Abstract
Cell-to-cell adhesions are crucial in maintaining the structural and functional integrity of cardiac cells. Little is known about the mechanosensitivity and mechanotransduction of cell-to-cell interactions. Most studies of cardiac mechanotransduction and myofibrillogenesis have focused on cell-extracellular matrix (ECM)-specific interactions. This study assesses the direct role of intercellular adhesion, specifically that of N-cadherin-mediated mechanotransduction, on the morphology and internal organization of neonatal ventricular cardiac myocytes. The results show that cadherin-mediated cell attachments are capable of eliciting a cytoskeletal network response similar to that of integrin-mediated force response and transmission, affecting myofibrillar organization, myocyte shape, and cortical stiffness. Traction forces mediated by N-cadherin were shown to be comparable to those sustained by ECM. The directional changes in predicted traction forces as a function of imposed loads (gel stiffness) provide the added evidence that N-cadherin is a mechanoresponsive adhesion receptor. Strikingly, the mechanical sensitivity response (gain) in terms of the measured cell-spread area as a function of imposed load (adhesive substrate rigidity) was consistently higher for N-cadherin-coated surfaces compared with ECM protein-coated surfaces. In addition, the cytoskeletal architecture of myocytes on an N-cadherin adhesive microenvironment was characteristically different from that on an ECM environment, suggesting that the two mechanotransductive cell adhesion systems may play both independent and complementary roles in myocyte cytoskeletal spatial organization. These results indicate that cell-to-cell-mediated force perception and transmission are involved in the organization and development of cardiac structure and function.
Keywords: cell-to-cell interaction, myofibrillogenesis, extracellular matrix, cell biomechanics
numerous studies in cell mechanobiology have shown that the stiffness of the material on which or within which cells grow has sizeable, specific effects on cell morphology, cytoskeletal structure, motility, differentiation, and proliferation (16, 39, 43, 50, 62). Mechanical cues, which can act together with or in opposition to chemical stimuli, are potentially associated with pathological conditions that occur in cancer, fibrotic disease, abnormal wound healing, and cardiac remodeling. The mechanical microenvironment of the cell in vivo is defined by its attachment to the extracellular matrix (ECM) and to other cells via forces generated and transmitted across cell-to-cell junctions constituting a network assembly of cells. Nearly all studies of cell mechanobiology in vitro have focused on the attachment of subconfluent cells to ECM ligands such as fibronectin, collagen, and laminin. These studies have shown that in cardiac cells the response to substrate stiffness is not monotonic and that attributes such as cell shape (aspect ratio, circularity), myofibrillar maturation, or expression of specific transcription factors have optima at a specific substrate stiffness and may depend on the nature of the adhesive ligand (14, 27). Because most of these studies focus on cell-ECM mechanotransduction, little is known about mechanical signaling between cells. Whether the changes that occur subsequent to cell-to-cell contact are chemically or mechanically triggered, i.e., whether they are initiated from signals activated by cadherins that override signals from integrins, or whether they result from the fact that cell-to-cell contacts can sense and transduce mechanical forces, is unknown. This study assesses directly the role of intercellular adhesions, specifically N-cadherin-mediated mechanotransduction, on the morphology and internal organization of neonatal ventricular cardiac myocytes.
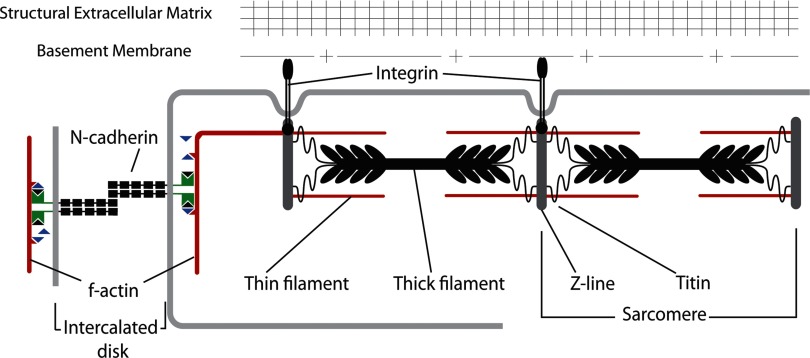
The study looks at two cell-based subadhesion systems, namely cell-to-cell (N-cadherins) and the control, cell-ECM (integrins). Changes or disruptions to these adhesion systems can have adverse effects on myocyte morphology and are often associated with hypertrophic and dilated cardiac myopathy (29, 30, 51). In myocytes, cell-ECM interactions occur mainly via integrins, which are localized at costameres and focal adhesions, whereas N-cadherins are transmembrane adhesion proteins that mediate cell-to-cell adherens junctions (AJ) through their extracellular domains and form part of the intercalated discs that link myocytes together end to end (Fig. 1) (55). N-cadherins are homophilic Ca2+-dependent cell-to-cell adhesion molecules belonging to the classic cadherin subfamily (56). AJ are stabilized by the dynamic binding of the intracellular cadherin domain to the actin cytoskeleton via molecular complexes consisting of α-catenins (1), β-catenins (28), p120, and other molecular partners. The N-cadherin/catenin complex serves as an attachment site for myofibrils spanning adjacent cells (23). The precise composition of proteins mediating the membrane-myofibril/cytoskeleton mechanical connection is unclear (42), but it is distinct from the protein complexes that form cell-ECM junctions. Cadherin-mediated cell-to-cell interactions do not simply glue cells together; they also coordinate complex processes such as cell polarization, migration, and differentiation by modulating the actin cytoskeleton to coordinate these basic cell functions. Perturbing the assembly of actin with cytochalasin D inhibits cadherin-mediated adhesions (34). The formation of cadherin clusters between cells requires myosin II-driven contraction, which results in the formation of a cortical actin network oriented tangentially (Fig. 1) to the plasma membrane (25).
Fig. 1.
Schematic representation of N-cadherin-mediated cell-cell interaction, localized to the intercalated disc of myocytes, forms a mechanical continuum for the transmission of myofibrillar forces from one cell to another.
Cadherin-mediated adhesion is essential for myofibril continuity across the myocyte plasma membrane, but its role in the assembly of the contractile apparatus is still debated (36). In addition, normal propagation of the cardiac electrical impulse relies on specific alignment and low electrical impedance via gap junctions between neighboring myocytes (31). Previous experiments have shown that conditional knockout of the N-cadherin gene in the adult mouse heart or blocking of N-cadherins in myocytes in vitro can lead to disruption of cardiomyocyte-intercalated disc structure and misaligned myofibrils, reduction in the gap-junction protein connexin 43, impaired mechanical function, and arrhythmia-induced sudden death (23, 30).
Cell-to-cell and cell-ECM adhesions are both wired directly to the cytoskeleton, enabling dynamic interplay between them. The cardiac ECM itself is a dynamic entity composed of basement membrane adhesion proteins such as fibronectin, collagen type IV, laminin, and proteoglycans (17), which serve as a crucial interface between myocytes and structural ECM proteins like collagen types I and III (1), all of which continuously remodel during cardiac development. Furthermore, the levels of collagen type I and III rapidly increase in neonatal rat myocardium after birth (10), along with myocyte hypertrophic changes (48), resulting in a dramatic increase in passive elastic modulus (26). These changes in tissue composition and biophysical properties can alter the myofibrillar architecture and myocyte function (48). Changes in cell shape along with matrix biophysical and chemical properties are also known to be associated with heart failure in response to extracellular cues (e.g., pressure-volume overload). However, it is not known how these changes in extracellular biophysical and chemical cues alter the cell-to-cell mechanical perception, which in turn can modulate myocyte structure and function by signaling the addition of sarcomeres and regulating gap-junction communication.
In cardiac disease, especially hypertrophic, dilated cardiac myopathy and at the border zones of an infarct, severe disarray of myocytes in cardiac tissue has been noted (2, 3). The spatial disarray of cardiac myocytes is characterized by increased lateral localization of the N-cadherin and gap junctions, which can serve as a trigger of arrhythmias (6). Moreover, hypertrophy/dilated cardiac myopathy is almost always accompanied by changes in the composition and mechanical properties of the ECM. Deletion of N-cadherin leads to upregulation of β1-integrins in myocytes (9), and, in vascular endothelial cells, E-cadherin modulates focal adhesion size and cell spreading (12). N-cadherin-mutated mouse embryos show severe cardiac adhesion defects as well as malformed heart tubes, suggesting its crucial role in cardiac development (45). These findings provide needed evidence that cross talk between these adhesions is essential to maintain tissue homeostasis and function. Whether these changes in myocyte orientation, shape, myofibrillar alignment, and relocalization of adhesion molecules are brought about by changes in mechanoperception of the cardiac cell, through N-cadherin cell-to-cell adhesion receptors, and/or cross talk with integrins remains to be fully elucidated. The aim of this study was to demonstrate whether myocyte morphology and internal organization are responsive to N-cadherin-mediated stiffness perception and to compare the results to those obtained with ECM (collagen type I and fibronectin). To elucidate the differential effects of imposed mechanical loads on myocyte morphology and cytoskeletal organization mediated by N-cadherin adhesions (Fig. 1), an assay system consisting of N-cadherin-Fc cross linked to polyacrylamide (PA) gels of varying stiffnesses was used. This model system enabled the study of N-cadherin-mediated mechanosensitivity without the active participation of the ECM. Our results show that neonatal ventricular rat myocytes (NVRM) display differential morphological characteristics (spread area, aspect ratio, myofibril assembly/polarization), traction forces, and cortical stiffness in response to N-cadherin-coated adhesive substrates of varying levels of rigidity mimicking weak or strong cell-to-cell interactions. The cytoskeletal response of myocytes on an N-cadherin adhesive microenvironment was characteristically different from that on ECM for the same ranges of substrate rigidity. The implication is that cell-to-cell-mediated contact and force transmission are directly involved in the organization and development of myocyte structure and function. Furthermore, these results suggest that N-cadherin-dependent cell-to-cell-mediated mechanotransduction is involved in cardiac tissue development and remodeling.
MATERIALS AND METHODS
Cell isolation.
