Abstract
End-tidal carbon dioxide tension (PetCO2) is reduced during an orthostatic challenge, during heat stress, and during a combination of these two conditions. The importance of these changes is dependent on PetCO2 being an accurate surrogate for arterial carbon dioxide tension (PaCO2), the latter being the physiologically relevant variable. This study tested the hypothesis that PetCO2 provides an accurate assessment of PaCO2 during the aforementioned conditions. Comparisons between these measures were made: 1) after two levels of heat stress (N = 11); 2) during combined heat stress and simulated hemorrhage [via lower-body negative pressure (LBNP), N = 8]; and 3) during an end-tidal clamping protocol to attenuate heat stress-induced reductions in PetCO2 (N = 7). PetCO2 and PaCO2 decreased during heat stress (P < 0.001); however, there was no group difference between PaCO2 and PetCO2 (P = 0.36) nor was there a significant interaction between thermal condition and measurement technique (P = 0.06). To verify that this nonsignificant trend for the interaction was not due to a type II error, PetCO2 and PaCO2 at three distinct thermal conditions were also compared using paired t-tests, revealing no difference between PaCO2 and PetCO2 while normothermic (P = 0.14) and following a 1.0 ± 0.2°C (P = 0.21) and 1.4 ± 0.2°C (P = 0.28) increase in internal temperature. During LBNP while heat stressed, measures of PetCO2 and PaCO2 were similar (P = 0.61). Likewise, during the end-tidal carbon dioxide clamping protocol, the increases in PetCO2 (7.5 ± 2.8 mmHg) and PaCO2 (6.6 ± 3.4 mmHg) were similar (P = 0.31). These data indicate that mean PetCO2 reflects mean PaCO2 during the evaluated conditions.
Keywords: heat stress, orthostatic challenge, brain blood flow, carbon dioxide
arterial carbon dioxide tension (PaCO2) is a primary regulator of cerebral perfusion, with hypercapnia and hypocapnia inducing increases and decreases in cerebral perfusion, respectively (7, 23). Studies using end-tidal carbon dioxide tension (PetCO2) to estimate PaCO2 during passive heat stress (i.e., increase in internal temperature ∼1.2–1.5°C) suggest that heat stress reduces PaCO2 ∼4–8 mmHg (3, 5, 15, 26). A potential limitation to these studies is the assumption that PetCO2 is an accurate surrogate for PaCO2, the latter being the physiologically relevant variable. In normothermic individuals, PetCO2 provides an accurate assessment of PaCO2 in supine, resting conditions (8, 9, 22). Upon assumption of the upright posture, however, there is a decrease in cardiac output that is accompanied by a ventilation/perfusion mismatch leading to much greater reductions in PetCO2 relative to PaCO2 (8, 9, 22). Thus, in this condition, there is a discrepancy between the values obtained from two measures of carbon dioxide tension such that reductions in PetCO2 overestimate reductions in PaCO2 (8, 9, 22). This issue has recently been minimized in normothermic individuals through the identification of a correction factor that can be used to predict PaCO2 from measures of PetCO2 (8). To our knowledge, however, there is no information regarding the relationship of PetCO2 to PaCO2 in individuals with elevated internal temperatures. This information is important because heat stress-induced reductions in PaCO2 likely contribute to reductions in cerebral blood flow (3, 5, 15, 26) and ultimately reduced orthostatic tolerance in this thermal condition (4, 12, 25). Because PetCO2 is commonly used as an index of PaCO2 in heat-stressed individuals (3, 5, 15, 26), it is important to identify the relationship between these variables in this thermal condition.
