Abstract
A substantial body of evidence suggests that nicotine adversely affects cerebral blood flow and the blood-brain barrier and is a risk factor for stroke. The present study investigated the effect of nicotine on cerebrovascular endothelium under basal and ischemia/reperfusion injury under in vivo condition. Nicotine (2 mg/kg sc) was administered to mice over 14 days, which resulted in plasma nicotine levels of ∼100 ng/ml, reflecting plasma concentrations in average to heavy smokers. An analysis of the phenotype of isolated brain microvessels after nicotine exposure indicated higher expression of inflammatory mediators, cytokines (IL-1β, TNF-α, and IL-18), chemokines (CCL2 and CX3CL1), and adhesion molecules (ICAM-1, VCAM-1, and P-selectins), and this was accompanied by enhanced leukocyte infiltration into brain during ischemia/reperfusion (P < 0.01). Nicotine had a profound effect on ischemia/reperfusion injury; i.e., increased brain infarct size (P < 0.01), worse neurological deficits, and a higher mortality rate. These experiments illuminate, for the first time, how nicotine regulates brain endothelial cell phenotype and postischemic inflammatory response at the brain-vascular interface.
Keywords: inflammation
cigarette smoking is widely recognized as a major modifiable risk factor for stroke (5, 12). There is a dose-response relationship between cigarette consumption and stroke risk, whereas smoking cessation leads to a prompt stroke risk reduction (33). Chronic exposure to tobacco or nicotine, a major active component of cigarettes, can cause cerebral vasoconstriction, decrease cerebral blood flow (CBF) and enhance ischemic brain injury following transient middle cerebral artery occlusion (MCAO) in rats (13, 38). In addition, some recent findings have indicated that cigarette smoking, and particularly nicotine, has a profound proinflammatory effect, causing a chronic inflammatory state with increased levels of circulating leukocytes, C-reactive protein, and fibrinogen, as well as enhanced leukocyte rolling and adhesion in the cerebral microcirculation and chemoattractant activity for neutrophil migration (7, 17, 47).
Proinflammatory effects of nicotine have been described at several target sites, including the vascular interface, leukocytes, and respiratory and intestinal epithelia. At these targets, nicotine alters expression of proinflammatory mediators, directly or indirectly aggravating the outcome of inflammation (2, 27, 34, 35). There is also evidence that nicotine can induce ICAM-1 and VCAM-1 expression on human umbilical vein endothelial cells (HUVEC) (2). At the level of the central nervous system and the blood-brain barrier (BBB), the effects of nicotine are still unclear. Some studies have indicated that nicotine regulates leukocyte rolling and adhesion mediated by P-selectin and CD18 (47). Nicotine may also alter nitric oxide levels, a molecule critical in regulating cerebrovascular tone and endothelial cell-leukocyte interactions (14).
Postischemic inflammation is considered a significant contributor to secondary brain injury after ischemic stroke (9, 30). The central event in postischemic inflammation is the recruitment of neutrophils that arrive first at the site of inflammation, followed by monocyte/macrophages. This is a multifactorial process involving chemotactic signals that promote the directional migration of leukocytes, adhesion, receptor/ligand interaction at the microvascular endothelial surface, and matrix metalloproteinase production needed for extracellular matrix breakdown and leukocyte extravasation (9, 30). Leukocyte infiltration into the ischemic territory is also associated with activation of microglia and astrocytes that have the potential to contribute further to the inflammatory cascade (9). Despite the fact that smoking and nicotine are indicated as risk factors for stroke and that nicotine is involved in the upregulation of some essential proinflammatory mediators (e.g., IL-8, IL-1β, TNF-α, and ICAM-1) during ischemia/reperfusion (I/R) injury in kidney, liver, coronary artery endothelial cells, and HUVEC, the effect of nicotine on cerebral postischemic inflammation is still unclear (3, 44).
The present study investigates the impact of nicotine on the postischemic inflammatory response at the BBB. Our results show that exposing the BBB to nicotine levels reflecting those in plasma of average to heavy smokers upregulates a broad range of cytokines, chemokines, and adhesion molecules at the vascular interface and significantly alters the inflammatory response during basal and I/R conditions.
MATERIALS AND METHODS
All procedures were performed in strict accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of University of Michigan.
Nicotine treatment.
C56BL/6 mice (22–28 g) were anesthetized with ketamine/xylazine (100 mg/kg and 10 mg/kg ip) and miniosmotic pumps (infusion rate 0.5 μl/h; model 2002 Azlet osmotic pump; DURECT, Cupertino, CA) were implanted in a subcutaneous pocket created by making a small incision in the skin between the scapulae. The pumps were filled with normal saline (0.9% NaCl, vehicle) or nicotine (Sigma Aldrich, St Louis, MO) dissolved in saline at a concentration sufficient to deliver 0.5, 2.0, and 5.0 mg/kg of nicotine over 14 days. Nicotine levels were evaluated in plasma samples collected by cardiac puncture (4–6 mice per group). Nicotine was measured using a liquid chromatography/tandem mass spectrometry (23) by the Biomedical Mass Spectrometry facility at the University of Michigan using a Finnigan TSQ Quantum Ultra AM. The duration of exposure, 14 days, was chosen on the basis of our evaluation of physiological parameters of nicotine-treated mice and their survival rate after transient MCAO. Fourteen days of exposure provided a stable level of nicotine in plasma and did not affect animal physiological parameters.
MCAO.
Experiments were performed on male C57BL/6 (22–25 g) mice (Jackson Laboratory, Bar Harbor, MA). Mice were anesthetized with ketamine and xylazine (100 and 10 mg/kg ip). Body temperature was maintained at 37 ± 0.5°C by means of a heating blanket and a heating lamp during the entire experimental procedure. Focal cerebral ischemia was induced by left MCAO using an intraluminal filament technique (10). Briefly, the common carotid artery was exposed through a midline incision in the neck. A 6–0 silicon suture was next introduced into the external carotid artery and advanced into the internal carotid artery a distance of 10–11 mm from the common carotid artery bifurcation according to animal weight. MCAO was confirmed by a Laser Doppler Flow probe (Model BPM System; Vasomedics, St. Paul, MN) positioned at 3 mm posterior and 5 mm lateral to bregma. After 30 min of MCAO, the mice were reperfused by suture withdrawal and then allowed to awake from anesthesia. Sham-operated animals underwent all procedures except the actual MCAO. Physiological parameters (Po2, Pco2, pH, blood glucose, and regional CBF) were monitored before, during, and after MCAO. A reperfusion period was 3 days. During reperfusion, neurological deficits were evaluated with the following scoring scheme: 0, no deficits; 1, flexion of the torso and contralateral forelimb when lifted by the tail; 2, contralateral forelimb weakness upon application of pressure to the side of the body; 3, circling to the affected side; 4, no spontaneous locomotor activity.
Brain water content and electrolytes.
Brain water content was measured by the wet/dry weight method. Samples were taken from ischemic and nonischemic hemispheres. After decapitation under deep isoflurane anesthesia, brains were weighed wet and then oven dried at 100°C for 48 h and reweighed. Brain water content (%) was calculated as [(wet weight − dry weight)/wet weight] × 100%. (10).
Morphometric measurement of infarct volume.
Animals were euthanized from 1 to 5 days after transient MCAO, and the brain was removed and sliced. Slices were incubated in 2% 2,3,5-triphenyltetrazolium chloride (Sigma Aldrich) solution for 1 h at 37°C. The area of infarction in each slice was determined by a computerized image-analysis system, and the volume of infarction was calculated by multiplying the distance between sections. In addition, to account for cerebral edema or resolution of the infarct, an indirect measurement of infarction was performed. Infarct volume was calculated as [contralateral hemisphere volume − (ipsilateral hemisphere volume − measured injury volume)] (10). Cresyl violet staining of 200-μm-thick serial sections was also used to examine infarct size after 3 days of reperfusion (10).
RT2 profiler PCR array and real-time PCR.
