Abstract
A microscale Western blotting system based on separating sodium-dodecyl sulfate protein complexes by capillary gel electrophoresis followed by deposition onto a blotting membrane for immunoassay is described. In the system, the separation capillary is grounded through a sheath capillary to a mobile X-Y translation stage which moves a blotting membrane past the capillary outlet for protein deposition. The blotting membrane is moistened with a methanol and buffer mixture to facilitate protein adsorption. Although discrete protein zones could be detected, bands were broadened by ~1.7-fold by transfer to membrane. A complete Western blot for lysozyme was completed in about one hour with 50 pg mass detection limit from low microgram per milliliter samples. These results demonstrate substantial reduction in time requirements and improvement in mass sensitivity compared to conventional Western blots. Western blotting using capillary electrophoresis shows promise to analyze low volume samples with reduced reagents and time, while retaining the information content of a typical Western blot.
INTRODUCTION
Affinity interactions coupled with separation methods are important techniques for life science research and biotechnology. Affinity chromatography, Southern blotting, and gel-mobility shift assays are examples of widely used techniques that combine separation methodology with selective binding to improve information content and selectivity. Of the techniques that combine separations and affinity interactions, Western blotting is perhaps the most widely used. The method is routinely used to assay proteins in complex mixtures and frequently used as a confirmatory test for clinical assays and regulatory tests.
In a Western blot, proteins are separated by size using sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a membrane by electro-blotting.1-3 The membrane is treated with blocking protein and then probed sequentially with primary and secondary antibody (conjugated with a label) to detect target protein. The technique is powerful because it provides selective protein detection based on size and antibody binding in a semi-quantitative assay. The method is also characterized by simplicity, reliability, and facile methods development.
Despite the great utility of Western blots, they have several well-known limitations. Western blots are manually intensive and time-consuming, generally requiring 4 to 24 h when taking into account gel preparation, separation, electro-blotting, and multiple incubations. Sensitivity is typically in the nanogram range making them incompatible with sample limited analysis. Analyis of large proteins is hindered by difficulty of transferring them from slab gels.4 Finally, the method has not been miniaturized, which wastes materials and reduces sensitivity.
Recently, efforts to reduce time and reagent requirements and introduce automation for Western blotting have been reported. A semi-automatic platform has been developed that uses vacuum filtration to pull blocking, antibody and reagent solutions through the membrane to decrease time required for the immunoassay procedure.5 Microfluidics technology has been used to create flow channels for applying blocking and antibody solutions over the surface of the blot, which is advantageous in reducing reagent consumption and optimizing conditions.10 These advances improve the immunoassay, but do not address other aspects of the Western such as separation and blotting. A more comprehensive approach is integration of separation with faster blotting in a microfluidic format.6, 7 In this method, antibodies pre-immobilized in the chip selectively capture protein targets from a high-speed gel separation. In one example, 500 nM free prostate specific antigen was separated, transferred and blotted in < 5 min with a signal-to-noise ratio of 40. This promising technique drastically reduces timescales and offers potential for automation; but the requirement for microfabrication, pre-labeling analyte proteins with fluorophore, and pre-loading of antibody presents barriers for some applications. Further development is expected to lower these barriers.
Interestingly, Western blots based on capillary electrophoresis (CE) have not been reported even though a variety of affinity-CE techniques8, 9 10 have been developed including immunoassays.11, 12 The closest approximation of a CE-Western blot that has been reported uses capillary isoelectric focusing to separate proteins which are then immobilized by photoactivated cross-linking to the capillary surface.10 The captured proteins are detected in the capillary by immunoassays performed by flushing antibodies and reagents through the capillary. The method provides excellent sensitivity and separation; but, the instrument used is expensive and the method does not provide the size-based separation of a classical Western blot.
Although a CE-based Western blot has not been described, several relevant technologies have been reported. For example, methods for capturing proteins separated by CE onto a membrane have been described.13-16 Most of this work was directed towards off-column mass spectrometry detection, although one study did use antibody detection.13 None of this prior work on membrane capture of proteins separated by CE used size-based separations; therefore, it did not provide the platform needed for Western blotting. Of course, size-based separation of proteins in capillary and chip-based gel electrophoresis formats has been demonstrated.17, 18 These studies show that smaller channel dimensions reduces sample volumes and enables higher electric fields (typically up to about 300 V/cm) relative to slab gels.
