Abstract
Perturbations in the normal sequence of ventricular activation can create regions of early and late activation, leading to dysynchronous contraction and areas of dyskinesis. Dyskinesis occurs across the left ventricular (LV) wall, and its presence may have important consequences on cardiac structure and function in normal and failing hearts. Acutely, dyskinesis can trigger inflammation and, in the long term (6 wk and above), leads to LV remodeling. The mechanisms that trigger these changes are unknown. To gain further insight, we used a canine model to evaluate transumural changes in myocardial function and inflammation induced by epicardial LV pacing. The results indicate that 4 h of LV suprathreshold pacing resulted in a 30% local loss of endocardial thickening. Assessment of neutrophil infiltration showed a significant approximately fivefold increase in myeloperoxidase activity in the epicardium versus the midwall/endocardium. Matrix metalloproteinase-9 activity increased ∼2 fold in the epicardium and ROS generation increased ∼2.5-fold compared with the midwall/endocardium. To determine the effects that electrical current alone has on these end points, a group of animals was subjected to subthreshold pacing. Significant increases were observed only in epicardial myeloperoxidase levels. Thus, the results indicate that transmural dyskinesis induced by suprathreshold epicardial LV activation triggers a localized epicardial inflammatory response, whereas subthreshold stimulation appears to solely induce the trapping of leucocytes. Suprathreshold pacing also induces a loss of endocardial function. These results may have important implications as to the nature of the mechanisms that trigger the inflammatory response and possibly long-term remodeling in the setting of dysynchrony.
Keywords: cell adhesion molecules
a normal sequence of cardiac activation results in a rapid synchronized contraction of the ventricles. Perturbations in this normal sequence can create regions of early and late activation, leading to a dysynchronous contraction pattern and areas of local dyskinesis (25). Dyskinesis occurs across the left ventricular (LV) wall, and its presence may have important short- and long-term consequences on cardiac structure and function in the normal and failing heart (2). It has been reported that the induction of ventricular dysynchrony in canine hearts leads to LV remodeling (22). Using a chronic model of ventricular dysynchrony induced by epicardial pacing, van Oosterhout et al. (23) demonstrated that early-activated LV regions became significantly thinner, whereas the late-activated region thickened significantly. Patients with ventricular dysynchrony also develop ventricular remodeling (22). However, there are no reports as to the probable causes underlying these events or of the transmural character of the early-activated region LV remodeling responses.
We (8) have previously reported that short-term suprathreshold LV pacing (4 h) significantly increased neutrophil infiltration (i.e., inflammation), as determined by myeloperoxidase (MPO) activity, at the early activated dyskinetic site relative to a remote site. These increases in MPO activity correlated with significant increases in ROS generation, matrix metalloproteinase (MMP), activity and collagen degradation (8). Inflammatory responses correlated with no apparent loss in LV contraction after the 4 h of LV pacing, as determined using uniaxial sonimicrometer crystals placed at the midwall. However, no information is available as to the transmural distribution of inflammatory responses or contractile function when LV pacing is sustained for a period of hours.
In a review (5) of 74 cases of patients with permanent pacing, severe chronic inflammation and scarring were observed at the pacing (i.e., electrode) site under macroscopic and histological examination. These observations led to the development and widespread use of steroid-eluting electrodes for long-term pacing to locally minimize these types of responses (21). In this context, it is also possible that electrical stimulation per se may be sufficient to stimulate inflammatory responses. The use of subthreshold stimuli as a means to stimulate the LV may allow investigators to explore the role that electrical impulses alone have on the development of inflammatory responses.
To gain further insight into the effects of electromechanical ventricular dysynchrony on inflammatory end points, contractile function, and their possible roles in mediating long-term LV remodeling, the following objectives were pursued: 1) evaluate the transmural distribution of epicardial activation-induced inflammatory responses; 2) compare subthreshold versus suprathreshold epicardial activation effects on these end points; and 3) assess progressive changes in transmural LV function in early-activated regions over the course of LV pacing. For this purpose, a canine model of epicardial LV pacing (4 h) was used to determine transmural differences in LV function and inflammatory responses.
METHODS
Animal Experiments
All procedures were approved by the Institutional Animal Care and Use Committee and conformed with published National Institutes of Health guidelines for animal research.
Instrumentation
A total of 23 adult male dogs weighing between 12 and 28 kg were used. For terminal experiments, animals were anesthetized with pentobarbital sodium (25–30 mg/kg iv), intubated, and mechanically ventilated using a Harvard respirator. A continuous intravenous infusion of pentobarbital sodium (0.15–0.25 mg·kg−1·h−1) was used to maintain a surgical plane of anesthesia. The heart was exposed via a medial sternotomy and left thoracotomy at the fifth intercostal space and supported in a pericardial cradle. Pairs of pacing wire (30-gauge copper wire in loops) were sutured to the left or right atrium and on the LV epicardial surface. LV pressure (LVP) was recorded with a pigtail micromanometer catheter (Millar Instruments, Houston, TX) inserted into the left femoral artery and advanced into the LV. Central aortic pressure (AoP) was measured with a fluid-filled catheter placed in the descending aorta. AoP, LVP, and surface ECGs were monitored throughout the study. In animals instrumented for LV function, three columns (i.e., bead array sets) of three to four 0.8-mm-diameter gold beads were inserted across the LV anterior wall between the first and second diagonal branches of the left anterior descending coronary artery (2). After bead set implantation, 1.7-mm-diameter surface gold beads were sewn on the epicardium (Epi) above each column. Gold beads (2 mm diameter) were sutured to the apical dimple (apex bead) and on the epicardium at the bifurcation of the left anterior descending coronary artery and left circumflex coronary artery (base bead) to provide end points for the LV long axis. Animals were positioned in a biplane X-ray system, and synchronous 125-Hz biplane cineradiographic images of bead displacement were digitally acquired with mechanical ventilation suspended at end expiration. LVP, AoP, and ECG were recorded simultaneously. In a subgroup of dogs, animals underwent a thoracotomy 2 wk before the initiation of LV pacing. In this group, animals were anesthetized with intravenous propofol (6 mg/kg), intubated, and mechanically ventilated with isoflurane (0.5–2.5%) and medical oxygen (2 l/min) to maintain a surgical plane of anesthesia. The heart was exposed via a left thoracotomy at the fifth intercostal space. After a pericardial dissection, a transmural bead array was implanted. The chest was closed, and animals were allowed to recover. Two weeks postthoracotomy, animals underwent a terminal experiment as described above.
