Abstract
Exercise-induced bronchoconstriction (EIB) is common; however, key aspects of its pathogenesis are still unclear. We investigated the feasibility of adapting an established animal model of asthma to investigate the earliest stages of EIB. The hypothesis was that a single exposure to a normally innocuous, and brief, exercise challenge could trigger EIB symptoms in rats previously sensitized to ovalbumin (OVA) but otherwise unchallenged. Brown-Norway rats were sensitized by intraperitoneal injection of OVA at 0 and 2 wk. At week 3, animals were exposed to either aerosolized OVA (SS) or exercise (EXS). A trained, blinded, clinical observer graded EIB by respiratory sounds. Plasma and lung cytokine levels were analyzed. No control rats with or without exercise (EX, CON) showed evidence of EIB. Eighty percent of the SS group demonstrated abnormal breath sounds upon exposure to aerosolized OVA. Approximately 30% of EXS rats sensitized to OVA but exposed only to exercise had abnormal breath sounds. Lung tissue levels of TNF-α, IL-1α, growth-related oncogene/keratinocyte/chemoattractant, and IFN-γ were significantly higher (P < 0.001) in the SS group, relative to all other groups. Changes in most of these cytokines were not notable in the EXS rats, suggesting a different mechanism of EIB. Remarkably, IFN-γ, but not the other cytokines measured, was significantly elevated following brief exercise in both sensitized and unsensitized rats. Exercise led to detectable breathing sound abnormalities in sensitized rats, but less severe than those observed following classical OVA challenge. Precisely how this immune crossover occurs is not known, but this model may be useful in elucidating essential mechanisms of EIB.
Keywords: running, cytokine, leukocytes, inflammation
in allergic asthma, a wide range of triggers stimulate an immediate airway response, leading to a cascade of inflammation, bronchoconstriction, and mucus hypersecretion, culminating in a clinical asthma attack. Exercise-induced asthma (EIA) is a common, often serious, manifestation of respiratory compromise, occurring in a high proportion of adults and children with known allergies (50, 54). Unlike exposure to known antigens (e.g., pollen, airborne particulate matter), the triggering factors in EIA remain enigmatic, and its mechanism is the object of substantial controversy (1). Over the past decade, there has been a rekindling of interest in the immunobiological consequences of physical exercise, such that it is now clearly established that exercise leads to a uniquely balanced, rapid stimulation of immune/inflammatory cells and mediators (11), some of which [such as the family of intracellular adhesion molecules (31)] are known to play a role in the asthma cascade.
We hypothesized that the exercise response could become unbalanced in the presence of experimentally induced allergen-activation of the immune system in animals that had never been challenged. We predicted that, in this case, a normally innocuous exercise challenge could trigger bronchoconstriction. We expected, therefore, to find both clinical evidence of airway hyperresponsiveness (AHR), in this case abnormal breathing sounds, and a pattern of cellular and mediator inflammation in the lung following exercise that would mimic a classic antigen challenge in a sensitized organism.
To test this, we employed a well-known murine model of allergic asthma, in which rats are sensitized parenterally with ovalbumin (OVA) (7, 35, 36, 45, 52). However, in contrast to most previous studies, the animals were not subjected to further treatments or challenges until being acutely tested for responsiveness to either 1) exercise or 2) aerosol challenge using the specific allergen OVA. If our hypothesis were true, then the animals rendered “allergic” by OVA sensitization could be induced to have bronchoconstriction by these separate challenges. Moreover, the pattern of cytokine and inflammatory cell changes in the lung would be similar following exercise and antigen challenge.
A barrier to using animal models for simulating human asthma lies in the difficulty of assessing AHR in a timely manner. In human asthma, EIA usually occurs within minutes of the onset of exercise and is detected typically by pulmonary function testing and/or auscultation of breath sounds (predominantly wheezing) (66). In murine models, however, the inflammatory cytokine and cellular response to a specific challenge are typically not measured for hours and often requires anesthesia and intubation (18, 23, 24, 39). There is a growing body of literature demonstrating minimally invasive approaches to assess airway function in the rodent (e.g., 19, 20). However, even with the rodent plethysmography, a period of ∼60 min is required for “reaclimitization.” (2) Our goal was to study the initiating events in the development of EIA. Therefore, all data were collected in a narrow time frame, immediately following the first and only exposure to a stimulus, be it exercise or aerosolized OVA. Breath sounds auscultated by stethoscopes or other devices have been used in rats effectively to model diseases in humans, particularly to assess acute responses to infectious or toxic agents (51, 55, 57). Moreover, despite the theoretical promise of digital signal processing of lung sounds, human auscultation remains the diagnostic tool of choice (3, 16).
The strength of OVA-induced airway inflammation in rats is strain dependent (27), and there is one strain of Brown-Norway rat in which alterations in breath sounds following OVA challenge in sensitized animals do occur and roughly parallel acute bronchoconstriction in humans (61, 69). The focus of this study was on exercise-associated AHR, and an acute assessment of change in airway function was essential. Consequently, the primary outcome variable was to determine whether an exercise challenge would lead to airway narrowing and accompanying changes in breath sounds (determined by auscultation) in rats sensitized to OVA but otherwise unperturbed. A secondary set of variables was selected to determine the immune and inflammatory changes in the lung that could shed insight onto the underlying mechanisms responsible for exercise-induced AHR, were it to occur.
MATERIALS AND METHODS
All methods and procedures were approved by the University of California Irvine Institutional Animal Care and Use Committee.
Animals
Male Brown-Norway rats, 120 g, 2–3-wk old, were obtained from Harlan Laboratories (Indianapolis, IN). This rat strain is suitable for the study of allergen-induced airway reactions (45). Rats were randomly assigned to one of four experimental groups with n = 10−22 per group: 1) CO: control group animals, which were not sensitized to ovalbumin (OVA; saline injection), were not exercised, but did receive aerosolized saline in parallel with the acute OVA challenge described below (n = 10); 2) EX: rats that were not sensitized to OVA (saline injection) and were acutely challenged with one bout of progressive exercise (n= 15); 3) SS: rats that were sensitized to OVA and were acutely challenged with aerosolized OVA (n = 22); and 4) EXS: rats that were sensitized to OVA, were acutely challenged with one bout of progressive exercise, and received no aerosolized OVA (n = 20).
