabolic signaling pathways that regulate cardiac remodeling, LV hypertrophy (LVH), and diastolic dysfunction through interstitial fibrosis (16). Experimental studies (14, 21, 36) have shown beneficial effects of MR antagonist therapy on myocardial fibrosis independent of blood pressure effects. It has been suggested that these blood pressure-independent, antifibrotic effects of MR antagonists are mediated, in part, through reductions in myocardial oxidative stress (14, 21, 36, 43).
Cardiac tissue remodeling manifests early as impairments in diastolic relaxation time (e.g., diastolic dysfunction) characterized by stiffness and is characteristically seen in conditions of pressure overload and inappropriate activation of the RAAS (27, 37, 41). Thus, diastolic dysfunction is promoted by hemodynamic and hormonal influences that promote hypertrophy and cardiac fibrosis. Thereby, we hypothesized that blockade of the MR would improve diastolic dysfunction through improvements in oxidative stress and interstitial fibrosis independently of changes in systolic blood pressure (SBP) in a rodent model of RAAS activation, the transgenic (mRen2)27 (R2) rat. This hypertensive, insulin-resistant model displays increased tissue ANG II and circulating aldosterone and manifest increases in tissue oxidative stress and interstitial fibrosis (36, 43, 44). To evaluate the blood pressure-independent effects of MR antagonism on diastolic function, we compared a low, nonpressure-lowering dose of spironolactone (Sp) (36, 43) with that of a conventional blood pressure-lowering dose (15, 21).
MATERIALS AND METHODS
Animals and Treatments
All animal procedures were approved by the University of Missouri Animal Care and Use Committee and housed in accordance with National Institutes of Health guidelines. Male R2 rats (5–6 wk of age) and age-matched Sprague-Dawley (SD) rats were randomly assigned to untreated groups (R2C and SDC, respectively) or treated with Sp at either a low dose or a conventional dose. R2 and SD rats were implanted with a timed-release Sp pellet designed to deliver either 5 or 150 mg Sp via the subcutaneous route for 21 days. The approximate doses for the low-dose and conventional-dose Sp treatments were 1 and 30 mg·kg−1·day−1, respectively.
Plasma Aldosterone
Rat blood samples were collected from the lumen of the LV at autopsy after experimental treatment and stored at −20°C. The plasma aldosterone content was determined using a radioimmunoassay from Siemens (Los Angeles, CA) using the manufacturer's protocol. Radioactivity was read using a multicrystal γ-counter (Gamma-C 12 DPC, Berthold, Germany).
SBP, Body Weight, and Homeostatic Model Assessment of Insulin Resistance
Restraint conditioning was initiated on the day of initial blood pressure measurements. SBP was measured in triplicate, on separate occasions throughout the day, using the tail-cuff method (Harvard Systems, Student Oscillometric Recorder) before the initiation of treatment and on days 19 or 20 before euthanization. Total body weight was obtained before the initiation of treatment and at the time of death. A venous blood sample was collected from a subset of fasting rats in each treatment group at the end of the study, and plasma was stored at −80°C. Glucose and insulin were measured by an automated hexokinase G-6-PDH assay and an ELISA kit specific for rat insulin, respectively. Homeostatic model assessment was calculated by taking the product of the glucose (in mmol/l) and insulin (in μU/ml) values and dividing by 22.
In Vivo Cine MRI
Noninvasive MRI scans were performed within 72 h of the end of the treatment period using a Varian 7-T horizontal-bore MRI (Varian, Palo Alto, CA) equipped with a 60-mm birdcage radiofrequency coil as previously described (36, 44, 47). Animals were weighed and anesthetized using 1.8–2.7% isoflurane on a nose cone nonrebreathing system supplying continuous oxygen. ECG and respiratory monitoring and gating were performed with a small animal monitoring system (SA Instruments, Stony Brook, NY). Warm air was circulated through the MRI bore to maintain the animal's body temperature. ECG/respiratory gated gradient echo sequences were acquired with 1-mm slice thickness and 65 × 45 and 45 × 45 mm2 fields of view for the LV in long- and short-axis images, respectively. Septal wall thickness measurements were determined on the midventricular axial image immediately after the R wave and with an averaging of 5. LV functional parameters were determined using a series of cine images of the LV in the long-axis view acquired at 16 equally spaced time points throughout the entire cardiac cycle with a frame rate of 8–12 ms/frame. At each time point, the endocardial borders were traced to measure the LV chamber area using VnmrJ software (Varian) by two experienced MRI readers. LV volumes (LVVs) at each phase were calculated with the following modified ellipsoid equation: 8A2/(3πL), where A is the endocardial area and L is the length of the LV long-axis chamber. The LVV curve was plotted as LVVs versus time in one cardiac cycle. LV EF was measured as follows: EF = (EDV − ESV)/EDV × 100%, where EDV is end-diastolic volume and ESV is end-systolic volume. The first derivatives of the LVV against time were calculated to extract the diastolic filling rates and relaxation time. The diastolic initial filling rate is defined as the slope of the first four time points of the early diastolic curve. The diastolic peak filling rate is defined as the maximum derivative of the LVV curve. The diastolic relaxation time is defined as the time duration from the end of the systolic phase to the peak filling phase.