Experimental Protocol
Atrial pacing was performed using atrial electrodes. Atrioventricular sequencial pacing (referred to as LV pacing) was accomplished using atrial and LV electrodes with an atrioventricular delay of 40–60 ms. All pacing protocols were conducted using a square-wave, constant-voltage electronic stimulator (Model SD9 Square Pulse Stimulator, Grass Instruments, Quincy, MA; or AV pacing system model 5311B, Medtronics, Minneapolis, MN) at a frequency 10–20% above the baseline heart rate to suppress native sinus rhythm. Stimulation parameters (voltage: 10% above or below threshold and duration: 8 ms) were kept constant in each animal. Zetabradine (0.5 mg/kg) was administered to dogs with heart rates of >110 beats/min to reduce the intrinsic heart rate. During suprathreshold LV pacing, electromechanical dyskinesis was verified by the widening of the QRS complex and attenuation of −dP/dt. The recordings of physiological parameters in the nonpaced group took place immediately after instrumentation (control atrial), at 4 h, and at 4 h and 5 min (late atrial). In the sub- and suprathreshold paced groups, recordings took place immediately after instrumentation with atrial pacing (control atrial), at 4 h after continuous LV pacing, and then immediately after the cessation of LV pacing (i.e., during atrial pacing = late atrial). Additional hourly recordings took place in all animals between the beginning atrial and 4-h timepoints. Data are presented only for the initial and final time points.
Euthanization Protocol and Tissue Collection
After 4 h of atrial or LV pacing, animals were euthanized with pentobarbital sodium (200 mg/kg), and hearts were rapidly excised and cooled in iced saline. Myocardial samples of ∼1.5 cm2 and the full transmural thickness were taken from the bead implantation site and two adjacent regions (lateral and basal to the bead set) as well as from the posterior wall. To examine transmural differences in inflammatory responses, each of these four samples was rapidly semifrozen until firm and sectioned into 1-mm-thick slices from the Epi to the endocardium (Endo; typically 6–9 slices/region), and samples were snap frozen in liquid nitrogen and stored at −80°C for immunohistochemistry and biochemical assays. For later analysis, these sections were combined into three approximately equal groups of two to three slices each [Epi, midwall (Mid), and Endo].
Ventricular Function
The three-dimensional (3-D) coordinates of the implanted gold beads were obtained from the biplane radiographic data corrected for magnification and spherical distortion from a single entire cardiac cycle selected at beginning atrial pacing, 4 h of LV pacing, and late atrial in each animal (1). In brief, with this data on bead position, continuous, nonhomogenous transmural distributions of 3-D finite strains were computed (6). Six independent finite strains [circumferential strain (E11), longitudinal strain (E22), radial strain (E33), circumferential-longitudinal shear (E12), longitudinal-radial shear (E23), and circumferential-radial shear (E13)] were computed in the local cardiac coordinate axis (circumferential, longitudinal, and radial, respectively) system (X1, X2, and X3). The three normal strain components reflect myocardial stretch or shortening along the circumferential (E11), longitudinal (E22), or radial (E33) cardiac axes (1). Finite strains were calculated for each frame (125 frames/s) as a deformed configuration with end diastole as the reference state at three wall depths: 25% (sub-Epi), 50% (Mid), and 75% (sub-Endo) wall depth from the epicardial surface. End diastole was defined as the time of the peak of the ECG R-wave for atrial pacing and the ventricular pacing artifact for the LV epicardial pacing. We chose the V-spike rather than the peak of the R-wave as the reference state for LV epicardial pacing because the former reflects the timing of the activation of the pacing site, as opposed to the latter, which represents the timing of activation of the whole ventricle (2). End systole was derived from the dichrotic notch of the central AoP.
Immunohistochemistry
Tissue samples were snap frozen in cryoembedding media (OCT) and stored at −80°C. Using a cryotome cryostat, the frozen tissue was sectioned into 7-μm sections. A streptavidin-alkaline phosphatase-based protocol with Vector red (Vector Labs) was used for immunohistochemically staining our tissue (26). Briefly, blocking was performed with 10% serum (from the animal source of the secondary antibody to be used) in 0.1% bovine serum albumin and Tris-buffered saline. Primary antibodies were subsequently added and incubated for 60 min at room teperature. Preimmune sera of the animal species in which the primary antibody was developed were used as negative controls. Biotinylated IgG was used as a secondary antibody, and incubation was carried out for 60 min. This step was followed by an incubation with alkaline phosphatase-conjugated streptavidin for 30 min.
MPO Activity
Tissue samples were homogenized in ice-cold buffer (50 mM KH2PO4 and 0.5% hexadecyltrimethylammonium bromide; pH 6.0). Homogenates were incubated on ice for 30 min and centrifuged at 4°C for 5 min. After centrifugation, supernatants were reacted with 0.8 mM tetramethylbenzidine (Sigma, St. Louis, MO) and 0.006% H2O2 in 50 mM KH2PO4. Kinetic absorbance measurements of MPO activity were immediately monitored at 655 nm (readings every 40 s for ∼20 min). Substrate cleavage rates were determined from the linear regions of the kinetic curve. Data were normalized to protein concentrations determined a BCA protein assay kit (Pierce, Rockford, IL).
Glutathione (GSSG/GSH) Assay
GSH and GSSG levels were determined as previously described (18). Briefly, tissue samples were homogenized in ice-cold homogenization buffer [154 mM KCl, 5 mM diethylenetriaminepentaacetic acid (DPTA), and 0.1 M potassium phosphate; pH 6.8]. After centrifugation, an aliquot was removed for protein determination using the BCA protein assay kit. Immediately after an aliquot was taken, one volume of cold acid buffer (40 mM HCl, 10 mM DPTA, 20 mM ascorbic acid, and 10% trichloroacetic acid) was added to the homogenate. The suspension was centrifuged, and the resulting supernatant solution was centrifuged through a 0.45-m microcentrifuge filter (Millipore, Bedford, MA). GSH and GSSG levels were determined using the fluorophore o-phtaladehyde, and the ratio was normalized to protein.
