Abstract
Although the receptor for advanced glycation end products (RAGE) has been used as a biological marker of alveolar epithelial cell injury in clinical studies, the mechanism for release of soluble RAGE from lung epithelial cells has not been well studied. Therefore, these studies were designed to determine the mechanism for release of soluble RAGE after lipopolysaccharide (LPS) challenge. For these purposes, alveolar epithelial cells from rat lungs were cultured on Transwell inserts, and LPS was added to the apical side (500 μg/ml) for 16 h on day 7. On day 7, RAGE was expressed predominantly in surfactant protein D-negative cells, and LPS challenge induced release of RAGE into the medium. This response was partially blocked by matrix metalloproteinase (MMP) inhibitors. Transcripts of MMP-3 and MMP-13 were upregulated by LPS, whereas RAGE transcripts did not change. Proteolysis by MMP-3 and MMP-13 resulted in soluble RAGE expression in the bronchoalveolar lavage fluid in the in situ rat lung, and this reaction was inhibited by MMP inhibitors. In human studies, both MMP-3 and -13 antigen levels were significantly correlated with the level of RAGE in pulmonary edema fluid samples. These results support the conclusion that release of RAGE is primarily mediated by proteolytic damage in alveolar epithelial cells in the lung, caused by proteases in acute inflammatory conditions in the distal air spaces.
Keywords: receptor for advanced glycation end products, matrix metalloproteinase, acute lung injury, pulmonary edema
alveolar epithelial cells have several important functions, including maintaining a tight barrier, regulating surfactant production, and removing excess alveolar fluid by vectorial ion transport as well as a role in regulating intra-alveolar inflammation and coagulation (13). Injury to the alveolar epithelium is an important feature of acute lung injury (ALI) (20, 23, 40). For the clinical evaluation of epithelial cell injury, biomarkers of epithelial cell origin have been reported to reflect the severity or prognosis of ALI (9, 15, 19). However, most of them are expressed primarily by alveolar type II cells, whereas alveolar type I cell covers most of the alveolar epithelial surface in the lung.
The receptor for advanced glycation end products (RAGE) is a cell surface protein that belongs to the immunoglobulin superfamily. Although the expression of RAGE was demonstrated also in the endothelial cells in large vessels and in neurological tissue, RAGE expression is most abundant in the lung (2). In the lung, RAGE expression was demonstrated on the basal membranes of alveolar type I epithelial cells (33). Two recent genomewide association studies for lung function demonstrated that the gene locus for RAGE (advanced glycation end products receptor; AGER) acts as one of the important determinants of pulmonary function (16, 30). We reported that RAGE can be used as a biomarker of alveolar epithelial injury in an experimental study and in patients with ALI or acute respiratory distress syndrome (ARDS) (36). Subsequent studies also found that plasma RAGE had prognostic value for short-term outcomes in patients undergoing lung transplantation (5, 7, 26). Also, with use of an isolated perfused human lung model, there was an inverse correlation between RAGE levels in the samples from air space and alveolar fluid clearance in two studies (3, 14), and our most recent clinical study reported the elevated RAGE levels in plasma had pathogenic and prognostic value in patients with ALI (6). However, the mechanism of release of RAGE from alveolar epithelial cells has not been well established.
Therefore, the objective of this study was to study the mechanism for release of soluble RAGE in cultured alveolar epithelial cells. For this purpose, we studied whether soluble RAGE is released from cultured rat alveolar epithelial cells by lipopolysaccharide (LPS), and we examined the role of proteolysis in release of RAGE by inhibition of matrix metalloproteinases (MMPs). We also tested the hypothesis that MMP-3 and MMP-13 may play a role in release of RAGE by inflammatory stimuli using the in situ rat lung. For clinical relevance, we also tested for a correlation between the levels of MMP-3 and MMP-13 and the concentrations of RAGE in human pulmonary edema fluid samples.
MATERIALS AND METHODS
Animal Studies
All protocols were approved by the institutional animal care committee of Tokyo Medical and Dental University.
Antibodies.
For immunocytochemistry, anti-surfactant protein-D (SP-D) monoclonal antibody (Acris Antibodies, Hiddenhausen, Germany) was used as a type II epithelial cell marker in this study. For RAGE expression analysis, anti-mouse RAGE polyclonal antibody (AF1179, R&D Systems, Minneapolis, MN) was used for immunoblot and immunocytochemistry. For ELISA assay of rat samples, anti-human RAGE monoclonal antibody (MAB11451, R&D Systems) was used as a capture antibody, and biotinylated AF1179 (BAF1179, R&D Systems) was used as a detection antibody. AF1179, BAF1179, and MAB11451 have cross-reactivity to rat RAGE. For MMP expression analysis, anti-human MMP-1 rabbit polyclonal antibody (no. 29574, AnaSpec, San Jose, CA), anti-human MMP-3 rabbit polyclonal antibody (BS1238, Bioworld Technology, Minneapolis, MN), and anti-human MMP-13 rabbit polyclonal antibody (Ab-5, Thermo Fisher Scientific, Fremont, CA) were used. These antibodies for MMP expression have cross-reactivity to rat MMP-1, -3, and -13, respectively. In the immunocytochemistries, a mouse IgG1 control (MAB002, R&D Systems), a rabbit IgG control (AB105-C, R&D Systems), and a goat IgG control (AB108-C, R&D Systems) were used as negative controls for the first antibodies to exclude nonspecific staining.
Primary culture of rat alveolar epithelial cells.
Primary cultures of rat alveolar epithelial cells were prepared as previously described (10). Briefly, Sprague-Dawley rats (6 wk) were tracheostomized under pentobarbital anesthesia (40 mg/kg ip). The rats were then euthanized by exsanguination under deep anesthesia (pentobarbital 100 mg/kg iv), and the lungs were removed en bloc. After the bronchoalveolar lavage (BAL) by solution I (140 mM NaCl, 5 mM KCl, 2.5 mM phosphate buffer, 10 mM HEPES, 6 mM d-glucose, and 2 mM EGTA) and solution II (140 mM NaCl, 5 mM KCl, 2.5 mM phosphate buffer, 10 mM HEPES, 2 mM CaCl2, and 1.3 mM MgSO4), lungs were treated with elastase (Worthington Biochemical, Lakewood, NJ). The lung tissue was minced and filtrated by 140- and 30-μm nylon mesh filters. Filtrated cells were centrifuged, and the cell pellet was resuspended into Dulbecco's modified Eagle's medium (DMEM, GIBCO, Invitrogen, Carlsbad, CA) and incubated on the bacteriological plate at 37°C for 1 h. Unattached cells were collected and seeded on 12-mm Transwell (product no. 3401, Corning International, Tokyo, Japan) at 2.5 × 106 cells/well. Medium was exchanged every 2 or 3 days using DMEM containing 10% fetal bovine serum (FBS, GIBCO, Invitrogen) unless the cells were treated with experimental conditioning medium.
