Abstract
During pulmonary edema, the alveolar space is exposed to a hypoxic environment. The integrity of the alveolar epithelial barrier is required for the reabsorption of alveolar fluid. Tight junctions (TJ) maintain the integrity of this barrier. We set out to determine whether hypoxia creates a dysfunctional alveolar epithelial barrier, evidenced by an increase in transepithelial electrical conductance (Gt), due to a decrease in the abundance of TJ proteins at the plasma membrane. Alveolar epithelial cells (AEC) exposed to mild hypoxia (Po2 = 50 mmHg) for 30 and 60 min decreased occludin abundance at the plasma membrane and significantly increased Gt. Other cell adhesion molecules such as E-cadherin and claudins were not affected by hypoxia. AEC exposed to hypoxia increased superoxide, but not hydrogen peroxide (H2O2). Overexpression of superoxide dismutase 1 (SOD1) but not SOD2 prevented the hypoxia-induced Gt increase and occludin reduction in AEC. Also, overexpression of catalase had a similar effect as SOD1, despite not detecting any increase in H2O2 during hypoxia. Blocking PKC-ζ and protein phosphatase 2A (PP2A) prevented the hypoxia-induced occludin reduction at the plasma membrane and increase in Gt. In summary, we show that superoxide, PKC-ζ, and PP2A are involved in the hypoxia-induced increase in Gt and occludin reduction at the plasma membrane in AEC.
Keywords: tight junctions, protein phosphatases 2A, reactive oxygen species, zonula occludens 1
a hypoxic enviroment is present at the alveolar space during pulmonary edema and contributes to the dysfunction of the alveolar epithelium (8, 11). The integrity of the epithelial barrier is compromised by cell death and cell adhesion disruption. Given that hypoxia does not cause cell death in alveolar epithelial cells (AEC) (31), loss of the alveolar epithelial barrier integrity during hypoxia is due to the disruption of cell adhesion molecules, such as tight junctions (TJ). TJ have been described as “kissing points” between cells, since the intercellular space is just 15–20 nm wide. They are the most apically positioned junction and hence are considered to delineate the apical and basolateral surfaces of the epithelial cell (3). They are composed of the transmembrane integral proteins occludin, claudins, and junctional adhesion molecule (JAM), which are linked to the cytoskeleton through interactions with cytoplasmic peripheral proteins, including the zonula occludens (ZO-1, ZO-2, and ZO-3) and actin-binding proteins (16). In addition, they determine the ion selectivity and conductance of the paracellular pathway (15). Claudin-knockout models have demonstrated that claudins are necessary and sufficient for TJ formation. Conversely, the role of occludin remains unclear. Clayburgh et al. (7) reported that occludin internalization was associated with development of epithelial barrier dysfunction, despite no endocytosis of ZO-1, claudins, and adherens junction proteins. Furthermore, Morgan et al. (24) reported in a model of multiple sclerosis that occludin dephosphorylation correlated with increased endothelial conductance and disease progression.
In the lung, alveolar epithelial disruption results in a dysfunctional epithelium and the development of pulmonary edema. This alveolar flooding creates a hypoxic environment that downregulates sodium transport, impairing the ability of the lung to reabsorb fluid (10, 26, 37). Although alveolar fluid reabsorption (AFR) is a key step in the recovery from pulmonary edema (36), a functional epithelial barrier is required. Bouvry et al. (2) reported that exposure of primary rat AEC to severe and mild hypoxia, 0.5 and 3% O2, respectively, for 18 h disrupted the cytoskeleton and TJ. Sodium transport inhibition and disruptions of the cytoskeleton and TJ after prolonged hypoxia likely contribute to prevent the resolution of pulmonary edema, increasing the morbidity and mortality of patients with acute lung injury (ALI). Although it has been reported that prolonged hypoxia disrupts the TJ in AEC, the time and signaling events involved in their disruption have not been elucidated. We set out to determine the signaling events of hypoxia on the alveolar epithelial TJ. In this study we report that, within 30 min, mild hypoxia (Po2 = 50 mmHg) induces a decrease of occludin abundance at the plasma membrane in AEC, without affecting the abundance of other TJ proteins such as claudins and ZO-1, nor disrupting the architecture of AEC monolayer. In addition, we show that AEC exposed to hypoxia did not increase hydrogen peroxide (H2O2), whereas it increased superoxide (O2−) concentrations. The hypoxia-induced occludin reduction at the plasma membrane in AEC was prevented by overexpressing antioxidant enzyme superoxide dismutase 1 (SOD1) but not SOD2. Conversely, overexpression of catalase prevented the hypoxia effects on AEC, despite no increase in H2O2 in our hypoxia model. Blocking PKC-ζ and protein phosphatase 2A (PP2A) prevented the hypoxia-induced occludin reduction at the plasma membrane and increase in transepithelial electrical conductance (Gt).