Gelatin Zymography
Samples were homogenized in 10 mM HEPES (pH 7.5), 150 mM NaCl, 0.2 mM EDTA, and 25% glycerol with protease inhibitor cocktail (Sigma). Samples (10 μg protein) were loaded onto a 10% SDS-PAGE gel substituted with 0.1% gelatin and then stained with 0.5% Coomassie blue. MMP-2 and MMP-9 protein standards were loaded on all gels. Bands of MMP-2 and/or MMP-9 gelatinolytic activity were digitally quantified with ImageJ and normalized to standards.
Study Groups
Three following groups of animals were studied.
Nonpaced group.
This group consisted of a total of nine animals, four of which were instrumented with bead sets.
Subthreshold group.
A total of five animals underwent 4 h of subthreshold (10% below threshold) LV pacing in the absence of bead sets.
Suprathreshold group.
A total of nine dogs underwent 4 h of suprathreshold (10% above threshold) LV pacing. Three of the animals had no bead sets. Of the six animals with bead sets, four were implanted acutely and two were implanted chronically.
Preliminary Analysis of Inflammatory End Points
Initial evaluations of inflammatory parameters were performed to compare responses in basal versus lateral regions. Results indicated no statistical differences in any of the measured inflammatory parameters; thus, biochemical results from these two regions were averaged and referred to as “areas around the bead set.” Similar comparisons were made within each study group comparing transmural responses of inflammatory parameters. Results indicated no statistical differences between Mid and Endo samples in any of the measured inflammatory parameters; thus, results were combined and referred to as Mid/Endo and compared versus Epi.
Initial experiments (n = 4) were performed to investigate the effects that instrumentation (i.e., acute implantation of bead set arrays) had on suprathreshold pacing-induced LV inflammation between the bead set site, areas around the bead set. and the posterior wall (control region). Epi MPO values were 0.6 ± 0.5 (bead site), 2.0 ± 1.04 (areas around), and 0.75 ± 0.3 (posterior wall). Thus, the introduction of bead arrays into the myocardium prevented the “injured” region from developing an LV pacing-induced inflammatory response. Thus, we only present data from areas around the bead set and posterior wall. Altogether, the results from the above-discussed analyses allowed us to group data as presented in the results.
Data Analyses and Statistics
Results are expressed as means ± SE. Comparisons between means were analyzed, as appropriate, by Student's t-test, one-way ANOVA, or two-way ANOVA followed by Bonferroni post hoc analysis. P values of <0.05 were considered statistically significant.
RESULTS
Inflammatory Tissue Responses
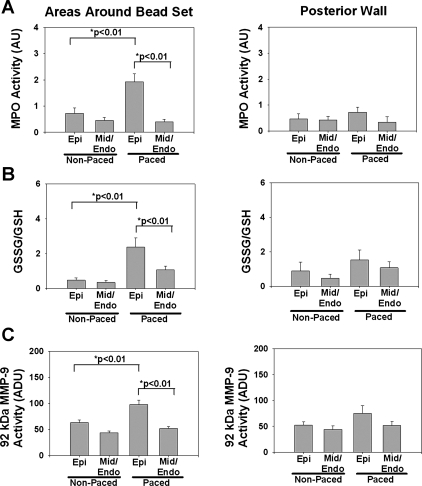
Figure 1 shows representative immunohistochemical sections obtained from the Epi in the areas around the bead set of an animal subjected to 4 h of LV superthreshold pacing. The results demonstrate more ICAM-1, MMP-9, and CD18 staining in the areas around the bead set versus the posterior wall, which served as a control area. In the suprathreshold paced group, in areas around the bead set, there was a significant approximately fivefold upregulation of MPO activity in the Epi compared with the Mid/Endo (P < 0.01, Epi vs. Mid/Endo; Fig. 2A). Epi MPO activity in the suprathreshold group compared with the nonpaced group increased approximately threefold (P < 0.01, suprathreshold vs. nonpaced; Fig. 2A). Figure 2B shows oxidative stress results from the different groups. Results are shown as the ratio of GSSG to GSH, which is a measure of total tissue oxidative stress. Oxidative stress in the suprathreshold paced group increased in the Epi compared with the Mid/Endo of areas around the bead set by ∼2.5-fold (P < 0.01, Epi vs. Mid/Endo; Fig. 2B) and ∼6-fold in Epi suprathreshold versus nonpaced groups (P < 0.01). Gelatin zymography of tissue homogenates from control or suprathreshold animals only revealed bands corresponding to 92-kDa MMP-9 and 72-kDa MMP-2; 86-kDa MMP-9 was not visible. As determined by densitometric analysis, no notable differences were identified in 72-kDa MMP-2 levels. Densitometric analysis indicated increased 92-kDa MMP-9 activity in the areas around the bead set of the suprathreshold paced group Epi of ∼2-fold (P < 0.01, Epi vs. Mid/Endo; Fig. 2C) and ∼1.7-fold in Epi suprathreshold versus nonpaced groups (P < 0.01). No significant transmural changes in suprathreshold or nonpaced groups were observed in inflammatory parameters in the posterior wall.
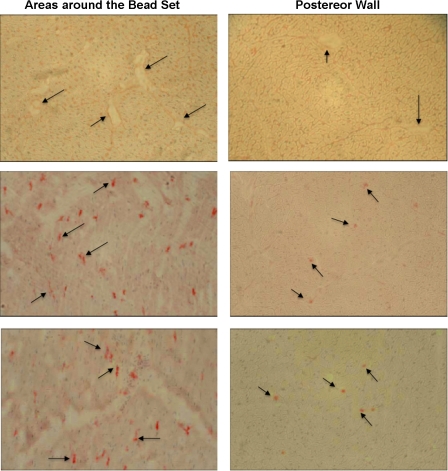
Fig. 1.