Immunocytochemistry.
Cells on Transwell were fixed with 4% formaldehyde, permeabilized with 0.2% Triton X (Sigma Aldrich Japan, Tokyo, Japan) except in cell surface expression studies and incubated in blocking solution containing 1% bovine serum albumin (KPL, Gaithersburg, MD). After staining with primary antibody and secondary antibody (Alexa Fluor 568 donkey anti-goat IgG, Alexa Fluor 647 chicken anti-rabbit IgG, Alexa Fluor 488 chicken anti-mouse IgG, Molecular Probes, Eugene, OR), Transwell membranes were mounted on slides and images were obtained by confocal laser scanning microscopy (LSM510 Carl Zeiss MicroImaging) and processed by Zeiss LSM Image Browser 4.2. (Carl Zeiss MicroImaging).
LPS stimulation and MMP inhibitor studies.
Primary rat alveolar epithelial cells were cultured on Transwells as described above, and medium was exchanged with FBS-free DMEM on day 5. On day 7, LPS (Escherichia coli 0111:B4, Sigma Aldrich Japan) was added to the medium of apical side at a concentration of 100 or 500 μg/ml, then cells were cultured for 16 h. In some experiments, alveolar epithelial cells were cultured in DMEM with 10% FBS by day 7 of culture and LPS was added to the media for 16 h on day 7. Media from the apical and basal sides were collected separately, and each of them was centrifuged (4°C, 1,500 g, 10 min). Soluble RAGE levels in the supernatant were analyzed by immunoblot and ELISA. For ELISA, a standard curve with linear regression was created for recombinant rat RAGE-Fc chimera protein (1616-RG, R&D Systems) with correlation coefficient between 0.98 and 0.99. The quantity of RAGE released into the media was calculated from the concentration of RAGE multiplied by the volume of the media. In some experiments, an MMP inhibitor was added to medium from day 5 to day 7 to investigate the role of MMPs in the release of soluble RAGE into the medium. MMP inhibitors used in this study were MMP-inhibitor 1 (MMPI, Kamiya Biomedical, Seattle, WA; an inhibitor of MMP-1, -2, -3, -7, and -13), TNF-α processing inhibitor-0 (TAPI-0, Biomol International, Plymouth Meeting, PA; an inhibitor of MMP-1, -3, -9, and -13), and CL82198 (Biomol International; a selective MMP-13 inhibitor). In some experiments, cells were treated with aprotinin [A6279 without dilution (5–10 trypsin inhibitor units/ml) from Sigma Aldrich Japan] and E-64 (50 μM) in place of MMP inhibitors, to study contribution of serine proteases or cysteine proteases to the RAGE release by LPS stimulation.
mRNA extraction and real-time PCR.
Total RNA was isolated from alveolar epithelial cell cultured on Transwell for 7 days by silica membrane column (High Pure RNA Isolation Kit, Roche Diagnostics, Mannheim, Germany). cDNA was synthesized from total RNA by using Transcriptor 1st strand cDNA Synthesis Kit (Roche Diagnostics). The expression of MMP-3, MMP-13, and RAGE were analyzed by real-time PCR using LC480 Probe Master (Roche Diagnostics). Primers were designed as shown in Table 1. RAGE forward AGCTTCAGTCTGGGCCTTC and RAGE reverse CAGCTGAATGCCCTCTGG correspond to the sequence of exon 6 and 7, which covered the extracellular domain. The abundance was standardized by comparison with the β-actin mRNA expression.
Table 1.
Forward and reverse primers for real-time PCR analysis
| Target | Sequence |
|---|---|
| RAGE | |
| Forward | AGCTTCAGTCTGGGCCTTC |
| Reverse | CAGCTGAATGCCCTCTGG |
| MMP-3 | |
| Forward | GAGAACTTTCCAGGCATTGG |
| Reverse | CCGCTGAAGAAGTAAAGAAACC |
| MMP-13 | |
| Forward | GGACAAGCAGCTCCAAAGG |
| Reverse | GGTCCAGACCGAGGGAGT |
| β-Actin | |
| Forward | CCCGCGAGTACAACCTTCT |
| Reverse | CGTCATCCATGGCGAACT |
MMP challenge in the in situ lung model.
To study whether MMP-3- or MMP-13-induced proteolysis causes expression of soluble isotype of RAGE in BAL, three experimental solutions were prepared. For MMP-3, 1) MMP-3 (0.1 μg/ml, recombinant human MMP-3 catalytic domain, AnaSpec. Fremont CA), 2) MMP-3 (0.1 μg/ml) with MMPI (200 μM) as an inhibitor for MMP-3, and 3) normal saline. For MMP-13, 1) MMP-13 (1 μg/ml, recombinant human MMP-13 catalytic domain, ProtEra, Firenze, Italy), 2) MMP-13 (1 μg/ml) with a specific inhibitor for MMP-13 (60 μM of CL82198), and 3) normal saline. In each set of experiments, Sprague-Dawley rats (n = 9) were anesthetized with pentobarbital (40 mg/kg ip) and tracheostomized with 14 G cannula (Surflow, Terumo, Tokyo, Japan). The rats were euthanized by exsanguination via the abdominal aorta under deep anesthesia (pentobarbital 100 mg/kg iv), and 3 ml of the experimental solution was instilled via tracheal cannula. The tracheal cannula was then clamped, and body temperature of the animal was maintained between 36 and 37°C by infrared lamp. After a 30-min interval, BAL was done with 5 ml PBS with protease inhibitor (Halt, Pierce Biotechnology, Rockford, IL). BAL samples were analyzed by immunoblot.
LPS-induced lung injury model study.
To study whether MMP-3- or MMP-13-induced proteolysis causes expression of soluble isotype of RAGE in BAL in in vivo LPS-induced lung injury model, male Sprague-Dawley rats (180 g) were anesthetized with ether, and a single dose of LPS (10 mg/kg) in 180 μl of saline, with or without a MMP inhibitor [MMPI (200 μM) or CL82198 (60 μM)], was administered by intratracheal instillation. In control animals, the same volume of intratracheal saline was instilled intratracheally. The animals were euthanized 6 h after instillation under deep anesthesia (pentobarbital 150 mg/kg iv), and BAL was done with 5 ml PBS with protease inhibitor (Halt, Pierce Biotechnology). BAL samples were analyzed by immunoblot.
Human Studies
Measurement of RAGE, MMP-3, and MMP-13 level in the pulmonary edema fluid.