All animals were maintained under virus/antigen-free conditions at the University of California, Irvine animal facility on a 12:12-h light-dark cycle. Animals were given food and water ad libitum.
Exercise Familiarization
Two weeks before sensitization, the animals of all four groups were familiarized with the treadmill twice weekly for 5 min at slow speed. In this way, the rats were accustomed to the treadmill but not trained.
Sensitization
Animals in the SS and EXS groups received a 1.0 ml ip injection of OVA (1 mg/ml; Sigma Chemical, St. Louis, MO) and aluminum hydroxide [Al(OH)3] (100 mg/ml; BDH Laboratory Supply, Poole, UK) in a 0.9% saline solution during week 1 and repeated 1 wk later. Rats in the CO and EX groups received intraperitoneal injections of similar volumes of 0.9% saline.
Acute Challenge—OVA
One week after the second sensitization treatment the rats assigned to the SS group were acutely challenged with 5% aerosolized OVA. OVA was aerosolized for 10 min using a DeVilbiss PulmoMate 4650D Nebulizer and an airflow of 8 l/ min. Following the challenge, rats were returned to their cages for a 10-min recovery and observation period, during which time, pulmonary auscultation was performed.
Acute Challenge—Exercise
One week after the second sensitization treatment, the rats assigned to the EXS group were acutely challenged with one bout of progressive exercise. The exercise challenge lasted 8 min. During the first 4 min, the treadmill speed increased to 33.52 m/min and remained at that speed until the completion of the challenge. The grade was 15° for all 8 min of the exercise challenge. In our experience, this level of exercise was close to the maximal running capability for this strain of rat. Following the challenge, rats were returned to their cages for a 10-min recovery and observation period, during which time pulmonary auscultation was performed.
Rats in the EX group, which had not been sensitized to OVA, underwent the acute exercise challenge as described for the EXS group prior to pulmonary auscultation.
Control Group
Rats in the CO were not sensitized to OVA but received IP injections of normal saline. In the 3rd wk, interleaved with the SS and EXS groups, the CO group animals received an acute exposure to aerosolized saline prior to pulmonary auscultation.
Pulmonary Auscultation
A single observer masked to the groups' identity scored all breathing sounds following the acute challenge of saline, OVA, or exercise. The observer was an asthma specialist board-certified in Pediatrics and Allergy and Immunology with substantial experience in assessing neonatal/pediatric breath sounds. In addition, the observer spent considerable time auscultating untreated rats at rest and after exercise. The observer used a digital stethoscope (Thinklabs, Centennial, CO) designed for infant auscultation with a tunable diaphragm that assured high acoustic sensitivity. Respiratory rate in newborns with lung disease can be in excess of 60 respirations/min, in the rat, the respiratory rates are in the range of around 100 per min.
During the recovery period, the rats were examined three times over a 10-min period, and each examination lasted 1 min. The examination sites coincided anatomically with anterior, lateral, and posterior projections of the rat lung and the anterior surface of the trachea. The final examination was performed immediately after the animal received an IP injection of pentobarbital sodium (50 mg/kg ip) for euthanasia.
Auscultated sounds were classified as 0, clear breath sounds with no abnormalities; 1, increased breath sounds without elements of tone or pitch; and 2, increased breath sounds with elements of tone or pitch.
Blood Sampling and Analysis of Circulating and Lung Tissue Analytes
After the induction of deep anesthesia, and prior to the cessation of breathing, blood was collected from the left ventricle via the diaphragm into EDTA-treated tubes. The numbers of neutrophils, monocytes, and lymphocytes in the circulating blood were measured using standard techniques. Cytokine levels in rat plasma and lung tissue were measured using either multiplex assays or ELISA (see below). Protein samples were extracted from the right lung middle lobe tissue. The right lung middle lobes were snap-frozen in dry ice and stored at −80°C until further analysis. The snap-frozen lungs were thawed, weighed, and homogenized at 4°C. Total protein concentrations in the lung tissue were diluted to a final protein concentration of 40 μg/ml.
Multiplex assay.
Chemiluminescence technology (41) was used to detect multiple mediators enabling the simultaneous examination of a series of interrelated cytokines implicated in the pathogenesis of asthma and bronchoconstriction, namely, IL-1α (9), IL-1β (4), IL-2 (26), IL-4 (30), IL-5 (32), IL-6 (43), IL-10 (44, 60), IL-12 (58), IL-18 (68), IFN-γ (13, 29, 40), TNF-α (65), granulocyte-macrophage colony stimulating factor (10), growth-related oncogene/keratinocyte/chemoattractant (GRO/KC) (38, 47), and monocyte chemoattractant protein-1 (62). When cytokine levels were lower than the detection limit, the value assigned was 0.001. Detection limits ranged between a low of 1.3–6.0 pg/ml to an upper limit of 5,000 pg/ml, depending upon the specific analyte.
ELISA.
Myeloperoxidase (MPO), a product of neutrophils known to play a role in asthma pathogenesis (49), was measured in plasma and lung tissue (10 μg protein) by specific ELISA (HyCult Biotechnology, Uden, The Netherlands). Histamine content [histamine has long been recognized as a critical, early-onset mediator of asthma (52)] was measured in plasma and lung tissue (10 μg protein) using immunoassay (Oxford Biomedical Research, Oxford, MI). For ELISA, measurements beyond detection level were treated as missing values (two in lung histamine, eight for lung MPO). Finally, ELISA was used to detect ovalbumin-specific IgE (OVA sIgE; Cusabio Biotech, Newark, DE). The OVA sIgE assay is semiquantitative and indicates only “positive” or “negative” presence of OVA sIgE in plasma.