NVRM were harvested from the hearts of 1- to 3-day-old euthanized Sprague-Dawley rat pups using a cell isolation kit (Cellutron Life Technology, Baltimore, MD) as described previously (27). Cells were isolated from the muscle tissue of the two ventricles. The cardiac myocytes were preplated for 1 to 2 h to purify the myocyte population. The cells were cultured at a density of 10,000 cells/cm2 in high serum (10% fetal bovine serum) medium (Cellutron) on the various gel substrates for 24 h. The medium was changed to low serum (2% fetal bovine serum) and maintained for another 24 h. This time period was sufficient to allow the cells to spread completely after the isolation procedure. For myosin inhibition assay, cells were cultured with 15 mM 2,3-butanedione 2-monoxime (BDM) (Sigma-Aldrich, St. Louis, MO) for 30 min.
PA gel preparation.
PA gels of desired stiffness were made by varying acrylamide and bisacrylamide stock solution (Bio-Rad Laboratories, Hercules, CA) concentrations as described previously (61). The shear modulus for each gel formulation was determined from rheological measurements conducted on a Bohlin CS-10 rheometer (Malvern Instruments, Westborough, MA) (Supplemental Fig. S1; supplemental material for this article is available online at the American Journal of Physiology Heart and Circulatory Physiology website). Rheological testing of PA gels showed shear modulus values close to those previously reported for similar combinations of acrylamide and bisacrylamide (61). For simplicity, we refer to these gels as 100 Pa, 300 Pa, 5 kPa, 10 kPa, and 30 kPa.
To polymerize the acrylamide solutions, 1.5 μl of tetramethylethylene diamine (Fisher BioReagents, Fairlawn, NJ) and 5 μl of 10% ammonium persulfate (Fisher BioReagents) were added to make up a total volume of 1,000 μl in 1× PBS. A 50-μl droplet was deposited on a 12-mm glass coverslip pretreated with 3-aminopropyltrimethoxysilane (Sigma-Aldrich) and gluteraldehyde (Sigma-Aldrich). A 12-mm top coverslip (Fisher Scientific, Pittsburgh, PA) was placed on the PA solution and removed after polymerization. The gel was then rinsed with 50 mM HEPES, and the cross-linker sulfo-N-sulfosuccinimidyl-6-(4′-azido-2′-nitrophenylamino) hexanoate (0.5 mg/ml in 50 mM HEPES buffer pH 8) (Thermo Fisher Scientific, Waltham, MA) was deposited on top of the gel. The gels were placed on and under ultraviolet light and irradiated for 5 min or until the solution turned dark.
Anti Fc antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) at a concentration of 2.5 μg/cm2 was added and incubated on the gel overnight at 4°C on a shaker. Recombinant human N-cadherin Fc chimera (R&D Systems, Minneapolis, MN), shown previously to closely mimic N-cadherin presentation to that of another adjacent cell in terms of dimerization, glycosylation, and structural properties (34), was used as a ligand. It was added at a saturating concentration of 5 μg/cm2 and incubated for a minimum of 2 h. As a control, the ECM proteins, rat tail collagen type I (BD Biosciences, Bedford, MA), and human fibronectin (Invitrogen, San Diego, CA) were added onto the gel at concentrations of 0.1 mg/ml and 0.05 mg/ml, respectively, and incubated overnight at 4°C or for 4 h at room temperature. All protein-laminated gel substrates were washed with PBS and blocked with 1.5% BSA at room temperature for 30 min. The substrates were allowed to equilibrate at 37°C in PBS for 30 min before cell seeding. To confirm the specificity of myocyte binding, cells were plated onto anti-Fc and rabbit polyclonal N-cadherin antibody (Santa Cruz Biotechnology, Santa Cruz, CA).
For comparison, N-cadherin Fc protein was also coated on silanized 12-mm glass coverslips as described previously (19). Glass coverslips were washed with sulfuric acid and immersed in a mixture of 1:1 acetone and methanol (Fisher Scientific). The coverslips were then coated with Sigmacote (Sigma Aldrich) and air dried. A solution of anti-Fc antibody in 0.1 M borate buffer pH 8 was added at a concentration of 2.5 μg/cm2 and incubated overnight. N-cadherin Fc was then added at a saturating concentration of 5 μg/cm2 and incubated for a minimum of 2 h. Substrates were saturated with 1.5% BSA in 0.1 M borate buffer for 5 min at room temperature and placed in the incubator to equilibrate for 30 min before cell seeding. To relate these studies to conventional ECM-mediated cell adhesion assays, 12-mm glass coverslips were dipped in a mixture of type I collagen and fibronectin as noted above.
Cell fixation and immunostaining.
After 48 h in culture, cells were fixed with 4% paraformaldehyde (Sigma) for 10 min and then washed three times with PBS and incubated for 15 min with 50 mM glycine (Sigma) to reduce background fluorescence. Substrates were permeabilized with 0.1% Triton X-100 in PBS for 10 min and washed three times for 5 min in TBS. Following permeabilization, substrates were blocked with 1% BSA for 30 min and washed once with TBS. Substrates were then incubated for 1 h at room temperature with mouse monoclonal anti-α-actinin to visualize sarcomere assembly and confirm cell phenotype (1:400; Sigma). Rabbit polyclonal antibody against β-catenin was used to visualize its distribution and presence in cardiac myocytes plated on cadherin-coated surfaces (1:400; Abcam, Cambridge, MA). To confirm the presence of N-cadherin in NVRM cell clusters formed on ECM-coated substrates as well as on N-cadherin Fc-coated substrates, both scaffolds were incubated with rabbit polyclonal antibody against N-cadherin (1:100; Santa Cruz Biotechnology). Rabbit polyclonal antibody against vinculin was used to visualize focal complexes in myocytes on ECM and N-cadherin substrates (1:200; Abcam). Rabbit polyclonal antibody against cellular fibronectin (1:100; Santa Cruz Biotechnology) was used to test for the presence of cellular fibronectin on N-cadherin-coated substrates. All primary antibody solutions were prepared in TBS with 1% BSA; the negative controls were made devoid of primary antibodies. Scaffolds were washed three times with TBS before secondary antibody was applied. Secondary antibodies, antimouse Alexa 488 (1:800; Invitrogen), and antirabbit Alexa 647 (1:800, Invitrogen) were prepared in TBS solution containing 1% BSA. Phalloidin-tetramethylrhodamine B isothiocyanate (1 μg/ml; Sigma) was used to visualize F-actin formation and bisbenzimide (1 μg/ml; Sigma) to visualize the cell nucleus. Substrates were incubated in secondary antibody for 1 h and washed three times with TBS containing 0.1% Tween. Scaffolds were mounted with antifade medium (Vectashield; Vector Laboratories, Burlingame, CA) and visualized under a conventional microscope (Carl Zeiss, Thornwood, NY). Images were acquired using proprietary software (AxioVision; Carl Zeiss).
Measurement of myocyte elastic modulus by atomic force microscopy.
Elasticity was measured using an atomic force microscope as described previously (9). The individual myocyte modulus of elasticity was measured with a DAFM-2X bioscope (Veeco, Woodbury, NY) mounted on an Axiovert 100 microscope (Carl Zeiss) using silicon nitride cantilevers (196 μm long, 23 μm wide, 0.6 μm thick) with a pyramid tip for indentation. The spring constant of the cantilever, calibrated by resonance measurements, was typically 0.06 N/m (DNP probe; Veeco). Correlation between cell and gel stiffness was determined by indenting the cells at three distinct points and the gel at three points adjacent to the attached cell. To quantify stiffness (elastic modulus), the first 500–800 nm of tip deflection from the horizontal (Δd) was fit with the Hertz model modified for a cone
| (1) |
where k and Δz are the bending rigidity and the vertical indentation of the cantilever; E is Young's modulus; α is the cone tip angle; and ν is the Poisson ratio. Young's modulus is the ratio between the stress applied to the material and the resulting strain (δz/z). The Poisson ratio is defined as the ratio of compression strain in the direction normal to the applied stress and the extensional strain in the direction of the applied stress and is taken to be 0.5 for all samples.
Measurement of myocyte-exerted traction stresses using traction-force microscopy.
The traction forces exerted by myocytes on N-cadherin-/ECM-coated substrates of various levels of rigidity were computed by measuring the displacement of 0.2 μm fluorescent beads (Molecular Probes, Invitrogen) embedded within the gels as described previously (35, 54). Briefly, images of bead motion near the substrate surface, distributed in and around the contact region of a single cell (before and after cell detachment with 0.5% trypsin EDTA), were acquired (Zeiss Observer Z1 Microscope) and converted into displacement vectors using the LIBTRC 2.4 software (developed by Prof. Micah Dembo, Boston University, Boston, MA). To compute the contractile traction force of the myocytes, time-lapse (20 frames/s) images of the bead displacements (resting and contracted cell state) were acquired and processed. An estimate of cell traction stresses was computed from the substrate displacement fields using the LIBTRC 2.4 software, as described previously (11). The average traction force (F) exerted by the cell on the substrate is the integral of the traction stress magnitude over the cell contact region.
Quantitative image and statistical analysis.
All samples were imaged using a Zeiss Observer Z1 microscope. For each scaffold, a minimum of 10 images were acquired at ×20 from different regions. Approximately 50 cells per scaffold were analyzed. For detailed morphological analysis, high-resolution images were acquired at ×63. The percentages of striated fractions and myofibril alignment of cardiac myocyte population on N-cadherin- and ECM-coated substrates were evaluated from these high-resolution images. A two-sample t-test assuming unequal variances (α = 0.05) was performed to demonstrate significance between the striated fraction and myofibril alignment on N-cadherin- and ECM-coated substrates of equal stiffness. To calculate cell-spread area and aspect ratio, images were analyzed using a dedicated MATLAB program (The MathWorks, Natick, MA) that quantifies (in pixel intensity units) the contrast between the fluorescent cells and the dark background. For each image, the degree of contrast at the cell border was defined and outlined by an unbiased observer. The aspect ratio of the cell was calculated using measurements of the longest and shortest chains of pixels that crossed the center of each cell. A histogram was produced from data for each substrate to determine the distribution of cell areas and aspect ratios. ANOVA was performed (α of 0.05) to show a significant statistical variation in cell areas and aspect ratios between the entire population of cells in each matrix category. A two-sample t-test assuming unequal variances (α = 0.05) was performed to test significance between the cell areas and aspect ratios between two substrates of different stiffness (where P < 0.05 was considered significant). The same test was used to compare cell areas and aspect ratios of cells on ECM scaffolds to those on N-cadherin substrates of equal stiffness.