In this work, we describe a capillary gel electrophoresis (CGE)-Western blot that builds on these previous developments. A gel-filled capillary is interfaced with a blotting membrane, which captures protein as it migrates from the capillary and eliminates the need for a separate electro-blotting step. The use of CE leads to better mass sensitivity as well as faster separation when compared to slab gel Western blots. CE also allows use of entangled polymer solutions, instead of cross-linked gels. Entangled polymer solutions facilitate automation and reduce time for gel preparation because capillaries can be emptied and refilled by pumping as necessary to maintain consistent separation performance. This is unlike cross-linked gels which must be formed within the capillary.19, 20 Faster separation, the elimination of an electro-blotting step, and recent improvement in commercial immunoassay instrumentation combine for a reduction in total Western blot analysis time to under two hours. The technique is promising for improved throughput, automation, and mass sensitivity while retaining the information content and ease of use of a traditional Western blot.
EXPERIMENTAL SECTION
Materials and Reagents
Fluorescein isothiocyanate (FITC) and FITC-labeled insulin were purchased from Invitrogen (Carlsbad, CA). ECL Plus chemifluorescence kit and polyvinylidene fluoride (PVDF) membranes were purchased from GE Healthcare (Piscataway, NJ) and GE Osmonics (Minnetonka, MN) respectively. Rabbit anti-lysozyme was purchased from Millipore (Bedford, MA) and rabbit anti-carbonic anhydrase from Genway Biotech (San Diego, CA). FITC-labeled bovine serum albumin (BSA), and the secondary antibody, horseradish peroxidase-conjugated anti-rabbit IgG produced in goat, were purchased from Sigma (St. Louis, MO). Other unlabeled proteins and all other chemicals were purchased from Sigma. Fused silica capillaries were from Polymicro (Phoenix, AZ). All solutions were made using Milli-Q (Millipore) 18 MΩ deionized water. Phosphate buffer solution (PBS) was 100 mM Na2HPO4 adjusted to pH 7.5 with 100 mM NaH2PO4. Electrophoresis buffer for free solution CE was 100 mM Tris adjusted to pH 8.8 with HCl. Sieving media was a proprietary solution of entangled polymers designed for resolution of proteins from 10 kDa to 225 kDa (part number 390953 from Beckman-Coulter, Brea, CA). This media had a kinematic viscosity of 78 cp at 40 °C as measured using a glass semi-micro viscometer (Cannon Instrument, State College, PA, USA).
Sample Preparation
Proteins were denatured by heating at 70 °C for 5 min in denaturation buffer consisting of PBS, 3% sodium dodecyl sulfate (SDS) and 5% β-mercaptoethanol (BME). In some instances, protein samples were then dialyzed against PBS using mini-dialysis cups (Pierce Biotechnology, Rockford, IL). Protein samples for injection were diluted from these sample stocks with 25 mM Tris adjusted to pH 8.8 with HCl. In some experiments, proteins were first labeled with FITC, then denatured and dialysed. FITC prepared at 1 mg/mL in dimethyl sulfoxide was diluted to a final concentration of 100 μg/mL and incubated for 1 h with protein at 1-5 mg/mL in PBS for labeling.
Apparatus and Procedure for CE Western Blot
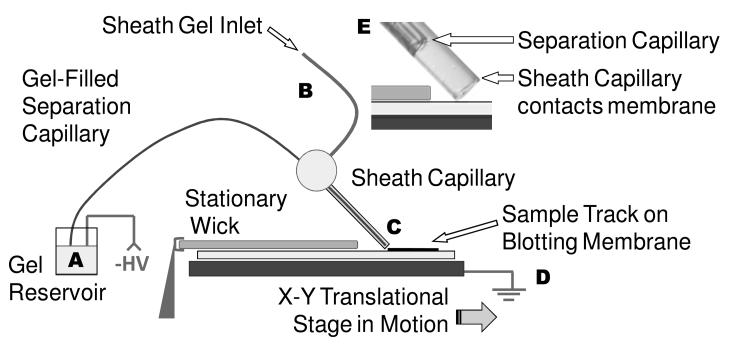
A diagram of the apparatus used to couple the separation capillary to the blotting membrane is shown in Figure 1. High voltage was applied at the separation capillary inlet reservoir and grounded at the stage. The separation capillary outlet was surrounded by a gel-filled sheath capillary that made contact with a PVDF membrane secured to the X-Y translational stage. During injection and separation the sheath capillary, fixed at an angle of approximately 45°, was positioned to make light contact with the membrane (determined by slowly lowering the capillary until it bent). The stage moved the blot past the capillary at a rate of 3 mm/min, which was the slowest setting possible for the motorized stage. A LabVIEW program controlled stage motion and application of high voltage. The maximum distance traveled by the stage, as constrained by the stepper motor track length, was 13 cm. Typically, a 0.5 cm by 12 cm blotting membrane was mounted on the stage, though a separation may require a shorter length. The footprint of the apparatus, including the stage, stepper motors, and capillary positioner is about 40 cm by 40 cm.