The purpose of this study was to test the hypothesis that PetCO2 provides an accurate assessment of PaCO2 during a moderate heat stress and during a more severe heat stress sufficient to decrease PetCO2 (aim 1) as well as during a heat stress combined with an orthostatic challenge (aim 2). Lastly, restoration of PetCO2 to preperturbation baseline is often used to assess the role of carbon dioxide on various physiological responses (3, 9). This procedure relies on the assumption that clamping-induced increases in PetCO2 are accompanied by comparable increases in PaCO2, but this assumption has not been assessed in heat-stressed individuals. Therefore, aim 3 tested the hypothesis that PetCO2 accurately tracks PaCO2 when the heat stress-induced reductions in PetCO2 are attenuated through PetCO2 clamping.
METHODS
Eleven healthy normotensive subjects participated in this study. Average (mean ± SD) subject characteristics were as follows: age, 34 ± 12 yr; height, 172 ± 7 cm; and weight, 70 ± 7 kg. Subjects were not taking medications and were free of any known cardiovascular, metabolic, or neurological diseases. Subjects were informed of the purpose and risks of the study before providing their informed written consent. The protocol and consent were approved by the Institutional Review Boards at the University of Texas Southwestern Medical Center at Dallas and Texas Health Presbyterian Hospital Dallas. Subjects refrained from alcohol, caffeine, and intense exercise for 24 h before the study.
Instrumentation and Measurements
Following arrival to the laboratory, each subject swallowed a telemetry pill for the measurement of intestinal temperature (HQ, Palmetto, FL). Mean skin temperature was measured from the weighted average of six thermocouples attached to the skin (24). Each subject was fitted with a water-perfused tube-lined suit (Med-Eng, Ottawa, Canada) and was placed in a lower body negative pressure (LBNP) chamber, sealed at the iliac crest, while in the supine position. The suit covered the entire body except for the head, face, hands, one forearm, and feet and permitted the control of skin and internal temperatures by adjusting the temperature of the water perfusing the suit. Heart rate was continuously obtained from an electrocardiogram (HP Patient Monitor; Agilent, Santa Clara, CA) interfaced with a cardiotachometer (CWE, Ardmore, PA). A 20-gauge catheter was inserted in the radial artery of the nondominant arm using sterile techniques under local anesthesia. The cannula was connected to a pressure transducer (Maxxim Medical, Athens, TX) that was positioned at the level of the heart. This catheter was used for direct continuous assessment of beat-by-beat arterial pressure as well as for blood samples for subsequent analysis of PaCO2.
Experimental Protocol
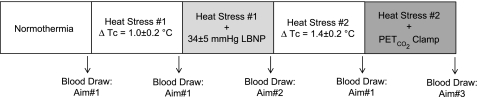
An outline of the entire experimental protocol is provided in Fig. 1. For all analyses, the alpha level was set at 0.05, and the results are reported as means ± SD.
Fig. 1.
Protocol schematic. The arrows indicate the timing of the blood draw for analysis of arterial carbon dioxide tension for each of the indicated aims. End-tidal carbon dioxide tension (PetCO2) was averaged over the 30-s period when arterial blood was drawn. Tc, core temperature.
Aim 1: PetCO2 vs. PaCO2 During Normothermia and Two Levels of Heat Stress
Following instrumentation, subjects rested quietly in the supine position while normothermic water (34°C) circulated through the suit. After a 10-min steady-state rest period, subjects were fitted with a nose clip and breathed room air through a mouth piece for 5 min while thermal, hemodynamic, and PetCO2 (VitalCap Capnograph Monitor; Oridion, Needham, MA) data were collected during spontaneous respiration. During the last minute of this period, blood was obtained from the arterial catheter and stored in a heparinized syringe on ice until subsequent analysis of PaCO2 was performed in triplicate (Gem Premier 3000; Instrumentation Laboratory, Lexington, MA). Following completion of normothermic data collection, heat stress began by circulating 49°C water through the suit. When internal temperature increased ∼1.0°C above baseline temperature, the PetCO2 and PaCO2 measures were repeated (heat stress 1). Subjects continued to be heat stressed until PetCO2 was reduced by at least 3 mmHg relative to normothermia. Once this decrease in PetCO2 was attained, the temperature of the water circulating the suit was slightly decreased in an effort to attenuate the rate of rise in internal temperature, as well as further decreases in PetCO2, at which point the PetCO2 and PaCO2 measures were repeated (heat stress 2).