To extract mechanosensitivity of the spread area (A) compared with substrate stiffness (S), the spread area data were curve fitted (nonlinear) to a Boltzmann sigmoidal function
| (2) |
where Y is the spread area; x is substrate stiffness; Ab is the baseline/lower asymptote; At is the saturation/upper asymptote; x0 is the midrange value/pivot point; and w is the dynamic x-data range. The gain sensitivity is defined as the slope (dA/dS) of the fitted sigmoidal function.
The surface protein density of N-cadherin- and ECM-coated substrates was evaluated by immunostaining the substrates with antibodies against N-cadherin, fibronectin, and collagen, respectively. The densitometric mean for each image was quantified using proprietary AxioVision software (Carl Zeiss). Five random images of each gel surface were taken, analyzed, and averaged. The intensity of the unlabeled gels, which represents autofluorescence, was subtracted from the average intensity of the labeled gels.
RESULTS
N-cadherin mediates tractional forces in myogenic cells plated on N-cadherin-Fc-coated microposts, and these forces are similar in magnitude to those generated at integrin-mediated adhesions (18); therefore, this study compared and evaluated the response of myocytes to N-cadherin- and ECM-based adhesion systems. With this in mind, NVRM cells were plated on PA gels of varying stiffnesses coated with N-cadherin to determine whether N-cadherin is involved in mediating cell mechanosensitivity. The results were compared with those from myocytes cultured on ECM under the same conditions.
Myocyte structure and morphology on collagen type I- and fibronectin-coated substrates are stiffness dependent.
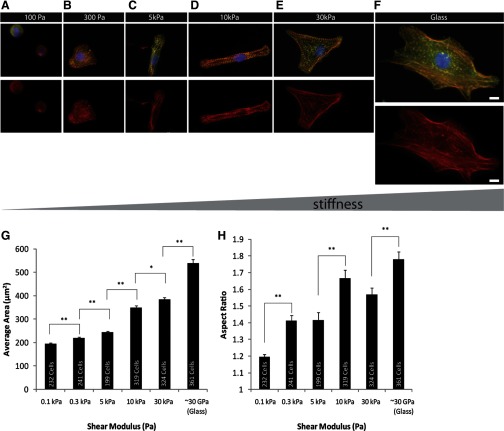
Previous studies have shown that varying substrate rigidity from tissue-like stiffness (5–10 kPa) to that of a scarred infarct (>50 kPa) can alter the maturation of myofibril assembly and contractile function in neonatal ventricular or embryonic cardiomyocytes (14, 27). The results of NVRM plated on ECM show (Fig. 2, A–H) similar behavior and response to changes in the stiffness of the substratum. To show the progression of myofibril assembly as a function of substrate stiffness, cells were immunostained for muscle-specific α-actinin. At high substrate stiffness (30 kPa, glass), myocytes showed an increase in spread area accompanied by reduced sarcomere assembly (Fig. 2, E and F).
Fig. 2.
Neonatal ventricular rat myocytes (NVRM) were plated (48 h) on collagen type I-coated (0.1 mg/ml) and fibronectin-coated (0.05 mg/ml) polyacrylamide (PA) gels of varying stiffnesses. A and B: on soft gels (shear modulus G ∼100 Pa, 300 Pa), cells appeared rounded, with poor sarcomere organization. C and D: at physiological stiffness (G ∼5 kPa, 10 kPa), cells exhibited striated F-actin-stained (red) and α-actinin-stained (green) fibers and increased spread area while maintaining a high aspect ratio. E and F: on a stiff surface (G ∼30 kPa, glass), cells exhibited prominent F-actin filaments devoid of striations, consistent with a stress fiber-like appearance. Scale bars = 10 μm for all images. G: comparative bar graph of cell-spreading area on extracellular matrices (ECM) of varying stiffnesses reveals an increase in spread area as a function of increasing stiffness. The spread areas of myocytes showed a statistically significant increase with increasing substrate stiffness from 0.1 kPa to glass. H: aspect ratio of cardiac myocytes responsive to varying stiffnesses. Significant differences in aspect ratio were observed between 0.1 kPa and 0.3 kPa. The aspect ratio was significantly higher for 10 kPa (physiological stiffness) compared with 0.3 kPa, 5 kPa, and 30 kPa. Group comparisons were made using ANOVA, and significance was determined using the t-test between two substrates of different stiffness (e.g., 0.3 kPa vs. 5 kPa, pairs shown in brackets). *P < 0.05; **P < 0.01. Number of samples is shown inside each vertical column (n = 199–361). Error bars indicate standard error.
Myocytes grown on substrates having material properties mimicking physiological stiffness (∼5–10 kPa) exhibited significantly lower spread area than those grown on stiffer PA gels (Fig. 2, C and D) as determined by the t-test (P < 0.05). At these intermediate levels of stiffness, numerous cells, such as those seen in Fig. 2D, had much higher aspect ratios, a morphology not commonly observed on either soft or stiff substrates. The average aspect ratio was considerably higher for cells exposed to a myocardial environment of normal stiffness than for cells grown on much softer and much stiffer substrates within PA gels (Fig. 2H).
On considerably softer but equally adhesive substrates (100 Pa, 300 Pa), cells exhibited a much more rounded geometry and a highly disorganized myofibrillar architecture (Fig. 2, A, B, and H). When viewed with a confocal microscope, these cells displayed striated actin filaments that were suggestive of partially formed sarcomeres, but they were not uniformly assembled or polarized.
Myocytes plated on N-cadherin-coated substrate demonstrate morphological features similar to those observed on ECM-coated substrates.
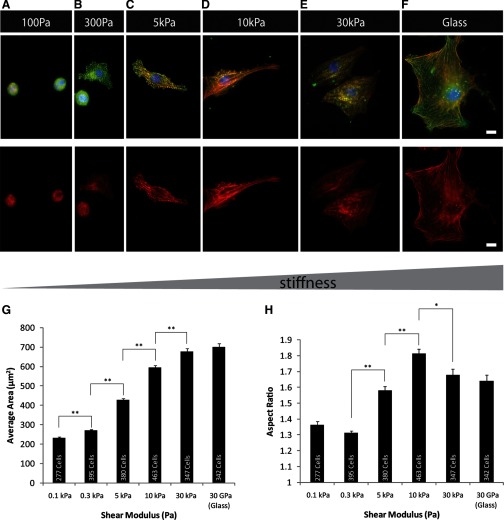
The cell-spreading area as well as the aspect ratio for myocytes cultured on N-cadherin-coated substrates showed a dependence on substrate stiffness similar to that seen on ECM (Fig. 3, A–H). Cells on stiff surfaces (30 kPa, glass) displayed a characteristic radial shape (Fig. 3, E and F) as previously described for myogenic cells (C2C12 myoblast) (19) and disarrayed myofibril structures. F-actin staining revealed stress fiber-like structures. The cell-spread area was characteristically greater on stiff surfaces (30 kPa, glass) than on soft materials (Fig. 3G). Myocytes grown on surfaces of intermediate stiffness coated with N-cadherin (5 kPa, 10 kPa) displayed well-formed sarcomeres that were less aligned than those on gels with similar stiffness coated with ECM (Fig. 3, C and D), and beating behavior was observed on surfaces of soft and intermediate stiffnesses (Supplemental Fig. S2). In comparison, myocytes grown on N-cadherin-coated soft substrates (100 Pa) displayed rounded shapes and a significantly (P < 0.01) reduced spread area.
Fig. 3.
NVRM were plated (48 h) on N-cadherin-Fc-coated PA gels of varying stiffnesses. A and B: on soft gels (shear modulus G ∼100 Pa), cells appeared rounded, with poor sarcomere organization. C and D: at physiological stiffness (G ∼5 kPa, 10 kPa), cells exhibited striated F-actin and α-actinin fibers and increased spread area while maintaining a high aspect ratio. E and F: on stiff surfaces (G ∼30 kPa, glass), cells exhibited prominent F-actin filaments devoid of striations, consistent with stress fiber-like appearance. F-actin (red); α-actinin (green). Scale bars = 10 μm for all images. G: comparative bar graph of cell spreading area on gels of differing stiffness reveals an increase in spread area with increase in gel stiffness. The significance in difference was measured for each individual data point using the t-test. No significance in difference in spread area of myocytes was observed between 30 kPa PA gel and glass. H: average aspect ratio of cardiac myocytes on gels of varying stiffness has relative maximum at 10 kPa; the significance in difference was measured using the t-test. Group comparisons were made using ANOVA, and significance was determined using the t-test between two substrates of different stiffness (e.g., 0.3 kPa vs. 5.0 kPa, pairs shown in brackets). *P < 0.05; **P < 0.01. Number of samples is shown inside each vertical column; (n = 277–463 cells). Error bars indicate standard error.
β-Catenin staining was performed to establish its colocalization in cadherin-mediated adhesion complexes on cadherin-coated substrates (Supplemental Fig. S3). No discernable differences in distribution of β-catenin were observed as a function of substrate stiffness.
A remarkably similar trend in cell-spread area and aspect ratio was seen for myocytes plated on varying substrates on ECM and N-cadherin (Fig. 2, G and H, and Fig. 3, G and H, respectively). This trend suggests that N-cadherin and integrins behave similarly in terms of their ability to remodel the global cell architecture via forces transduced through them, even though their connections to the cytoskeleton and their mechanisms of force transduction are likely to be different.