Representative immunohistochemical images (×20) obtained from epicardial cross-sections of an animal subjected to 4 h of suprathreshold left ventricular (LV) pacing. Tissues were taken from areas around the bead set and from the posterior wall epicardium, which served as the control region. Top images show antibodies against ICAM-1, middle images show CD-18 (anti-polymorphonuclear neutrophils), and bottom images show anti-matrix metalloproteinase (MMP)-9. Arrows denote positive Vector red immunoreactivity for the antigen of interest.
Fig. 2.
Acute myocardial inflammatory responses induced by electromechanical dysynchrony in nonpaced (n = 7) and paced (n = 8) hearts in epicardial (Epi) and midwall (Mid)/endocardial (Endo) regions. A: myeloperoxidase (MPO) activity. B: oxidized stress (GSSG/GSH) levels. C: 92-kDa MMP-9 activity in areas around the bead set and posterior wall of nonpaced or LV paced animals. Values are means ± SE.
Hemodynamics
There were no significant differences in heart rate, LV systolic pressure, LV end-diastolic pressure, or ±dP/dt between study groups at the three time points measured in any group. However, peak +dP/dt was significantly depressed at 4 h of LV pacing and late atrial compared with early pacing and control atrial pacing (P < 0.05; Table 1).
Table 1.
Hemodynamic values for the nonpaced, subthreshold, and suprathreshold groups
| Control Atrial | 4 h of LV Pacing | Late Atrial | |
|---|---|---|---|
| Nonpaced group | |||
| HR, beats/min | 120 ± 5 | N/A | 121 + 5 |
| LVSP, mmHg | 137 ± 6 | N/A | 121 ± 6 |
| LVEDP, mmHg | 13 ± 2 | N/A | 10 ± 3 |
| +dP/dt, mmHg/s | 2,632 ± 354 | N/A | 2,617 ± 191 |
| −dP/dt, mmHg/s | −2,524.2 ± 234 | N/A | −2,410 ± 283 |
| Subthreshold group | |||
| HR, beats/min | 116 ± 2 | 115 ± 2 | 115 ± 2 |
| LVSP, mmHg | 122 ± 6 | 109 ± 5 | 108 ± 6 |
| LVEDP, mmHg | 8 ± 2 | 7 ± 1 | 6 ± 2 |
| +dP/dt, mmHg/s | 3,457 ± 481 | 2,393 ± 252 | 2,929 ± 241 |
| −dP/dt, mmHg/s | −3,903 ± 796 | −2,815 ± 267 | −2,545 ± 259 |
| Suprathreshold group | |||
| HR, beats/min | 108 ± 5 | 114 ± 3 | 113 ± 3 |
| LVSP, mmHg | 129 ± 6 | 118 ± 4 | 127 ± 4 |
| LVEDP, mmHg | 9 ± 1 | 8 ± 1 | 8 ± 1 |
| +dP/dt, mmHg/s | 2,538 ± 403 | 2,013 ± 220* | 2,030 ± 281* |
| −dP/dt, mmHg/s | −2,640 ± 322 | −2,173 ± 176 | −2,334 ± 146 |
Values are means ± SE; n = 9 for the nonpaced group, 5 for the subthreshold group, and 9 for the suprathreshold group. HR, heart rate; LVSP, left ventricular (LV) systolic pressure; LVEDP, LV end-diastolic pressure; +dP/dt, increase of LV pressure; −dP/dt, decrease of LV pressure; N/A, not applicable.
P < 0.05 vs. the control atrial period.
Effects of Suprathreshold Pacing on Transmural LV Function
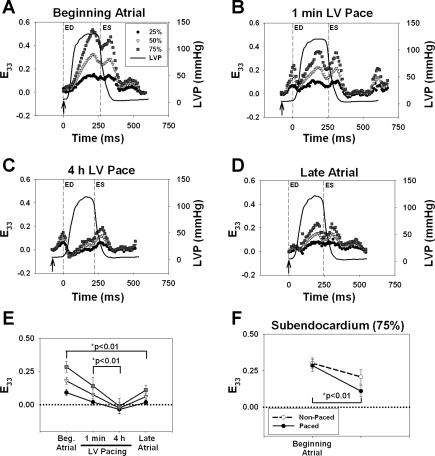
Figure 3, A–D, shows a representative time course of wall thickening (E33) in one contraction at 25%, 50%, and 75% depth in an animal subjected to LV suprathreshold pacing. Arrows indicate peak QRS in atrial runs and the time of stimulus in the paced runs. During the control atrial period (Fig. 3A), E33 demonstrated a transmural gradient thickening pattern with peak E33 strains near end systole. In the suprathreshold group at 1 min of LV pacing (Fig. 3B), E33 demonstrated early wall thickening during diastole. As shown in Fig. 3C, at 4 h of LV pacing compared with late atrial pacing, E33 also demonstrated early wall thickening during diastole, but the degree of wall thickening decreased from 1 min of LV pacing to 4 h of LV pacing at all depths. As shown in Fig. 3D, during late atrial pacing, the early activated site partly recovered a normal wall thickening pattern. These results contrast with those observed in the bead instrumented nonpaced animals (n = 4), where wall thickening patterns observed during the beginning atrial period remained essentially unaltered as a function of time (data not shown).
Fig. 3.
Representative time course of wall thickening (E33) at the early activated site (A–D). Sub-Epi, Mid, and sub-Endo represent 25%, 50%, and 75% wall depth. LV pressures (LVP) are included as well as the times for end diastole (ED) and end systole (ES), as shown by the dashed lines. The reference frame for strain calculation was ED for atrial pacing or the timing of the stimulus artifact for LV pacing (denoted by arrows). A: beginning atrial. B: 1 min of LV pacing. C: 4 h of LV pacing. D: late atrial. E: end-systolic E33 strains in paced animals at beginning atrial, 1 min of LV pacing, 4 h of LV pacing, and late atrial periods. Values are means ± SE; n = 6. Statistical differences were only observed in subendocardial E33 strains (■). F: Endo end-systolic E33 strains in nonpaced (n = 4) and paced (n = 6) animals at the beginning atrial and late atrial periods. Values are means ± SE. Statistical differences were only observed in sub-Endo E33 strains in paced animals (●).