Data analysis for aim 1.
Data were sampled at 50 Hz via a data-acquisition system (Biopac System, Santa Barbara, CA). For normothermia and both levels of heating, data during the 60-s period before the blood draw, for each respective thermal condition, were averaged, the exception being that PetCO2 was averaged over the 30-s period when arterial blood was drawn. Hemodynamic data among the three conditions (i.e., baseline normothermia, heat stress 1, and heat stress 2) were analyzed via one-way repeated-measures ANOVA, followed by a Tukey post hoc analysis when a main effect was identified. Values of PetCO2 and PaCO2 were evaluated via a two-way repeated-measures ANOVA, with main factors of carbon dioxide measurement method and thermal condition. Comparisons between PaCO2 and PetCO2 measures, during the three thermal conditions, were also evaluated using the methods of differences as described by Bland-Altman (2). The Bland-Altman plot was subsequently analyzed by linear regression analysis to determine if the slope of the relationship was greater than zero, which would indicate that a proportional bias was present [i.e., difference between two methods changes as the average values from the two methods becomes smaller or larger (6, 14, 16)]. Furthermore, a fixed bias is present if the 95% confidence limit of the Bland-Altman plot does not include zero (14, 16). Lastly, a linear regression analysis was performed to further characterize the relationship between PaCO2 and PetCO2 measures during the three thermal conditions.
Aim 2: PetCO2 vs. PaCO2 During a Simulated Hemorrhage Challenge While Heat Stressed
Immediately after the PetCO2 and PaCO2 measures during moderate heating (i.e., heat stress 1), eight subjects were exposed to 4 min of ∼30 mmHg LBNP. PetCO2 and PaCO2 were measured during the final minute of LBNP.
Data analysis for aim 2.
Absolute values of PetCO2 and PaCO2 during the LBNP challenge were compared using a paired t-test as well as by linear regression analysis (N = 8). In a subset of subjects (N = 5), this level of LBNP was sufficient to further reduce PetCO2 at least 2 mmHg below the heat stress value (i.e., pre-LBNP). In these individuals, the magnitude of the reduction in PetCO2 and PaCO2 during LBNP relative to the pre-LBNP values was compared using a paired t-test and linear regression analysis.
Aim 3: Does PetCO2 Track PaCO2 When the Heat Stress-Induced Reductions in PetCO2 are Attenuated?
Immediately after the PetCO2 and PaCO2 measures during pronounced heating (i.e., heat stress 2), a subset of subjects (N = 7) was exposed to a PetCO2 clamping protocol. This was accomplished using a computer-controlled gas blender, sequential gas delivery, and rebreathing circuit (RespirAct; Thornhill Research, Toronto, Canada), which has been described in detail elsewhere (3, 10, 17–19). The Respiract device was programmed to return PetCO2 to tensions measured during normothermia while simultaneously maintaining normoxia via administration of a mixture of nitrogen, oxygen, and carbon dioxide gases in a closed-loop sequential rebreathing circuit. Once this was achieved, the aforementioned PetCO2 and PaCO2 measures were repeated.
Data analysis for aim 3.
The magnitude of the increase in PetCO2 and PaCO2 during the clamping protocol relative to preclamped heat stress values was compared using a paired t-test and linear regression analysis.