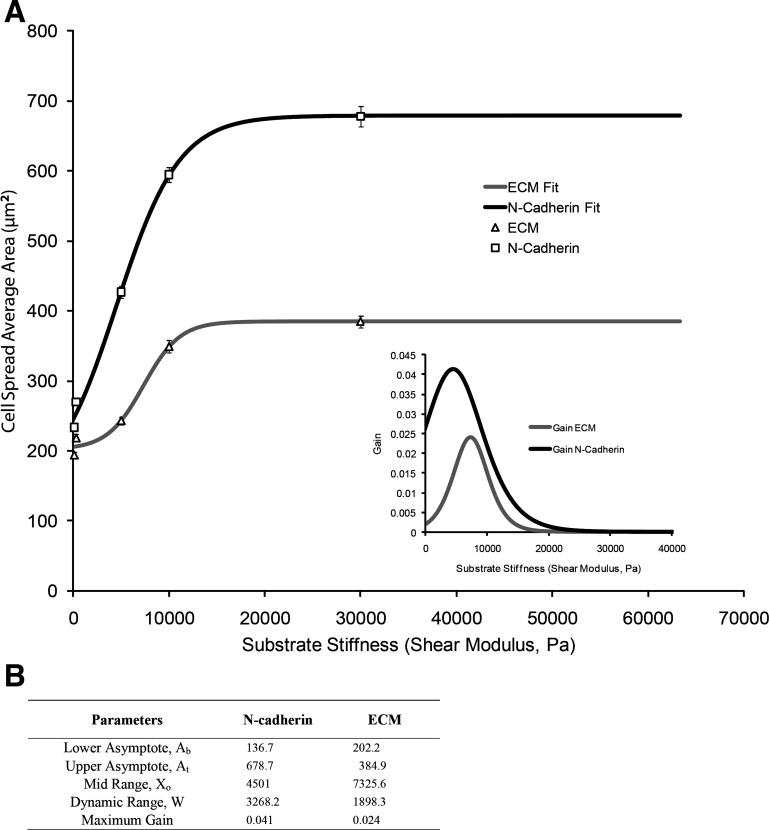
To further elaborate on and quantify the differential sensitivities of the respective mechanical sensors, substrate stiffness and spread area were analyzed within the framework of a classic sigmoidal load-response curve (Fig. 4). The slope of the curve represents the effective system gain of the mechanotransduction process for N-cadherin-mediated spread-area change. The sensitivity response was consistently higher for N-cadherin than for integrins at all ranges of the imposed mechanical load (substrate stiffness). In particular, the peak sensitivity gain for N-cadherin was approximately twofold higher than that for integrin, corresponding to a substrate stiffness of 4.5 kPa and 7.3 kPa, respectively.
Fig. 4.
A: Boltzmann curve is fitted for spread area of N-cadherin and ECM compared with substrate stiffness. Inset: gain (dA/dS) vs. substrate stiffness. B: Boltzmann curve parameters and their corresponding fit values.
Immunofluorescence staining of N-cadherin substrates showed uniform protein coating on polyacrylamide substrates (Supplemental Fig. S1B). Because PA gels were saturated with the protein coating, no discernable differences in ligand density (Supplemental Fig. S1B) were observed between N-cadherin- and ECM-coated substrates. Furthermore, to rule out the possibility that myocytes released their own matrix and engaged in integrin-mediated adhesions on N-cadherin-coated PA gel substrates, they were stained for cellular and plasma fibronectin. Image analysis confirmed that no fibronectin was bound to N-cadherin-coated substrates (Supplemental Fig. S4). These results are in agreement with those from previous studies that used a similar model system (33).
Cardiac myocyte striation and myofibrillar alignment are a function of substrate stiffness and adhesion type.
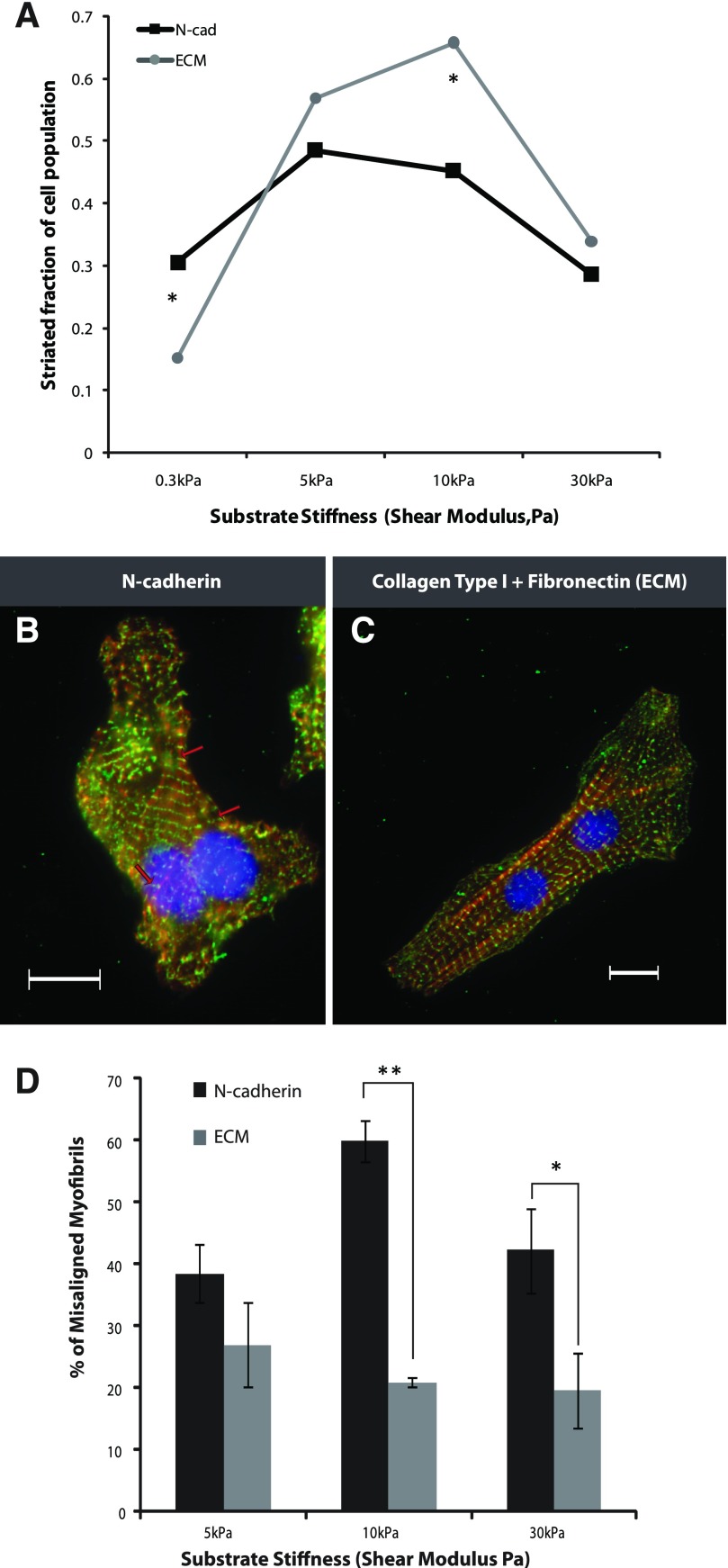
Myocyte striation and myofibrillar alignment were quantified on N-cadherin- and ECM-coated substrates. To assess the assembly of myofibrils as a function of matrix elasticity and adhesion type, NVRM were plated on soft (300 Pa), intermediately stiff (5 kPa, 10 kPa), and hard (30 kPa) N-cadherin- or ECM-coated substrates for 48 h and stained for sarcomeric α-actinin (Fig. 5). For each substrate, high magnification images (×63) of cells were taken to quantify the fraction of cells on each gel that exhibited the typical striated myofibrils throughout the cytoplasm. Myocytes displayed optimal striations at intermediate stiffness for both adhesion systems (Fig. 5A). With softer substrates, the fraction of striated myocytes was significantly (P < 0.05) higher for N-cadherin, indicating that the myocyte cytoskeletal response to N-cadherin-mediated adhesions is sensitive to lower forces, thereby allowing greater spreading and myofibrillar assembly. On stiffer substrates, increased spreading of the myocyte cytoskeleton on cadherin leads to a smaller population of striated cells compared with cells bound to ECM (Fig. 5A).
Fig. 5.
A: striated population of cardiac myocytes cultured on N-cadherin- and ECM-coated substrates was calculated using high magnification images (×63). Myocytes displaying 40% striation across their cytoplasm and sarcomere length ∼1.7 μm were considered striated. The striated cell population was significantly higher for myocytes on 0.3-kPa N-cadherin-coated gels than on ECM-coated gels. On 10-kPa substrates, the striated population for ECM was significantly higher than that for N-cadherin. Each data set point represents an average of 30 cells. Myocytes cultured on substrates of intermediate stiffness (10 kPa) coated with N-cadherin (B) displayed fragmented domains of myofibril alignment (red arrows), whereas those cultured on ECM (C) cells established a fully aligned and polarized myofibril continuum. D: percentage of misaligned myofibrils was calculated for myocytes on N-cadherin- and ECM-coated substrates of varying stiffness; myofibrils deviating more than 20° from the longest axis of the cell were considered misaligned. The percentage of misaligned myofibrils was significantly higher for N-cadherin than ECM for 10-kPa and 30-kPa gels. Each column represents an average of 15 cells. *P < 0.05, **P < 0.01; the difference between data sets was determined by the t-test. Error bars indicate standard error.
Myofibrillar alignment on cadherin-coated substrates was fragmented into distinct domains (Fig. 5B, red arrows); for ECM substrates, cells successfully established an aligned and polarized myofibril cytoskeletal continuum (Fig. 5C). Further analysis of myofibrillar alignment to the long axis of the myocyte showed a significant difference (determined using the t-test, P < 0.05) between myofibrillar misalignment on stiffer N-cadherin substrates (10 kPa, 30 kPa) compared with ECM-coated substrates even though the aspect ratio of myocytes on the two adhesion systems was the same (Fig. 5D). These results indicate that, regardless of the global anisotropy of cell shape, myofibrils on N-cadherin show fragmented polarization indicative of the myocyte cytoskeletal response to local anisotropy.