To study the correlation between RAGE levels and MMP antigen levels, we measured the levels of MMP-3 and MMP-13 antigen in the pulmonary edema fluid in which we measured RAGE levels in our previous study (36). Samples were selected randomly from a stored sample bank of pulmonary edema fluid from patients with ALI/ARDS and hydrostatic pulmonary edema. Eligibility for inclusion in the study was based solely on availability of an adequate stored volume of edema fluid for measurement of RAGE, MMP-3, and MMP-13 levels. Patients with ALI or ARDS were identified on the basis of the American European Consensus Conference definitions (1). Patients with hydrostatic pulmonary edema were identified as previously described (39). Undiluted pulmonary edema fluid were collected as previously described (39). This study was approved by the UCSF Committee on Human Research. The concentrations of RAGE, MMP-3, and MMP-13 antigen were measured in duplicate by commercially available ELISA kits (R&D Systems).
Data Analysis
Continuous variables were compared by Student's t-test or by analysis of variance with Scheffé's test for multiple comparisons. All data are reported as means ± SD, and statistical significance was defined as P < 0.05.
RESULTS
RAGE Expression on Cultured Rat Alveolar Epithelial Cells
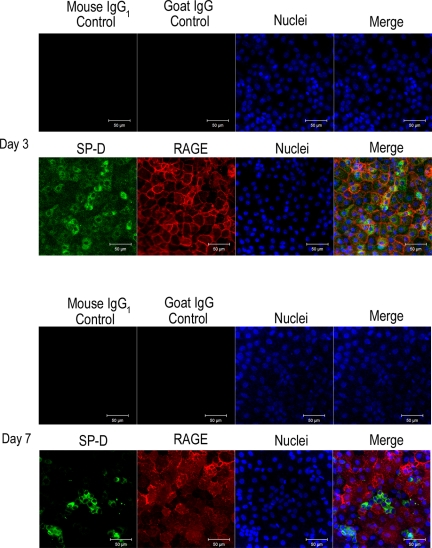
To verify RAGE expression on cultured rat alveolar epithelial cells, cells cultured on Transwell membranes were stained with anti-RAGE antibody (AF1179) and anti-SP-D antibody (as a marker of type II alveolar epithelial cell phenotype) on days 3 and 7. On day 3 of culture, most (81%) of the cells expressed SP-D, reflecting that the cells had a type II cell phenotype at the beginning of cell culture, and SP-D expression was maintained in a small fraction (16%) of the cells, covering 13% of the total surface area, by day 7 of culture (Fig. 1). RAGE expression was observed in the cellular junctions on day 3 (Fig. 1) and was augmented especially in the SP-D-negative cells by day 7 (Fig. 1).
Fig. 1.
Expression of receptor for advanced glycation end products (RAGE, red), and surfactant protein-D (SP-D, green) in the rat alveolar epithelial cells cultured on Transwell. Nuclei are stained with 4,6-diamidino-2-phenylindole (DAPI, blue). RAGE was expressed in the cellular junction on day 3, and RAGE expression was positive predominantly in SP-D-negative cells on day 7. Mouse IgG1 control and goat IgG control were used for negative control treatments for SP-D and RAGE, respectively. Each result was obtained as a representative result from consecutive 4 preparations in each study condition.
Release of Soluble RAGE by LPS Challenge
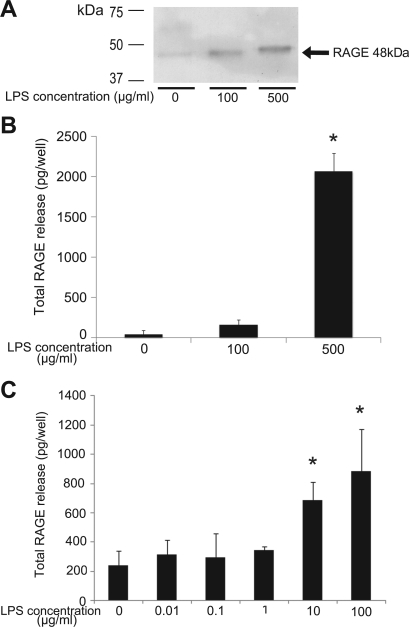
To study the mechanism for release of soluble RAGE into the alveolar space in this in vitro model, we stimulated the cultured rat alveolar epithelial cells with LPS and measured RAGE levels in the media by immunoblot and ELISA (Figs. 2 and 3). As shown in Fig. 2, the results from ELISA matched the results from immunoblot analysis using AF1179. In the medium of the cells treated with LPS in the serum-free conditions, the quantity of RAGE released from cells was dose dependently higher than in the control cells (n = 4, Fig. 2B). Supplementation of 10% FBS facilitated the release of RAGE induced by LPS stimulation in lower concentration (Fig. 2C).
Fig. 2.
Analysis of abundance of soluble RAGE into the media from cultured alveolar epithelial cells stimulated by lipopolysaccharide (LPS) in serum-free conditions (A and B) and in serum-supplemented conditions (C). A: representative results of immunoblot analysis for the RAGE level in the apical side media from consecutive 4 experiments in each study condition. B and C: quantity of soluble RAGE calculated from the RAGE concentration multiplied by the volume of media. LPS increased release of soluble RAGE from cells dose dependently both in serum-free conditions (B) and in serum-supplemented conditions (C) (n = 4 in each preparation, *P < 0.05 vs. control).
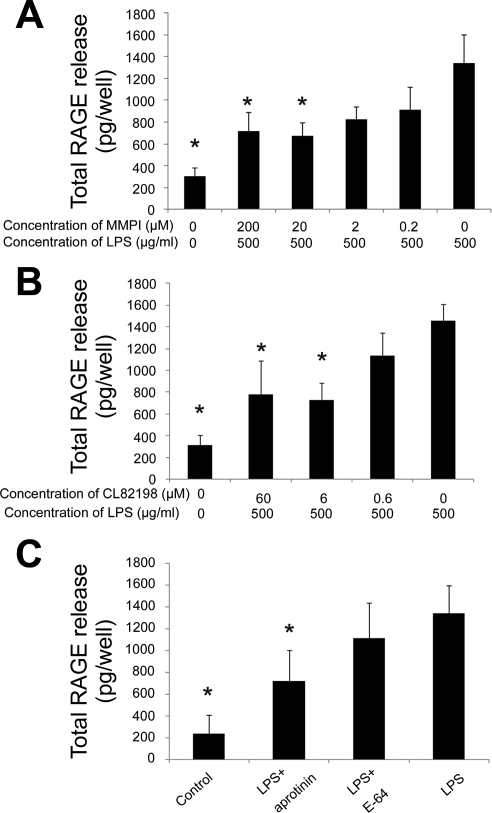
Fig. 3.
Effect of protease inhibitors on RAGE release from the cultured rat alveolar epithelial cells stimulated by LPS (500 μg/ml). Each figure demonstrates the quantity of RAGE released into the media calculated from the RAGE concentration analyzed by ELISA. When matrix metalloproteinase (MMP) inhibitor-1 (MMPI, inhibitor of MMP-1, -2, -3, -7, -13; A) or CL82198 (a selective MMP-13 inhibitor; B) was added to the medium, release of RAGE under LPS stimulation was dose dependently decreased (n = 4 in each preparation, *P < 0.05 vs. LPS 500 μg/ml without MMP inhibitors). Maximum effect was observed at 20 μM for MMPI and 6 μM for CL82198. C: effect of serine protease inhibitor aprotinin (5–10 trypsin inhibitor units/ml) and cysteine protease inhibitor E-64 (50 μM) on RAGE release induced by LPS treatment. Aprotinin significantly decreased the total amount of RAGE released from alveolar epithelial cells, whereas E-64 did not show significant effect (n = 4 in each preparation, *P < 0.05 vs. LPS).
