Abstract
Increasing arterial blood pressure (AP) decreases ventilation, whereas decreasing AP increases ventilation in experimental animals. To determine whether a “ventilatory baroreflex” exists in humans, we studied 12 healthy subjects aged 18–26 yr. Subjects underwent baroreflex unloading and reloading using intravenous bolus sodium nitroprusside (SNP) followed by phenylephrine (“Oxford maneuver”) during the following “gas conditions:” room air, hypoxia (10% oxygen)-eucapnia, and 30% oxygen-hypercapnia to 55–60 Torr. Mean AP (MAP), heart rate (HR), cardiac output (CO), total peripheral resistance (TPR), expiratory minute ventilation (VE), respiratory rate (RR), and tidal volume were measured. After achieving a stable baseline for gas conditions, we performed the Oxford maneuver. VE increased from 8.8 ± 1.3 l/min in room air to 14.6 ± 0.8 l/min during hypoxia and to 20.1 ± 2.4 l/min during hypercapnia, primarily by increasing tidal volume. VE doubled during SNP. CO increased from 4.9 ± .3 l/min in room air to 6.1 ± .6 l/min during hypoxia and 6.4 ± .4 l/min during hypercapnia with decreased TPR. HR increased for hypoxia and hypercapnia. Sigmoidal ventilatory baroreflex curves of VE versus MAP were prepared for each subject and each gas condition. Averaged curves for a given gas condition were obtained by averaging fits over all subjects. There were no significant differences in the average fitted slopes for different gas conditions, although the operating point varied with gas conditions. We conclude that rapid baroreflex unloading during the Oxford maneuver is a potent ventilatory stimulus in healthy volunteers. Tidal volume is primarily increased. Ventilatory baroreflex sensitivity is unaffected by chemoreflex activation, although the operating point is shifted with hypoxia and hypercapnia.
Keywords: baroreceptors, ventilation, blood pressure
In intact animals (dogs) hyperpnea follows occlusion of the common carotid arteries; it is wholly reflex in origin because the effect is the same even though all branches of the carotids have been previously tied excepting only the lingual arteries, and because section of the sinus nerves completely abolishes the reaction.
Carl F. Schmidt (45)
detection of arterial blood pressure (AP) by the arterial baroreceptors causes changes in heart rate (HR), sympathetic vasoconstriction, adrenal function, and renal sympathetic activity (2, 5). Evidence primarily derived from animal experiments suggests that the carotid sinus baroreflex also affects respiratory function (7, 26, 45, 48). Rapidly increasing blood pressure causes decreased ventilation, whereas decreasing blood pressure causes increased ventilation. While these ventilatory effects were at one time attributed to increased or decreased perfusion of the arterial chemoreceptors (26, 32), subsequent investigations in experimental preparations have shown that exclusion of the carotid body from the carotid sinus area does not alter the ventilatory response in vagotomized dogs (7) and sinoaortic denervation eliminates the pressure-sensitive ventilation response in dogs (47), whereas the carotid body chemoreceptor in cats is insensitive to blood pressure change (3).
Additional studies have shown, however, that arterial baroreflexes and chemoreflexes may interact in controlling ventilation (24). For example, Heistad et al. (23) used hemorrhage to show that baroreflex unloading potentiates carotid chemoreflex activation by nicotine, whereas baroreflex loading by transfusion blunts this response. These findings suggest modulation of the chemoreflex by the baroreflex at the level of the central nervous system (23).
Most past studies were performed in anesthetized, instrumented experimental animals, often after vagotomy, or in isolated tissue obtained from such animals. To date, there has been only one relevant study (55) of ventilation and arterial baroreflex in humans that focused on the effects of peripheral and central adrenergic stimulation on chemoreflex activation, i.e., how the arterial baroreflex modulates chemoreflex activity. Interestingly, increased ventilation follows the administration of epinephrine, norepinephrine, and isoproterenol, but decreased ventilation follows phenylephrine and ANG II (25). Indeed, other pilot data have shown decreased ventilation with increased blood pressure in humans (11), However, these responses were regarded as small and unimportant, and the effects of hypotension on baroreflex-mediated ventilation and its modulation by chemoreflex activation have not been investigated in humans. Our reason for studying a “ventilatory baroreflex” takes clinical origin from observations made of hyperpnea and hypocapnia with baroreflex unloading during upright tilt. Hyperpnea is exaggerated in patients with orthostatic intolerance who have the greatest degree of thoracic hypovolemia and thus might experience enhanced arterial baroreflex unloading (50).
Therefore, we used the modified Oxford method, in which baroreflex unloading and loading were produced by pharmacologically induced blood pressure decreases and increases. We hypothesized that ventilation increases during hypotension and decreases during hypertension. We further hypothesized that this ventilatory baroreflex has a reciprocal relationship with chemoreflex activation such that chemoreflex activation enhances baroreflex-activated ventilation.
METHODS
Subjects.
We enrolled 12 healthy young volunteer subjects aged 18–26 yr (median = 22 yr, 6 women and 6 men). Average weight was 70 ± 14 kg, average height was 169 ± 10 cm, and average body mass index was 23 ± 4 kg/m2 (means ± SD). All measurements were made with the subjects supine. Resting supine systolic blood pressure was 118 ± 4 mmHg, resting supine diastolic blood pressure was 65 ± 2 mmHg, and resting HR was 63 ± 3 beats/min.
All subjects were free of all cardiorespiratory and autonomic ailments and systemic illnesses. Subjects were not taking medications and were nonsmokers. There were no trained athletes or bedridden subjects. Informed consent was obtained. All protocols were approved by the Committee for the Protection of Human Subjects of New York Medical College.
Subjects refrained from eating for at least 4 h and had no caffeinated beverages for at least 12 h before being tested. An intravenous catheter was placed in the left antecubital vein. We used a Finometer (FMS) to assess beat-to-beat AP and to estimate cardiac output (CO) via ModelFlow, which models the circulation as an adaptive Windkessel. Electrodes were placed for ECG measurement of HR and rhythm. Respirations were monitored by pneumotachography (Hans Rudolph, Shawnee, KS), which is described in detail below. End-tidal CO2 (ETco2) was measured with a combined capnograph using nasal prongs connected to a side stream capnograph while O2 saturation was measured using standard pulse oximetry (Capnocheck Sleep Capnograph/Oximeter, Tri-Anim Health, and Sylmar).
Inhalation apparatus.
Ventilatory responses to eucapneic hypoxia and hyperoxic hypercapnia were obtained using the “dynamic end-tidal forcing” technique (51). This technique allows for the manipulation of the end-tidal concentration of one gas while maintaining the end-tidal concentration of the other gas constant.