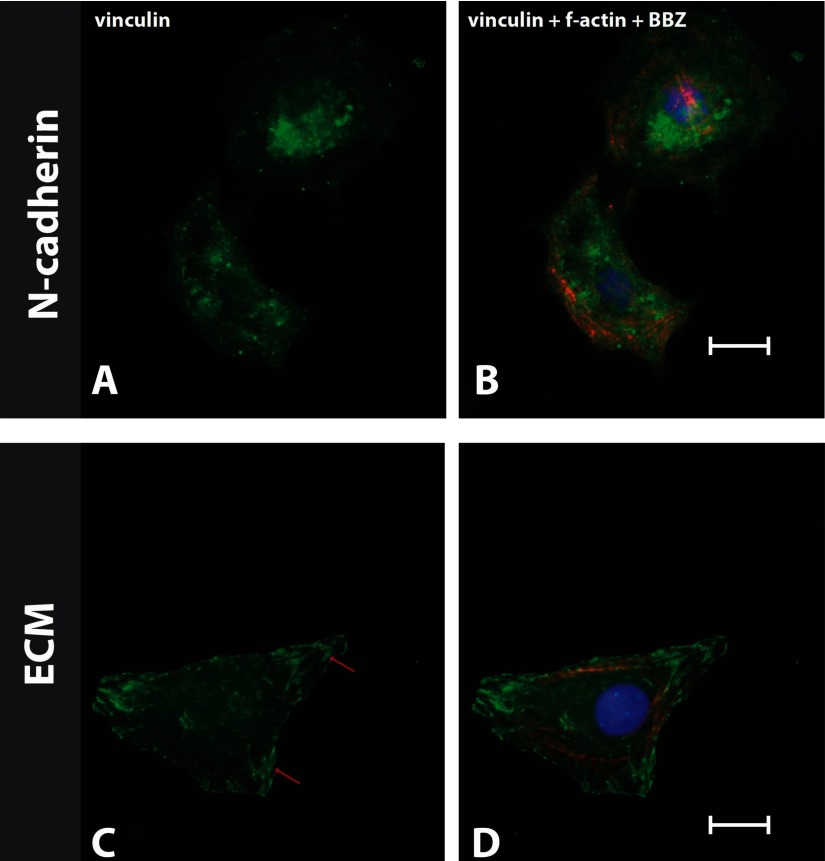
To verify that the adhesion complexes formed on N-cadherin-coated substrates were not formed through focal adhesion complexes (characteristic of ECM-mediated adhesions), myocytes were stained for vinculin on stiff (30 kPa) substrates (Fig. 6, A–D). The staining revealed that vinculin localizes in focal adhesions on ECM-coated stiff substrates (30 kPa) (Fig. 6, C and D) but that no focal adhesion complexes formed on N-cadherin-coated stiff substrates (Fig. 6, A and B). Taken together, these results indicate that myocyte sarcomere formation is partly independent of the type of adhesion. However, the structural integrity in terms of myofibril polarization and two-dimensional (2D) distribution was characteristically different for the two adhesion subsystems.
Fig. 6.
Myocytes were plated on N-cadherin- and ECM-coated stiff substrates (30 kPa) for 48 h. The cells on N-cadherin (A and B) and ECM (C and D) were stained for vinculin (left), F-actin, and nucleus [bisbenzimide (BBZ); right]. Red arrows (C) indicate the localization of vinculin on focal adhesions, which were not seen on N-cadherin-coated substrates (A).
Spontaneously beating single cells were observed on both N-cadherin- and ECM-coated gels of varying stiffnesses. The observed percentage of beating cells increased with decreasing stiffness [40% (0.3 kPa), 30% (10 kPa), and 20% (30 kPa) of cells (n = 60)]. No significant differences in percent of cells beating were seen between N-cadherin- and ECM-coated gels as determined using the t-test (comparisons were made between N-cadherin and ECM groups for the same stiffness).
N-cadherin-mediated traction forces are responsive to substrate stiffness.
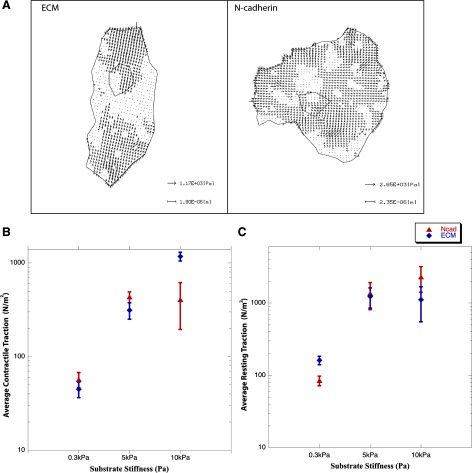
The amount of force exerted through N-cadherin- and ECM-coated substrates was estimated using traction force microscopy analysis of the displacement of beads embedded in gels of varying degrees of stiffness. Mapping of the distribution of the estimated traction stresses on 5-kPa stiff substrates showed a greater polarization of forces exerted on ECM-coated substrates compared with N-cadherin-coated substrates (Fig. 7A). These results correlate well with our morphological data, which show that myofibrillar alignment is lower for myocytes on N-cadherin-coated substrates (Fig. 5D). The average contractile forces of myocytes cultured on ECM-coated substrates increased with increasing stiffness (Fig. 7B). However, the contractile traction forces on N-cadherin substrates plateaued at 5 kPa, correlating with the observed changes in myocyte striation (Fig. 5A). On physiologically meaningful substrate stiffness (10 kPa), the contractile traction forces exerted on ECM- (611 ± 100 nN) were significantly (P < 0.05) higher than those exerted on N-cadherin- (295.5 ± 88.5 nN) coated PA gels (Fig. 7B), which was consistent with the relatively greater sarcomeric assembly and myofibrillar organization (Fig. 5). The resting traction forces exerted by myocytes on N-cadherin- and ECM-coated substrates increased with substrate stiffness. No significant differences were observed between the resting traction forces on N-cadherin- and ECM-coated substrates of the same stiffness (Fig. 7C) (determined by the t-test). At high PA gel stiffness (30 kPa, shear modulus), bead displacement was not optically detectable, which precluded our ability to correlate structural adaptation to the resultant traction forces. The directional changes in traction forces as a function of imposed loads (gel stiffness) provide the critical evidence that N-cadherin is a mechanoresponsive adhesion receptor and is also capable of mediating traction forces similar to those mediated by ECM.
Fig. 7.
Traction-force microscopy measurements. A: distribution of estimated traction stresses (Pa) in myocytes cultured for 48 h on 5-kPa shear modulus PA gels showing greater polarization of generated traction stresses on ECM- compared with N-cadherin-coated substrates. B: estimated contractile traction measured as a function of substrate stiffness changes (0.3 kPa, 5.0 kPa, and 10.0 kPa) shows a corresponding increase in force generation for N-cadherin and ECM; significant changes were observed (P < 0.05) for all ranges of stiffness on ECM and only between 0.3 kPa and 5.0 kPa for N-cadherin. C: resting traction computed as a function of substrate stiffness shows a similar trend; significant changes were observed (P < 0.05) only between 0.3 kPa and 5.0 kPa for both ECM and N-cadherin. Group comparisons were made using ANOVA, and significance was determined using the t-test. Data set points represent 5 cells/point; error bars indicate standard error.
Myocyte cortical stiffness increases with increasing substrate stiffness on N-cadherin.
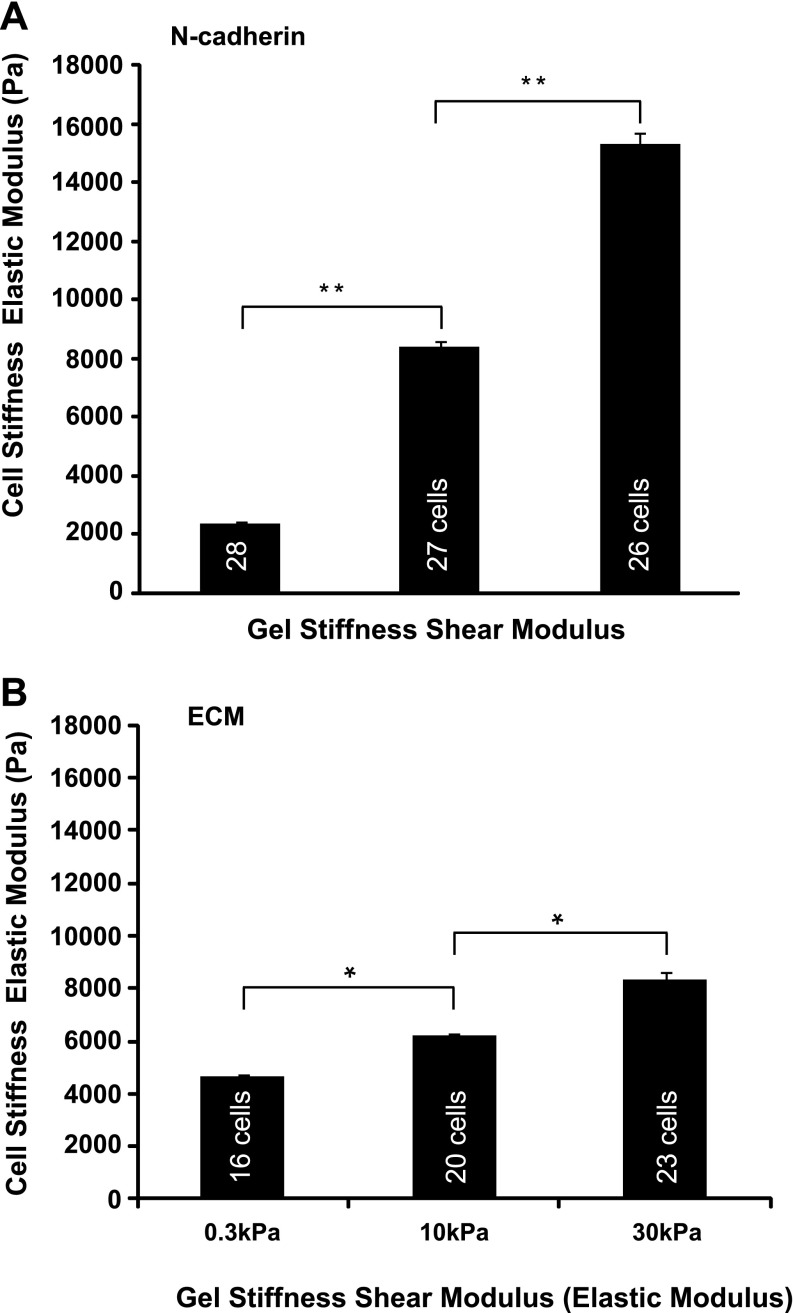
Atomic force microscopy measurements of cell stiffness of myocytes on N-cadherin-coated substratum revealed an apparent mechanical adaptation to the change in substratum stiffness. Myocytes displayed an increase in both area (Fig. 3G) and cortical stiffness (Fig. 8A) when cultured on gels of different stiffness coated with N-cadherin, much like fibroblasts on ECM-coated gels that have modulated their own stiffness in accordance with that of the substratum (54). This striking mechanoadaptation demonstrates that the cells increase their effective elastic modulus when presented with a stiff cadherin-coated substratum, behavior similar to that seen on ECM. Myosin inhibition with BDM after cell spreading showed a lowering of the cell elastic modulus from 15 kPa to 2.7 kPa ± 0.5 kPa on stiffer gels (30 kPa). No changes in overall spread area (average area, 650 ± 30 μm2, n = 50) and in striated fraction (striated fraction, 0.21 ± 0.02, n = 50) were seen after the addition of BDM, indicating that, during the time the experiments were performed, myosin inhibition did not significantly alter myocyte structure. These combined results suggest that the relative stiffening (resting tension) seen in cells may be attributed to myosin-dependent contractility. The overt mechanical response of myocytes to cadherin substratum uncovers a link between the mechanical resistance/load of the substratum and the global mechanical load-bearing properties of the cytoskeleton.
