Abstract
Objectives
To examine the association between cerebral hyperintensities and cerebrovascular risk factors (CVRF) among middle-aged and older adults with major depressive disorder (MDD).
Methods
Thirty patients (aged 55–77 years) with MDD and no history of stroke participated in a magnetic resonance imaging assessment to assess for the presence of cerebral hyperintensities and underwent a physical examination to assess stroke risk as indexed by the Framingham Stroke Risk Profile (FSRP). In addition, intima medial thickness (IMT) was measured in the left and right carotid arteries.
Results
Higher FSRP levels were associated with total greater cerebral hyperintensities (r = 0.64), as well as greater subependymal hyperintensities (r = 0.47), confluent periventricular changes (r = 0.46), and tended to be associated with subcortical gray matter hyperintensities (r = 0.34). A quadratic relationship was observed between IMT and total cerebral hyperintensities (b = 4.84), and higher IMT levels were associated with greater subependymal hyperintensities (r = 0.40).
Conclusions
Higher levels of CVRF are associated with graded increases in cerebral hyperintensities among middle-aged and older adults with MDD.
Keywords: Cerebrovascular risk factors, vascular disease, cerebral hyperintensities, major depression
Major depressive disorder (MDD) is the leading cause of disability in the United States1 and has been associated with greater cerebrovascular risk factors (CVRF) and subclinical vascular disease.2 Older individuals with MDD are often characterized by increased frequency of cerebral hyperintensities seen on magnetic resonance imaging (MRI), as well as a later onset of first depressive episode.3 Although previous studies among healthy adults have demonstrated a dose–response association between CVRF and cerebral hyperintensities,4 the graded nature of this relationship has not been evaluated in patients with MDD in which case–control methodologies have been widely used. Therefore, we investigated the relationship between CVRF and cerebral hyperintensities among 30 adults with MDD, aged 55 years and older. We hypothesized that higher levels of CVRF would be associated with greater cerebral hyperintensities.
METHODS
Study Design
The sample examined in this report represents a subset of individuals from a larger clinical trial among middle-aged and older adults with MDD.5 The current analysis is limited to those individuals (N = 30) who volunteered for MRI assessments at baseline. The inclusion criteria includes age at least 55 years with MDD based on clinician-diagnosed Diagnostic and Statistics Manual fourth edition criteria. Exclusion criteria have been detailed elsewhere.5
Cerebrovascular Risk Factors
Framingham Stroke Risk Profile
The Framingham Stroke Risk Profile (FSRP), a risk assessment tool used to assess the risk of incident stroke, was used as an index of CVRF.6 Risk factors used to assess stroke risk include systolic blood pressure (SBP), use of antihypertensive therapy, diabetes mellitus, cigarette smoking, cardiovascular disease, and atrial fibrillation.6 Details of our CVRF assessments have been previously reported.7
Intima Medial Thickness of the Carotid Arteries
Carotid artery intima medial thickness (IMT) was assessed by high-resolution B-mode ultrasound using an Acuson Aspen (Mountain View, CA) vascular imaging system with 10-MHz linear array transducer. Ultrasound examinations of the far wall of the left and right common carotid arteries were used to acquire longitudinal images spanning 2 cm proximal to the carotid bulb. IMT of the far wall of the left and right common carotid arteries was measured over a 1-cm segment using Carotid Analyzer 5.0.5 (Medical Imaging Applications LLC, Iowa City, IA) edge detection software. Far wall measurements only were used as near wall measurements have been shown to have limited reliability.
Hyperintensities in the Brain
Magnetic Resonance Imaging
Participants were assessed using a 1.5 Tesla whole-body MRI system with standard head coil and an anterior neck coil for the carotid vascular imaging. The examination included a standard clinical assessment of the brain that gathered T1-weighted, T2-weighted, and proton-density-weighted images for assessment of brain pathology. Calibration standards for T2 (80 and 120-msec T2 values) were included within the field of view. The first set of images was obtained with an axial, multisection, T1-weighted pulse sequence (TR= 500 msec, TE= 15 msec) with a 256 × 256 matrix, 5-mm section thickness with a 7.5-mm distance between section centers, a 20-cm field of view, and 1 excitation per phase-encoding increment. This was followed by a long TR (2500 msec), double-echo (TE= 30 and 80 msec) spin-echo data-acquisition sequence using the same field of view, section thickness, and spacing, with flow compensation gradients, 256 × 256 matrix, using conjugate synthesis (half-Fourier). Saturation of spins outside the imaging volume (standard gap 15 mm) and flow compensation (gradient moment nulling) were used to eliminate artifacts due to flowing blood and CSF. Aliasing in the frequency-encoding axis was eliminated by oversampling and filtration. The studies were filmed and archived by a trained technologist.