Figure 3E shows averaged end-systolic E33 strains from all suprathreshold animals (n = 6). All other strain components are shown in Table 2. The data demonstrated a significant loss of end-systolic E33 from 1 min to 4 h of LV pacing. Loss of wall thickening was more severe in the Endo than in the Epi (P < 0.05). The results also showed limited recovery of E33 during the late atrial time point (P < 0.02; Fig. 3E). Figure 3F shows that Endo loss of E33 was largely unrelated to the effects of instrumentation and 4 h of anesthesia, as there was no significant difference between control atrial and late atrial pacing in nonpaced animals (dashed line).
Table 2.
Cardiac strain data for the nonpaced and paced groups
| Beginning Atrial | 1 min of LV Pacing | 4 h of LV Pacing | Late Atrial | |
|---|---|---|---|---|
| Nonpaced group | ||||
| 25% | ||||
| E11 | −0.039 ± 0.012 | N/A | N/A | 0.056 ± 0.035 |
| E22 | −0.050 ± 0.004 | N/A | N/A | −0.024 ± 0.005 |
| E33 | 0.047 ± 0.031 | N/A | N/A | −0.030 ± 0.032 |
| E12 | −0.016 ± 0.025 | N/A | N/A | 0.013 ± 0.027 |
| E23 | −0.028 ± 0.023 | N/A | N/A | −0.015 ± 0.018 |
| E13 | 0.021 ± 0.019 | N/A | N/A | 0.030 ± 0.007 |
| 50% | ||||
| E11 | −0.099 ± 0.019 | N/A | N/A | 0.026 ± 0.033 |
| E22 | −0.078 ± 0.006 | N/A | N/A | −0.046 ± 0.008 |
| E33 | 0.163 ± 0.029 | N/A | N/A | 0.078 ± 0.010 |
| E12 | −0.016 ± 0.025 | N/A | N/A | 0.016 ± 0.025 |
| E23 | −0.007 ± 0.020 | N/A | N/A | −0.008 ± 0.015 |
| E13 | 0.024 ± 0.032 | N/A | N/A | 0.040 ± 0.025 |
| 75% | ||||
| E11 | −0.160 ± 0.027 | N/A | N/A | −0.052 ± 0.021 |
| E22 | −0.073 ± 0.009 | N/A | N/A | −0.049 ± 0.017 |
| E33 | 0.300 ± 0.037 | N/A | N/A | 0.207 ± 0.050 |
| E12 | −0.007 ± 0.020 | N/A | N/A | 0.014 ± 0.013 |
| E23 | 0.038 ± 0.029 | N/A | N/A | 0.006 ± 0.012 |
| E13 | 0.044 ± 0.041 | N/A | N/A | 0.048 ± 0.036 |
| Paced group | ||||
| 25% | ||||
| E11 | −0.038 ± 0.022 | 0.020 ± 0.0244 | 0.035 ± 0.025* | 0.012 ± 0.023 |
| E22 | −0.050 ± 0.018 | −0.019 ± 0.020 | −0.003 ± 0.011 | −0.022 ± 0.012 |
| E33 | 0.091 ± 0.024 | 0.020 ± 0.024 | −0.036 ± 0.030* | 0.016 ± 0.020† |
| E12 | 0.011 ± 0.016 | 0.002 ± 0.009 | −0.017 ± 0.014 | 0.002 ± 0.009 |
| E23 | 0.012 ± 0.019 | −0.025 ± 0.013 | −0.032 ± 0.016 | −0.005 ± 0.011 |
| E13 | −0.002 ± 0.008 | −0.002 ± 0.011 | 0.003 ± 0.015 | 0.006 ± 0.011 |
| 50% | ||||
| E11 | −0.082 ± 0.020 | −0.011 ± 0.029 | 0.029 ± 0.032* | −0.016 ± 0.026 |
| E22 | −0.085 ± 0.017 | −0.037 ± 0.024 | −0.008 ± 0.015 | −0.034 ± 0.010 |
| E33 | 0.179 ± 0.025 | 0.075 ± 0.044 | −0.026 ± 0.044* | 0.060 ± 0.027 |
| E12 | 0.009 ± 0.015 | 0.005 ± 0.008 | −0.011 ± 0.011 | 0.002 ± 0.007† |
| E23 | 0.033 ± 0.018 | −0.008 ± 0.015 | −0.033 ± 0.016 | 0.010 ± 0.013 |
| E13 | 0.008 ± 0.020 | 0.003 ± 0.009 | 0.009 ± 0.020 | 0.020 ± 0.018 |
| 75% | ||||
| E11 | −0.142 ± 0.011 | −0.059 ± 0.030 | 0.011 ± 0.035* | −0.064 ± 0.020 |
| E22 | −0.097 ± 0.010 | −0.046 ± 0.024 | −0.005 ± 0.014 | −0.038 ± 0.004 |
| E33 | 0.284 ± 0.040 | 0.141 ± 0.064 | −0.013 ± 0.058* | 0.109 ± 0.038† |
| E12 | 0.002 ± 0.013 | 0.008 ± 0.008 | 0.003 ± 0.006 | 0.001 ± 0.011 |
| E23 | 0.054 ± 0.028 | 0.003 ± 0.023 | −0.037 ± 0.020 | 0.024 ± 0.025 |
| E13 | 0.005 ± 0.040 | 0.006 ± 0.017 | 0.018 ± 0.031 | 0.029 ± 0.031 |
Values are means ± SE; n = 4 for the nonpaced group and 6 for the paced group. Shown are averaged three-dimensional end-systolic strains for nonpaced and paced animals. 25%, subepicardium; 50%, midwall; 75%, subendocardium; E11, circumferential strain; E22, longitudinal strain; E33, radial strain; E12, circumferentrial-longitudinal shear strain; E23, longitudinal-radial shear strain; E13, circumferential-radial shear strain.
P < 0.02 vs. the 1 min of LV pacing period;
P < 0.01 vs. the beginning atrial period.