Fig. 8.
Cell stiffness measured by atomic force microscopy of cardiac myocytes cultured for 48 h on N-cadherin-laminated (A) and ECM (B) substrates of varying stiffnesses. The substrate was saturated with N-cadherin or ECM. Group comparisons were made using ANOVA, and significance was determined using the t-test (e.g., 0.3 kPa vs. 10.0 kPa, pairs shown in brackets). *P < 0.05, **P < 0.01. Number of samples is shown inside each vertical column (n = 16–28 cells). Error bars indicate standard error.
Neonatal myocyte cortical stiffness also increased with increasing stiffness of ECM-coated substrates; however, the change in cortical stiffness values relative to substrate stiffness was lower than that observed for N-cadherin (Fig. 8B). This result is in agreement with the change in spread area relative to substrate stiffness seen for N-cadherin compared with ECM.
DISCUSSION
Cell-to-cell interactions regulate a diverse range of cellular phenomena including lineage specifications, cell sorting, compaction, wound healing, and tumor formation (2, 32, 47, 58). Merely changing the plating densities of mesenchymal stem cells, fibroblasts, or endothelial cells can elicit differences in cell shape and cytoskeleton tension regardless of the stiffness of the substrate on which they were grown (39, 61). Additionally, cell-to-cell interactions play a major role in the remodeling of gap junctions of myocytes at the border zone in myocardial infarct tissue (38). One of the earliest adherens couplers involved in the formation of cell-to-cell junctions between myocytes is N-cadherin and its associated proteins (β-catenin, α-catenin) (21). Deletion of N-cadherin leads to the dissolution of the intercalated disc and is rescued partly by introducing other types of cadherins (30, 36). Mouse embryos lacking N-cadherin are unable to form a cohesive cardiac tissue (45). In addition to cadherins, other components of the intercalated disc, such as desmosomes and zonula occludens, play an integral role in maintaining the structural and mechanical organization of cardiac tissue during and after development. In contrast, the deletion of desmoplakin, one of the various proteins that bind desmosomal cadherins to intermediates filaments, also shows structural defects in embryonic myocytes but does not lead to the complete dissolution of the intercalated disc (7). However, relatively little is known about the direct role that cadherins play in mediating myocyte spatial and internal organization.
This study assesses the direct role of mechanotransduction by N-cadherin-mediated intercellular adhesions on the morphology and internal organization of neonatal ventricular cardiac myocytes. A well-established 2D engineered substrate model system with controlled elasticity was used to probe the mechanotransductive attributes of myocyte response to N-cadherin-functionalized surfaces. The results show that cadherin-mediated cell attachments are capable of eliciting a cytoskeletal mechanical remodeling response similar to that of integrin, affecting myocyte shape, myofibrillar organization, traction forces, and cortical stiffness.
Present concepts used to explain cardiac muscle myofibrillogenesis invoke a three-step process that begins with the formation of premyofibrils, followed by nascent myofibrils leading to mature myofibrils (46), and the regulated control of cell spreading appears to promote myofibrillar formation and maturation. Cell spreading is responsive to substrate stiffness as well as to the type of ECM adhesion (35, 41, 43). Consistent with the results presented in this study, the process of spreading is considered a readout of the mechanosensitive features of adhesion. Neonatal ventricular myocytes, when cultured on relatively stiff (30 kPa, glass) ECM or N-cadherin-coated substrates, display an increase in spread area, a low aspect ratio, and stress fiber-like F-actin assemblies; myofibril maturation and sarcomere formation are inhibited. These results are in agreement with previous reports that sarcomere assembly as well as myofibril maturation is inhibited in neonatal ventricular myocytes on stiff (> 30 kPa) substrates (15, 27). Studies have also shown that myocytes grown on ECM mimicking stiff substrates have a higher resting tension and a lower sarcomeric contractile force. Moreover, an increase in contractility on stiff surfaces causes a greater strain-induced change within the cell (intracellular strain is greater than extracellular strain on stiff surfaces), resulting in unfavorable protein conformation, inhibiting myofibril assembly (14). In contrast, inhibition of contractility via RhoA/Rock inhibitors such as C3 toxin/Fasudil and blebbistatin (myosin II inhibitor) enable a lower tension on these stiff substrates, thereby promoting myofibrillar assembly (27, 53).
On soft N-cadherin or ECM substrates designed to mimic weak cell-to-cell or cell-ECM mechanical interactions, myofibril maturation/formation fails to occur, and cells appear rounded. Consistent with these observations, other studies have shown a decrease in muscle cell-spread area, aspect ratio, resting tension, a lower adhesive force, and reduced striation on soft ECM-mimicking substrates (14, 15, 24, 27).
On the other hand, myocytes cultured on substrates of tissue-like intermediate (∼5–10 kPa) stiffness show a progressively elongated shape, increased striation, and a mature myofibrillar assembly. This result strongly suggests that an adhesion-contractile balance is a prerequisite for favorable myofibrillogenesis. Clearly, these data show that neonatal myocytes are highly adaptive and can remodel their shape, spread area, and striation on N-cadherin-coated substratum.
In these experiments, the spread areas on cadherin-coated substrates were significantly higher than those seen on ECM-coated substrates, which suggests that the molecular signaling mechanisms involved in the N-cadherin- and integrin-mediated mechanoreceptor function are different in terms of their cytoskeletal end result. The differential sensitivities of the respective adhesion systems, substrate stiffness, and spread area, were analyzed using a sigmoidal load-response curve. The sensitivity response was consistently higher for N-cadherin than for integrins at all ranges of the imposed mechanical load (substrate elasticity). In particular, the peak sensitivity gain for N-cadherin was approximately twofold higher than that for integrin, corresponding to a substrate elasticity of 4.5 kPa and 7.3 kPa, respectively. Strikingly, these values fall within the range of physiological cardiac tissue stiffness. The observations are particularly noteworthy because they suggest that N-cadherin-mediated mechanoreceptor function may play a critical role in how cardiac cells respond selectively to mechanical perturbation to their adhesive microenvironment. It is important to recognize that the ligand densities for N-cadherin and ECM substrates were the same (Supplemental Fig. S1B) as measured using previously established methods (13, 60). It must be noted, however, that the receptor engagement may be different, which may in part account for the greater apparent mechanosensitivity. The intriguing possibility is that cardiac cells respond differentially to cell-to-cell and cell-ECM adhesion systems, suggesting that persistent cross talk between them is required for integrated cytoskeletal remodeling and tissue function.
The striated population of myocytes was significantly greater on soft N-cadherin-coated substrates (300 Pa) than on ECM of equivalent stiffness, whereas on stiffer ECM-coated substrates (5 kPa, 30 kPa), the percentage of striation was higher. This distinct myofibrillar assembly/cytoskeletal response to the resistance force experienced by the two adhesion systems may be a result of a common molecular machinery that is driven by cytoskeletal regulators responding differentially. Other studies have indicated that various physiological processes like neuronal cell branching and myoblast differentiation are also differentially regulated by N-cadherin adhesions, although through common global cytoskeleton regulators such as RhoA; however, their sensitivity through cadherin-mediated forces was not examined (8, 20). The general trend of myocytes exhibiting striation was similar for both N-cadherin- and ECM-mediated adhesions, showing a maxima in striated myocyte populations at intermediate stiffness [N-cadherin (5 kPa) and ECM (10 kPa)]. This observation provides evidence that myocyte striation is relatively independent of adhesion type but remains sensitive to mechanical forces, be they generated by cell-to-cell or cell-ECM contacts. This study provides direct evidence that N-cadherin-mediated adhesions can modulate cardiac myocyte morphology and internal organization.
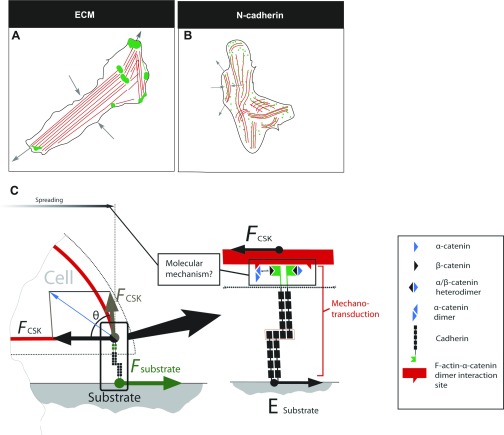
Recent work has shown that myofibril alignment and integration of the cytoskeleton are required for the proper functioning of cardiac tissue (30, 36). It is therefore assumed that proper alignment of the myocyte within the tissue is brought about by mechanical force transduction between the cell-to-cell adhesion subsystems. This study addresses directly myocyte/intracellular cytoskeletal polarization. In the range of tested stiffnesses (5, 10, and 30 kPa), myofibril alignment was significantly lower in myocytes presented to isotropic N-cadherin-coated substrates than in those presented to ECM-coated substrates. On cadherin substrates, the myofibrillar cytoskeleton was fragmented into distinct domains, whereas, on ECM-mimicking 2D substrates, cells established a fully aligned and polarized cytoskeletal continuum. Mathematical models have shown that stress fiber polarization is a function of substrate stiffness and anisotropic cell spreading (59, 62). Cells on stiff ECM-coated substrates form adhesion complexes that mature and assemble to form larger focal adhesions and are less dynamic in nature; as such, they dominate the smaller adhesion complexes and thereby contribute to anisotropic stress fiber formation. Taken together, these observations are consistent with the notion that, with increasing stiffness, actin fibers bundle in the direction of applied force through these formed focal adhesions (Fig. 9A). In contrast, on soft substrates, the adhesions are much more dynamic and give rise to randomly oriented and misaligned myofibrils.