Isolated microvessels and whole brain with or without nicotine treatment were collected at the end of experiments. For analysis of I/R injury, isolated brain microvessels and brain area (penumbra) around the ischemic lesion (1 mm thick) were collected using “pinch-out” method (10). The corresponding contralateral region was also collected. For isolated microvessels brain tissue was mechanically dissociated and homogenized in the Dounce type of homogenizer. After washing with Hanks balanced solution, the myelin and erythrocytes were cleaned by 18% Dextran solution and Percol gradient retrospectively (9). Total RNA was prepared using TRIZOL (Invitrogen, Carlsbad, CA). Single-strand cDNA from 2 μg total RNA was synthesized using RT2 first strand kit, and real-time PCR was performed according to the RT2 Profiler PCR Array System (SABioscience, Frederick, MD) using SYBR Green PCR Master Mix in an Eppendorf Mastercycler (Eppendorf, Hauppauge, NY). The PCR arrays Mouse Inflammatory Cytokines and Receptors (SABioscience) were repeated three times and the data analyzed using PCR Array analysis software (SABioscience). In addition, real-time PCR analysis was performed to compare RNA expression of select genes (IL-1β, TNF-α, IL-18, CX3CL1, CCL2, CXCL5, CD40, CCL4, and IL-6ra) between nicotine-treated and non-treated experimental groups with or without I/R injury. All primer sets were from SABioscience.
Cytokine antibody array.
The Mouse Cytokine Antibody Array 3 (RayBiotech, Norcross GA) was used to simultaneously detect and semiquantify 62 cytokines in samples collected from all experimental groups. For tissue, samples were homogenized in 1.8 ml Tris buffer solution (pH 8.5) supplemented with 1% Triton X-100. Protein level was evaluated using a bicinchoninic acid protein assay (Thermo Fisher Scientific, Rockford, IL) and for each sample was adjusted to 2 mg/ml. The cytokine antibody array was performed according to the manufacturer's instructions. Membranes were developed with the Pierce ECL substrate kit (Thermo Fisher Scientific) and underwent densitometric analysis using ImageJ analysis software (NIH, Bethesda, MD). The relative level of inflammatory cytokines was evaluated using software provided by the manufacturer. In addition, IL-1β, IL-1α, IL-6, IL-12, IFN-γ, TNF-α, CCL5, CCL2, CCL3, CXCL12, and CCL11 protein levels were quantified by ELISA assay kit (SABioscience).
Quantitative immunohistochemistry.
Brain samples were fixed in 4% paraformaldehyde for 18 h and then cryoprotected with sequential immersions in 10% and 20% sucrose solutions and then cut into 50-μm-thick coronal sections with a freezing microtome. After that samples were preincubated in blocking solution (5% bovine serum albumin, 5% normal goat serum, 0.05% Tween and PBS), and then incubated overnight with primary antibody rat anti-mouse Ly6G antibody (BD Bioscience, San Jose, CA) and anti-myeloperoxidase (MPO) antibody (HyCult Biotechnology, Uden, The Netherlands) at 4°C. Reaction was visualized by Texas red-conjugated anti-rat (Sigma-Aldrich) or anti-rabbit antibody (Vector Laboratory, Burlingame, CA). All samples were viewed on a confocal microscope (LSM 510, Zeiss, Jena, Germany). For quantification, 50 coronal brain slices (25 slices in front and 25 behind the “middle line” of the visible lesion). Microscope data were acquired with a ×10 objective numerical aperture with constant laser power (45% of laser power), pinhole, zoom, focus, gain, and duration of image capturing. A total of 20 images was randomly selected and captured per slide. The immunolabeled cells were counted in areas surrounding ischemic lesion. Five mouse brains per group were analyzed. In the sham-operated group, brain areas were analyzed corresponding to those analyzed in ischemic mice. Slides were coded so that the counter was blind to the identity of the slides being counted.
Statistical analysis.
All values are expressed as means ± SD. One-way ANOVA followed by Bonferroni post hoc analysis, Chi-squared tests (neurological scores), and Kaplan-Meier survival curves were used (Prism analysis software). A P value <0.05 was regarded as statistically significant.
RESULTS
Plasma nicotine and cotinine concentrations.
Initial studies were performed where mice received either one of three doses of nicotine (0.5, 2.0 or 5.0 mg/kg sc) or vehicle (0.9% NaCl) for 14 days. In the nicotine-treated groups, at days 3, 7, and 13 the plasma nicotine levels were 0.52 ± 0.03, 2.7 ± 0.1, and 4.5 ± 0.9 ng/ml in the 0.5 mg/kg group, 67 ± 12, 98 ± 10, and 99 ± 3 ng/ml in the 2.0 mg/kg group, and 87 ± 10, 155 ± 14, and 184 ± 21 ng/ml in the 5.0 mg/kg group. The plasma concentrations of the nicotine metabolite cotinine at the same time points were 3.2 ± 1, 6.3 ± 1.1, and 10.2 ± 2.1 ng/ml in the 0.5 mg/kg group, 96 ± 5, 139 ± 7, and 169 ± 19 ng/ml in the 2.0 mg/kg group, and 201 ± 23, 302 ± 34, and 523 ± 36 ng/ml in the 5.0 mg/kg group (Supplemental Fig. S1, A and B; supplemental material for this article is available online at the American Journal of Physiology Heart and Circulatory Physiology website). These nicotine and cotinine levels are similar to those found in moderate, average, and heavy smokers (>5 mg/ml, 10–100 ng/ml, and <200 ng/ml) on the basis of epidemiological and biochemical studies (20). All nicotine-treated mice did not show changes in pH, Po2, Pco2, and glucose, but they did have significantly reduced body weight from 10 days of nicotine treatment (e.g., 89% and 75% of initial body weight at 10 and 14 days, respectively in the 2 mg/kg group; Supplemental Fig. S1C). In addition, the mice did not display symptoms of nicotine intoxication like excitability, shivering, tremor, or diarrhea even in the experimental group, which received the high dose of the nicotine. For most of our further experiments we primarily focused on the 2 mg/kg nicotine group.
Proinflammatory effects of nicotine at the BBB.
To examine the potential proinflammatory action of nicotine at the BBB, mice were divided into two groups that received either nicotine (2 mg/kg sc) or vehicle (0.9% NaCl) for 14 days. The effect of nicotine on the BBB inflammatory phenotype was examined at the gene and protein levels. Pro- and anti-inflammatory gene expression was evaluated using a Real-Time Profile PCR Array to analyze expression of 87 cytokines, cytokines receptors, chemokines, chemokines receptor, and other genes involved in the inflammatory response. Three independent samples of isolated microvessels or whole brain tissue from nicotine-treated mice (n = 3) were compared with control (nonexposed) mice. Isolated microvessels were not additionally trypsin digested to preserve the BBB in situ. Thus samples contained astrocyte foot processes as well as perivascular macrophages and pericytes, as confirmed by immunohistochemistry (data not shown).