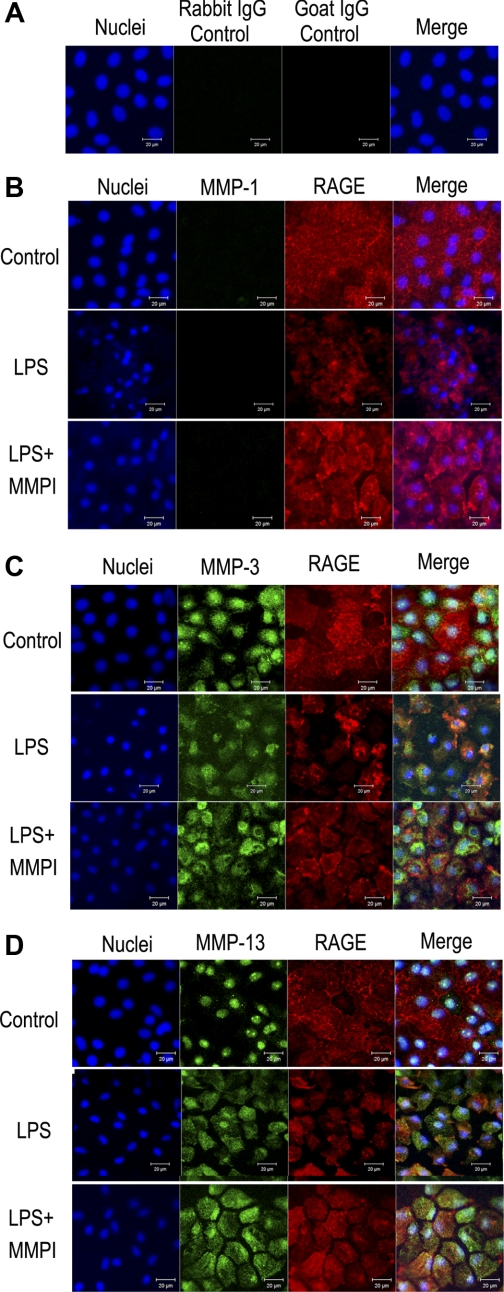
When MMPI, an inhibitor of MMP-1, -2, -3, -7, and -13, was added to the medium, the quantity of RAGE released by LPS stimulation was dose dependently decreased (Fig. 3A). TAPI-0 (1 μM), an inhibitor of MMP-1, -3, -9, and -13, also significantly decreased the release of RAGE caused by LPS stimulation (data not shown). Then we studied the protein expression of MMP-1, -3, and -13 because these MMPs were inhibited by both MMPI and TAPI-0. Immunocytochemical study demonstrated that both MMP-3 (Fig. 4C) and MMP-13 (Fig. 4D) were expressed in the cultured alveolar epithelial cells, whereas MMP-1 expression was negative (Fig. 4B). LPS stimulation loosened the cellular attachment and weakened the RAGE expression, and we found that MMP-3 and -13 were coexpressed with RAGE in alveolar epithelial cells under LPS challenge (Fig. 4, C and D). With inhibition of protease activity by MMPI, RAGE expression was maintained and coexpression of RAGE with MMP-3 and MMP-13 was more obviously demonstrated (Fig. 4, C and D). Interestingly, both MMP-3 and -13 were expressed mainly in the nuclei under control conditions, and LPS augmented extranuclear expression of these MMPs. To study RAGE expression on the cellular surface of cultured alveolar epithelial cells, we stained RAGE on the cells without membrane permeabilization. Again RAGE expression was loosened by LPS, and MMPI partially inhibited this reaction (Fig. 5). In culture media, LPS stimulation increased the level of pro-MMP-3 (Fig. 6A), and active form of MMP-13 (Fig. 6B). Furthermore, to study whether LPS challenge induced MMP-3, MMP-13, and RAGE expression in cultured alveolar epithelial cells, we measured the level of transcript in the cultured alveolar epithelial cells under LPS challenge. The results of real-time PCR demonstrated that LPS challenge (100 μg/ml for 4 h) resulted in the increase in the transcript of MMP-3 and -13 (Fig. 6, C and D). On the other hand, the abundance of RAGE transcript was not changed by LPS stimulation, and both MMPI and CL82198 had no effect on the abundance of RAGE transcript (Fig. 6E).
Fig. 4.
Expression of MMP-1 (B), MMP-3 (C), and MMP-13 (D) in the rat alveolar epithelial cells cultured on Transwell. Cells were also stained with anti-RAGE antibody (red), and nuclei are stained with DAPI (blue). A: results of negative control stain with rabbit IgG control and goat IgG control for anti-MMP antibodies (rabbit IgG) and anti-RAGE antibody (goat IgG). Each result was obtained as a representative result from consecutive 4 preparations in each study condition. And in each set of images, top row demonstrates control condition, middle row demonstrates cells treated with LPS (500 μg/ml), and bottom row demonstrates cells treated with LPS (500 μg/ml) and MMP-inhibitor 1 (MMPI, 200 μM) (LPS+MMPI), where MMPI was added to prevent loss of membrane-bound RAGE and detachment of cells. In control cells, MMP-3 and MMP-13 were expressed and their spatial expression overlapped with DAPI stain. In both LPS cells and LPS+MMPI cells, RAGE stain demonstrates loosening of cellular attachment and change in the spatial expression of MMP-3 and MMP-13. MMP-1 was negative in control cells, LPS cells, and LPS+MMPI cells.
Fig. 5.
RAGE expression on the cell surface: control condition (A), cells treated LPS (500 μg/ml) (B), and cells treated with LPS (500 μg/ml) supplemented with MMP-inhibitor 1 (MMPI, 200 μM) (LPS+MMPI) (C). In each setting, cells were fixed with formalin and stained with anti-RAGE antibody (red) without permeabilization with Triton X, and nuclei were stained with DAPI (blue). Each result was obtained as a representative result from consecutive 4 preparations in each study condition.
Fig. 6.
