Abstract
Sixteen years ago, mutations in cardiac TnT and α-tropomyosin were linked to Familial Hypertrophic Cardiomypathy, thus transforming the disorder from a disease of the β-myosin heavy chain to a disease of the cardiac sarcomere. From the outset, studies suggested that mutations in the regulatory thin filament caused a complex, heterogeneous pattern of ventricular remodeling with wide variations in clinical expression. To date, the clinical heterogeneity is well matched by an extensive array of nearly 100 independent mutations in all components of the cardiac thin filament. Significant advances in our understanding of the biophysics of myofilament activation coupled to the emerging evidence that thin filament linked cardiomyopathies are progressive suggests that a renewed focus on the most proximal events in both the molecular and clinical pathogenesis of the disease will be necessary to achieve the central goal of using genotype information to manage affected patients. In this review we will examine the existing biophysical and clinical evidence in support of a more proximal definition of thin filament cardiomyopathies. In addition, new high resolution, integrated approaches will be presented to help define the way forward as the field works towards developing a more robust link between genotype and phenotype in this complex disorder.
Keywords: familial cardiomyopathy, thin filaments, Ca2+ kinetics
Introduction
The publication of the original study in 1990 establishing the genetic linkage of the β-myosin heavy chain gene (βMyHC) to the familial form of hypertrophic cardiomyopathy (HCM) was, like all seminal scientific findings, both a beginning and an end1. It represented an “endpoint” in that it provided a definitive answer to a question that had been discussed and debated for over thirty years. The initial description of a patient with HCM by Teare in 1958 had noted that multiple family members were also affected by this “unexplained” ventricular hypertophy2. The linkage results also prompted a spirited round of “of course!” from muscle biologists as it seemed to make perfect sense that a mutation in the enyzymatic domain of the primary cardiac motor protein could lead to a massive hypertrophic response in the heart. Thus began the molecular era of HCM.
From the outset it was clear that the oft-noted clinical heterogeneity of HCM was likely to be matched by considerable genetic heterogeneity, as suggested by the initial observation that the inheritance of HCM and the initial DNA marker loci were discordant3. Subsequent genome-wide linkage analysis initially identified three additional disease loci. As considerable phenotypic overlap between the affected families was noted, additional components of the cardiac sarcomere were selected as strong disease gene candidates. The subsequent identification of the 15q2 locus was facilitated by the finding that the murine α-Tropomyosin (TM) gene mapped to a region of chromosome 9 immediately adjacent to a syntenic portion of human chromosome 15, representing both the first use of the mouse in the study of HCM, and the extension of the disorder to mutations in the thin filament4. It is interesting to note that the chromosomal locations of several of the human thin filament proteins including the cardiac isoform of Troponin T (cTnT) were not known prior to the linkage studies and thus the studies identifying α-TM and cTnT as important disease loci also provided basic information regarding genomic location, a significant advance. This interrelationship between elucidating the pathogenesis of a complex primary cardiac genetic disorder and understanding the basic biology of the cardiac thin filament has continued to this day.
Linking Genotype to Phenotype in HCM: A Moving Target
The extension of the causative mutations to the regulatory thin filament changed the definition of HCM from a disease of the cardiac motor to a disease of the cardiac sarcomere and greatly expanded the breath of the potential molecular pathogenic mechanisms. In the following decade causative mutations were linked to the genes encoding the remaining cardiac thin filament proteins5–7. One of the earliest hypotheses regarding disease mechanism posited that the varied clinical prognoses in the familial form of HCM (FHC) could be directly linked to mutations in independent protein components of the sarcomere. While the original goal to link genotype to phenotype remains, it is now clear that the overall complexity of both the potential molecular mechanisms at the level of the cardiac sarcomere and our evolving understanding of the dynamic myocellular and ventricular remodeling that occurs in patients with thin filament mutations will require a far more subtle understanding on both levels.
Before addressing the potential mechanisms whereby thin filament protein mutations can cause cardiomyopathies it is important to consider the complexity of the end cardiac phenotype. The basic definition of HCM is deceptively simple and dates back to the original findings by Teare of unexplained “asymmetric ventricular hypertrophy”2. Thus, HCM represents a classic diagnosis of exclusion where LV remodeling occurs in the absence of known external causes. This straightforward clinical definition obscures the vast complexity of both the pathologic and physiologic findings in HCM, many of which were documented in a comprehensive 1968 Circulation paper by Frank and Braunwald documenting a striking variation in LV wall thickness between affected patients8. While it is now well-established that “extreme” degrees of LV hypertrophy (maximal wall thickness > 30mm) represent a significant risk factor for sudden cardiac death (SCD), one of the more intriguing clinical observations was that the degree of functional impairment in patients with HCM was not necessarily directly related to the degree of LV hypertrophy9. In particular, abnormalities in relaxation parameters that were out of proportion to wall thickness were noted in several studies and led to the rather prescient prediction that there was likely to be an unknown impairment in function at the myocellular level10. These observations have been extended to FHC where significant differences in LV mass were found in patients with independent gene mutations, different mutations within the same gene and surprisingly, within affected families carrying the same gene mutation11. Subsequently, mutations in sarcomeric genes (including the thin filament proteins cTnT and cTnI) have been linked to both dilated (DCM) and restrictive (RCM) cardiomyopathies12–14. This seemingly endless degree of phenotypic variability has limited meaningful genotype-phenotype correlations for most mutations, calling into question the feasibility of using genotype information in patient management15.
The most likely mechanism(s) whereby mutations in the same functional domain or even identical mutations can lead to different patterns of ventricular remodeling would be the effects of modifier genes and/or environment that influences the downstream, secondary cellular responses to thin filament mutations. It is also possible that sarcomeric gene variants or polymorphisms may act as secondary phenotypic modifiers. More recently it has become apparent that 5–6% of patients with FHC have either double (two mutations in the same gene) or compound (two or more mutations in multiple genes) genotypes16. While most of the involved multiple mutations are in β-MyHC or MyBP-C, the resultant effects include earlier presentation and often rapid disease progression. These findings have established the importance of including a complete panel of sarcomeric genes in screening strategies. There is, however, an additional and perhaps central issue that confounds our ability to match genotype to phenotype in FHC, the progressive nature of the cardiac pathogenic process. In the past several years, a series of longitudinal studies of patients with thin filament protein mutations have documented the dynamic nature of the ventricular remodeling, including late-onset HCM, progression from HCM to DCM, stable HCM with worsening restrictive physiology and primary DCM13, 17. Thus, it is apparent that focusing only on the end phenotype as the supposed “link” to the molecular mechanism is not only limiting, but also likely to be misleading. Surmounting this significant limitation will require additional longitudinal studies of early stage or “genotype-positive, phenotype-negative” cohorts on the clinical side. The potential power of this approach was recently demonstrated in a study by Ho, et al, where early profibrotic signaling was detected in patients with MYH7 and MYBPC3 mutations prior to the onset of hypertrophic growth or detectable fibrosis18. Of note, differential activation patterns were observed between patients carrying independent gene mutations, thus providing a potential mechanism to explain gene mutation-specific patterns of ventricular remodeling. Overall, the predicted result of focusing on the earliest stages of cardiac remodeling (prior to the activation of potentially complex signaling cascades) would be that the observed molecular and biophysical effects of thin filament mutations would provide a more robust mechanistic link between genotype and phenotype. Moreover, from the standpoint of developing more specific therapeutic options for this complex disorder, it is likely to be far easier to alter the natural history of an early pathogenic process as opposed to achieving the regression of an end-stage cardiomyopathy. Just as a more “proximal” assessment of the clinical disease process is likely to reveal a less variant phenotype, to directly determine the potentially unique role of thin filament mutations in FHC, it is important to begin with the assumption that the primary biophysical insult at the level of the cardiac sarcomere remains the single pathogenic constant among all patients with a given mutation. Thus, by definition, the mechanism of FHC begins with the cardiac crossbridge cycle.
The cardiac thin filament as a dynamic multisubunit regulatory “machine”
The fundamental source of cardiac force is the crossbridge and represents a complex mechanochemical interaction between the S1 head of the myosin heavy chain (MyHC) and the thin filament backbone, comprised of cardiac actin (reviewed in19). Myosin is an enzymatic energy-transducing molecule. It converts energy in the pyrophosphate bond of ATP to mechanical energy. While questions remain as to whether this energy transduction is direct or the result of a “stochastic ratchet”, it is clear that each step of the myosin motion over the actin backbone drives the hydrolysis of ATP20. The actomyosin interaction can occur in the absence of the troponin (cTn) complex and tropomyosin (TM). The essential regulation of contraction, however, wherein the hemodynamic demands of the body are directly transduced to the cardiac sarcomere requires the presence of the complete regulatory thin filament21. As shown in Figure 1, the basic functional unit of the cardiac thin filament is a highly evolved multi-protein machine comprised of seven actin monomers, one troponin complex (cTnI:cTnT:cTnC) and one tropomyosin coiled-coil dimer. As originally noted by Ebashi in his elegant 1968 study, the role of Ca2+ as the activator of striated muscle contraction is fully dependent on Ca2+ binding to the Tn complex22. Forty years later, there remains considerable debate as to the molecular mechanisms that underlie the dynamic changes in structure and function that govern myofilament activation following Ca2+ binding to cTnC. The initial hypothesis explaining the complex activation process was proposed by Huxley and proposed two specific positions (states) of TM that were dependent on Ca2+ binding to TnC23. In the Ca2+-off state (“blocked” or B-state), TM was predicted to sterically block the interaction of actin and myosin. Myofilament activation would subsequently occur via Ca2+ - binding induced changes in the protein-protein interactions within the Tn complex causing a significant azimuthal shift in the position of TM (to the “closed” or C-state) that would allow for actin-myosin binding and force generation. While a broad array of subsequent biophysical and kinetic studies supported the 2-state steric-blocking model, it became clear that myosin binding was required for full activation, e.g. Ca2+ binding alone was insufficient. This limitation of the 2-state model was directly addressed by McKillop and Geeves, where they showed that the kinetic and equilibrium data could be reconciled by the addition of a third TM position (the “open” state or M-state) that was induced by an isomerization promoting the transition of the initial weak actinmyosin interaction to a strong, force-generating crossbridge24. Subsequent structural studies have since confirmed and extended the 3-state model of myofilament activation25. As shown in Figure 2, the average position of TM in each of the three “states” determines the interaction between actin and myosin that in turn leads to force generation. Given that thin filament regulation is fully dependent on the precise coordinated movements of multiple proteins it is perhaps not surprising that a wide range of thin filament protein mutations can disrupt the function of this highly tuned molecular machine and cause complex cardiomyopathies.
Figure 1.
An Atomistic Model of the Human Cardiac Thin Filament in the Ca2+-activated State
Yellow = cTnT; Blue= cTnI; Red = cTnC; Green = α – Tropomyosin ; Silver/Gray = actin filament. For details of model development please see Data Supplement.
Figure 2.
The 3-State Model of Myofilament Activation
The 3 average positions of TM are depicted. In the Blocked State (red), TM resides at the outer actin domain, Ca2+ binding to cTnC results in an azimuthal shift to the weakly bound Closed State (yellow) in the actin inner domain and myosin binding drives the final shift to the force-producing Open state (green).
Mutations in thin filament proteins alter structure and dynamics to disrupt myofilament function and cause FHC
The original findings that mutations in thin filament proteins caused FHC led to an initial series of important in vitro investigations that identified many of the basic effects of disease mutations on myofilament activation. Impairments in ATPase activity, the Ca2+ sensitivity of force generation and the importance of mutant protein dosage on function were all detailed in early work from several independent groups26–28. Interestingly, the disease linkage discovery was also concurrent with the basic studies establishing the biophysics of the 3-state model of myofilament activation (reviewed in29) and contributed to a renewed interest in understanding the unique biology and structure of the cardiac sarcomere. This effort extended the original NMR and FRET-based approaches and led to the first high-resolution crystal structure of the human Ca2+ -activated cardiac thin filament core domain in a landmark study by Takeda, et al.30. Subsequent studies utilizing reconstituted thin filaments followed by 3D reconstruction confirmed and extended the Takeda model and the current extant structure of the thin filament represents the basis of any approach to link genotype to phenotype31. As noted above, while crystal structures are, by definition, static representations of atomic position at a given timepoint, proteins are inherently dynamic. The inherent flexibility of the thin filament (especially cTnT and TM) has been previously noted, in fact the flexibility of cTnT N-terminal region contributed to the difficulty in obtaining crystals of the Ca2+ -activated cTn core domain30. The concept of single protein flexibility can be addressed at multiple levels of resolution and is an important component of the basic concept of allostery, whereby local changes in structure are translocated to distant regions of the protein and affect function. More recently, studies in enzymes have revealed that mutations at a distance from the active site can significantly alter the rate of catalysis via “time dependent motions” (protein dynamics)32, 33. Work from our lab originally applied these computational approaches (Molecular Dynamics, MD) to evaluate local changes in protein flexibility caused by cTnT FHC mutations34. Since that time, the use of MD to extend the resolution of our molecular understanding of dynamic changes in thin filament function to the atomic level has become a component of an approach to evaluate the precise effects of independent thin filament mutations on structure and dynamics35, 36. In the next sections we will detail how these high resolution approaches to understanding the molecular pathogenesis of thin filament related FHC have begun to bridge the divide between genotype and phenotype.
cTnI and cTnC: The Ca2+ sensor and trigger for myofilament activation
As noted above, cTnI and cTnC together comprise a functional complex that directly transmits the Ca2+ binding status of the single functional regulatory site (Site 2) on cTnC to the thin filament via cTnI (Figure 3). It is this basic binding step that triggers the initial blocked (B) → closed (C) transition and is absolutely required for myofilament activation. As these interactions are fully interdependent, it is illustrative to consider the effects of disease-causing mutations on the functional cTnI:cTnC complex.
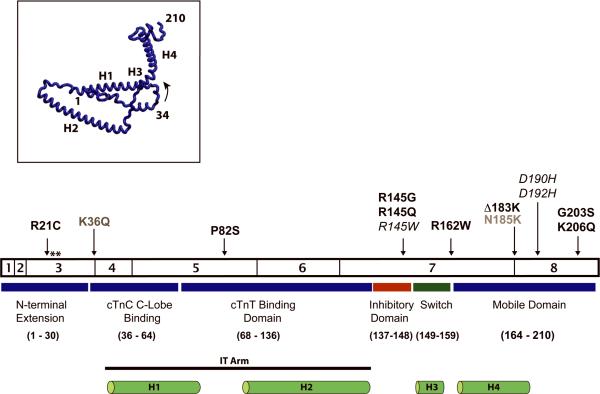
Figure 3.
