Abstract
The present study evaluated the hypothesis that developmental changes in hypothalamic sleep-regulatory neuronal circuits contribute to the maturation of sleep homeostasis in rats during the fourth postnatal week. In a longitudinal study, we quantified electrographic measures of sleep during baseline and in response to sleep deprivation (SD) on postnatal days 21/29 (P21/29) and P22/30 (experiment 1). During 24-h baseline recordings on P21, total sleep time (TST) during the light and dark phases did not differ significantly. On P29, TST during the light phase was significantly higher than during the dark phase. Mean duration of non-rapid-eye-movement (NREM) sleep bouts was significantly longer on P29 vs. P21, indicating improved sleep consolidation. On both P22 and P30, rats exhibited increased NREM sleep amounts and NREM electroencephalogram delta power during recovery sleep (RS) compared with baseline. Increased NREM sleep bout length during RS was observed only on P30. In experiment 2, we quantified activity of GABAergic neurons in median preoptic nucleus (MnPN) and ventrolateral preoptic area (VLPO) during SD and RS in separate groups of P22 and P30 rats using c-Fos and glutamic acid decarboxylase (GAD) immunohistochemistry. In P22 rats, numbers of Fos+GAD+ neurons in VLPO did not differ among experimental conditions. In P30 rats, Fos+GAD+ counts in VLPO were elevated during RS. MnPN neuronal activity was state-dependent in P22 rats, but Fos+GAD+ cell counts were higher in P30 rats. These findings support the hypothesis that functional emergence of preoptic sleep-regulatory neurons contributes to the maturation of sleep homeostasis in the developing rat brain.
Keywords: postnatal development, sleep-promoting systems, sleep homeostasis, preoptic hypothalamus
sleep in adult mammals is homeostatically regulated. Rats exhibit a gradual emergence of different components of sleep homeostasis during postnatal development (5, 14, 40, 47). Some aspects of sleep homeostasis are present in rats on postnatal day (P) 2, when sleep deprivation (SD) increases sleep time during postdeprivation recovery sleep (47). Postdeprivation increases in non-rapid-eye-movement (NREM) sleep delta (δ) power, a measure of the intensity of homeostatic response in adults (7), are observed in P24 rats but not in P20 rats (14). In developing rats, electroencephalogram (EEG) spectral power in the δ frequency (0.5–4.0 Hz) in NREM sleep progressively increases across P10–P20 (13, 22). However, the normal decline in NREM sleep δ-wave activity during the rest phase, typical of adult rats and reflective of adult sleep homeostasis, is absent in rats younger than P24 (13). The above findings suggest that sleep homeostasis in rats undergoes significant maturation between the third and fourth postnatal weeks.
Little is known about the development of sleep-regulatory neuronal systems in early postnatal life in mammals. Sleep in adult rats is associated with activation of GABAergic neurons in the preoptic hypothalamus, including the median preoptic nucleus (MnPN) and the ventral lateral preoptic area (VLPO) (41, 45, 16, 44, 20). Activation of GABAergic neurons in the VLPO and MnPN is a factor in the suppression of monoaminergic, cholinergic, and hypocretinergic arousal-regulatory systems during sleep (for review, see Refs. 38 and 46). Some evidence suggests that VLPO and MnPN have different functions in the homeostatic response to SD. Based on patterns of c-Fos protein expression, MnPN neurons are activated during periods of SD, suggesting involvement of MnPN neurons in mediating sleepiness and promoting sleep onset in response to SD (25). Maximal activation in VLPO neurons occurs during recovery sleep following SD, suggesting a role for VLPO neurons in regulating sleep continuity and sleep depth during recovery sleep (25). This hypothesized dichotomy of function (24, 46) is supported by electrophysiological (44, 45) and anatomical studies in adult animals (49, 48, 8).
The present study evaluated the hypothesis that developmental changes in hypothalamic sleep-regulatory neuronal circuits during the fourth postnatal week contribute to the maturation of sleep homeostasis in rats. In a longitudinal study (experiment 1), we quantified baseline diurnal distribution and consolidation of sleep-wakefulness states on P21 and P29, using electrographic measures of sleep and wakefulness. On P22 and P30, in these same rats, we quantified sleep-wake and EEG responses to SD. In experiment 2, we quantified activity of MnPN and VLPO GABAergic neurons in the conditions of spontaneous sleep, SD, and recovery sleep in separate groups of P22 and P30 rats using immunohistochemistry for c-Fos protein and glutamic acid decarboxylase (GAD).
MATERIALS AND METHODS
All experimental protocols were approved by the Animal Care and Use Committee at Veterans Affairs Greater Los Angeles and were conducted according to the guidelines of the National Research Council and the National Institute of Health.
Animals and Experimental Environment
A total of 60 Sprague-Dawley rats were cross-fostered among 23 different litters, and the littermates were assigned to different experimental groups. The litter sizes were culled to 8–10 pups within 3 days of birth. Day of birth was designated as P0. To habituate the animals to the SD procedures, all pups were handled for 10–15 min in their home cages, without separation from dam or litter, two to three times a day for the 7 days before the separation from dams. The pups were separated from dams on P18. The animals were first housed by pairs in small acrylic cages containing bedding from their home cage and transferred to the recording room. The cages were placed in environmental chambers (Fisher Scientific, Pittsburgh, PA) at an age-appropriate temperature (P18: 25°C; P19: 24°C; P20–P30: 22°C) (1, 13, 26). The light-dark cycle was kept the same as in the animal house (6:00 A.M.–6:00 P.M., with lights on at 6:00 A.M.). The pups were fed rat chow and water. To facilitate the feeding, food was crushed and mixed with the bedding. To verify that the pups could eat rat chow and reach water, they were video monitored within the first 90–120 min after the separation from dams. On P19, the pups were housed individually, still in cages containing bedding from their home cage. Equal numbers of male and female pups were randomly assigned to two series of experiments.
Daily handling of the pups for the SD procedures was continued after separation from the dam. To interrupt sleep episodes during SD protocols, rat pups were subjected to gentle arousing stimuli (tapping on the cage and/or slight movement of the cage) within 3–5 s of the first appearance of EEG signs of sleep (in experiment 1) or at the first appearance of behavioral signs of sleep (in experiment 2). Animals were exposed to the stimuli as often as necessary to maintain wakefulness within the entire SD period.
Experiment 1. Baseline Sleep-Wakefulness Cycle Architecture and Homeostatic Responses to SD on P21/P29 and P22/P30
Experimental procedures.