A: abundance of MMP-3 protein in the culture media. LPS stimulation (500 μg/ml on the apical side for 6 h) increased the expression of pro-MMP-3 (∼55 kDa, arrow). Each result was obtained as a representative result from consecutive 4 preparations in each study condition. B: abundance of MMP-13 protein in the culture media. LPS stimulation (500 μg/ml on the apical side for 6 h) increased the expression of active form of MMP-13 (∼48 kDa) whereas a weak pro-MMP-13 (∼60 kDa) band was observed in the control media. Each result was obtained as a representative result from consecutive 4 preparations in each study condition. C: abundance of MMP-3 transcript in the cultured alveolar epithelial cells. LPS stimulation (100 μg/ml on the apical side for 4 h) resulted in increase in the abundance of MMP-3 transcript (n = 6, *P < 0.05 vs. control). D: abundance of MMP-13 transcript in the cultured alveolar epithelial cells. LPS stimulation (100 μg/ml on the apical side for 4 h) resulted in increase in the abundance of MMP-13 transcript (n = 6, *P < 0.05 vs. control). E: abundance of RAGE transcript in the cultured alveolar epithelial cells. LPS stimulation (100 μg/ml on the apical side for 4 h) with or without MMP inhibitors [MMP-inhibitor 1 (MMPI, 200 μM) or CL82198 (60 μM)] resulted in no change in the abundance of RAGE transcript (n = 6 in each preparation).
To confirm the role of MMP-13 as a mechanism for release of RAGE, CL82198, a selective MMP-13 inhibitor, was added to the medium and CL82198 dose dependently decreased the quantity of RAGE released into the medium under LPS stimulation (Fig. 3B). We also studied two different classes of protease inhibitors: aprotinin (5–10 trypsin inhibitor units/ml) as a serine protease inhibitor and E-64 (50 μM) as a cysteine protease inhibitor. As shown in Fig. 3C, aprotinin partially inhibited RAGE release from LPS-stimulated cells, whereas E-64 had no significant effect for RAGE release induced by LPS.
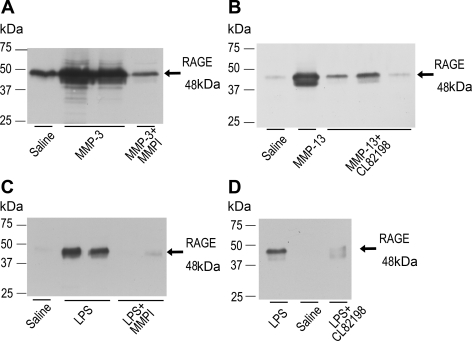
To verify whether proteolysis induced by MMP-3 or MMP-13 results in the expression of soluble RAGE in BAL, these MMPs were instilled into the rat lung in situ. Results of immunoblot for BAL demonstrated that soluble RAGE was expressed in the BAL from the rat lung treated with MMP-3 or MMP-13, but not in the BAL from the rat lung treated with MMP-3 antagonized by MMPI or MMP-13 antagonized by CL82198 (Fig. 7, A and B). We also studied the effect of MMP inhibitors on the RAGE release into alveolar space in the LPS-induced lung injury model. RAGE level in the BAL was decreased by the MMPI and CL82198 (Fig. 7, C and D), which suggested that RAGE release into the BAL was dependent on the MMP-mediated reactions in LPS-induced lung injury model.
Fig. 7.
A and B: RAGE expression in the bronchoalveolar lavage (BAL) from the lung treated with MMP-3 (A) and MMP-13 (B) in rat in situ lung model. Instillation of MMP-3 (0.1 μg/ml, 3 ml) and MMP-13 (1 μg/ml, 3 ml) into the airway resulted in the increase in the expression of RAGE in the BAL. This reaction was inhibited by MMPI in the MMP-3 study (A) and MMP-13-specific inhibitor CL82198 in the MMP-13 study (B). C and D: RAGE expression in the BAL from the in vivo rat LPS-induced lung injury model with or without MMP inhibitors [MMPI, 200 μM (C) and CL82198 60 μM (D)]. Treatment with MMPI and CL82198 decreased the release of RAGE in the BAL induced by LPS stimulation. Each result was obtained as a representative result from consecutive 3 preparations in each study condition.
Correlation Between the Level of RAGE and MMP Antigen in the Alveolar Edema Fluid From Patients With ARDS or Hydrostatic Pulmonary Edema
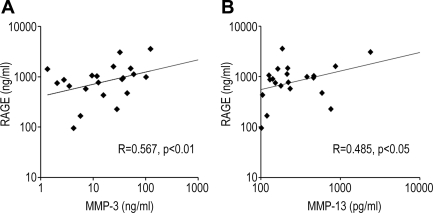
We measured 21 samples from patients with pulmonary edema in our previous study (36). RAGE levels in the alveolar edema fluid were significantly correlated with the level of MMP-3 antigen (R = 0.567, P < 0.01) and the level of MMP-13 antigen (R = 0.485, P < 0.05) (Fig. 8).
Fig. 8.
Correlation between the levels of MMP-3, MMP-13, and RAGE antigen in the pulmonary edema fluid from patients with acute respiratory distress syndrome or hydrostatic pulmonary edema. RAGE level in the edema fluid was significantly correlated with the level of MMP-3 antigen [R = 0.567, P < 0.01 (n = 21)] and MMP-13 antigen [R = 0.485, P < 0.05 (n = 21)].
DISCUSSION
The main results of these studies can be summarized as follows. Cultured alveolar epithelial cells expressed RAGE on day 7 of culture, and LPS stimulation induced release of soluble RAGE into the media by MMP-dependent mechanisms. Expression of MMP-3 and -13 in alveolar epithelial cells was augmented by LPS. Proteolysis by MMP-3, as well as MMP-13, resulted in soluble RAGE expression in the BAL in the in situ rat lung, and this reaction was inhibited by MMP inhibitors. In human studies, both MMP-3 and -13 antigen level was significantly correlated with the level of RAGE in pulmonary edema fluid samples.
RAGE Expressed in the Rat Alveolar Epithelial Cell Primary Culture Cells
Although number of studies supported RAGE expression in type I alveolar epithelial cells in the lung, there is uncertainty in the mechanism of RAGE release into the plasma and alveolar fluid in the status of lung injury. To study whether alveolar epithelial cells have potential to release RAGE by inflammatory stimuli, we used rat alveolar epithelial cell monolayers. As a first step, we studied RAGE expression on the cultured alveolar epithelial cell monolayer, because cultured alveolar epithelial cell is generally thought to have type II epithelial cell phenotype initially. In the present studies, cultured alveolar epithelial cells increased their expression of RAGE in both abundance and the pattern of expression over 7 days of culture (Fig. 1). In the early phase of the culture, more than 80% of the cells had a type II cell phenotype and expressed RAGE only in the cellular junctions. However, RAGE expression was observed predominantly in the SP-D-negative cells, which covered 87% of the surface area on day 7. These results were consistent with the previous study (8), which demonstrated that human freshly isolated alveolar epithelial cells have the potential to express RAGE and expression and abundance are dependent on their transdifferentiation from type II to type I cell phenotype; type II-like cells have characteristics of weak or no expression of RAGE whereas type I-like cells have RAGE expression on the basal membrane. Considering these results, we confirmed that cultured alveolar epithelial cell monolayer on day 7 is an appropriate model for the study for the mechanism of RAGE release from type I-like alveolar epithelial cells.