Figure 1.
Instrument overview. Sample is injected at the inlet of the separation capillary (A). The protein mixture migrates the gel-filled capillary under an electric field that is generated by the application of negative high voltage (A) and ground (D). Proteins exit the capillary as it drags over the surface, and deposit on the blotting membrane (C). A translational stage moves the blot past the end of capillary to preserve the protein separation on the membrane. Gel pumped through a sheath capillary (B) that surrounds the latter portion of the separation capillary and and makes direct contact with the blotting membrane (E). The blotting membrane (and wick overlay) are moistened with 50:50 (v:v) methanol: electrophoresis buffer.
Polyimide-coated fused silica capillaries were used for both the separation and sheath capillaries. The separation capillary (20 cm in length, 50 μm ID, 185 μm OD) was threaded through a sheath capillary (6.5 cm long, 250 μm ID, 360 μm OD) and fixed in place using a PEEK tee (VICI Valco Instruments, Houston, TX). The sheath capillary extended past the separation capillary by 0.5 mm. During separations electrophoresis gel was pumped through the sheath capillary at 10 nL/min using a syringe pump (Fusion 400, Chemyx, Stafford, TX). Separation capillaries were conditioned by sequential rinsing with 0.1 M NaOH, 0.1 M HCl, and water for 10, 5, and 2 min respectively, followed by pumping gel through the capillary (10 min) as recommended by the manufacturer. Gel-filled capillaries were used for 3 to 5 injections before regeneration with further conditioning.
The PVDF membrane mounted on the stage was kept wet with a wick that overlaid the membrane prior to contact with the capillary. The wick was kept stationary so that the membrane on the stage moved out from under it just before contact with the capillary. Both the membrane and wick were wetted with 50:50 (v:v) methanol and electrophoresis buffer. After a region of membrane passed the capillary it was allowed to dry.
Detection on Membrane
Fluorescent proteins were detected on the membrane by direct imaging using a Typhoon 9410 variable mode imager (GE Healthcare) in fluorescence mode. For Western blot analyses, immunoassays were performed per instructions of the manufacturer using a SNAP i.d. unit (Millipore), which partially automates antibody incubation and wash steps. In this system, 10 mL of blocking solution, 1 mL volume of antibody solutions, and tens of mL of rinse buffer, are poured over the surface of the blot (28 cm2 in area in this case) in the normal sequence. After incubation (10 min for each antibody step) solution is forced through the PVDF membrane using a vacuum pump. Design of the unit, including a cartridge which holds the blot stationary, prevents membrane drying. The primary antibody sera to each target protein was diluted to 1:1700 and the secondary antibody was diluted to 1:33000. All dilutions were made with electrophoresis buffer. 0.5 % nonfat dry milk and 0.5% Tween 20 were used in the blocking and rinse steps, respectively, per manufacturer suggestions. Signal generation, using hydrogen peroxide and acridan ester substrates in a chemifluorescence kit (ECL Plus, GE Healthcare), was catalyzed by horseradish peroxidase-conjugated secondary antibody.
Capillary Gel Electrophoresis
For comparison to the X-Y translational stage apparatus, experiments were conducted using a P/ACE MDQ capillary electrophoresis unit equipped with an LIF detector (Beckman-Coulter, Fullerton, CA, USA). The detector used 5 mW of 488 nm light from an Ar+ laser (Model: IMA101015B0S; Melles Griot, Carlsbad, CA, USA) for excitation. Emission was detected after passing through a 488 nm notch filter and a 520 ± 10 nm band-pass filter. Capillaries had an effective length of 20 cm, 50 μm ID, and 360 μm OD. Separation capillaries were treated as described above for the membrane capture experiments. Fluorescence imaging of proteins migrating from separation capillary outlet into the sheath capillary region was performed using an inverted epi-fluorescence microscope (IX71, OlympusAmerica, Inc., Melville, NY) and CCD camera (C9100-13, Hamamatsu Photonic Systems, Bridgewater, NJ) as described in detail elsewhere.21 In these experiments the capillaries were of similar dimensions to those of the CE-Western blot, except that the sheath capillary extended 5 cm beyond the separation capillary and was grounded in a gel reservoir rather than to a moving surface.