Suprathreshold Versus Subthreshold Pacing
Table 3 shows the QRS duration during atrial, subthreshold, and suprathreshold pacing. There was no significant change in QRS duration during subthreshold pacing, whereas suprathreshold pacing significantly widened the QRS complex (P < 0.05). To determine if there was evidence for mechanical activation in subthreshold groups, the displacement of the two electrodes during the cardiac cycle (i.e., uniaxial shortening) was computed from X-ray images acquired during the cardiac cycle. The data demonstrate that during atrial pacing, no change in electrode displacement was observed between the time of end diastole to peak +dP/dt (15.9–15.9 mm). Suprathreshold pacing showed a decrease in electrode displacement (16.2–15.5 mm), which was associated with early activation. Subthreshold pacing produced a pattern similar to atrial pacing, in which there was no change in electrode displacement (15.6–15.7 mm). Thus, these results demonstrate that subthreshold pacing did not produce detectable shortening during the cardiac cycle.
Table 3.
QRS duration during atrial, subthreshold, and suprathreshold runs
Values are means ± SE.
P < 0.05 vs. the suprathreshold run.
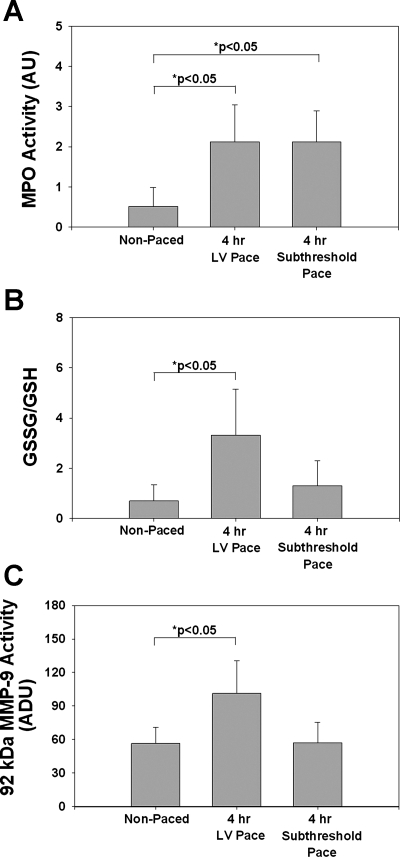
The data from subthreshold animals indicated significant increases in MPO activity compared with nonpaced animals (2.12 ± 0.34 vs. 0.52 ± 0.21 arbitrary density units, P < 0.05; Fig. 4A). Interestingly, there were no significant differences in oxidative stress (Fig. 4B), 92-kDa MMP-9 activity (Fig. 4C), and 72-kDa MMP-2 activity (data not shown). These results suggest that electrical stimulation of the myocardium initiates the recruitment of inflammatory cells to the site of pacing but that local dyskinesis is needed to trigger the downstream inflammatory responses.
Fig. 4.
Acute myocardial inflammatory responses in nonpaced (n = 9), suprathreshold (n = 9), and subthreshold (n = 5) hearts. A: MPO activity. B: GSSG/GSH levels. C: 92-kDa MMP-9 activity in areas around the bead set. Values are means ± SE.
DISCUSSION
Previous studies have shown that Epi activation leads to an acute local inflammatory response and that with chronic Epi activation, there is wall thinning in early activated areas. The unique results of the present study indicate that despite the presence of transmural dyskinesis induced by epicardial LV activation, the local inflammatory response is limited to the Epi. Moreover, subthreshold stimulation induces Epi increases in MPO but not in tissue oxidative stress or MMP-9 activity, implying that subthreshold currents induce epicardial leucocyte trapping but not activation. Moreover, and perhaps more importantly, suprathreshold pacing of only 4 h of duration was associated with a loss of endocardial function, which does not immediately recover.
A major finding of the present study is that the inflammatory response was localized to the Epi (outer 1/3) layer of the LV wall. The Epi localization of the inflammatory response has important implications regarding its mechanistic origins. Since changes in local deformation at early activated sites are transmural (2), this virtually eliminates abnormal local deformation as the direct cause of the inflammatory response. The Epi inflammatory results are similar to our previous study, in that there were two- to threefold increases in neutrophil infiltration at the early activated site observed in transmural tissue samples and in tissue oxidative stress. However, increases in 92-kDa MMP-9 activity were more pronounced in our previous study (∼40-fold) compared with the present study (∼2-fold). The differences likely arise from variations used in the implementation of the zymography technique and the large number of samples examined. A recent study (12) reported on increases in MMP-9 activity of ∼0.6-fold (60%) in pig hearts undergoing 60 min of LV pacing followed by 2 h of atrial pacing. Thus, the magnitudes of increases in pig myocardium MMP-9 are more in line with those reported by this study.
Possible mechanisms that may explain the inflammatory response and its limitation to the Epi include dysynchrony-induced changes in blood flow patterns triggering the upregulation of cell adhesion molecules (CAMs) and myocardial stretch-induced release of ANG II. Our results showed an increase in ICAM-1 expression (Fig. 1) in the Epi of the areas around the bead set in animals that underwent 4 h of suprathreshold pacing. Alterations in Epi microvascular flow patterns with LV pacing may induce this expression of CAMs and, thus, trigger inflammation. The effects that mechanical forces exert on endothelial cell (EC) CAM gene expression and protein production have gained vast attention due to its relationship with atherogenesis (14, 16, 17). It has been shown that endothelial CAM expression (e.g., ICAM-1 and P-selectin) can be modulated by mechanical stimuli, which are recognized through “stress-sensitive promoter elements” (11). Hemodynamic forces, such as fluid shear stress, are known to regulate vascular wall structure/function, including EC cell shape, nitric oxide and prostacycin production, EC proliferation, and CAM expression (3, 4, 7, 19). Studies (17, 19) have demonstrated that exposure of ECs to “normal” laminar flow patterns produce low levels of CAMs and enhance the production of antiadhesive factors. However, when ECs are exposed to altered patterns of flow, CAM expression/production significantly increases (7, 19). This perturbed flow-induced increase in CAM expression/production can then become the trigger for inflammation. However, this response would need to be exclusive to the Epi layer so as to become a viable explanation. Perhaps early contraction of the Epi induces a reversal of the normal Endo-to-Epi venous flow in small vessels, and this phase reversal is enough to stimulate the inflammatory response in the Epi. Although there is no evidence for altered microvascular flows, this mechanism remains the most plausible explanation for the Epi inflammatory changes observed in the present study.