Lesion ratings were performed by two experienced clinicians, blinded to participants’ treatment assignment, using the Boyko lesion classification system,8 which assesses cerebral hyperintensities as follows: frontal caps: 0 = absent, 1 = present; sub-ependymal hyperintensities: 0 = absent, 1 = thin, 2 = thick >2 mm; confluent periventricular changes: 0 = absent, 1 = hypointense to gray matter and hyperintense to CSF on proton density images, 2 = isointense to gray matter and hyperintense to CSF on proton density images, 3 = hyperintense to CSF on T2 weighted images, 4 = hyperintense to CSF on T2-weighted images and isointense to CSF on T1-weighted images; deep white matter hyperintensities: 0 = absent, 1 = punctate, 2 = rounded <5 mm, 3 = irregular >5 mm, 4 = confluent lesions; and subcortical gray matter hyperintensities: 0 = absent, 1 = punctate, 2 = rounded <5 mm, 3 = irregular >5 mm, 4 = confluent lesions. Prior studies from our group have consistently demonstrated interrater reliability of >0.80 on each of the hyperintensity item ratings. All participants were screened by the technologist for the presence of cardiac pacemakers, neurostimulants, metallic implants, metal in the orbit, aneurysm clips, or any other circumstance for which MRI was contraindicated.
Assessment of Depression
The presence of MDD was determined by a trained clinical psychologist using the Diagnostic Interview Schedule9 and the 17-item Hamilton Depression Rating Scale to assess MDD severity. Assessments were performed by licensed clinical psychologists blinded to the treatment condition. All participants met criteria for MDD based on Diagnostic and Statistical Manual-IV.
Statistical Analysis
Data Reduction
To minimize the number of statistical models examining the association between CVRF and cerebral hyperintensities, all hyperintensities were summed to create a continuous criterion variable in our primary model. Supplementary analyses were conducted within each lesion area only if the primary model demonstrated a significant relationship between CVRF and total cerebral hyperintensities.
Data Analysis
For the purposes of our primary analysis, total cerebral hyperintensities served as our criterion variable in a linear (Pearson) correlation analysis with FSRP levels as the sole predictor. To determine the relationship between cerebral hyperintensities and IMT, we estimated a general linear model with the continuous form of cerebral hyperintensities as the response, IMT as the predictor of primary interest, and age as an adjustment covariate. We examined model assumptions using graphical methods, modifying the model as necessary.
Secondary analyses were conducted within each lesion location. Because only one participant did not exhibit frontal caps during their MRI assessment, we were unable to conduct secondary analyses within this lesion area. Spearman rank correlation was used to examine the association among FRSP levels, IMT, and cerebral hyperintensities within each lesion area due to non-normality of the distributions within individual lesion locations. In all analyses, FSRP levels and IMT were scaled using the interquartile range, which allows the parameter estimate to be interpreted as comparing a “typical” person in the middle of the upper half of the predictor distribution with a “typical” person in the middle of the lower half of the predictor distribution.
RESULTS
Sample Characteristics
Thirty individuals participated in MRI assessments, ranging in age from 55 to 77 years (mean = 58.8 years, SD = 4.8). The primary reasons for not participating in MRI assessments were age <55 years (79%), participant declined (13%), or medical contraindications to participation (3.4%). Participants were mild-to-moderately depressed (mean Hamilton Depression score = 16.3, SD = 5.3) and did not exhibit any gross cognitive deficits. The majority of participants were female (66.7%), European American (83.3%), and almost half were college educated (47.7%). Participants were generally in good health, as indexed by relatively low levels of SBP (mean SBP = 126 mmHg, SD = 17) and LDL to HDL cholesterol ratio (mean ratio = 2.3, SD = 0.9), FSRP levels (mean FSRP score = 5.1, SD = 2.7), and relatively low severity of cerebral hyperintensities (mean cerebral hyperintensities = 3.1, SD = 2.2). No participants reported a history of stroke, and the sample was also relatively free of cardiovascular comorbidities such as diabetes (6.7%), arthritis (33.3%), and current tobacco use (23.3%).