Cardiac TnI Exons, Structure and Functional Domains
Listed mutations are discussed in text, ** denotes Ser 23/24 PKA-dependent phosphorylation sites. Mutations in grey have been linked to primary DCM and mutations in italic have been linked to RCM. Inset: cTnI secondary structure
Since the first reported linkage study in 1997, mutations in cTnI represent the most extensively characterized clinical subset in FHC5. While the original linkage study did not provide clinical profiles, a subsequent report focusing on an in-frame deletion of a residue directly adjacent to the second actintropomyosin binding site of cTnI (ΔLys183) provided a comprehensive characterization of 25 individuals across 7 families carrying the mutation37. This study was notable for high disease penetrance, age-independent SCD, extreme variability of the observed ventricular remodeling both with respect to degree of LVH and regional distribution and evidence for progressive LV dilatation post age 40. The broad range of clinical phenotypes caused by the same gene mutation, including individuals within the same family, was surprising. The clinical characterization was made more complex by the observation that mutations in cTnI contributed to the development of restrictive physiology both within HCM families and as an apparent rare primary manifestation13, 14, 38. Important considerations regarding the interpretation of many of the initial family studies are the persistent effects of small family size and referral bias. Subsequent studies directly addressed this concern by assessing the frequency of sarcomeric gene mutations in large populations of unrelated patients with HCM. While the overall frequency of thin-filament mutations ranged from 3–8%, the landmark kin-cohort study by Mogensen, et al., provided a uniquely detailed, longitudinal characterization of the striking phenotypic variation in cTnI-related FHC, derived from 748 HCM families, encompassing 100 mutation carriers with 13 different cTnI mutations39. The basic findings regarding the distribution of disease-causing mutations (largely exons 7 and 8), the presence of HCM and RCM within single families and the wide range of ages for initial disease presentation (2nd to 8th decade of life) largely confirmed the results of previous reports. Of particular note, the 10-year follow up period again established the plasticity of the ventricular remodeling with clear disease progression. More recently, two cTnI mutations were definitively linked to autosomal dominant DCM that was notable for rapid clinical progression post presentation (Lys36Gln, Asn185Lys)40. Finally, while ~ 8 independent cTnC missense substitutions have been associated with primary cardiomyopathies, with the exception of the Gly159Asp DCM mutation, the clinical data are restricted to individual patients or very small families, thus greatly limiting the phenotypic characterization7. In the main, it is apparent that the end cardiac phenotypes caused by mutations in cTnI:cTnC are complex, progressive and, often strongly dependent on when the disease is first diagnosed, an important consideration for establishing mechanistic links between genotype and clinical phenotype.
To begin to elucidate the structural mechanism(s) of cTnI:cTnC cardiomyopathies, it is useful to first examine the distribution of cTnI mutations within the known functional domains as shown in Figure 3. The basic structure of cTnI represents a common motif among the three proteins of the cTn complex. Highly alpha-helical domains are separated by linker sequences of varying lengths and convey a high degree of flexibility to the overall structure30. Approximately 35 independent mutations in cTnI have been linked to FHC. A striking degree of structural “clustering” is apparent with >75% of the known mutations falling within either the highly charged Inhibitory Region (cTnIIn: residues 137–148) or the proposed Mobile region that contains the second actin-TM binding domain (cTnIreg: residues 164–210). Both of these regions were poorly defined within the extant crystal structure of the Ca2+ -activated cTn core domain, likely due to their high inherent motility. This mutational clustering within the highly flexible domains of the troponin core is a recurrent observation and may reflect a “tolerance” of the thin filament to functional alterations in dynamic components of the regulatory thin filament. These are regions that change in orientation and position as part of normal activation and thus mutations within these domains likely alter these dynamic movements without fully abolishing the regulation of thin filament activation (reviewed in41). Conversely, the regions that form rigid structural components of the cTn core and/or extended protein-protein interactions are virtually devoid of known mutations. A case in point is the highly conserved cTnI components of the rigid IT arm comprised of the H1 (cTnC-C-lobe bound) and H2 (cTnT-bound) helices where the Pro82Ser cTnI mutation is located within the flexible linker that bridges the two helices and thus is unlikely to significantly disrupt the overall structure of the extended IT arm42. In contrast, the HCM/DCM-linked mutations in cTnC are evenly dispersed throughout the complex bi-lobed structure comprised of multiple α-helices connected by flexible linkers36.
A broad range of in vitro studies have been performed to assess the primary functional effects of cTnI:cTnC mutations on the regulation of myofilament activation. It is informative to consider these studies in the context of two of the basic physiologic roles of the complex, (1) to directly translate the binding of Ca2+ to Site II of cTnC and trigger the onset of the B to C to M state transitions and (2) to transduce an increase in hemodynamic demand via PKA-induced phosphorylation of Ser 23/24 in the N-terminal domain of cTnI to the cardiac sarcomere.
Cardiac TnC has three discrete Ca2+ binding sites and initial myofilament activation is triggered solely via oscillatory Ca2+ binding to Site II in the regulatory N-lobe. The resultant exposure of a hydrophobic “patch” in the N-lobe that binds to the cTnI switch domain leads to the release of the adjacent inhibitory and 2nd actin-TM binding domains, facilitating the movement of TM and subsequent formation of weak actomyosin crossbridges (Figure 3). As noted above, these two domains contain >75% of all cTnI mutations and several groups have investigated the functional effects of disrupting these regions of the protein. An initial report by Elliott, et al., showed that both the cTnI Arg145Gly and Arg162Trp mutations led to a significant increase in the Ca2+ sensitivity of ATPase regulation and a decrease in the inhibition of the actin-TM activated ATPase activity43. These findings were confirmed and extended in an extensive study utilizing cTn exchange into skinned rabbit muscle fibers to directly measure the Ca2+-sensitivity of force development44. Virtually all of the cTnI mutations studied (Arg145Gly, Arg145Gln, Arg162Trp, ΔLys183 and Lys206Gln), spanning all three of the C-terminal cTnI functional domains caused a fundamental increase in Ca2+ sensitivity, a result consistent with an increase in myofilament activation at submaximal [Ca2+]. The predicted physiologic result would be a significant impairment in cardiac relaxation, a finding that was fully confirmed at the whole-heart level in the subsequent Arg145Gly transgenic mouse model45. These studies established the basic observation that mutations in cTnI linked to a “hypertrophic” (or more accurately, “non-dilated”) phenotype resulted in an increase in the Ca2+ sensitivity of myofilament activation, though the precise mechanism(s) remained unclear.
The more recent discovery that a subset of cTnI mutations may cause a restrictive phenotype has raised the general question of whether the magnitude of the shift in Ca2+ sensitivity is predictive of severity. An important limitation in interpreting the magnitude of the Ca2+ shift to imply cardiac stiffness is that there is significant variation in the literature values between different laboratories and experimental systems. To directly address both the potential mechanism(s) underlying the observed increases in Ca2+ sensitivity and whether these changes could be linked to genotype-specific remodeling, Kobayashi and Solaro measured the actin-S1 ATPase activity and Ca2+ binding affinity of mutant cTnI molecules in reconstituted thin filament systems with varying degrees of biological complexity46. The underlying premise was that the impaired inhibition of activation at low [Ca2+] would alter the average TM position, and bias the equilibrium towards the C and M states. Four independent cTnI mutants were studied, two from the Inhibitory domain, Arg145Gly (HCM) and Arg145Trp (RCM) and two from the 2nd actin-TM binding domain, Asp190His (RCM) and Asp192His (RCM). Interestingly, while all four mutations exhibited a similar degree of leftward shift in the pCa-actin-S1 ATPase assay, only the Arg145Gly/Trp mutations caused a significant decrease in inhibitory activity. Moreover, all four mutations increased Ca2+ binding to the thin filament with an apparent destabilization of the Ca2+ free state, partially accounting for the observed increase in sensitivity. Thus, while no significant differences were found between RCM and HCM – associated mutations that would directly account for the different phenotypes, the mutations in the 2nd actin-TM binding domain alone (Asp190His and Asp192His) were predicted to have a more limited effect on the equilibrium thin filament shift (e.g. from B to C but not M), establishing the distinct functional role of the highly mobile C-terminal end of cTnI. More recently, these biophysical studies were strongly supported by Galinska, et al, using electron microscopy and 3D reconstruction to demonstrate that the extreme C-terminal domain of cTnI (immediately adjacent to the Asp190His and Asp192His mutations) acts to stabilize tropomyosin in the Ca2+-activated state47. The basic mechanism posits an electrostatic-mediated search function for the mobile domain of cTnI via a “fly-casting” mechanism once it is released from it's B-state position (bound to actin) after Ca2+ binds to TnC48. As a corollary to this role in the Ca2+ activated state it is likely that a successful “search” would facilitate a rapid return to the B-state (relaxation) when Ca2+ is released. Therefore, as predicted by Kobayashi and in a subsequent paper by Mathur, et al, mutations in the C-terminal domain of cTnI lead to an apparent increase in the Ca2+-sensitivity of ATPase activity by altering the expected equilibrium position of TM, and this mechanism, in part, represents the most proximal cause of cTnI-related cardiomyopathies at the myofilament level46, 49.
The maintenance of cardiac output via the beat to beat regulation of contractility and relaxation is a central component of cardiovascular function21. The PKA-mediated phosphorylation of cTnI at Ser 23/24 in response to β-adrenergic activation represents a basic regulatory mechanism to directly transduce this signal to the myofilament level. The combined physiologic effects of cTnI Ser 23/24 phosphorylation include a decrease in the Ca2+ sensitivity of force generation, an increase in the off rate of Ca2+ from cTnC Site II, an increase in the cross bridge cycling rate and an increase in the rate of relaxation (reviewed by Metzger and Westfall in50). It had long been noted that patients with HCM exhibited blunted or abnormal cardiac responses to β-adrenergic stimulation. The discovery that mutations in cTnI caused HCM (and more recently, DCM) raised the important question of whether this integral function of cTnI as a modulator of the β-adrenergic response was altered by disease mutations. There are at least two possible mechanisms whereby cTnI:cTnC mutations might interfere with this basic function. First, mutations could alter the PKA-mediated phosphorylation “potential” at Ser 23/24 leading to a decrease in the expected level of phosphorylation for a given stimulus. Second, the expected physiologic response to the phosphorylation event may be altered by mutation. Initial studies by Deng, et al addressed the latter question utilizing reconstituted thin filaments in acto-S1 ATPase assays to assess the effects of the cTnI Arg145Gly, Gly203Ser and Gly206Gln mutations51, 52. While the Arg145Gly mutants exhibited the expected increase in the Ca2+sensitivity of ATPase activity, the expected desensitization post bisphosphorylation was not observed. Neither of the C-terminal mutations had an effect on Ca2+ sensitivity in the dephosphorylated state. It is interesting to speculate that the predicted effects of the Arg145Gly mutation would be particularly severe as the increase in Ca2+ sensitivity coupled to the loss of the PKA-mediated desensitization would affect both baseline diastolic function and the ability to recruit additional cardiac reserve in the context of increased hemodynamic demand. Of note, a subsequent analysis of the Arg21Cys cTnI mutation by Gomes et al., revealed a significant increase in the Ca2+ sensitivity of force development in skinned fibers at baseline and a minimal desensitization in response to PKA phosphorylation, similar to the findings for Arg145Gly53. In contrast, however, the impaired response was caused by a decrease in the level of Ser23/24 phosphorylation, consistent with a potential local change in the phosphorylation potential, perhaps in part due to the loss of the Arg residue at 21 that is part of the PKA recognition sequence. Thus, cTnI mutations can exhibit direct effects on the post-translational regulation of myofilament activation.
More recently, significant advances have been made in elucidating both the structure of the N-terminal domain of cTnI and the nature of the inter- and intra- molecular interactions within the cTnI:cTnC complex that are modulated by phosphorylation at Ser23/24. As noted previously, several regions of the Ca2+-activated cTn core domain including the cardiac-specific N-terminal domain (residues 1–30) were not included in the crystal complex30. The lack of high-resolution structure for this crucial domain has limited our understanding of the regulatory mechanism. A broad range of biophysical studies utilizing an array of methodologies including FRET, ESR, and solution NMR had established that the N-terminal domain is highly flexible, maintains a relatively weak affinity for the N-lobe of cTnC in the dephosphorylated state and that phosphorylation at Ser23/24 induces a significant conformational change of the N-terminus (reviewed in54). A recent study by Howarth et al, utilized in solution NMR of fragments of the N-terminus of cTnI in multiple states including bisphorylated, unphosphorylated and - DD substituted followed by sequence analysis and comparison with existing structures to develop an atomic level model of troponin55. In this model, the bisphorylation of cTnI at Ser 23/24 induces a lengthening of the adjacent C-terminal helix (residues 21–30) that further decreases the affinity of the N-terminus for the N-lobe of cTnC and in doing so, realigns the extended acidic domain, thus facilitating the interaction with the highly basic inhibitory domain via an acute bend in the molecule. The net affect is an increase in the Ca2+ off rate (desensitization) that increases the rate of relaxation. These complex intra and inter-molecular interactions provide mechanistic insight for the observed increase in Ca2+ sensitivity and loss of the expected desensitization with bisphosphorylation observed in the Arg145Gly cTnI studies. It is expected that the loss of a central basic residue at residue 145 would destabilize the electrostatic interactions between the N-terminus and the inhibitory domain at baseline, leading to activation at submaximal Ca2+ and the effects of bisphosphorylation would likewise be attenuated by this impaired interaction.