P19 rats (n = 6) were anesthetized with methoxyflurane gas inhalation and surgically implanted with chronic cortical EEG and dorsal neck electromyogram (EMG) electrodes for assessment of the sleep-wakefulness states. Leads of flexible Teflon insulated wires were inserted under the skull in the frontal and parietal cortices and in neck muscles. To minimize the weight of the assembly to be anchored to the skull of the rat pups, no connectors were used, and the EEG/EMG wires were directly affixed on the skull with sterile dental acrylic. After the completion of surgery, the pups were returned to the home incubators and connected to the recording system. The EEG/EMG wires were long enough to reach the rotating commutators affixed to the counterweighted beam. The wires were lightly suspended above the animals. This system allowed the animals' unimpeded movement throughout the chambers. The wires were connected to Grass amplifiers (15A94 Quad Neuroamplifiers; Grass Technologies: Astro-Med Industrial Park, West Warwick, RI) throughout the entire experimental period. EEG potentials were band-pass filtered (0.5–30 Hz), and EMG data were high-pass filtered (>10 Hz). The signals were digitized at 256-Hz sampling rate and stored on a computer hard drive. For manual scoring of sleep-wake, the EEG and EMG channels were displayed on the computer screen using Spike 2 software (CED system; Cambridge Electronic Design, Great Britain; scoring and spectral analysis scripts were provided by R. Nienhuis). A 24-h baseline sleep-wake recording for these rats was first conducted on P21. On P22, the rats were subjected to 2 h SD at Zeitgeber time (ZT, hours after the lights on) 1–3. After the completion of the SD protocol, the rats were recorded for 1 h postdeprivation recovery sleep. A second baseline recording was accomplished on P29. On P30, the rats were again subjected to 2 h SD at ZT1–3 and recorded for postdeprivation recovery sleep at ZT3–4.
An additional group of rats (n = 6) was surgically implanted with EEG/EMG electrodes on P27, 2 days before the 24-h baseline sleep-wake recording scheduled for P29. This group (ConP29) served as a control for a “postsurgical effect” on the architecture of the sleep-wakefulness cycle considering the short time period (2 days of the postsurgery recovery) allowed before conducting the first baseline recording, in the longitudinal study.
Experiment 2. Fos Immunoreactivity in MnPN and VLPO GABAergic Neurons During Spontaneous Sleep, SD, and Recovery Sleep in P22 and P30 Rats
Experimental groups and procedures.
P22 (n = 24) and P30 (n = 24) rats were assigned to the experimental conditions of spontaneous sleep, SD, and recovery sleep. Twelve rats from each age group were permitted spontaneous sleep-wakefulness starting at ZT1, for either 2 (n = 6) or 3 (n = 6) h and killed at ZT3 and ZT4, respectively. Six P22 and six P30 rats were subjected to 2 h SD (starting at ZT1) and killed immediately after the completion of the SD protocol, at ZT3. Additional P22 and P30 rats (n = 6 for each age group) were subjected to 2 h SD and then allowed 1 h postdeprivation recovery sleep before death at ZT4.
To estimate time spent in total sleep and wakefulness, each individual rat pup was video recorded throughout the entire experimental period. A video camera (Sony 1/3 Inch Super HAD CCD) was mounted on the top of the recording chamber. Data were stored on a personal computer for off-line analysis.
Immediately after the end of all experiments, rats were given a lethal dose of anesthetic followed by perfusion (see below).
Immunohistochemistry.
Under deep pentobarbital anesthesia (100 mg/kg), animals were transcardially perfused with physiological PBS (0.01 M, pH: 7.4) followed by 4% paraformaldehyde (PFA) in phosphate buffer (0.1 M, pH: 7.4). The brains were then removed and postfixed in the same 4% PFA solution for 15 min and transferred to 30% sucrose in 0.1 M phosphate buffer (pH: 7.4) at 4°C until they sank. Brain tissue was processed for double immunostaining for Fos, the protein product of the immediate-early gene c-fos (10, 37), and GAD. Coronal sections, 40 μm, were cut through the MnPN and the VLPO on a freezing microtome. Free-floating sections were processed for Fos protein staining first. Sections were incubated overnight in a rabbit anti-c-Fos primary antiserum [AB-5(4), 1:10,000; Calbiochem] on a shaking table at room temperature. Sections were processed with biotinylated goat anti-rabbit IgG (1:900; Vector Laboratories, Burlingame, CA) for 1.5 h at room temperature, followed by reaction with avidin-biotin complex (Vector Elite kit, 1:200; Vector Laboratories). Sections were developed with nickel-diaminobenzidine tetrahydrochloride (DAB), which produced a black reaction product in cell nuclei. There was no nuclear staining in the absence of primary antiserum. To stain for GAD, we used mouse anti-GAD67 monoclonal antibody (MAB5406, lot 24100380; Chemicon, Temecula, CA). This antibody (raised against recombinant GAD67 protein) is specific for GAD67 and shows no reactivity to GAD65 by Western blot. The antibody has been used successfully for Western blot and immunohistochemistry on rat and mouse tissue. The sections were incubated in the primary antibody (1:500) at 4°C over 72 h, processed with biotinylated anti-mouse IgG (BA-2001, 1:400; Vector Laboratories), and developed with DAB to produce a brown reaction product. Omission of the GAD primary antibody resulted in the absence of specific staining. We always performed matched immunological processing of tissue simultaneously on pairs of brains from the different experimental groups. After the staining, sections were mounted on gelatin-coated microscope slides, dehydrated in graded alcohols (50, 70, 95, and 100%), cleared in xylene, and coverslipped with DPX mounting medium (Electron Microscopy Sciences, Hatfield, PA).
Data Analysis
Characteristics of sleep and wakefulness.
For experiment 1, sleep-wakefulness polygraphic recordings were scored visually. An individual expert in rodent sleep state classification determined the sleep-wakefulness states of the rat pups based on the behavioral state occupying the majority of 10-s epochs of EEG/EMG recordings. The scorer was blind to experimental condition and group identity of the animals. Wakefulness was defined by the presence of low-amplitude and high-frequency EEG activity combined with elevated neck muscle tone. NREM sleep was defined by the presence of high-amplitude slow-wave EEG combined with low EMG tone relative to wake. Epochs of transitions from NREM to REM sleep, which were defined by the appearance of high-amplitude spindles and theta activity intermixed with EEG slow-wave activity, were scored as NREM sleep. REM sleep was identified by the presence of a moderate-amplitude EEG with dominant theta frequency activity coupled with tonic low muscle tone in the EMG, interrupted by phasic muscle twitches.
To characterize the developmental changes in diurnal distribution of behavioral states, mean total amounts of wakefulness, NREM sleep, and REM sleep (as percentage of total recording time) were calculated for consecutive 2-h intervals across the 24-h baseline recordings and for the 12-h light and dark phases, on P21 and P29. To assess the continuity of wakefulness and NREM sleep on P29 and P21, mean durations of wakefulness and NREM sleep bouts were calculated for the light and dark periods of 24-h baseline recordings (1 epoch of a qualifying state, i.e., 10 s, was considered the minimum bout length).
The magnitude of the compensatory sleep responses to SD after the release from the deprivation protocol in both age groups was assessed by total durations of the sleep-wakefulness states, EEG slow wave amplitude (SWA), and mean lengths of NREM sleep and rapid-eye-movement (REM) sleep bouts.
EEG records of consecutive 10-s epochs of NREM sleep, for the entire postdeprivation recovery sleep and corresponding baseline sleep at ZT3–4, were subjected to a fast-Fourier transform routine to obtain EEG power spectra in the delta frequency range (0.5–4.0 Hz; SWA). Epochs containing EEG artifacts, which were recognized during visual scoring, were excluded from spectral analysis of all the EEG recordings. The average SWA value for all NREM sleep epochs subjected to spectral analysis was determined for each animal, and individual group mean SWA values were calculated.