LPS-Induced RAGE Release From Cultured Alveolar Epithelial Cells Into the Media
Previous animal experiments and human studies indicated that RAGE is detected in samples from the alveolar space and the plasma. There are two possible mechanisms explaining release of soluble RAGE into the blood or alveolar space: 1) increase in the splice variant mRNA for soluble isoform and 2) proteolytic shedding from membrane-bound isotype. For the splice variant theory, Yonekura and colleagues (46) demonstrated a splice variant of human RAGE mRNA for endogenous soluble RAGE. However, Hudson and colleagues (18) recently demonstrated that the transcriptional expression frequency of this splice variant in human lung mRNA library was 7%, whereas the prevalence of the full length RAGE mRNA was 80%. In support of the proteolysis theory, Hanford and colleagues (17) demonstrated that there was no splice variant responsible for soluble isoform of RAGE in mouse lung, and soluble RAGE was produced by carboxy terminal truncation. Recently, Raucci and colleagues (29) demonstrated that human plasma concentration of splice variant RAGE consisted of only 20% of the total soluble isoform and that the metalloproteinase 10 (ADAM10) promoted proteolytic cleavage of RAGE in HeLa cells and HEK293 cells transfected with full-length RAGE expression plasmids.
In the present study, LPS stimulation resulted in release of RAGE from cultured rat alveolar epithelial cells into the media (Figs. 2 and 3) and in the in vivo LPS-induced lung injury model. The results demonstrated several lines of evidence that support the hypothesis that proteolysis caused by MMP-3 and -13 contributed to soluble RAGE release into the media. First, both MMP-3 and -13 transcripts were induced by LPS challenge, whereas RAGE transcript did not change in the same preparation. These results indicate induction of MMP-3 and -13 by the inflammatory stimuli (LPS) whereas transcription for RAGE was not changed by the LPS challenge. Secondly, the protein expression of MMP-3 and MMP-13 was documented in the cultured epithelial cells and LPS augmented the cytosolic expression of these proteases. In the culture media, LPS induced increase in the level of pro-MMP-3 and active MMP-13. Thirdly, a broad-spectrum MMP inhibitor and a MMP-13-specific inhibitors decreased the quantity of RAGE released into the media in the cultured cell model without affecting abundance in the RAGE transcript (Fig. 3, A and B, and 6E). Fourthly, proteolysis by MMP-3 or MMP-13 resulted in soluble RAGE expression in the BAL in the in situ rat lung. In this in situ rat lung model study, MMP-3 induced RAGE expression was inhibited by a broad-spectrum MMP inhibitor, and MMP-13 induced RAGE expression was inhibited by an MMP-13-specific inhibitor. Furthermore, LPS-induced RAGE expression in the BAL in vivo was also inhibited by these MMP inhibitors (Fig. 7, C and D). Finally, both MMP-3 and -13 antigen levels were significantly correlated with the level of RAGE in the human pulmonary edema fluid samples (Fig. 8), suggesting that soluble RAGE was released from injured alveolar epithelial cells by proteolytic reaction caused by proteases including MMP-3 and -13. The correlation with both hydrostatic and lung injury edema fluid is consistent with our prior studies that showed that there is proinflammatory activity in the edema fluid of patients with acute hydrostatic edema as well as lung injury edema (28).
MMPs are a large family of proteolytic enzymes related with degeneration of extracellular matrix and tissue remodeling. MMP-13 belongs to the family of interstitial collagenases, and upregulation of this protease is related with diseased conditions with matrix degeneration, including osteoarthritis (22, 32), rheumatoid arthritis (45), and aortic aneurysm (44). Although the contribution of MMP-13 to lung injury has not been studied yet, in an in vitro alveolar epithelial cell culture model, MMP-13 has the capacity to decrease cellular attachment of alveolar epithelial cells and induce a decrease in cytoskeleton stiffness (27). Furthermore, MMP-3 is a member of the stromelysin family, and it is thought to be involved in diseases with extracellular matrix degradation. For lung injury, Warner and colleagues (41) demonstrated that MMP-3 activity was augmented in the BAL fluid from the lung injured by IgG immune complex, and MMP-3-deficient mice had less severely injured lung in the IgG immune complex induced lung injury (41) and in the macrophage inhibitory protein-2-induced injury model (25, 41). Also, the presence of MMP-3 in the BAL from patients with ALI reflected disease severity and higher mortality in patients with ALI (12), and MMP-3 levels in the BAL from neonates with bronchopulmonary dysplasia (BPD) was significantly higher than from neonates without BPD (38). Because MMP-3 also activates MMP-13 in a concentration-dependent manner (21), these MMPs might synergistically degrade extracellular matrix in the injured lung. Because full-length membrane-bound RAGE is expressed on the basal side of type I alveolar epithelial cells (11, 33), soluble RAGE might be shed from alveolar epithelial cells during the course of MMP-induced matrix degradation.
Interestingly, several studies recently demonstrated that a number of ligands for RAGE induced increased expression of MMP-3 and MMP-13 in some cell types (24, 43). Considering that acute inflammatory responses could induce release of HMGB1 and S100A12 into alveolar spaces in the lung (37, 42), there is a possibility that these inflammatory ligands stimulate the production of MMP-3 and MMP-13 in alveolar epithelial cells via membrane-bound RAGE. Even if this is not the case, MMP expression could be induced by some inflammatory mediators including IL-6 or TNF-α (21). Our new results from pulmonary edema fluid from patients demonstrated a significant correlation between the level of these MMPs and the levels of soluble RAGE, suggesting that acute inflammation upregulated production of these MMPs in the lung and resulted in increased cleavage of extracellular part of RAGE with matrix degradation. Because membrane-bound RAGE could participate in some inflammatory process by binding its ligands (31, 34) and soluble RAGE could inhibit those reactions as a decoy receptor, proteolytic release of soluble RAGE might participate in a negative feedback after excessive inflammation (4). However, confirmation of this hypothesis is beyond the scope of this study, and further study is needed to discuss contribution of RAGE-mediated inflammatory reaction in ALI.
There are some limitations to this study. In the in vivo setting, there are several sources of proteases during inflammation (e.g.. neutrophils, monocytes, and interstitial fibroblasts). Thus, in the intact lung, other proteases could be involved in proteolytic release of RAGE from alveolar epithelial cells. As shown in Fig. 3C, LPS-induced release of RAGE from in vitro cell monolayers was partially inhibited by aprotinin, which suggested contribution of serine proteases to the release of RAGE from alveolar epithelial cells. In this sense, the present study demonstrated that both MMP-3 and MMP-13 were key proteases in the release of RAGE from alveolar epithelial cells; however, there is a possibility that these MMPs are members of protease cascades that induce release of RAGE. Secondly, the expression of RAGE and MMPs could be regulated by the surrounding matrix and cells other than epithelial cells. Furthermore, as we demonstrated that supplementation of serum modified the sensitivity to LPS stimulation (Fig. 2C), some humoral factors might modify the reaction of alveolar epithelial cells. In this sense, the present study demonstrates the response of alveolar epithelial cells alone to LPS challenge, whereas alveolar epithelial cells in vivo could be regulated by additional stimuli or humoral factors from circulating blood or surrounding structures.