Measurement of Cardiac Tissue Oxidative Stress
ROS formation was evaluated in LV sections by chemiluminescense and further by immunostaining for 3-nitrotyrosine content, a marker of peroxynitrite formation, as previously described (36, 43, 44, 47).
Measurement of NADPH Oxidase
NADPH oxidase activity.
Total enzyme activity was determined in LV tissue as previously described (43, 44, 47). Briefly, NADPH oxidase activity was determined by measuring the conversion of a radical detector (Cayman Chemical, Ann Arbor, MI) using spectrophotometric (450 nm) techniques.
NADPH oxidase 2 and Rac1 immunostaining.
LV sections were immunostained and quantitated as previously described (44, 47).
LVH
At autopsy, the LV plus septum (LV + S) was dissected free of the right ventricle, atria, and great vessels. (LV + S) normalized to body weight is a commonly used index of LVH in nonobese rodent models.
Light Microscopic Analysis for Cardiomyocyte Hypertrophy and Myocardial Fibrosis
Tissue samples were excised from similar sections of the lateral wall of the LV and processed for light microscopy. Samples from five rats from each of the four treatment groups were analyzed as follows. Paraffin sections (5 μm) were deparaffinized, rehydrated, and stained with Verhoeff-Van Gieson (VVG) stain, which stains collagen type III and elastin fibers pink, to evaluate interstitial and perivascular fibrosis, as previously described (44). Ten nonoverlapping ×40 images (1,392 × 1,040 pixels) were captured and analyzed by an individual blinded to the animal and treatment group. Perivascular fibrosis was measured as the percent area of the vascular wall of coronary arterioles, including the intima, media, and adventitia, presenting fibrosis on VVG staining. To quantify only collagen staining in either the periarterial or interstitial areas, all other colors were filtered out with the aid of MetaVue software. The area within each of the 10 images was quantified by MetaVue, and an average value was generated for each rat and expressed as arbitrary units. The accuracy of this technique has been confirmed by immunostaining of the myocardium with antibodies to collagen. To evaluate cardiomyoctye hypertrophy, digital images were captured on cardiomyocytes in cross-section, and the cross-sectional area was determined with the aid of image-analysis software. To quantify cardiomyocyte size, ∼20 cells from 2 separate sites were measured per sample, and only cardiomyocytes with a well-defined cellular membrane and visible nucleus were measured. The average size of all measured cardiomyocytes within a sample was determined and expressed in units of cross-sectional area (in μm2).
Statistical Analysis
All values are expressed as means ± SE. Statistical analyses were performed using a t-test for aldosterone measures and two-way ANOVA with Tukey's post hoc test for all other outcomes (SigmaStat 3.1, Systat Software, Chicago, IL).
RESULTS
Sp Effects on Experimental Parameters
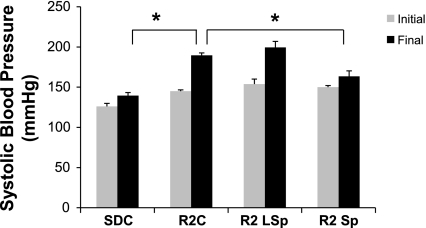
There were increases in circulating aldosterone in R2C rats compared with SDC rats (936 ± 198 vs. 501 ± 122 pg/ml, P < 0.05), and the higher plasma aldosterone level in R2C rats was associated with increased SBP compared with SDC rats (P < 0.05; Table 1). These data confirm previous reports demonstrating hyperaldosteronemia in the hypertensive Ren2 rat. Conventional-dose Sp but not low-dose Sp reduced SBP (Table 1 and Fig. 1).
Table 1.
Experimental parameters
| Groups |
|||||
|---|---|---|---|---|---|
| Parameter | ANOVA | SDC | R2C | R2 LSp | R2 Sp |
| n | 6 | 5 | 5 | 5 | |
| Age, wk | 0.041 | 8.7 ± 0.3 | 8.2 ± 0.2 | 8.8 ± 0.4 | 9 ± 0.3 |
| SBP, mmHg | |||||
| Initial | <0.001 | 126 ± 4 | 145 ± 2* | 154 ± 6* | 150 ± 2* |
| Final | <0.001 | 140 ± 4 | 190 ± 3* | 199 ± 7* | 163 ± 7*† |
| Body weight, g | |||||
| Initial | ND | 103 ± 19 | 109 ± 2 | 138 ± 13 | 123 ± 4 |
| Final | ND | 256 ± 10 | 255 ± 7 | 267 ± 11 | 263 ± 6 |
| HOMA-IR | ND | 0.06 ± 0.02 | 0.15 ± 0.06 | 0.08 ± 0.01 | 0.02 ± 0.0 |
Values are means ± SE; n, sample size. Rats were divided into the following groups: Sprague-Dawley control (SDC), transgenic (mRen2)27 (R2) control (R2C), R2 rats treated with low-dose spironolactone (R2 LSp), and R2 rats treated with conventional-dose spironolactone (R2 Sp). Systolic blood pressure (SBP) and body weight were measured immediately before treatment (initial) and at the end of the treatment period (final). ND indicates that no differences were observed by ANOVA.
P < 0.05 vs. the SDC group;
P < 0.05 vs. the R2 LSp group.