MATERIALS AND METHODS
Antibodies and reagents.
Western blots (WB) were done with rabbit polyclonal antibodies against ZO-1, claudin 18 (C-Term), claudin 3, claudin 4, and claudin 5 (Invitrogen Laboratories, Carlsbad, CA). WB were done with mouse monoclonal antibodies against occludin (Invitrogen Laboratories), PP2A (Millipore, Bedford, MA), and PKC-ζ (Santa Cruz Biotechnology, Santa Cruz, CA).
The following compounds were used: bisindolylmaleimide (BIS; Calbiochem EMD Biosciences, La Jolla, CA); protein A/G plus-agarose (Santa Cruz); streptavidin and sulfo-NHS-SS-biotin (Pierce Biotechnology, Rockford, IL); Vectashield mounting media (Vector Laboratories, Burlingame, CA); heavy water (deuterium oxide 99.9 atom %D; Sigma-Aldrich, St. Louis, MO). All other reagents were commercial products of the highest grade available.
Isolation of lung alveolar epithelial type II cells.
Primary AEC were isolated from pathogen-free male Sprague-Dawley rats weighing 200–225 g as previously described (12, 27). Briefly, the lungs were perfused via the pulmonary artery, lavaged, and digested with elastase (30 U/ml; Worthington Biochemical) for 20 min at 37°C. The ATII cells were purified by differential adherence to immunoglobulin G-coated dishes. Counting and viability of the ATII cells were assessed by exclusion of Trypan blue stain. Cells were plated with Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2.5 μg/ml amphotericin. All AEC were placed on filters with the purpose of developing cell polarity. Experiments were performed 3 days after isolation.
Cell surface biotinylation.
After exposing the cells to hypoxia or normoxia, the samples were placed on ice. The cells were washed twice with ice-cold PBS with CaCl2 (1 mM), MgCl2 (0.5 mM) and incubated with 1 mg/ml sulfo-NHS-SS-biotin for 20 min at 4°C in the dark. The cells were washed with PBS (containing 100 mM glycine) and scraped in the following buffer: 50 mM Tris pH 8, 150 mM NaCl, 1% Nonidet P-40, 1% deoxycholic acid, and protease inhibitors (2 μg/ml leupeptin, 100 μg/ml PMSF, and 100 μg/ml N-tosyl-l-phenylalanine chloromethyl ketone). The cell suspension was centrifuged at 12,000 g at 4°C for 5 min. The supernatants were transferred to clean tubes and incubated (75 μg protein) with high-capacity streptavidin agarose resin (Thermo Scientific) overnight at 4°C with end-over-end rotation. The beads were then washed three times with the following washing solution: 500 mM NaCl, 20 mM Tris pH 7.4, and 0.2% BSA and one last wash with 10 mM Tris. The beads were resuspended in sample buffer. Electrophoresis, WB analysis with occludin and E-cadherin antibodies, and analysis of densitometry scans were performed.
Hypoxia exposure.