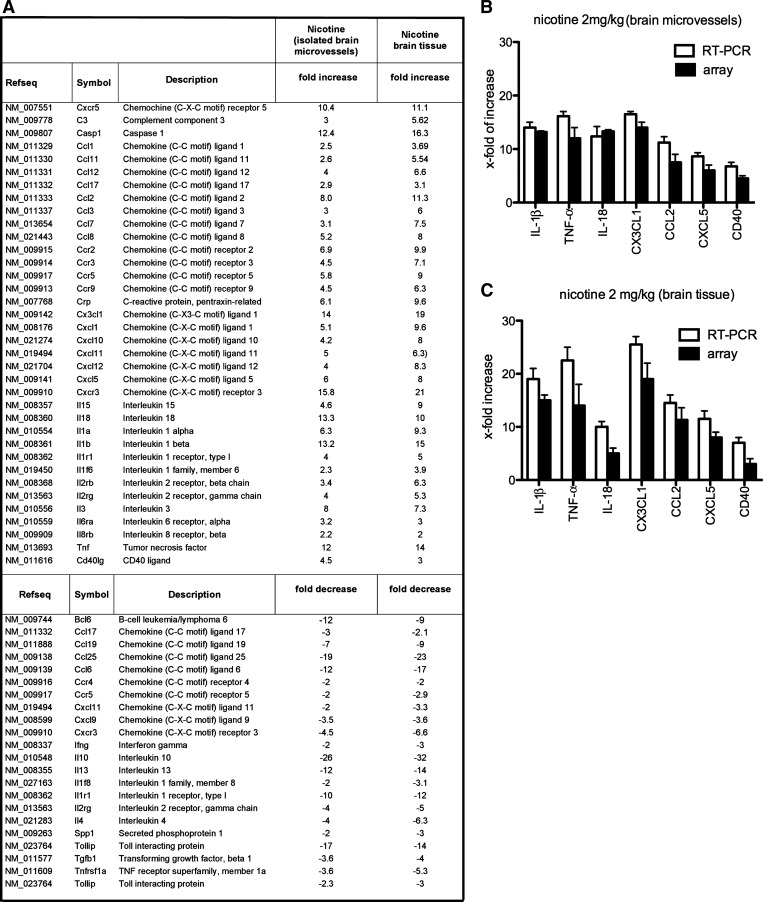
Genes were evaluated on the basis of the criteria of at least a 2.5-fold up- or downregulation compared with control and that they were regulated in more than 70% of comparisons. Using these stringent selection criteria, we identified 36 genes that were upregulated (2.9- to 15.8-fold) and 21 genes that were downregulated (2.7- to 12-fold) in microvessels (Fig. 1A), and 37 genes upregulated (2.7- to 36-fold) and 21 genes downregulated (2.6- to 20-fold) in brain tissue (Fig. 1A). The significantly upregulated genes were cytokines IL-18, IL-1β, TNF-α, IL-1α (13-, 13-, 12-, and 6-fold increases, respectively) and chemokines CX3CL1, CCL2, CXCL5, CCL8, and CXCL1 (14-, 8-, 6-, 5-, and 5-fold increases, respectively), indicating a strong proinflammatory response at the BBB in the presence of nicotine. In addition, there was higher expression of caspase-1 (12-fold increase), complement C3 and CD40 ligand (4.5-fold), suggesting not only a proinflammatory but also a proatherogenic effect of nicotine. There was also a significant downregulation of some anti-inflammatory cytokines and signaling molecules such as Bcl6, CCL25, CCL6, IL-13, IL-10, and Toll interacting protein (Tollip) (12-, 9-, 26-, 12-, and 17-fold decreases, respectively) in the brain as well as the BBB of mice exposed to nicotine. Thus nicotine causes an imbalance in pro- and anti-inflammatory responses that may induce vascular injury (Fig. 1A).
Fig. 1.
A: list of the transcripts modulated by nicotine in brain tissue and isolated brain microvessels from mice treated with nicotine (2 mg/kg) for 14 days. Fold increase/decrease indicate the level of up- or downregulation of transcripts compared with control (vehicle-treated mice). Three independent samples were analyzed by RT2 real-time PCR array. B and C: quantitative real-time PCR for IL-1β, TNF-α, IL-18, CX3CL1, CCL2, CXCL5, and CD40 was carried out on RNA from isolated brain microvessels (B) and brain tissue (C) from nicotine-treated (n = 3) and vehicle-treated mice (n = 3). Expression of target genes was normalized to control vehicle-treated mice. Values are presented as means ± SD. The fold changes obtained with RT-PCR were similar to those obtained by the PCR array.
To confirm the PCR array findings, single real-time RT-PCR analyses were performed for select proinflammatory mediators. IL-1β, TNF-α, IL-18, CX3CL1, CCL2, CXCL5, and CD40L all showed significant increases in expression after nicotine treatment compared with vehicle treatment (Fig. 1, B and C).
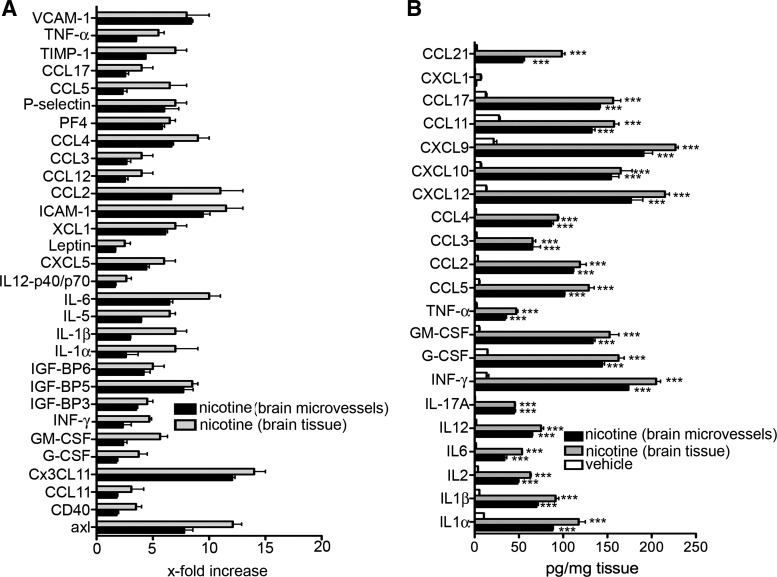
In addition to gene expression, the protein levels of proinflammatory mediators were analyzed. The analysis included total secreted amount of cytokines/chemokines (observed by ELISA) and protein expression of microvessels and brain tissue (antibody based protein array). Following the pattern of gene expression, there was significant level of secreted proinflammatory cytokines/chemokines [e.g., IL-1α, IL-1β, IL-12, TNF-α, CCL2, IL-6, INF-γ, granulocyte/macrophage colony-stimulating factor (GM-CSF), G-CSF, and CXCL12] in brain tissue and BBB after exposure to nicotine (P < 0.001) compared with controls (Fig. 2B). In response to nicotine, brain tissue and the BBB in situ also had 5- to 15-fold increases in a set of platelet-dependent chemokines/cytokines and proteins like CCL17, CXCL12, PF4, CCL3, CXC3CL1, and CD40, as well as the adhesion molecules ICAM-1, P-selectin, and VCAM-1 (Fig. 2A). This strongly supports a proatherogenic effect of nicotine at the level of the BBB. Similar pattern of pro- and anti-inflammatory cytokines expression was also presented in brain microvessels and brain tissue of mice exposed to low and high doses of nicotine for 14 days although that magnitude of cytokine expression was directly correlated to the dose of nicotine (data not shown).
Fig. 2.
Cytokine antibody array (A) and ELISA (B) analysis of isolated brain microvessels and brain. For the cytokine array, 62 cytokines, adhesion molecules, and chemokines were assayed in 3 samples. Values after nicotine treatment (2 mg/kg; 14 days) were normalized to brain microvessels or brain tissue isolated from vehicle-treated mice. For the ELISA, samples (brain microvessels or brain tissue) were taken after mice had been treated with nicotine (2 mg/kg) or vehicle for 14 days. Values represent means ± SD from 3 independent brain samples. ***P < 0.001. GM-CSF, granulocyte/macrophage colony-stimulating factor; TIMP, tissue inhibitor of matrix metalloproteinase; INF, interferon.
Effect of nicotine on brain I/R injury.