CVRF and Cerebral Hyperintensities
Framingham Stroke Risk Profile
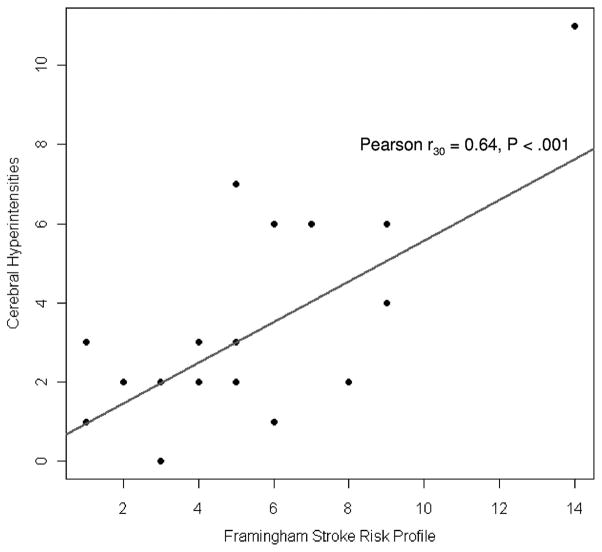
Higher FSRP levels were associated with total greater cerebral hyperintensities (Pearson r30 = 0.64, p <0.001; Fig. 1). After controlling for age, we found a significant correlation between increasing SBP levels and total cerebral hyperintensities (t27 = 2.18, b = 1.22, p = 0.038). Supplementary analyses within each lesion area demonstrated that higher FSRP levels were associated with greater subependymal hyperintensities (Spearman r30 = 0.47, p = 0.009) and confluent periventricular changes (Spearman r30 = 0.46, p = 0.011), but were not statistically significantly associated with subcortical gray matter hyperintensities (Spearman r30 = 0.34, p = 0.066) or deep white matter hyperintensities (Spearman r30 = 0.23, p = 0.215).
FIGURE 1.
Framingham Stroke Risk Profile Levels and Cerebral Hyperintensities
Intima Medial Thickness
On examining residuals from our primary model, we observed strong evidence of nonlinearity between cerebral hyperintensities and IMT. Thus, we estimated a model that included both a quadratic and linear term for IMT, along with the age covariate. The quadratic relation was statistically significant (t26 = 2.08, b = 4.84, p = 0.047), such that the cerebral hyperintensities increased exponentially in the presence of higher levels of IMT. In addition, supplementary analyses demonstrated that higher levels of IMT were associated with greater subependymal hyperintensities (Spearman r30 = 0.40, p = 0.030), but were not statistically significantly related to confluent periventricular changes (Spearman r30 = 0.25, p = 0.183), subcortical gray (Spearman r30 = −0.10, p = 0.595), or deep white matter hyperintensities (Spearman r30 = 0.01, p = 0.957).
DISCUSSION
Results from this analysis demonstrate that greater CVRF are associated with greater cerebral hyperintensities among middle-aged and older adults with MDD. These results extend previous findings2 by demonstrating that a graded relationship exists between CVRF and cerebral hyperintensities among individuals with MDD.