Fig. 1.
Initial and final systolic blood pressure for control and spironolactone (Sp)-treated Sprague-Dawley (SD) and transgenic (mRen2)27 (R2) rats. Rats were divided into the following groups: SD control (SDC), R2 control (R2C), R2 rats treated with low-dose Sp (R2 LSp), and R2 rats treated with conventional-dose Sp (R2 Sp). *P < 0.05 vs. R2C rats.
The presence of insulin resistance is an important predictor of diastolic dysfunction in models of pressure overload and hypertension (19, 20). While there was a trend in the homeostatic model assessment of insulin resistance data suggesting decreased systemic insulin resistance with increasing doses of Sp in this experiment, there were no significant changes in insulin sensitivity or in body weight at the end of treatment (Table 1).
Sp Improves LV Diastolic Function
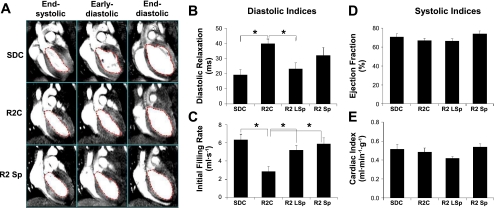
There were no changes in heart rate, LV volumes (EDV and ESV), or systolic indexes (cardiac index and EF) in either strain or with treatment (Table 2). However, there was observable diastolic dysfunction determined by an increased LV diastolic relaxation time and a reduced initial filling rate in R2 rats compared with SDC rats (P < 0.05; Table 2 and Fig. 2). Despite the absence of a reduction in SBP, low-dose Sp treatment in R2 rats resulted in improved diastolic function, characterized by an increased initial filing rate and a reduced diastolic relaxation time compared with R2C rats (P < 0.05; Table 2 and Fig. 2). The improvement in diastolic dysfunction with the blood pressure-lowering conventional dose of Sp, like the low dose of Sp, resulted in an increased initial filling rate (P < 0.05); however, the reduction in diastolic relaxation time did not reach statistical significance (Table 2 and Fig. 2).
Table 2.
Systolic and diastolic indexes by MRI
| Groups |
|||||
|---|---|---|---|---|---|
| Parameter | P Value (by ANOVA) | SDC | R2C | R2 LSp | R2 Sp |
| n | 6 | 7 | 5 | 5 | |
| Age at MRI, wk old | ND | 8.3 ± 0.3 | 8.9 ± 0.3 | 7.9 ± 0.4 | 8.4 ± 0.2 |
| Heart rate and cardiac volumes | |||||
| Heart rate, beats/minute | ND | 387 ± 18 | 364 ± 17 | 396 ± 12 | 373 ± 8 |
| End-diastolic volume, μl | ND | 448 ± 32 | 537 ± 40 | 427 ± 28 | 508 ± 23 |
| End-systolic volume, μl | ND | 128 ± 11 | 177 ± 19 | 145 ± 18 | 133 ± 18 |
| Systolic indexes | |||||
| Cardiac index, ml·min−1·g body wt−1 | ND | 0.51 ± 0.05 | 0.49 ± 0.04 | 0.42 ± 0.02 | 0.54 ± 0.03 |
| Ejection fraction, % | ND | 71 ± 3 | 67 ± 2 | 67 ± 2 | 74 ± 3 |
| Diastolic indexes | |||||
| Diastolic relaxation time, ms | 0.001 | 19.4 ± 3.4* | 39.9 ± 2.9 | 23.3 ± 3.9* | 32.1 ± 5.2 |
| Peak filling rate, μl/s | ND | 8.18 ± 0.46 | 8.27 ± 1.03 | 6.94 ± 0.44 | 7.94 ± 0.56 |
| Initial filling rate, μl/s | 0.001 | 6.34 ± 0.61* | 2.87 ± 0.54 | 5.24 ± 0.49* | 5.90 ± 0.67* |
Values are means ± SE; n, sample size. ND indicates that no differences were observed in the ANOVA main effect.
P < 0.05 vs. the R2C group.
Fig. 2.
A: typical cine MRI of SDC (top), R2C (middle), and R2 Sp rats after SP treatment (bottom). A total of 16 cine MRI frames were recorded in 1 entire cardiac cycle for each rat. The frames shown here are frames 7, 10, and 16, representing end-systolic, early diastolic, and end-diastolic phases, respectively. B–E: indexes of diastolic (B and C) and systolic (D and E) function derived from the analysis of cine MRI images. *P < 0.05 vs. R2C rats.