To investigate the effects of nicotine on ischemic brain damage, we first examined infarct volume and neurological deficits in nicotine- and vehicle-treated mice (exposure time was 14 days) subjected to transient MCAO with reperfusion times lasting up to 10 days. Physiological parameters (pH, Po2, Pco2, glucose level, and regional CBF) before MCAO and after 30 min of reperfusion were not significantly different between nicotine- and vehicle-treated groups (Supplemental Table S1). However, there were marked differences in neurological outcome and survival (Fig. 3, A and B). Nicotine-treated mice had worse neurological deficits. On a scale of 0–4, 80% of the mice showed no spontaneous locomotor activity (score 4) or circling to the affected side (score 3) evaluated from days 0–5 of reperfusion. In contrast, a score of 0–2 was found in 80% of vehicle-treated mice. There was also a lower survival rate in nicotine-treated mice. Forty percent of nicotine-treated mice survived until day 3 of reperfusion, and none survived to day 5, compared with 100% survival in vehicle-treated mice at days 3 and 5. Furthermore, infarct volume was greater with nicotine treatment compared with vehicle-treated mice in dose-dependent manner [day 3: vehicle-treated mice 113.9 ± 7.6 mm3 vs. nicotine-treated mice 132.7 ± 16.3 mm3, P < 0.001 (2 mg/kg group) and 137.2 ± 13.8 mm3, P < 0.001 (5 mg/kg group); Fig. 3C]. Analyzing the regional distribution of the infarct, we found that in all experimental groups infarct lesion was present in cortex and striatum. However, the nicotine-treated mice had bigger striatal and cortical infarcts compared with vehicle-treated mice (Fig. 3D). Again, there was a close correlation with the dose of nicotine and infract size in nicotine-treated mice. Mice exposed to average (2 mg/kg group) and high (5 mg/kg group) doses of nicotine had significantly increased striatal (P < 0.001) and cortical (P < 0.05 and P < 0.001, respectively). In addition, nicotine-treated mice had increased brain water content (edema) in the ischemic (but not nonischemic) hemisphere compared with vehicle-treated mice at day 3 of reperfusion in a dose-dependent manner (Fig. 3E). Because of the potential confounding effects of edema and infarct resolution, an indirect measure of infarct volume was also used. Using this measure, average and high dose of nicotine-treated mice still showed significantly greater infarct volumes compared with vehicle-treated mice (P < 0.001; Fig. 3F). Taken together, these data demonstrate that nicotine worsens neurological deficits and increases infarct volume, contributing to the progression of postischemic injury.
Fig. 3.
A: Kaplan-Meier survival curve in mice exposed to either nicotine (2 mg/kg) or vehicle (0.9% NaCl) for 14 days followed by induction of middle cerebral artery occlusion (MCAO). B: summary of neurological scores in nicotine- (2 mg/kg) and vehicle-treated mice at day 3 after transient MCAO. No neurological deficit scores are 0; maximal deficit score is 4. Vehicle (n = 15) and nicotine (n = 15), *P < 0.05 by Chi-squared test. C: 2,3,5-triphenyltetrazolium chloride-stained coronal sections of brain illustrating typical infarcts (arrows) 3 days after reperfusion in nicotine- and vehicle-treated mice. Bar graph showing infarct volumes at day 3 after transient MCAO in vehicle (n = 10) and nicotine (0.5, 2.0, and 5.0 mg/kg) (n = 7) mice. D: cortical and striatal infarct volume in same experimental animals as in C. Values are means ± SD. ***P < 0.001. E: brain edema formation after MCAO was evaluated by measuring the brain water content in ischemic and contralateral hemispheres at day 3 after transient MCAO. Values are means ± SD for nicotine-treated mice (0.5, 2.0, and 5.0 mg/kg; n = 7) vehicle-treated mice (n = 7). F: indirect measure (to correct for edema/infarct resolution) of total infarct volume at day 3 after transient MCAO in vehicle- and nicotine-treated (0.5, 2.0, and 5.0 mg/kg) mice. **P < 0.01.
Effects of nicotine on the expression of inflammatory mediator after ischemia.
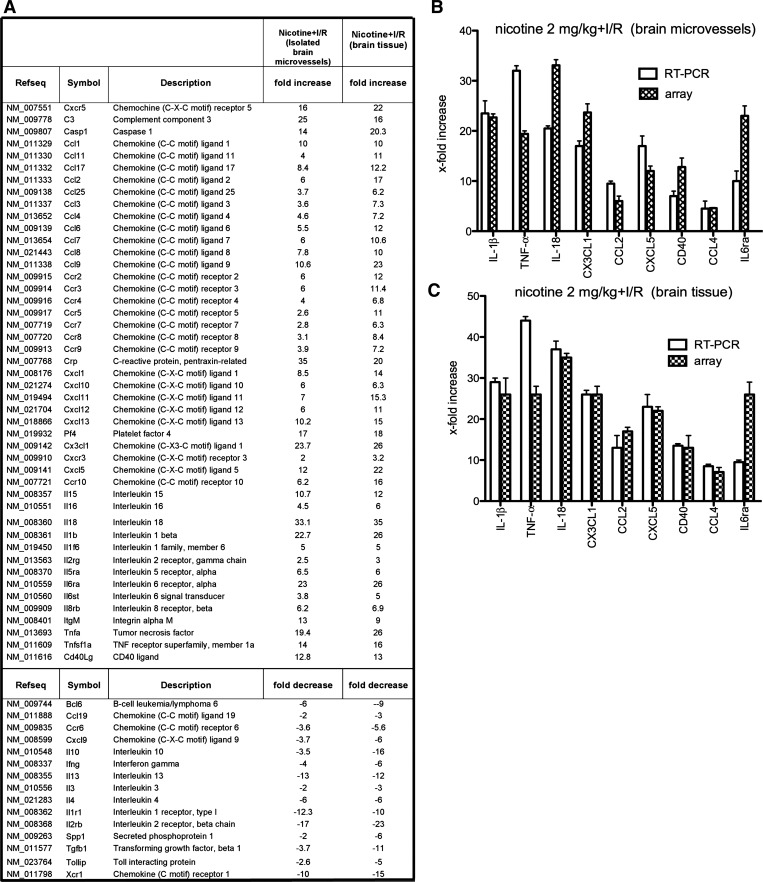
Transient focal ischemia leads to increased expression of several proinflammatory genes, and the resulting inflammation significantly contributes to stroke outcome (10). Using a Proinflammatory Cytokines & Receptors GeneArray, we analyzed the profile of proinflammatory gene expression in the core of ischemic lesion and in surrounding penumbra as well as in the isolated microvessels from the penumbra at day 3 of reperfusion in nicotine (2 mg/kg group)- and vehicle-treated mice. The following groups were examined: 1) chronically nicotine-treated MCAO mice (nicotine + I/R); 2) vehicle-treated MCAO mice (vehicle + I/R), and 3) vehicle-treated sham-operated mice treated (vehicle). In general, nicotine acted as strong proinflammatory agent with an increase (2- to 35-fold) in mRNA levels of 46 proinflammatory cytokines/chemokines during reperfusion compared with vehicle-treated MCAO mice (Fig. 4A). For example, nicotine-treated MCAO mice had increased RNA levels of chemokines CCL2, CCL7, CCL9, CXCL13, CX3CL1, and CXCL5 (6- to 10-fold increase in brain microvessels and 10- to 22-fold increase in brain tissue) compared with vehicle-treated MCAO mice (Fig. 4A). Nicotine-treated MCAO mice also had increased levels of the cytokines IL-15, IL-18, IL-1β and TNF-α (10- to 33-fold increase in brain microvessels and 12- to 35-fold increase in brain tissue), as well as complement C3, caspase 1, and CD40L (up to 25-fold increase in microvessels and brain tissue) compared with vehicle-treated MCAO mice. There were also significant decreases in the expression of some anti-inflammatory cytokines or receptors in nicotine-treated MCAO mice, including IL-10, IL-1r1, TGF-β1, and Tollip (up to 12-fold decrease in brain microvessels and up to 16-fold decrease in brain tissue). These results were confirmed by single real-time RT-PCR analysis (Fig. 4).
Fig. 4.
A: list of the genes modulated by nicotine in isolated brain microvessels and brain tissue from around the ischemic area (penumbra) of mice treated with nicotine (2 mg/kg) for 14 days followed by transient MCAO and reperfusion for 3 days. Fold increase/decrease indicates level of up- or downregulation of genes compared with control (mice treated with vehicle for 14 days, followed by transient MCAO and reperfusion for 3 days). Three independent samples were analyzed by real-time RT-PCR array. B and C: quantitative real-time PCR for IL-1β, TNF-α, IL-18, CX3CL1, CCL2, CXCL5, CD40, CCL4, and IL-6ra was carried out on RNA of isolated brain microvessels (B) or brain tissue (C) from nicotine-treated mice with brain ischemia/reperfusion (I/R) injury (n = 3) or vehicle-treated mice with brain I/R injury (n = 3). Expression of target genes was normalized to control (vehicle-treated mice with brain I/R injury). Values are presented as means ± SD. The real-time PCR confirmed the changes in these genes detected by the PCR array although there were some differences in the absolute level of upregulation with the two techniques.