Quantification of Cell Infiltrate in Lung Tissue
Sections of the left lung were removed and placed in 4% buffered paraformaldehyde and were subsequently processed for routine embedding with paraffin, and transverse 5-μm sections were stained with hematoxylin and eosin and Wright's stains for visualization of eosinophils and neutrophils. Periodic acid-Schiff stain was used for visualization of mast cells. Additional pieces of lung tissue were cryoprotected in 30% sucrose and subsequently processed for the immunocytochemical localization of polymorphonuclear leukocytes (PMNs) in the lung [purified rabbit anti-rat PMN Ab, (Cedarlane Laboratory, Burlington, NC)]. Sections were cut at 50 μm on a freezing microtome and incubated overnight in primary antibody (1/4,000 with 2% normal goat serum and 1% Triton-X100). Sections were subsequently developed with the alkaline phosphatase Vectastain kit, AK5001 (Vector Laboratories, Burlingame CA). Quantification of neutrophil, eosinophil, and mast cell infiltrate in lung tissue was determined by manually counting the number of cells in a 320 × 320 μm area using a Leitz microscope (×312.5). A 10 × 10 eyepiece grid reticle was used to facilitate cell counts. The number of cells from 3 or 4 areas of lung was averaged for each animal.
Data Analysis
In this study, we have found that some cytokines and inflammatory mediators were below detection, and the distribution of some studied variables did not follow normal assumption. Thus, we applied nonparametric method (Kruskal-Wallis test) to all studied cytokines, inflammatory mediators, and cell counts for overall difference among the four study groups and followed by post hoc paired comparison with Bonferroni's multiple-comparison adjustment. Fisher's exact test was utilized to examine the association between pulmonary auscultation and study group. Data were presented with median and range (minimum-maximum) or count and percentage. All analyses were performed using SAS 9.2 (Cary, NC, USA), and the significance level was set at 0.05.
RESULTS
Airway Hyperresponsiveness-Pulmonary Auscultation
A shown in Table 1, there was a significant association between pulmonary auscultation and study group (P < 0.0001). As expected, the rats in the CO and EX groups showed no change in auscultator breath sounds in response to the aerosolized saline or acute exercise challenges, respectively. Sixteen (80%) rats in the SS group showed abnormal breathing with element of tone or pitch, while six (27.3%) rats in the EXS group showed abnormal breathing, two of which were as severe as the rats in the SS group.
Table 1.
Distribution of breathing sounds
| 0 Clear breath sounds with no abnormalities | 1 Increased breath sounds without elements of tone or pitch | 2 Increased breath sounds with elements of tone or pitch | Total | |
|---|---|---|---|---|
| Control | 10 (100%) | 0 | 0 | 10 |
| EX | 15 (100%) | 0 | 0 | 15 |
| SS | 4 (20%) | 0 | 16 (80%) | 20 |
| EXS | 16 (72.7%) | 4 (18.2%) | 2 (9.1%) | 22 |
EX, control rat with exercise; SS, aerosolized ovalbumin; EXS, exercise.
Cytokine Levels in the Circulation
Relative to controls, a significant elevation in several plasma cytokines were observed in the EX, SS, and EXS groups as outlined in Table 2. However, no significant differences in circulating leukocytes, cytokine levels, MPO, or histamine levels between the two OVA-exposed groups (SS and EXS) were found.
Table 2.
Plasma cytokines
| Control (n = 10) |
Ex (n = 15) |
SS (n = 20) |
EXS (n = 22) |
Kruskal-Wallis Test |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Median | Range | Median | Range | Median | Range | Median | Range | P value | Paired Comparison* |
| IL-1β | b.d. | (b.d., 0.8) | 2.8 | (b.d., 8.37) | 0.30 | (b.d., 2.56) | 0.4 | (b.d., 2.48) | <0.0001 | 1,2,3,4,5 |
| IL-4 | b.d. | (b.d., 2.24) | 1.59 | (b.d., 3.85) | 1.59 | (b.d., 4.31) | 1.59 | (b.d., 42.86) | 0.012 | 1,2,3 |
| IL-18 | b.d. | (b.d., 13.8) | 13.14 | (2, 31.22) | 7.55 | (0.74, 34.97) | 14.47 | (4.32, 25.36) | <0.0001 | 1,2,3 |
| IFNγ | b.d. | (b.d., 1.52) | 0.86 | (0.12, 3.13) | 1.52 | (b.d., 4.06) | 1.52 | (0.28, 427.34) | 0.0002 | 1,2,3 |
| TNFα | b.d. | (b.d., 0.74) | 0.62 | (0.4, 0.79) | 0.68 | (0.37, 0.85) | 0.62 | (0.43, 0.91) | 0.0003 | 1,2,3 |
b.d., below detection.
Significant difference (P < 0.0083) of paired comparison between control (CON) and EX (1), CON and EXS (2), CON and SS (3), EX and EXS (4), and EX and SS (5).
Lung Cytokines, MPO, and Histamine
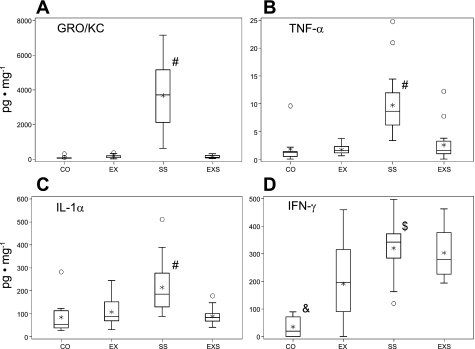
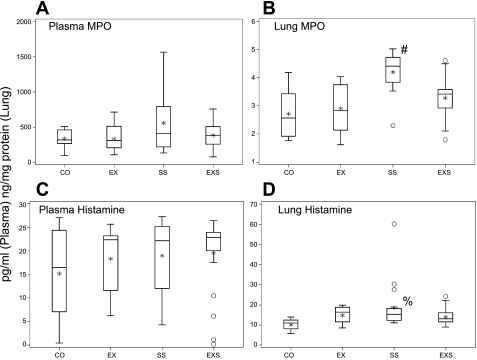
Of the inflammatory mediators and cytokines examined in lung tissue following OVA challenge, rats in the SS group were found to have significantly higher levels of IL-1α, TNF-α, and GRO/KC than all the other three groups and higher level in IFN-γ than control and EX groups (Fig. 1). The level in GRO/KC in SS group was remarkably high (3,729.9 [623.3–7,140.3] pg/mg) in proportion to the levels observed in the other cytokines (<512 pg/mg) and other groups. Similarly, lung MPO and histamine were significantly elevated only in the SS rats (Fig. 2).
Fig. 1.