For the groups of experiment 2, time spent in total sleep and wake during the experimental sessions was assessed by off-line analysis of video recordings. Sleep was defined as a state of immobility with body and limbs partly outstretched and with eyes closed. Slight movements of the body, position change, and twitches (of the eyes, whiskers, ear, or paws) when the animal was recumbent with eyes closed were accounted to the behavioral correlates of REM sleep and included in total sleep time. In this experiment, therefore, time spent in REM sleep was not quantified. Opened eyes, eating, drinking, and coordinated movements such as grooming, locomotion, stretching, and yawning were accounted to the behavioral characteristics of wakefulness (33).
Fos+ and Fos+GAD double-labeled cell counts.
An individual who was blind to the experimental condition of the animals conducted cell counts. The Neurolucida computer-aided plotting system (MicroBrightField, Williston, VT) was used to identify and quantify neurons that were labeled for c-Fos immunoreactivity and for Fos+GAD immunoreactivity. Section outlines were drawn under ×20 magnification. Fos-immunoreactive (Fos-IR) and Fos+GAD-IR neurons were mapped in the section outlines under ×400 magnification. All cell counts were calculated for constant rectangular grids corresponding to four areas of interest. Grid sizes for infant rats were scaled based on the grids used in our previous studies in adult rats (19, 23, 25). (1) The rostral MnPN (rMnPN) grid was a 510 × 510-μm square centered on the apex of the third ventricle rostral to the decussation of the anterior commissure and to bregma (anterior, 0.1 mm) (2). The caudal MnPN (cMnPN) grid was placed immediately dorsal to the third ventricle at the level of the decussation of the anterior commissure, extending 130 μm laterally and 510 μm dorsally just caudal to bregma (anterior, −0.26 mm). The VLPO counting grid was placed at the level of 140 μm or more caudal to the organum vasculosum of the lamina terminalis (approximately anterior, from −0.3 to −0.7 mm relative to bregma) and was subdivided into core and extended sections (4). The VLPO core box was 255 μm wide by 255 μm high, placed along the base of the brain, with its far border 340 μm lateral to the lateral edge of the optic chiasm. (5) The medial extended VLPO box was medial to the VLPO core, 340 μm wide by 255 μm high. The dorsal extended VLPO box was 170 μm wide by 255 μm high, positioned above the VLPO core and medial extended VLPO boxes and centered over their border. For both the rMnPN and the cMnPN, cell counts were made in three sections and averaged to yield a single value for each rat. For VLPO, cell counts were made bilaterally in three sections containing the largest part of the VLPO. Those six counts were then averaged to yield a single value for both the VLPO core and extended VLPO (medial and dorsal sections combined) for each rat.
Statistics
All results are reported as means ± SE. For experiment 1, differences in the amounts of the sleep-wakefulness states between ConP29 and P29 rats were assessed with Student's t-test for independent samples. Differences in the mean durations of wakefulness or NREM sleep bouts during the light and dark phases between P21 and P29 and differences in the amounts of sleep-wakefulness states between the light and dark periods of the 24-h baseline recordings performed on P21 and on P29 were assessed with paired Student's t-test. Differences in the measured sleep parameters such as percent wakefulness, percent NREM sleep; percent REM sleep; NREM sleep EEG SWA, and mean durations of NREM sleep and REM sleep bouts during spontaneous sleep and recovery sleep following SD on P21, P22, P29, and P30 were assessed with one-way repeated-measures ANOVAs followed by pairwise multiple-comparison procedures (Holm-Sidak method).
For experiment 2, the differences in total sleep and total wakefulness times between spontaneous sleep and recovery sleep groups of P22 and P30 rats were assessed with independent Student's t-test. Two-way ANOVA was performed to assess the effects of the experimental condition (spontaneous sleep, SD, and recovery sleep following the SD) and age (P22 and P30) on total Fos+ or Fos+/GAD+ cell counts in MnPN and VLPO sites. Significant main effects were followed by Tukey's honest significant difference (HSD) post hoc test. Significant interactions were analyzed by simple main effects (1-way ANOVA).
RESULTS
Diurnal distribution and consolidation of sleep-wakefulness states on P21 and P29 (experiment 1).
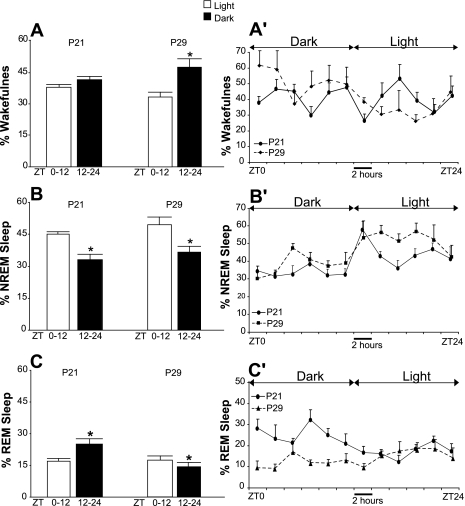
Rats exhibited prominent differences in electrographic measures of baseline sleep-wakefulness cycle organization on P29 compared with P21 (experiment 1). Figure 1 presents the mean percentages of time spent in wakefulness, NREM sleep, and REM sleep during the 12-h light vs. dark periods (Fig. 1, A–C) and across consecutive 2-h time periods of the 24-h baseline recordings (Fig. 1, A′–C′) on P21 and P29. As shown, rats exhibited no light-dark differences in mean total wakefulness and total sleep amounts during 24-h baseline recordings on P21. The 24-h distributions of NREM sleep and REM sleep in these animals exhibited light-dark differences. REM sleep time was significantly higher during the dark compared with the light period, whereas NREM sleep amounts were significantly higher during the light period compared with the dark period. On P29, wakefulness was elevated in the dark vs. the light phase, and both NREM and REM sleep were higher in the light phase compared with the dark phase (Fig. 1). Episodes of sleep and wakefulness were more consolidated on P29 compared with P21. The mean duration of wakefulness bouts during the dark period on P29 was significantly higher compared with P21 (5.1 ± 0.75 vs. 1.9 ± 0.1 min, P < 0.05, paired t-test). The mean duration of NREM sleep bouts on P29 vs. P21 was significantly increased for both the light and the dark periods of the 24-h baseline recordings (the light period, 5.0 ± 1.0 vs. 2.6 ± 0.5 min; the dark period, 3.2 ± 0.67 vs. 1.6 ± 0.2 min, P < 0.05, paired Students's t-test).
Fig. 1.
