Abstract
Post-myocardial infarction (MI), chemokine homing of inflammatory cells into the injured left ventricle (LV) regulates ventricular remodeling, in part by stimulating the extracellular matrix response. The CC chemokine receptor 5 (CCR5) is a key chemokine receptor expressed on macrophages, and CCR5 ligands are highly upregulated post-MI. We hypothesized that deletion of CCR5 would attenuate adverse remodeling by decreasing inflammatory cell recruitment. Accordingly, we examined LV function, macrophage recruitment and activation, and collagen content in wild-type (WT, n = 25) and CCR5 null (n = 33) mice at 7 days post-MI. Both groups had similar infarct sizes (44 ± 2% in WT and 42 ± 2% in CCR5 null; P = 0.37). However, the LV remodeling index (end diastolic volume/LV mass) increased to a larger extent in CCR5 null (1.28 ± 0.08 μl/mg for CCR5 null and 1.02 ± 0.06 μl/mg for WT; P < 0.05). Although numbers of infiltrated macrophages were similar in WT and CCR5 null mice, CCR5-deficient macrophages isolated from the infarct zone displayed >50% decrease in gene expression levels of proinflammatory activation markers (interleukin-1β, interleukin-6, and tumor necrosis factor-α), as well as anti-inflammatory activation markers (arginase 1, CD163, mannose receptor, and transforming growth factor-β1) compared with WT (all P < 0.05). Concomitant with the reduced macrophage activation, heat shock protein-47 and collagen type I precursor levels in the infarct region decreased in the CCR5 null (1.2 ± 0.3 units in the CCR5 null and 2.3 ± 0.4 units in the WT; P < 0.05), while collagen fragments increased (88.3 ± 5.9 units in the CCR5 null and 32.7 ± 8.5 units in the WT; P < 0.05). We conclude that CCR5 deletion impairs LV remodeling by hindering macrophage activation, which stimulates an imbalance in collagen metabolism and increases the remodeling index.
Keywords: inflammation, matrix metalloproteinases, CC chemokines
cc chemokines control immune responses by regulating the recruitment and activation of leukocytes. CC chemokine ligand 2 (CCL2; monocyte chemotactic protein-1), CCL3 (macrophage inflammatory protein-1α), CCL4 (macrophage inflammatory protein-1β), and CCL5 (regulated on activation normal T-expressed and presumably secreted) chemokines, in particular, have monocyte chemotactic activities, and neutralization of these chemokines and downstream signaling results in reduced macrophage infiltration to sites of inflammation (2, 3, 15). In vivo, CCL2, CCL3, and CCL5 have been implicated in left ventricular (LV) remodeling, as levels of these CC chemokines increase post-myocardial infarction (MI) in both animal models and humans, and serum levels increase further in patients who progress to heart failure (5, 6, 24).
CC chemokines receptor 5 (CCR5) is the major coreceptor for human immunodeficiency virus infection of macrophages and is the natural receptor for CCL2, CCL3, CCL4, and CCL5, although the affinity of CCR5 for CCL2 is low (14, 29, 30). Post-MI, CCR5 and its four ligands are prominently expressed within 24 h (7, 16). CCR5 has been suggested to mediate macrophage infiltration into healing myocardium (14, 19). In the post-MI LV, macrophages are a key source of matrix metalloproteinases (MMPs) that break down extracellular matrix (ECM) components and growth factors, such as transforming growth factor (TGF)-β1, that activate fibroblasts to deposit ECM and facilitate scar formation (8, 11, 13, 21, 28, 31). We hypothesized that CCR5 deletion would attenuate adverse LV remodeling by decreasing macrophage infiltration and reducing the inflammatory response. Accordingly, we examined the effect of CCR5 deletion after experimental MI, focusing on LV function, macrophage infiltration and activation, fibroblast activation, collagen content, and MMP levels.
MATERIALS AND METHODS
All animal procedures were conducted according to the “Guide for the Care and Use of Laboratory Animals” (National Institutes of Health publication no. 85-23, revised 1996) and were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio.
Mice.
Control groups consisted of 3- to 6-mo-old C57BL/6 wild-type (WT; n = 13) and CCR5 null (n = 13) mice of both sexes at day 0. WT (n = 44) and CCR5 null mice (n = 60), 3–6 mo old of both sexes, were used for a 7-day MI study. MI was induced by surgical ligation of the left anterior descending coronary artery, as described previously (20). After 7 days of permanent ligation, 31 WT mice and 42 null mice survived. Of these, 25 WT mice and 33 null mice underwent echocardiographic assessments at day 7 post-MI. WT (n = 15) and null (n = 20) LV were used for histology, and WT (n = 6) and null (n = 9) LV were used for macrophage isolation. For immunoblotting, 10 WT controls, 11 null controls, 11 WT post-MI, and 23 null post-MI were used.
At 7 days post-MI, LV tissue was collected. Tissue from animals that died spontaneously before the 7-day time point was not included in this study. The mice were anesthetized with 2% isoflurane, and the coronary vasculature was flushed with cardioplegic solution (or DMEM for the animals used for macrophage isolation). The hearts were excised, and the LV and right ventricle (RV) were separated and weighed individually. The LV was sectioned into apex, midcavity, and base slices and stained with 1% 2,3,5-triphenyltetrazolium chloride (Sigma) for infarct sizing (25). The infarct and remote regions from the apex and base were separated, snap frozen, and stored at −80°C for biochemical analysis, or were used for macrophage isolation. The midcavity was fixed in 10% zinc formalin for histological examination. The lungs were also removed and weighed.
Echocardiographic measurements.