RESULTS
Thermal and hemodynamic data. Before any thermal perturbations, internal and mean skin temperatures were 37.0 ± 0.4 and 34.5 ± 0.5°C, respectively. Heat stress 1 and heat stress 2 increased mean skin temperature to 38.5 ± 0.6 and 38.7 ± 0.9°C, respectively (both variables P < 0.001 relative to normothermia), and the magnitude of this increase was similar between the two heat stress conditions (P = 0.66). Internal temperature was increased to 38.0 ± 0.5°C during heat stress 1 (P < 0.001 relative to normothermia) and was further elevated to 38.4 ± 0.4°C during heat stress 2 (P < 0.001 relative to normothermia and heat stress 1). Heart rate was increased from 63 ± 8 beats/min during normothermia to 102 ± 18 and 106 ± 14 beats/min during heat stress 1 and heat stress 2, respectively (both variables P < 0.001 relative to normothermia). Mean arterial pressure was reduced from a normothermic value of 89 ± 7 mmHg to 77 ± 7 and 76 ± 6 mmHg during heat stress 1 and heat stress 2, respectively (both variables P < 0.001 relative to normothermia).
Aim 1: PetCO2 Provides an Accurate Assessment of PaCO2 During Normothermia and Two Levels of Heat Stress
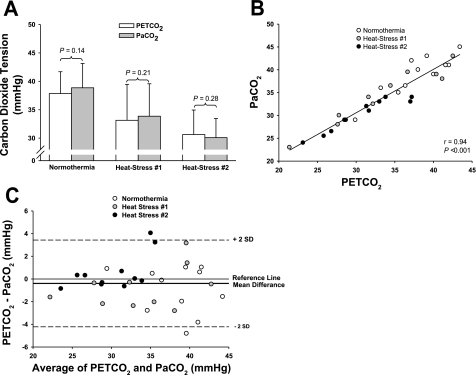
The two-way repeated-measures ANOVA revealed a significant main effect of thermal condition in reducing carbon dioxide tension (P < 0.001); however, this reduction was similar between measurement techniques [main effect of carbon dioxide measurement technique (PetCO2 and PaCO2; P = 0.36)]. While not significant, there was a trend toward an interaction between thermal condition and measurement technique (P = 0.06). To verify that this nonsignificant trend was not due to a type II error, PetCO2 and PaCO2 values at each of the three thermal conditions were also compared using paired t-tests. The results were consistent with the two-way repeated-measures ANOVA in that there was no difference between PetCO2 and PaCO2 while normothermic (P = 0.14), during heat stress 1 (P = 0.21), and during heat stress 2 (P = 0.28; Fig. 2A). Linear regression analysis demonstrated a significant correlation between measures of PetCO2 and PaCO2 when the three thermal conditions were analyzed together (r = 0.94, P < 0.001; Fig. 2B). The Bland-Altman plot revealed a small bias (bias, −0.4 mmHg) between measures of PetCO2 and PaCO2 when the three thermal conditions were analyzed together (Fig. 2B). Neither a proportional (r = 0.01, P = 0.94, slope −0.004) nor a fixed (95% confidence interval = −4.2 mmHg, 3.4 mmHg) bias was detected from the Bland-Altman plot (Fig. 2C).
Fig. 2.
Relationship between PetCO2 and arterial carbon dioxide tension (PaCO2) during normothermia, as well as elevations in core body temperature of 1.0 ± 0.2°C (heat stress 1) and 1.4 ± 0.2°C (heat stress 2). Measures of PetCO2 (white bars) and PaCO2 (gray bars) were similar for each thermal condition (P values are from paired t-tests; A). The linear regression analysis (B) and the Bland-Altman plot (C) also revealed a close relationship between PaCO2 and PetCO2 within the three thermal conditions, which was particularly evident in the hypocapnic range (B and C). For the Bland-Altman plot, there was a small bias between measurement devices (bias, −0.4 mmHg) and 95% confidence limits of −4.2 and 3.4 (B).