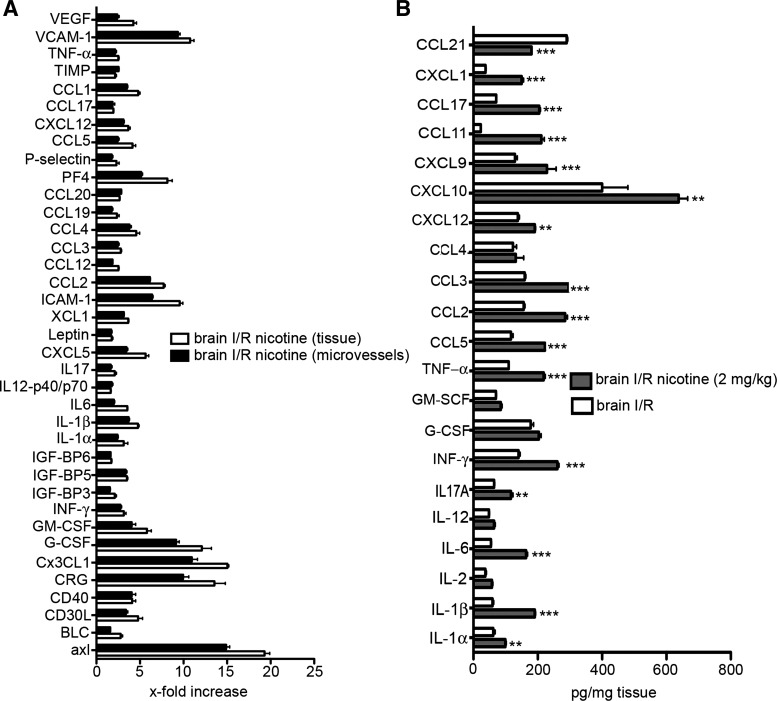
Further analysis was focused on the protein levels of secreted or expressed proinflammatory mediators in the area of penumbra as well as in the isolated brain microvessels. Nicotine had a profound effect on microvessel and brain tissue levels as manifested by 2- to 25-fold increases in proinflammatory mediators compared with vehicle-treated MCAO mice (Fig. 5A). In particular, adhesion molecules (such as VCAM-1, ICAM-1, P-selectin, and CX3CL1) and cytokines/chemokines (such as GM-CSF, G-CSF, cytokine responsive gene (CRG), CCL2, CXCL4, and CXCL10) had enhanced protein levels in nicotine-treated MCAO mice. There were significant increases (P < 0.001) in the production of cytokines (IL-6, IL-17, IL-1β, and TNF-α) and chemokines (CCL2, CCL5, CXCL10, CCL17, and CCL11) after brain I/R injury in the mice exposed to nicotine compared with vehicle-exposed MCAO mice (Fig. 5B). Thus nicotine has a proinflammatory effect, and this, in the case of brain I/R injury, aggravates the expression of most proinflammatory mediators, which in turn may contribute to the enhanced brain I/R injury in nicotine-treated mice.
Fig. 5.
A: cytokine antibody array of brain microvessels and brain tissue from mice exposed to nicotine (2 mg/kg) for 14 days followed by a MCAO for 3 days. For the cytokine array, 62 cytokines, adhesion molecules, and chemokines were assayed in 3 samples. The values were normalized to brain microvessels or brain tissue from the ischemic penumbra from vehicle-treated mice that underwent MCAO with reperfusion. B: ELISA analysis of penumbral brain samples collected from mice treated with nicotine or vehicle for 14 days followed by a MCAO for 3 days. Values represent means ± SD from n = 3 independent experiments of brain samples or brain microvessels. **P < 0.01, *** P < 0.001 comparing vehicle-treated and nicotine-treated mice that underwent MCAO with reperfusion. BMEC, brain microvascular endothelial cell.
Effects of nicotine on leukocyte infiltration after ischemia.
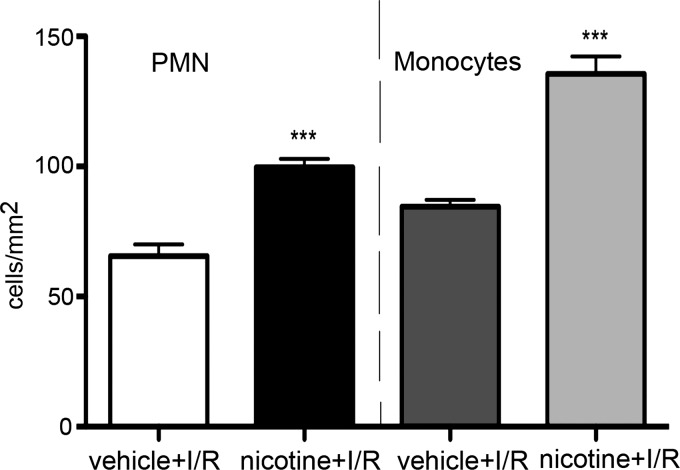
Immunohistochemical analysis of brain tissues from mice exposed to nicotine (2 mg/kg group) or vehicle in vivo did not show significant infiltration of neutrophils (MPO+ cells) or monocytes (Ly6G+) in the absence of ischemia (data not shown). However, the ischemia-induced infiltration of neutrophils and monocytes in brain parenchyma was significantly increased in mice exposed to nicotine vs. vehicle (P < 0.001) evaluated at days 3 and 5 after MCAO (Fig. 6). These data further confirm that nicotine has a profound effect on reperfusion injury and the postischemic inflammatory response.
Fig. 6.
Immunohistochemical analysis of polymorphonuclear leukocytes (PMNs) and monocytes infiltration into brain of mice exposed to nicotine (2 mg/kg) or vehicle for 14 days before MCAO. Analysis was performed at day 3 of reperfusion. Anti-MPO (PMN) and anti-Ly6G (monocytes) antibodies were used. ***P < 0.001 vs. vehicle-treated group.
DISCUSSION
Little is known about the effects of nicotine on the BBB, particularly in the setting of postischemic inflammation. The present study analyzed those potential effects and found that 1) nicotine altered the BBB phenotype to a proinflammatory one; 2) this alteration is associated with worsening of I/R injury (increased infarct volume, mortality, neurological deficits, and inflammation); and 3) the nicotine-induced proinflammatory response is characterized by significant increases in proinflammatory cytokine/chemokine expression and production although it did not alter the specific type (e.g., neutrophil vs. monocyte) of the postischemic inflammatory response. These findings are discussed below.
We would like first to address issues regarding our model system. We are aware that nicotine is just one component of cigarettes, there being ∼4,000 compounds detectable in tobacco tar. However, there are some obstacles to study those components. There are still limitations in detection assays for most of the compounds, affecting our ability to determine their effects. On the other hand, several epidemiological and biochemical studies on the basis of the habit of smokers have determined plasma nicotine levels, showing that nonsmokers or passive smokers have less than 10 ng/ml, that modest or average smokers have nicotine levels between 10–100 ng/ml, whereas heavily smokers have nicotine levels of more than 100 ng/ml (6, 20). Using this “empirical” classification, we chose nicotine doses of 0.5, 2.0, and 5.0 mg/kg, which achieved plasma nicotine concentrations corresponding to passive, average, and heavy smokers. Prolonged exposure to these selected doses may best represent the effects that nicotine, one of the major compounds in tobacco, could have on cerebral endothelium and brain I/R injury in smokers. It should be noted, however, that other compounds in cigarette smoke might potentially modulate the effects of nicotine. Nicotine replacement therapies, (i.e., nicotine chewing gum, inhaler, or skin patches) utilize slightly lower doses of pure nicotine in 6-wk cigarette cessation programs (5, 6). However, there is an increasing body of evidence that they have an effect similar to the one described for nicotine. Although there are lower doses of nicotine in plasma than average smokers, clinical and epidemiological studies indicate that pure nicotine usage may have severe effects on patients with cardiovascular disease and may cause some inflammatory reactions (24). This suggests our model may in part represent the potential changes, which can occur during nicotine replacement therapy and offer some new directions regarding dosage and time of exposure. It is also important to take into account that under certain conditions nicotine can have more anti-inflammatory and protective effects as indicated in several recent studies (20, 28), and this study aimed to clarify whether absorbed nicotine would have damaging or beneficial effects.