Echocardiograms were acquired under spontaneous respiration with 0.5–2% isoflurane in a 100% oxygen mix. Electrocardiogram and heart rate were monitored throughout the imaging procedure using a surface electrocardiogram. All images were acquired with the use of a Vevo 770 High-Resolution In Vivo Imaging System (Visual Sonics) and were taken at a heart rate >400 beats/min to achieve physiologically relevant measurements. Measurements were taken from the two-dimensional parasternal long-axis and short-axis (m-mode) recordings of the LV. For each parameter, three images from consecutive cardiac cycles were measured and averaged. The LV remodeling index was calculated as the end-diastolic volume to LV mass ratio (1).
Histology.
Midventricular transverse sections of the LV were embedded in paraffin, sectioned at 5 μm, and stained using hematoxylin and eosin. Picrosirius red staining was used to visualize collagen content. Immunohistochemistry was performed with the use of the Vectastain ABC kit (Vector Laboratories). HistoMark Black (KPL 54–75-00) was used to visualize positive staining, with eosin as a counterstain. Antibodies specific for macrophages (Mac 3, Cedarlane CL8943AP; 1:100 dilution) and neutrophils (Antibodies Direct, Serotec MCA 771G; 1:100 dilution) were used to selectively detect macrophages and neutrophils, and staining levels were quantified using Image-Pro software (Media Cybernetics) to calculate percentage of total area stained positive. Negative controls included no primary and IgG-matched isotype antibodies.
Macrophage isolation.
To isolate macrophages from the 7-day LV infarct, LV tissue from C57BL/6 (3 female, 3 male) and CCR5 null (5 female, 4 male) was minced and dissociated into single-cell suspension using liberase blendzyme (0.25 mg/ml, Roche Applied Science). Cells were washed and resuspended in cold phosphate-buffered saline (PBS) supplemented with 0.5% BSA and 2 mM EDTA and applied over preseparation filters (Miltenyi Biotec 130-041-407). Cell number was determined, and the concentration was adjusted to 1 × 107 cells per 80 μl buffer. The cells were incubated with CD11b microbeads (Miltenyi Biotec 130-049-601) for 15 min, and the positive cells were isolated using magnetic MS columns following the manufacturer's recommendations (Miltenyi Biotec 130–042-201). RNA extraction was immediately performed on the isolated cells using PureLink RNA Mini Kit (Invitrogen 12183–018A), and cDNA was synthesized using High Capacity RNA to cDNA Kit (Applied Biosystems 4387406).
Real-time PCR.
To assess macrophage mRNA expression of proinflammatory M1 and anti-inflammatory M2 macrophage markers, quantitative RT-PCR for the following was performed: arginase-1, CD-163, interleukin (IL)-1β, IL-6, IL-10, mannose receptor 1, MMP-9, TGF-β1, and tumor necrosis factor-α (TNF-α). Quantitative RT-PCR was performed using Power SYBR Green PCR Master Mix (ABI 4367659) and the primer sequences listed in Table 1. Expression levels were normalized for cell number, and GAPDH expression was used as housekeeping control.
Table 1.
RT-PCR primer sequences
| Probe | Forward | Reverse |
|---|---|---|
| Arginase-1 | 5′-ACGGCAGTGGCTTTAACCTT-3′ | 5′-GCGCATTCACAGTCACTTAGG-3′ |
| CD-163 | 5′-CCAAGCTGTGAAGGCACTAAA-3′ | 5′-ACGGTTTGGCAGGACAATC-3′ |
| GAPDH | 5′-CCATGGAGAAGGCTGGGG-3′ | 5′-CAAAGTTGTCATGGATGACC-3′ |
| IL-1β | 5′-ATAACCTGCTGGTGTGTGACG-3′ | 5′-GGTGGAGAGCTTGCAGCTCAT-3′ |
| IL-6 | 5′-TGATGGATGCTACCAAACTGG-3′ | 5′-TCTGAAGGACTCTGGCTTTGTC-3′ |
| IL-10 | 5′-CAGTGGAGCAGGTGAAGAGTGA-3′ | 5′-CCTGGAGTCCAGCAGACTCAAT-3′ |
| Mannose R1 | 5′-ATGAAGATCACAAGCGCTGC-3′ | 5′-TGACACCCAGCGGAATTTC-3′ |
| MMP-9 | 5′-GCATACTTGTACCGCTATGG-3′ | 5′-TAACCGGAGGTGCAAACTGG-3′ |
| TGF-β1 | 5′-ATTCAGCGCTCACTGCTCTT-3′ | 5′-CGGTTCATGTCATGGATGG-3′ |
| TNF-α | 5′-CTTCTCATTCCTGCTTGTGG-3′ | 5′-GGCCATAGAACTGATGAGAGG-3′ |
IL, interleukin; MMP-9, matrix metalloproteinase-9; TGF-β1, transforming growth factor-β1; TNF-α, tumor necrosis factor-α.
Protein extraction.
Soluble proteins were extracted from the infarct region of the LV (LVI) and noninfarct region (LVC) by homogenizing the sample in PBS containing 1× Complete Protease Inhibitor Cocktail (Roche). Insoluble proteins (proteins that were pelleted after centrifugation of the soluble fraction) were extracted using Sigma Reagent 4 (7 M urea, 2 M thiourea, 40 mM Trizma base, and the detergent 1% C7BzO) and 1× protease inhibitor cocktail. Protein concentrations were determined using the Bradford assay. Due to the high urea content in Reagent 4, insoluble protein extracts were diluted 1:40 with water for Bradford assay compatibility. Total protein (10 μg) for each fraction of all samples was run on one-dimensional SDS gels stained with Coomassie blue to verify protein concentration and loading accuracy.
Immunoblotting.