Aim 2: PetCO2 Provides an Accurate Assessment of PaCO2 During a Simulated Hemorrhage Challenge While Heat Stressed
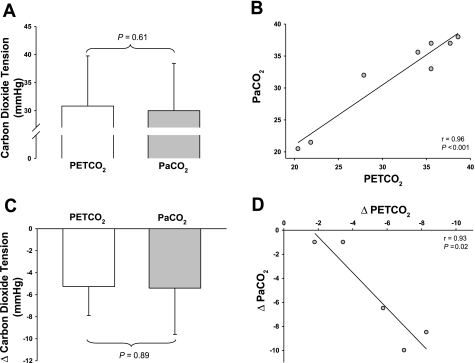
A paired t-test revealed no difference between absolute values of PetCO2 (31.4 ± 7.2 mmHg) and PaCO2 (31.8 ± 7.0 mmHg) during LBNP while subjects were heat stressed (P = 0.61; Fig. 3A). This finding was supported by linear regression analysis which revealed a significant correlation between absolute values of PetCO2 and PaCO2 during LBNP (r = 0.96, P < 0.001; Fig. 3B). Likewise, a paired t-test revealed that the magnitude of the reduction in PetCO2 (5.3 ± 2.6 mmHg) and PaCO2 (5.4 ± 4.2 mmHg) in the subset of subjects (N = 5) who exhibited decreases in PetCO2 during LBNP was also similar (P = 0.89; Fig. 3C), which was further confirmed by linear regression analysis (r = 0.93, P = 0.02; Fig. 3D).
Fig. 3.
Relationship between PetCO2 and PaCO2 during combined lower body negative pressure (LBNP) and heat stress 1. Absolute PetCO2 and PaCO2 during LBNP while subjects were heat stressed were similar, as identified by a paired t-test (N = 8; A) and linear regression analysis (N = 8; B). Likewise, the magnitude of the reduction in PetCO2 (5.3 ± 2.6 mmHg) and PaCO2 (5.4 ± 4.2 mmHg) tensions to LBNP was similar, as identified by a paired t-test (N = 5; C) and linear regression analysis (N = 5; D), indicating that PetCO2 tension accurately reflects PaCO2 tension during these perturbations.
Aim 3: The Magnitude of the Increase in PetCO2 and PaCO2 During a PetCO2 Clamping Procedure is Similar in Heat-Stressed Subjects
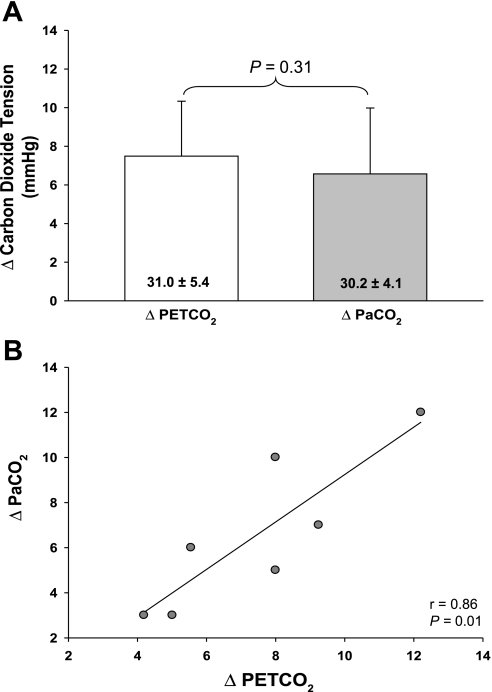
The PetCO2 clamping procedure was successful at returning PetCO2, which decreased during heat stress 2, to normothermic levels (normothermia: 38 ± 4 mmHg; heat stress preclamp: 31 ± 5 mmHg; heat stress clamp: 38 ± 5 mmHg; P < 0.001 between heat stress values, whereas there was no difference between normothermia and heat stress + clamp values; P = 0.97). Importantly, the magnitude of the increase in PaCO2 that occurred during this clamping procedure was similar to the increase in PetCO2 (P = 0.31; Fig. 4A). This finding was confirmed by linear regression analysis (r = 0.86, P = 0.01; Fig. 4B).
Fig. 4.