Mean percentages of time spent in wakefulness (A), non-rapid-eye-movement (NREM) sleep (B), and rapid-eye-movement (REM) sleep (C) during the light (open bars) and dark (filled bars) periods (12 h each) of the baseline recording for six rats on postnatal day (P) 21 and these same rats on P29 (experiment 1). A′–C′: mean percentages of time spent in wakefulness (A′), NREM sleep (B′), and REM sleep (C′) during consecutive 2-h intervals across 24-h baseline recordings on P21 (solid line) and on P29 (broken line). The calculations are done based on electroencephalogram (EEG)/electromyogram (EMG) scoring. On P21, the rats exhibited significantly different amounts of NREM sleep [t(10)−6.1; P < 0.001] and REM sleep [t(10)4.2; P = 0.002] during the light vs. the dark period, whereas the percentage of time spent in wakefulness did not differ significantly between the light and dark phases. On P29, the rats showed diurnality in all three behavioral states [wakefulness: t(10)4.3, P = 0.002; NREM sleep: t(10)−2.4, P = 0.037; and REM sleep: t(10)−2.9, P = 0.014]. *P < 0.05.
Baseline architecture of the sleep-wakefulness cycle of ConP29 rats (postoperative recovery control), which were implanted with EEG/EMG on P27, did not significantly differ from the sleep-wakefulness pattern of rats that were operated on P19, then assigned to the longitudinal study and recorded for 24-h baseline sleep-wakefulness cycle on P29 (see Table 1).
Table 1.
The 24-h sleep-wake cycle architecture in ConP29 and P29 rats (experiment 1)
| Sleep-Wake States | Light Period in ConP29 Rats | Light Period in P29 Rats | Dark Period in ConP29 Rats | Dark Period in P29 Rats |
|---|---|---|---|---|
| Wakefulness, % | 32.8 ± 2.9 | 33.1 ± 2.7 | 49.9 ± 2.1 | 47.5 ± 3.9 |
| NREM sleep, % | 51.1 ± 2.6 | 49.4 ± 3.5 | 36.7 ± 2.3 | 37.7 ± 2.9 |
| REM sleep, % | 16.1 ± 0.8 | 17.5 ± 2.1 | 13.4 ± 1.6 | 14.8 ± 2.0 |
Values are expressed as means ± SE. P, postnatal day; ConP29, postoperative recovery control rats; P29, rats assigned to the longitudinal study; NREM, nonrapid eye movement; REM, rapid eye movement. Data are assessed by the analysis of electroencephalogram (EEG)/electromyogram (EMG) recordings. Shown are the percentages of time spent in wakefulness, NREM sleep, and REM sleep during the 12 h light and 12 h dark periods of the baseline recordings. Values of the sleep-wakefulness states for the light and for the dark phases are compared between the two groups of rats recorded on P29 (ConP29, n = 6 vs. P29, n = 6). No significant group differences in percentages of sleep-wakefulness states were found.
Homeostatic Responses to SD on P22 and P30 (experiment 1)
During the SD period, experimental animals exhibited a significantly lower percentage of time spent in NREM sleep compared with the relevant baseline values (on P21-P22, 9.2 ± 1.5 vs. 54.2 ± 3.7%, P < 0.001; on P29–30, 7.7 ± 1.2 vs. 66.6 ± 2.1%, P < 0.001, paired Students's t-test). No REM sleep was achieved during SD procedures on P22 and P30. The mean percentages of NREM sleep during SD did not significantly differ on P30 and P22.
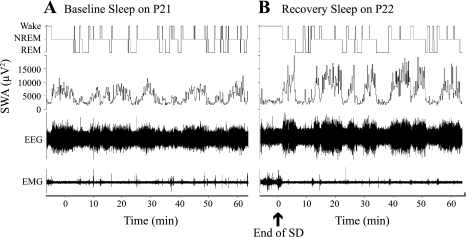
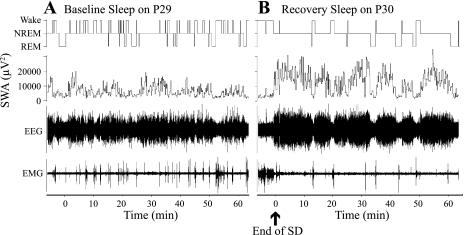
Examples of the sleep-wakefulness organization during postdeprivation recovery sleep on P22 and P30 vs. corresponding baseline recordings on P21 and P29, respectively, for representative rats are shown on Figs. 2 and 3.
Fig. 2.
Examples of the effect of a 2-h total sleep deprivation (SD) performed on P22 on baseline sleep-wakefulness cycle architecture and NREM sleep EEG slow wave amplitude (SWA) (experiment 1). A, top: hypnogram of 60-min continuous recording of baseline sleep-wake on P21; second panel, NREM sleep EEG SWA values for P21 (μV2). B, top: hypnogram of 60-min continuous recording of recovery sleep following the SD on P22; second panel, NREM sleep EEG SWA values for P22 (ηV2).
Fig. 3.
Examples of the effect of a 2-h total SD performed on P30 on baseline sleep-wakefulness cycle architecture and NREM sleep EEG SWA (experiment 1). A, top: hypnogram of 60-min continuous recording of baseline sleep-wake on P29; second panel shows NREM sleep EEG SWA values for P29 (μV2). B, top: hypnogram of 60-min continuous recording of recovery sleep following the SD on P30; second panel shows NREM sleep EEG SWA values for P30 (ηV2).
On both P22 and P30, rats exhibited compensatory increases in total NREM sleep time and NREM sleep EEG SWA during recovery sleep at ZT3–4 following the SD compared with the relevant baseline (spontaneous sleep-wake at the same circadian time on P21 and P29, respectively) (Table 2). Increased sleep consolidation, assessed by the mean duration of NREM sleep bouts, during the recovery sleep vs. baseline sleep was seen only on P30 (Table 2).
Table 2.
Sleep-wake characteristics during spontaneous sleep and recovery sleep recorded in the same rats at different stages of postnatal development (experiment 1)
| Sleep-Wake Characteristics at ZT3–4 | Spontaneous sleep-Wake on P21 | Recovery Sleep-Wake on P22 | Spontaneous Sleep-Wake on P29 | Recovery Sleep-Wake on P30 Rats |
|---|---|---|---|---|
| Wakefulness, % | 41.7 ± 2.4* | 18.3 ± 1.3* | 29.4 ± 2.0* | 9.6 ± 1.3* |
| NREM sleep, % | 42.5 ± 2.6* | 64.8 ± 2.3* | 56.6 ± 1.9* | 78.7 ± 1.5* |
| REM sleep, % | 15.8 ± 0.8 | 16.9 ± 2.6 | 14.0 ± 0.6 | 11.7 ± 0.8* |
| EEG SWA, μV2 | 2,794.0 ± 102.4* | 4,295.6 ± 141.1 | 4,631.2 ± 70.4 | 6,760.3 ± 104.8* |
| Mean duration of NREM sleep episodes, min | 1.6 ± 0.1 | 1.7 ± 0.1 | 2.5 ± 1.2* | 4.3 ± 1.4* |
| Mean duration of REM sleep episodes, min | 1.4 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.2 | 1.1 ± 0.1 |
Values are means ± SE; n = 6 rats in each group. ZT, Zeitgeber time. Data are assessed by the analysis of EEG/EMG recordings. EEG SWA, slow-wave activity represented by power density in the 0.5- to 4.0-Hz band.
Different from all other groups (repeated-measures ANOVA followed by pairwise multiple comparison procedures, Holm-Sidak method).