Sp Improves LV Oxidative Stress
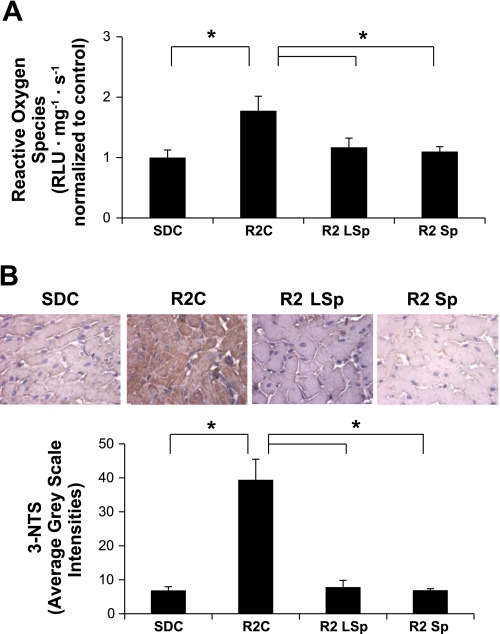
Antagonism of the MR is thought to improve diastolic dysfunction through improvements in oxidant stress through nongenomic actions (17, 19, 20). In this regard, the increases in the level of ROS, measured by chemiluminescence, or 3-nitrotyrosine, measured by immunostaining, in the LV of R2C rats compared with SDC rats were improved with both doses of Sp (Fig. 3, A–C).
Fig. 3.
Markers of oxidative stress in cardiac tissue from control and Sp-treated SD and R2 rats. A: bar graph showing chemiluminescence-derived measures of total ROS formation. B: representative images (top) of 3-nitrotryisone (3-NTS) content, a marker for peroxynitrite formation, with average grayscale intensities (bottom). *P < 0.05 vs. R2C rats.
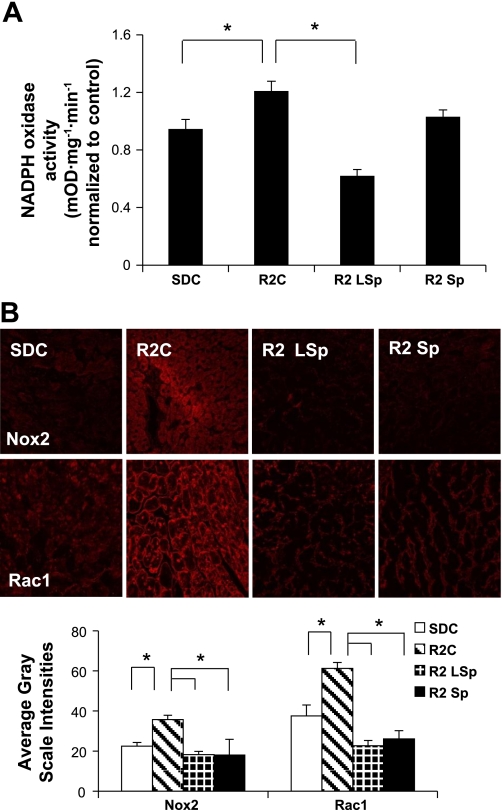
Oxidative stress in this model is driven by the enzyme complex NADPH oxidase (27). As previously reported (1, 33), NADPH oxidase activity was elevated in R2C rats compared with SDC rats (Fig. 4A). There were elevations in NADPH oxidase subunits (NADPH oxidase 2 and Rac1) in R2C rats compared with SDC rats (Fig. 4, A and B). Interestingly, only low-dose Sp improved NADPH oxidase activity but not conventional dose, whereas both doses of Sp improved the level of subunits.
Fig. 4.
Measures of NADPH oxidase. A: bar graph showing means ± SE values of total NADPH oxidase enzyme activity in cardiac tissue. B: representative confocal images (top) of NADPH oxidase subunits [NADPH oxidase 2 (Nox2) and Rac1] with measures of average grayscale intensities (bottom). *P < 0.05 vs. R2C rats.
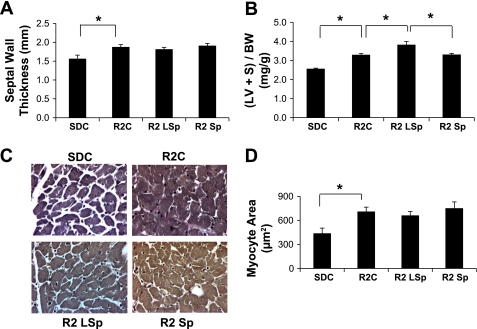
Sp Does Not Improve Measures of Hypertrophy
Increases in LV mass are inversely associated with diastolic relaxation times (8). Our data showing an increased relaxation time and a decreased initial filling rate in R2C rats (Fig. 2) and increases in markers of hypertrophy in R2C rats compared with SDC rats, including septal wall thickness, LV weight, and myocyte size, is consistent with this notion (P < 0.05; Fig. 5). On the other hand, both low and conventional doses of Sp did not improve markers of hypertrophy in R2 rats, yet improved diastolic function.
Fig. 5.
Markers of cardiac tissue hypertrophy for control and Sp-treated R2 rats. A and B: bar graphs showing increased septal wall thickness via cine MRI (A) and left ventricle + septum weight normalized to body weight [(LV + S)/BW; B] in R2C, R2 LSp, and R2 Sp rats compared with SDC rats. C: representative light micrographs of cardiomyocytes in cross section. D: bar graph showing average cardiomyoctye size expressed as cross-sectional area (in μm2). *P < 0.05 for paired comparisons.