We used the following three different gas conditions:
1. Eucapneic normoxia (breathing room air).
2. Eucapneic hypoxia (10% O2 balanced with N2 while maintaining eucapnia by adding CO2 to the circuit as needed).
3. Hypercapnic hyperoxia (30% O2 plus added CO2 sufficient to raise ETco2 to 55–60 Torr); 30% O2 (hyperoxia) was added to exogenous CO2.
Hypercapnic hyperoxia was used to stimulate central chemoreceptors through hypercapnia while minimizing the effect of CO2 on peripheral chemoreceptors by using hyperoxia. Eucapneic hypoxia was used to primarily stimulate peripheral chemoreceptors. This experimental scheme permitted separation of the effects of hypoxia and hypercapnia.
Subjects breathed through a face mask connected to a pneumotachograph (Hans Rudolph), from which we obtained ventilatory data. An open circuit was used to control inspired gases during each of these gas conditions. The other end of the pneumotachograph was connected through a low-resistance two-way nonrebreathing valve, which had two outlets. One outlet was an exhaust to room air, whereas the other outlet was connected to a 30-liter breathing bag (Rusch, Research Triangle Park, NC). The gas in the bag was constantly replenished from cylinders of O2, CO2, and N2 and were mixed to the desired composition using a gas rotometer flowmeter (model FL-6GP, Omega Engineering, Stamford, CT). The breathing bag was always fully inflated at the start of inspiration to insure a constant flow during the increased ventilation of hypoxia and hypercapnia.
Two-stage gas flow regulators (Western Medica, Westlake, OH) connected to each of the three air cylinders (O2, CO2, and N2) were used to achieve constant gas flow at the desired concentration of the air mixture balanced with N2 (5% CO2, 10% O2, and 30% O2 balanced by N2). The inhaled O2 concentration was measured continuously and directly by an oxygen sensor (S-3A/I Oxygen Analyzer with N-22M Sensor, AEI Technologies, Naperville, IL) attached near the face mask. The pneumotachograph was calibrated using a 3-liter syringe, and the oxygen sensor was calibrated using known concentrations of O2 before each testing session.
For eucapneic normoxia, the valve at the bag was regulated so that subjects breathed in room air. For eucapneic hypoxia, the level of O2 was titrated so that all subjects breathed 10% O2 (monitored by oxygen sensor) for 8 min. During this hypoxia, subjects achieved an arterial O2 saturation (SaO2) of ∼80–85%, as assessed by pulse oximetry. For hypercapnic hyperoxia, the level of O2 was 30%, and CO2 was added to achieve an ETco2 of 55–60 mmHg, which was at least 10 Torr above the eucapneic baseline.
Protocol.
Testing began at 10 AM in a temperature-controlled room. Subjects were familiarized with the procedures used in the study before any instrumentation. Thereafter, subjects were instrumented. After an initial 20 min of acclimatization, subjects were monitored during a 30-min rest period in which ETco2 was measured. Mean ETco2 over the last 5 min of the rest period was defined as eucapnia for each subject. Subjects then underwent three measurement periods separated by 30-min rest periods. Each measurement period corresponded to one of the following gas conditions presented in random order: room air, eucapneic hypoxia, and hypercapnic hyperoxia.
Patients had a 5-min baseline data collection period preceding the alteration of gas conditions. When gas conditions were altered, patient breathing, HR, and blood pressure were allowed to reach steady states over 5 min. Baseline ventilation and hemodynamic assessments for each gas condition were obtained during minute 4. We then used the modified Oxford method (49) to generate decreases in blood pressure (arterial baroreflex unloading) by intravenous bolus administration of 100 μg sodium nitroprusside (SNP) followed 60 s later by 150 μg phenylephrine to produce AP restitution and mild hypertension (arterial baroreflex loading). The Oxford maneuver produced decreases in blood pressure below a baseline of ∼15–25 mmHg followed by increases of ∼10–15 mmHg above baseline (4). Prior work has shown that repeated baroreflex trials using the modified Oxford method and separated by a 30-min rest period are reproducible (44). Also, 30 min of rest are sufficient to restore ventilation and hemodynamics to baseline conditions (57).
Data analysis.
Baseline beat-to-beat data were collected during the 3 min preceding the alteration of gas conditions. Data were collected throughout gas conditions and continuously during hypotensive-hypertensive Oxford maneuvers. Data included ECG rate and rhythm, CO, systolic and diastolic blood pressures (via Finometer), mean AP [MAP; calculated from systolic AP and diastolic AP using the following formula: MAP = (systolic AP + 2 × diastolic AP)/3], SaO2, ETco2, expiratory minute ventilation (VE), and respiratory rate. Total peripheral resistance (TPR) was computed using the following formula: TPR = MAP/CO and was expressed in Woods units (mmHg·l−1·min−1). Data were digitized at 200 Hz with custom signal processing software and analyzed offline.
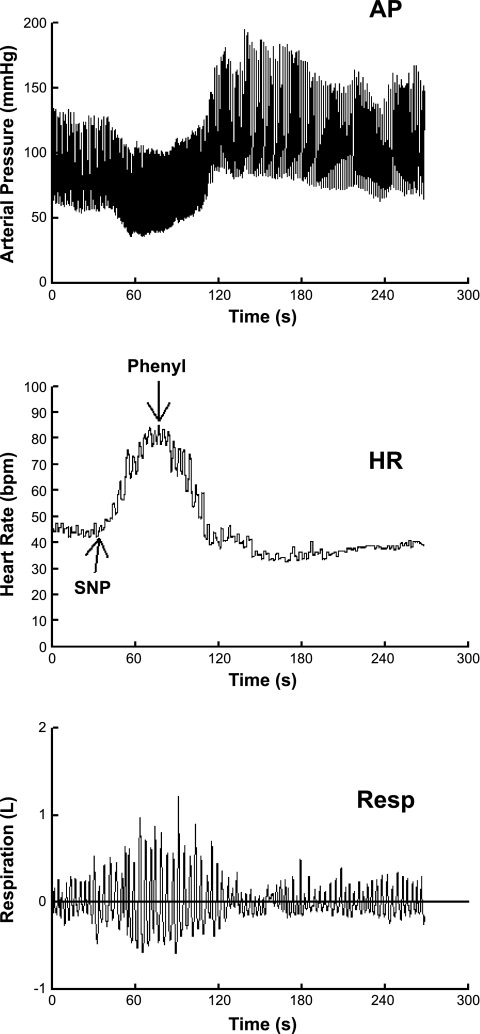
Representative changes in AP, HR, and respirations are shown in Fig. 1. In response to decreasing blood pressure caused by SNP, HR and respiratory volume increased; in response to increasing blood pressure caused by phenylephrine, HR and respiratory volume decreased.