Lung cytokine and chemokine levels. Box-and-whisker plot of lung cytokine/chemokine in the four experimental groups: control (CO; n = 10), control with exercise (EX; n = 15), aerosolized ovalbumin (SS; n = 20), and SS and exercise (EXS; n = 22). The box represents the first quartile, the median, and the third quartile; the whisker extends to 1.5 times interquartile range (the difference between 1st and 3rd quartiles), the asterisk is the mean, and the circles are outliers. Significant overall group differences were found in growth related oncogene/keratinocyte/chemoattractant (GRO/KC; P < 0.0001), TNFα (P = 0.0003), IL-1α (P < 0.0001) and IFN-γ (P < 0.0001). Post hoc paired comparison results: #SS group significantly higher than all other three groups; $SS group significantly higher than EX group; and &Control group significantly lower than all other three groups.
Fig. 2.
Lung and plasma myeloperoxidase (MPO) and histamine levels. Box-and-whisker plot of MPO and histamine levels in plasma and lung, among the four experimental groups: CO (n = 10, 9, 10, 9 as ordered in figure), EX (n = 15, 14, 15, 15), SS (n = 20, 18, 20, 20), and EXS (n = 21, 18, 22, 21). The box represents the first quartile, the median, and the third quartile; the whisker extends to 1.5 times interquartile range (the difference between 1st and 3rd quartiles), the asterisk is the mean, and the circles are outliers. Overall group differences were only found in the lung MPO (P < 0.0001) and lung histamine (P = 0.0076). Post hoc paired-comparison results. #SS group significantly higher than all other 3 groups, and %SS group significantly higher than control.
Lung Polymorphonuclear Leukocytes
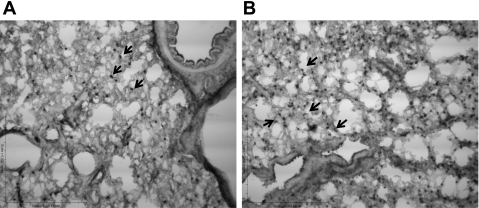
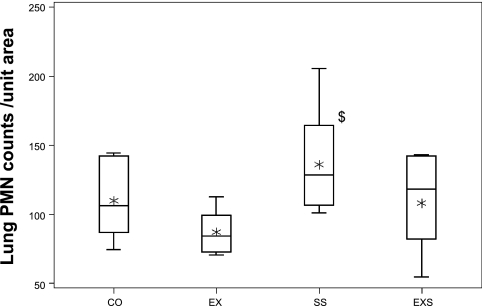
A subset of lung tissues (6 in CON, 7 in EX, 8 in SS, and 8 in EXS) was evaluated with regard to the number of eosinophils or mast cells, and no differences were found among the groups. However, assessment of the number of PMNs identified in the lungs by immunocytochemistry (Fig. 3) indicated that the number of PMNs in the lung was significantly different among the four groups (P = 0.028), where the SS group (130.8 [100.8–205.3]) is higher than the number found in the EX group (84.3 [70.3–112.8]) (P = 0.0065) (Fig. 4). No differences were found between CO (106.3 [74.3–144.3]) and/or EXS (120.5 [54.3–143.3]) groups.
Fig. 3.
Lung PMN localization. A: controls are shown. B: ovalbumin (OVA)-sensitized and OVA-challenged rat (SS) increased the number of PMN (arrows) in the lungs. ×20 Magnification.
Fig. 4.
Lung polymorphonuclear leukocyte (PMN) counts. Box-and-whisker plot of PMN counts per unit area in the four experimental groups: CO (n = 6), EX (n = 7), SS (n = 8), and EXS (n = 8). The box represents the first quartile, the median, and the third quartile; the whisker extends to 1.5 times interquartile range (the difference between 1st and 3rd quartiles), and the asterisk is the mean. There is a significant overall group difference in the PMN counts per unit area (P = 0.028). Post hoc paired comparison results: $SS group significantly more counts than control.
OVA-Specific IgE
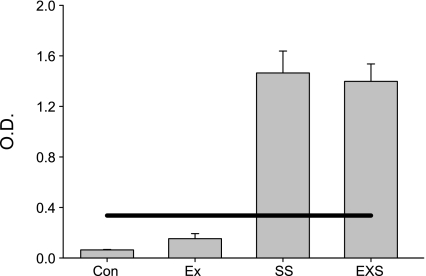
Illustrated in Fig. 5 are the results of the OVA-specific IgE analysis of plasma. As shown, OVA-specific IgE was not detectable in the control groups, whereas both groups sensitized to OVA were positive for OVA-specific IgE, verifying the efficiency of the OVA sensitization protocol.
Fig. 5.
OVA-specific IgE. Animals exposed to ovalbumin show positive levels of OVA-specific IgE in the circulation using a commercially available semiquantitative OVA-specific IgE ELISA. The solid line represents the outside diameter (O.D.) for a positive test. No OVA-specific IgE was detected in the control groups.
DISCUSSION
A single brief bout of heavy exercise is now known to lead to a remarkable set of airway and inflammatory responses, many of which are known to be involved in exercise-induced bronchoconstriction (EIB) (e.g., 11, 64). In this study, we showed that while exercise itself altered key immune mediators, conservative evidence for AHR only occurred in animals that had been previously sensitized to a seemingly unrelated antigen, ovalbumin (Fig. 5). Alterations in breath sounds did not occur in all OVA-sensitized, exercise-challenged animals, only in 27%. These initial observations are consistent with clinical manifestations of EIB and exercise-associated anaphylaxis in humans. These phenomena occur commonly, but not invariably, in individuals with a history of asthma and atopy (8, 21, 46), consistent with our finding that some sort of prior immunogenic sensitization is necessary for exercise to lead to AHR. Finally, our observations suggest that some individuals may experience distinct mechanisms for allergen-induced and exercise-induced asthma response, reflecting studies made in adults and children by Weiler-Ravell and Godfrey in 1981 (67).
In animal models, there are a number of studies focused on eucapnic hyperventilation and airway drying (possible associated triggers of EIB) and AHR (e.g., 18, 28, 34). However, this is one of the few studies to examine clinically detectable airway responses and lung cytokine, leukocyte, and mediator responses to a brief exercise or allergic challenge in rats at a time interval (10 min postchallenge) that mimics the phenomenon of EIB in humans (59). The exercise challenge was designed to reflect the 6–8 min of intense exercise recommended for stimulating EIB in humans (12), and much shorter in duration than is typically used in rodent exercise studies classified as “brief” or “single-bout” (e.g., 22, 25). Moreover, the response to the exercise challenge was assessed within 10 min.