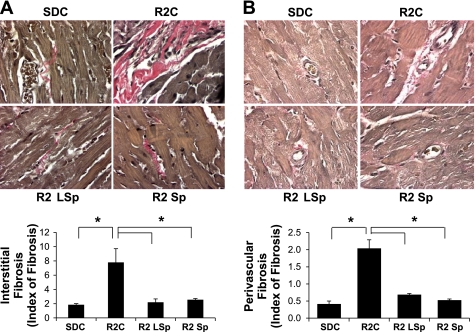
Sp Improves Fibrosis
The development of interstitial fibrosis leads to progressive stiffening of the ventricular wall, which significantly contributes to diastolic dysfunction. There were increases in interstitial fibrosis and perivascular fibrosis in R2C rats compared with SDC rats (Fig. 6, A and B, respectively), and both doses of Sp normalized the level of fibrosis in R2 rats.
Fig. 6.
Markers of fibrosis for control and Sp-treated R2 rats. A and B: representative light micrographs (top) showing collagen fiber staining (pink) upon Verhoeff-van Gieson staining in the interstitium (A) and adventitia (B) surrounding coronary arterioles with bar graphs of relative degrees of fibrosis (bottom). *P < 0.05 vs. R2C rats.
DISCUSSION
The results from the present investigation suggest that diastolic dysfunction occurs in conjunction with elevated plasma aldosterone levels and increased cardiac measures of oxidative stress, hypertrophy, and fibrosis in young transgenic R2 rats. Furthermore, antagonism of the MR with a low dose of Sp improved diastolic dysfunction and oxidative stress/fibrosis without reductions in SBP in these transgenic animals compared with conventional-dose Sp treatment. Finally, our finding that both doses of Sp had no impact on measures of hypertrophy but did have a comparable impact on myocardial fibrosis suggests the early LV relaxation abnormalities mediated through MR signaling are predominantly due to fibrotic rather than hypertrophic or pressure-dependent changes. These data are the first in vivo experiments using a RAAS-dependent model that support a blood pressure-independent effect of MR signaling in mediating diastolic dysfunction in association with increases in NADPH oxidase activity/ROS and the accompanying interstitial and perivascular fibrosis.
Our finding that antagonism of the MR with a low dose of Sp reduced the diastolic relaxation time and increased the initial filling rate characteristic of diastolic dysfunction without changes in SBP in the transgenic R2 rat extend findings from other laboratories demonstrating improvement in diastolic function with MR blockade independently of effects on blood pressure (15, 17, 18). Although previous work on blood pressure-independent effects of MR antagonism and diastolic function have supported an essential role for salt and/or nephrectomy in promoting MR-dependent cardiac fibrosis and diastolic dysfunction (2, 15, 17, 18, 29, 31, 38), this is the first such study in a RAAS-dependent model. Because we began treatment before the onset of diastolic dysfunction, our data further suggest that low-dose Sp treatment is effective at preventing the development of diastolic dysfunction through improvements in myocardial fibrosis and oxidant stress in young transgenic rats.
Aldosterone actions on the MR have been demonstrated in cardiomyocytes and fibroblasts, the two primary cell types that contribute to hypertrophy and fibrosis in the development of diastolic dysfunction (14, 15, 17, 18, 22, 38). The putative mechanisms underlying the beneficial effects of MR blockade on diastolic dysfunction include reductions in LV interstitial fibrosis and LVH (15, 17, 18). In this context, the LV of transgenic R2 rats exhibited perivascular and interstitial fibrosis as well as increases in septal wall thickness, LV weight, and myocyte size.
The improvements in fibrosis observed with both doses of Sp treatment are consistent with previous literature showing that MRs are present in both cardiomyocytes and fibroblasts (38). The development of LV fibrosis is derived from an imbalance in the synthesis and degradation of the extracellular matrix, which is predominantly composed of collagens (types I and III). The myocardial and pericoronary fibroblasts that synthesize these collagens have been demonstrated to have MRs (3, 41). These findings corroborate our findings of fibrosis, both interstitial and perivascular, in the transgenic R2 rat that was improved with both doses of Sp.
Our findings showing improvements in fibrosis but not measures of hypertrophy with Sp treatment suggest that conditions that cause fibroblasts to abnormally synthesize collagen can be separated from the pathophysiological conditions activating cardiomyocyte hypertrophy. Indeed, MR-dependent fibrosis can occur in the both hypertrophied LV in which workload is increased as well as in the nonhypertrophied LV with a normal workload (1). This suggests that despite the presence of the MR in both cardiomyocytes and fibroblasts, the pathways that regulate the expansion of fibrous tissue are distinct from that of from myocyte growth. The hypertrophic activity of cardiomyoctyes may be regulated by numerous interacting factors, including hemodynamic (ventricular overload and high coronary perfusion pressure) as well as humoral (ANG II as well as aldosterone) factors (27, 33, 41). In our model, the RAAS is activated by elevations in tissue ANG II as well as circulating aldosterone, and results from our previous studies (6, 44, 45) supports that blockade of the ANG II type 1 receptor (AT1R) improves measures of hypertrophy. Therefore, our findings that Sp treatment improves fibrosis but not hypertrophy is not entirely surprising and suggest that tissue ANG II may have a more predominant effect on hypertrophic responses in this model than aldosterone actions on the MR.