Cultured polarized AEC were placed in an incubation chamber (Ruskinn Microaerophilic System) flushed with a gas mixture for the indicated times at 37°C or in a self-made chamber (Supplemental Fig. S1; the online version of this article contains supplemental data) that consisted of a plate with inserts, that were placed in a 37°C bath and flushed with different gas mixtures (5% CO2, 1.5 or 16% O2, and nitrogen balanced). An oxygen sensor was placed in the chambers to determine the oxygen concentration in the air. Within 45 s, oxygen levels would drop to the expected value, either 1.5 or 16%, and they would remain steady throughout the entire experiment. pH, partial pressure of oxygen (Po2), and carbon dioxide (Pco2) concentrations were determined in the media on the apical side. A 1-ml sample of apical media was obtained and using a blood gas analyzer (ABL 810, Flex Radiometer, Radiometer Medical), we determined pH, Po2, and Pco2 on different conditions (normoxia or hypoxia) at 30 and 60 min. Prior to all the measurements, the machine electrodes were calibrated per the manufacturer's recommendation.
Gt measurement.
Primary rat AEC were plated on inserts and, on day 3 after isolation, cells were treated with different oxygen concentrations (1.5 or 16%) for different time intervals (30 and 60 min). Electrical resistance was measured with the Millicell Electrical Resistance System (Millipore), and conductance was calculated as its reciprocal.
Adenovirus infection.
AEC (95% confluent) were infected with 20 pfu/cell of adenoviruses containing the plasmid of interest in DMEM without serum; e.g., SOD2 (Ad5CMVMnSOD), SOD1 (Ad5CMVCuZnSOD), and catalase (Ad5CMVCat) (20, 39, 40), purchased through University of Iowa, Viral Core, and small-t and vector adenovirus with cytomegalovirus (CMV) promoter, a gift of Dr. K. Rundell (19). After 2 h, the medium was removed and replaced by fresh medium with bovine serum. Experiments were performed the next day of infection. WB for respective proteins (SOD1, SOD2, catalase, and small-t) were performed to determine infection efficiency.
Immunofluorescence.
After treatments polarized AEC were fixed with 4% paraformaldehyde (PFA) in PBS for 15 min and quenched with 10 mM glycine in PBS for 10 min at room temperature. After fixation cells were permeabilized and blocked with 0.3% Triton X, 0.3% BSA, 15 μl/ml goat serum in PBS for 30 min. Immunofluorescence (IF) labeling was performed by exposing cells to primary antibodies overnight at 4°C, using monoclonal anti-occludin, polyclonal anti-ZO-1 (Invitrogen) and polyclonal anti-PKC-ζ (Santa Cruz Biotechnology). After being washed with PBS, cells were exposed to secondary antibody Cy3-coupled goat anti-rabbit and FITC-coupled goat anti-mouse (Invitrogen) in the dark for 30 min. Cells were visualized and photographed under confocal fluorescence microscope.
Propidium iodide.
After 60 min of treatment with normoxic or hypoxic conditions (in a plastic chamber), polarized AEC were treated with 10 μg/ml of propidium iodide and incubated in the dark for 10 min. Cells were then photographed under fluorescence microscope and a percentage of fluorescent cells was registered compared with a positive control (100% ethanol for 5 min).
PP2A activity.
Polarized AEC were exposed to hypoxia or normoxia at different time points (15, 30, 60 min). Cells were then harvested and assayed to determine PP2A activity. This assay (R and D Systems DuoSet IC) was performed according to manufacturer's instructions. Briefly, an antibody specific for the catalytic subunit of PP2A binds both active and inactive PP2A. After washing away of unbound material, a synthetic phosphopeptide substrate is added that is dephosphorylated by active PP2A to generate free phosphate and unphosphorylated peptide. The free phosphate is detected by a sensitive dye-binding assay using malachite green and molybdic acid. PP2A activity is expressed relative to control.
ROS measurements.