Fig. 1.
Representative changes in arterial blood pressure (AP; top), heart rate [HR; in beats/min (bpm); middle], and respirations (Resp; bottom) are shown. In response to decreasing blood pressure caused by sodium nitroprusside (SNP), HR and respiratory volume increased; in response to increasing blood pressure caused by phenylephrine (Phenyl), HR and respiratory volume decreased.
Respiratory volumetric changes were converted to VE using a differencing procedure in which the volume of air exhaled was calculated for each breath and divided by the total time (in s) of that breath and then multiplied by 60 (s/min). As calculated from raw pneumotachometer data, this equaled the integrated flow during expiration divided by the breath duration from the onset of inspiration to the offset of expiration (= onset of the next breath) multiplied by 60. Initially we used systolic, diastolic, and mean APs aligned to VE. A time delay between MAP and ventilation would be expected based on neuromechanical issues. This was observed. Usually this varied between 0 and 3 s. Delays were related to the time to onset of the first breath after the start of decreasing blood pressure due to SNP used in the Oxford method. Delays were compensated by shifting respirations such that the onset of the change in respiration corresponded to the onset of the change in pressure. This is similar to practices used to align muscle sympathetic nerve recordings to corresponding RR intervals (13). We performed a Quicksort (27) on pressures where corresponding VE values were reordered in concert with corresponding pressures.
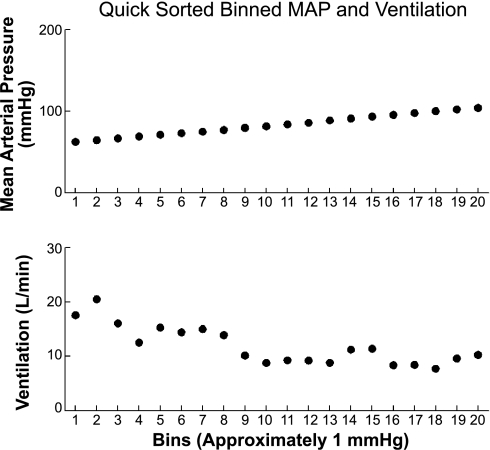
This Quicksort procedure requires further explanation. The first step was the alignment of synchronous AP with respirations, which was performed automatically by our software. Next, we took the MAP corresponding to each cardiac cycle and assigned that pressure to the entire RR interval. We then calculated the VE corresponding to each breath and assigned that value to the entire breath. Thus, we obtained a graphical sequence of MAP steps of length equal to RR intervals and a second sequence of VE steps of length equal to respiratory intervals. Each MAP step had a portion of a VE step corresponding to it (within the particular RR interval) or even overlapped adjacent VE steps. In either case, a time average of the VE falling within the particular RR interval was performed. After averaging was complete, each MAP step (RR interval) and its corresponding VE were collapsed to a single point value to obtain digital sequences of MAP and corresponding digital sequences of VE. The final stage of the procedure required sorting data on the basis of MAP from smallest to largest pressure. As MAP point values were sorted and rearranged, their corresponding VE point values were rearranged. We then binned the MAP into pressure bins of 1 mmHg. A number of points in the MAP sequence may have fallen into any given bin and were averaged to obtain an averaged bin pressure. We then averaged all VE points corresponding to the MAP binned pressures to obtain an averaged bin VE. This yielded sequences of MAP and VE as shown in Fig. 2. We performed a weighted least-squares fit of VE against MAP where the weights were the number of points that fell into a specific bin divided by the total number of points in all bins.
Fig. 2.
Sampled, sorted, and binned points for AP [here, mean AP (MAP)] and corresponding binned values of expiratory minute ventilation (VE) are shown. After pressures were sorted, corresponding VE values were reordered with corresponding pressures, and discrete data points were obtained by “binning” blood pressures within 1-mmHg intervals and computing a weighted average over VE falling within each bin. Points were included from the start of the decrease in AP through the SNP-induced minimum until the maximum AP was reached after phenylephrine administration.
Point values were included from the start of decrease in AP through the SNP-induced minima until the maximum AP was reached after phenylephrine. The arrangement of sampled, sorted, and binned points for AP (here, MAP) and corresponding values of VE are shown in Fig. 2.
Ventilatory baroreflex curves were constructed for each subject and for each gas condition by plotting VE on the ordinate and blood pressure on the abscissa in response to the Oxford maneuver. A logistic (sigmoidal) curve was fitted for each patient and each gas condition using the Levenberg-Marquardt nonlinear least-squares algorithm (35). An r2 value of 0.85 or greater was obtained with each fit.
The logistic curve is determined by four parameters as follows:
where a0 is the lower asymptote of the fit, a0 + a1 is the maximum asymptote of the fit, a2 is the value of AP at maximum slope (midpoint of the linear portion of the curve), and a1/(4 × a3) determines the maximum slope.
Initial fits were obtained for VE as a function of systolic, diastolic, and mean APs. It was soon found that MAP yielded the best fits and was therefore used for comparisons The parameters of the sigmoidal fits were identical for systolic, diastolic, and mean APs except for a shift of the center (corresponding to the coefficient a2).
A sigmoidal nonlinear least-squares curve fit of VE as a function of MAP was calculated for each subject and each gas condition over the entire pressure range. Much of the data values fell on the “linear portion” of these curves, but not always. Therefore, we averaged fitted sigmoidal curves for a given gas condition over all subjects.
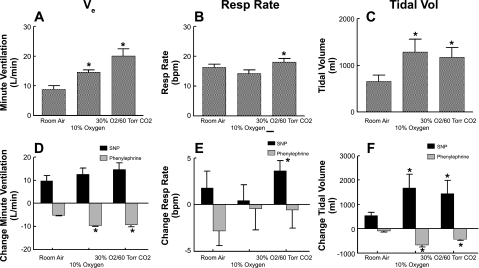
Data are also presented in compound graphics in which VE, respiratory rate, and tidal volume are plotted in A–C for all gas conditions, whereas maximum and minimum changes in these measurements in response to SNP and phenylephrine are shown in D–F (Fig. 3).
Fig. 3.
Ventilatory findings during each gas condition. A: VE. B: respiratory rate. C: tidal volume. D–F: maximum increases and decreases during the Oxford maneuver of VE (D), respiratory rate (E), and tidal volume (F). Increased ventilation (panel A) was primarily determined by increased tidal volume (C). Changes of VE during the Oxford maneuver (D) were inversely related to VE (A) such that the product of VE and the change in VE with SNP was not different across gas conditions. *P < 0.05 compared with room air.