Cardiac Troponin C was the last thin filament protein to be linked to cardiomyopathic remodeling. The initial study identified the Gly159Asp cTnC mutation in a screening study of 235 consecutive patients with idiopathic dilated cardiomyopathy56. To date, this study represents the only extended phenotypic characterization of a cTnC mutation as the subsequent mutations were largely limited to individual patients and thus must be interpreted conservatively from the phenotypic standpoint7. The clinical profile of affected Gly159Asp patients was notable for a relatively severe phenotype with multiple affected individuals across generations requiring cardiac transplantation for severe systolic failure and, as previously reported for both the ΔLys210 mutation in cTnT and the Asp230Asn mutation in TM, several of the cTnC Gly159Asp patients responded to traditional medical management (angiotensin-converting enzyme inhibitors and β-blockers), an intriguing observation for a known structural mutation12, 56, 57. While initial reconstituted actin-TM ATPase studies suggested that the Gly159Asp cTnC DCM mutation exhibited the “expected” baseline Ca2+ desensitization and a decrease in maximal activation, subsequent studies in more biologically complex skinned fiber systems revealed no significant shifts in the Ca2+ dependence of force generation nor ATP hydrolysis rate at baseline58, 59. Moreover, extensive analysis of cTnI Gly159Asp in the context of cTnI 23/24 phosphorylation revealed a loss of the expected Ca2+ desensitization of force generation while the cross bridge cycling rate was increased, thus representing a molecular uncoupling of these two basic myofibrillar responses to PKA-mediated phosphorylation. As shown by Biesiadecki, et al, the observed effects on Ca2+ binding to cTn mirrored the fiber-based studies in that cTnC-Gly159Asp: cTnI-DD complexes did not lead to the expected phosphorylation-mediated decrease in cTnC Ca2+ binding59. This result suggests that the previously noted increase in cTnC −cTnI binding affinity due to the cTnC Gly159Asp mutation may impair the effects of Ser23/24 phosphorylation to weaken the interaction of the N-terminal extension of cTnI and the N-lobe of cTnC (and decrease Ca2+ affinity) as described above. These results were confirmed by a later study that revealed a significant uncoupling between Ser 23/24 phosphorylation of cTnI and the Ca2+ sensitivity of force generation in skinned cardiac myocytes isolated from a patient carrying the Gly159Asp cTnC mutation60. Thus, while the mechanism whereby this primary effect on the Ca2+ sensor for tuning the physiologic response to increased hemodynamic demand would lead to a primary dilated cardiomyopathy remains unclear, the development of more integrated and specific approaches to address precise changes in thin filament dynamics caused by independent mutations has begun to elucidate the complex links between biophysics and physiology in FHC.
Cardiac TnT: altering the flexibility and orientation of the central thin filament adapter has far-ranging effects on myofilament activation
Cardiac TnT was one of the first thin filament proteins to be linked to FHC and while over 30 mutations have been identified to date, our understanding regarding the molecular mechanism(s) that underlie this subset remain limited, in part due to the lack of a high-resolution structure of the N- and C-terminal domains. As noted by Tobacman in his extensive 1996 review, cTnT represents the “glue” of the thin filament as it links the cTnI:cTnC complex to TM:actin61. In our laboratory, it's the homerun protein because it “touches them all”; the intricate and highly dynamic array of protein-protein interactions that determine myofilament activation are fully dependent on the highly flexible cTnT molecule.
While cTnT was one of the first thin filament proteins to be linked to FHC, relatively few large family studies are available and thus the clinical characterization remains somewhat in flux. In particular, the question of whether patients carrying a subset of cTnT mutations exhibit a high frequency of SCD in the context of relatively mild LVH has been of particular interest as it suggests that the disease mechanism is inherently myocellular as opposed to being dependent on ventricular morphology, a central issue regarding the ability to non-invasively diagnose the disorder. This potential “dissociation” between the degree of ventricular morphology and the risk of sudden or disease-related death in cTnT-linked FHC was first noted by Watkins et al, in the original clinical characterization of 8 independent mutations across 26 kindreds62. While several subsequent studies did not fully support this observation, given the continuing limitations regarding the relatively low frequency of cTnT mutations, small family size and referral bias, the definitive conclusion remains elusive63. Again, as with cTnI:cTnC mutations it is important to note that if mild LVH (e.g. subclinical and/or asymptomatic) is a distinguishing characteristic, patients with this clinical profile would be underrepresented in any HCM clinic, especially those with large surgical referral cohorts where many of the patients have obstructive physiology (rarely observed in patients with thin filament mutations). Two subsequent reports identified a second independent mutation at Residue 92 of cTnT, (Arg92Trp)64, 65. In the study by Moolman, et al, the patient profile was similar to the previously described cTnT mutations, with minimal LVH, low disease penetrance via echocardiagram and a high frequency of sudden cardiac death, especially in males. More recently, a long-term follow up study based on the same patient cohort revealed late-onset (> age 35) hypertrophy of the IVS in 46% of carriers, with no evidence for progression to a dilated phenotype17. While patients with the cTnT Arg92Gln/Trp mutations exhibit a relatively consistent early-onset clinical phenotype, this is not the case with several of the more C-terminal mutations, for example Arg278Cys, where late-onset, symptomatic septal hypertrophy has been observed66. Finally, a recent report by Menon, et al, described a multigenerational family carrying the Ile79Asn cTnT mutation exhibiting a fully mixed HCM/RCM phenotype67. Thus, similar to the complexity evidenced by patients carrying mutant cTnI proteins, patients with cTnT-related FHC defy easy morphological characterizations, require extended follow-up and “mild hypertrophy” does not necessarily correlate with a “low” risk for SCD.
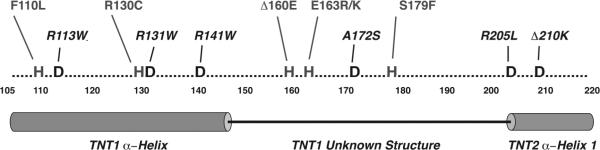
In 2000, Kamisago, et al, published the first linkage of primary DCM to an in-frame single amino acid deletion of cTnT Lys210 (ΔLys210)12. The clinical findings were notable for early onset DCM without any evidence for precedent hypertrophy, thus suggesting that the pathogenic pathways for dilated and non-dilated forms of cTnT-linked cardiomyopathies were likely to be distinct, a hypothesis that continues to be actively investigated to this day. Subsequent family studies identified several additional DCM-linked cTnT mutations (Arg131Trp, Arg141Trp, Arg205Leu and Asp270Asn)56, 68. Unlike the mixed morphologies noted in the HCM cohorts, many of the DCM-linked families exhibited high penetrance and severity, with early cardiac transplantation a not infrequent outcome. Of note, mutations that cause primary DCM are relatively common in cTnT, comprising ~ 20% of all known mutations. Moreover, as shown in Figure 4, the distribution of the HCM and DCM mutations are tightly interspersed within the C-terminal domain of TNT1 and the immediate N-terminal domain of TNT2, an intriguing finding for such disparate clinical entities that likely reflects the highly tuned function of this integral adapter protein within the cardiac sarcomere.
Figure 4.
Distribution of HCM and DCM – linked Mutations in the cTnT N-terminal Domain
Residues 105 to 220 of cTnT are shown. This region of cTnT is highly conserved and the structure of the protein between residues 150 – 200 is poorly defined. Mutations that lead to hypertrophic or non-dilated ventricular remodeling are shown in gray and are contiguous to DCM -causing mutations (black).
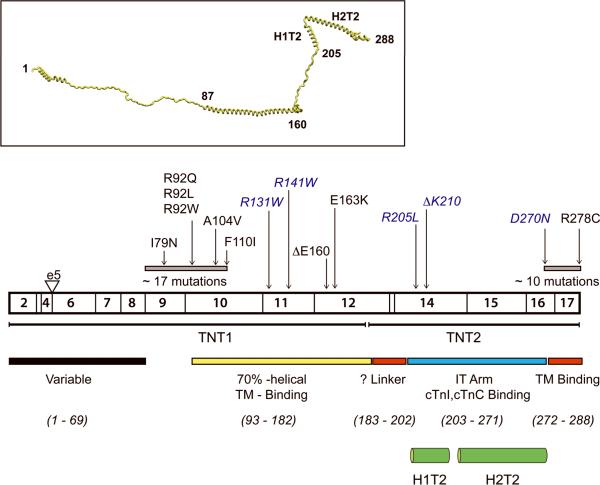
As shown in Figure 5, similar to the findings for cTnI:cTnC, disease-causing mutations in cTnT are highly clustered within two primary locations, all located in regions of the protein that are predicted to be highly mobile. The “classic” organization of cTnT into two major functional domains (TNT1, TNT2) is largely based on chymotryptic digest studies and likely demarcate regions of the protein that are accessible to proteolysis69. The TNT2 domain is directly bound to cTnI:cTnC and comprises a significant portion of the rigid IT arm (residues 203–271). This region was included in the Ca2+-activated core cTn crystal structure and consists of two α-helices (H1: 204–220, H2: 226–271) connected via a short 5 amino acid linker sequence30. Several of the known TNT2 mutations occur at the extreme N-terminal portion of the H1 helix and have been exclusively associated with DCM12, 39, 70. The remaining regions of the protein include the extended N-terminal (comprised of the N-terminal “hypervariable” region and the largely α-helical TNT1) and the extreme C-terminal (C-TnT) domains that were either not included in the cTn complexes that were crystallized or could not be resolved in the extant structures, likely due to the high degree of inherent protein flexibility required to modulate the physical position of TM in response to Ca2+ binding. In the main, these unresolved domains include over 90% of the known disease-causing mutations in cTnT and this lack of structural information remains an important limitation to elucidating the molecular mechanisms of this complex and often severe disease subset. The basic functional role of cTnT is to directly modulate the average position of TM between the 3 known states of myofilament activation. The response of the thin filament to both basal and increased hemodynamic demand is highly regulated by Ca2+ binding, post-translational modifications, the direct effects of myosin and stretch. These inputs are modulated locally by direct interactions between cTnI and cTnC within the globular core domain of cTn. The critical mechanism(s) whereby changes in these local protein-protein interactions are transduced to the elongated N-terminal domain of cTnT, the region directly responsible for both modulating the position of TM along the actin groove and stabilizing the head-to-tail array of contiguous TM molecules is unclear. Conversely, cTnT mutations in the N-terminal domain, in particular, occur at a great physical distance from the regulatory cTnI:cTnC complex. Thus, the disease mechanism underlying this large subset of cTnT – linked FHC mutations is likely to be strongly influenced by the allosteric propagation of the local changes in cTnT function to the rest of the thin filament “machine”.
Figure 5.
Human Cardiac cTnT Exons, Structure and Functional Domains
Listed mutations are discussed in text, Mutations in blue have been linked to DCM. Undefined structures (as per Takeda, et al) are red and black, gray boxes represent clusters of known mutations.
Inset: Predicted cTnT secondary structure
The central role of cTnT in modulating myofilament activation coupled to the large number of known mutations (with distinct clinical phenotypes) has lead to a broad array of studies by our lab and others, utilizing biochemical, biophysical, computational and animal model methodologies. Despite the lack of a high resolution structure for much of cTnT, this in silico to in vivo approach has begun to provide important mechanistic insight. Missense substitutions, single in-frame amino acid deletions and premature termination mutations have all been linked to cTnT-related FHC. As shown in Figure 5, there is significant mutational clustering between residues 69 – 110, with 17 independent mutations and three “hotspots” at residues 92, 94 and 110. While no primary DCM mutations have been mapped to this region, the observed clinical phenotypes are highly variable both within single families (Ile79Asn) and between independent substitutions at the same amino acid (Arg92Gln/Trp/Leu)62, 71. This clustering suggests that the N-terminal domain plays a complex modulatory role, whereby mutations alter but do not destroy normal function. Initial biophysical studies had described the elongated troponin tail as a Ca2+-independent anchor between cTn and the TM-actin complex72. In 1999, Hinkle, et al, used a series of full-length and truncated bovine cTnT molecules in binding studies to show that the residues 1–153 of the cTnT tail domain promoted TM binding to actin in the absence of cTnI:cTnC73. This observation along with previous reports raised the important question of whether cTnT independently contributed to the stabilization of TM in the low Ca2+ B-state. In a subsequent study, myosin-activated ATPase, regulated in vitro motility and myosin binding assays were utilized to demonstrate that residues 1–153 of cTnT alone exerted a strong inhibitory effect on the interaction of myosin with the rest of the thin filament74. Moreover, electron microscopy and 3D reconstruction on reconstituted thin filaments plus cTnT 1–153 performed in the absence of Ca2+ revealed that cardiac TM was localized to the outer domain of actin, consistent with a strengthening of the TM-actin interaction and suggested that the N-terminal tail played an important role in the inhibition of myofilament activation. Disruption of the native inhibitory function of the N-terminal tail by FHC-linked mutations would thus likely alter the distribution of TM between the B and C states under conditions of low Ca2+ and be manifest as an increase in the Ca2+ sensitivity of myofilament activation, a prediction that was fully confirmed by an extensive series of studies by multiple groups using reconstituted systems, both solution-based and in skinned fibers27, 28, 75, 76. It is important to note that while these findings are consistent, as previously shown for mutations in cTnI:cTnC, measurements of Ca2+ sensitivity, per se, are relatively low resolution and represent the complex outcome of multiple inputs and are thus somewhat limited in the context of determining precise mechanisms for non-dilated mutations.
Two independent studies had directly examined the biophysical effects of N-terminal mutations on TM-dependent properties of cTnT in both fragments (residues 70–170) and full length protein complexes77, 78. Several mutations that immediately preceded the crucial TM binding domain at residues 112–136 (Arg92Gln/Trp/Leu, Ala104Val and Phe110Ile) all decreased cTnT TM affinity and were less effective at promoting TM-actin binding. These effects were likely due to changes in α-helical stability that would be predicted to significantly alter the flexibility of the N-terminal tail. Interestingly, mutations outside this range (Ile79Asn, Δ160Glu and Glu163Lys) did not disrupt TM-dependent properties. Thus, similar to the findings for the C-terminal cTnI mutations, the degree of flexibility within this crucial cTnT domain acts to regulate the dynamic interactions with TM that, in part, determines the average position of TM as it transitions between the 3 states. While the original studies could predict the qualitative effects on N-terminal flexibility, to further delineate the potential impact of individual mutations on flexibility and structural dynamics at the atomic level, our lab has applied Molecular Dynamics (MD) approaches79. Mutation-specific alterations in peptide flexibility for each of the three known hotspot substitutions at Residue 92, Arg92Gln/Trp/Leu were observed34. Of note, patients with each of these substitutions have been indentified and exhibit mutation-specific patterns of ventricular remodeling. Average snapshots of the predicted motion reveal a striking “hinge” at Residue 104 (Arg92Leu >> Arg92Trp ~ Arg92Gln), 12 amino acids distant from the mutation site, a region that is known to be important for protein solubility and the site of a mutation linked to complex cardiac remodeling (Ala104Val). Based on this finding we recently performed additional analyses to determine how the mutations at Residue 92 lead to helical unwinding at Residue 104. Examination of carbon-carbon distances (n+4) for the WT and Arg92Trp, Arg92Leu revealed that the Trp and Leu substitutions induce a local helical electrostatic compaction and a subsequent helical expansion in the Residue 104 region. Thus the “bunching” of residues in the helix turns at the mutation site eventually results in a relative expansion of the helix turns in the first turn composed of hydrophobic residues resulting in mutation-specific unwinding and an increase in flexibility35. These findings are fully consistent with both the previous biophysical studies and our original hypothesis that the predicted dynamic changes induced by the residue 92 mutations would alter the structural character in the hinge region while maintaining the overall helical character of the peptide, thus “modulating” as opposed to destroying thin filament function. In addition, the observation that the molecular effects of single amino acid substitutions can be propagated throughout the helix to effect structural and dynamic changes at a significant physical distance suggests that mutations at independent residues can lead to similar effects on function. In this manner, the mutations clustered immediately proximal to the cTnT-TM binding domain at 115–139 may have similar effects on function by acting at a distance to alter the Residue 104 hinge region, a conclusion that could not easily be obtained via experiment.