The data tended to support one of our hypotheses, namely, a substantial percentage of Brown-Norway rats previously sensitized to OVA showed rapid, clinically detectable AHR following a brief exercise challenge. However, the extent of the clinically detectable AHR as shown in Table 1, was not as robust as we found when OVA-sensitized rats were challenged with aerosolized OVA. Thus, these data provide evidence that classic sensitization to a known allergen renders the organism somewhat more sensitive to seemingly unrelated AHR triggers, such as exercise. In contrast, our data did not substantiate our second hypothesis, namely, that in sensitized rats, the lung cytokine, cellular, and inflammatory mediator responses to an exercise challenge would parallel the changes observed with the aerosolized OVA challenge.
Auscultation with a stethoscope in humans and even in the rat is rapid, simple, and reliable. Moreover, in the context of our study, auscultation has the advantage of high-specificity for acute AHR, even though its sensitivity to subtle changes in airway resistance may not be as high as direct measurements of airway resistance by plethysmography or other methods. For example, airway resistance assessments (using the interrupter resistance technique, which requires subject cooperation) in children challenged with methacholine typically show worsening of airways resistance at considerably lower doses of methacholine than when wheezing is ultimately auscultated (33). Thus, our use of auscultation erred, if at all, by underestimating (and surely not overestimating) the true presence of AHR immediately following exercise in the rat model.
These data contrast, to some extent, to earlier and recent elegant work focused on exercise and OVA sensitization. In a series of studies, Pastva et al. (52) and Hewitt et al. (23, 24) examined how brief exercise and exercise training modulated subsequent lung inflammatory responses to OVA in sensitized rats. These studies demonstrated a generally moderating effect of exercise on subsequent lung inflammatory responses to acute allergen challenges specifically by decreasing NF-κB nuclear translocation and IκBα phosphorylation, thereby diminishing key proinflammatory control pathways. In addition, these workers showed that exercise training ameliorates AHR through β2-adrenergic receptor mechanisms.
This seeming contradiction, namely, the results of our study showing an acute AHR effect of an exercise challenge in OVA-sensitized rats and the Pastva and Hewitt studies showing a moderating effect of exercise on OVA-induced lung inflammation in sensitized mice, in fact, parallels observations in humans. While single bouts of exercise, as noted, constitute a major trigger of bronchoconstriction in many allergic individuals, a growing number of studies show that exercise training moderates airway inflammation in humans and improves asthma control (e.g., 6, 17, 48).
Our data provide some insight into plausible mechanisms to explain this paradoxical role of exercise vs. exercise training in AHR. While sensitized rats challenged with OVA (SS) demonstrated, as expected, clinical AHR and increased lung IFN-γ, a remarkable finding of this study was the significant elevation of IFN-γ in the lungs of unsensitized rats challenged with exercise, animals that did not show any clinical signs of AHR. The role of IFN-γ in acute asthma in humans remains quite controversial (37), with some studies suggesting that the IFN-γ is eosinophil dependent (29) and others showing that AHR-associated IFN-γ is independent of eosinophils and emanates largely from resident pulmonary macrophages (70). In stable asthmatic children, IFN-γ in the exhaled breath condensate is elevated relative to healthy controls (56). Our finding that exercise in unsensitized rats led to increased IFN-γ in the lung most likely resulted from exercise associated secretion by “resident” leukocytes or macrophages (the number of leukocytes did not increase Fig. 5). However, in the unsensitized animal, the IFN-γ was not sufficient to unleash the asthma “cascade,” while in the sensitized rat, exercise-associated IFN-γ was associated with acute clinically detectable AHR. We speculate that repeated stimulation of lung IFN-γ by exercise training might lead to a down-regulation of certain elements of inflammation and render the sensitized animal less sensitive to subsequent classic allergen-induced pulmonary inflammation.
In this context, our data highlight the unique nature of exercise-associated AHR in the sensitized rats. In contrast to our hypothesis, the lung cytokine response to the exercise challenge in OVA-sensitized rats did not parallel the lung cytokine response to the OVA challenge in OVA-sensitized rats, even though AHR was detectable clinically in both groups. Lung TNF-α, IL-1, IFN-γ, GRO-KC, histamine, and MPO were all elevated in the SS group following the challenge, while IFN-γ was the only one of the tested cytokines to increase in the EXS group. Thus, our data do not provide a ready explanation for the mechanism of AHR in the EXS animals. We did observe that the clinical AHR response was muted in the EXS animals compared with SS animals, suggesting that exercise did not elicit a “full-blown” acute inflammatory response, and, as noted, consistent with this, the lung cytokine response in the EXS animals was not nearly as robust as in the SS animals. It is becoming increasingly clear that the AHR response is not an “all or nothing” response; indeed, airway inflammation can be dissociated from AHR (23).
In considering the mechanisms of the exercise-associated AHR that we found in the EXS rats, noninflammatory mechanisms may come into play. For example, Quarcoo et al. (53) showed that stress (induced, for example, by foot shock) exacerbates the AHR caused by exposure to OVA in sensitized animals. These authors suggest that stress and allergy both lead to stimulation of afferent nerve fibers lining the mucosal epithelium that secrete tachykinins, such as substance P and neurokinin A. These mediators were not studied in the present set of experiments.
Immune cell accumulation in the lung (neutrophils, macrophages, lymphocytes, and eosinophils) is a well-recognized cellular component of AHR (63). In the bulk of studies in the Brown-Norway rat, accumulation of inflammatory cells in the lung is measured between 12 and 24 h after the challenge. However, in our model of the acute (i.e., within 10 min) response to the exercise or OVA challenge, the only change in pulmonary cellularity that we observed at this early time point following challenge was a tendency for the number of lung neutrophils to increase in the OVA-sensitized rats that were also challenged with OVA, compared with the CO and EX groups. Recently, Shan et al. (59) studied the impact of an OVA aerosolized challenge to OVA-sensitized Brown-Norway rats. They measured a variety of cells, including neutrophils and eosinophils in bronchoalveolar lavage fluid at 1, 3, 6, and 24 h following the challenge. Remarkably, they found a very rapid increase in neutrophils, but there was no appreciable increase in eosinophils until the 24-h time point. These data, along with our finding of very early increase in the number of neutrophils accumulating in the lungs of the SS animals, are consistent with the selective and early increase in the levels of inflammatory cytokines and the remarkable increase in the lung tissue levels of the neutrophil chemoattractant GRO/KC that we found in the SS animals, relative to all other groups.