Relationship between PetCO2 and PaCO2 tensions during the clamping procedure directed toward restoring PetCO2 tension to preheat stress levels. The clamping procedure similarly increased PetCO2 (7.5 ± 2.8 mmHg) and PaCO2 (6.6 ± 3.4 mmHg) tensions relative to their respective preclamp heat stress values, indicated numerically in each column (i.e., heat stress 2; A). This finding is further confirmed by linear regression analysis, which identified a significant correlation between PetCO2 and PaCO2 (B). These finding indicate that PetCO2 tension accurately reflects PaCO2 tension during this clamping procedure.
DISCUSSION
PetCO2 is reduced in individuals with an elevated internal temperature, and the reduction in PetCO2 is exacerbated when this thermal stress is combined with an orthostatic challenge (3, 15, 26). These physiological occurrences likely contribute to decreases in cerebral perfusion and thus the reduction in orthostatic tolerance that occur during heat stress (1, 4, 11–13, 25). The precise understanding of the relationship between carbon dioxide tension and the cerebral vasculature is dependent on the assumption that PetCO2 accurately reflects PaCO2, the latter of which is the physiologically relevant variable. The current results indicate that PetCO2 provides an accurate noninvasive index of PaCO2 during normothermic conditions, differing degrees of heat stress, and during a simulated hemorrhage challenge combined with heat stress. Furthermore, the magnitude of the increase in PaCO2 upon returning PetCO2 to normothermic levels (i.e., the PetCO2 clamping protocol) is similar to that of PetCO2. These data suggest that PetCO2 provides an accurate assessment of PaCO2 under the testing conditions without the added cost and risk of arterial catheterization.
In normothermic supine resting individuals, PetCO2 provides an accurate reflection of PaCO2 (8, 9, 22), which is consistent with the present data (Fig. 2, A, B, and C). That said, a discrepancy in the relationship between these variables has been reported following the assumption of the upright posture in normothermic individuals, such that reductions in PetCO2 are greater than PaCO2 (8, 9). This response is due to changes in distribution of blood flow throughout the lungs secondary to postural reductions in cardiac output, which subsequently alters the ventilation-perfusion ratio (8). In the current study, subjects were exposed to a simulated orthostatic challenge during heat stress conditions by LBNP. Unlike what is observed during normothermia (8, 9), the present results indicate that PetCO2 is similar to PaCO2 during the LBNP challenge (Fig. 3, A and B), and, furthermore, the magnitude of the reductions in PetCO2 and PaCO2 during this challenge are similar (Fig. 3, C and D). While speculative, it is possible that differing normothermic and heat stress responses to an orthostatic stress are related to the differences in cardiac output between thermal conditions. Cardiac output was not measured in the current study; however, values of 10–13 l/min have been reported during similar degrees of heat stress (20, 21, 26). The imposed LBNP during heat stress reduces cardiac output by ∼3 l/min (26), resulting in final cardiac outputs of ∼7–10 l/min. In contrast, during an orthostatic challenge in normothermic conditions, cardiac output is reduced by ∼1.5 l/min (26), resulting in a final cardiac output of ∼4 l/min. Thus it is possible that, despite greater reductions in cardiac output during LBNP while heat stressed, the prevailing cardiac output (i.e., ∼7–10 l/min) and thus blood flow distribution throughout the lungs remains sufficient to maintain similarities between measures of PetCO2 and PaCO2, relative to when cardiac output has been reduced to ∼4 l/min during LBNP while normothermic.
Despite differences in PetCO2 and PaCO2 during normothermic head up tilt (8, 9), Immink et al. (9) reported that the magnitude of increase in both of these variables was similar when PetCO2 was clamped at the pretilt level. Additionally, using the same clamping device as in the current study, Ito et al. (10) recently reported that the accuracy of PetCO2 as an estimate of PaCO2 was sustained in seated normothermic individuals across a wide range of hypocapnic and hypercapnic stimuli. The present findings are in agreement with the cited findings given that the magnitude of the increase in PetCO2 and PaCO2 was similar during the clamping protocol (Fig. 4, A and B).