Soluble and insoluble protein levels were quantified by immunoblotting using the following antibodies: bone morphogenetic protein 1 (BMP-1; Abcam ab38953), heat shock protein-47 (HSP-47; Epitomics 3198–1), MMP-2 (a gift from Dr. Bjorn Steffensen), MMP-8 (Calbiochem PC493), MMP-9 (Abcam ab38898), MMP-13 (Abcam ab75606), MMP-14 (Abcam ab53712), rabbit anti-collagen type I (Cedarlane CL50141AP and Cosmo LSL-LB-1102), and rabbit anti-collagen type III (Cosmo LSL-LB-1300). Equal quantities of total protein (10 μg) were loaded on 26-well 4–12% Criterion Bis-Tris gels (Bio-Rad). Equal protein transfer was verified using Ponceau staining of the nitrocellulose membranes. Immunoblotting was performed as previously described (4). Molecular Imaging Software (Kodak) was used to measure densitometry, which was normalized to the 43-kDa Ponceau-stained band.
Statistical analyses.
Data are reported as means ± SE. Two group comparisons were performed using Student's t-test. One-way ANOVA followed by Student Newman-Keuls post hoc test was used for comparisons of more than two groups. A P < 0.05 was considered significant.
RESULTS
7-Day mortality post-MI.
During the 7 days post-MI, WT mice had a mortality rate of 29.5% (13 of 44 mice), and the CCR5 mice had a mortality rate of 30.0% [18 of 60 mice; P = nonsignificant (NS)]. In the WT group, 61.5% of the deaths (8 of 13) were confirmed cardiac ruptures, whereas, in the CCR5 null group, 66.6% of the deaths (12 of 18) were due to rupture (P = NS). Nonrupture-related deaths, most probably the result of heart failure or arrhythmias, accounted for 38.5% of the deaths (5 of 13) in the WT mice and 33.3% of the deaths in the CCR5 null mice (P = NS). In the WT group, the male mortality rate was 47.8%, and the female mortality rate was 9.5%. CCR5 null mice showed similar rates, at 45.2% for the males and 13.8% for the females (P = NS).
Echocardiographic and morphometric analyses.
Echocardiographic, necropsy, and infarct size analyses for WT and CCR5 null mice at 7 days post-MI are shown in Table 2. The echocardiographic and morphometric analyses were analyzed separately by sex, but there were no differences seen for the WT male vs. female or null male vs. female comparisons. Therefore, the sexes were combined for further analyses. The control day 0 groups demonstrated no differences in LV mass, RV mass, and lung mass between WT and null. In response to a similar extent of initial myocardial injury (infarct sizes were 44 ± 2% for the WT and 42 ± 2% for the CCR5 null, P = NS), both groups showed significant increases in LV mass and LV-to-body weight ratio compared with the day 0 controls (P < 0.05).
Table 2.
Echocardiography and necropsy results
| WT Control | CCR5 Null Control | WT 7-day MI | CCR5 Null 7-day MI | |
|---|---|---|---|---|
| n | 13 | 13 | 25 | 33 |
| Body weight, g | 25.1 ± 0.2 | 24.0 ± 1.1 | 23.6 ± 0.7 | 21.6 ± 0.6 |
| Heart rate, beats/min | 460 ± 10 | 482 ± 16 | 492 ± 13 | 490 ± 9 |
| End-diastolic dimension, mm | 3.62 ± 0.09 | 3.81 ± 0.08 | 5.37 ± 0.20* | 5.67 ± 0.18* |
| End-systolic dimension, mm | 2.40 ± 0.08 | 2.60 ± 0.10 | 4.90 ± 0.22* | 5.14 ± 0.21* |
| Posterior wall thickness, mm | 1.08 ± 0.04 | 1.07 ± 0.03 | 0.52 ± 0.04* | 0.55 ± 0.04* |
| Fractional shortening, % | 34 ± 1 | 32 ± 2 | 9 ± 1* | 10 ± 1* |
| End-diastolic volume, μl | 42 ± 3 | 39 ± 2 | 97 ± 9* | 119 ± 10* |
| End-systolic volume, μl | 15 ± 1 | 15 ± 2 | 82 ± 9* | 102 ± 10* |
| Ejection fraction, % | 64 ± 2 | 61 ± 3 | 18 ± 2* | 17 ± 2* |
| LV mass, mg | 74 ± 3 | 75 ± 4 | 93 ± 3* | 92 ± 3* |
| RV mass, mg | 18 ± 1 | 18 ± 1 | 22 ± 1 | 24 ± 1* |
| Lung wet weight, mg | 138 ± 10 | 126 ± 3 | 194 ± 15 | 226 ± 18* |
| LV mass-to-BW ratio, mg/g | 3.0 ± 0.1 | 3.1 ± 0.1 | 4.2 ± 0.1* | 4.3 ± 0.1* |
| LV remodeling index, μl/mg | 1.02 ± 0.06 | 1.25 ± 0.08† | ||
| Infarct size, % | 44 ± 2 | 42 ± 2 |
Values are means ± SE; n, no. of mice. Control is day 0 samples.
WT, wild type; CCR5, CC chemokines receptor 5; MI, myocardial infarction; LV, left ventricle; RV, right ventricle; BW, body weight. LV remodeling index is end-diastolic volume/LV mass.
P < 0.05 vs. control;
P < 0.05 vs. WT MI.
WT and CCR5 null post-MI mice also demonstrated significant increases in end-diastolic dimensions and end-systolic dimensions, and a decrease in posterior wall thickness and percent fractional shortening compared with controls (P < 0.05 for all). Both WT and CCR5 null mice had significant increases in end-diastolic volumes and end-systolic volumes post-MI compared with day 0 controls. Ejection fraction significantly decreased in WT and null mice post-MI compared with day 0 controls (all P < 0.05). The calculated LV remodeling index (end-diastolic volume-to-LV mass ratio) significantly increased in the CCR5 null MI mice compared with WT MI mice (P < 0.05). Consistent with this result, RV mass and lung wet weight increased in the CCR5 null post-MI, but not the WT post-MI group, compared with the day 0 controls (P < 0.05). The increases in RV and lung wet weights indicate pulmonary edema, consistent with more adverse remodeling in the MI mice with targeted deletion of CCR5.