T-wave inversion and ST segment depression after periods of ventricular stimulation were often apparent in our experiments. In the suprathreshold group, T-waves recorded after 4 h of ventricular pacing were either inverted or had a smaller amplitude (vs. those recorded before pacing). T-wave changes after ventricular pacing have been studied for many years (13), and there is insight into their molecular triggers. In brief, ANG II release and the secondary generation of ROS (i.e., proinflammatory events) at the site of ventricular pacing appears associated with short-term memory responses (13). However, it is not clear whether the electrophysiological effects are due to changes localized to the Epi in early activated areas where inflammation is observed. Indeed, it seems unlikely that local stretch is responsible for the release of ANG II given that in early-activated areas, there is early shortening and not stretch. Furthermore, there is substantial prestretch in late-activated areas (i.e., posterior wall), which are more likely sites for the molecular changes observed in cardiac memory studies. Thus, it seems likely that the inflammatory response and electrophysiological changes are caused by Epi activation via alternate mechanisms.
It is also possible that the stimulus to pacing-induced inflammation is indeed transmural (e.g., dyskinesis) but that the Endo is protected from this effect. It has been demonstrated that Epi pacing induces the reversal of the transmural mechanical activation sequence and depresses sheet extension and wall thickening at end systole (2). It can be speculated that the reversal of the mechanical activation sequence can lead to reduced Endo perfusion. Goto et al. (9) showed that cardiac contraction predominantly affects sub-Endo vessels and impedes sub-Endo more than sub-Epi flow (9). Thus, during early contraction, Endo blood flow may be further impeded, leading to brief periods of flow-function imbalance. Thus, the Endo may be spared the inflammatory response by undergoing repeated episodes of “preconditioning.” Indeed, Vanagt et al. (24) demonstrated that repeated episodes of LV pacing can precondition the myocardium and protect it from ischemia. The absence of inflammatory responses in areas mechanically injured by instrumentation supports the possibility that inflammation may be suppressed.
The results from this study indicate that 4 h of suprathreshold LV pacing leads to a progressive loss of Endo systolic wall thickening as a function of time. The loss was still evident immediately after the return to atrial pacing. Loss of wall thickening was unrelated to the effects of surgical instrumentation or implantation of columns of beads since no differences were observed in animals without or with chronically implanted beads. This result is in contrast with our previous report (8), where no significant changes in Mid uniaxial contraction occurred after LV pacing. However, the uniaxial in-plane measurements from the previous study did not reflect wall thickening, which was the only functional parameter adversely affected in the present study.
There are several possibilities that may explain the loss of function. A mechanism that can account for loss of contractile function relates to increases in MMP activity and the resulting degradation of collagen (8, 12). Damage to the extracellular matrix superstructure can lead to myocyte slippage and loss of contractile function (8, 20). However, the inflammatory response was limited to the Epi, whereas the loss of function was in the Endo, making a cause-and-effect scenario unlikely. There is evidence that metabolism and blood flow are altered in chronically early-activated regions (15). Prinzen and Reneman (15) proposed that the reduction of blood flow is directly proportional to reduced local work in early-activated regions. Their study (15) demonstrated a reduction in blood flow of ∼20%. However, currently, no results are available on the transmural distribution of blood flow in early-activated areas. In another study, the reduction in regional work estimated from the use of a mathematical model of ventricular contraction was found to be proportional to the reduction in blood flow (10). Thus, the authors concluded that there was not a mismatch between blood flow and function in early-activated regions. Although we did not measure blood flow, this conclusion is not supported by our findings on the progressive decrease in Endo function during LV pacing and, in particular, by the persistent depression after the return to normal activation. There remains the possibility that a loss in the stability of the experimental preparation over time may explain some of the loss of Endo E33.
The finding that subthreshold stimulation is sufficient to induce only Epi increases in MPO was not expected and may be the determining factor in the Epi localization of the inflammatory response. This result suggests that electrical stimuli may be sufficient to upregulate CAM and adhere inflammatory cells, such as neutrophils, but not to lead to their activation and degranulation. Thus, the effects of suprathreshold stimulation indicate that dyskinesis may be necessary to then trigger a full inflammatory response beyond that initiated by subthreshold electrical stimulation. Alternatively, the use of a greater electrical current during suprathreshold stimulation may also be sufficient to trigger the degranulation of neutrophils. This hypothesis is supported by the results of Mukherjee et al. (12), where 60 min of LV pacing in the presence of dyskinesis was sufficient to trigger MMP activation and collagen degradation.
If acute inflammatory responses are associated with long-term structural changes in the remodeled LV, it is reasonable to speculate that the remodeling of the LV is to differ transmurally. A published study (23) characterized the thinning of the LV wall in early-activated regions. However, no studies have examined the transmural distribution of the remodeling responses. If the inflammatory events were initially responsible for localized remodeling, then long-term studies assessing the transmural distribution of LV remodeling would predict that thinning of the LV wall would be mostly localized to the outer one-third of the chamber. These issues warrant the pursuit of long-term studies.
In conclusion, our results demonstrate that subthreshold LV pacing is sufficient to induce the trapping of neutrophils. However, in the presence of dyskinesis (as triggered by suprathreshold stimulation), neutrophil trapping is accompanied by an inflammatory response at early-activated sites. Suprathreshold pacing also triggers the loss of Endo function. The mechanisms behind these events and their relationship with long-term remodeling await further investigation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant Hl-43617 (to F. J. Villarreal). Predoctoral support for K. G. Yamazaki was provided by NHLBI Training Grant T32-HL-107444.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors are grateful to Maraliz Fischler-Barraza, Maria Rivas, and Irina Ellrott for technical assistance. The authors are also thankful to Prof. J. Omens for help with the experimental design and data analysis.