In this vein, the observed myocyte hypertrophy and interstitial fibrosis is consistent with the well-delineated effects of both ANG II and aldosterone (4, 5, 7, 10, 13, 28, 46). In addition to direct effects of mineralocorticoids in the heart, an interaction with ANG II is important in the pathogenesis of cardiac fibrosis (1, 3, 33, 42). Indeed, AT1R expression and protein levels are increased in the LV of aldosterone-treated rats (4, 10, 13). Previous work from several laboratories (4, 10, 13, 24, 39, 46) has suggested that MR activation potentiates the cardiac fibrotic effects of AT1R signaling by enhancing oxidative stress induced by ANG II. Indeed, it is well documented that pressure overload-induced heart failure enhances oxidative stress, in part through the activation of tissue AT1Rs and MRs (3, 14–16, 18, 21, 36, 41, 42). Additionally, there is substantial evidence that oxidative stress, in turn, promotes hypertrophic and proinflammatory/profibrotic changes in the myocardium (2, 15, 17, 18, 29, 30, 32, 38). However, the observation that MR blockade attenuated interstitial and periarterial fibrosis and corrected diastolic dysfunction without effects on myocyte hypertrophy underscores the seminal role of MR in promoting diastolic dysfunction through increased fibrosis and myocardial stiffness.
The balance between ROS generation and elimination plays a pivotal role in maintaining normal cardiac function, and studies (16, 18, 39) support that excessive myocardial ROS contribute to impairments in cardiac function. There is an emerging body of evidence that ANG II and aldosterone potentiate each other's action in stimulating myocardial NADPH oxidase activity (4, 10, 13). We (4, 10, 13, 44, 45, 47) have previously reported that the primary source of ROS in R2 rats is from increased NADPH oxidase activity rather than from mitochondrial sources. Data from this investigation are consistent with other reports that ANG II and aldosterone activation of NADPH oxidase occurs through the phosphorylation and activation of p47phox and Rac1 (10, 13, 43) as well as c-Src-dependent mechanisms (4). Here, we demonstrate that treatment with a subdepressor dose of Sp resulted in reductions in NADPH oxidase activity and subunits independently of changes in blood pressure. The intermediate effect on reductions in NADPH oxidase activity observed with conventional-dose Sp may be due to the antiandrogenic properties that this compound has relative to more specific blockade of the MR with eplerenone (11, 34). Data have suggested that testosterone deficiency may have a specific effect on increasing NADPH oxidase activity that is antagonized by Sp but not eplerenone (34). Further data have suggested that Sp promotes pathways that regulate apoptosis by increasing JNK/ERK signaling and caspase activation under conditions of testosterone deficiency (11, 34).
Our finding that low-dose Sp treatment ameliorated NADPH oxidase-induced generation of ROS is important in that increases in ventricular load and mechanical strain have also been shown to activate NADPH oxidase and contribute to cardiac structural and functional remodeling (12, 40, 41). This provides further credence that antagonism of the MR specifically improved diastolic function through improvements in oxidative stress and fibrosis.
In conclusion, early heart failure with preserved EF is estimated to comprise 50% of all heart failure disease, and the best therapeutic strategy to prevent diastolic heart disease remains controversial (23, 27, 35, 41). Hypertension, obesity, and aging are risk factors for diastolic heart disease (23, 27, 35), but the precise mechanisms that transition the heart from normal to diastolic dysfunction remain unclear. To this point, elevated aldosterone levels are associated with increased mortality in patients with hypertension and diastolic heart disease (9, 36). The observations in this study in rats with elevated plasma aldosterone, as well as other studies (13, 15, 17, 18), suggest that MR antagonism prevents myocardial fibrosis and the development of diastolic heart disease independently of hemodynamic factors. Collectively, studies have suggested that mineralocorticoids cause extracellular matrix deposition by the transcription of collagen genes and by augmenting the effects of ANG II on the transcription of these genes (1, 3, 33, 42). However, a recent report (18) has indicated that mineralocorticoids, acting through nongenomic mechanisms, promote the transition to myocardial fibrosis and diastolic heart disease in a hypertensive rodent model. The results of the present investigation suggest that increased NADPH oxidase activity and resulting increases in oxidative stress is a key mechanism by which this MR activation promotes myocardial fibrosis and associated diastolic dysfunction.
Thus, our investigation presents novel information that elucidates a potential mechanism whereby aldosterone actions on the MR and the generation of oxidative stress contribute to cardiac fibrosis and diastolic dysfunction independently of changes in blood pressure. The efficacy of Sp treatment demonstrated in this study translates to a clinically relevant therapeutic agent as a strategy for an emerging dilemma (e.g., diastolic dysfunction) in the clinical care of the cardiovascular patient.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-73101-01A1, by Veterans Affairs Merit System Grants 0018 (to J. R. Sowers) and CDA-2 (to A. T. Whaley-Connell), and by the University of Missouri Research Board (to A. T. Whaley-Connell).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors acknowledge the technical contributions of Rebecca Schneider, Nathan Rehmer, and Mona Garro as well as students Safwan Hyder and Bennett Krueger. The authors thank Brenda Hunter for assistance in preparing the manuscript.