We analyzed a broad panel of cytokines and chemokines using multiplex techniques. As noted in a recent comparison study (15), “the most appropriate use for these tests may currently be as screening tools for the selection of promising markers for analysis by more sensitive techniques.” We found distinct differences in circulating levels of cytokines in control compared with the challenged rats. Interestingly, each of the three challenges (i.e., OVA challenge in sensitized rats, exercise challenge in unsensitized rats, and exercise challenge in sensitized rats) led to elevated circulating levels of IL-1β, IL-4, IL-18, IFN-γ, and TNF-α. Increasingly, asthma in humans is seen as a condition in which chronic inflammation (either as a “spillover” from lung involvement or reflecting an as yet poorly understood systemic defect) leads to elevations in circulating levels of inflammatory cytokines (42, 49, 50). Identifying causal relationships between the finding of an elevated cytokine concentration and AHR still remains elusive. However, our data support the emerging concept that acute exacerbations of AHR are often associated with increased circulating biomarkers of inflammation. Moreover, systemic manifestations of the immune responses associated with both exercise and allergic challenges are in some ways similar. Finally, the precision of the multiplex approach was insufficient in the current study to assess the impact of changes in cytokines reflecting Th1 (e.g., IFN-γ) and Th2 (e.g., IL-4). Th1 and Th2 responses have been clearly implicated in asthma pathogenesis (42).
Perspectives and Significance
In summary, we found that Brown-Norway rats that had been sensitized, via OVA injection, show a modest degree of AHR in response to a seemingly unrelated trigger, brief exercise. The rapid onset of the change in breath sounds following the exercise challenge is similar to exercise-induced bronchoconstriction observed clinically in humans. Exercise, even in the absence of prior sensitization to OVA, led to increased lung IFN-γ, without an increase in immune cell infiltration, suggesting that that resident leukocytes or macrophages were responsible. Our findings indicate that some individuals may experience common mechanisms and cross-responsiveness to exercise or allergen following sensitization, whereas other individuals, may experience distinct signaling mechanisms to allergen or exercise that would result in no overlap of response. This model may prove useful in better understanding, and, ultimately preventing, exercise-induced bronchoconstriction, a serious and sometimes life-threatening consequence of asthma in humans (5, 14).
GRANTS
This work was supported by National Institutes of Health grant P01HD048721.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the technical assistance of Georgia Lucy-Bachman for the assays.
REFERENCES
- 1. Anderson SD. How does exercise cause asthma attacks? Curr Opin Allergy Clin Immunol 6: 37–42, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Baekey DM, Feng P, Decker MJ, Strohl KP. Breathing and sleep: measurement methods, genetic influences, and developmental impacts. ILAR J 50: 248–261, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Bahoura M. Pattern recognition methods applied to respiratory sounds classification into normal and wheeze classes. Comput Biol Med 39: 824–843, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Baines KJ, Simpson JL, Scott RJ, Gibson PG. Immune responses of airway neutrophils are impaired in asthma. Exp Lung Res 35: 554–569, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Becker JM, Rogers J, Rossini G, Mirchandani H, D'Alonzo GE., Jr Asthma deaths during sports: report of a 7-year experience. J Allergy Clin Immunol 113: 264–267, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Bonsignore MR, La GS, Cibella F, Scichilone N, Cuttitta G, Interrante A, Marchese M, Veca M, Virzi M, Bonanno A, Profita A, Morici G. Effects of exercise training and montelukast in children with mild asthma. Med Sci Sports Exerc 40: 405–412, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Careau E, Bissonnette EY. Adoptive transfer of alveolar macrophages abrogates bronchial hyperresponsiveness. Am J Respir Cell Mol Biol 31: 22–27, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Castells MC, Horan RF, Sheffer AL. Exercise-induced anaphylaxis. Curr Allergy Asthma Rep 3: 15–21, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Caucig P, Teschner D, Dinges S, Maxeiner JH, Reuter S, Finotto S, Taube C, von Stebut E. Dual role of interleukin-1alpha in delayed-type hypersensitivity and airway hyperresponsiveness. Int Arch Allergy Immunol 152: 303–312, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Chiba T, Kanda A, Ueki S, Ito W, Yamaguchi K, Kamada Y, Takeda M, Tanigai T, Oyamada H, Kayaba H, Chihara J. Possible novel receptor for PGD2 on human bronchial epithelial cells. Int Arch Allergy Immunol 143 Suppl 1: 23–27, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Cooper DM, Radom-Aizik S, Schwindt CD, Zaldivar F. Dangerous exercise—lessons learned from dysregulated inflammatory responses to physical activity. J Appl Physiol 103: 700–709, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med 161: 309–329, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Dharajiya N, Vaidya SV, Murai H, Cardenas V, Kurosky A, Boldogh I, Sur SA. FcgammaRIIb inhibits allergic lung inflammation in a murine model of allergic asthma [Online]. PLoS ONE 5: e9337, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DiDario AG, Becker JM. Asthma, sports, and death. Allergy Asthma Proc 26: 341–344, 2005 [PubMed] [Google Scholar]