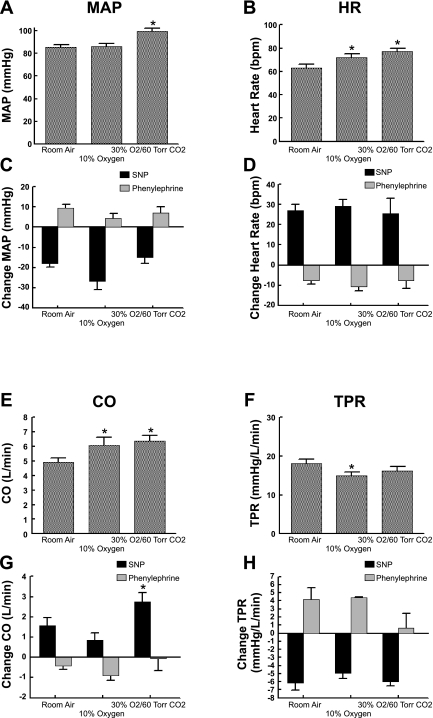
Hemodynamic data averaged over all subjects are similarly depicted. Averaged MAP, HR, systolic and diastolic pressure, CO, and TPR were graphically displayed for all gas conditions in A, B, E, and F, whereas changes in response to SNP and phenylephrine are shown in C, D, G, and H (Fig. 4).
Fig. 4.
Circulatory findings during each gas condition. A: MAP during gas conditions. B: HR. C and D: respective changes of MAP (C) and HR (D) during the Oxford maneuver. E: cardiac output (CO; E) during gas conditions. F: total peripheral resistance (TPR). G and H: respective changes of CO (G) and TRP (H) during the Oxford maneuver. MAP increased during hypercapnia but not during hypoxia (A). There were no significant differences in responses of MAP to SNP during the different gas conditions (C). Decreased MAP was associated with a large increase in HR (D). HR increased during hypoxia and hypercapnia (B). CO increased during hypoxia and hypercapnia (E), whereas TPR decreased (F). During phenylephrine, TPR increased, although the increase was blunted during hypercapnia (H). *P < 0.05 compared with room air.
Statistics.
Because there were no discernible differences between the data from men and women, data from the two groups were combined for statistical analysis. Measurements across the conditions were computed for each individual. One-way ANOVA for repeated measures was used for comparing data across baseline gas conditions. Two-way ANOVA for repeated measures was used for Oxford measurements comparing changes in response to SNP and phenylephrine across gas conditions. When appropriate, post hoc comparisons were performed using Tukey's test. VE, respiratory rate, and tidal volume were averaged across each gas condition, and the change from room air was assessed by paired t-test. Differences were considered significant when P < 0.05. All values and error bars indicate means ± SE. Results were calculated using SPSS software (version 11.0).
RESULTS
Arterial saturation and ETco2.
While resting supine and breathing room air, subjects had an O2 saturation that varied from 96% to 99% (mean: 97 ± 1%) with an ETco2 varying from 38 to 46 Torr (mean: 42 ± 2 Torr), which was defined as “eucapnia” for a given individual. While breathing 10% O2, O2 saturation decreased significantly to 80–85% (mean: 83 ± 1, P < 0.001) while ETco2 was kept unchanged by bleeding 5% CO2 into the mask as needed to maintain eucapnia. O2 saturation was uniformly 100% when subjects received the 30% O2-CO2 mixture intended to maintain CO2 in the 55- to 60-Torr range.
Respiratory results: VE and respiratory rate.
Figure 3 shows ventilatory findings during each gas condition. VE increased from 8.8 ± 1.3 l/min in room air to 14.6 ± 0.8 l/min during hypoxia and to 20.1 ± 2.4 l/min during hypercapnia. During SNP administration, VE increased compared with baseline by 9.7 ± 2.4 l/min in room air, by 12.7 ± 2.6 l/min with hypoxia, and by 14.6 ± 2.9 l/min with hypercapnia (P < 0.01 compared with room air). During phenylephrine, VE decreased by 5.1 ± 1.1 l/min in room air, by 9.5 ± 0.5 l/min during hypoxia (P < .01), and by 9.2 ± 1.0 l/min (P < 0.01) by phenylephrine during hypercapnia.
Respiratory rate increased from 16 ± 1.0 breaths/min in room air to 18 ± 1.0 breaths/min (P < 0.05) during hypercapnia. Respiratory rate during hypoxia was not different from room air at 16 ± 1.0 breaths/min. Respiratory rate was not significantly affected by the Oxford maneuver.
VE and respiratory rate data corresponded to an increase in tidal volume from 660 ± 126 ml in room air to 1,289 ± 270 ml (P < 0.025 compared with room air) in hypoxia and to 1,171 ± 205 ml (P < 0.025 compared with room air) in hypercapnia. During SNP administration, tidal volume increased from baseline by 541 ± 125 ml in room air, by 1,675 ± 567 ml in hypoxia (P < .025 compared with room air), and by 1,445 ± 541 ml in hypercapnia (P < 0.025 compared with room air). During phenylephrine administration, tidal volume decreased from baseline by 92 ± 53 ml in room air, by 657 ± 90 ml in hypoxia (P < 0.01 compared with room air), and by 433 ± 50 ml (P < 0.01 compared with room air) in hypercapnia.
Circulatory results: HR, AP, CO, and TPR.
MAP did not increase during hypoxia but was significantly (P < 0.01) increased from 85 ± 3 to 101 ± 3 mmHg during hypercapnia, as shown in Fig. 4. During the Oxford maneuver, MAP was decreased below baseline with SNP and increased above baseline for phenylephrine, but there were no significant differences in these decreases or increases during the different gas conditions. The decrease in MAP was associated with a large increase in HR during SNP, shown in Fig. 4D. The baseline value of HR increased during hypoxia from 63 ± 3 to 73 ± 3 beats/min (P < .05) and was further significantly (P < 0.025) increased during hypercapnia to 77 ± 3 beats/min.
CO increased significantly from 4.9 ± 0.3 l/min in room air to 6.1 ± 0.6 l/min (P < 0.01) during hypoxia and to 6.4 ± 0.4 l/min (P < 0.01) during hypercapnia. During SNP, CO increased from baseline by a significantly larger amount for hypercapnia (2.74 ± 0.45 vs. 1.57 ± 0.39 l/min for room air, P < 0.05) but was relatively unaffected during hypoxia. TPR was decreased from 18 ± 1 Woods units in room air to 15 ± 1 Woods units (P < 0.025) during hypoxia and to 16 ± 1 Woods units (P < 0.01) during hypercapnia. In response to SNP, TPR decreased for all gas conditions to the same degree. During phenylephrine, TPR increased only for room air and hypoxia, whereas there was no change during hypercapnia.