Effect of SD on Behavioral Sleep and Fos and Fos/GAD-Immunoreactivity in MnPN and VLPO Sites (Experiment 2)
All SD rats manifested significant increases in total sleep time during recovery sleep compared with the time-matched spontaneous sleep. Table 3 presents the percentages of time spent in sleep and wakefulness during postdeprivation recovery vs. spontaneous sleep for the groups of P22 and P30 animals, based on behavioral scoring.
Table 3.
Sleep-wake characteristics at ZT3-4 during baseline and post-SD recovery sleep conditions in P22 and P30 rats (experiment 2)
| Experimental Conditions and Groups | Total Sleep, % | Wakefulness, % |
|---|---|---|
| Spontaneous Sleep-wake in P22 rats (n = 6) | 64.7 ± 1.2 | 35.3 ± 1.2 |
| Recovery Sleep-wake in P22 rats (n = 6) | 89 ± 2.7* | 11 ± 2.7* |
| Spontaneous Sleep-wake in P30 rats (n = 6) | 67.3 ± 2.9 | 32.7 ± 2.9 |
| Recovery Sleep-wake in P30 rats (n = 6) | 85.3 ± 3.2* | 14.7 ± 3.2* |
Values are means ± SE; n, no. of rats. The data were assessed by behavioral scoring, based on video recordings. Percentages of total sleep and wakefulness were calculated for the last 1-h video-recording period, before the rats were killed. Values of recovery sleep, in each age group, are compared with spontaneous sleep values. Percentage of wakefulness was significantly different between the two experimental conditions [in P22: t(10)9.8, P < 0.001; in P30: t(10)5.3, P < 0.001]. Percentage of total sleep during recovery vs. spontaneous sleep-wakefulness cycle was also significantly different [in P22: t(10)–9.8, P < 0.001; in P30: t(10)–5.3, P < 0.001].
Statistically significant difference.
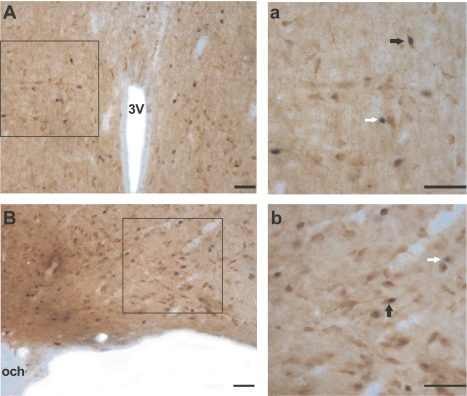
Figure 4 presents examples of Fos single-labeled and Fos/GAD double-labeled neurons in the MnPN and VLPO of P30 rats.
Fig. 4.
Photomicrographs showing examples of Fos single-labeled (white arrows) and Fos/GAD double-labeled (black arrows) neurons within rostral median preoptic nucleus (MnPN, Aa) and ventrolateral preoptic area (VLPO, Bb) from P30 rats that, during 2 h before death, were predominantly asleep (experiment 2). a and b (magnification ×40) correspond to the areas marked as black squares in A and B (magnification ×20, respectively). The Fos protein is stained black and confined to the nucleus. The glutamic acid decarboxylase (GAD) immunoreactive (IR) neurons are stained brown, and the staining is evident throughout the soma and the proximal dendrites. 3V, third ventricle; och, optic chiasm. Scale bar, 50 μm.
Mean numbers of Fos-IR and Fos/GAD-IR neurons in MnPN and VLPO sites, across different experimental conditions in different age groups, are presented on Figs. 5 and 6, respectively.
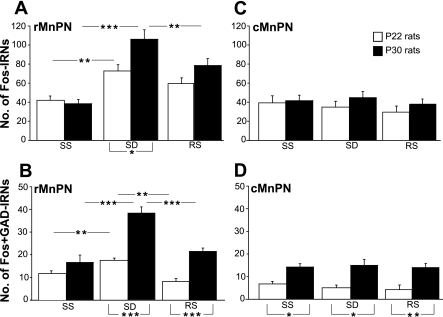
Fig. 5.
The mean numbers of total Fos immunoreactive neurons (IRNs, A and C) and Fos+GAD IRNs (B and D) in rostral and caudal parts of the MnPN (rMnPN and cMnPN, respectively) in experiment 2. Open bars, P22 rats; filled bars, P30 rats. SS, spontaneously sleeping animals; SD, sleep-deprived animals; RS, post-SD recovery sleep group. All results are reported as means ± SE. Statistical comparisons were performed by 2-way ANOVA. Significant main effects were followed by Tukey's honest significant difference (HSD) post hoc test. Analyses for simple main effects (1-way ANOVAs), required in case of the presence of significant interaction and defensible in case of the absence of significant main effects and interaction, were then applied to the data. Statistically significant differences: *P < 0.05; **P < 0.01; and ***P < 0.001.
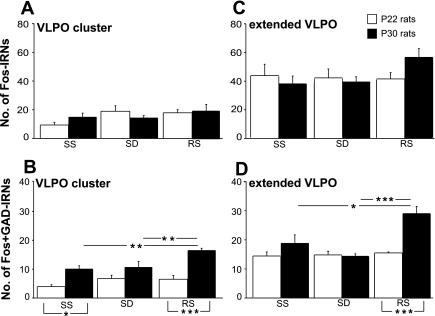
Fig. 6.
The mean nos. of total Fos IRNs (A and C) and Fos+GAD IRNs (B and D) in the VLPO cluster and extended VLPO in experiment 2. Statistically significant differences: *P < 0.05, **P < 0.01, and ***P < 0.001.
Rostral MnPN.
A two-way ANOVA (2 × 3 factorial design with age and experimental condition as between-subject factors) revealed statistically significant age times experimental condition interactions for the mean numbers of Fos+ neurons [F(2,30) = 3.8, P < 0.05] and Fos+/GAD+ neurons [F(2,30) = 12.3, P < 0.001]. The effect of experimental condition on Fos-IR and Fos/GAD-IR cell counts was significant for both P22 and P30 groups [Fos-IR: F(2,15) = 7.1, P < 0.01 and F(2,15) = 20.8, P < 0.001, respectively; Fos/GAD-IR: F(2,15) = 16.4, P < 0.001, F(2,15) = 34.3, P < 0.001, simple main effect analysis, 1-way ANOVAs]. The mean numbers of Fos-IR and Fos/GAD-IR neurons, within both P22 and P30 groups, were elevated in the condition of SD compared with spontaneous sleep (Tukey HSD post hoc test; see Fig. 5, A and B, for significance). P30 rats, but not P22 rats, exhibited increased Fos-IR in the condition of SD compared with the recovery sleep condition (Fig. 5A). Fos/GAD-IR cell counts were elevated in the SD condition compared with spontaneous sleep and the postdeprivation recovery condition in P30 and compared with spontaneous sleep in P22 rats (Fig. 5B). P30 compared with P22 rats exhibited significantly higher Fos-IR cell counts in the condition of SD [F(1,10) = 7.1, P < 0.05] and significantly higher Fos/GAD-IR cell counts in the condition of SD and recovery sleep [F(1,10) = 45.8 and F(1,10) = 55.6, P < 0.001, respectively, 1-way ANOVAs] (Fig. 5B).