While the vast complexity of the cardiac remodeling and clinical phenotypes observed in the hypertrophic or non-dilated patient cohorts has been mirrored by the biophysical findings, the DCM linked cTnT mutations are, in general more consistent on both levels. The first cTnT mutation to be linked to DCM was the in-frame deletion of a single Lys residue at 21012. As noted above, many of the DCM-linked thin filament mutations cause a relatively severe, early onset dilated cardiomyopathy that, in most cases, appears to be a primary event, as opposed to transitioning through a hypertrophic phase56. Initial biophysical studies showed that, unlike virtually all of the HCM-linked thin filament mutations, the DCM-linked ΔLys210 and Arg141Trp mutations led to a primary decrease in the Ca2+ sensitivity of tension when exchanged into skinned rabbit fibers, a result that would likely affect the ability of the heart to generate force80, 81. This surprising observation was subsequently expanded to nearly all of the known cTnT DCM mutations in a study by Mirza, et al82. Utilizing reconstituted human thin filament proteins in actin-TM activated ATPase and in vitro motility assays performed at both 100% and the expected physiologic 1:1 ratios of WT and mutant cTnT they demonstrated that the DCM-linked mutations all caused varying degrees of Ca2+ desensitization and decreases in thin filament activation. Thus the prevailing hypothesis for the clear discordance in cardiac remodeling between HCM and DCM was that the primary pathogenic mechanism is determined by the changes in Ca2+ sensitivity at the myofilament level. This was directly tested by Du, et al by generating a knock-in murine model of the cTnT ΔLys21083.Both the heterozygous (+/ΔLys210) and homozygous (ΔLys210/ΔLys210) mice were studied from the myocellular to whole heart levels, with the ΔLys210/ΔLys210 animals exhibiting a particularly severe early-onset and rapidly progressive course. Several important observations stand out. First, isolated fibers from both lines exhibited a decrease in Ca2+ sensitivity without a significant decrease in maximal isometric tension in the context of normal cTnI phosphorylation. Second, the amplitude of the Ca2+ transient and SR Ca2+ load were elevated in both mutant mouse lines, a potential compensatory change to a perceived lack of Ca2+ “responsiveness” at the myofilament level. Lastly, in a direct test of the potential mechanism, pimobendan, a potent PDE III inhibitor and direct myofilament cTnC Ca2+-sensitizer was orally administered to both mutant lines starting at 1 month of age. Treated ΔLys210/ΔLys210 mice exhibited a significant abrogation of virtually all of the observed pathogenic parameters including cardiac remodeling, increased Ca2+ transients and improved survival despite persistence of the initial decrease in Ca2+ sensitivity at the myofilament level. While this reversal is supportive of the basic hypothesis, both the severity of the ΔLys210/ΔLys210 phenotype (with rapid activation of apoptotic activity and precedent episodes of Torsades de Pointes and VF) and the mild response to pimobendan in the +/ΔLys210 mice suggest that multiple downsteam pathogenic signaling pathways are highly activated in the homozygous animals. Regardless, the ability to disrupt the pathogenic cascade with pimobendan is an encouraging and original observation from the clinical standpoint. Despite this high degree of consensus regarding the role of a decrease in the Ca2+ sensitivity of force development as a primary cause of DCM (in a field that is usually notable for the lack thereof), the structural mechanism(s) remain unclear. As is shown in Figure 4 virtually all of the known DCM-linked cTnT mutations fall within the C-terminal portion of TNT1 or the extreme N-terminal portion of TNT2 and are closely interspersed with known non-dilated mutations including a hotspot at 160–163. Residues distal to 203 were included in the core domain structure and comprise the initial component of the H1T2 helix (and the IT arm) and thus may directly affect Ca2+ sensitivity via interactions with the C-lobe of cTnC, though direct evidence is lacking. In contrast, while the TNT1 domain is thought to be ~ 70% α-helical, the C-terminal end of the region includes a highly charged sequence (158RREEEENRR166) that is likely to be accessible to solvent and may unwind to form a long flexible linker to TNT2. This domain is very highly conserved and a better understanding of the structure, function and of this unresolved region would provide a framework for determining the molecular mechanism that underlies the development of HCM vs DCM in cTnT-linked cardiomyopathies.
TM: The “gatekeeper” of myofiment activation
Tropomyosin and cardiac actin form a unique functional unit that plays both structural and dynamic roles in sarcomeric function. Moreover, both of these integral thin filament proteins impact the thin filament beyond the single basic 7:1:1 functional unit. The head to tail array of contiguous TM coiled-coil dimers is a central component of the cooperative activation of the myofilaments and actin interacts with the myocellular cytoskeleton via attachments at the Z-band, thus mutations in either of these thin filament proteins have the potential for far-reaching and complex effects on cardiac function.
While TM was one of the first thin filament proteins to be linked to FHC, it remains an infrequent cause of the disease, accounting for ~ 3% of sarcomeric gene mutations. Many of the available studies rely on relatively small clinical cohorts, and suggest a strong influence of genetic modifiers and environment on the end phenotype. A case in point is the Asp175Asn mutation, identified in the original linkage study and subsequently found in both Northern European and Japanese kindreds. Initial clinical profiles of the initial patient population described a highly variable pattern of mild-moderate LVH and an overall favorable prognosis with infrequent SCD4, 62. These results were supported by an extensive study of three independent families with the Asp175Asn mutation that revealed varying degrees of LVH (ranging from mild to moderate and fully echo penetrant by adulthood), consistent patterns of myofibrillar disarray and fibrosis, yet an overall stable clinical course and minimal SCD84. In contrast, two small studies from a Japanese HCM cohort detailed a strikingly different prognosis for patients carrying the Asp175Asn mutation, characterized by mixed age of onset HCM with progressive remodeling leading to dilatation and frequent SCD85, 86. A subsequent large single family study of a novel TM mutation, Ala95Val, revealed yet another seemingly “private” clinical pattern with mild hypertrophy and a high frequency of SCD87. HCM-linked mutations in cardiac actin are also rare and lead to heterogeneous phenotypes not dissimilar to those seen in TM-related disease. In both cases, sporadic mutations are common and often exhibit early onset, severe phenotypes.6, 88. Interestingly, the Glu99Lys mutation in cardiac actin has been linked to the development of apical hypertrophy in two independent studies, and this atypical pattern of cardiac remodeling is thought to represent the influence of specific, unknown genetic modifiers(s)6, 89. Thus, mutations in these two integral components of the thin filament contribute to complex clinical patterns that are strongly modulated by genetic and environmental factors, an observation that was validated experimentally by two independent transgenic mouse models of the Glu180Gly TM mutation where the resultant animals exhibited highly divergent phenotypes that were attributed to the effects of different genetic backgrounds90, 91.
Mutations in both TM and cardiac actin have been linked to familial DCM. Olson, et al utilized a candidate gene approach to screen for TPM1 mutations in 350 unrelated patients with idiopathic DCM and indentified two novel mutations, Glu40Lys and Glu54Lys92. While the family sizes were small and the age of presentation variable, both mutations led to a primary dilated LV phenotype with no evidence for an intermediary hypertrophic stage. A similar approach by the same group had previously identified two mutations in cardiac actin associated with primary DCM, Arg312His and Glu361Gly93. Definitive DCM linkage via segregation analysis was recently established for a novel TM mutation (Asp230Asn) in a extensive study by Lakdawala, et al where two large independent multigenerational families carrying the Asp230Asn mutations were identified and studied longitudinally57. Many of the initial clinical observations were similar to those noted for the Δ210Lys DCM mutation in cTnT, including early onset primary ventricular dilatation in children and young adults and a high frequency of SCD. The extended clinical follow up of these unique kindreds also revealed a bimodal pattern of disease expression with distinct “peaks” of presentation in early childhood (severe LV dilatation) and middle age (mild-moderate LV dilatation). Of particular note, as previously described in other thin filament related cases of DCM, many of the affected children responded to aggressive supportive medical therapy with stabilization of the disease process and significant recovery of LV systolic function by early adulthood. The authors posited that the presence of the Asp230Asn TM conferred a susceptibility to secondary cardiac injury, for example, amplifying the precipitous loss of systolic function that occurs in the context of myocarditis. This is perhaps a structural analogue to a loss of cardiac reserve that is thought to arise in the context of sarcomeric gene mutations that decrease the energetic efficiency of the heart94. It is possible that this potential “second-hit” mechanism for the Asp230Asn TM mutation represents another example of the unique sensitivity of TM and cardiac actin mutations to extra-sarcomeric modifiers including environmental, genetic and external pathogenic stimuli. As noted above, these two proteins are very highly conserved, both play essential structural and regulatory roles and disease-causing mutations are relatively rare. While speculative, some TPM1 and ACTC mutations may only be tolerated when their baseline effects on sarcomeric function are relatively mild and thus more severe manifestations occur only in the context of additional factors. The findings from the recently published mutant cardiac actin Glu316Gly transgenic mouse model generally support this hypothesis in that the 50% replacement mouse exhibited a minimal baseline phenotype at all levels studied and required β-agonist infusion to uncover a pathogenic response95.
Taken to it's logical conclusion and given both the ordered structure and the dynamics of the movements that govern the Ca2+ regulation of myofilament activation it is somewhat surprising that mutations in either TM or cardiac actin are tolerated in nature. Indeed, the relatively low incidence of FHC- related mutations in TM and cardiac actin likely reflect a relative intolerance to structural and/or functional disruption of this essential interaction. Tropomyosin represents a classic coiled-coil semi-rigid structure comprised of two α helices arranged in parallel (reviewed in96). Each TM α-helix is defined by a continuous series of 40 short-range heptad repeats (7 residues, a-b-c-d-e-f-g) that extend over two helical turns. Residues at positions a/d are generally apolar and act to internally stabilize the dimer while acidic residues at e/g of the heptad repeats interact with the basic residues on the flat surface of actin to form an electrostatic “sliding surface”, facilitating the TM movements that determine the 3 states of myofilament activation and contribute to stability of the overall structure. These basic amino acids also define a long-range periodicity ~ 40 residues long that matches the pitch of consecutive actin monomers. Each TM supercoil is composed of 7 such “periods” that are not 100% regular in spacing and contain small clusters of core alanine residues at positions a/d that serve to locally destabilize/narrow the structure, leading to a “stagger” and resultant bend in the coiled-coil that is central to the actin-TM interaction97, 98. An elegant series of mutational and “period-swapping” studies by Singh and Hitchcock further established that both the acidic surface residues and the instability conferred by the alanine clusters are required for TM-actin binding. Moreover, this requirement defines a hierarchy whereby periods 1 and 5 represent primary and 2–3, 6–7 are secondary actin binding sites99. This hypothesis requires an inherent local flexibility as the primary sites serve as pseudo-anchors while the secondary sites “search” for optimal interactions with actin, a dynamic process that is likely central to myofilament activation and may be directly affected by TM-related disease mutations. Finally, while the interactions between a single TM coiled-coil and 7 actin monomers defines the basic contractile functional unit, TM forms a contiguous array of head-to-tail dimers that form complex overlapping structures and strongly stabilize the actin filament throughout its length. Although the overall structure of these crucial TM overlap regions remains unclear, they play a central role in the cooperativity that is essential to the coordination of contractile function at the molecular level100, 101.
As shown in Figure 6, most of the known HCM - related mutations in TM are clustered within or flanking two major regions of the protein, the N-terminus (Gly62Glu, Ala63Val, Lys70Tyr, Val95Ala) and period 5 (Ile172Thr, Asp175Asn, Glu180Gly, Glu180Val, Leu185Arg, Glu192Lys). Two of the original TM mutations identified (Asp175Asn, Glu180Gly) occupy positions in the heptad repeat predicted to stabilize the coiled-coil via electrostatic interactions and are within a domain of TM that weakly binds to the globular head region of Tn (via TNT2). They have been extensively studied both in vitro and via transgenic animal models. To date, the findings have been strikingly consistent across both species and experimental approaches. Both Asp175Asn and Glu180Gly increase the Ca2+ sensitivity of myofilament activation102, 103. Similar results were obtained for all of the known HCM –linked mutations tested, thus fully recapitulating one of the central observations regarding the effects of HCM-linked thin filament protein mutations. Based in part on the predicted effects of e/g substitutions on TM stability, several groups determined whether there were mutation-specific effects on thermal properties and/or actin binding. Again, there was strong consensus across methodological approaches as several groups showed that the Glu180Gly substitution had a greater effect (e.g. a decrease in actin binding or an increase in thermal unfolding) than Asp175Asn104, 105. Due to the heterogeneity in the known patient populations it remains unclear as to whether these measureable mutation-specific differences in TM local structure specifically influence clinical outcomes. Results from transgenic animal models of the Glu180Gly and Asp175Asn TM mutations (both maintained on the FVB/N background), however, strongly suggest that differential downstream pathogenic signaling pathways are activated by the independent mutations, as the phenotype of the Glu180Gly mice is markedly more severe and accelerated as compared to the Asp175Asn mice at similar levels of transgene expression90, 106. While the basic mechanisms involved are unclear, recent advances in understanding the complex functional role of period 5 of TM (where both mutations reside) can guide future studies. One possible mechanism, the so-called “additive effect” could be applied to many of the thin filament protein mutations, whereby independent impairments in specific aspects of thin filament activation lead to more severe phenotypes based on cumulative functional disruption that may overwhelm myocellular compensatory responses. In the current example, as Glu180Gly alters both of the known functional roles of period 5 (actin binding and Ca2+ sensitivity) while Asp175Asn largely affects Ca2+ sensitivity, the overall effects of Glu180Gly may cause more severe remodeling via significant disruption of local flexibility. Moreover, as previously noted, mutations that lead to a greater effect on component protein-protein interactions such that primary structure is significantly disrupted may have a disproportionate effect on end phenotype.
Figure 6.
Human Cardiac TM Exons, Structure and Periods
Grey shaded periods represent the two primary actin-binding regions. Listed mutations are discussed in text, Mutations in blue have been linked to DCM.