Macrophage infiltration and activation and fibroblast response.
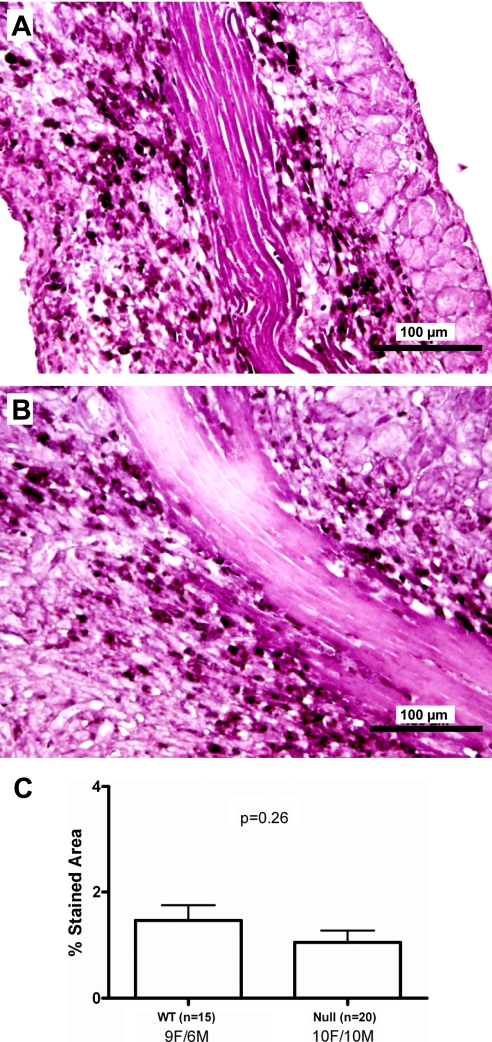
Because CCR5 has been suggested to be a key receptor influencing macrophage migration, midcavity sections of the LV from WT MI (9 female, 6 male) and CCR5 null MI (10 female, 10 male) groups were immunostained for macrophages. No differences were detected in macrophage-stained areas of WT MI and CCR5 null MI LV sections [1.5 ± 0.3% for WT (n = 15) and 1.1 ± 0.2% for CCR5 null (n = 20; P = NS)], indicating similar number of macrophages infiltrated into the infarct region of both groups by day 7 post-MI (Fig. 1).
Fig. 1.
Macrophage infiltration is similar in the CC chemokine receptor 5 (CCR5) wild-type (WT) and CCR5 null mice at day 7 post-myocardial infarction (MI). Immunohistochemical analysis of left ventricular (LV) midcavity sections at 7 days post-MI indicate similar percent stained area (black stain indicated by the arrows) in the WT LV infarct (A) compared with CCR5 null infarct (B). C: percent macrophage stained area was 1.5 ± 0.3% in the WT MI (n = 15) compared with 1.1 ± 0.2% in the CCR5 null MI (n = 20; P = 0.26). Values are means ± SE.
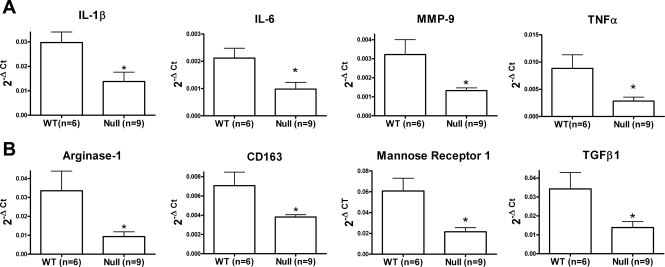
Since macrophage numbers were similar, we examined the activation phenotypes of macrophages isolated from the LV infarct region. We isolated 4.1 ± 2.4 × 105 infarct macrophages from the WT mice (n = 6) and 2.7 ± 1.1 × 105 infarct macrophages from the CCR5 null mice (n = 9; P = NS). The fact that we isolated similar numbers of cells from both groups is consistent with the immunohistochemistry results. CCR5 null macrophages showed decreased gene expression levels of inflammatory markers IL-1β, IL-6, MMP-9, and TNF-α (Fig. 2A), as well as anti-inflammatory markers arginase-1, CD163, mannose receptor-1, and TGF-β1 (Fig. 2B). These results indicate that CCR5 deletion impaired macrophage activation in the ischemic myocardium.
Fig. 2.
Macrophage activation decreases in the CCR5-deficient mice post-MI. Macrophages isolated from the LV infarct of CCR5 null mice at day 7 post-MI show decreased expression of M1 activation markers [interleukin (IL)-1β, IL-6, matrix metalloproteinases (MMP)-9, and tumor necrosis factor (TNF)-α; A] and M2 activation markers [arginase-1, CD163, mannose receptor 1, and transforming growth factor (TGF)-β1; B]. Values are means ± SE. Sample sizes are n = 6 for WT 7 days post-MI mice, and n = 9 for CCR5 null 7 days post-MI mice. Ct, cycle threshold. *P < 0.05 compared with WT.
Because macrophage activation was impaired in the CCR5 null mice, we immunostained the infarct region for neutrophils at day 7 to see if neutrophil removal was reduced. Both WT and CCR5 null sections showed similarly low levels of staining at day 7, indicating that macrophage phagocytic abilities were not altered by CCR5 deletion.