REFERENCES
- 1. Brilla CG, Pick R, Tan LB, Janicki JS, Weber KT. Remodeling of the rat right and left ventricles in experimental hypertension. Circ Res 67: 1355– 1364, 1990 [DOI] [PubMed] [Google Scholar]
- 2. Brilla CG, Weber KT. Reactive and reparative myocardial fibrosis in arterial hypertension in the rat. Cardiovasc Res 26: 671– 677, 1992 [DOI] [PubMed] [Google Scholar]
- 3. Brilla CG, Zhou G, Matsubara L, Weber KT. Collagen metabolism in cultured adult rat cardiac fibroblasts: response to angiotensin II and aldosterone. J Mol Cell Cardiol 26: 809– 820, 1994 [DOI] [PubMed] [Google Scholar]
- 4. Callera GE, Touyz RM, Tostes RC, Yogi A, He Y, Malkinson S, Schiffrin EL. Aldosterone activates vascular p38MAP kinase and NADPH oxidase via c-Src. Hypertension 45: 773– 779, 2005 [DOI] [PubMed] [Google Scholar]
- 5. DeAngelis N, Fiordaliso F, Latini R, Calvillo L, Funicello M, Gobbi M, Mennini T, Masson S. Appraisal of the role of angiotensin II and aldosterone in ventricular myocyte apoptosis in adult normotensive rat. J Mol Cell Cardiol 34: 1655– 1665, 2002 [DOI] [PubMed] [Google Scholar]
- 6. DeMarco VG, Johnson MS, Habibi J, Pulakat L, Gul R, Hayden MR, Tilmon RD, Dellsperger KC, Winer N, Whaley-Connel AT, Sowers JR. Comparative analysis of telmisartan and olmesartan on cardiac function in the TG(mRen2)27 rat. Am J Physiol Heart Circ Physiol 300: H181– H190, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fiebeler A, Schmidt F, Muller DN, Park JK, Dechend R, Bieringer M, Shagdarsuren E, Breu V, Haller H, Luft FC. Mineralocorticoid receptor affects AP-1 and nuclear factor-κB activation in angiotensin II-induced cardiac injury. Hypertension 37: 787– 793, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Fouad FM, Slominski JM, Tarazi RC. Left ventricular diastolic function in hypertension: relation to left ventricular mass and systolic function. J Am Coll Cardiol 3: 1500– 1506, 1984 [DOI] [PubMed] [Google Scholar]
- 9. Guder G, Bauersachs J, Frantz S, Weismann D, Allolio B, Ertl G, Angermann CE, Stork S. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation 115: 1754– 1761, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J 20: 1546– 1548, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Juliet PA, Hayashi T, Daigo S, Matsui-Hirai H, Miyazaki A, Fukatsu A, Funami J, Iguchi A, Ignarro LJ. Combined effect of testosterone and apocynin on nitric oxide and superoxide production in PMA-differentiated THP-1 cells. Biochim Biophys Acta 1693: 185– 191, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Kapuku GK, Seto S, Mori H, Mori M, Utsunomia T, Suzuki S, Oku Y, Yano K, Hashiba K. Impaired left ventricular filling in borderline hypertensive patients without cardiac structural changes. Am Heart J 125: 1710– 1716, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Keidar S, Kaplan M, Pavlotzky E, Coleman R, Hayek T, Hamoud S, Aviram M. Aldosterone administration to mice stimulates macrophage NADPH oxidase and increases atherosclerosis development: a possible role for angiotensin-converting enzyme and the receptors for angiotensin II and aldosterone. Circulation 109: 2213– 2220, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi N, Yoshida K, Nakano S, Ohno T, Honda T, Tsubokou Y, Matsuoka H. Cardioprotective mechanisms of eplerenone on cardiac performance and remodeling in failing rat hearts. Hypertension 47: 671– 679, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Kuster GM, Kotlyar E, Rude MK, Siwik DA, Liao R, Colucci WS, Sam F. Mineralocorticoid receptor inhibition ameliorates the transition to myocardial failure and decreases oxidative stress and inflammation in mice with chronic pressure overload. Circulation 111: 420– 427, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Li JM, Gall NP, Grieve DJ, Chen M, Shah AM. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension 40: 477– 484, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Mak GJ, Ledwidge MT, Watson CJ, Phelan DM, Dawkins IR, Murphy NF, Patle AK, Baugh JA, McDonald KM. Natural history of markers of collagen turnover in patients with early diastolic dysfunction and impact of eplerenone. J Am Coll Cardiol 54: 1674– 1682, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Mohammed SF, Ohtani T, Korinek J, Lam CS, Larsen K, Simari RD, Valencik ML, Burnett JC, Jr, Redfield MM. Mineralocorticoid accelerates transition to heart failure with preserved ejection fraction via “nongenomic effects”. Circulation 122: 370– 378, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mureddu GF, Greco R, Rosato GF, Cella A, Vaccaro O, Contaldo F, de SG. Relation of insulin resistance to left ventricular hypertrophy and diastolic dysfunction in obesity. Int J Obes Relat Metab Disord 22: 363– 368, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Nagano N, Nagano M, Yo Y, Iiyama K, Higaki J, Mikami H, Ogihara T. Role of glucose intolerance in cardiac diastolic function in essential hypertension. Hypertension 23: 1002– 1005, 1994 [DOI] [PubMed] [Google Scholar]