Considerations/Limitations
While not significant, there was a trend toward an interaction between the thermal condition and measurement technique in aim 1 (P = 0.06). This is most likely the result of a slightly greater decrease in PaCO2 (8.8 ± 4.6 mmHg) during heat stress 2 relative to the decrease in PetCO2 (7.2 ± 4.2 mmHg). Nonetheless, when paired comparisons were made at each thermal condition, the measures of CO2 were similar regardless of the measurement technique (Fig. 2).
The Bland-Altman plot in Fig. 2C depicts the relationship of the difference between the two measurement techniques (y-axis) and the average between the two techniques (x-axis) during resting normothermia and two levels of heat stress. This plot further reveals a close relationship between measures of PetCO2 and PaCO2, as demonstrated by a small measurement bias of −0.4 mmHg. A proportional bias exists if the slope of the relationship between the difference of two measurements and the mean of the two measurements is significantly different from zero [i.e., the difference between the two methods changes as the average values from the two methods becomes smaller or larger (6, 14, 16)]. In the current study, a proportional bias was not detected (r = 0.01, P = 0.94, slope −0.004). That being said, it appears that the relationship between the PetCO2 and PaCO2 weakens at higher CO2 tensions as indicated by a wider dispersion of data points with respect to the measurement bias (in both the positive and negative direction). Therefore, it is possible that greater differences would be identified if comparisons between carbon dioxide measurement methods were evaluated in the hypercapnic range.
Perspectives and Significance
In conclusion, absolute PetCO2 and PaCO2 were similar during normothermic conditions, differing degrees of heat stress, as well as during LBNP combined with heat stress. Furthermore, the heat stress-induced reductions in PetCO2 and PaCO2 were of similar magnitude, and subsequent further decreases in PetCO2 and PaCO2 imposed by LBNP were also of a similar magnitude. Based on these findings, PetCO2 measures can be used to estimate PaCO2 during heat stress studies that use the imposed perturbations. These findings are important to the clinical community who are interested in the impact of changes in carbon dioxide concentration on physiological responses, and to researchers who do not have access to skilled personnel necessary for arterial catheter placement and to the subjects by reducing the risks associated with experimentation where estimates of PaCO2 are needed.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants HL-61388, HL-84072, and HL-092761 and by the Research and Education Institute of Texas Health Resources.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Jena Langlois, Peggy Fowler, and Cindi Foulk for technical assistance and the subjects for their willing participation in this project.
REFERENCES
- 1. Allan JR, Crossley RJ. Effect of controlled elevation of body temperature on human tolerance to +G z acceleration. J Appl Physiol 33: 418–420, 1972 [DOI] [PubMed] [Google Scholar]
- 2. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1: 307–310, 1986 [PubMed] [Google Scholar]
- 3. Brothers RM, Wingo JE, Hubing KA, Crandall CG. The effects of reduced end-tidal carbon dioxide tension on cerebral blood flow during heat stress. J Physiol 587: 3921–3927, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cui J, Wilson TE, Crandall CG. Orthostatic challenge does not alter skin sympathetic nerve activity in heat-stressed humans. Auton Neurosci 116: 54–61, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Fan JL, Cotter JD, Lucas RA, Thomas K, Wilson L, Ainslie PN. Human cardiorespiratory and cerebrovascular function during severe passive hyperthermia: effects of mild hypohydration. J Appl Physiol 105: 433–445, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Hart EC, Joyner MJ, Wallin BG, Karlsson T, Curry TB, Charkoudian N. Baroreflex control of muscle sympathetic nerve activity: a nonpharmacological measure of baroreflex sensitivity. Am J Physiol Heart Circ Physiol 298: H816–H822, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ide