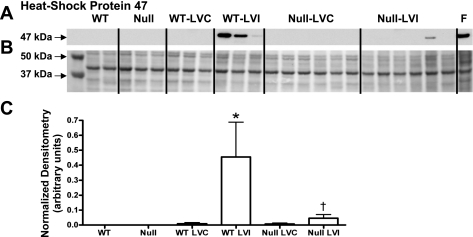
Given that macrophage activation influences fibroblast activation, and attenuated macrophage activation could reduce the fibrotic response during myocardial healing post-MI, we quantified fibroblast activation using the procollagen-specific chaperone, HSP-47. By immunoblot analysis, HSP-47 was found to be significantly decreased in the infarct region of CCR5 null mice compared with the WT infarct region (Fig. 3, A and C). These results indicate that impaired macrophage activation in the absence of CCR5 hinders the downstream fibrotic response post-MI.
Fig. 3.
Heat shock protein (HSP)-47 decreases in the CCR5 null infarcts post-MI. A and C: HSP-47 protein levels increase in the WT infarct region compared with controls. In the absence of CCR5, post-MI HSP-47 protein levels remain unchanged from day 0 controls, and HSP-47 levels were decreased in the null infarct region compared with WT infarct region. B: Ponceau S stained membrane was used as protein loading control. F, mouse myofibroblast cell extract. Values are means ± SE. Sample sizes are n = 10 for WT control; n = 11 for WT LV noninfarct (LVC) remote region; n = 11 for WT LV infarct (LVI) infarct region; n = 11 for null control; n = 23 for null LVC remote region; and n = 23 for null LVI infarct region. *P < 0.05 compared with matching control WT or null; †P < 0.05 compared with WT LVI.
Collagen content.
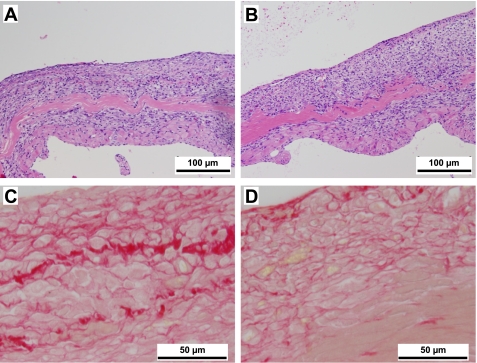
To qualify collagen content, the midcavity sections of the LV from WT MI and CCR5 null MI were stained with picrosirius red. Hematoxylin and eosin staining revealed no obvious differences in the infarct regions of the WT and CCR5 null mice (Fig. 4, A and B) however, picrosirius red staining demonstrated that total collagen content was qualitatively decreased in the CCR5 null infarct (Fig. 4D) compared with the WT infarct (Fig. 4C).
Fig. 4.
CCR5 deletion attenuates collagen deposition at day 7 post-MI. LV midcavity sections of WT (A) and CCR5 null mice (B) post-MI, stained with hematoxylin and eosin, show similar infarct characteristics (×20 magnification). Picrosirius red staining of WT (C) and CCR5 null sections (D) show higher total collagen levels in the WT group compared with CCR5 null infarct (×100 magnification).
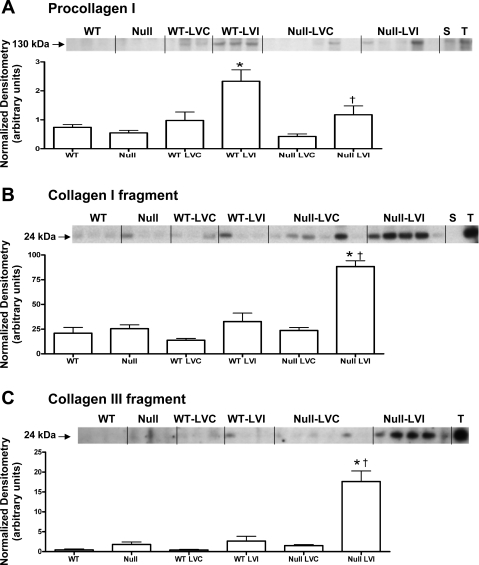
To quantify the collagen content in WT and CCR5 null LV protein extracts, we measured procollagen type I levels by immunoblotting. Procollagen type I was decreased in the infarct region of the CCR5 null mice compared with WT; LVI levels (Fig. 5A). Consistent with this finding, the CCR5 null LVI samples showed increased generation of type I and type III collagen fragments (Fig. 5, B and C, respectively).
Fig. 5.
CCR5 deletion reduces procollagen levels and increases collagen fragments post-MI. A: procollagen I levels increase in the WT infarcts, and this increase is attenuated in the CCR5 null infarcts. Fragment of type I (B) and type III (C) collagens increases in the CCR5 null infarcts, compared with WT infarcts, suggesting increased collagen degradation post-MI in CCR5-deficient mice. LVC, remote region; LVI, infarct region; S, spleen extract positive control; T, mouse liver tumor extract positive control. Values are means ± SE. Sample sizes are n = 10 for WT controls; n = 11 for WT LVC remote region; n = 11 for WT LVI infarct region; n = 11 for null controls; n = 23 for null LVC remote region; and n = 23 for null LVI infarct region. *P < 0.05 compared with matching control WT or null; †P < 0.05 compared with WT LVI.
MMPs.