Inset: Predicted TM secondary structure, P2 = Period 2, P5 = Period 5 (regions where mutations are clustered)
Based on the known structure, mutations in the N- and C- terminal overlapping domains of TM would be predicted to have distinct effects on myofilament structure and activation. Heller, et al directly addressed the basic question of whether the HCM-linked Ala63Val and Lys70Thr mutations in the TM N-terminal domain would affect cooperative activation via changes in flexibility within the crucial head-to-tail overlap region107. Similar to the previously described Asp175 and Glu180Gly mutations, both Ala63Val and Lys70Thr increased the Ca2+ sensitivity of actin-activated ATPase and thin filament sliding speed. Surprisingly, despite significant decreases in folding stability that would likely alter the flexibility of the mutant N-terminus and indirectly affect the C-terminal region of TM at a considerable distance, no measurable effects on cooperativity were observed. While it is possible that mutations that significantly affect cooperativity are simply non viable, the similarity of the observations among the HCM-linked mutations in different functional domains argues that current approaches do not have sufficient molecular resolution given the complexity of the overall complex. Of note, all the known primary DCM-linked TM mutations are located at either end of the molecule. While the available clinical information is limited, the Glu40Lys and Glu54Lys mutations in TM have extensively studied at both the in vitro and in vivo levels. These mutations do not appear to cause the bimodal distribution of clinical expression exhibited by other thin filament DCM mutations and may cause disease via unique mechanisms. The Glu40Lys and Glu54Lys substitutions cause a significant increase in the local positive potential at position e of the heptad (2 turns apart) and would be predicted to alter protein stability. Mirza et al, evaluated the potential structural effects of the N-terminal DCM mutations via CD and differential scanning calorimetry and found both local destabilization (40/54) and distant stabilization of the helix (54 alone), suggesting that Glu54Lys may affect TM structure at a considerable distance that may account for the more complex functional disruption108. The complexity of the biophysical findings for the N-terminal DCM mutants were matched by the Glu54Lys murine model, recapitulating some of the clinical phenotype albeit with a profound dosage effect on survival109. In the main, the potential mechanistic links between the biophysical effects at the molecular level and the observed changes in myofilament function remain obscure for both the HCM and DCM -linked mutations in TM, especially regarding predicted structural effects and flexibility where there is considerable overlap between mutations. One potential explanation for the difficulties in linking predicted local changes in TM structure to the observed functional effects lies in the potential for the propagation of structural effects through the helix as originally observed via MD for cTnT N-terminal mutations. These effects “at a distance” were supported by the findings of Heller and Mirza. More recently, Nirody, et al, used EM and MD to evaluate the effects of amino acid substitutions on TM flexibility at the atomistic level. Not only did they find that the substitution of Leu for a highly conserved Asp at residue 137 caused a significant increase in both flexibility and the curvature of TM, but that the bulk of the increase in TM curvature occurred around residue 175 (within a major mutational clustering site) with only minor changes in the local structure at observed about residue 137110. Thus, the predicted effects on myofilament activation would be expected to be more consistent with the functional role of the more distant site, a highly testable hypothesis that reinforces the utility of coupling computational approaches to experiment in order to obtain information regarding both local and propagated changes in protein function and dynamics caused by thin filament mutations. This recurring observation that independent mutations in disparate regions of the thin filament “machine” can cause similar functional effects from a distance suggests that a new classification of mutations based on their effects on myofilament activation as opposed to protein site may provide a more robust organization for future study.
Bridging the gap between biophysics and physiology: animal models
As noted here and in multiple prior reviews, the availability of transgenic and knock-in animal models of thin filament cardiomyopathies are essential to translating the primary molecular effects of mutations in sarcomeric proteins to the resultant impact on cardiac function111, 112. A case in point is the potential link between thin filament protein mutations and SCD. For many of the patient cohorts, the degree of LVH did not directly correlate with the risk of SCD, a central concern for clinical risk stratification and management. Animal model studies of thin filament mutations have identified two main primary mechanisms, myocellular energetics and Ca2+ regulation. First, independent single amino acid substitutions at cTnT Residue 92 in the N-terminal domain have been shown to directly increase the energetic cost of contraction at all workloads as measured via 31P NMR spectroscopy in contracting hearts113, 114. Of particular note, despite near-identical increases in the Ca2+ sensitivity of force generation for each of the three independent Residue 92 mutations, the measured increases in the free energy of ATP hydrolysis, (representing a baseline increase in energy utilization required to perform the identical workload) were mutation-specific with regards to magnitude and likely due to precise changes in local protein-protein dynamics within the N-terminal tail domain. The net effect would be a significant decrease in contractile reserve that would be dramatically accentuated in the context of increased hemodynamic demand and potentially trigger SCD115. Moreover, the long-term energetic compromise could contribute to cardiomyopathic remodeling. Both clinical NMR and animal studies suggest that abnormal cardiac energetics may represent a more general mechanism in HCM linked to sarcomeric gene mutations and thus represent a potentially important therapeutic target116.
A second central disease mechanism involves the complex and dynamic role of myocellular Ca2+ handling in thin filament cardiomyopathies. Alterations in the cellular distribution and myofibrillar response to Ca2+ have been documented at every level of investigation, from changes in the Ca2+ sensitivity of ATPase activity in reconstituted myofilaments to abnormal Ca2+ transients in murine transgenic myocytes to altered Ca2+ dependency of force generation in human patient samples. It is not entirely surprising that disrupting the function of the myofilament Ca2+ sensing “machine” would directly alter the Ca2+ regulation of activation. In fact, the oft-noted (and measured) changes in the Ca2+ sensitivity of force generation have been touted as a general disease paradigm based on the observed end-stage cardiac remodeling, whereby an increase leads to a “hypertrophic” and a decrease to a dilated response117. The latter observation has been a more consistent finding, with the prediction that the decrease in Ca2+ sensitivity (coupled to a decrease in the crossbridge recycling rate) would lead to a baseline primary defect in force generation that causes a compensatory ventricular dilatation to maintain cardiac output. Likewise, the observed increase in Ca2+ sensitivity for many of the HCM-linked thin filament mutations has been posited to result in a compensatory hypertrophic response. Given the overall lack of correlation between the magnitude of the increase in Ca2+ sensitivity and the degree of LVH, not to mention the existence of families with a broad range of ventricular remodeling in the context of a single mutation (as recently shown for cTnT Ile79Asn) the “compensatory” hypertrophy hypothesis remains unproven67, 118. Moreover, as noted in the cTnC Gly159Asp discussion above, recent work by the Solaro and Marston labs has shown that the supposed link between DCM and a decrease in Ca2+ sensitivity may be an oversimplification due to the important influence of cTnI phosphoryation59, 60. This does not mean that the observed effects of thin filament mutations on Ca2+ sensitivity do not contribute to disease pathogenesis. Jagatheesan, et al, used a transgenic chimeric TM mouse model to genetically reverse the increased Ca2+ sensitivity of force generation in their TM Glu180Gly mouse model, demonstrating that this property could serve as a primary therapeutic target119. Along similar lines, increasing myofilament Ca2+ sensitivity via the Ca2+ sensitizer EMD 57033 lowered the arrhythmia threshold of WT mice while decreasing Ca2+ sensitivity in mutant cTnT mice with blebbistatin exhibited a protective effect by increasing the arrhythmia threshold120. Thus, the observed primary alterations in Ca2+ sensitivity at the myofilament level are at the very least, an indication that a central aspect of the Ca2+-dependent sarcomeric activation is disrupted by thin filament mutations. The precise mechanistic role, however, remains unclear, though recent experimental advances to measure cTnC Ca2+ affinity in more complex systems will significantly advance our understanding of this central question121.
As noted by Alves, et al in their recent review, the effects of sarcomeric mutations on Ca2+-dependent functions are not limited to the myofilament level122. Analysis of isolated adult ventricular myocytes from several mutant thin filament mouse models have revealed significant downstream alterations in myocellular Ca2+ kinetics. Two mutant cTnT models have been extensively studied, the Arg92Trp/Leu transgenic mice and the Δ210Lys knock-in, linked to HCM and DCM respectively83, 123, 124. At baseline, skinned fibers isolated from the Arg92Trp mice had revealed an increase in the Ca2+ sensitivity of force generation and fibers from the ΔLys210 mice exhibited a decrease. Complex alterations in Ca2+ homeostasis were noted in both model systems and several observations are particularly noteworthy (for the following discussion the comparisons are between the models with matched mutant protein levels, the Arg92Trp-50% and the ΔLys210 +/−). First, while the peak amplitudes of the Ca2+ transients were decreased in the Arg92Trp mice, they were increased in myocytes isolated from the ΔLys210 animals. This is an intriguing finding as it suggests that the altered Ca2+ sensitivity at the myofilament level is directly coupled to the downstream response. For example, a decrease in myofilament Ca2+ sensitivity may be “sensed” by the cell as insufficient cytoplasmic Ca2+ and thus the transient is increased in response (and vice versa). The mechanism of the sensing is unclear, but the close coupling is supported by a second observation, that these changes in Ca2+ kinetics occur at early timepoints (< 2 months of age) and in the case of the Arg92Trp mice, significantly change over time. While the initial changes in myocellular Ca2+ are likely compensatory, chronic disregulation of Ca2+ handling could cause multiple downstream pathogenic effects including secondary activation of Ca2+ - regulated signaling pathways, cardiac remodeling, decreased arrhythmia thresholds and eventually apoptosis. Some of these secondary effects have been observed in mutant thin filament protein murine models and partial disease regression has been achieved with Ca2+ blocker treatment125. Therefore it is clear that a better understanding of the underlying Ca2+-mediated mechanisms at both the myofilament and cellular levels is clinically relevant and represents an excellent therapeutic target for this currently untreatable disorder.
Conclusion and Future Directions
In the main, our basic understanding of how thin filament mutations cause complex cardiomyopathies has significantly evolved on both the molecular and clinical levels. On the molecular side, mutation-induced local changes in protein dynamics and structure that disrupt the normal Ca2+ activated distribution of TM have been shown to have wide ranging effects on virtually every aspect of myofilament activation. These effects disrupt basal function, the ability of the sarcomere to respond to hemodynamic demands, and downstream Ca2+ handling. The role of phosphorylation, both PKA and more recently, PKC-mediated in the context of thin filament mutations is particularly important and again, must be evaluated in the context of a dynamic system as phosphorylation is often a transient effect, especially during the early compensatory response to contractile dysfunction. On the clinical side, there is renewed interest in both the earliest stages of the disease process and the overall time course of disease progression. For example, Ho, et al, recently showed that patients with preclinical HCM (sarcomeric gene mutation-positive, no overt LVH) exhibited abnormal relaxation with preserved systolic function with a later progression to both diastolic and systolic abnormalities126. It is important to note that the latter observations required newer methodologies to measure longitudinal peak systolic strain and systolic strain rate as LVEF was not sufficiently sensitive, thus demonstrating how a higher “resolution” approach to an established clinical parameter can potentially provide novel insight into disease progression. Coupled to the aforementioned findings regarding preclinical activation of profibrotic signaling in patients with FHC linked to MYBPC3 and MYH7 mutations, it is clear that study of these cohorts will be very informative and these approaches should be extended both to patients with thin filament mutations and available animal models. Along similar lines, the potential role of abnormal protein processing via the ubiquitin proteosome system has recently been identified as a contributor to abnormal remodeling in HCM, representing another potential point of intervention127. Finally, as recently noted by Kaski et al (and in support of earlier studies), the growing literature regarding the surprisingly high frequency of sarcomeric gene mutations in infants and children with HCM strongly suggests that our current view regarding the pathogenesis and progression of FHC needs to be reconsidered, especially regarding age of presentation128. This renewed focus on the earliest stages of the disorder will undoubtedly provide a more “proximal” phenotypic characterization. Armed with a robust molecular understanding and coupled to a less “end-stage” phenotype, the still elusive link(s) between genotype and phenotype will be uncovered. Of note, a recently published, “white paper” discussing the proposed research priorities for the next stage of HCM investigations addresses many of the issues raised in the current review and expands on others, it outlines an exciting way forward for this complex field129. In the end, this improved and integrated approach to studying thin filament cardiomyopathies will lead to novel and perhaps mutation or gene-specific approaches to interrupt the progression of this complex and currently untreatable disorder.
Generation of the human Ca2+-activated thin filament model (Shown in Figure 1).
The figure was obtained by docking cardiac troponin T to overlapping tropomyosin molecules, orienting the cardiac troponin and tropomyosins to the actin backbone and performing a 50ps simulation in equilibrium at 70 K using CHARMM33bl1. Cardiac troponin was constructed using an atomistic model of the entire cTn complex from PDB entry 1J1E, an atomistic model of TM based on Holmes' coordinates, an overlapping model of TM-TM based on PDB entry 2Z5I and cTn – TM interactions based on PDB entry 2Z5H2, 3. Missing regions of cardiac troponin were constructed using secondary structure prediction (PSIPRED) and homology with chicken fs Tn4–6. The native stoichiometry of the thin filament 7:1:1, (actin:tropomyosin:troponin) may be more accurately described as 14:2:1 (actin:tropomyosin:troponin) where one troponin requires two overlapping tropomyosins to properly bind, thus requiring 14 actin monomers and 2 tropomyosins per each cTn complex. The actin backbone for the overlapping tropomyosins was created using Holmes' coordinates and creating a 360 degree rotation about the long axis in the diagram, resulting in a 25.7 degree azimuthal rotation per monomer with a linear displacement of 57.25 Angstroms between monomer centers of mass. Recent evidence suggests a more likely rotation in the diagram would be an approximately 334 degree rotation about the long axis7, 8.