RESULTS AND DISCUSSION
SDS-protein complex capture from CGE
Our approach to CE-based Western blot was to capture SDS-protein complexes onto a membrane as they migrated from the column using the system shown in Figure 1 and then detect them using conventional immunoassay. Initial experiments were directed at identifying conditions that would allow protein capture with minimal band spreading using fluorescently-labeled proteins as a model. Figure 2 illustrates images from the direct detection on a PVDF membrane of captured FITC-labeled proteins that had been separated by CGE as SDS-complexes. PVDF membrane was chosen as the blotting membrane because of its reported high binding capacity and mechanical strength22; however it is anticipated this method could also be used with different substrates. Earlier work with nitrocellulose did allow for electrophoresis, though the membrane was brittle and difficult to manipulate (data not shown). With this work utilizing PVDF membranes proteins are captured in discrete zones, preserving the separation. Furthermore, proteins migrated according to log molecular weight as expected for CGE of SDS-complexes (Figure 2).
Figure 2.
Size-dependent separation of standard FITC-labeled proteins. (A) 3 proteins, prepared in stock samples of 100-300 μg/mL. The molecular weight for unlabeled protein is noted beside each observed peak. (B) Plotting log MW as a function of mobility yields a linear plot for these FITC-labeled proteins.
Reproducibility of migration time (measured as distance on the membrane) was good with FITC-labeled BSA and insulin peaks observed at 34.3 ± 0.8 min and 18.8 ± 0.6 min respectively (n = 3). These samples separated on a commercial CE-LIF instrument with the same nominal conditions (capillary length, i.d., buffer, and electric field) had longer migration times at 41.5 ± 1.5 min and 23.14 ± 0.02 min (n = 3) respectively. A likely cause of this difference was a lack of provision for cooling on the blotting instrument, compared to active cooling to 22-24 °C on the commercial apparatus. Supporting this conclusion, we found that separations performed without temperature control (ambient temperature was 26 °C) and with on-column detection yielded migration times (29.9 ± 0.2 and 16.5 ± 0.1 min (n = 3) for BSA and insulin respectively) that were more similar to those obtained by membrane capture.
Although previous work had already demonstrated capturing native proteins on surfaces after separation by free solution CE13, 14, 23, we found that several modifications to conditions used in those reports were necessary to capture proteins separated as SDS complexes by CGE and achieve detection of discrete bands as shown in Figure 2. In particular, it was necessary to wet the capture membrane with a methanol: electrophoresis buffer mixture (a 50:50 v:v mixture was used). Without methanol present during capture either no bands or diminished signal was observed. Methanol helps to wet the PVDF membrane and to dissociate the SDS-protein complex which improves adsorption onto the hydrophobic membrane.24 A wick overlaid the membrane prior to capture, and helped to prevent evaporation of the methanol mixture during CGE separation.
A second modification from previous work was use of a gel-filled sheath capillary around the outside of the separation capillary (Figure 1). Without a sheath capillary, electrophoresis current was unstable and bubble formation was observed in the separation capillary a few minutes after voltage was applied. If gel in the sheath was static, i.e. without flow, migration times were irregular and the current was only stable for about 15 min before drift occurred. Pumping the sheath fluid at 10 nL/min alleviated these problems. Higher sheath gel flow rates (50-200 nL/min) were found to smear protein on the blot. The underlying cause of these effects were not investigated, but they are consistent with electrolysis induced alterations in electrolyte at the outlet. Specifically, it has been observed that when the outlet vial has a small volume, such as the thin layer of buffer on the blotting membrane, OH− formed at the outlet may migrate towards the inlet to cause changes in pH and conductivity in the capillary.25 The sheath capillary will dilute OH− formed and create a low field region that slows the OH− migration towards the inlet. With a supplemental flow, the OH− may be prevented from entering the separation capillary at all. The sheath flow capillary also prevents a sharp electric field boundary along with buffer change at the outlet of the separation capillary which may also contribute to the stability observed.