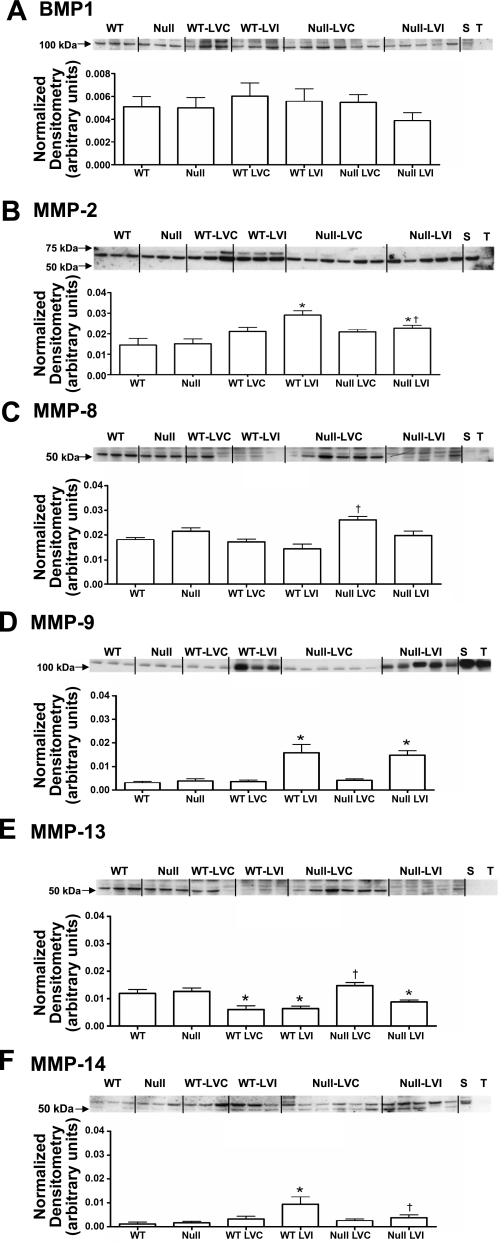
MMPs are critical components of post-MI inflammation, and MMP levels regulate ECM content during scar formation (21, 35). In addition to the ECM degrading MMPs, BMP-1 is a metalloproteinase that stimulates collagen cross-linking by activating lysyl oxidase (22). Therefore, we quantified protein levels of BMP-1 and MMP-2, -8, -9, -13, and -14 in the LV protein extracts of WT and CCR5 null day 0 controls (WT and null, respectively) and remote and infarct regions (LVC and LVI) of day 7 MI samples.
For both groups, BMP-1 levels in LV extracts were unchanged from controls at 7 days post-MI (Fig. 6A). MMP-2 levels increased in the infarct region of WT mice and increased to a lesser extent in the infarct regions of the CCR5 null group (Fig. 6B). MMP-8 levels were higher in the remote region of the CCR5 null compared with the WT remote region (Fig. 6C). MMP-9 levels increased similarly in the infarct regions of both groups compared with the respective day 0 controls (Fig. 6D). MMP-13 levels decreased post-MI in both remote and infarct regions of WT, but decreased only in the infarct region of the CCR5 null (Fig. 6E). MMP-14 increased post-MI in the WT, but not CCR5 null, infarct region (Fig. 6F). To summarize, overall protease levels were reduced in the infarct region of the CCR5 null mice compared with the WT mice.
Fig. 6.
Metalloproteinase levels in WT and CCR5 null mice post-MI. A: bone morphogenetic protein (BMP)-1 levels remained unchanged among all groups. B: MMP-2 increased in both WT and null LVI infarct regions, compared with the respective day 0 controls. However, the increase was attenuated in the null infarct region compared with WT infarct region. C: MMP-8 levels increased in the null LVC remote region compared with the WT LVC remote region. D: MMP-9 protein levels increased similarly in both WT and null LVI infarct regions, compared with the respective day 0 controls. E: MMP-13 levels decreased in WT LVC remote and WT LVI infarct regions, compared with WT day 0 levels. In the null, MMP-13 decreased in the LVI infarct region, compared with the null day 0 levels. Higher MMP-13 levels were found in the null LVC remote region compared with the WT LVC remote region levels. F: MMP-14 protein levels increased in the WT LVI infarct region, and MMP-14 levels were significantly lower in the null LVI infarct region compared with the WT LVI infarct region. Values are means ± SE. Sample sizes are n = 10 for WT controls; n = 11 for WT LVC remote region; n = 11 for WT LVI infarct region; n = 11 for null controls; n = 23 for null LVC remote region; and n = 23 for null LVI infarct region. *P < 0.05 compared with matching control WT or null; †P < 0.05 compared with respective WT LVC or WT LVI.
DISCUSSION
CCR5 is a key receptor expressed on macrophages that regulates inflammatory responses. We examined the functional consequences of CCR5 gene deletion on post-MI remodeling, with respect to echocardiographic parameters, macrophage infiltration and activation, collagen content, and metalloproteinase levels. The significant and unique findings of this study were that permanent MI induction in CCR5 null mice resulted in 1) decreased macrophage activation; 2) decreased total collagen with a concomitant increase in collagen fragments in the infarct; and 3) increased remodeling index at day 7 post-MI.
CCR5 is a proinflammatory receptor and a coreceptor for human immuodeficiency virus-1 (HIV-1) virus infection of macrophages (30). A 32-bp deletion in the CCR5 gene in a subpopulation of humans results in an inactive, truncated receptor. This deletion confers resistance to HIV and suggests a positive role for CCR5 deletion in infectious diseases. In diseases with a chronic inflammatory component (e.g., arthritis, asthma, and atherosclerotic lesions), patients homozygous or heterozygous for the 32-bp mutation are also protected as a result of an attenuated inflammatory response (12). Inflammation, however, is a necessary and essential component of the healing process post-MI, and macrophages are a primary factor (10, 18). Macrophage infiltration into the infarct area regulates phagocytosis of necrotic myocytes and apoptotic neutrophils, collagen metabolism (both degradation and synthesis), and fibroblast activation (17). Whether CCR5 deletion would benefit cardiac wound healing had not previously been evaluated in detail.
In our study, CCR5 null mice showed an increased remodeling index compared with the WT. Consistent with signs of adverse remodeling and an early progression to heart failure, CCR5 null mice had increased RV and lung wet weight. The increases in RV and lung weights indicate pulmonary edema, consistent with more adverse remodeling in the MI mice with targeted deletion of CCR5. In agreement with these findings, Dobaczewski and colleagues (7) demonstrated, in an ischemia-reperfusion model, that CCR5 null mice have increases in LV end-diastolic dimensions, end-diastolic volumes, and decreased fractional shortening at 7 days postreperfusion. The monocyte chemoattractant protein-1 (CCL2) null mice and CCR2 null mice both demonstrate no survival benefit compared with WT at 28 days post-MI but attenuated ventricular dysfunction (9, 14).