The miniosmotic pump delivery system was chosen because it provides a stable level of nicotine in plasma, as can also be seen in smokers. In contrast, other systems (nicotine delivered though water, inhalation device, etc.) show very unstable levels of nicotine in plasma and are often associated with hypoxic episodes and stress of repeated application, effects eliminated with the miniosmotic delivery system (40, 41). It is important also to pinpoint that, via the miniosmotic delivery of nicotine, mice did not exhibit any signs of intoxication (behavioral and pathophysiological symptoms) for 14 days. However, prolonging exposure more than 14 days did have an impact on some physiological symptoms as well as on the rate of survival after MCAO. Finally, it is also important to address that in our experimental groups we did not find evidence of alterations in blood flow during nicotine treatment. This could be the result of the young age of mice and the relatively short duration (14 days) of nicotine exposure. Future analysis of the cerebral blood vessel wall with nicotine exposure should address this point.
The pro- or anti-inflammatory effects of nicotine have been the subject of controversy and discussion. Nicotine as the major component of tobacco is, on the one side, denoted as a strong proinflammatory mediator enhancing the inflammatory responses by regulating monocyte, interacting with endothelial cells, controlling leukocyte rolling and adhesion, inducing massive leukocyte infiltration, and upregulating proinflammatory factors (IL-8, IL-1β, TNF-α, ICAM-1, and P-selectins) (26, 39, 43, 47). However, there is also compelling evidence that nicotine can display opposite effects. Acting through nicotinic acetylcholine receptors on neurons, nicotine can have anti-inflammatory effects protecting, for example, against neural damage during inflammation associated with Parkinson's disease or traumatic brain injury (19, 37, 31, 32). Obviously, the microenvironment, types of additive stimuli, as well as the targeted cells (endothelial cells, neurons, glial cells, or leukocytes) significantly impact upon the effects of nicotine, adding complexity to any analysis of the contribution of nicotine as an inflammatory factor.
At the cerebrovascular level, several proinflammatory factors including cytokines (IL-6, TNF-α, and IL-1β), matrix metalloproteinases (MMP-2, MMP-9, and MMP-13), inducible NO synthase, adhesion molecules (ICAM-1, VCAM-1, and selectins), and angiotensin I and II receptors are indicated as being involved in the inflammatory response triggered by tobacco smoking (18, 25, 42). These data point to the ability of cigarette smoke and nicotine to modulate the complex interplay of signaling, adhesion molecules, and extracellular matrix remodeling that control the vascular inflammatory response and, therefore, increase the risk for the pathogenesis and progression of atherogenesis and vascular impairments. Our results extend previous findings and highlight a very similar proinflammatory pattern after administration of nicotine alone at a dose found in the plasma of average to heavy smokers. So as not to focus on a specific group of proinflammatory mediators, we analyzed a broad set of different pro- and anti-inflammatory cytokines, chemokines, and adhesion molecules (87 at the gene level and 62 at the protein level) in isolated cerebral microvessels (BBB in situ) and brain tissue.
The presence of nicotine induced in the BBB and brain parenchyma mostly a nonspecific, acute inflammatory response mirrored in expression of cytokines IL-1β, TNF-α, IL-6, and IL-6Ra. This is in strong agreement with recently published studies by two laboratories, which studied the direct effect of cigarette particles on cerebral blood vessels and brain endothelial cells (20, 42). Expression of these cytokines could imply higher sensitivity of the cerebrovascular endothelium on cytokine stimulation and may be responsible for triggering and supporting the expression of other proinflammatory mediators. Our study implicated a variety of other cytokines (e.g., IL-2, IL-12, GM-CSF, and G-SCF), chemokines (e.g., CCL2, CCL9, CCL11, CCL17, and CXCL5), adhesion molecules (ICAM-1, VCAM-1, P-selectins, and CX3CL1), and molecules such as caspase-1, CD40L, and C3, which could play role in further promoting the development of a proinflammatory and proatherogenic phenotype of brain endothelial cells by nicotine. In addition, nicotine caused a significant downregulation of anti-inflammatory mediators, creating a proanti-inflammatory imbalance in the cells, which may have a profound effect on vascular function. A possible result of these effects could be activation of various pathophysiological programs such as matrix remodeling, apoptosis, changes in vascular hemodynamics (sheer stress, flow pattern) at the vascular interface, and an alteration in the intravascular environment from a hemodynamically stable state to a procoagulant and prooxidant state favoring an exaggerated response to vascular injury and prompting the development of ischemic events. Support for these observations is found in a recent study by Vikman and colleagues, which clearly pinpointed that lipid-soluble cigarette-smoking particles may induce the upregulation of MMP-9 and MMP-13, important in remodeling of the extracellular arterial wall (42). Taking into consideration that the studied dose of nicotine corresponds to the level found in average to heavy smokers and the proinflammatory response of brain endothelial cells in vivo, nicotine should be considered as a severe factor for developing the biological phenomena known as vascular aging, fueling the development of stroke. In the support of this concept is our finding of significant upregulation of proatherogenic factors such as caspase-1, C3, and CD40L, which in combination with other proinflammatory mediators make substrate for the aging type of vascular dysfunction.
Besides affecting the brain endothelium under resting conditions, nicotine also affected the development of brain I/R injury. Although it is well known that cigarette smoking/nicotine is a major risk factor for stroke, extensive analysis on the detrimental effects of nicotine on ischemic injury is still lacking. Using a two-pronged approach examining infarct volume and neurological deficits after MCAO, we found that nicotine enhanced infarct size and worsened neurological status. Furthermore, the brain infarct size was closely associated with the exposed dose of nicotine, pinpointing that increased levels of nicotine directly aggravate brain injury. The potential reason for the enhanced ischemic injury may be that prior nicotine treatment induces a low inflammatory response, which can be a solid substrate for a profound response to ischemic injury at the BBB and in brain parenchyma. Analyzing the changes in brain tissue, our results indicated a significant upregulation in expression of IL-1β, TNF-α, and IL-6 at the mRNA and protein levels after nicotine exposure, whereas anti-inflammatory cytokines IL-10 or IL-1ra were significantly downregulated. Taking into consideration that IL-10 and IL-1ra act as neuroprotective mediators (prevent apoptotic events and glutamate excitotoxicity), particularly after brain I/R injury, the imbalance in pro- and anti-inflammatory mediators generated by nicotine may affect the susceptibility of neurons and glial cells to injury, and this could play a role in expanding infarct size in nicotine-treated animals. The adverse effects on brain parenchyma may be enhanced by effects at the level of the BBB, the first defense against noxic stimuli. Alterations in the BBB proinflammatory phenotype even before ischemic onset, as well as the profound effect during the reperfusion injury, may facilitate potential stroke onset and enhance the final ischemic outcome. Therefore, the actions of nicotine could be defined as “breaking the system of defense” at the level of BBB and brain tissue, which in turn affects neuronal viability and worsens the outcome. The increased infarct size and worse neurological deficits in nicotine-treated mice was not a surprising outcome considering its profound proinflammatory effects.
Nicotine has been implicated in BBB changes leading to brain edema formation. It is known that nicotine alters the Na+, K+, 2Cl cotransporter 1 (NKCC1) on the abluminal (brain facing) surface of the BBB during in vitro hypoxia/aglycemia conditions, affecting the development of both cytotoxic and vasogenic brain edema (1, 16, 29, 49). Nicotine also affects the tight-junction complexes between brain endothelial cells, which may contribute to vasogenic brain edema (16). Although our study did not focus on BBB permeability, the increased water content in the injured hemisphere of nicotine-treated mice supports these findings. In addition, our evidence regarding the proinflammatory alterations in BBB with nicotine treatment and the known effects of proinflammatory mediators on vasogenic edema suggest that the extensive brain edema in nicotine-treated mice may result from the enhanced postischemic inflammatory response (36, 45). The exacerbation of brain edema may contribute to worsening of stroke outcome by nicotine, particularly to the neurological deficit and high mortality rate.