Band broadening
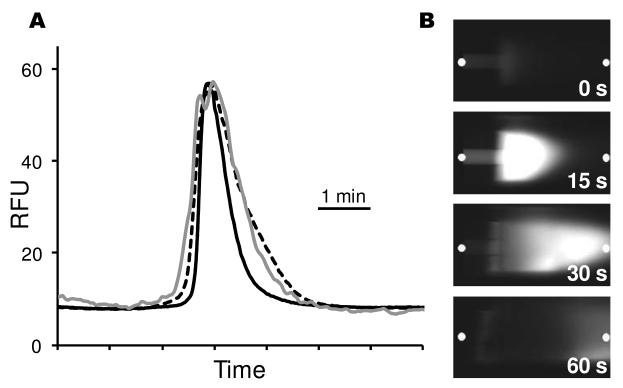
Although proteins are detected in discrete bands, we found that the system produced bands that were approximately 1.7 fold broader than on-column detection. To determine the source of band broadening, zones detected on membrane were compared to those detected on-column and within the sheath-flow capillary. For these experiments, the membrane capture experiment was performed as before (e.g., Figure 2A). A separate measurement was done to detect on-column and in the sheath simultaneously using an imaging system under similar separation conditions (i.e., sample composition, column length, column i.d., buffer, and electric field). Separate experiments were required because of the impracticality of detecting on-capillary while also capturing zones on a membrane. For both measurements, FITC-BSA was used as a test compound. Peak variance for FITC-BSA increased from 230 s2 on capillary to 610 s2 in the sheath (350 μm downstream of the separation capillary) to 670 s2 on the membrane (see Figure 3A). These results indicate that the majority of band broadening in transfer from the capillary to membrane occurs within the sheath itself. Images of the transfer from capillary to sheath (Figure 3B) provide insight into some of the reasons for this band broadening. The parabolic flow induced in the sheath, evident from the bullet shaped zone, adds band broadening. (Recall that flow was important to maintain stable currents). The decreased electric field in the sheath capillary, due to the wider bore and lower electrical resistance compared the separation capillary, may also contribute to the band broadening. These results suggest that fabrication of a sheath with narrower bore, perhaps through microfabrication methods, would decrease band broadening and improve resolution. The imaging results also show that the zone is not focused by the sheath flow, as is observed with higher flow rates. 26
Figure 3.
Measurements of band broadening inside sheath capillary and on membrane. (A) Comparison of peak width for on-column detection (black line), in sheath 350 μm beyond the exit of the separation capillary (dashed line), and on membrane after traveling through 500 μm of sheath (gray line). The on-column and in sheath measurements were taken from the same separation. The membrane data was from a separate injection. All separations used 150 μg/mL FITC-BSA as the sample separated at 300 V/cm with an effective capillary length of 20 cm. Capillaries were not thermostatted. (B) Selected images of protein exiting the separation capillary and entering sheath capillary. Time zero represents time zone first appears at exit of separation capillary and is 32 minutes after sample injection. The white dots indicate where signal was measured to construct Figure 3A.
When SDS-protein complexes migrate onto the membrane, most of the spreading on the blot appears to be laterally across the surface, rather than down into the membrane. Evaluation of spreading in the dimension perpendicular to the motion of the capillary indicates a typical trace width of approximately 450 μm, about 200 μm larger than the width of the sheath capillary inner diameter (Figure 4). Yet in the vertical dimension protein was not detected beyond the first 150 μm thick membrane when deposition was performed onto a stack of membranes. This result is in contrast to previous work that indicated some protein penetrated a first membrane and bound the second.13 In that work, 20 ng of protein was deposited whereas in this work picograms of proteins were deposited, so it could be that the spread through the membrane is too low for detection via immunoassay. These results support the idea of rapid protein capture.
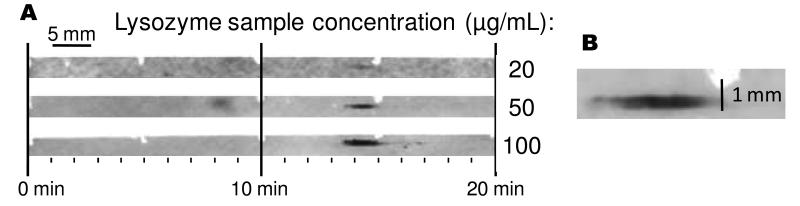
Figure 4.
CE-based Western blot of lysozyme at 3 different concentrations. Samples were separated at 300 V/cm and the resulting membranes probed with antibody using an automated system for applying reagents. Analysis time for an individual assay was about 60 min, though the immunoassay and detection were performed in parallel. Enlargement shows that zones spread perpendicular to the deposition track. The sheath capillary was 250 μm in diameter but the zone is 450 μm wide.
Improvements in separation efficiency may be possible with this method. Provision for cooling or use of narrower-bore capillaries would minimize heating effects in the gel. Fabrication of lower volume sheath capillaries may also avoid extra-column broadening. Finally, some improvement may be obtained in membrane capture. Focusing the electric field near the capillary outlet 27, 28 or using vacuum deposition following a liquid junction29 have been used to ameliorate spreading during deposition with free solution CE; however, the interfaces previously used with free solution CE are unlikely to be compatible with gel-based separations because of the lack of electroosmotic flow and sensitivity to the outlet buffer composition for CGE.