In the absence of CCR5, macrophage accumulation and neutrophil egression did not change in our permanent occlusion model, a finding also supported by the ischemia-reperfusion study performed by Dobaczewski and colleagues (7). Combined, these data indicate that CCR5 deletion is not critical for macrophage infiltration into infarcted myocardium, in contrast to CCR2 null macrophages, which show a decreased and delayed accumulation (6).
After 7 days of permanent left anterior descending ligation, CCR5 null macrophages showed blunted activation, as evidenced by lower expression of IL-1β, IL-6, MMP-9, and TNF-α, as well as arginase-1, CD163, mannose receptor 1, and TGF-β1. In agreement with our study, the Frangogiannis laboratory showed in their ischemia-reperfusion model that CCR5 null mononuclear cells had reduced expression of IL-1β and the anti-inflammatory cytokine IL-10 compared with their WT counterparts (7). The decreased expression of proinflammatory and anti-inflammatory markers on macrophages could be the result of CCR5 effects on T-cell function. Previously, Yi and colleagues (33) demonstrated that CD4+ T-cell-activated macrophages recognize and reject xenografts in the absence of other immune effector cells. In a follow-up study to evaluate the signaling pathway, isolated CCR5-deficient macrophages from xenograft recipients showed an impaired activation phenotype, suggesting that CCR5 signaling mediates CD4+ T-cell-macrophage activation (34). Interestingly, in the CCR5 ischemia-reperfusion MI study, CCR5 deficiency was strongly associated with a decrease in regulatory CD4+ T cell recruitment, indicating that CCR5 signaling directs both T-cell recruitment and subsequent T-cell activation of macrophages (7).
After ischemia-reperfusion, global expression of inflammatory and anti-inflammatory markers increased in the infarcts of CCR5 null mice. In addition, the ratio of IL-1β to IL-10 or TGF-β1 also increased in the CCR5 null mice, indicating enhanced inflammation. As a result, MMP-2 and -9 activities were increased in the CCR5 null at 72 h of reperfusion. In contrast, after 7 days of permanent ligation, the CCR5 null infarcts in our study showed significantly decreased MMP-2 protein levels, while MMP-9 levels were increased similarly in both WT and CCR5 infarcts. These differences could be attributed to differences in macrophage responses to the ischemia-reperfusion and permanent ligation stimuli or to the kinetic differences between the 72-h and 7-day time points.
HSP-47 levels were suppressed in the CCR5 null infarct region. HSP-47 is a procollagen-specific chaperone that serves as a pro-fibrotic marker (27). Consequent with the reduced HSP-47 levels, procollagen I protein levels were decreased and type I and type III collagen fragments increased. This suggests that fibroblast activation was impaired in the CCR5 null mice. Cardiac fibroblasts respond to several inflammatory cytokines released by macrophages, including IL-1β, IL-6, TNF-α, and TGF-β1. Therefore, it is possible that infarct healing in the CCR5 null mice is impaired as a result of reduced macrophage activation, leading to decreased fibroblast activation that results in an imbalance between collagen degradation and synthesis (26, 32). Similar results were seen by Van Amerongen and colleagues (31), who used clodronate liposomes to deplete macrophages in a cryoinjury model of LV remodeling. Their results demonstrated that macrophage depletion reduced collagen deposition and impaired myocardial wound healing, consistent with the macrophage response being a mediator of myocardial reparative functions, particularly the fibrotic response (23).
There were several limitations of the study. These include the following: 1) the data presented here for macrophage infiltration were obtained at 7 days post-MI, and no other temporal data were acquired; 2) the MMP levels reported represent total protein, which does not equal activity and may explain why collagen fragment levels increased; and 3) the animals used in this study were 3–6 mo old, and results are likely to be different in older animals.
In conclusion, we demonstrate in this study that CCR5 deletion, while beneficial in infectious diseases and chronic inflammatory processes, such as HIV transmission, arthritis, and atherosclerosis, may actually impart negative consequences in the setting of acute inflammation and post-MI remodeling. CCR5 null mice showed impaired wound healing concomitant with reduced macrophage activation. CCR5 inhibitors are clinically used to treat HIV patients, but the model that emerges from this study suggests that CCR5 inhibition needs to be assessed in the post-MI setting. Future studies to examine the role of CCR5 inhibition or polymorphisms in post-MI responses in larger animal models and patients are also warranted. Our data suggest that CCR5 deletion imparts a net negative consequence in the setting of acute inflammation and post-MI remodeling by attenuating macrophage activation and decreasing the fibrotic response.
GRANTS
We acknowledge funding from the National Institutes of Health (NIH) (R01 HL-75360), American Heart Association (AHA) Grant-in-Aid (0855119F), the Max and Minnie Tomerlin Voelcker Fund, and a Veteran's Administration Merit Award to M. L. Lindsey; T32 (HL07446) and AHA (09POST2150178) to R. Zamilpa; and NIH (AI48644, AR 052755) and a Veterans Affairs Merit Award to S. S. Ahuja.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Bjorn Steffensen at the University of Texas Health Science Center at San Antonio for the MMP-2 antibody.