- 15. Djoba Siawaya JF, Roberts T, Babb C, Black G, Golakai HJ, Stanley K, Bapela NB, Hoal B, Parida S, van HP, Walzl G. An evaluation of commercial fluorescent bead-based luminex cytokine assays. PLoS ONE 3: e2535, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Falk TH, Chan WY. Modulation filtering for heart and lung sound separation from breath sound recordings. Conf Proc IEEE Eng Med Biol Soc 2008: 1859–1862, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Fanelli A, Cabral AL, Neder JA, Martins MA, Carvalho CR. Exercise training on disease control and quality of life in asthmatic children. Med Sci Sports Exerc 39: 1474–1480, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Freed AN. Models and mechanisms of exercise-induced asthma. Eur Respir J 8: 1770–1785, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Glaab T, Hecker H, Stephan M, Baelder R, Braun A, Korolewitz R, Krug N, Hoymann HG. Comparison of non-invasive measures of cholinergic and allergic airway responsiveness in rats. Acta Physiol (Oxf) 186: 301–308, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Glaab T, Hoymann HG, Hohlfeld JM, Korolewitz R, Hecht M, Alarie Y, Tschernig T, Braun A, Krug N, Fabel H. Noninvasive measurement of midexpiratory flow indicates bronchoconstriction in allergic rats. J Appl Physiol 93: 1208–1214, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Goldflam K, Silvers CT. Exercise-induced anaphylaxis as a cause of syncope. J Emerg Med In Press. [DOI] [PubMed] [Google Scholar]
- 22. Gwag T, Lee K, Ju H, Shin H, Lee JW, Choi I. Stress and signaling responses of rat skeletal muscle to brief endurance exercise during hindlimb unloading: a catch-up process for atrophied muscle. Cell Physiol Biochem 24: 537–546, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Hewitt M, Creel A, Estell K, Davis IC, Schwiebert LM. Acute exercise decreases airway inflammation, but not responsiveness, in an allergic asthma model. Am J Respir Cell Mol Biol 40: 83–89, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hewitt M, Estell K, Davis IC, Schwiebert LM. Repeated bouts of moderate-intensity aerobic exercise reduce airway reactivity in a murine asthma model. Am J Respir Cell Mol Biol 42: 243–249, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoene M, Lehmann R, Hennige AM, Pohl AK, Haring HU, Schleicher ED, Weigert C. Acute regulation of metabolic genes and insulin receptor substrates in the liver of mice by one single bout of treadmill exercise. J Physiol 587: 241–252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holgate S, Smith N, Massanari M, Jimenez P. Effects of omalizumab on markers of inflammation in patients with allergic asthma. Allergy 64: 1728–1736, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Hylkema MN, Hoekstra MO, Luinge M, Timens W. The strength of the OVA-induced airway inflammation in rats is strain dependent. Clin Exp Immunol 129: 390–396, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jammes Y, Barthelemy P, Fornaris M, Grimaud C. Cold-induced bronchospasm in normal and sensitized rabbits. Respir Physiol 63: 347–360, 1986 [DOI] [PubMed] [Google Scholar]
- 29. Kanda A, Driss V, Hornez N, Abdallah M, Roumier T, Abboud G, Legrand F, Staumont-Salle D, Queant S, Bertout J, Fleury S, Remy P, Papin JP, Julia V, Capron M, Dombrowicz D. Eosinophil-derived IFN-gamma induces airway hyperresponsiveness and lung inflammation in the absence of lymphocytes. J Allergy Clin Immunol 124: 573–82, 582, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Kang DH, Weaver MT. Airway cytokine responses to acute and repeated stress in a murine model of allergic asthma. Biol Psychol 84: 66–73, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Kelly M, Hwang JM, Kubes P. Modulating leukocyte recruitment in inflammation. J Allergy Clin Immunol 120: 3–10, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Kim J, McKinley L, Natarajan S, Bolgos GL, Siddiqui J, Copeland S, Remick DG. Anti-tumor necrosis factor-alpha antibody treatment reduces pulmonary inflammation and methacholine hyper-responsiveness in a murine asthma model induced by house dust. Clin Exp Allergy 36: 122–132, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Kivastik J, Gibson AM, Primhak RA. Methacholine challenge in pre-school children—which outcome measure? Respir Med 101: 2555–2560, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Koyama S, Ohtsuka A, Horie T. Eucapnic hyperventilation-induced bronchoconstriction in rabbits. Tohoku J Exp Med 168: 611–619, 1992 [DOI] [PubMed] [Google Scholar]
- 35. Kruschinski C, Skripuletz T, Bedoui S, Raber K, Straub RH, Hoffmann T, Grote K, Jacobs R, Stephan M, Pabst R, von Hörsten S. Postnatal life events affect the severity of asthmatic airway inflammation in the adult rat. J Immunol 180: 3919–3925, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Kumar RK, Herbert C, Foster PS. The “classical” ovalbumin challenge model of asthma in mice. Curr Drug Targets 9: 485–494, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Kumar RK, Webb DC, Herbert C, Foster PS. Interferon-gamma as a possible target in chronic asthma. Inflamm Allergy Drug Targets 5: 253–256, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Kuo CH, Jan RL, Chu YT, Wang WL, Huang MY, Huang CH, Chen TH, Hung CH. Prostaglandin I(2) analogues enhance growth-related oncogene-alpha expression in human monocyte-derived dendritic cells. Inflammation 33: 334–343, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Labonte I, Hassan M, Risse PA, Tsuchiya K, Laviolette M, Lauzon AM, Martin JG. The effects of repeated allergen challenge on airway smooth muscle structural and molecular remodeling in a rat model of allergic asthma. Am J Physiol Lung Cell Mol Physiol 297: L698–L705, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Lavoie JP, Maghni K, Taha R, Yang XX, Lang GM, Sehon AH, Hamid QA, Martin JG. Conjugates of ovalbumin and monomethoxypolyethylene glycol abolish late allergic responses and decrease IL-4 and IL-5 mRNA expression in the rat. Pulm Pharmacol Ther 16: 361–369, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kuchel GA. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J Gerontol A Biol Sci Med Sci 63: 879–884, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol 10: 838–848, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lugogo NL, Kraft M, Dixon AE. Does obesity produce a distinct asthma phenotype? J Appl Physiol 108: 729–734, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Manise M, Schleich F, Gusbin N, Godinas L, Henket M, Antoine N, Corhay JL, Louis R. Cytokine production from sputum cells and blood leukocytes in asthmatics according to disease severity. Allergy 65: 889–896, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Martin JG, Tamaoka M. Rat models of asthma and chronic obstructive lung disease. Pulm Pharmacol Ther 19: 377–385, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Martin-Munoz MF, Pagliara L, Antelo MC, Madero JR, Barrio MI, Martinez MC, Martin EM. Exercise-induced asthma in asthmatic children. Predisposing factors. Allergol Immunopathol (Madr) 36: 123–127, 2008 [PubMed] [Google Scholar]