- 21. Nagata K, Obata K, Xu J, Ichihara S, Noda A, Kimata H, Kato T, Izawa H, Murohara T, Yokota M. Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and failure in low-aldosterone hypertensive rats. Hypertension 47: 656– 664, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Ohtani T, Ohta M, Yamamoto K, Mano T, Sakata Y, Nishio M, Takeda Y, Yoshida J, Miwa T, Okamoto M, Masuyama T, Nonaka Y, Hori M. Elevated cardiac tissue level of aldosterone and mineralocorticoid receptor in diastolic heart failure: beneficial effects of mineralocorticoid receptor blocker. Am J Physiol Regul Integr Comp Physiol 292: R946– R954, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355: 251– 259, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Park YM, Park MY, Suh YL, Park JB. NAD(P)H oxidase inhibitor prevents blood pressure elevation and cardiovascular hypertrophy in aldosterone-infused rats. Biochem Biophys Res Commun 313: 812– 817, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 348: 1309– 1321, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 341: 709– 717, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 289: 194– 202, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Robert V, Heymes C, Silvestre JS, Sabri A, Swynghedauw B, Delcayre C. Angiotensin AT1 receptor subtype as a cardiac target of aldosterone: role in aldosterone-salt-induced fibrosis. Hypertension 33: 981– 986, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Rocha R, Martin-Berger CL, Yang P, Scherrer R, Delyani J, McMahon E. Selective aldosterone blockade prevents angiotensin II/salt-induced vascular inflammation in the rat heart. Endocrinology 143: 4828– 4836, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, McMahon EG, Delyani JA. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol 283: H1802– H1810, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, McMahon EG, Delyani JA. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol 283: H1802– H1810, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Rossi GP, Di BV, Ganzaroli C, Sacchetto A, Cesari M, Bertini A, Giorgi D, Scognamiglio R, Mariani M, Pessina AC. Excess aldosterone is associated with alterations of myocardial texture in primary aldosteronism. Hypertension 40: 23– 27, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Sadoshima J, Izumo S. Molecular characterization of angiotensin II–induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res 73: 413– 423, 1993 [DOI] [PubMed] [Google Scholar]
- 34. Sanchez-Mas J, Turpin MC, Lax A, Ruiperez JA, Valdes CM, Pascual-Figal DA. Differential actions of eplerenone and spironolactone on the protective effect of testosterone against cardiomyocyte apoptosis in vitro. Rev Esp Cardiol 63: 779– 787, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Sowers JR, Whaley-Connell A, Epstein M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med 150: 776– 783, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stas S, Whaley-Connell A, Habibi J, Appesh L, Hayden MR, Karuparthi PR, Qazi M, Morris EM, Cooper SA, Link CD, Stump C, Hay M, Ferrario C, Sowers JR. Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin-angiotensin-aldosterone system stimulation of NADPH oxidase and cardiac remodeling. Endocrinology 148: 3773– 3780, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Stier CT, Jr, Chander PN, Rocha R. Aldosterone as a mediator in cardiovascular injury. Cardiol Rev 10: 97– 107, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart : role of oxidative stress. Am J Pathol 161: 1773– 1781, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 49: 241– 248, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Wang H, Shimosawa T, Matsui H, Kaneko T, Ogura S, Uetake Y, Takenaka K, Yatomi Y, Fujita T. Paradoxical mineralocorticoid receptor activation and left ventricular diastolic dysfunction under high oxidative stress conditions. J Hypertens 26: 1453– 1462, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Weber KT. Fibrosis and hypertensive heart disease. Curr Opin Cardiol 15: 264– 272, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 83: 1849– 1865, 1991 [DOI] [PubMed] [Google Scholar]
- 43. Wei Y, Whaley-Connell AT, Habibi J, Rehmer J, Rehmer N, Patel K, Hayden M, DeMarco V, Ferrario CM, Ibdah JA, Sowers JR. Mineralocorticoid receptor antagonism attenuates vascular apoptosis and injury via rescuing protein kinase B activation. Hypertension 53: 158– 165, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Whaley-Connell A, Govindarajan G, Habibi J, Hayden MR, Cooper SA, Wei Y, Ma L, Qazi M, Link D, Karuparthi PR, Stump CS, Ferrario CM, Sowers JR. Angiotensin-II mediated oxidative stress promotes myocardial tissue remodeling in the transgenic TG (mRen2) 27 Ren2 rat. Am J Physiol Endocrinol Metab 293: E355– E363, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Whaley-Connell A, Habibi J, Cooper SA, DeMarco VG, Hayden MR, Stump CS, Link D, Ferrario C, Sowers JR. Effect of renin inhibition and AT1R blockade on myocardial remodeling in the transgenic Ren2 rat. Am J Physiol Endocrinol Metab 295: E103– E109, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao W, Ahokas RA, Weber KT, Sun Y. ANG II-induced cardiac molecular and cellular events: role of aldosterone. Am J Physiol Heart Circ Physiol 291: H336– H343, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Zhou X, Ma L, Habibi J, Whaley-Connel AT, Hayden MR, Tilmon RD, Brown AN, DeMarco VG, Sowers JR. Nebivolol improves diastolic dysfunction and myocardial tissue remodeling through reductions in oxidative stress in the Zucker obese rat. Hypertension 55: 880– 888, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]