The relationship between cerebral health and depression has received increasing attention, initially through “vascular depression” hypothesis by Alexopoulos et al. and recently by Taylor et al. who recommended a new diagnostic classification of subcortical ischemic depression.2 Although population-based studies have demonstrated that greater CVRF are associated with greater white matter hyperintensities,4 to our knowledge, this is the first study to demonstrate a graded relationship between CVRF and cerebral hyperintensities among middle-aged and older individuals with MDD, despite similar findings among case–control studies.10 This finding is important because it provides preliminary data that cerebral damage may be observed at relatively low levels of cerebrovascular risk among older adults with depression, indicating that the risk of depression and cerebral health may be continuous, in contrast to the threshold relationship previously postulated.2 Taken together with recent findings that the onset of depression and number of previous episodes are associated with subclinical atherosclerosis, but not current depression severity,11 the current findings provide evidence that risk of depression associated with vascular health exists on a continuum, such that treatment of vascular risk factors may help prevent the occurrence of depression in later life. Although preliminary, replication of these findings could have important implications for treatment strategies among individuals at risk for late-life depression and provides further evidence that managing vascular risk factors is an important treatment target warranting further investigation.12
We note that this preliminary report has several limitations. Because only 30 of the 54 qualified individuals chose to participate in the MRI portion of our study, the generalizability of our findings is limited. In addition, our MRI protocol did not use a FLAIR sequence, which would have improved the sensitivity of our scans to structural damage. Finally, participants exhibited relatively low levels of cerebral hyperintensities and few subependymal hyperintensities and confluent periventricular changes, in particular. Therefore, the current analyses may have been underpowered to examine the relationship between CVRFs and cerebral hyperintensities within specific lesion areas, such as deep white matter hyperintensities. Therefore, the present findings must be viewed as preliminary and should be examined in larger samples with a greater prevalence of cardiovascular disease.
In summary, higher levels of CVRF are associated with greater cerebral hyperintensities among middle-aged and older adults with MDD. Future studies should investigate the prospective association between CVRF and cerebral hyperintensities to elucidate the causal relationship between these factors. Moreover, future studies would benefit from the inclusion of more sensitive measures of cerebral white matter damage, such as functional MRI and diffusion tensor imaging, in assessing structural white matter changes among individuals with MDD.
Acknowledgments
The work was supported by grants MH 49679 and HL080664–01A1 from the National Institutes of Health and M01-RR-30 from the General Clinical Research Center Program, National Center for Research Resources, National Institutes of Health. Presented at the American Psychosomatic Society Annual Conference, Chicago, March 7, 2009. Dr. P. Murali Doraiswamy has over the past 2 years received grants (through Duke University) or honoraria from Novartis, Forest, BristolMyersSquibb, Pfizer, Otsuka, Lundbeck, AVID, Neuronetrix, and Schering. He owns shares in Sonexa. Dr. David Steffens is a consultant to Johnson & Johnson, received financial support for travel expenses to present at a national meeting from Bristol-Myers Squibb and receives research funding from the National Institute of Mental Health.
References
- 1.Simon GE. Social and economic burden of mood disorders. Biol Psychiatry. 2003;54:208–215. doi: 10.1016/s0006-3223(03)00420-7. [DOI] [PubMed] [Google Scholar]
- 2.Taylor WD, Steffens DC, Krishnan KR. Psychiatric disease in the twenty-first century: the case for subcortical ischemic depression. Biol Psychiatry. 2006;60:1299–1303. doi: 10.1016/j.biopsych.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 4.Jeerakathil T, Wolf PA, Beiser A, et al. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69:587–596. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dagostino RB, Wolf PA, Belanger AJ, et al. Stroke risk profile—adjustment for antihypertensive medication—the Framingham-Study. Stroke. 1994;25:40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 7.Smith PJ, Blumenthal JA, Babyak MA, et al. Cerebrovascular risk factors, vascular disease, and neuropsychological outcomes in adults with major depression. Psychosom Med. 2007;69:578–586. doi: 10.1097/PSY.0b013e31812f7b8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyko OB, Alston SR, Fuller GN, et al. Utility of postmortem magnetic resonance imaging in clinical neuropathology. Arch Pathol Lab Med. 1994;118:219–225. [PubMed] [Google Scholar]
- 9.Robins LN, Helzer JE, Croughan J, et al. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 10.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and depressive symptoms in elderly adults. Arch Gen Psychiatry. 2000;57:1071–1076. doi: 10.1001/archpsyc.57.11.1071. [DOI] [PubMed] [Google Scholar]
- 11.Smith PJ, Blumenthal JA, Babyak MA, et al. Intima-media thickness and age of first depressive episode. Biol Psychol. 2009;80:361–364. doi: 10.1016/j.biopsycho.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flicker L. Vascular factors in geriatric psychiatry: time to take a serious look. Curr Opin Psychiatry. 2008;21:551–554. doi: 10.1097/YCO.0b013e32830ef736. [DOI] [PubMed] [Google Scholar]