REFERENCES
- 1. Bevan AK, Hutchinson KR, Foust KD, Braun L, McGovern VL, Schmelzer L, Ward JG, Petruska JC, Lucchesi PA, Burghes AHM, Kaspar BK. Early heart failure in the SMN Delta 7 model of spinal muscular atrophy and correction by postnatal scAAV9-SMN delivery. Hum Mol Genet 19: 3895– 3905, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bless NM, Huber-Lang M, Guo RF, Warner RL, Schmal H, Czermak BJ, Shanley TP, Crouch LD, Lentsch AB, Sarma V, Mulligan MS, Friedl HP, Ward PA. Role of CC chemokines (macrophage inflammatory protein-1 beta, monocyte chemoattractant protein-1, RANTES) in acute lung injury in rats. J Immunol 164: 2650– 2659, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Bursill CA. The role of chemokines in atherosclerosis: recent evidence from experimental models and population genetics. Curr Opin Lipidol 15: 145– 149, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Dai Q, Escobar GP, Hakala KW, Lambert JM, Weintraub ST, Lindsey ML. The left ventricle proteome differentiates middle-aged and old left ventricles in mice. J Proteome Res 7: 756– 765, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol 164: 665– 677, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 96: 881– 889, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol 176: 2177– 2187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dreier R, Grassel S, Fuchs S, Schaumburger J, Bruckner P. Pro-MMP-9 is a specific macrophage product and is activated by osteoarthritic chondrocytes via MMP-3 or a MT1-MMP/MMP-13 cascade. Exp Cell Res 297: 303– 312, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Frangogiannis NG, Dewald O, Xia Y, Ren G, Haudek S, Leucker T, Kraemer D, Taffet G, Rollins BJ, Entman ML. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation 115: 584– 592, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Frantz S, Bauersachs J, Ertl G. Post-infarct remodelling: contribution of wound healing and inflammation. Cardiovasc Res 81: 474– 481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest 114: 623– 633, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gonzalez P, Alvarez R, Batalla A, Reguero JR, Alvarez V, Astudillo A, Cubero GI, Cortina A, Coto E. Genetic variation at the chemokine receptors CCR5/CCR2 in myocardial infarction. Genes Immun 2: 191– 195, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest 116: 59– 69, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaikita K, Hayasaki T, Okuma T, Kuziel WA, Ogawa H, Takeya M. Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am J Pathol 165: 439– 447, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keepers TR, Gross LK, Obrig TG. Monocyte chemoattractant protein 1, macrophage inflammatory protein 1 alpha, and RANTES recruit macrophages to the kidney in a mouse model of hemolytic-uremic syndrome. Infect Immun 75: 1229– 1236, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kobusiak-Prokopowicz M, Orzeszko J, Mazur G, Mysiak A, Orda A, Poreba R, Mazurek W. Chemokines and left ventricular function in patients with acute myocardial infarction. Eur J Intern Med 18: 288– 294, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol 130: 147– 158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leor J, Rozen L, Zuloff-Shani A, Feinberg MS, Amsalem Y, Barbash IM, Kachel E, Holbova R, Mardor Y, Daniels D, Ocherashvilli A, Orenstein A, Danon D. Ex vivo activated human macrophages improve healing, remodeling, and function of the infarcted heart. Circulation 114: I94– I100, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Liehn EA, Merx MW, Postea O, Becher S, Djalali-Talab Y, Shagdarsuren E, Kelm M, Zernecke A, Weber C. Ccr1 deficiency reduces inflammatory remodelling and preserves left ventricular function after myocardial infarction. J Cell Mol Med 12: 496– 506, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, McClister DM, Jr, Su H, Gannon J, MacGillivray C, Lee RT, Sinusas AJ, Spinale FG. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol 290: H232– H239, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Lindsey ML, Zamilpa R. Temporal and spatial expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases following myocardial infarction. Cardiovasc Ther. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lopez L, Cook EF, Horng MS, Hicks LS. Lifestyle modification counseling for hypertensive patients: results from the National Health and Nutrition Examination Survey 1999–2004. Am J Hypertens 22: 325– 331, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 121: 2437– 2445, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parissis JT. Serum profiles of C-C chemokines in acute myocardial infarction: possible implication in postinfarction left ventricular remodeling. J Interferon Cytokine Res 22: 223– 229, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA, Braunwald E. Myocardial infarct size and ventricular function in rats. Circ Res 44: 503– 512, 1979 [DOI] [PubMed] [Google Scholar]
- 26. Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther 123: 255– 278, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Sasaki H, Sato T, Yamauchi N, Okamoto T, Kobayashi D, Iyama S, Kato J, Matsunaga T, Takimoto R, Takayama T, Kogawa K, Watanabe N, Niitsu Y. Induction of heat shock protein 47 synthesis by TGF-beta and IL-1 beta via enhancement of the heat shock element binding activity of heat shock transcription factor 1. J Immunol 168: 5178– 5183, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Tao ZY, Cavasin MA, Yang F, Liu YH, Yang XP. Temporal changes in matrix metalloproteinase expression and inflammatory response associated with cardiac rupture after myocardial infarction in mice. Life Sci 74: 1561– 1572, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Thomas JC. Characterization of the CCR5 chemokine receptor gene. Biochem Mol Biol Educ 32: 191– 195, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Tuttle DL, Harrison JK, Anders C, Sleasman JW, Goodenow MM. Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1. J Virol 72: 4962– 4969, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJA. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol 170: 818– 829, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol 7: 30– 37, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Yi S, Hawthorne WJ, Lehnert AM, Ha H, Wong JK, van Rooijen N, Davey K, Patel AT, Walters SN, Chandra A, O'Connell PJ. T cell-activated macrophages are capable of both recognition and rejection of pancreatic islet xenografts. J Immunol 170: 2750– 2758, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Yi S, Ouyang L, Ha H, O'Hara JM, Chandra AP, Akima S, Hawthorne W, Patel AT, Stokes R, O'Connell PJ. Involvement of CCR5 signaling in macrophage recruitment to porcine islet xenografts. Transplantation 80: 1468– 1475, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Zamilpa R, Lindsey ML. Extracellular matrix turnover and signaling during cardiac remodeling following MI: causes and consequences. J Mol Cell Cardiol 48: 558– 563, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]