The ventilatory baroreflex.
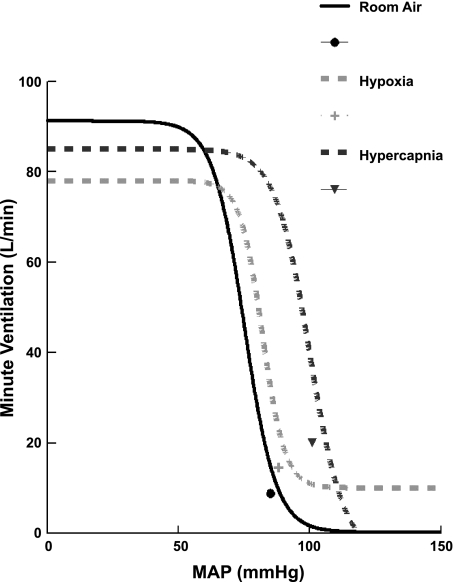
Figure 5 shows the relationship between Ve and MAP for all subjects during each of the three different gas conditions. The curves were generated in the following manner: a sigmoidal nonlinear least-squares fit was performed for each subject and every gas condition. Most data values fell on the linear portion of these curves, but not always. Therefore, we averaged all data generated by fitted sigmoidal curves over all subjects for a given gas condition.
Fig. 5.
Relationship between VE and MAP for all subjects during each of the three different gas conditions. The solid circle corresponds to room air, the light gray plus sign corresponds to hypoxia, and the dark gray inverted triangle corresponds to hypercapnia. The point adjacent to each curve is the “operating point” corresponding to the average values of VE and MAP during baseline gas conditions. There were clear differences in the curves and changes in operating points, but there was no significant difference in the slopes of composite averages under the different gas conditions.
The point adjacent to each curve is the “operating point,” corresponding to the average over all subjects of VE (ordinate) and the average over all subjects of MAP (abscissa) during baseline gas conditions (i.e., before the Oxford maneuver). There were clear differences in the curves and changes in operating points, but there were no significant differences in the slopes of composite averages under the different gas conditions. The shift in the operating point is the consequence of increased MAP and ventilation with hypercapnia and the increased ventilation but not MAP with hypoxia. The slopes, thresholds, saturations, response ranges, and operating points for each gas condition are shown in Table 1.
Table 1.
Calculated sensitivity (slopes), threshold, saturation, response range, and operating points for each gas condition
| Room Air | Hypoxia | Hypercapnia | |
|---|---|---|---|
| Sensitivity | |||
| Slope, l·min−1·mmHg−1 | −3.8 ± 0.7 | −4.0 ± 0.4 | −3.4 ± 0.6 |
| Threshold, l/min | 0.3 ± 9 | 10 ± 4 | −3 ± 6 |
| Saturation, l/min | 91 ± 10 | 78 ± 16 | 84 ± 10 |
| Response range, l/min | 88 ± 13 | 70 ± 16 | 85 ± 14 |
| Operating point | |||
| Mean arterial pressure, mmHg | 85 ± 2 | 86 ± 3 | 101 ± 3* |
| Expiratory minute ventilation, l/min | 8.8 ± 1.3 | 14.6 ± 0.8* | 20.1 ± 2.4* |
Values are means ± SE.
P < 0.05 compared with room air.
DISCUSSION
The ventilatory baroreflex exists and is independent of chemoreflexes.
A ventilatory component of the arterial baroreflex appears in experimental animal literature (7, 26, 45, 48) but was relatively small compared with chemoreflex ventilatory regulation. Rather, the arterial baroreflex was thought to serve a role as a modulator of chemoreflex activity (55).
The principal and new findings of this investigation are the detection of physiologically important and statistically significant changes in ventilation due to baroreflex unloading in healthy young human subjects. These ventilatory changes are on the order of 100% increases in VE during SNP administration across all gas conditions. Thus, the changes in ventilation due to baroreflex unloading (the ventilatory baroreflex) are relatively large and independent of chemoreflex activation. These findings are physiologically important because a relatively small and brief decrease in MAP of only 10–20% by SNP caused a rapid 100% increase in ventilation. Decreases in ventilation and tidal volume were also observed with phenylephrine during hypoxia and hypercapnia but were absent in room air, primarily due to the experimental design (SNP was administered first, which decreased MAP, followed by the administration of phenylephrine, which essentially restored MAP). Despite this, estimates obtained from the slopes of the ventilatory baroreflex function curves indicate that similar increases in MAP across gas conditions should produce similar decreases in VE across gas conditions.
Past evidence, primarily accrued in nonhuman mammals, strongly implicates the arterial baroreflexes in this ventilatory response to AP and precludes the involvement of the chemoreflexes (2, 8, 26, 45, 46). Similar experiments have not been performed in humans because of the need to isolate or differentially ablate the carotid body or carotid sinuses. However, in our present study, the relative effects of change in MAP on change in ventilation (i.e., the slope; Fig. 5) are relatively unaffected by gas condition, which suggests that the activation of chemoreflexes does not affect changes in ventilation with changing blood pressure. Several caveats are necessary. First, the sensitivity of the baroreflex curve was measured at its predicted maximum slope based on curve fitting. The maximum slope is midway between threshold and saturation and is seldom encompassed by raw data points of specific curve fits. Slope/sensitivity is thus a predicted quantity. Second, although the predicted sensitivity of the entire baroreflex curve was not altered by the gas stimulus, the gain of that response immediately around the operating point can be different, based on the location of the operating points, as shown in Fig. 5. The shift in the operating point is a consequence of increased MAP and ventilation with hypercapnia and increased ventilation but not increased MAP with hypoxia. Thus, while the sensitivity of the responses to pressor-depressor stimuli is independent of chemoreflex activation, the complete response is dependent on the operating point.
An alternative explanation for our observations might be direct chemical stimulation of chemoreceptors by SNP and inhibition by phenylephrine. However, past work has indicated that nitric oxide donors inhibit rather than stimulate chemoreflexes (54), whereas phenylephrine has no direct effect on chemoreflex activity in humans (25).
Experimental stimulation of central and peripheral chemoreceptors.