Fig. 9.
A conceptual drawing of myofibril alignment (red) on ECM- (A) and N-cadherin (B)-coated substrates. On ECM-coated substrates, focal adhesions (clustering of integrins and focal adhesion kinase complexes, green) are known to form on stiff substrates. Black arrows indicate the compaction of myofibrils in association with the overall anisotropy (gray arrow). Myocytes on N-cadherin (B) substrates form continuous adhesions, shown in green; myofibril alignment and compaction (black arrows) respond to the local anisotropy leading to global myofibril misalignment. C: conceptual representation of force transmission through N-cadherin adhesion receptors; FCSK is a traction force applied by the cell on the substratum via N-cadherin adhesion receptor, triggering intracellular biomolecular events [through linker proteins to the cytoskeleton (CSK)]; Fsubstrate is a reaction force, which is a function of substrate rigidity.
As observed in these experiments, the adhesion complexes formed on N-cadherin-coated substrates are not focal or large. In particular, staining for vinculin and β-catenin confirms the distributed nature of these complexes, in contrast to the focal adhesions seen on ECM. The adherens complexes formed on cadherins are smaller and more diffuse, which is consistent with their high, dynamic turnover (44). Therefore, the response of the cytoskeleton to cadherin-mediated adhesions is distinct and dynamic and invokes a lamellipodium-like cytoskeleton (40). Consistent with these results, the estimated contractile traction stress vectors on physiologically mimetic stiffness substrates were mainly locally polarized on N-cadherin, whereas on ECM, a more prominent effective dipole was made visible. Myofibril organization on N-cadherin-coated substrates appears to be in a state of dynamic disarray and exhibits only local polarization (Fig. 9B), independent of the stiffness of the substrate. These results indicate that myofibril alignment and polarization on isotropically stiff substrates are directly dependent on the characteristic attributes of the adhesion complexes formed (cell-to-cell or cell-ECM). The noted alignment of myofibrils on N-cadherin substrates is brought about by local intracellular anisotropy and is dictated by the specific nature of the cell-to-cell adhesion complexes. This result points to the specific role of N-cadherins in bringing about fine spatiotemporal structural end-to-end adjustments of myocytes in normal heart syncytium. Moreover, these results suggest that changes in cell-to-cell mechanoperception (load) during development and disease can lead to alterations in alignment and assembly of contractile and other sarcomeric proteins, e.g., sarcomeric addition.
N-cadherin-mediated traction forces proved to be responsive to substrate stiffness. In particular, the average resting forces mediated by N-cadherin were of similar magnitude to those sustained by ECM. The directional changes in predicted traction forces as a function of imposed loads (gel stiffness) provide the evidence that N-cadherin-mediated force transmission is responsible for altering the internal organization of the myocyte cytoskeleton. The contractile forces for myocytes plated on ECM-coated substrates increased with stiffness and correlated well with myocyte striation data. Other studies have also established this correlation for ECM substrates (14, 15, 26). Contractile forces measured through cadherin adhesions also increased with stiffness; however, they plateaued after 5 kPa, which is consistent with the morphological data, which show optima in striation at 5 kPa. In contrast, the contractile forces were significantly lower on 10-kPa stiff N-cadherin substrates and were associated with a relatively lower striated fraction and alignment of myofibrils compared with integrin-mediated traction. These findings further support the concept that myocyte cytoskeletal adaptation is uniquely responsive to the forces perceived by the two adhesion systems. The implication is that the two adhesion systems may contribute uniquely to the adaptation response of myocytes in conditions associated with various forms of cardiac myopathies (dilated-axial cell lengthening; hypertrophic-transverse cell growth).
Not surprisingly, myocytes plated on N-cadherin-coated surfaces increase their cortical stiffness as a function of increasing substrate rigidity, a response that has been seen in other cell types on ECM, such as mesenchymal stem cells (16) and fibroblasts (54). The cardiomyocyte stiffness on N-cadherin-coated substrate of physiologically realistic stiffness (∼10 kPa), measured here as Emyocytes ∼8 kPa, is in agreement with results from other studies showing ENVRM ∼11 kPa, Emyotubes ∼8–18 kPa or the native myocardium, which is E ∼15 − 30 kPa (3, 7, 14, 15). Other studies have shown that myocardial stiffness is altered dramatically after birth (from ∼12 kPa to ∼39 kPa), implicating a commensurate change in substrate stiffness during cardiogenesis (26). The in vivo microenvironment of myocytes is a complex three-dimensional (3D) weave of ECM proteins, which may account for the relatively higher stiffness measurements observed. The 2D cell culture model system used in this study is inherently limited in its ability to recapitulate completely the in vivo 3D ECM complexity of the tissue. In a 2D system, the adhesions are predominantly polarized to the bottom surface of the cell, unlike what a cardiac cell experiences in vivo, where adhesions can be formed all along the cell surfaces (basal, apical, and lateral), creating a highly tethered morphological layout. In general, cells in a 3D culture system exhibit smaller lamellipodia, a higher aspect ratio, and less prominent stress fibers (4, 5). The ventral binding of myocytes to functionalized N-cadherin-engineered surfaces also differs from the geometry of cell-to-cell contacts between polarized myocytes in vivo, where their localization is predominantly end to end. This geometric difference likely accounts for the lower sarcomere alignment on these surfaces but is unlikely to affect the range of stiffnesses over which N-cadherin responds. Inevitable differences occur between any simplified system and the chemically and spatially more complex in vivo setting, but there is compelling evidence that nonmuscle cells as well as cardiac myocytes respond to mechanical cues at least qualitatively in a similar fashion in both 3D and 2D substrates (22, 52, 60). Actin-myosin contractility plays a major role in cell-to-cell as well as cell-matrix adhesion responses. Myosin-mediated contractility is required for cadherin initiation, clustering, and maintenance (2). Inhibition of myosin contractility with BDM significantly lowers the cortical stiffness of myocytes on N-cadherin-coated substrates (30 kPa), indicating that myocyte contractility on cadherin substrates is modulated by myosin. The significant finding is that cells adapt their cytoskeletal properties in response to their mechanical environment, irrespective of whether the adhesion is cell-to-cell or cell-ECM mediated, although the physical adaptation is sensitive to the adhesion type. The relatively greater changes in spread area and cortical stiffness observed for N-cadherin-coated substrates compared with those on ECM, accompanied by relatively similar resting traction forces, strongly suggest that myocyte cytoskeleton reorganization is more mechanosensitive to cadherin adhesions than to integrins (12, 18, 33).
The precise molecular mechanisms that regulate cadherin-cytoskeleton interactions are still being investigated and debated (40). Proteins that could be involved in mechanosensing are p120, vinculin, and α-catenin, which are found to localize at cadherin-mediated adhesions on N-cadherin substrates (19). Vinculin, a well-known regulator of integrin-mediated focal adhesions and a potential mechanotransducer (50), is also found at cell-to-cell junctions. Vinculin also plays a crucial role in cardiac myocyte mechanotransduction (49) and is found at costameres as well as in the intercalated disc (57). Moreover, vinculin is a crucial downstream effector of myosin VI in E-cadherin-mediated adhesions (37). It remains to be seen how mechanical forces transmitted via N-cadherin engage the various sensors involved in the regulation and maintenance of cytoskeletal organization (Fig. 9C). An important remaining challenge is to identify not only the proteins and signals involved in mechanosensing but also the physical principles that enable cells to measure stiffness and force with the precision required to use these inputs to control their phenotype and fate.
This study is a major departure from the idea that only ECM-mediated forces play an important role in myofibrillogenesis, cardiac function, and disease; our studies show that cell-to-cell-mediated forces should also be taken into consideration.
Cell shape-dependent functions are a result of complex mechanical interactions (force transduction, transmissions) between the cytoskeleton architecture and external conditions, be they cell-to-cell AJ or cell-ECM adhesion contact mediated. What remains to be elucidated is how the imposed changes in the external microenvironment are partitioned and/or shared to affect a distinct myocyte remodeling response (e.g., sarcomere serial/parallel addition). In particular, understanding these issues is critical for probing the mechanical signaling targets that contribute to the cardiac remodeling (micro-macro scale) associated with various forms of dilated and hypertrophic myopathies, myocardial infarction, heart failure, and reverse remodeling.
GRANTS
This work was funded in part by the Pennsylvania Department of Health and the National Institutes of Health by grant GM083272 from the National Institute of General Medical Sciences.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Qi Wen and Fitzroy Byfield (University of Pennsylvania) for assistance and advice in atomic force microscope cell studies and Gregory Botta (Drexel University) for initial assistance in cell isolation. We thank Victor Lin (Drexel University College of Medicine) for assistance with cell culture and data analysis.