Caudal MnPN.
A two-way ANOVA did not reveal significant interactions between the factors age and experimental condition for the mean numbers of total Fos+ neurons and Fos+/GAD+ neurons. Neither significant main effects of experimental condition nor age were found for the mean number of Fos+ neurons (Fig. 5C), whereas the main effect of age was significant for Fos+/GAD+ cell counts [F(1,30) = 37.9, P < 0.001]. Fos/GAD-IR cell counts were elevated in P30 compared with P22 rats (Fig. 5D).
VLPO cluster.
A two-way ANOVA did not reveal significant main effects of factors age and experimental condition and significant interactions between the factors for the mean numbers of Fos+ neurons. The mean number of Fos+/GAD+ cells varied significantly across experimental conditions [F(2,30) = 7.2, P < 0.01] and between age groups [F(1,30) = 42.7, P < 0.001], and no significant interaction between factors was found [F(2,30) = 3.1, P = 0.06]. In P30 rats, Fos+/GAD+ cell counts were higher in the recovery sleep condition compared with both spontaneous sleep and SD conditions (Fig. 6B). The mean numbers of Fos/GAD-IR neurons in P22 rats did not differ significantly across the experimental conditions. Significant differences in Fos/GAD-IR cell counts between P22 and P30 rats were found in spontaneous sleep and postdeprivation recovery conditions (P < 0.05 and P < 0.001, respectively, Tukey's post hoc test; see Fig. 6B).
Extended VLPO.
A two-way ANOVA did not reveal significant interactions between the factors for the mean numbers of Fos+ neurons. Significant interactions between the factors age and experimental condition were found for the mean numbers of Fos+/GAD+ neurons [F(2,30) = 8.5, P < 0.01]. Simple main effect analysis showed an effect of experimental condition in P30 rats [F(2,15) = 11.9, P < 0.001, 1-way ANOVAs] but not in P22 rats (Fig. 6D). Post hoc comparisons showed that Fos/GAD-IR, in P30 rats, was higher in the condition of recovery sleep vs. SD and spontaneous sleep (P < 0.001 and P < 0.05, respectively, Tukey post hoc test). Fos/GAD-IR cell counts in P30 compared with P22 rats were higher in the condition of postdeprivation recovery sleep [F(1,10) = 31.0, P < 0.001].
DISCUSSION
This is the first study to report that developmental changes in the functional activity of hypothalamic sleep-promoting systems parallel the maturation of sleep-wake regulation during the fourth postnatal week in rats. Maturational changes were observed in the degree of consolidation of spontaneous sleep and wakefulness, and in compensatory responses to acute SD. Mean durations of NREM sleep episodes and of wakefulness episodes under baseline conditions were significantly longer on P29 compared with P21 (experiment 1), indicating increased consolidation of both wakefulness and sleep during the fourth postnatal week. These results are in agreement with earlier reports (6, 21, 29). Although increases in NREM sleep time and increases in NREM sleep EEG delta power during recovery sleep following SD were observed on both P22 and P30, increases in NREM sleep bout length during postdeprivation recovery sleep were present only on P30. On P22, there was no activation of GABAergic neurons in the VLPO during either spontaneous sleep or recovery sleep compared with wakefulness (experiment 2). On P30, Fos-GAD+ cell counts in the VLPO during postdeprivation recovery sleep were significantly elevated compared with the other conditions. MnPN GABAergic neuronal activity was state-dependent in P22 rats, but total Fos-GAD+ cell counts in the MnPN were higher in P30 rats. These findings provide evidence that the transition from infant to adult patterns of sleep regulation reflects the maturation of hypothalamic neuronal circuits that regulate sleep.
The diurnal distribution of sleep-wake states changed significantly between P21 and P29 (experiment 1). On P21, amounts of total sleep and wakefulness were evenly distributed across the 12:12-h light-dark periods. By P29, the adult pattern of elevated total sleep time in the light phase vs. the dark phase had emerged. These changes were due largely to diurnal reorganization of REM sleep expression. REM sleep amounts were higher in the dark vs. light phase on P21. The pattern was reversed by P29, with higher REM sleep amounts during the light vs. the dark phase. These results are in agreement with two previous studies of diurnal distribution of sleep-wake states in rats at similar postnatal ages (1, 26).
SD procedures significantly decreased the percentage of time spent in NREM sleep on both P22 and P30 compared with relevant baseline and completely prevented REM sleep in both age groups (experiment 1). A homeostatic discharge of accumulated need for sleep was evident from the enhancement of %NREM sleep and of NREM sleep EEG SWA activity during the initial 1-h interval of the recovery period (see Table 2 and Figs. 2–3) in all SD rats. On P22, rats exhibited some, but not all, components of the sleep homeostatic response to SD that were present on P30. The mean duration of NREM sleep bouts during postdeprivation recovery sleep on P30 was significantly increased compared with relevant baseline, whereas no change in NREM sleep bout duration during recovery sleep was observed on P22. Frank et al. (14) also failed to find increases in NREM bout duration in P20 and P24 rats during recovery sleep following SD induced by gentle handling. This finding suggests that sleep homeostatic mechanisms by P22 are not sufficiently mature to produce compensatory increases in NREM sleep consolidation in response to acute SD.
Increased sleep continuity in response to acute total SD has recently been reported in P2 rats (47). In that study, post-SD recovery sleep continuity was assessed from the mean duration of sleep episodes estimated from EMG recordings. Hence, quiet (NREM) and active (REM) sleep states were not differentiated in P2 rats. Todd et al. (47) hypothesized that compensatory increases in sleep bout durations in P2 rats reflected an active sleep rebound and an increase in active sleep bout duration. What we report here are increases in average NREM sleep bout duration following SD on P30 and the absence of such an increase on P22 rats.
On P29, EEG SWA activity during spontaneous NREM sleep was significantly increased compared with P21 (Table 2). This finding suggests that the previously reported progressive increases in the NREM sleep EEG δ-wave activity across P10–P20 (13) continue through the fourth postnatal week.
In P22 rats, Fos-IR in GABAergic neurons of rMnPN was highest in the condition of SD (Fig. 5B). This finding is in agreement with our previous report in adult rats (25). Unlike adult rats, cell counts in cMnPN on P22 did not differ across the experimental conditions. The pattern of Fos-IR in MnPN sites across experimental groups was similar in P30 and P22 rats, but the numbers of Fos-positive GABAergic neurons were higher in P30 vs. P22 rats, suggesting further maturation of the MnPN sleep-promoting system (Fig. 5, B and D). VLPO GABAergic neurons exhibited adult-like elevations in Fos-IR during recovery sleep vs. spontaneous sleep and SD on P30, but not on P22 (Fig. 6, B and D), suggesting functional emergence of VLPO sleep regulatory neurons by P30.