Central and peripheral chemoreceptors have the dominant role in the control of respiration, as shown in Fig. 3. Our experimental design was to, as near as possible, selectively stimulate peripheral and central receptors. The peripheral receptors respond primarily to arterial hypoxia and, to a lesser extent, to hypercapnia (40). The central receptors are far more sensitive to hypercapnia (increase in hydrogen ions) (12). The peripheral chemoreceptors are contained within the carotid and aortic bodies, whereas the central chemoreceptors are distributed mainly in the medulla (6). Both hypoxia and hypercapnia are potent ventilatory stimuli. Hypercapnia exerts its central effects via medullary respiratory centers by changes in the hydrogen ion concentration. Thus, to examine ventilatory effects of central chemoreceptor stimulation, we used hyperoxic hypercapnia. This setting of hyperoxia along with hypercapnia was used to minimize the effect of peripheral chemoreceptors (38) and maximize the effects on central chemoreceptors. Hypoxia exerts its effects mainly via the peripheral receptors comprising the carotid and aortic bodies. Thus, to examine the ventilatory effects of peripheral chemoreceptor stimulation, we used eucapnic hypoxia to minimize the effects of central chemoreceptors and maximize the effects of peripheral chemoreceptors.
Ventilation increases by change in tidal volume.
The change in ventilation caused by the ventilatory baroreflex is produced primarily by an increase or decrease in tidal volume with smaller changes in respiratory rate. This is different from experimental studies in animals, which have typically demonstrated a larger increase in respiratory rate than in tidal volume (7, 26, 45, 47). However, most of these studies were performed after vagotomy. The presence of an intact vagus suppresses respiratory rate when lungs are greatly inflated through the Hering-Breuer reflex (56) and may account for the differences recorded in intact humans versus surgically altered experimental animals.
Mild to moderate hypoxia and hypercapnia do not result in important amounts of tachypnea (53), and our results are consistent with these findings. Thus, the increase in ventilation caused by hypoxia alone is unassociated with an increase in respiratory rate. The degree of O2 desaturation we measured during hypoxia was moderate, and thus the hypoxemia was modest as well. Prior work supports the relative constancy of respiratory rate during moderate transient hypoxia (58), although the respiratory rate increases during severe hypoxemia. Similarly, the increase in ventilation due to hypercapnia resulted primarily from increased tidal volume. However, a small hypercapnic increase in respiratory rate was observed. This may result from a relatively larger hypercapnic stimulus (compared with hypoxic stimulus) exceeding a 10-Torr increase in ETco2, resulting in a larger increase in tidal volume and a small increase in respiratory rate compared with hypoxia.
Hemodynamic results.
MAP was increased during hypercapnia, whereas HR was increased during hypoxia and hypercapnia. This has been reported by some investigators (10, 29, 36) but not by others (21, 36). We found that TPR was reduced and CO increased, which would seem at odds with the known potentiating effects of hypoxia and hypercapnia on the sympathetic nervous system (36). However, all gas conditions were acute and transient rather than chronic and intermittent. Acute mild hypoxia increases CO in dogs (1) and humans (42) while reducing TPR (“hypoxic vasodilation”) (24). The effects of chronic intermittent hypoxia are quite different, as this results in decreased CO and increased TPR (20). Similarly, acutely increased CO2 produces vasodilation and an increase in CO, largely from local direct vasodilatory effects at the tissue vascular level (30, 37). Intermittent hypercapnia results in overriding sympathetic activation and vascular remodeling, producing the opposite: increased TPR and decreased CO.
How does baroreflex unloading produce hyperventilation?.
Although sympathetic activity was not specifically measured during present experiments, baroreflex unloading of healthy subjects results in sympathoexcitation. Sympathoexcitation alone may result in hyperventilation (14). In addition, the arterial baroreceptors send specific projections to the respiratory area (18, 33, 43). It may be inferred that these projections can directly modulate respiratory activity. Finally, both sympathetic activation and parasympathetic withdrawal stimulate peripheral and central chemoreflex activity provoking hyperventilation and hypocapnia (22, 34).
Perspective.
Arterial baroreflex unloading is pleiotropic and now includes enhanced ventilation as one of its effects in humans. Such increased ventilation commonly causes hypocapnia in diverse forms of orthostatic intolerance, as occur in autonomic failure (52), simple faint (31) and chronic orthostatic intolerance (41), and may also explain posturally potentiated “idiopathic” hyperventilation syndromes (19). Should hypocapnia occur as a result of hyperventilation, cerebral blood flow will decrease and may contribute to impairment of cognition and consciousness. Although CO2 was controlled throughout the present experiments, our results suggest that baroreflex loading might improve orthostatic hyperpnea; indeed, we have anecdotal experience using phenylephrine to alleviate such hyperpnea. The consequences of sustained baroreflex unloading as might occur in hypovolemic shock are subjects for further investigation.
Limitations.
First, the initial reduction in blood pressure after SNP administration is often very rapid, decreasing thereafter at a lesser rate before the administration of phenylephrine. This rapid reduction in pressure occurs over a limited number of respiratory cycles on the order of six to seven breaths. Using the present analysis, multiple cardiac cycles at different pressures can be binned with the same value of VE. This is a limitation of the analytic approach.
Second, most important, analyses of threshold, saturation, and response range highlight a significant concern regarding methods and underscore the limitations of the Oxford method in assessing ventilatory changes in human subjects.
The key question arises of whether we are entitled to fit our data to sigmoidal curves. This is particularly important because the sensitivity of such a curve is often taken as the maximum slope or peak gain, which occurs in the midst of “the most linear portion” of the curve, as defined by Hunt and Farquhar (28), midway between asymptotes. The results shown in Fig. 5 indicate that it would require unacceptable hypotension to produce a complete sigmoidal curve in the average patient, although they do reach at the least a portion of the most linear portion of the responding range.
However, if we assume that the sigmoidal model is correct for all gas conditions, then we are comparing predicted rather than measured maximum slopes. Most of the time, the raw data do not encompass the point of maximum slope, and, therefore, we are not able to empirically verify the accuracy of those slopes. Although sigmoidal modeling fits the data well (better than any linear or polynomial fit), if we do not reach pressures at which the maximum slope occurs, then the modeled predicted maximum slope should be questioned. Complete verification of sigmoidal shapes might be obtained in an animal study. More complete curves might also be produced by using prolonged infusions.
The mean age of the group studied was relatively young. Young adults are healthy, whereas age and illness modify reflex function. Thus, while a younger age group may be ideal for investigations of healthy physiological function, the applicability of these findings may be limited to this age group.
The data from male and female subjects were combined. Preliminary analyses showed no qualitative or quantitative differences by sex. However, known differences in circulatory and autonomic function occur by sex (17). Nevertheless, changes in ventilation with blood pressure were directionally similar in our subjects regardless of sex. Also, we studied females without regard to menstrual cycle. The phase of the menstrual cycle might alter ventilatory baroreflex mechanisms. However, recent work suggests that neither the cardiovagal nor sympathetic baroreflexes are affected by menstrual cycle (9, 15).