Nicotine also affects reperfusion injury, and our study pinpoints a major effect on the postischemic inflammatory response. That response is a critical event in reperfusion injury after ischemic stroke. It is designated as an acute inflammatory response manifested by significant upregulation of inflammatory molecules. In patients, cytokines (TNF-α, IL-1β, IL-8, and IL-18) and soluble adhesion molecules (L-, E-, and P-selectin, sICAM-1, and sVCAM-1) are elevated in blood and CSF from the first day of stroke. In animals, there is upregulation of cytokines (IL-1β, TNF-α, and IL-6) and chemokines (CCL2, CCL3, CCL5, and CCL4) after MCAO, and this is associated with significant infiltration of neutrophils and monocytes into brain parenchyma (10, 30). Analyzing the expression of proinflammatory mediators in our ischemic model, we found expression of a variety of cytokines (i.e., IL-1β, TNF-α, CCL2, CXCL5, and CX3CL1) not only in the brain parenchyma but also in the BBB in situ (isolated microvessels). This pinpoints that the inflammatory response develops at the level of the BBB and vascular interface as well as the parenchyma. The inflammatory events at the BBB and brain parenchyma cause further aggravation of ischemic lesion by promoting infiltration of neutrophils and monocytes into the area around the ischemic lesion. Nicotine actions under these ischemia/reperfusion conditions can be characterized as detrimental. Whereas mice exposed chronically to nicotine did not have changes in the pattern of cytokines/chemokines and adhesion molecules expression, there were marked changes in magnitude of the response, with some cytokines and chemokines reaching three- to fivefold increases. A similar pattern was also found for infiltrating leukocytes where nicotine-treated animals had more infiltrating cells (both neutrophils and monocytes) compared with vehicle-treated mice. Thus, although the effect of nicotine on the postischemic inflammatory response is marked, it can be characterized as affecting the magnitude and not the type of response.
It is also important to address the issue that the delivery of nicotine was stopped during I/R injury, mimicking what is expected in patients with a stroke. Under in vivo conditions, we expect that some nicotine will remain in the circulation for several days after miniosmotic pump removal, but it will not be at as high a concentration. However, even with this reduced level of exposure during I/R, prior nicotine treatment still exacerbated the inflammatory response during I/R injury. Presumably, alterations in the proinflammatory phenotype at the brain vascular interface attributable to prior nicotine treatment continue to aggravate the inflammatory response and I/R injury. However, future studies should address whether high concentrations of nicotine during I/R affect injury. The dose dependency of the actions of nicotine and the mechanism by which nicotine has proinflammatory effects also need further study.
In summary, nicotine exerts marked effects on the expression of inflammatory mediators at the level of the BBB, changing the brain endothelium to a proinflammatory phenotype. This phenotype change may affect stroke occurrence and our results show that it does enhance ischemia-induced brain injury in a dose-dependent manner. We did not find any sign of an anti-inflammatory or protective role of nicotine at the level of BBB, and our conclusion is that nicotine at the BBB has an exclusively proinflammatory role. This study provides new insights into how to develop new therapeutic strategies for stroke in smokers. In addition, although this study focuses on stroke, the results have implications for other neurological disorders involving an inflammatory component and for the postischemic inflammatory response that occurs in tissues other than brain.
GRANTS
This work was supported by grant R21 NS062299 (A. Andjelkovic) from the National Institutes of Health and University of Michigan Cardiovascular Center (CVC) McKay Research Grant (A. Andjelkovic).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
REFERENCES
- 1. Abbruscato TJ, Lopez SP, Roder K, Paulson JR. Regulation of blood-brain barrier Na,K,2Cl-cotransporter through phosphorylation during in vitro stroke conditions and nicotine exposure. J Pharmacol Exp Ther 310: 459– 468, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Albaugh G, Bellavance E, Strande L, Heinburger S, Hewitt CW, Alexander JB. Nicotine induces mononuclear leukocyte adhesion and expression of adhesion molecules, VCAM and ICAM, in endothelial cells in vitro. Ann Vasc Surg 18: 302– 307, 2004. [DOI] [PubMed] [Google Scholar]
- 3. Albaugh G, Kann B, Strande L, Vemulapalli P, Hewitt C, Alexander JB. Nicotine induces endothelial TNF-alpha expression, which mediates growth retardation in vitro. J Surg Res 99: 381– 384, 2001. [DOI] [PubMed] [Google Scholar]
- 4. Andjelkovic AV, Stamatovic SM, Keep RF. The protective effects of preconditioning on cerebral endothelial cells in vitro. J Cereb Blood Flow Metab 23: 1348– 1355, 2003. [DOI] [PubMed] [Google Scholar]
- 5. Apelberg BJ, Onicescu G, Avila-Tang E, Samet JM. Estimating the risks and benefits of nicotine replacement therapy for smoking cessation in the United States. Am J Public Health 100: 341– 348, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benowitz NL, Jacob P, 3rd, Herrera B. Nicotine intake and dose response when smoking reduced-nicotine content cigarettes. Clin Pharmacol Ther 80: 703– 714, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Burns DM. Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis 46: 11– 29, 2003. [DOI] [PubMed] [Google Scholar]
- 8. Cohn ZA. The isolation and cultivation of mononuclear phagocytes. Methods Enzymol 32: 758– 765, 1974. [DOI] [PubMed] [Google Scholar]
- 9. Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol 62: 127– 136, 2003. [DOI] [PubMed] [Google Scholar]
- 10. Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke 38: 1345– 1353, 2007. [DOI] [PubMed] [Google Scholar]
- 11. Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Effects of the chemokine CCL2 on blood-brain barrier permeability during ischemia-reperfusion injury. J Cereb Blood Flow Metab 26: 797– 810, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Dietrich WD, Busto R, Bethea JR. Postischemic hypothermia and IL-10 treatment provide long-lasting neuroprotection of CA1 hippocampus following transient global ischemia in rats. Exp Neurol 158: 444– 450, 1999. [DOI] [PubMed] [Google Scholar]
- 13. Domino EF, Minoshima S, Guthrie S, Ohl L, Ni L, Koeppe RA, Zubieta JK. Nicotine effects on regional cerebral blood flow in awake, resting tobacco smokers. Synapse 38: 313– 321, 2000. [DOI] [PubMed] [Google Scholar]
- 14. Gerzanich V, Ivanova S, Simard JM. Early pathophysiological changes in cerebral vessels predisposing to stroke. Clin Hemorheol Microcirc 29: 291– 294, 2003. [PubMed] [Google Scholar]
- 15. Hankey GJ. Risk factor management to prevent stroke. Adv Neurol 92: 179– 185, 2003. [PubMed] [Google Scholar]
- 16. Hawkins BT, Abbruscato TJ, Egleton RD, Brown RC, Huber JD, Campos CR, Davis TP. Nicotine increases in vivo blood-brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res 1027: 48– 58, 2004. [DOI] [PubMed] [Google Scholar]
- 17. Hawkins BT, Brown RC, Davis TP. Smoking and ischemic stroke: a role for nicotine? Trends Pharmacol Sci 23: 78– 82, 2002. [DOI] [PubMed] [Google Scholar]