REFERENCES
- 1. Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci 107: 3655– 3663, 1994. [DOI] [PubMed] [Google Scholar]
- 2. Adams CL, Nelson WJ. Cytomechanics of cadherin-mediated cell-to-cell adhesion. Curr Opin Cell Biol 10: 572– 577, 1998. [DOI] [PubMed] [Google Scholar]
- 3. Azeloglu EU, Costa KD. Cross-bridge cycling gives rise to spatiotemporal heterogeneity of dynamic subcellular mechanics in cardiac myocytes probed with atomic force microscopy. Am J Physiol Heart Circ Physiol 298: H853– H860, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Beningo KA, Dembo M, Wang YL. Responses of fibroblasts to anchorage of dorsal extracellular matrix receptors. Proc Natl Acad Sci USA 101: 18024– 18029, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beningo KA, Wang YL. Double-hydrogel substrate as a model system for three-dimensional cell culture. Methods Mol Biol 370: 203– 212, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol 290: H2196– H2203, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Bierkamp C, Mclaughlin KJ, Schwarz H, Huber O, Kemler R. Embryonic heart and skin defects in mice lacking plakoglobin. Dev Biol 180: 780– 785, 1996. [DOI] [PubMed] [Google Scholar]
- 8. Bixby JL, Zhang R. Purified N-cadherin is a potent substrate for the rapid induction of neurite outgrowth. J Cell Biol 110: 1253– 1256, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Byfield FJ, Wen Q, Levental I, Nordstrom K, Arratia PE, Miller RT, Janmey PA. Absence of filamin A prevents cells from responding to stiffness gradients on gels coated with collagen but not fibronectin. Biophys J 96: 5095– 5102, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carver W, Terracio L, Borg TK. Expression and accumulation of interstitial collagen in the neonatal rat heart. Anat Rec 236: 511– 520, 1993. [DOI] [PubMed] [Google Scholar]
- 11. Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J 76: 2307– 2316, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dzamba BJ, Jakab KR, Marsden M, Schwartz MA, DeSimone DW. Cadherin adhesion, tissue tension, and noncanonical Wnt signaling regulate fibronectin matrix organization. Dev Cell 16: 421– 432, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J 86: 617– 628, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Engler AJ, Carag-Kriefer C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci 121: 3794– 3802, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol 166: 877– 887, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 126: 677– 689, 2006. [DOI] [PubMed] [Google Scholar]
- 17. Fomovsky GM, Thomopoulos S, Holmes JW. Contribution of extracellular matrix to the mechanical properties of the heart. J Mol Cell Cardiol 48: 490– 496, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ganz A, Lambert M, Saez A, Silberzan P, Buguin A, Mege RM, Ladoux B. Traction forces exerted through N-cadherin contacts. Biol Cell 98: 721– 730, 2006. [DOI] [PubMed] [Google Scholar]
- 19. Gavard J, Lambert M, Grosheva I, Marthiens V, Irinopoulou T, Riou JF, Bershadsky A, Mege RM. Lamellipodium extension and cadherin adhesion: two cell responses to cadherin activation relying on distinct signalling pathways. J Cell Sci 117: 257– 270, 2004. [DOI] [PubMed] [Google Scholar]
- 20. Gavard J, Marthiens V, Monnet C, Lambert M, Mege RM. N-cadherin activation substitutes for the cell contact control in cell cycle arrest and myogenic differentiation: involvement of p120 and beta-catenin. J Biol Chem 279: 36795– 36802, 2004. [DOI] [PubMed] [Google Scholar]
- 21. Geisler SB, Green KJ, Isom LL, Meshinchi S, Martens JR, Delmar M, Russell MW. Ordered assembly of the adhesive and electrochemical connections within newly formed intercalated disks in primary cultures of adult rat cardiomyocytes (Abstract). J Biomed Biotechnol 2010: 624719, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J 90: 3012– 3018, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goncharova EJ, Kam Z, Geiger B. The involvement of adherens junction components in myofibrillogenesis in cultured cardiac myocytes. Development 114: 173– 183, 1992. [DOI] [PubMed] [Google Scholar]
- 24. Griffin MA, Sen S, Sweeney HL, Discher DE. Adhesion-contractile balance in myocyte differentiation. J Cell Sci 117: 5855– 5863, 2004. [DOI] [PubMed] [Google Scholar]
- 25. Ivanov DB, Philippova MP, Tkachuk VA. Structure and functions of classical cadherins. Biochemistry (Mosc) 66: 1174– 1186, 2001. [DOI] [PubMed] [Google Scholar]
- 26. Jacot JG, Martin JC, Hunt DL. Mechanobiology of cardiomyocyte development. J Biomech 43: 93– 98, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jacot JG, McCulloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J 95: 3479– 3487, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jou TS, Stewart DB, Stappert J, Nelson WJ, Marrs JA. Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc Natl Acad Sci USA 92: 5067– 5071, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Keller RS, Shai SY, Babbitt CJ, Pham CG, Solaro RJ, Valencik ML, Loftus JC, Ross RS. Disruption of integrin function in the murine myocardium leads to perinatal lethality, fibrosis, and abnormal cardiac performance. Am J Pathol 158: 1079– 1090, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kostetskii I, Li J, Xiong Y, Zhou R, Ferrari VA, Patel VV, Molkentin JD, Radice GL. Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure. Circ Res 96: 346– 354, 2005. [DOI] [PubMed] [Google Scholar]
- 31. Kresh JY. Cell replacement therapy: the functional importance of myocardial architecture and intercellular gap-junction distribution. J Thorac Cardiovasc Surg 131: 1310– 1313, 2006. [DOI] [PubMed] [Google Scholar]
- 32. Krieg M, Arboleda-Estudillo Y, Puech PH, Kafer J, Graner F, Muller DJ, Heisenberg CP. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol 10: 429– 436, 2008. [DOI] [PubMed] [Google Scholar]
- 33. Ladoux B, Anon E, Lambert M, Rabodzey A, Hersen P, Buguin A, Silberzan P, Mège RM. Strength dependence of cadherin-mediated adhesions. Biophys J 98: 534– 542, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lambert M, Padilla F, Mege RM. Immobilized dimers of N-cadherin-Fc chimera mimic cadherin-mediated cell contact formation: contribution of both outside-in and inside-out signals. J Cell Sci 113: 2207– 2219, 2000. [DOI] [PubMed] [Google Scholar]
- 35. Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J 79: 144– 152, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luo Y, Radice GL. Cadherin-mediated adhesion is essential for myofibril continuity across the plasma membrane but not for assembly of the contractile apparatus. J Cell Sci 116P: 1471– 1479, 2003. [DOI] [PubMed] [Google Scholar]
- 37. Maddugoda MP, Crampton MS, Shewan AM, Yap AS. Myosin VI and vinculin cooperate during the morphogenesis of cadherin cell cell contacts in mammalian epithelial cells. J Cell Biol 178: 529– 540, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Matsushita T, Oyamada M, Fujimoto K, Yasuda Y, Masuda S, Wada Y, Oka T, Takamatsu T. Remodeling of cell-to-cell and cell-extracellular matrix interactions at the border zone of rat myocardial infarcts. Circ Res 85: 1046– 1055, 1999. [DOI] [PubMed] [Google Scholar]
- 39. McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6: 483– 495, 2004. [DOI] [PubMed] [Google Scholar]
- 40. Mege RM, Gavard J, Lambert M. Regulation of cell-to-cell junctions by the cytoskeleton. Curr Opin Cell Biol 18: 541– 548, 2006. [DOI] [PubMed] [Google Scholar]
- 41. Mooney DJ, Langer R, Ingber DE. Cytoskeletal filament assembly and the control of cell spreading and function by extracellular matrix. J Cell Sci 108: 2311– 2320, 1995. [DOI] [PubMed] [Google Scholar]
- 42. Nelson WJ, Drees F, Yamada S. Interaction of cadherin with the actin cytoskeleton. Novartis Found Symp 269: 159– 68; discussion 168–77, 223–230, 2005. [PubMed] [Google Scholar]
- 43. Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA 94: 13661– 13665, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perret E, Benoliel AM, Nassoy P, Pierres A, Delmas V, Thiery JP, Bongrand P, Feracci H. Fast dissociation kinetics between individual E-cadherin fragments revealed by flow chamber analysis. EMBO J 21: 2537– 2546, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol 181: 64– 78, 1997. [DOI] [PubMed] [Google Scholar]
- 46. Rhee D, Sanger JM, Sanger JW. The premyofibril: evidence for its role in myofibrillogenesis. Cell Motil Cytoskeleton 28: 1– 24, 1994. [DOI] [PubMed] [Google Scholar]
- 47. Ruiz SA, Chen CS. Emergence of patterned stem cell differentiation within multicellular structures. Stem Cells 26: 2921– 2927, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Russell B, Motlagh D, Ashley WW. Form follows function: how muscle shape is regulated by work. J Appl Physiol 88: 1127– 1132, 2000. [DOI] [PubMed] [Google Scholar]
- 49. Samarel AM. Costameres, focal adhesions, and cardiomyocyte mechanotransduction. Am J Physiol Heart Circ Physiol 289: H2291– H2301, 2005. [DOI] [PubMed] [Google Scholar]
- 50. Schwartz MA. Cell biology. The force is with us. Science 323: 588– 589, 2009. [DOI] [PubMed] [Google Scholar]
- 51. Shai SY, Harpf AE, Babbitt CJ, Jordan MC, Fishbein MC, Chen J, Omura M, Leil TA, Becker KD, Jiang M, Smith DJ, Cherry SR, Loftus JC, Ross RS. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res 90: 458– 464, 2002. [DOI] [PubMed] [Google Scholar]
- 52. Shapira-Schweitzer K, Seliktar D. Matrix stiffness affects spontaneous contraction of cardiomyocytes cultured within a PEGylated fibrinogen biomaterial. Acta Biomater 3: 33– 41, 2007. [DOI] [PubMed] [Google Scholar]
- 53. Skwarek-Maruszewska A, Hotulainen P, Mattila PK, Lappalainen P. Contractility-dependent actin dynamics in cardiomyocyte sarcomeres. J Cell Sci 122: 2119– 2126, 2009. [DOI] [PubMed] [Google Scholar]
- 54. Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J 93: 4453– 4461, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sparrow JC, Schock F. The initial steps of myofibril assembly: integrins pave the way. Nat Rev Mol Cell Biol 10: 293– 298, 2009. [DOI] [PubMed] [Google Scholar]
- 56. Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol 7: 619– 627, 1995. [DOI] [PubMed] [Google Scholar]
- 57. Tokuyasu KT, Dutton AH, Geiger B, Singer SJ. Ultrastructure of chicken cardiac muscle as studied by double immunolabeling in electron microscopy. Proc Natl Acad Sci USA 78: 7619– 7623, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Trinkaus JP, Lentz JP. Direct observation of type-specific segregation in mixed cell aggregates. Dev Biol 89: 115– 136, 1964. [DOI] [PubMed] [Google Scholar]
- 59. Walcott S, Sun SX. A mechanical model of actin stress fiber formation and substrate elasticity sensing in adherent cells. Proc Natl Acad Sci USA 107: 7757– 7762, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Winer JP, Janmey PA, McCormick ME, Funaki M. Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng 15: 147– 154, 2009. [DOI] [PubMed] [Google Scholar]
- 61. Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton 60: 24– 34, 2005. [DOI] [PubMed] [Google Scholar]
- 62. Zemel A, Rehfeldt F, Brown AE, Discher DE, Safran SA. Cell shape, spreading symmetry and the polarization of stress-fibers in cells (Abstract). J Phys Condens Matter 22: 194110, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.