Polarized AEC were treated with normoxia or hypoxia for 30 and 60 min. Intracellular H2O2, peroxyl radicals (ROO−), and peroxynitrite anion (ONOO−) were measured by dichlorofluorescein diacetate (DCFH-DA; Invitrogen). Briefly, after treatments, polarized AEC were treated with dichlorofluorescein (DCFH) 40 nM for 20 min at 37°C in darkness. Cells were then washed and resuspended in PBS and kept on ice. Flow cytometry was performed to assess the level of reactive oxygen species (ROS) (32). Extracellular H2O2 was measured by the following method: Polarized AEC were exposed in phenol red-free HBSS (1.0 ml) supplemented with glucose (6.5 mM), HEPES (1 mM), sodium bicarbonate (6 mM), p-hydroxyphenyl acetic acid (pHPA; 1.6 mM), and horseradish peroxidase (HRP; 95 μg/ml). After treatments, amount of H2O2 was measured at an excitation wavelength 323 nm and emission 400 nm. H2O2 reacts with HRP, forming compound I, which in turn reacts with pHPA, forming a stable fluorescent dimer, pHPA2 (34). Superoxide (O2−) was measured by NADPH assay: Briefly, after treatments, 10–30 μg of protein cell lysate were mixed with NADPH (1 mM) and lucigenin. Chemiluminescence was measured for 10 min at 1-min intervals (23).
Lanthanum nitrate.
After treatment, polarized AEC were incubated with 2.5% glutaraldehyde overnight at 4°C. After being washed three times with cacodylate, and an additional three times with 0.2 M sym-collidine pH 7.4, cells were then incubated with a 1:1 solution of 0.2 M lanthanum nitrate and 0.2 M sym-collidine with 2% osmium tetroxide on the apical side. 0.2 M sym-collidine was maintained without lanthanum nitrate in the basolateral side of the cells. The cells were then dehydrated with a graded ethanol series and embedded in resin. Sections were cut and observed under transmission electron microscope (TEM).
Statistics.
Comparison between experimental groups was made with two-way ANOVA and t-test. P < 0.05 was considered significant. Each n represents an independent isolation of AEC. In addition, each n was done in triplicate.
RESULTS
Po2 in AEC exposed to 1.5 and 16% inspired oxygen fraction (FiO2) after 30 and 60 min is higher than predicted. Polarized AEC were exposed to different FiO2 (1.5 and 16%) for a maximum of 60 min and the actual oxygen concentrations were determined in the media on the apical side. A 1-ml sample of apical medium was obtained and Po2, Pco2, and pH were determined by use of a blood gas analyzer (ABL 810, Flex Radiometer, Radiometer Medical). As shown in Table 1, AEC exposed to FiO2 of 1.5% had an actual Po2 exposure at 30 and 60 min of ∼50 mmHg, whereas AEC exposed to FiO2 of 16% had an actual Po2 exposure at 30 and 60 min of ∼135 mmHg. Other values such as pH and Pco2 were not significantly different.
Table 1.
pH, Po2, and Pco2 in AEC exposed to 1.5 and 16% FiO2 after 30 and 60 min
| FiO2 |
||
|---|---|---|
| 1.5% (n = 5) | 16% (n = 5) | |
| After 30 min | ||
| pH | 7.36 ± 0.04 | 7.35 ± 0.02 |
| O2, mmHg | 50 ± 5 | 136 ± 5 |
| CO2, mmHg | 54 ± 3 | 53 ± 2 |
| After 60 min | ||
| pH | 7.37 ± 0.3 | 7.34 ± 0.3 |
| O2, mmHg | 50 ± 2 | 133 ± 5 |
| CO2, mmHg | 53 ± 3 | 55 ± 3 |
Values are means ± SE. Po2, partial pressure of oxygen; Pco2, partial pressure of carbon dioxide; AEC, alaveolar epithelial cells; FiO2, inspired oxygen fraction.
Hypoxia increases Gt and decreases occludin abundance at the plasma membrane in AEC.