In conclusion, our study confirms the expression and release of RAGE in cultured alveolar epithelial type-I like cells and supports the hypothesis that RAGE can be a biomarker of alveolar epithelial injury, an observation that has been supported by several clinical studies (5, 6) and a recent experimental study (35). In addition, these in vitro and in situ lung studies and measurements in human pulmonary edema fluid indicate that RAGE released from alveolar epithelial cells in the presence of acute inflammation may reflect proteolytic damage, in part by MMP-3 and MMP-13.
GRANTS
This research was funded by Grants-in-Aid 19390404 from the Japanese Society for the Promotion of Science, Japan (T. Uchida) and National Heart, Lung, and Blood Institute Grants HL51854 and HL51856 (M. A. Matthay).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We appreciate the assistance of Andrew Manies in the preparation of this manuscript.
REFERENCES
- 1.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149: 818–824, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Brett J, Schmidt AM, Yan SD, Zou YS, Weidman E, Pinsky D, Nowygrod R, Neeper M, Przysiecki C, Shaw A, Migheli A, Stern D. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol 143: 1699–1712, 1993 [PMC free article] [PubMed] [Google Scholar]
- 3.Briot R, Frank JA, Uchida T, Lee JW, Calfee CS, Matthay MA. Elevated levels of the receptor for advanced glycation end products, a marker of alveolar epithelial type I cell injury, predict impaired alveolar fluid clearance in isolated perfused human lungs. Chest 135: 269–275, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckley ST, Ehrhardt C. The receptor for advanced glycation end products (RAGE) and the lung. J Biomed Biotechnol 2010: 917108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calfee CS, Budev MM, Matthay MA, Church G, Brady S, Uchida T, Ishizaka A, Lara A, Ranes JL, deCamp MM, Arroliga AC. Plasma receptor for advanced glycation end-products predicts duration of ICU stay and mechanical ventilation in patients after lung transplantation. J Heart Lung Transplant 26: 675–680, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calfee CS, Ware LB, Eisner MD, Parsons PE, Thompson BT, Wickersham N, Matthay MA. Plasma receptor for advanced glycation end-products and clinical outcomes in acute lung injury. Thorax 63: 1083–1089, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christie JD, Shah CV, Kawut SM, Mangalmurti N, Lederer DJ, Sonett JR, Ahya VN, Palmer SM, Wille K, Lama V, Shah PD, Shah A, Weinacker A, Deutschman CS, Kohl BA, Demissie E, Bellamy S, Ware LB. Plasma levels of receptor for advanced glycation end products, blood transfusion, and risk of primary graft dysfunction. Am J Respir Crit Care Med 180: 1010–1015, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demling N, Ehrhardt C, Kasper M, Laue M, Knels L, Rieber EP. Promotion of cell adherence and spreading: a novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell Tissue Res 323: 475–488, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Eisner MD, Parsons P, Matthay MA, Ware L, Greene K. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax 58: 983–988, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang X, Song Y, Zemans R, Hirsch J, Matthay MA. Fluid transport across cultured rat alveolar epithelial cells: a novel in vitro system. Am J Physiol Lung Cell Mol Physiol 287: L104–L110, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Fehrenbach H, Kasper M, Tschernig T, Shearman MS, Schuh D, Muller M. Receptor for advanced glycation endproducts (RAGE) exhibits highly differential cellular and subcellular localisation in rat and human lung. Cell Mol Biol (Noisy-le-grand) 44: 1147–1157, 1998 [PubMed] [Google Scholar]
- 12.Fligiel SE, Standiford T, Fligiel HM, Tashkin D, Strieter RM, Warner RL, Johnson KJ, Varani J. Matrix metalloproteinases and matrix metalloproteinase inhibitors in acute lung injury. Hum Pathol 37: 422–430, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Folkesson HG, Matthay MA. Alveolar epithelial ion and fluid transport: recent progress. Am J Respir Cell Mol Biol 35: 10–19, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank JA, Briot R, Lee JW, Ishizaka A, Uchida T, Matthay MA. Physiological and biochemical markers of alveolar epithelial barrier dysfunction in perfused human lungs. Am J Physiol Lung Cell Mol Physiol 293: L52–L59, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank JA, Parsons PE, Matthay MA. Pathogenetic significance of biological markers of ventilator-associated lung injury in experimental and clinical studies. Chest 130: 1906–1914, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hancock DB, Eijgelsheim M, Wilk JB, Gharib SA, Loehr LR, Marciante KD, Franceschini N, van Durme YM, Chen TH, Barr RG, Schabath MB, Couper DJ, Brusselle GG, Psaty BM, van Duijn CM, Rotter JI, Uitterlinden AG, Hofman A, Punjabi NM, Rivadeneira F, Morrison AC, Enright PL, North KE, Heckbert SR, Lumley T, Stricker BH, O'Connor GT, London SJ. Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat Genet 42: 45–52, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanford LE, Enghild JJ, Valnickova Z, Petersen SV, Schaefer LM, Schaefer TM, Reinhart TA, Oury TD. Purification and characterization of mouse soluble receptor for advanced glycation end products (sRAGE). J Biol Chem 279: 50019–50024, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudson BI, Carter AM, Harja E, Kalea AZ, Arriero M, Yang H, Grant PJ, Schmidt AM. Identification, classification, and expression of RAGE gene splice variants. FASEB J 22: 1572–1580, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Ishizaka A, Matsuda T, Albertine KH, Koh H, Tasaka S, Hasegawa N, Kohno N, Kotani T, Morisaki H, Takeda J, Nakamura M, Fang X, Martin TR, Matthay MA, Hashimoto S. Elevation of KL-6, a lung epithelial cell marker, in plasma and epithelial lining fluid in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 286: L1088–L1094, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Lee JW, Fang X, Dolganov G, Fremont RD, Bastarache JA, Ware LB, Matthay MA. Acute lung injury edema fluid decreases net fluid transport across human alveolar epithelial type II cells. J Biol Chem 282: 24109–24119, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leeman MF, Curran S, Murray GI. The structure, regulation, and function of human matrix metalloproteinase-13. Crit Rev Biochem Mol Biol 37: 149–166, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Loeser RF, Yammani RR, Carlson CS, Chen H, Cole A, Im HJ, Bursch LS, Yan SD. Articular chondrocytes express the receptor for advanced glycation end products: Potential role in osteoarthritis. Arthritis Rheum 52: 