References
1. Brooks BR, Bruccolerii RE, Olafson BD, States DJ, Swaminathan S, Karplus M. Charmm: A program for macromolecular energy, minimization, and dynamics calculations. J Computational. Chem. 1983;4:187–217
2. Takeda S, Yamashita A, Maeda K, Maéda Y. Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form. Nature. 2003;424:35–41
3. Murakami K, Stewart M, Nozawa K, Tomii K, Kudou N, Igarashi N, Shirakihara Y, Wakatsuki S, Yasunaga T, Wakabayashi T. Structural basis for tropomyosin overlap in thin (actin) filaments and the generation of a molecular swivel by troponin-T. Proc Natl Acad Sci USA. 2008;105:7200–7205
4. Jones DT. Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol. 1999;292:195–202
5. Vinogradova MV, Stone DB, Malanina GG, Karatzaferi C, Cooke R, Mendelson RA, Fletterick RJ. Ca(2+)-regulated structural changes in troponin. Proc Natl Acad Sci USA. 2005;102:5038–5043
6. Bryson K, McGuffin LJ, Marsden RL, Ward JJ, Sodhi JS, Jones DT. Protein structure prediction servers at university college london. Nucleic Acids Res. 2005;33:W36–38
7. Oda T, Iwasa M, Aihara T, Maeda Y, Narita A. The nature of the globular- to fibrous-actin transition. Nature. 2009;457:441–445
8. Holmes KC. Structural biology: Actin in a twist. Nature. 2009;457:389–390
Acknowledgments
Sources of Funding: NIH/NHLBI (HL075619)
Footnotes
Disclosures: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geisterfer-Lowrance AA, Kass S, Tanigawa G, Vosberg HP, McKenna W, Seidman CE, Seidman JG. A molecular basis for familial hypertrophic cardiomyopathy: A beta cardiac myosin heavy chain gene missense mutation. Cell. 1990;62:999–1006. doi: 10.1016/0092-8674(90)90274-i. [DOI] [PubMed] [Google Scholar]
- 2.Teare D. Asymmetrical hypertrophy of the heart in young adults. Br.Heart J. 1958;20:1–8. doi: 10.1136/hrt.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon SD, Jarcho JA, McKenna W, Geisterfer-Lowrance A, Germain R, Salerni R, Seidman JG, Seidman CE. Familial hypertrophic cardiomyopathy is a genetically heterogeneous disease. J. Clin. Invest. 1990;86:993–999. doi: 10.1172/JCI114802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thierfelder L, Watkins H, MacRae C, Lamas R, McKenna W, Vosberg HP, Seidman JG, Seidman CE. Alpha-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: A disease of the sarcomere. Cell. 1994;77:701–712. doi: 10.1016/0092-8674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 5.Kimura A, Harada H, Park JE, Nishi H, Satoh M, Takahashi M, Hiroi S, Sasaoka T, Ohbuchi N, Nakamura T, Koyanagi T, Hwang TH, Choo JA, Chung KS, Hasegawa A, Nagai R, Okazaki O, Nakamura H, Matsuzaki M, Sakamoto T, Toshima H, Koga Y, Imaizumi T, Sasazuki T. Mutations in the cardiac troponin I gene associated with hypertrophic cardiomyopathy. Nature. 1997;16:379–382. doi: 10.1038/ng0897-379. [DOI] [PubMed] [Google Scholar]
- 6.Olson TM, Doan TP, Kishimoto NY, Whitby FG, Ackerman MJ, Fananapazir L. Inherited and de novo mutations in the cardiac actin gene cause hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2000;32:1687–1694. doi: 10.1006/jmcc.2000.1204. [DOI] [PubMed] [Google Scholar]
- 7.Landstrom AP, Parvatiyar MS, Pinto JR, Marquardt ML, Bos JM, Tester DJ, Ommen SR, Potter JD, Ackerman MJ. Molecular and functional characterization of novel hypertrophic cardiomyopathy susceptibility mutations in TNNC1-encoded troponin C. J Mol Cell Cardiol. 2008;45:281–288. doi: 10.1016/j.yjmcc.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank S, Braunwald E. Idiopathic hypertrophic subaortic stenosis. Clinical analysis of 126 patients with emphasis on the natural history. Circulation. 1968;37:759–788. doi: 10.1161/01.cir.37.5.759. [DOI] [PubMed] [Google Scholar]
- 9.Spirito P, Maron BJ. Relation between extent of left ventricular hypertrophy and diastolic filling abnormalities in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1990;15:808–813. doi: 10.1016/0735-1097(90)90278-w. [DOI] [PubMed] [Google Scholar]
- 10.Maron BJ, Bonow RO, Cannon RO, 3rd, Leon MB, Epstein SE. Hypertrophic cardiomyopathy. Interrelations of clinical manifestations, pathophysiology, and therapy (1) N Engl J Med. 1987;316:780–789. doi: 10.1056/NEJM198703263161305. [DOI] [PubMed] [Google Scholar]
- 11.Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet JP, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M. Hypertrophic cardiomyopathy: Distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107:2227–2232. doi: 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- 12.Kamisago M, Sharma SD, DePalma SR, Solomon S, Sharma P, McDonough B, Smoot L, Mullen MP, Woolf PK, Wigle ED, Seidman JG, Seidman CE. Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N.Engl.J.Med. 2000;343:1688–1696. doi: 10.1056/NEJM200012073432304. [DOI] [PubMed] [Google Scholar]
- 13.Mogensen J, Kubo T, Duque M, Uribe W, Shaw A, Murphy R, Gimeno JR, Elliott P, McKenna WJ. Idiopathic restrictive cardiomyopathy is part of the clinical expression of cardiac troponin I mutations. J. Clin. Invest. 2003;111:209–216. doi: 10.1172/JCI16336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubo T, Gimeno JR, Bahl A, Steffensen U, Steffensen M, Osman E, Thaman R, Mogensen J, Elliott PM, Doi Y, McKenna WJ. Prevalence, clinical significance, and genetic basis of hypertrophic cardiomyopathy with restrictive phenotype. J Am Coll Cardiol. 2007;49:2419–2426. doi: 10.1016/j.jacc.2007.02.061. [DOI] [PubMed] [Google Scholar]
- 15.Elliott P, McKenna WJ. How should hypertrophic cardiomyopathy be classified?: Molecular diagnosis for hypertrophic cardiomyopathy: Not ready for prime time. Circ Cardiovasc Genet. 2009;2:87–89. doi: 10.1161/CIRCGENETICS.108.835645. discussion 89. [DOI] [PubMed] [Google Scholar]
- 16.Ingles J, Doolan A, Chiu C, Seidman J, Seidman C, Semsarian C. Compound and double mutations in patients with hypertrophic cardiomyopathy: Implications for genetic testing and counselling. J Med Genet. 2005;42:e59. doi: 10.1136/jmg.2005.033886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Revera M, van der Merwe L, Heradien M, Goosen A, Corfield VA, Brink PA, Moolman-Smook JC. Long-term follow-up of R403W MYH7 and R92W TNNT2 HCM families: Mutations determine left ventricular dimensions but not wall thickness during disease progression. Cardiovascular journal of Africa. 2007;18:146–153. [PMC free article] [PubMed] [Google Scholar]
- 18.Ho CY, Lopez B, Coelho-Filho OR, Lakdawala NK, Cirino AL, Jarolim P, Kwong R, Gonzallez A, Colan SD, Seidman JG, Diez J, Seidman CE. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N.Eng.J.Med. J1 - NEJM. 2010;363:552–563. doi: 10.1056/NEJMoa1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooke R. Actomyosin interaction in striated muscle. Physiol. Rev. 1997;77:671–697. doi: 10.1152/physrev.1997.77.3.671. [DOI] [PubMed] [Google Scholar]
- 20.Takano M, Terada TP, Sasai M. Unidirectional brownian motion observed in an in silico single molecule experiment of an actomyosin motor. Proc. Natl. Acad. Sci. U.S.A. 2010;107:7769–7774. doi: 10.1073/pnas.0911830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solaro RJ, Rarick HM. Troponin and tropomyosin: Proteins that switch on and tune in the activity of cardiac myofilaments. Circ Res. 1998;83:471–480. doi: 10.1161/01.res.83.5.471. [DOI] [PubMed] [Google Scholar]
- 22.Ebashi S, Kodama A, Ebashi F. Troponin 1. Preparation and physiological function. J. Biochem. 1968;64:465–477. doi: 10.1093/oxfordjournals.jbchem.a128918. [DOI] [PubMed] [Google Scholar]
- 23.Hitchcock SE, Huxley HE, Szent-Gyorgyi AG. Calcium sensitive binding of troponin to actintropomyosin: A two-site model for troponin action. J Mol Biol. 1973;80:825–836. doi: 10.1016/0022-2836(73)90212-x. [DOI] [PubMed] [Google Scholar]
- 24.McKillop DF, Geeves MA. Regulation of the interaction between actin and myosin subfragment 1: Evidence for three states of the thin filament. Biophys J. 1993;65:693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pirani A, Vinogradova MV, Curmi PMG, King WA, Fletterick RJ, Craig R, Tobacman LS, Xu C, Hatch V, Lehman W. An atomic model of the thin filament in the relaxed and Ca2+-activated states. J Mol Biol. 2006;357:707–717. doi: 10.1016/j.jmb.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 26.Lin D, Bobkova A, Homsher E, Tobacman LS. Altered cardiac troponin T in vitro function in the presence of a mutation implicated in familial hypertrophic cardiomyopathy. J Clin Invest. 1996;97:2842–2848. doi: 10.1172/JCI118740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morimoto S, Yanaga F, Minakami R, Ohtsuki I. Ca2+-sensitizing effects of the mutations at Ile-79 and Arg-92 of troponin T in hypertrophic cardiomyopathy. Am J Physiol. 1998;275:C200–207. doi: 10.1152/ajpcell.1998.275.1.C200. [DOI] [PubMed] [Google Scholar]
- 28.Redwood C, Lohmann K, Bing W, Esposito GM, Elliott K, Abdulrazzak H, Knott A, Purcell I, Marston S, Watkins H. Investigation of a truncated cardiac troponin T that causes familial hypertrophic cardiomyopathy: Ca(2+) regulatory properties of reconstituted thin filaments depend on the ratio of mutant to wild-type protein. Circ.Res. 2000;86:1146–1152. doi: 10.1161/01.res.86.11.1146. [DOI] [PubMed] [Google Scholar]
- 29.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 30.Takeda S, Yamashita A, Maeda K, Maéda Y. Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form. Nature. 2003;424:35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]
- 31.Galińska-Rakoczy A, Engel P, Xu C, Jung H, Craig R, Tobacman LS, Lehman W. Structural basis for the regulation of muscle contraction by troponin and tropomyosin. J Mol Biol. 2008;379:929–935. doi: 10.1016/j.jmb.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saen-Oon S, Quaytman-Machleder S, Schramm VL, Schwartz SD. Atomic detail of chemical transformation at the transition state of an enzymatic reaction. Proc Natl Acad Sci U S A. 2008;105:16543–16548. doi: 10.1073/pnas.0808413105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nashine VC, Hammes-Schiffer S, Benkovic SJ. Coupled motions in enzyme catalysis. Curr Opin Chem Biol. 2010 doi: 10.1016/j.cbpa.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ertz-Berger BR, He H, Dowell C, Factor SM, Haim TE, Nunez S, Schwartz SD, Ingwall JS, Tardiff JC. Changes in the chemical and dynamic properties of cardiac troponin t cause discrete cardiomyopathies in transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 2005;102:18219–18224. doi: 10.1073/pnas.0509181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guinto JM,P, Schwartz S, Tardiff JC. Computational characterization of mutations in cardiac troponin t known to cause familial hypertrophic cardiomyopathy. Journal of Theoretical and Computational Chemistry. 2007;6:413–419. doi: 10.1142/S0219633607003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim CC, Yang H, Yang M, Wang CK, Shi J, Berg EA, Pimentel DR, Gwathmey JK, Hajjar RJ, Helmes M, Costello CE, Huo S, Liao R. A novel mutant cardiac troponin C disrupts molecular motions critical for calcium binding affinity and cardiomyocyte contractility. Biophys J. 2008;94:3577–3589. doi: 10.1529/biophysj.107.112896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kokado H, Shimizu M, Yoshio H, Ino H, Okeie K, Emoto Y, Matsuyama T, Yamaguchi M, Yasuda T, Fujino N, Ito H, Mabuchi H. Clinical features of hypertrophic cardiomyopathy caused by a Lys183 deletion mutation in the cardiac troponin I gene. Circulation. 2000;102:663–669. doi: 10.1161/01.cir.102.6.663. [DOI] [PubMed] [Google Scholar]
- 38.Kaski JP, Syrris P, Burch M, Tome-Esteban MT, Fenton M, Christiansen M, Andersen PS, Sebire N, Ashworth M, Deanfield JE, McKenna WJ, Elliott PM. Idiopathic restrictive cardiomyopathy in children is caused by mutations in cardiac sarcomere protein genes. Heart. 2008;94:1478–1484. doi: 10.1136/hrt.2007.134684. [DOI] [PubMed] [Google Scholar]
- 39.Mogensen J, Murphy RT, Kubo T, Bahl A, Moon JC, Klausen IC, Elliott PM, McKenna WJ. Frequency and clinical expression of cardiac troponin I mutations in 748 consecutive families with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;44:2315–2325. doi: 10.1016/j.jacc.2004.05.088. [DOI] [PubMed] [Google Scholar]
- 40.Carballo S, Robinson P, Otway R, Fatkin D, Jongbloed JDH, de Jonge N, Blair E, van Tintelen JP, Redwood C, Watkins H. Identification and functional characterization of cardiac troponin I as a novel disease gene in autosomal dominant dilated cardiomyopathy. Circ. Res. 2009;105:375–382. doi: 10.1161/CIRCRESAHA.109.196055. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman RM, Sykes BD. Disposition and dynamics: Interdomain orientations in troponin. Adv Exp Med Biol. 2007;592:59–70. doi: 10.1007/978-4-431-38453-3_7. [DOI] [PubMed] [Google Scholar]
- 42.Frazier A, Judge DP, Schulman SP, Johnson N, Holmes KW, Murphy AM. Familial hypertrophic cardiomyopathy associated with cardiac beta-myosin heavy chain and troponin I mutations. Pediatr Cardiol. 2008;29:846–850. doi: 10.1007/s00246-007-9177-9. [DOI] [PubMed] [Google Scholar]