We did not study baroreceptor unloading that may occur in the absence of blood pressure changes, but rather used brief hypotension to unload the arterial baroreceptors. Baroreflex unloading without hypotension may occur during orthostasis and might produce a sustained effect without obvious changes in systemic AP (16), although perfusion pressure at the level of the peripheral and central baroreflexes is subject to hemostatic unloading due to upright positioning.
Hypoxia, but not hypercapnia, has been shown to produce sustained changes in peroneal microneurographic recording of muscle sympathetic nerve activity (MSNA) (39, 57) that would have continued through the recovery period between gas conditions. MSNA recording was not part of the present study, and we cannot speculate what those results would have been in our subjects. However, sustained elevation of MSNA was produced during a longer hypoxic exposure time (20 vs. 8 min) than used in our experiments (39, 57). Also, these investigators showed that increased MSNA persisted after hypoxia, although VE, leg blood flow, leg vascular resistance, HR, and blood pressure returned to baseline levels within 20 min, as did our ventilation and hemodynamic measurements.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants 1-F30-HL-097380, 1-R21-HL-091948, 1-RO1-HL-074873, and 1-RO1-HL-087803 and by American Heart Association (Northeast Affliate) Grant 0735603T.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank the members of the Department of Pediatrics, especially its Chairman, Dr. Leonard Newman, and the Division of Pediatric Cardiology, especially its Director, Dr. Michael H. Gewitz, for the unflagging support. The authors also acknowledge our intellectual debt to our mentors, Dr. Thomas H. Hintze, Dr. Gabor Kaley, Dr. David Robertson, and Dr. Phillip Low for the constant inspiration and stimulation.
REFERENCES
- 1. Adachi H, Strauss W, Ochi H, Wagner HN., Jr The effect of hypoxia on the regional distribution of cardiac output in the dog. Circ Res 39: 314– 319, 1976 [DOI] [PubMed] [Google Scholar]
- 2. Aviado DM, Jr, Schmidt CF. Reflexes from stretch receptors in blood vessels, heart and lungs. Physiol Rev 35: 247– 300, 1955 [DOI] [PubMed] [Google Scholar]
- 3. Biscoe TJ, Bradley GW, Purves MJ. The relation between carotid body chemoreceptor discharge, carotid sinus pressure and carotid body venous flow. J Physiol 208: 99– 120, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonyhay I, Freeman R. Sympathetic nerve activity in response to hypotensive stress in the postural tachycardia syndrome. Circulation 110: 3193– 3198, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Bouckaert JJ, Heymans C. Carotid sinus reflexes. Influence of central blood-pressure and blood supply on respiratory and vaso-motor centres. J Physiol 79: 49– 66, 1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bruce EN, Cherniack NS. Central chemoreceptors. J Appl Physiol 62: 389– 402, 1987 [DOI] [PubMed] [Google Scholar]
- 7. Brunner MJ, Sussman MS, Greene AS, Kallman CH, Shoukas AA. Carotid sinus baroreceptor reflex control of respiration. Circ Res 51: 624– 636, 1982 [DOI] [PubMed] [Google Scholar]
- 8. Brunner MJ, Wallace A, MacAnespie CL. Interaction of carotid chemoreceptor and baroreceptor reflexes in anesthetized dogs. Am J Physiol Regul Integr Comp Physiol 254: R1– R10, 1988 [DOI] [PubMed] [Google Scholar]
- 9. Cooke WH, Ludwig DA, Hogg PS, Eckberg DL, Convertino VA. Does the menstrual cycle influence the sensitivity of vagally mediated baroreflexes? Clin Sci (Lond) 102: 639– 644, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Cooper VL, Pearson SB, Bowker CM, Elliott MW, Hainsworth R. Interaction of chemoreceptor and baroreceptor reflexes by hypoxia and hypercapnia–a mechanism for promoting hypertension in obstructive sleep apnoea. J Physiol 568: 677– 687, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cunningham DJ, Howson MG, Pickering TG, Sleight P, Petersen ES. The effect of raising arterial blood pressure on ventilation in man. J Physiol 204: 89P, 1969 [PubMed] [Google Scholar]
- 12. Dahan A, DeGoede J, Berkenbosch A, Olievier IC. The influence of oxygen on the ventilatory response to carbon dioxide in man. J Physiol 428: 485– 499, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delius W, Hagbarth KE, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand 84: 65– 81, 1972 [DOI] [PubMed] [Google Scholar]
- 14. Folgering H. The pathophysiology of hyperventilation syndrome. Monaldi Arch Chest Dis 54: 365– 372, 1999 [PubMed] [Google Scholar]
- 15. Fu Q, Okazaki K, Shibata S, Shook RP, Van Gunday TB, Galbreath MM, Reelick MF, Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol 587: 2019– 2031, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fu Q, Shibata S, Hastings JL, Prasad A, Palmer MD, Levine BD. Evidence for unloading arterial baroreceptors during low levels of lower body negative pressure in humans. Am J Physiol Heart Circ Physiol 296: H480– H488, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol 289: R109– R116, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Gabriel M, Seller H. Excitation of expiratory neurones adjacent to the nucleus ambiguus by carotid sinus baroreceptor and trigeminal afferents. Pflügers Arch 313: 1– 10, 1969 [DOI] [PubMed] [Google Scholar]
- 19. Gardner W. Orthostatic increase of respiratory gas exchange in hyperventilation syndrome. Thorax 55: 257– 259, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gilmartin GS, Lynch M, Tamisier R, Weiss JW. Chronic intermittent hypoxia in humans during 28 nights results in blood pressure elevation and increased muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol 299: H925– H931, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Halliwill JR, Minson CT. Effect of hypoxia on arterial baroreflex control of heart rate and muscle sympathetic nerve activity in humans. J Appl Physiol 93: 857– 864, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Heistad D, Abboud FM, Mark AL, Schmid PG. Effect of baroreceptor activity on ventilatory response to chemoreceptor stimulation. J Appl Physiol 39: 411– 416, 1975 [DOI] [PubMed] [Google Scholar]