Footnotes
1 Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1. Ashikaga H, Criscione JC, Omens JH, Covell JW, Ingels NB., Jr Transmural left ventricular mechanics underlying torsional recoil during relaxation. Am J Physiol Heart Circ Physiol 286: H640– H647, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ashikaga H, Omens JH, Ingels NB, Jr, Covell JW. Transmural mechanics at left ventricular epicardial pacing site. Am J Physiol Heart Circ Physiol 286: H2401– H2407, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bongrazio M, Baumann C, Zakrzewicz A, Pries AR, Gaehtgens P. Evidence for modulation of genes involved in vascular adaptation by prolonged exposure of endothelial cells to shear stress. Cardiovasc Res 47: 384– 393, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Braddock M, Schwachtgen JL, Houston P, Dickson MC, Lee MJ, Campbell CJ. Fluid shear stress modulation of gene expression in endothelial cells. News Physiol Sci 13: 241– 246, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Chatelain P, Adamec R, Cox JN. Morphological changes in human myocardium during permanent pacing. Virchows Arch 407: 43– 57, 1985 [DOI] [PubMed] [Google Scholar]
- 6. Costa KD, Takayama Y, McCulloch AD, Covell JW. Laminar fiber architecture and three-dimensional systolic mechanics in canine ventricular myocardium. Am J Physiol Heart Circ Physiol 276: H595– H607, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Dancu MB, Berardi DE, Vanden Heuvel JP, Tarbell JM. Asynchronous shear stress and circumferential strain reduces endothelial NO synthase and cyclooxygenase-2 but induces endothelin-1 gene expression in endothelial cells. Arterioscler Thromb Vasc Biol 24: 2088– 2094, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Garcia RA, Brown KL, Pavelec RS, Go KV, Covell JW, Villarreal FJ. Abnormal cardiac wall motion and early matrix metalloproteinase activity. Am J Physiol Heart Circ Physiol 288: H1080– H1087, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Goto M, Flynn AE, Doucette JW, Jansen CM, Stork MM, Coggins DL, Muehrcke DD, Husseini WK, Hoffman JI. Cardiac contraction affects deep myocardial vessels predominantly. Am J Physiol Heart Circ Physiol 261: H1417– H1429, 1991 [DOI] [PubMed] [Google Scholar]
- 10. Kerckhoffs RC, McCulloch AD, Omens JH, Mulligan LJ. Effects of biventricular pacing and scar size in a computational model of the failing heart with left bundle branch block. Med Image Anal 13: 362– 369, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Methe H, Balcells M, Alegret Mdel C, Santacana M, Molins B, Hamik A, Jain MK, Edelman ER. Vascular bed origin dictates flow pattern regulation of endothelial adhesion molecule expression. Am J Physiol Heart Circ Physiol 292: H2167– H2175, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Mukherjee R, Zavadzkas JA, Rivers WT, McLean JE, Chang EI, Bouges S, Matthews RG, Koval CN, Stroud RE, Spinale FG. Short-term disruption in regional left ventricular electrical conduction patterns increases interstitial matrix metalloproteinase activity. Am J Physiol Heart Circ Physiol 299: H217– H224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ozgen N, Rosen MR. Cardiac memory: a work in progress. Heart Rhythm 6: 564– 570, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Papadaki M, Eskin SG. Effects of fluid shear stress on gene regulation of vascular cells. Biotechnol Prog 13: 209– 221, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Prinzen FW, Augustijn CH, Arts T, Allessie MA, Reneman RS. Redistribution of myocardial fiber strain and blood flow by asynchronous activation. Am J Physiol Heart Circ Physiol 259: H300– H308, 1990 [DOI] [PubMed] [Google Scholar]
- 16. Resnick N, Gimbrone MA., Jr Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J 9: 874– 882, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Resnick N, Yahav H, Schubert S, Wolfovitz E, Shay A. Signalling pathways in vascular endothelium activated by shear stress: relevance to atherosclerosis. Curr Opin Lipidol 11: 167– 177, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Senft AP, Dalton TP, Shertzer HG. Determining glutathione and glutathione disulfide using the fluorescence probe o-phthalaldehyde. Anal Biochem 280: 80– 86, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res 91: 769– 775, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Spinale FG, Tomita M, Zellner JL, Cook JC, Crawford FA, Zile MR. Collagen remodeling and changes in LV function during development and recovery from supraventricular tachycardia. Am J Physiol Heart Circ Physiol 261: H308– H318, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Tomaske M, Gerritse B, Kretzers L, Pretre R, Dodge-Khatami A, Rahn M, Bauersfeld U. A 12-year experience of bipolar steroid-eluting epicardial pacing leads in children. Ann Thorac Surg 85: 1704– 1711, 2008 [DOI] [PubMed] [Google Scholar]
- 22. van der Land V, Germans T, van Dijk J, Zwanenburg JJ, Spreeuwenberg M, Marcus JT, Kamp O, Gotte MJ, van Rossum AC. The effect of left bundle branch block on left ventricular remodeling, dyssynchrony and deformation of the mitral valve apparatus: an observational cardiovascular magnetic resonance imaging study. Int J Cardiovasc Imaging 23: 529– 536, 2007 [DOI] [PubMed] [Google Scholar]
- 23. van Oosterhout MF, Prinzen FW, Arts T, Schreuder JJ, Vanagt WY, Cleutjens JP, Reneman RS. Asynchronous electrical activation induces asymmetrical hypertrophy of the left ventricular wall. Circulation 98: 588– 595, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Vanagt WY, Cornelussen RN, Poulina QP, Blaauw E, Vernooy K, Cleutjens JP, van Bilsen M, Delhaas T, Prinzen FW. Pacing-induced dys-synchrony preconditions rabbit myocardium against ischemia/reperfusion injury. Circulation 114: I264– 269, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Vernooy K, Verbeek XA, Peschar M, Prinzen FW. Relation between abnormal ventricular impulse conduction and heart failure. J Interv Cardiol 16: 557– 562, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Villarreal FJ, MacKenna DA, Omens JH, Dillmann WH. Myocardial remodeling in hypertensive Ren-2 transgenic rats. Hypertension 25: 98– 104, 1995 [DOI] [PubMed] [Google Scholar]