- 18. Hossain M, Sathe T, Fazio V, Mazzone P, Weksler B, Janigro D, Rapp E, Cucullo L. Tobacco smoke: a critical etiological factor for vascular impairment at the blood-brain barrier. Brain Res 1287: 192– 205, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kostrzewa RM, Segura-Aguilar J. Novel mechanisms and approaches in the study of neurodegeneration and neuroprotection. A review. Neurotox Res 5: 375– 383, 2003. [DOI] [PubMed] [Google Scholar]
- 20. Lawson GM, Hurt RD, Dale LC, Offord KP, Croghan IT, Schroeder DR, Jiang NS. Application of serum nicotine, and plasma cotinine concentrations to assessment of nicotine replacement in light, moderate, and heavy smokers undergoing transdermal therapy. J Clin Pharmacol 38: 502– 509, 1998. [DOI] [PubMed] [Google Scholar]
- 21. Liu S, Xu GY, Johnson KM, Echetebu C, Ye ZS, Hulsebosch CE, McAdoo DJ. Regulation of interleukin-1beta by the interleukin-1 receptor antagonist in the glutamate-injured spinal cord: endogenous neuroprotection. Brain Res 22: 63– 74, 2008. [DOI] [PubMed] [Google Scholar]
- 22. Miller GJ, Bauer KA, Cooper JA, Rosenberg RD. Activation of the coagulant pathway in cigarette smokers. Thromb Haemost 79: 549– 553, 1998. [PubMed] [Google Scholar]
- 23. Naidong W, Shou W, Chen YL, Jiang X. Novel liquid chromatographic-tandem mass spectrometric methods using silica columns and aqueous-organic mobile phases for quantitative analysis of polar ionic analytes in biological fluids. J Chromatogr B Biomed Sci Appl 754: 387– 399, 2001. [DOI] [PubMed] [Google Scholar]
- 24. Nicholson JA, Smith D, Scott MH. Nicotine gum causing pancreatitis: a case report. Pancreas 39: 116, 2010. [DOI] [PubMed] [Google Scholar]
- 25. Nordskog BK, Fields WR, Hellmann GM. Kinetic analysis of cytokine response to cigarette smoke condensate by human endothelial, and monocytic cells. Toxicology 212: 87– 97, 2005. [DOI] [PubMed] [Google Scholar]
- 26. Nordskog BK, Blixt AD, Morgan WT, Fields WR, Hellmann GM. Matrix-degrading and pro-inflammatory changes in human vascular endothelial cells exposed to cigarette smoke condensate. Cardiovasc Toxicol 3: 101– 117, 2003. [DOI] [PubMed] [Google Scholar]
- 27. Nowak D, Ruta U, Piasecka G. Nicotine increases human polymorphonuclear leukocytes chemotactic response–a possible additional mechanism of lung injury in cigarette smokers. Exp Pathol (Jena) 39: 37– 43, 1990. [DOI] [PubMed] [Google Scholar]
- 28. Park HJ, Lee PH, Ahn YW, Choi YJ, Lee G, Lee DY, Chung ES, Jin BK. Neuroprotective effect of nicotine on dopaminergic neurons by anti-inflammatory action. Eur J Neurosci 26: 79– 89, 2007. [DOI] [PubMed] [Google Scholar]
- 29. Paulson JR, Yang T, Selvaraj PK, Mdzinarishvili A, Van der Schyf CJ, Klein J, Bickel U, Abbruscato TJ. (2010) Nicotine exacerbates brain edema during in vitro and in vivo focal ischemic conditions. J Pharmacol Exp Ther 332: 371– 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petty MA, Wettstein JG. Elements of cerebral microvascular ischaemia. Brain Res Rev 36: 23– 34, 2001. [DOI] [PubMed] [Google Scholar]
- 31. Picciotto MR, Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer's and Parkinson's disease. Front Biosci 13: 492– 504, 2008. [DOI] [PubMed] [Google Scholar]
- 32. Ravikumar R, Flora G, Geddes JW, Hennig B, Toborek M. Nicotine attenuates oxidative stress, activation of redox-regulated transcription factors and induction of proinflammatory genes in compressive spinal cord trauma. Brain Res Mol Brain Res 124: 188– 198, 2004. [DOI] [PubMed] [Google Scholar]
- 33. Schweitzer JR, Koehler PJ, Voogd AC, Franke CL. Searching for prognostic variables for secondary worsening after ischaemic stroke. J Neurol 257: 1552– 1556. 2010. [DOI] [PubMed] [Google Scholar]
- 34. Sikora L, Rao SP, Sriramarao P. Selectin-dependent rolling, and adhesion of leukocytes in nicotine-exposed microvessels of lung allografts. Am J Physiol Lung Cell Mol Physiol 285: L654– L663, 2003. [DOI] [PubMed] [Google Scholar]
- 35. Speer P, Zhang Y, Gu Y, Lucas MJ, Wang Y. Effects of nicotine on intercellular adhesion molecule expression in endothelial cells and integrin expression in neutrophils in vitro. Am J Obstet Gynecol 186: 551– 556, 2002. [DOI] [PubMed] [Google Scholar]
- 36. Stamatovic SM, Dimitrijevic OB, Keep RF, Andjelkovic AV. Inflammation and brain edema: new insights into the role of chemokines and their receptors. Acta Neurochir (Wien) 96: 444– 50, 2006. [DOI] [PubMed] [Google Scholar]
- 37. Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev 17: 259– 273, 2007. [DOI] [PubMed] [Google Scholar]
- 38. Terborg C, Birkner T, Schack B, Witte OW. Acute effects of cigarette smoking on cerebral oxygenation, and hemodynamics: a combined study with near-infrared spectroscopy and transcranial Doppler sonography. J Neurol Sci 205: 71– 75, 2002. [DOI] [PubMed] [Google Scholar]
- 39. Ueno H, Pradhan S, Schlessel D, Hirasawa H, Sumpio BE. Nicotine enhances human vascular endothelial cell expression of ICAM-1 and VCAM-1 via protein kinase C, p38 mitogen-activated protein kinase, NF-kappaB, and AP-1. Cardiovasc Toxicol 6: 39– 50, 2006. [DOI] [PubMed] [Google Scholar]
- 40. Ulrich YM, Hargreaves KM, Flores CM. A comparison of multiple injections versus continuous infusion of nicotine for producing up-regulation of neuronal [3H.]-epibatidine binding sites. Neuropharmacology 36: 1119– 1125, 1997. [DOI] [PubMed] [Google Scholar]
- 41. Valentine JD, Hokanson JS, Matta SG, Sharp BM. Self-administration in rats allowed unlimited access to nicotine. Psychopharmacology (Berl) 133: 300– 304, 1997. [DOI] [PubMed] [Google Scholar]
- 42. Vikman P, Xu CB, Edvinsson L. Lipid-soluble cigarette smoking particles induce expression of inflammatory and extracellular-matrix-related genes in rat cerebral arteries. Vasc Health Risk Manag 5: 333– 41, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang L, Kittaka M, Sun N, Schreiber SS, Zlokovic BV. Chronic nicotine treatment enhances focal ischemic brain injury and depletes free pool of brain microvascular tissue plasminogen activator in rats. J Cereb Blood Flow Metab 17: 136– 146, 1997. [DOI] [PubMed] [Google Scholar]
- 44. Wang Y, Wang Z, Zhou Y, Liu L, Zhao Y, Yao C, Wang L, Qiao Z. Nicotine stimulates adhesion molecular expression via calcium influx and mitogen-activated protein kinases in human endothelial cells. Int J Biochem Cell Biol 38: 170– 182, 2006. [DOI] [PubMed] [Google Scholar]
- 45. Xiao F. Bench to bedside: brain edema and cerebral resuscitation: the present and future. Acad Emerg Med 9: 933– 946, 2002. [DOI] [PubMed] [Google Scholar]
- 46. Yang T, Roder KE, Bhat GJ, Thekkumkara TJ, Abbruscato TJ. Protein kinase C family members as a target for regulation of blood-brain barrier Na,K,2Cl-cotransporter during in vitro stroke conditions and nicotine exposure. Pharm Res 23: 291– 302, 2006. [DOI] [PubMed] [Google Scholar]
- 47. Yong T, Zheng MQ, Linthicum DS. Nicotine induces leukocyte rolling and adhesion in the cerebral microcirculation of the mouse. J Neuroimmunol 80: 158– 164, 1997. [DOI] [PubMed] [Google Scholar]
- 48. Yuan Y, Fleming BP. A method for isolation and fluorescent labeling of rat neutrophils for intravital microvascular studies. Microvasc Res 40: 218– 229, 1990. [DOI] [PubMed] [Google Scholar]
- 49. Zidovetzki R, Chen P, Fisher M, Hofman FM, Faraci FM. Nicotine increases plasminogen activator inhibitor-1 production by human brain endothelial cells via protein kinase C-associated pathway. Stroke 30: 651– 655, 1999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.