K, Eliasziw M, Poulin MJ. The relationship between middle cerebral artery blood velocity and end-tidal Pco2 in the hypocapnic-hypercapnic range in humans. J Appl Physiol 95: 129–137, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Immink RV, Secher NH, Roos CM, Pott F, Madsen PL, Lieshout JJ. The postural reduction in middle cerebral artery blood velocity is not explained by PaCO(2). Eur J Appl Physiol 96: 609–614, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Immink RV, Truijen J, Secher NH, Van Lieshout JJ. Transient influence of end-tidal carbon dioxide tension on the postural restraint in cerebral perfusion. J Appl Physiol 107: 816–823, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Ito S, Mardimae A, Han J, Duffin J, Wells G, Fedorko L, Minkovich L, Katznelson R, Meineri M, Arenovich T, Kessler C, Fisher JA. Non-invasive prospective targeting of arterial P(CO2) in subjects at rest. J Physiol 586: 3675–3682, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson JM, Niederberger M, Rowell LB, Eisman MM, Brengelmann GL. Competition between cutaneous vasodilator and vasoconstrictor reflexes in man. J Appl Physiol 35: 798–803, 1973 [DOI] [PubMed] [Google Scholar]
- 12. Keller DM, Low DA, Wingo JE, Brothers RM, Hastings J, Davis SL, Crandall CG. Acute volume expansion preserves orthostatic tolerance during whole-body heat stress in humans. J Physiol 587: 1131–1139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lind AR, Leithead CS, McNicol GW. Cardiovascular changes during syncope induced by tilting men in the heat. J Appl Physiol 25: 268–276, 1968 [DOI] [PubMed] [Google Scholar]
- 14. Lipman RD, Salisbury JK, Taylor JA. Spontaneous indices are inconsistent with arterial baroreflex gain. Hypertension 42: 481–487, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Low DA, Wingo JE, Keller DM, Davis SL, Zhang R, Crandall CG. Cerebrovascular responsiveness to steady-state changes in end-tidal CO2 during passive heat stress. J Appl Physiol 104: 976–981, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ludbrook J. Statistical techniques for comparing measurers and methods of measurement: a critical review. Clin Exp Pharmacol Physiol 29: 527–536, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Mandell DM, Han JS, Poublanc J, Crawley AP, Kassner A, Fisher JA, Mikulis DJ. Selective reduction of blood flow to white matter during hypercapnia corresponds with leukoaraiosis. Stroke 39: 1993–1998, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Mandell DM, Han JS, Poublanc J, Crawley AP, Stainsby JA, Fisher JA, Mikulis DJ. Mapping cerebrovascular reactivity using blood oxygen level-dependent MRI in Patients with arterial steno-occlusive disease: comparison with arterial spin labeling MRI. Stroke 39: 2021–2028, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Prisman E, Slessarev M, Han J, Poublanc J, Mardimae A, Crawley A, Fisher J, Mikulis D. Comparison of the effects of independently-controlled end-tidal PCO(2) and PO(2) on blood oxygen level-dependent (BOLD) MRI. J Magn Reson Imaging 27: 185–191, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Rowell LB. Thermal stress. In: Human Circulation Regulation During Physical Stress. New York, NY: Oxford Univ Press, 1986, p. 174–212 [Google Scholar]
- 21. Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol 27: 673–680, 1969 [DOI] [PubMed] [Google Scholar]
- 22. Serrador JM, Hughson RL, Kowalchuk JM, Bondar RL, Gelb AW. Cerebral blood flow during orthostasis: role of arterial CO2. Am J Physiol Regul Integr Comp Physiol 290: R1087–R1093, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31: 1672–1678, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol 66: 1586–1592, 1989 [DOI] [PubMed] [Google Scholar]
- 25. Wilson TE, Cui J, Zhang R, Crandall CG. Heat stress reduces cerebral blood velocity and markedly impairs orthostatic tolerance in humans. Am J Physiol Regul Integr Comp Physiol 291: R1443–R1448, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wilson TE, Cui J, Zhang R, Witkowski S, Crandall CG. Skin cooling maintains cerebral blood flow velocity and orthostatic tolerance during tilting in heated humans. J Appl Physiol 93: 85–91, 2002 [DOI] [PubMed] [Google Scholar]