Immunoblotting after capillary separation
Figure 4 illustrates a CGE-Western blot for lysozyme at different concentrations. As with traditional slab-gel Western blots, this technique is semi-quantitative allowing for differences in protein concentration to be determined by the intensity of the band detected, as shown in Figure 4. An advantage of the CE method over traditional Western blots is elimination of a separate electro-blotting step because protein is directly delivered to the membrane as it is separated. This saves time as transfer requires up to 1 h in a conventional slab gel system. In this system, a smaller protein like lysozyme (MW = 14.3 kDa) migrated onto the membrane in about 15 min. Combined with the 30 min semi-automated commercial immunoassay platform and 10 min for incubation in detection reagents and fluorescence scanning, these Western blots were completed in less than 1 h, excluding sample preparation. Larger proteins, such as BSA, would extend total time to about 1.5 h.
Limits of detection
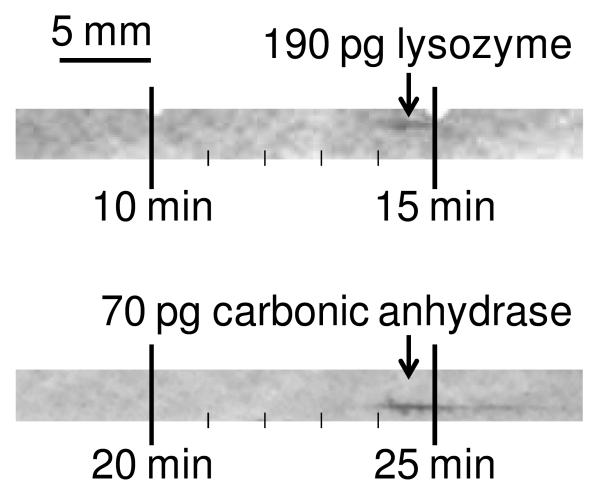
The limit of detection was investigated by performing CGE-Western blot of carbonic anhydrase and lysozyme at concentrations that produced weak signals (Figure 5). In these experiments, sample concentrations of 25 and 20 μg/mL resulted in peaks with signal-to-noise ratio (S/N) of 22 and 12 for carbonic anhydrase and lysozyme respectively. Assuming a linear response, this S/N corresponds to a LOD of 3 μg/mL or 10 pg injected for carbonic anhydrase and 5 μg/mL or 50 pg injected for lysozyme. (Amounts injected were estimated using injection duration, sample concentration and sample mobility.) The manufacturer reports the LOD for the chemifluorescence assay kit to be low picograms, so the sensitivity of our scheme is on par with the capabilities of the detection method. The good mass LOD for this system is attributed to use of small bore capillaries and small capture zones. Further, recovery may be higher in CGE because proteins are migrated from a low-volume sieving polymer matrix rather than electro-transferred out of a cross-linked slab gel for blotting.
Figure 5.
Immunoassay of unlabeled sample proteins at low levels. Estimated quantities of proteins calculated from injection length, elution time and sample concentrations are displayed for different proteins.
The rate of stage motion past the capillary may also impact LOD and resolution. Lower speeds are preferable to capture the band in a small zone and improve sensitivity by keeping the zone concentrated; however, if the speed is too low, zones would be captured with little distance between them, and resolution may be lost. In our experiments we used the lowest speed of the translational stage because no resolution was lost when compared to higher speeds, but the zones were more concentrated on the membrane. A stage with slower translational velocity therefore may allow better sensitivity.
Incorporation of size standards
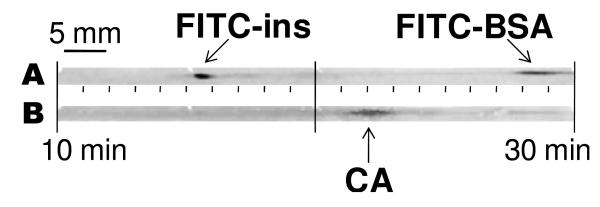
An important component of a Western blot is providing size information on the detected proteins in addition to immunoaffinity. The size of the target protein is estimated by comparison of its mobility to the mobility of a different proteins of known size separated in an adjacent lane. One approach for calibration in the CGE system is to add fluorescently-labeled protein size standards to the sample mixture and analyze the membrane by both direct fluorescence and immunoblotting. Results from such an experiment are illustrated in Figure 6. In the initial fluorescence scan, the added calibrants, FITC- insulin and FITC-BSA, are detected to provide reference positions on the membrane. The membrane is then probed with antibodies specific for the analyte protein, carbonic anhydrase. The signal for this protein is detected as a band between the reference bands, as anticipated because of its intermediate molecular weight. During the second analysis of the membrane, the standard proteins are barely detectable. This may be because the chemifluorescent signal from the secondary antibody conjugate is more intense than the fluorescent ladder proteins. The signal of the ladder proteins may also be reduced by photobleaching or rinsing prior to the second detection step.