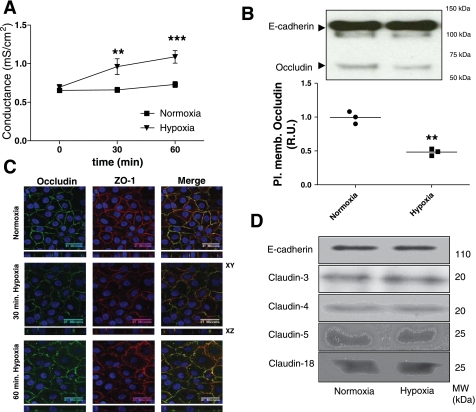
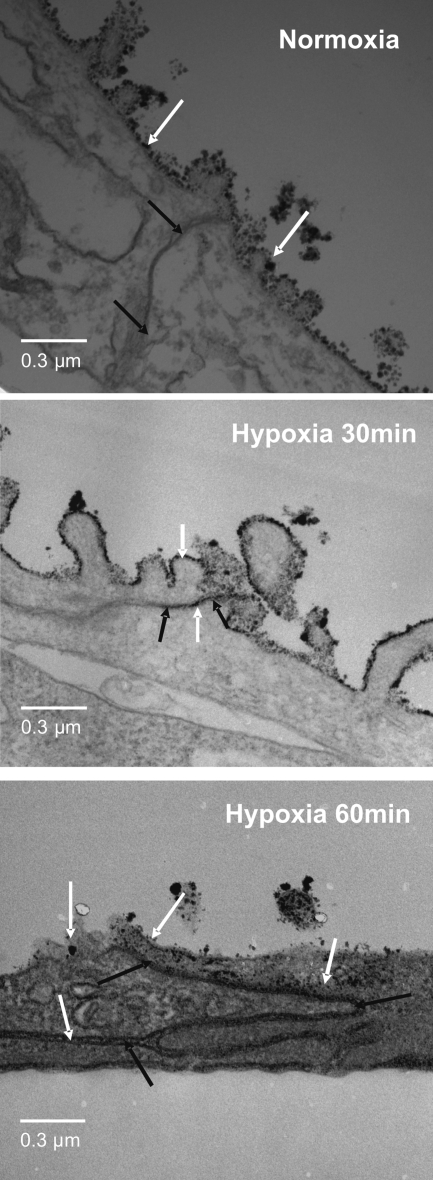
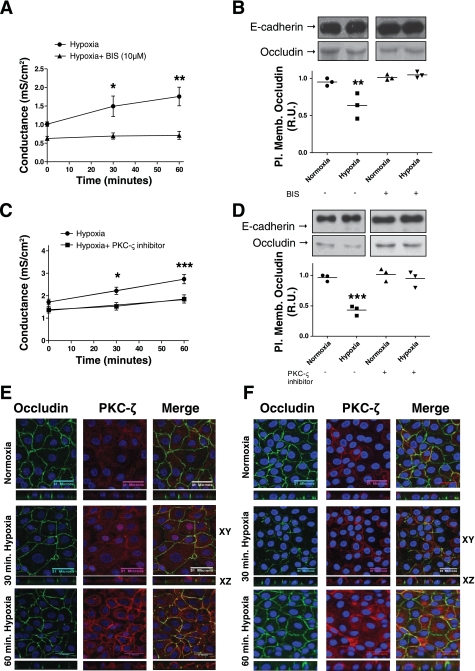
We examined whether hypoxia induced an increase in alveolar epithelial monolayer Gt. AEC were exposed to normoxia or hypoxia for 30 and 60 min. As shown in Fig. 1A, AEC exposed to normoxia had no effect on their Gt whereas AEC exposed to hypoxia had an increase in Gt at 30 min (P = 0.0016), compared with normoxic conditions, which became more statistically significant at 60 min (P = 0.0001). To determine whether this Gt increase was accompanied by a decrease in TJ proteins at the plasma membrane, AEC were exposed to normoxia or hypoxia, followed by labeling AEC with biotin and analyzing results by WB. In addition, experiments were complemented by microscopy analysis. AEC were fixed in 4% PFA and processed for IF by standard procedures. As shown in Fig. 1B, occludin abundance at the plasma membrane did not decrease in AEC exposed to normoxia, whereas AEC exposed to hypoxia for 30 min had a decrease in occludin abundance at the plasma membrane (P = 0.001). Also, Fig. 1C shows that polarized AEC exposed to hypoxia had a decrease in occludin colocalization with ZO-1, the development of increase intracellular vesicles and a less smooth plasma membrane at 30 and 60 min compared with 60 min of normoxia. In addition, no changes in claudin 3, 4, 5, 18, and E-cadherin were observed (Fig. 1D). Since the assessment of the integrity of the epithelial barrier includes measuring cell death and permeability to different molecular weight markers, we set out to determine whether AEC exposed to normoxia or hypoxia had changes in these parameters. As shown in Supplemental Fig. S2, cells exposed to normoxia or hypoxia did not have an increase in cell death determined by the proportion of propidium iodide- positive cells. Also, AEC exposed to normoxia or hypoxia did not show any increase in permeability to 4-kDa FITC dextran (data not shown). Given that Gt measures the permeability to all ions, without discerning between transcellular and paracellular permeability, La3+ movement through the TJ was evaluated in polarized AEC by TEM as described by Flynn et al. (15). La3+ is an electron-dense element with a radius of 0.4 nm and TJ are impermeable to it under normal conditions. As shown in Fig. 2, exposure of AEC to hypoxia for 30 and 60 min caused La3+ to pass through the intercellular space, whereas AEC exposed to normoxia for 60 min remained impermeable to La3+.