- 23. Heistad DD, Abboud FM, Mark AL, Schmid PG. Interaction of baroreceptor and chemoreceptor reflexes. Modulation of the chemoreceptor reflex by changes in baroreceptor activity. J Clin Invest 53: 1226– 1236, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heistad DD, Wheeler RC. Effect of acute hypoxia on vascular responsiveness in man. I. Responsiveness to lower body negative pressure and ice on the forehead. II. Responses to norepinephrine and angiotensin. III. Effect of hypoxia and hypocapnia. J Clin Invest 49: 1252– 1265, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heistad DD, Wheeler RC, Mark AL, Schmid PG, Abboud FM. Effects of adrenergic stimulation on ventilation in man. J Clin Invest 51: 1469– 1475, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heymans C, Bouckaert JJ. Sinus caroticus and respiratory reflexes: I. Cerebral blood flow and respiration. Adrenaline apnoea. J Physiol 69: 254– 266, 1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoare CA. Quicksort. Comp J 5: 10– 16, 1962 [Google Scholar]
- 28. Hunt BE, Farquhar WB. Nonlinearities and asymmetries of the human cardiovagal baroreflex. Am J Physiol Regul Integr Comp Physiol 288: R1339– R1346, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Kara T, Narkiewicz K, Somers VK. Chemoreflexes–physiology and clinical implications. Acta Physiol Scand 177: 377– 384, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Kiely DG, Cargill RI, Lipworth BJ. Effects of hypercapnia on hemodynamic, inotropic, lusitropic, and electrophysiologic indices in humans. Chest 109: 1215– 1221, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Lagi A, Cencetti S, Corsoni V, Georgiadis D, Bacalli S. Cerebral vasoconstriction in vasovagal syncope: any link with symptoms? A transcranial Doppler study. Circulation 104: 2694– 2698, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Lahiri S, De Laney RG. Relationship between carotid chemoreceptor activity and ventilation in the cat. Respir Physiol 24: 267– 286, 1975 [DOI] [PubMed] [Google Scholar]
- 33. Lipski J, McAllen RM, Spyer KM. The sinus nerve and baroreceptor input to the medulla of the cat. J Physiol 251: 61– 78, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mancia G. Influence of carotid baroreceptors on vascular responses to carotid chemoreceptor stimulation in the dog. Circ Res 36: 270– 276, 1975 [DOI] [PubMed] [Google Scholar]
- 35. Marquardt DW. A nonlinear algorithm. J Soc Industr Appl Math 11: 431– 441, 1963 [Google Scholar]
- 36. Marshall JM. Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev 74: 543– 594, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Matalon S, Nesarajah MS, Krasney JA, Farhi LE. Effects of acute hypercapnia on the central and peripheral circulation of conscious sheep. J Appl Physiol 54: 803– 808, 1983 [DOI] [PubMed] [Google Scholar]
- 38. Mohan RM, Amara CE, Cunningham DA, Duffin J. Measuring central-chemoreflex sensitivity in man: rebreathing and steady-state methods compared. Respir Physiol 115: 23– 33, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Morgan BJ, Crabtree DC, Palta M, Skatrud JB. Combined hypoxia and hypercapnia evokes long-lasting sympathetic activation in humans. J Appl Physiol 79: 205– 213, 1995 [DOI] [PubMed] [Google Scholar]
- 40. Nattie G, Li A. Multiple central chemoreceptor sites: cell types and function in vivo. Adv Exp Med Biol 605: 343– 347, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Novak V, Spies JM, Novak P, McPhee BR, Rummans TA, Low PA. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke 29: 1876– 1881, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Phillips BA, McConnell JW, Smith MD. The effects of hypoxemia on cardiac output. A dose-response curve. Chest 93: 471– 475, 1988 [DOI] [PubMed] [Google Scholar]
- 43. Richter DW, Seller H. Baroreceptor effects on medullary respiratory neurones of the cat. Brain Res 86: 168– 171, 1975 [DOI] [PubMed] [Google Scholar]
- 44. Rudas L, Crossman AA, Morillo CA, Halliwill JR, Tahvanainen KU, Kuusela TA, Eckberg DL. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. Am J Physiol Heart Circ Physiol 276: H1691– H1698, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Schmidt CF. Carotid sinus reflexes to the respiratory center. Am J Physiol 102: 94– 119, 1932 [Google Scholar]
- 46. Schmidt CF. Cycles in concepts of respiratory control. Arch Int Pharmacodyn Ther 140: 506– 513, 1962 [PubMed] [Google Scholar]
- 47. Schopp RT, Gilfoil TM, Youmans WB. Mechanisms of respiratory stimulation during hemorrhage. Am J Physiol 189: 117– 122, 1957 [DOI] [PubMed] [Google Scholar]
- 48. Shoukas AA, Brunner MJ, Frankle AE, Greene AS, Kallman CH. Carotid sinus baroreceptor reflex control and the role of autoregulation in the systemic and pulmonary arterial pressure-flow relationships of the dog. Circ Res 54: 674– 682, 1984 [DOI] [PubMed] [Google Scholar]
- 49. Smyth HS, Sleight P, Pickering GW. Reflex regulation of arterial pressure during sleep in man. A quantitative method of assessing baroreflex sensitivity. Circ Res 24: 109– 121, 1969 [DOI] [PubMed] [Google Scholar]
- 50. Stewart JM, Medow MS, Cherniack NS, Natelson BH. Postural hypocapnic hyperventilation is associated with enhanced peripheral vasoconstriction in postural tachycardia syndrome with normal supine blood flow. Am J Physiol Heart Circ Physiol 291: H904– H913, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Swanson GD, Bellville JW. Step changes in end-tidal CO2: methods and implications. J Appl Physiol 39: 377– 385, 1975 [DOI] [PubMed] [Google Scholar]
- 52. Thijs RD, van Dijk JG. Hyperventilation in autonomic failure. Int J Clin Pract 61: 695– 696, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Tzeng YC, Larsen PD, Galletly DC. Effects of hypercapnia and hypoxemia on respiratory sinus arrhythmia in conscious humans during spontaneous respiration. Am J Physiol Heart Circ Physiol 292: H2397– H2407, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Valdes V, Mosqueira M, Rey S, Del Rio R, Iturriaga R. Inhibitory effects of NO on carotid body: contribution of neural and endothelial nitric oxide synthase isoforms. Am J Physiol Lung Cell Mol Physiol 284: L57– L68, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Whelan RF, Young IM. The effect of adrenaline and noradrenaline infusions on respiration in man. Br J Pharmacol Chemother 8: 98– 102, 1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Widdicombe JG. Respiratory reflexes. Handbook of Physiology. Respiration. Betesda, MD: Am. Physiol. Soc., 1964, sect. 3, chapt. 24, p. 585– 630 [Google Scholar]
- 57. Xie A, Skatrud JB, Puleo DS, Morgan BJ. Exposure to hypoxia produces long-lasting sympathetic activation in humans. J Appl Physiol 91: 1555– 1562, 2001 [DOI] [PubMed] [Google Scholar]
- 58. Yasuma F, Hayano JI. Impact of acute hypoxia on heart rate and blood pressure variability in conscious dogs. Am J Physiol Heart Circ Physiol 279: H2344– H2349, 2000 [DOI] [PubMed] [Google Scholar]