Increases in c-Fos+ cell counts have been reported in the MnPN and VLPO of P2 rats during recovery sleep following SD (47). This is in contrast to our failure to detect sleep- related c-Fos expression in the VLPO of P22 rats. It is possible that sleep-related activation of VLPO neurons occurs transiently in the immediate postnatal period and reappears later in development. There are no published reports of sleep-related c-Fos expression during P3–P20 in rats to refute or confirm this possibility. Kinetics of c-Fos synthesis and degradation may also differ between P2 and older rats. Fos+ cell counts in VLPO and MnPN remained elevated 210 min following the end of SD in P2 rats (47), whereas sleep-related Fos expression in adult rats declines between the 1st and 2nd h of recovery sleep following SD (2, 23).
The timing of sleep-related changes in c-Fos expression has been previously estimated to be 60–90 min (42). In our previous study in adult rats, we found that the mean numbers of Fos+GAD-IR neurons in the preoptic area were significantly elevated after 1 h of recovery sleep and decreased from the 1st to the 2nd h of recovery sleep (23). Other estimates of the timing of peak c-Fos activation in response to stimuli range from 30 min (18, 27) to 120 min (35, 43). This timing can vary depending on neuronal phenotype (43). We consider 60 min as adequate time to assess c-Fos expression during recovery sleep following short-term SD (2, 23, 25), although we cannot rule out that c-Fos expression quantified in this present study may reflect neuronal activity occurring during both recovery sleep and the latter part of the SD period.
Taken together, the above findings suggest that parallel maturation of 1) sleep-wakefulness architecture, (2) spectrum of sleep homeostatic response to SD, and 3) activity in preoptic hypothalamic sleep-promoting systems occurs during the fourth postnatal week. We hypothesize that postnatal development of preoptic sleep-promoting neuronal circuits contributes to the transition from infant to adult patterns of sleep regulation in the rat. The hypothesized involvement of preoptic sleep-promoting systems in developmental changes in sleep architecture during infancy is supported by transection, lesion, and electrophysiological studies (28, 36).
Our current findings and previously published reports suggest that the fourth postnatal week is characterized by important developmental processes that contribute to the consolidation of wakefulness and NREM sleep. We hypothesize that neither sleep-promoting nor wake-promoting neuronal circuits are mature enough to secure stabilization of the 24-h sleep-wakefulness cycle at P21. The age groups in this study represent a developmental period characterized by increased synaptic connectivity of the noradrenergic and cholinergic systems to neocortical regions. Activity in both systems is associated with wake maintenance and arousal quality (4, 11). Noradrenergic projections to the cortex are present and innervating the appropriate cortical layers by P3 (12, 32). However, during the 3rd wk of postnatal development (P15–P21), an increase in norepinephrine and tyrosine hydroxylase content in the neocortex occurs, reaching adult levels at the beginning of week 4 (12, 32). Cortical cholinergic afferents increase in density throughout postnatal weeks 3–6 (9). Recent findings demonstrate that the numbers of hypocretin (orexin) immunoreactive cells in the rat hypothalamus are increased from postnatal week 2 to maturation (39) and that the adult pattern of immunoreactivity in the hypothalamic orexinergic system is not yet fully developed by postnatal week 4.
Developmental changes in adenosinergic systems in the rat may also contribute to the maturation of sleep homeostasis between the 3rd and 4th wk of postnatal development. Density of adenosine-1 receptors in mammalian brain increases during the first three postnatal weeks, reaching adult levels between P20 and P30 (17). The differences between infant and adult levels of sleep-wakefulness stabilization and the expression of sleep homeostatic responses to SD, therefore, may reflect developmental changes in kinetics of adenosine synthesis and degradation and/or neuronal responsiveness to extracellular adenosine.
The stabilization of the sleep-wakefulness cycle in P29–P30 rats may also reflect the maturation of circadian processes. Independent circadian processes, originating in the suprachiasmatic nucleus (SCN), may actively promote both wakefulness and sleep at different phases of the circadian cycle (for review, see Re. 34). Studies on postnatal ontogenesis of photoperiodic entrainment of the molecular core clockwork in the rat SCN demonstrate that the circadian clock is sensitive to light soon after birth. Light induces expression of c-Fos protein within SCN in P1 and P3 rat pups (3, 31). However, the ability of the SCN to recognize a photoperiod develops gradually; it starts at P10, and is not yet fully completed within the first 20 postnatal days (30).
Perspectives and Significance
In summary, multiple factors are likely to be involved in the postnatal development of sleep-wakefulness regulation in rats. Although the results described here emphasize developmental changes in the activity of preoptic GABAergic neurons, maturation of brain mechanisms that regulate waking brain activity and consolidation of waking may be of primary importance for normal development of sleep homeostasis throughout ontogeny. Future studies, using the same methodological approach, can further investigate the developmental elaboration of the neural circuits controlling sleep-wake processes and central mechanisms underlying maturation of sleep regulation throughout ontogeny. Sleep disturbances are common in diseases that occur in childhood, including autism, depression, and anxiety disorders. Identifying the mechanisms underlying sleep development should help to understand how delayed maturation of sleep homeostasis impacts subsequent behavior in childhood and adolescence.
GRANTS
This work was supported by the Department of Veterans Affairs, National Institute of Mental Health Grant MH-63323, and GNSF/ST09-722-6-274.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Alfoldi P, Tobler I, Borbely AA. Sleep regulation in rats during early development. Am J Physiol Regul Integr Comp Physiol 258: R634–R644, 1990 [DOI] [PubMed] [Google Scholar]
- 2. Basheer R, Sherin JE, Saper CB, Morgan JI, McCarley RW, Shiromani PJ. Effects of sleep on wake-induced c-fos expression. J Neurosci 17: 9746–9750, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bendova Z, Sumova A, Illnerova H. Development of circadian rhythmicity and photoperiodic response in subdivisions of the rat suprachiasmatic nucleus. Dev Brain Res 148: 105–112, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev 42: 33–84, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Blumberg MS, Middlemis-Brown JE, Johnson ED. Sleep Homeostasis in Infant Rats. Behav Neurosci 118: 1253–1261, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blumberg MS, Seelke MA, Lowen SB, Karlsson KE. Dynamics of sleep-wake cyclicity in developing rats. Proc Natl Acad Sci USA 102: 14860–14864, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borbely AA, Tobler I. Homeostatic and circadian principals in sleep regulation in the rat. In: Brain Mechanisms of Sleep, edited by McGinty D. New York, NY: Raven, 1985 [Google Scholar]
- 8. Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. J Neurosci 22: 977–990, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coyle JT, Yamamura HI. Neurochemical aspects of the ontogenesis of cholinergic neurons in the rat brain. Brain Res 118: 429–440, 1976 [DOI] [PubMed] [Google Scholar]