- 47. Matsuda A, Orihara K, Fukuda S, Fujinaga H, Matsumoto K, Saito H. Corticosteroid enhances TNF-α-mediated leukocyte adhesion to pulmonary microvascular endothelial cells. Allergy 63: 1610–1616, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Mendes FA, Almeida FM, Cukier A, Stelmach R, Jacob-Filho W, Martins MA, Carvalho CR. Effects of aerobic training on airway inflammation in asthmatic patients. Med Sci Sports Exerc 43: 197–203, 2011 [DOI] [PubMed] [Google Scholar]
- 49. Natarajan S, Kim J, Remick DG. Acute pulmonary lipopolysaccharide tolerance decreases TNF-alpha without reducing neutrophil recruitment. J Immunol 181: 8402–8408, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Parsons JP, Mastronarde JG. Exercise-induced asthma. Curr Opin Pulm Med 15: 25–28, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Parton R, Hall E, Wardlaw AC. Induction of abnormal respiratory sounds by capsaicin in rats previously infected with Bordetella pertussis. FEMS Immunol Med Microbiol 20: 139–144, 1998 [DOI] [PubMed] [Google Scholar]
- 52. Pastva A, Estell K, Schoeb TR, Atkinson TP, Schwiebert LM. Aerobic exercise attenuates airway inflammatory responses in a mouse model of atopic asthma. J Immunol 172: 4520–4526, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Quarcoo D, Pavlovic S, Joachim RA. Stress and airway reactivity in a murine model of allergic airway inflammation. Neuroimmunomodulation 16: 318–324, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Randolph C. An update on exercise-induced bronchoconstriction with and without asthma. Curr Allergy Asthma Rep 9: 433–438, 2009 [DOI] [PubMed] [Google Scholar]
- 55. Reinhold RW, Hoffman GM, Bolte HF, Rinehart WE, Rusch GM, Parod RJ, Kayser M. Subchronic inhalation toxicity study of caprolactam (with a 4-wk recovery) in the rat via whole-body exposures. Toxicol Sci 44: 197–205, 1998 [DOI] [PubMed] [Google Scholar]
- 56. Robroeks CM, Rijkers GT, Jobsis Q, Hendriks HJ, Damoiseaux JG, Zimmermann LJ, van Schayck OP, Dompeling E. Increased cytokines, chemokines and soluble adhesion molecules in exhaled breath condensate of asthmatic children. Clin Exp Allergy 40: 77–84, 2010 [DOI] [PubMed] [Google Scholar]
- 57. Sabaitis CP, Leong BK, Rop DA, Aaron CS. Validation of intratracheal instillation as an alternative for aerosol inhalation toxicity testing. J Appl Toxicol 19: 133–140, 1999 [DOI] [PubMed] [Google Scholar]
- 58. Schutze N, Lehmann I, Bonisch U, Simon JC, Polte T. Exposure to mycotoxins increases the allergic immune response in a murine asthma model. Am J Respir Crit Care Med 181: 1188–1199, 2010 [DOI] [PubMed] [Google Scholar]
- 59. Shan L, Kawakami T, Asano S, Noritake S, Yoshimoto D, Yamashita K, Kikkawa H, Kinoshita M, Matsubara S. Inverse relationship between Sec14l3 mRNA/protein expression and allergic airway inflammation. Eur J Pharmacol 616: 293–300, 2009 [DOI] [PubMed] [Google Scholar]
- 60. Silva RA, Vieira RP, Duarte AC, Lopes FD, Perini A, Mauad T, Martins MA, Carvalho CR. Aerobic training reverses airway inflammation and remodeling in asthma murine model. Eur Respir J 35: 994–1002, 2010 [DOI] [PubMed] [Google Scholar]
- 61. Sirois J, Bissonnette EY. Alveolar macrophages of allergic resistant and susceptible strains of rats show distinct cytokine profiles. Clin Exp Immunol 126: 9–15, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. St-Laurent J, Boulet LP, Bissonnette E. Cigarette smoke differently alters normal and ovalbumin-sensitized bronchial epithelial cells from rat. J Asthma 46: 577–581, 2009 [DOI] [PubMed] [Google Scholar]
- 63. Stenton GR, Ulanova M, Dery RE, Merani S, Kim MK, Gilchrist M, Puttagunta L, Musat-Marcu S, James D, Schreiber AD, Befus AD. Inhibition of allergic inflammation in the airways using aerosolized antisense to Syk kinase. J Immunol 169: 1028–1036, 2002 [DOI] [PubMed] [Google Scholar]
- 64. Suzuki K, Nakaji S, Yamada M, Totsuka M, Sato K, Sugawara K. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc Immunol Rev 8: 6–48, 2002 [PubMed] [Google Scholar]
- 65. Traves SL, Donnelly LE. Th17 cells in airway diseases. Curr Mol Med 8: 416–426, 2008 [DOI] [PubMed] [Google Scholar]
- 66. Vilozni D, Bentur L, Efrati O, Barak A, Szeinberg A, Shoseyov D, Yahav Y, Augarten A. Exercise challenge test in 3- to 6-year-old asthmatic children. Chest 132: 497–503, 2007 [DOI] [PubMed] [Google Scholar]
- 67. Weiler-Ravell D, Godfrey S. Do exercise- and antigen-induced asthma utilize the same pathways? Antigen provocation in patients rendered refractory to exercise-induced asthma. J Allergy Clin Immunol 67: 391–397, 1981 [DOI] [PubMed] [Google Scholar]
- 68. Wu H, Romieu I, Shi M, Hancock DB, Li H, Sienra-Monge JJ, Chiu GY, Xu H, del Rio-Navarro BE, London SJ. Evaluation of candidate genes in a genome-wide association study of childhood asthma in Mexicans. J Allergy Clin Immunol 125: 321–327, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xu KF, Vlahos R, Messina A, Bamford TL, Bertram JF, Stewart AG. Antigen-induced airway inflammation in the Brown Norway rat results in airway smooth muscle hyperplasia. J Appl Physiol 93: 1833–1840, 2002 [DOI] [PubMed] [Google Scholar]
- 70. Yang M, Kumar RK, Foster PS. Interferon-gamma and pulmonary macrophages contribute to the mechanisms underlying prolonged airway hyperresponsiveness. Clin Exp Allergy 40: 163–173, 2010 [DOI] [PubMed] [Google Scholar]