Fig. 1.
Hypoxia induces transepithelial electrical conductance (Gt) increase and occludin reduction at the plasma membrane in alveolar epithelial cells (AEC). A: polarized AEC were plated on inserts and exposed to normoxia (Po2 ∼135 mmHg) or hypoxia (Po2 ∼50 mmHg) for 60 min. Gt was measured at 30 and 60 min (n = 9 independent experiments in triplicate). B: polarized AEC were exposed to normoxia or hypoxia for 30 min. Surface biotinylation was performed and Western blot (WB) was developed against occludin and E-cadherin as loading control. Pl. memb., plasma membrane; R.U., relative units. C: AEC were exposed to normoxia or hypoxia for 30 and 60 min. Occludin (green) and zonula occludens (ZO)-1 (red) abundance was determined via immunofluorescence (IF). Panels show x, y, and z projections. D: AEC were exposed to normoxia or hypoxia for 30 min. Surface biotinylation was performed and WB was developed against E-cadherin and claudins 3, 4, 5, and 18. Shown are representative blots and quantitative analysis. MW, molecular mass. Graphs represent means ± SE; means with dots represent individual experiments (n = 3 independent experiments in triplicate). **P < 0.01 and ***P < 0.001 compared with control.
Fig. 2.
Hypoxia induces an increase in lanthanum staining at the tight junction (TJ) in AEC. Polarized AEC were exposed to hypoxia for 30 and 60 min. They were then fixed and stained with La3+ on the apical side and observed under transmission electron microscopy. Electron dense La3+ (white arrows) penetrate the TJ into the intercellular space (black arrows) after 30 and 60 min of hypoxia, but not after 60 min of normoxia (n = 3).
ROS participate in the hypoxia-induced Gt increase and occludin reduction in AEC.
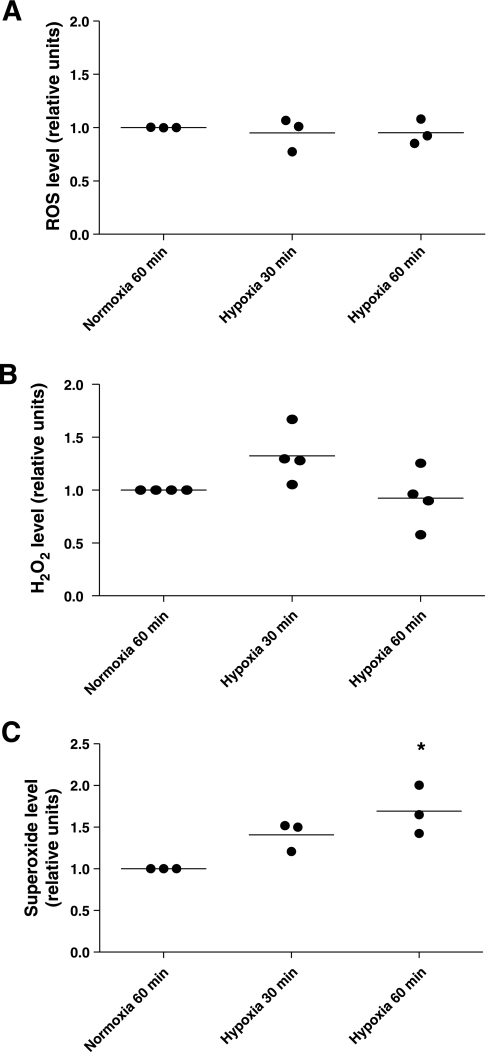
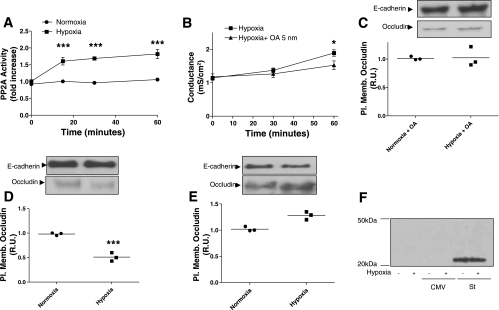
AEC generate mitochondrial ROS when exposed to severe hypoxia (10), but because our model shows that AEC are exposed only to mild hypoxia, we set out to determine whether a Po2 of 50 mmHg would increase ROS production. To our surprise, hypoxia did not increase total amount of ROS nor H2O2 levels (Fig. 3, A and B). Conversely, hypoxia increased superoxide levels in AEC at 30 and 60 min, this last being statistically significant (P = 0.0139) (Fig. 3C).