Figure 6.
Immunoassay of unlabeled peak with labeled size standards. (A) Initial fluorescence scan displays FITC-labeled size standards at approximately 16 and 28 minutes for insulin (FITC-ins) and bovine serum albumin (FITC-BSA). (B) Upon immunoassaying this PVDF membrane with anti-carbonic anhydrase IgG, the intermediate MW protein, carbonic anhydrase (CA), is detected at 22 minutes.
Potential for improvements
These experiments prove the feasibility of performing CE-based Western blots. The system allows separation and blotting to occur at the same time thus eliminating the time for electro-blotting. Although the described approach shows promise for reducing sample requirements, automating, and reducing time of Western blots, improvements in time of analysis, robustness, throughput, and LOD are possible. It will be interesting to test the system with active cooling of the separation capillary, use of a smaller i.d., or a different sieving matrix to determine if faster and higher efficiency separations can be obtained. Addition of cooling complicates the implementation of this technique, but it may serve to push the boundaries in separation efficiency and speed. As indicated by microfluidic gel sieving, extremely fast protein size separations are possible in miniaturized formats suggesting the possibility of reducing the separation and blotting time less than 1 min.7, 18 Throughput could also be improved by use of capillaries in parallel. Parallelization would also allow ladder proteins to be run in a separate capillary for sizing if desired. Further throughput improvements are possible by automating gel-regeneration, as has been done for DNA sequencing instruments. Entangled polymer gel may also be more amenable to transfer of large proteins. On-column sample preconcentration is possible in CGE30, and would likely further improve concentration LODs. It may be possible to manipulate the translational stage velocity to improve either resolution or sensitivity. Finally, the small size of the capillary and resulting tracks would suggest that it may be possible to store many electropherograms on a small membrane to reduce reagent consumption and improve throughput relative to a traditional slab-gel Western blot.
CONCLUSIONS
CE has been recognized as a powerful method for protein separations; however, it can be argued that widespread acceptance of CE for protein analysis has been inhibited by lack of an analog to Western blotting. This report demonstrates a route to CE-Western blotting. Many of the improvements expected for migration from a slab-gel to capillary format are demonstrated. The time of analysis is improved through faster separation, use of polymer solutions for sieving media, and elimination of the electro-blotting step. Mass detection limits of 10 pg were readily achieved with little optimization. The system has potential for substantial improvements in speed, throughput, reagent consumption, and sensitivity. Importantly, the method also retains the essential features of the traditional Western blot format by providing size (calibrated with standards) and immunoaffinity information in a semi-quantitative assay. Furthermore, methods development is the same as a Western blot so that optimization of detection and development of assays for different proteins can follow well-established procedures. This is exemplified by the fact that we were able to readily develop Western blots for 3 different proteins in the course of this work. With further development, this approach may find use for sample limited analyses or in situations where better speed, throughput or automation is necessary.
ACKNOWLEDGEMENTS
This work was supported by NSF Grant CHE-0809013, as well as the UM Reproductive Sciences Training Program (NIH T32 HD07048).
REFERENCES
- 1.Towbin H, Staehelin T, Gordon J. Proc. Natl. Acad. Sci. U. S. A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnette WN. Anal. Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 3.Gong MJ, Kim BY, Flachsbart BR, Shannon MA, Bohn PW, Sweedler JV. IEEE Sens. J. 2008;8:601–607. [Google Scholar]