- 10. Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods 29: 261–265, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Fadel J, Sarter M, Bruno JP. Age-related attenuation of stimulated cortical acetylcholine release in basal forebrain-lesioned rats. Neurosci 90: 793–802, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Foote SL, Morrison JH. Development of the noradrenergic, serotonergic, and dopaminergic innervation of neocortex. Curr Top Dev Biol 21: 391–423, 1987 [DOI] [PubMed] [Google Scholar]
- 13. Frank MG, Heller HC. Development of diurnal organization of EEG slow activity and slow wave sleep in the rat. Am J Physiol Regul Integr Comp Physiol 273: R472–R478, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Frank M, Morrissette R, Heller CH. Effects of sleep deprivation in neonatal rats. Am J Physiol Regul Integr Comp Physiol 275: R148–R157, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Franken P, Dijk DJ, Tobler I, Borbely AA. Sleep deprivation in the rat: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol Regul Integr Comp Physiol 261: R198–R208, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Gaus SE, Strecker RE, Tate BA, Parker RA, Saper CB. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience 115: 285–294, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Geiger JD, LaBella FS, Nagy JI. Ontogenesis of adenosine receptors in the central nervous system of the rat. Brain Res 315: 97–104, 1984 [DOI] [PubMed] [Google Scholar]
- 18. Giovannelli L, Shiromani PJ, Jirikowski GF, Bloom FE. Expression of c-fos protein by immunohistochemically identified oxytocin neurons in the rat hypothalamus upon osmotic stimulation. Brain Res 588: 41–48, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Gong H, Szymusiak R, King J, Steininger T, McGinty D. Sleep-related c-Fos protein expression in the preoptic hypothalamus: effects of ambient warming. Am J Physiol Regul Integr Comp Physiol 279: R2079–R2088, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Gong H, McGinty D, Guzman-Marin R, Chew KT, Stewart D, Szymusiak R. Activation of c-fos in GABAergic neurons in the preoptic area during sleep and in response to sleep deprivation. J Physiol (Lond) 556: 935–946, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gramsbergen A, Schwartze P, Prechtl HF. The postnatal development of behavioral states in the rat. Dev Psychobiol 3: 267–280, 1970 [DOI] [PubMed] [Google Scholar]
- 22. Gramsbergen A. The development of the EEG in the rat. Dev Psychobiol 9: 501–515, 1976 [DOI] [PubMed] [Google Scholar]
- 23. Gvilia I, Turner A, McGinty D, Szymusiak R. Preoptic area neurons and the homeostatic regulation of rapid eye movement sleep. J Neurosci 26: 3037–3044, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gvilia I, Szymusiak R. Regulation of nonREM Sleep: Interactions Among Sleep-Active Neurons in the Preoptic Hypothalamus. In: Current Advances in Sleep Biology: Regulation and Function, edited by Frank M. New York, NY: Nova Science, 2010 [Google Scholar]
- 25. Gvilia I, Xu F, McGinty D, Szymusiak R. Homeostatic regulation of sleep: a role for preoptic area neurons. J Neurosci 26: 9426–9433, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ibuka N. Ontogenesis of circadian sleep-wakefulness rhythms and developmental changes of sleep in the altricial rat and in the precocial guinea pig. Behav Brain Res 11: 185–196, 1984 [DOI] [PubMed] [Google Scholar]
- 27. Imaki T, Shibasaki T, Hotta M, Demura H. Early induction of c-fos precedes increased expression of corticotropin-releasing factor messenger ribonucleic acid in the paraventricular nucleus after immobilization stress. Endocrinology 131: 240–246, 1992 [DOI] [PubMed] [Google Scholar]
- 28. Karlsson KE, Kreider JC, Blumberg MS. Hypothalamic contribution to sleep-wake cycle development. Neuroscience 123: 575–582, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Kleitman N, Engelmann TG. Sleep characteristics of infants. J Appl Physiol 6: 269–282, 1953 [DOI] [PubMed] [Google Scholar]
- 30. Kovacikova Z, Sladek M, Laurinova K, Bendova Z, Illnerova H, Sumova A. Ontogenesis of photoperiodic entrainment of the molecular core clockwork in the rat suprachiasmatic nucleus. Brain Res 1064: 83–89, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Leard LE, Macdonald ES, Heller HC, Kilduff TS. Ontogeny of photic-induced c-fos mRNA expression in rat suprachiasmatic nuclei. NeuroReport 5: 2683–2687, 1994 [DOI] [PubMed] [Google Scholar]
- 32. Levitt P, Moore RY. Development of the noradrenergic innervation of neocortex. Brain Res 162: 243–259 1979 [DOI] [PubMed] [Google Scholar]
- 33. Maloney KJ, Cape EG, Gotman J, Jones BE. High-frequency electroencephalogram activity in association with sleep-wake states and spontaneous behaviors in the rat. Neuroscience 76: 541–55 1997 [DOI] [PubMed] [Google Scholar]
- 34. Mistlberger RE. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res Rev 49: 429–454, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Miyata S, Itoh T, Lin SH, Ishiyama M, Nakashima T, Kiyohara T. Temporal changes of c-fos expression in oxytocinergic magnocellular neuroendocrine cells of the rat hypothalamus with restraint stress. Brain Res Bull 37: 391–395, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Mohns EJ, Karlsson KA, Blumberg MS. The preoptic hypothalamus and basal forebrain play opposing roles in the descending modulation of sleep and wakefulness in infant rats. Eur J Neurosci 23: 1301–1310, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morgan JI, Curran T. Role of ion flux in the control of c-fos expression. Nature 322: 552–555, 1986 [DOI] [PubMed] [Google Scholar]
- 38. Saper CB, Cano G, Scammell TE. Homeostatic, circadian, and emotional regulation of sleep. J Comp Neurol 493: 477–478, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Sawai N, Ueta Y, Nakazato M, Ozava H. Developmental and aging changes of orexin-A and-B immunoreactive neurons in the mail rat hypothalamus. Neurosci Lett 468: 51–55, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Seelke AM, Blumberg MS. The microstructure of active and quiet sleep as cortical delta activity emerges in infant rats. Sleep 31: 691–699, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci 18: 4705–4721, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shiromani PJ, Malik M, Winston S, McCarley RW. Time course of Fos-like immunoreactivity associated with cholinergically induced REM sleep. J Neurosci 15: 3500–3508, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sim LJ, Morris M. Fos activation in cultured tyrosine hydroxylase and oxytocin immunoreactive neurons. Brain Res Bull 36: 399–404, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Suntsova N, Szymusiak R, Alam MN, Guzman-Marin R, McGinty D. Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J Physiol (Lond) 543: 665–667, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Szymusiak R, Alam MN, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res 803: 178–188, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Szymusiak R, Gvilia I, McGinty D. Hypothalamic Control of Sleep. Sleep Med 8: 291–301, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Todd WD, Gibson JL, Shaw CS, Blumberg MS. Brainstem and hypothalamic regulation of sleep pressure and rebound in newborn rats. Behav Neurosci 124: 69–78, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Uschakov A, Gong H, McGinty D, Szymusiak R. Sleep-active neurons in the preoptic area project to the hypothalamic paraventricular nucleus and the perifornical lateral hypothalamus. Eur J Neurosci 23: 3284–3296, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Uschakov A, Gong H, McGinty D, Szymusiak R. Efferent projections from the median preoptic nucleus to sleep- and arousal-regulatory nuclei in the rat brain. Neuroscience 150: 104–120, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Valatx JL, Jouvet M. Circadian rhythms of slow-wave sleep and paradoxical sleep are in opposite phase in genetically hypoprolactinemic rats. C R Acad Sci III 307: 789–794, 1988 [PubMed] [Google Scholar]