2376–2385, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthay MA, Robriquet L, Fang X. Alveolar epithelium: role in lung fluid balance and acute lung injury. Proc Am Thorac Soc 2: 206–213, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Nah SS, Choi IY, Yoo B, Kim YG, Moon HB, Lee CK. Advanced glycation end products increases matrix metalloproteinase-1, -3, and -13, and TNF-alpha in human osteoarthritic chondrocytes. FEBS Lett 581: 1928–1932, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Nerusu KC, Warner RL, Bhagavathula N, McClintock SD, Johnson KJ, Varani J. Matrix metalloproteinase-3 (stromelysin-1) in acute inflammatory tissue injury. Exp Mol Pathol 83: 169–176, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelaez A, Force SD, Gal AA, Neujahr DC, Ramirez AM, Naik PM, Quintero DA, Pileggi AV, Easley KA, Echeverry R, Lawrence EC, Guidot DM, Mitchell PO. Receptor for advanced glycation end products in donor lungs is associated with primary graft dysfunction after lung transplantation. Am J Transplant 10: 900–907, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Planus E, Galiacy S, Matthay M, Laurent V, Gavrilovic J, Murphy G, Clerici C, Isabey D, Lafuma C, d'Ortho MP. Role of collagenase in mediating in vitro alveolar epithelial wound repair. J Cell Sci 112: 243–252, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Pugin J, Verghese G, Widmer MC, Matthay MA. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med 27: 304–312, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Raucci A, Cugusi S, Antonelli A, Barabino SM, Monti L, Bierhaus A, Reiss K, Saftig P, Bianchi ME. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J 22: 3716–3727, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Repapi E, Sayers I, Wain LV, Burton PR, Johnson T, Obeidat M, Zhao JH, Ramasamy A, Zhai G, Vitart V, Huffman JE, Igl W, Albrecht E, Deloukas P, Henderson J, Granell R, McArdle WL, Rudnicka AR, Barroso I, Loos RJ, Wareham NJ, Mustelin L, Rantanen T, Surakka I, Imboden M, Wichmann HE, Grkovic I, Jankovic S, Zgaga L, Hartikainen AL, Peltonen L, Gyllensten U, Johansson A, Zaboli G, Campbell H, Wild SH, Wilson JF, Glaser S, Homuth G, Volzke H, Mangino M, Soranzo N, Spector TD, Polasek O, Rudan I, Wright AF, Heliovaara M, Ripatti S, Pouta A, Naluai AT, Olin AC, Toren K, Cooper MN, James AL, Palmer LJ, Hingorani AD, Wannamethee SG, Whincup PH, Smith GD, Ebrahim S, McKeever TM, Pavord ID, MacLeod AK, Morris AD, Porteous DJ, Cooper C, Dennison E, Shaheen S, Karrasch S, Schnabel E, Schulz H, Grallert H, Bouatia-Naji N, Delplanque J, Froguel P, Blakey JD, Britton JR, Morris RW, Holloway JW, Lawlor DA, Hui J, Nyberg F, Jarvelin MR, Jackson C, Kahonen M, Kaprio J, Probst-Hensch NM, Koch B, Hayward C, Evans DM, Elliott P, Strachan DP, Hall IP, Tobin MD. Genome-wide association study identifies five loci associated with lung function. Nat Genet 42: 36–44, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds PR, Schmitt RE, Kasteler SD, Sturrock A, Sanders K, Bierhaus A, Nawroth PP, Paine R, 3rd, Hoidal JR. Receptors for advanced glycation end-products targeting protect against hyperoxia-induced lung injury in mice. Am J Respir Cell Mol Biol 42: 545–551, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Sakao K, Takahashi KA, Arai Y, Saito M, Honjo K, Hiraoka N, Asada H, Shin-Ya M, Imanishi J, Mazda O, Kubo T. Osteoblasts derived from osteophytes produce interleukin-6, interleukin-8, and matrix metalloproteinase-13 in osteoarthritis. J Bone Miner Metab 27: 412–423, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Shirasawa M, Fujiwara N, Hirabayashi S, Ohno H, Iida J, Makita K, Hata Y. Receptor for advanced glycation end-products is a marker of type I lung alveolar cells. Genes Cells 9: 165–174, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Sternberg DI, Gowda R, Mehra D, Qu W, Weinberg A, Twaddell W, Sarkar J, Wallace A, Hudson B, D'Ovidio F, Arcasoy S, Ramasamy R, D'Armiento J, Schmidt AM, Sonett JR. Blockade of receptor for advanced glycation end product attenuates pulmonary reperfusion injury in mice. J Thorac Cardiovasc Surg 136: 1576–1585, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Su X, Looney MR, Gupta N, Matthay MA. Receptor for advanced glycation end-products (RAGE) is an indicator of direct lung injury in models of experimental lung injury. Am J Physiol Lung Cell Mol Physiol 297: L1–L5, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med 173: 1008–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, Kobayashi A, Maruyama I, Yamada S, Hasegawa N, Soejima J, Koh H, Ishizaka A. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med 170: 1310–1316, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Vento G, Tirone C, Lulli P, Capoluongo E, Ameglio F, Lozzi S, Cota F, Mosca F, Romagnoli C, Messana I, Castagnola M, Inzitari R. Bronchoalveolar lavage fluid peptidomics suggests a possible matrix metalloproteinase-3 role in bronchopulmonary dysplasia. Intensive Care Med 35: 2115–2124, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Verghese GM, Ware LB, Matthay BA, Matthay MA. Alveolar epithelial fluid transport and the resolution of clinically severe hydrostatic pulmonary edema. J Appl Physiol 87: 1301–1312, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 163: 1376–1383, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Warner RL, Beltran L, Younkin EM, Lewis CS, Weiss SJ, Varani J, Johnson KJ. Role of stromelysin 1 and gelatinase B in experimental acute lung injury. Am J Respir Cell Mol Biol 24: 537–544, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Wittkowski H, Sturrock A, van Zoelen MA, Viemann D, van der Poll T, Hoidal JR, Roth J, Foell D. Neutrophil-derived S100A12 in acute lung injury and respiratory distress syndrome. Crit Care Med 35: 1369–1375, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Yammani RR, Carlson CS, Bresnick AR, Loeser RF. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4: role of the receptor for advanced glycation end products. Arthritis Rheum 54: 2901–2911, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Ye S. Influence of matrix metalloproteinase genotype on cardiovascular disease susceptibility and outcome. Cardiovasc Res 69: 636–645, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Ye S, Patodi N, Walker-Bone K, Reading I, Cooper C, Dennison E. Variation in the matrix metalloproteinase-3, -7, -12 and -13 genes is associated with functional status in rheumatoid arthritis. Int J Immunogenet 34: 81–85, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, Yasui K, Takeuchi M, Makita Z, Takasawa S, Okamoto H, Watanabe T, Yamamoto H. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J 370: 1097–1109, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]