- 43.Elliott K, Watkins H, Redwood CS. Altered regulatory properties of human cardiac troponin I mutants that cause hypertrophic cardiomyopathy. J Biol Chem. 2000;275:22069–22074. doi: 10.1074/jbc.M002502200. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi-Yanaga F, Morimoto S, Harada K, Minakami R, Shiraishi F, Ohta M, Lu QW, Sasaguri T, Ohtsuki I. Functional consequences of the mutations in human cardiac troponin I gene found in familial hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2001;33:2095–2107. doi: 10.1006/jmcc.2001.1473. [DOI] [PubMed] [Google Scholar]
- 45.James J, Zhang Y, Osinska H, Sanbe A, Klevitsky R, Hewett TE, Robbins J. Transgenic modeling of a cardiac troponin I mutation linked to familial hypertrophic cardiomyopathy. Circ Res. 2000;87:805–811. doi: 10.1161/01.res.87.9.805. [DOI] [PubMed] [Google Scholar]
- 46.Kobayashi, Solaro R. Increased Ca2+ affinity of cardiac thin filaments reconstituted with cardiomyopathy-related mutant cardiac troponin I. J.Biol.Chem. J1 - JBC. 2006 doi: 10.1074/jbc.M509561200. [DOI] [PubMed] [Google Scholar]
- 47.Galińska A, Hatch V, Craig R, Murphy AM, Van Eyk JE, Wang C-LA, Lehman W, Foster DB. The C terminus of cardiac troponin I stabilizes the Ca2+-activated state of tropomyosin on actin filaments. Circ. Res. 2010;106:705–711. doi: 10.1161/CIRCRESAHA.109.210047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffman RM, Blumenschein TM, Sykes BD. An interplay between protein disorder and structure confers the Ca2+ regulation of striated muscle. J Mol Biol. 2006;361:625–633. doi: 10.1016/j.jmb.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 49.Mathur MC, Kobayashi T, Chalovich JM. Negative charges at protein kinase C sites of troponin I stabilize the inactive state of actin. Biophys J. 2008;94:542–549. doi: 10.1529/biophysj.107.113944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Metzger JM, Westfall MV. Covalent and noncovalent modification of thin filament action: The essential role of troponin in cardiac muscle regulation. Circ. Res. 2004;94:146–158. doi: 10.1161/01.RES.0000110083.17024.60. [DOI] [PubMed] [Google Scholar]
- 51.Deng Y, Schmidtmann A, Redlich A, Westerdorf B, Jaquet K, Thieleczek R. Effects of phosphorylation and mutation R145G on human cardiac troponin i function. Biochemistry. 2001;40:14593–14602. doi: 10.1021/bi0115232. [DOI] [PubMed] [Google Scholar]
- 52.Deng Y, Schmidtmann A, Kruse S, Filatov V, Heilmeyer LMG, Jaquet K, Thieleczek R. Phosphorylation of human cardiac troponin I G203S and K206Q linked to familial hypertrophic cardiomyopathy affects actomyosin interaction in different ways. J. Mol. Cell. Cardiol. 2003;35:1365–1374. doi: 10.1016/j.yjmcc.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Gomes AV, Harada K, Potter JD. A mutation in the N-terminus of troponin I that is associated with hypertrophic cardiomyopathy affects the Ca(2+)-sensitivity, phosphorylation kinetics and proteolytic susceptibility of troponin. J. Mol. Cell. Cardiol. 2005;39:754–765. doi: 10.1016/j.yjmcc.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 54.Kobayashi T, Solaro RJ. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu Rev Physiol. 2005;67:39–67. doi: 10.1146/annurev.physiol.67.040403.114025. [DOI] [PubMed] [Google Scholar]
- 55.Howarth JW, Meller J, Solaro RJ, Trewhella J, Rosevear PR. Phosphorylation-dependent conformational transition of the cardiac specific N-extension of troponin I in cardiac troponin. J Mol Biol. 2007;373:706–722. doi: 10.1016/j.jmb.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 56.Mogensen J, Murphy RT, Shaw T, Bahl A, Redwood C, Watkins H, Burke M, Elliott PM, McKenna WJ. Severe disease expression of cardiac troponin C and T mutations in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2004;44:2033–2040. doi: 10.1016/j.jacc.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 57.Lakdawala NK, Dellefave L, Redwood CS, Sparks E, Cirino AL, Depalma S, Colan SD, Funke B, Zimmerman RS, Robinson P, Watkins H, Seidman CE, Seidman JG, McNally EM, Ho CY. Familial dilated cardiomyopathy caused by an alpha-tropomyosin mutation: The distinctive natural history of sarcomeric dilated cardiomyopathy. J Am Coll Cardiol. 2010;55:320–329. doi: 10.1016/j.jacc.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Preston LC, Lipscomb S, Robinson P, Mogensen J, McKenna WJ, Watkins H, Ashley CC, Redwood CS. Functional effects of the DCM mutant Gly159Asp troponin C in skinned muscle fibres. Pflugers Arch. 2007;453:771–776. doi: 10.1007/s00424-006-0161-7. [DOI] [PubMed] [Google Scholar]
- 59.Biesiadecki BJ, Kobayashi T, Walker JS, Solaro RJ, de Tombe PP. The troponin C G159D mutation blunts myofilament desensitization induced by troponin I Ser23/24 phosphorylation. Circ. Res. 2007 doi: 10.1161/01.RES.0000267744.92677.7f. [DOI] [PubMed] [Google Scholar]
- 60.Dyer EC, Jacques AM, Hoskins AC, Ward DG, Gallon CE, Messer AE, Kaski JP, Burch M, Kentish JC, Marston SB. Functional analysis of a unique troponin C mutation, Gly159Asp, that causes familial dilated cardiomyopathy, studied in explanted heart muscle. Circulation Heart Failure. 2009;2:456–464. doi: 10.1161/CIRCHEARTFAILURE.108.818237. [DOI] [PubMed] [Google Scholar]
- 61.Tobacman LS. Thin filament-mediated regulation of cardiac contraction. Ann.Rev.Physiol. 1996;58:447–481. doi: 10.1146/annurev.ph.58.030196.002311. [DOI] [PubMed] [Google Scholar]
- 62.Watkins H, McKenna WJ, Thierfelder L, Suk HJ, Anan R, O'Donoghue A, Spirito P, Matsumori A, Moravec CS, Seidman JG. Mutations in the genes for cardiac troponin T and alpha-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med. 1995;332:1058–1064. doi: 10.1056/NEJM199504203321603. [DOI] [PubMed] [Google Scholar]
- 63.Van Driest SL, Ackerman MJ, Ommen SR, Shakur R, Will ML, Nishimura RA, Tajik AJ, Gersh BJ. Prevalence and severity of “benign” mutations in the beta-myosin heavy chain, cardiac troponin T, and alpha-tropomyosin genes in hypertrophic cardiomyopathy. Circulation. 2002;106:3085–3090. doi: 10.1161/01.cir.0000042675.59901.14. [DOI] [PubMed] [Google Scholar]
- 64.Moolman JC, Corfield VA, Posen B, Ngumbela K, Seidman C, Brink PA, Watkins H. Sudden death due to troponin T mutations. J Am Coll Cardiol. 1997;29:549–555. doi: 10.1016/s0735-1097(96)00530-x. [DOI] [PubMed] [Google Scholar]
- 65.Varnava AM, Elliott PM, Baboonian C, Davison F, Davies MJ, McKenna WJ. Hypertrophic cardiomyopathy: Histopathological features of sudden death in cardiac troponin T disease. Circulation. 2001;104:1380–1384. doi: 10.1161/hc3701.095952. [DOI] [PubMed] [Google Scholar]
- 66.Van Driest SL, Ellsworth EG, Ommen SR, Tajik AJ, Gersh BJ, Ackerman MJ. Prevalence and spectrum of thin filament mutations in an outpatient referral population with hypertrophic cardiomyopathy. Circulation. 2003;108:445–451. doi: 10.1161/01.CIR.0000080896.52003.DF. [DOI] [PubMed] [Google Scholar]
- 67.Menon SC, Michels VV, Pellikka PA, Ballew JD, Karst ML, Herron KJ, Nelson SM, Rodeheffer RJ, Olson TM. Cardiac troponin T mutation in familial cardiomyopathy with variable remodeling and restrictive physiology. Clin Genet. 2008;74:445–454. doi: 10.1111/j.1399-0004.2008.01062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li D, Czernuszewicz GZ, Gonzalez O, Tapscott T, Karibe A, Durand JB, Brugada R, Hill R, Gregoritch JM, Anderson JL, Quinones M, Bachinski LL, Roberts R. Novel cardiac troponin T mutation as a cause of familial dilated cardiomyopathy. Circulation. 2001;104:2188–2193. doi: 10.1161/hc4301.098285. [DOI] [PubMed] [Google Scholar]
- 69.Ohtsuki I. Molecular arrangement of troponin-T in the thin filament. J Biochem. 1979;86:491–497. doi: 10.1093/oxfordjournals.jbchem.a132549. [DOI] [PubMed] [Google Scholar]
- 70.Hershberger RE, Pinto JR, Parks SB, Kushner JD, Li D, Ludwigsen S, Cowan J, Morales A, Parvatiyar MS, Potter JD. Clinical and functional characterization of TNNT2 mutations identified in patients with dilated cardiomyopathy. Circ Cardiovasc Genet. 2009;2:306–313. doi: 10.1161/CIRCGENETICS.108.846733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Forissier JF, Carrier L, Farza H, Bonne G, Bercovici J, Richard P, Hainque B, Townsend PJ, Yacoub MH, Fauré S, Dubourg O, Millaire A, Hagège AA, Desnos M, Komajda M, Schwartz K. Codon 102 of the cardiac troponin T gene is a putative hot spot for mutations in familial hypertrophic cardiomyopathy. Circulation. 1996;94:3069–3073. doi: 10.1161/01.cir.94.12.3069. [DOI] [PubMed] [Google Scholar]
- 72.Heald RW, Hitchcock-DeGregori SE. The structure of the amino terminus of tropomyosin is critical for binding to actin in the absence and presence of troponin. J Biol Chem. 1988;263:5254–5259. [PubMed] [Google Scholar]
- 73.Hinkle A, Goranson A, Butters CA, Tobacman LS. Roles for the troponin tail domain in thin filament assembly and regulation. A deletional study of cardiac troponin T. J.Biol.Chem. J1 -JBC. 1999;274:7157–7164. doi: 10.1074/jbc.274.11.7157. [DOI] [PubMed] [Google Scholar]
- 74.Tobacman LS, Nihli M, Butters C, Heller M, Hatch V, Craig R, Lehman W, Homsher E. The troponin tail domain promotes a conformational state of the thin filament that suppresses myosin activity. J Biol Chem. 2002;277:27636–27642. doi: 10.1074/jbc.M201768200. [DOI] [PubMed] [Google Scholar]
- 75.Harada K, Potter JD. Familial hypertrophic cardiomyopathy mutations from different functional regions of troponin T result in different effects on the pH and Ca2+ sensitivity of cardiac muscle contraction. J. Biol. Chem. 2004;279:14488–14495. doi: 10.1074/jbc.M309355200. [DOI] [PubMed] [Google Scholar]
- 76.Chandra M, Tschirgi ML, Tardiff JC. Increase in tension-dependent atp consumption induced by cardiac troponin T mutation. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H2112–2119. doi: 10.1152/ajpheart.00571.2005. [DOI] [PubMed] [Google Scholar]
- 77.Palm T, Graboski S, Hitchcock-DeGregori SE, Greenfield NJ. Disease-causing mutations in cardiac troponin T: Identification of a critical tropomyosin-binding region. Biophys J. 2001;81:2827–2837. doi: 10.1016/S0006-3495(01)75924-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hinkle A, Tobacman LS. Folding and function of the troponin tail domain. Effects of cardiomyopathic troponin T mutations. J. Biol. Chem. 2003;278:506–513. doi: 10.1074/jbc.M209194200. [DOI] [PubMed] [Google Scholar]
- 79.Brooks BR, Bruccolerii RE, Olafson BD, States DJ, Swaminathan S, Karplus M. Charmm: A program for macromolecular energy, minimization, and dynamics calculations. J. Computational. Chem. 1983;4:187–217. [Google Scholar]
- 80.Morimoto S, Lu QW, Harada K, Takahashi-Yanaga F, Minakami R, Ohta M, Sasaguri T, Ohtsuki I. Ca(2+)-desensitizing effect of a deletion mutation delta K210 in cardiac troponin T that causes familial dilated cardiomyopathy. Proc Natl Acad Sci U S A. 2002;99:913–918. doi: 10.1073/pnas.022628899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu Q-W, Morimoto S, Harada K, Du C-K, Takahashi-Yanaga F, Miwa Y, Sasaguri T, Ohtsuki I. Cardiac troponin t mutation R141W found in dilated cardiomyopathy stabilizes the troponin T-tropomyosin interaction and causes a Ca2+ desensitization. J. Mol. Cell. Cardiol. 2003;35:1421–1427. doi: 10.1016/j.yjmcc.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 82.Mirza M, Marston S, Willott R, Ashley C, Mogensen J, McKenna W, Robinson P, Redwood C, Watkins H. Dilated cardiomyopathy mutations in three thin filament regulatory proteins result in a common functional phenotype. J. Biol. Chem. 2005;280:28498–28506. doi: 10.1074/jbc.M412281200. [DOI] [PubMed] [Google Scholar]
- 83.Du CK, Morimoto S, Nishii K, Minakami R, Ohta M, Tadano N, Lu QW, Wang YY, Zhan DY, Mochizuki M, Kita S, Miwa Y, Takahashi-Yanaga F, Iwamoto T, Ohtsuki I, Sasaguri T. Knock-in mouse model of dilated cardiomyopathy caused by troponin mutation. Circ. Res. 2007;101:185–194. doi: 10.1161/CIRCRESAHA.106.146670. [DOI] [PubMed] [Google Scholar]
- 84.Coviello DA, Maron BJ, Spirito P, Watkins H, Vosberg HP, Thierfelder L, Schoen FJ, Seidman JG, Seidman CE. Clinical features of hypertrophic cardiomyopathy caused by mutation of a “hot spot” in the alpha-tropomyosin gene. J Am Coll Cardiol. 1997;29:635–640. doi: 10.1016/s0735-1097(96)00538-4. [DOI] [PubMed] [Google Scholar]
- 85.Yamauchi-Takihara K, Nakajima-Taniguchi C, Matsui H, Fujio Y, Kunisada K, Nagata S, Kishimoto T. Clinical implications of hypertrophic cardiomyopathy associated with mutations in the alpha-tropomyosin gene. Heart. 1996;76:63–65. doi: 10.1136/hrt.76.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakajima-Taniguchi C, Matsui H, Nagata S, Kishimoto T, Yamauchi-Takihara K. Novel missense mutation in alpha-tropomyosin gene found in japanese patients with hypertrophic cardiomyopathy. J Mol Cell Cardiol. 1995;27:2053–2058. doi: 10.1016/0022-2828(95)90026-8. [DOI] [PubMed] [Google Scholar]