- 4.Kurien BT, Scofield RH. Methods. 2006;38:283–293. doi: 10.1016/j.ymeth.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 5.SNAP i.d. Protein Detection System User Guide. http://www.millipore.com/userguides/tech1/00103871 (accessed July 1, 2010)
- 6.He M, Herr AE. Anal. Chem. 2009;81:8177–8184. doi: 10.1021/ac901392u. [DOI] [PubMed] [Google Scholar]
- 7.He M, Herr AE. J. Am. Chem. Soc. 2010;132:2512–2513. doi: 10.1021/ja910164d. [DOI] [PubMed] [Google Scholar]
- 8.Ding YS, Lin BC, Huie CW. Electrophoresis. 2001;22:2210–2216. doi: 10.1002/1522-2683(20017)22:11<2210::AID-ELPS2210>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 9.Kiessig S, Reissmann J, Rascher C, Kullertz G, Fischer A, Thunecke F. Electrophoresis. 2001;22:1428–1435. doi: 10.1002/1522-2683(200105)22:7<1428::AID-ELPS1428>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 10.O'Neill RA, Bhamidipati A, Bi XH, Deb-Basu D, Cahill L, Ferrante J, Gentalen E, Glazer M, Gossett J, Hacker K, Kirby C, Knittle J, Loder R, Mastroieni C, MacLaren M, Mills T, Nguyen U, Parker N, Rice A, Roach D, Suich D, Voehringer D, Voss K, Yang J, Yang T, Vander Horn PB. Proc. Natl. Acad. Sci. U. S. A. 2006;103:16153–16158. doi: 10.1073/pnas.0607973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schou C, Heegaard NHH. Electrophoresis. 2006;27:44–59. doi: 10.1002/elps.200500516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roper MG, Shackman JG, Dahlgren GM, Kennedy RT. Anal. Chem. 2003;75:4711–4717. doi: 10.1021/ac0346813. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson KO, Palm A, Hjerten S. Anal. Biochem. 1992;201:211–215. doi: 10.1016/0003-2697(92)90330-a. [DOI] [PubMed] [Google Scholar]
- 14.Zhang HY, Caprioli RM. J. Mass Spectrom. 1996;31:1039–1046. doi: 10.1002/(SICI)1096-9888(199609)31:9<1039::AID-JMS398>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 15.Yeung KKC, Kiceniuk AG, Li L. J. Chromatogr., A. 2001;931:153–162. doi: 10.1016/s0021-9673(01)01200-6. [DOI] [PubMed] [Google Scholar]
- 16.Johnson T, Bergquist J, Ekman R, Nordhoff E, Schurenberg M, Kloppel KD, Muller M, Lehrach H, Gobom J. Anal. Chem. 2001;73:1670–1675. doi: 10.1021/ac0011888. [DOI] [PubMed] [Google Scholar]
- 17.Michels DA, Brady LJ, Guo A, Balland A. Anal. Chem. 2007;79:5963–5971. doi: 10.1021/ac0705521. [DOI] [PubMed] [Google Scholar]
- 18.Bousse L, Mouradian S, Minalla A, Yee H, Williams K, Dubrow R. Anal. Chem. 2001;73:1207–1212. doi: 10.1021/ac0012492. [DOI] [PubMed] [Google Scholar]
- 19.Zhang JY, Tran NT, Weber J, Slim C, Viovy JL, Taverna M. Electrophoresis. 2006;27:3086–3092. doi: 10.1002/elps.200500771. [DOI] [PubMed] [Google Scholar]
- 20.Quigley WWC, Dovichi NJ. Anal. Chem. 2004;76:4645–4658. doi: 10.1021/ac040100d. [DOI] [PubMed] [Google Scholar]
- 21.Dishinger JF, Reid KR, Kennedy RT. Anal. Chem. 2009;81(8):3119–3127. doi: 10.1021/ac900109t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurien BT, Scofield RH. J. Immunol. Methods. 2003;274:1–15. doi: 10.1016/s0022-1759(02)00523-9. [DOI] [PubMed] [Google Scholar]
- 23.Preisler J, Foret F, Karger BL. Anal. Chem. 1998;70:5278–5287. doi: 10.1021/ac9807823. [DOI] [PubMed] [Google Scholar]
- 24.Magi B, Liberatori S. In: Immunochemical protocols. Burns R, editor. Humana Press; Totowa, N.J.: 2005. pp. 236–237. [Google Scholar]
- 25.Timperman A, Tracht SE, Sweedler JV. Anal. Chem. 1996;68:2693–2698. doi: 10.1021/ac960166b. [DOI] [PubMed] [Google Scholar]
- 26.Chen DY, Dovichi NJ. J. Chromatogr., B: Biomed. Sci. Appl. 1994;657:265–269. doi: 10.1016/0378-4347(94)00014-x. [DOI] [PubMed] [Google Scholar]
- 27.Magnusdottir S, Heller C, Sergot P, Viovy JL. Electrophoresis. 1997;18:1990–1993. doi: 10.1002/elps.1150181118. [DOI] [PubMed] [Google Scholar]
- 28.Tracht S, Toma V, Sweedler JV. Anal. Chem. 1994;66:2382–2389. doi: 10.1021/ac00086a026. [DOI] [PubMed] [Google Scholar]
- 29.Rejtar T, Hu P, Juhasz P, Campbell JM, Vestal ML, Preisler J, Karger BL. J. Proteome Res. 2002;1:171–179. doi: 10.1021/pr015519o. [DOI] [PubMed] [Google Scholar]
- 30.Xu ZQ, Timerbaev AR, Hirokawa T. J. Chromatogr., A. 2009;1216:660–670. doi: 10.1016/j.chroma.2008.10.077. [DOI] [PubMed] [Google Scholar]