- 87.Karibe A, Tobacman LS, Strand J, Butters C, Back N, Bachinski LL, Arai AE, Ortiz A, Roberts R, Homsher E, Fananapazir L. Hypertrophic cardiomyopathy caused by a novel alpha-tropomyosin mutation (V95A) is associated with mild cardiac phenotype, abnormal calcium binding to troponin, abnormal myosin cycling, and poor prognosis. Circulation. 2001;103:65–71. doi: 10.1161/01.cir.103.1.65. [DOI] [PubMed] [Google Scholar]
- 88.Mogensen J, Klausen IC, Pedersen AK, Egeblad H, Bross P, Kruse TA, Gregersen N, Hansen PS, Baandrup U, Borglum AD. Alpha-cardiac actin is a novel disease gene in familial hypertrophic cardiomyopathy. J. Clin. Invest. 1999;103:R39–43. doi: 10.1172/JCI6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arad M, Penas-Lado M, Monserrat L, Maron BJ, Sherrid M, Ho CY, Barr S, Karim A, Olson TM, Kamisago M, Seidman JG, Seidman CE. Gene mutations in apical hypertrophic cardiomyopathy. Circulation. 2005;112:2805–2811. doi: 10.1161/CIRCULATIONAHA.105.547448. [DOI] [PubMed] [Google Scholar]
- 90.Prabhakar R, Boivin GP, Grupp IL, Hoit B, Arteaga G, Solaro JR, Wieczorek DF. A familial hypertrophic cardiomyopathy alpha-tropomyosin mutation causes severe cardiac hypertrophy and death in mice. J Mol Cell Cardiol. 2001;33:1815–1828. doi: 10.1006/jmcc.2001.1445. [DOI] [PubMed] [Google Scholar]
- 91.Michele DE, Gomez CA, Hong KE, Westfall MV, Metzger JM. Cardiac dysfunction in hypertrophic cardiomyopathy mutant tropomyosin mice is transgene-dependent, hypertrophy-independent, and improved by beta-blockade. Circ Res. 2002;91:255–262. doi: 10.1161/01.res.0000027530.58419.82. [DOI] [PubMed] [Google Scholar]
- 92.Olson TM, Kishimoto NY, Whitby FG, Michels VV. Mutations that alter the surface charge of alpha-tropomyosin are associated with dilated cardiomyopathy. J Mol Cell Cardiol. 2001;33:723–732. doi: 10.1006/jmcc.2000.1339. [DOI] [PubMed] [Google Scholar]
- 93.Olson TM, Michels VV, Thibodeau SN, Tai YS, Keating MT. Actin mutations in dilated cardiomyopathy, a heritable form of heart failure. Science. 1998;280:750–752. doi: 10.1126/science.280.5364.750. [DOI] [PubMed] [Google Scholar]
- 94.Ingwall JS. Transgenesis and cardiac energetics: New insights into cardiac metabolism. J Mol Cell Cardiol. 2004;37:613–623. doi: 10.1016/j.yjmcc.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 95.Song W, Dyer E, Stuckey D, Leung M-C, Memo M, Mansfield C, Ferenczi M, Liu K, Redwood C, Nowak K, Harding S, Clarke K, Wells D, Marston S. Investigation of a transgenic mouse model of familial dilated cardiomyopathy. J. Mol. Cell. Cardiol. 2010 doi: 10.1016/j.yjmcc.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 96.Brown JH, Cohen C. Regulation of muscle contraction by tropomyosin and troponin: How structure illuminates function. Adv Protein Chem. 2005;71:121–159. doi: 10.1016/S0065-3233(04)71004-9. [DOI] [PubMed] [Google Scholar]
- 97.Brown JH, Kim KH, Jun G, Greenfield NJ, Dominguez R, Volkmann N, Hitchcock-DeGregori SE, Cohen C. Deciphering the design of the tropomyosin molecule. Proc Natl Acad Sci U S A. 2001;98:8496–8501. doi: 10.1073/pnas.131219198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singh A, Hitchcock-DeGregori SE. Local destabilization of the tropomyosin coiled coil gives the molecular flexibility required for actin binding. Biochemistry. 2003;42:14114–14121. doi: 10.1021/bi0348462. [DOI] [PubMed] [Google Scholar]
- 99.Singh A, Hitchcock-DeGregori SE. Tropomyosin's periods are quasi-equivalent for actin binding but have specific regulatory functions. Biochemistry. 2007;46:14917–14927. doi: 10.1021/bi701570b. [DOI] [PubMed] [Google Scholar]
- 100.Greenfield NJ, Swapna GV, Huang Y, Palm T, Graboski S, Montelione GT, Hitchcock-DeGregori SE. The structure of the carboxyl terminus of striated alpha-tropomyosin in solution reveals an unusual parallel arrangement of interacting alpha-helices. Biochemistry. 2003;42:614–619. doi: 10.1021/bi026989e. [DOI] [PubMed] [Google Scholar]
- 101.Murakami K, Stewart M, Nozawa K, Tomii K, Kudou N, Igarashi N, Shirakihara Y, Wakatsuki S, Yasunaga T, Wakabayashi T. Structural basis for tropomyosin overlap in thin (actin) filaments and the generation of a molecular swivel by troponin-T. Proc Natl Acad Sci U S A. 2008;105:7200–7205. doi: 10.1073/pnas.0801950105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bottinelli R, Coviello DA, Redwood CS, Pellegrino MA, Maron BJ, Spirito P, Watkins H, Reggiani C. A mutant tropomyosin that causes hypertrophic cardiomyopathy is expressed in vivo and associated with an increased calcium sensitivity. Circ Res. 1998;82:106–115. doi: 10.1161/01.res.82.1.106. [DOI] [PubMed] [Google Scholar]
- 103.Michele DE, Albayya FP, Metzger JM. Direct, convergent hypersensitivity of calcium-activated force generation produced by hypertrophic cardiomyopathy mutant alpha-tropomyosins in adult cardiac myocytes. Nat Med. 1999;5:1413–1417. doi: 10.1038/70990. [DOI] [PubMed] [Google Scholar]
- 104.Golitsina N, An Y, Greenfield N, Thierfelder L, Iizuka K, Seidman J, Seidman C, Lehrer S, Hitchcock-DeGregori S. Effects of two familial hypertrophic cardiomyopathy-causing mutations on alpha-tropomyosin structure and function. Biochemistry. 1999;38:3850. doi: 10.1021/bi9950701. [DOI] [PubMed] [Google Scholar]
- 105.Kremneva E, Boussouf S, Nikolaeva O, Maytum R, Geeves MA, Levitsky DI. Effects of two familial hypertrophic cardiomyopathy mutations in alpha-tropomyosin, Asp175Asn and Glu180Gly, on the thermal unfolding of actin-bound tropomyosin. Biophys J. 2004;87:3922–3933. doi: 10.1529/biophysj.104.048793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Muthuchamy M, Pieples K, Rethinasamy P, Hoit B, Grupp IL, Boivin GP, Wolska B, Evans C, Solaro RJ, Wieczorek DF. Mouse model of a familial hypertrophic cardiomyopathy mutation in alpha-tropomyosin manifests cardiac dysfunction. Circ.Res. 1999;85:47–56. doi: 10.1161/01.res.85.1.47. [DOI] [PubMed] [Google Scholar]
- 107.Heller M, Nili M, Homsher E, Tobacman L. Cardiomyopathic tropomyosin mutations that increase thin filament Ca2+ sensitivity and tropomyosin N-domain flexibility. Journal of Biological. 2003 doi: 10.1074/jbc.M303408200. [DOI] [PubMed] [Google Scholar]
- 108.Mirza M, Robinson P, Kremneva E, Copeland On, Nikolaeva O, Watkins H, Levitsky D, Redwood C, El-Mezgueldi M, Marston S. The effect of mutations in alpha-tropomyosin (E40K and E54K) that cause familial dilated cardiomyopathy on the regulatory mechanism of cardiac muscle thin filaments. J Biol Chem. 2007;282:13487–13497. doi: 10.1074/jbc.M701071200. [DOI] [PubMed] [Google Scholar]
- 109.Rajan S, Ahmed RPH, Jagatheesan G, Petrashevskaya N, Boivin GP, Urboniene D, Arteaga GM, Wolska BM, Solaro RJ, Liggett SB, Wieczorek DF. Dilated cardiomyopathy mutant tropomyosin mice develop cardiac dysfunction with significantly decreased fractional shortening and myofilament calcium sensitivity. Circ. Res. 2007;101:205–214. doi: 10.1161/CIRCRESAHA.107.148379. [DOI] [PubMed] [Google Scholar]
- 110.Nirody J, Li X, Sousa D, Sumida J, Fischer S, Lehrer S, Lehman W. Electron microscopy and molecular dynamics on a D137L mutant of tropomyosin. Biophysical Journal (Meeting Abstracts) 2010;98:1–766. [Google Scholar]
- 111.Tardiff JC. Sarcomeric proteins and familial hypertrophic cardiomyopathy: Linking mutations in structural proteins to complex cardiovascular phenotypes. Heart failure reviews. 2005;10:237–248. doi: 10.1007/s10741-005-5253-5. [DOI] [PubMed] [Google Scholar]
- 112.Jagatheesan G, Rajan S, Wieczorek DF. Investigations into tropomyosin function using mouse models. J. Mol. Cell. Cardiol. 2010;48:893–898. doi: 10.1016/j.yjmcc.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Javadpour MM, Tardiff JC, Pinz I, Ingwall JS. Decreased energetics in murine hearts bearing the R92Q mutation in cardiac troponin T. J Clin Invest. 2003;112:768–775. doi: 10.1172/JCI15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.He H, Javadpour MM, Latif F, Tardiff JC, Ingwall JS. R-92L and R-92W mutations in cardiac troponin T lead to distinct energetic phenotypes in intact mouse hearts. Biophys J. 2007;93:1834–1844. doi: 10.1529/biophysj.107.107557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schwartz K, Mercadier J-J. Cardiac troponin T and familial hypertrophic cardiomyopathy: An energetic affair. J. Clin. Invest. 2003;112:652–654. doi: 10.1172/JCI19632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Crilley JG, Boehm EA, Blair E, Rajagopalan B, Blamire AM, Styles P, McKenna WJ, Ostman-Smith I, Clarke K, Watkins H. Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of hypertrophy. J Am Coll Cardiol. 2003;41:1776–1782. doi: 10.1016/s0735-1097(02)03009-7. [DOI] [PubMed] [Google Scholar]
- 117.Robinson P, Griffiths P, Watkins H. Dilated and hypertrophic cardiomyopathy mutations in troponin and {alpha}-tropomyosin have opposing effects on the calcium affinity of cardiac thin filaments. Circulation. 2007 doi: 10.1161/CIRCRESAHA.107.156380. [DOI] [PubMed] [Google Scholar]
- 118.Palmer BM, Fishbaugher DE, Schmitt JP, Wang Y, Alpert NR, Seidman CE, Seidman JG, VanBuren P, Maughan DW. Differential cross-bridge kinetics of FHC myosin mutations R403Q and R453C in heterozygous mouse myocardium. Am J Physiol Heart Circ Physiol. 2004;287:H91–99. doi: 10.1152/ajpheart.01015.2003. [DOI] [PubMed] [Google Scholar]
- 119.Jagatheesan G, Rajan S, Petrashevskaya N, Schwartz A, Boivin G, Arteaga GM, Solaro RJ, Liggett SB, Wieczorek DF. Rescue of tropomyosin-induced familial hypertrophic cardiomyopathy mice by transgenesis. Am J Physiol Heart Circ Physiol. 2007;293:H949–958. doi: 10.1152/ajpheart.01341.2006. [DOI] [PubMed] [Google Scholar]
- 120.Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, Potter JD, Knollmann BC. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest. 2008;118:3893–3903. doi: 10.1172/JCI36642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tikunova SB, Liu B, Swindle N, Little SC, Gomes AV, Swartz DR, Davis JP. Effect of calcium-sensitizing mutations on calcium binding and exchange with troponin C in increasingly complex biochemical systems. Biochemistry. 2010;49:1975–1984. doi: 10.1021/bi901867s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alves ML, Gaffin RD, Wolska BM. Rescue of familial cardiomyopathies by modifications at the level of sarcomere and Ca2+ fluxes. J. Mol. Cell. Cardiol. 2010;48:834–842. doi: 10.1016/j.yjmcc.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Haim TE, Dowell C, Diamanti T, Scheuer J, Tardiff JC. Independent FHC-related cardiac troponin T mutations exhibit specific alterations in myocellular contractility and calcium kinetics. J. Mol. Cell. Cardiol. 2007;42:1098–1110. doi: 10.1016/j.yjmcc.2007.03.906. [DOI] [PubMed] [Google Scholar]
- 124.Guinto PJ, Haim TE, Dowell-Martino CC, Sibinga N, Tardiff JC. Temporal and mutation-specific alterations in Ca2+ homeostasis differentially determine the progression of cTnT-related cardiomyopathies in murine models. Am J Physiol Heart Circ Physiol. 2009;297:H614–626. doi: 10.1152/ajpheart.01143.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Westermann D, Knollmann BC, Steendijk P, Rutschow S, Riad A, Pauschinger M, Potter JD, Schultheiss H-P, Tschöpe C. Diltiazem treatment prevents diastolic heart failure in mice with familial hypertrophic cardiomyopathy. Eur J Heart Fail. 2006;8:115–121. doi: 10.1016/j.ejheart.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 126.Ho CY, Carlsen C, Thune JJ, Havndrup O, Bundgaard H, Farrohi F, Rivero J, Cirino AL, Andersen PS, Christiansen M, Maron BJ, Orav EJ, Køber L. Echocardiographic strain imaging to assess early and late consequences of sarcomere mutations in hypertrophic cardiomyopathy. Circ Cardiovasc Genet. 2009;2:314–321. doi: 10.1161/CIRCGENETICS.109.862128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Predmore JM, Wang P, Davis F, Bartolone S, Westfall MV, Dyke DB, Pagani F, Powell SR, Day SM. Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies. Circulation. 2010;121:997–1004. doi: 10.1161/CIRCULATIONAHA.109.904557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kaski JP, Syrris P, Esteban MTT, Jenkins S, Pantazis A, Deanfield JE, McKenna WJ, Elliott PM. Prevalence of sarcomere protein gene mutations in preadolescent children with hypertrophic cardiomyopathy. Circ Cardiovasc Genet. 2009;2:436–441. doi: 10.1161/CIRCGENETICS.108.821314. [DOI] [PubMed] [Google Scholar]
- 129.Force T, Bonow RO, Houser SR, Solaro RJ, Hershberger RE, Adhikari B, Anderson ME, Boineau R, Byrne BJ, Cappola TP, Kalluri R, LeWinter MM, Maron MS, Molkentin JD, Ommen SR, Regnier M, Tang WH, Tian R, Konstam MA, Maron BJ, Seidman CE. Research priorities in hypertrophic cardiomyopathy: Report of a working group of the national heart, lung, and blood institute. Circulation. 2010;122:1130–1133. doi: 10.1161/CIRCULATIONAHA.110.950089. [DOI] [PMC free article] [PubMed] [Google Scholar]