Fig. 3.
Hypoxia increases superoxide production in AEC. Polarized AEC were exposed to normoxia or hypoxia 30 and 60 min. At the end of the respective conditions, AEC were placed at 4°C and reactive oxygen species (ROS) were measured by different methodologies. A: dichlorofluorescein diacetate [DCFH-DA; measures intracellular hydrogen peroxide (H2O2) peroxyl radicals (ROO−) and peroxynitrite anion (ONOO−); n = 3 independent experiments in triplicate]. B: measurement of extracellular H2O2 (n = 4 independent experiments in triplicate). C: NADPH assay that measures superoxide (O−) (n = 3 independent experiments in triplicate).
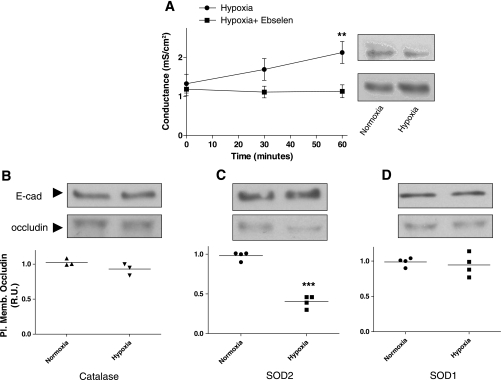
Since several authors have reported protective effects of several antioxidants during hypoxia, we set out to determine whether these antioxidants would protect against the hypoxia-induced occludin reduction in AEC in this model of mild hypoxia. We pretreated AEC with ebselen (20 μM) 30 min prior to treatment and exposed them to normoxia or hypoxia for 30 and 60 min. Despite the lack of H2O2 increase in this model, pretreatment of AEC with ebselen prevented the hypoxia-induced increase in Gt and occludin reduction at the plasma membrane (P = 0.0019) (Fig. 4A). Furthermore, we infected polarized AEC with adenoviral vectors encoding SOD1, SOD2, and catalase (20 pfu/cell), 24 h prior to exposure to normoxia or hypoxia for 30 min. All adenoviral infections were confirmed by WB analysis (data not shown) As shown in Fig. 4, C and D, the overexpression of SOD1, but not SOD2, protected against the hypoxia-induced decrease occludin abundance at the plasma membrane (P < 0.0001). In addition, overexpression of catalase prevented the hypoxia-induced occludin reduction at the plasma membrane (Fig. 4B), which is consistent with the protection conferred by pretreatment of AEC with ebselen. Furthermore, neither ebselen nor adenoviruses made any difference in the Gt or occludin abundance at the plasma membrane in AEC under basal conditions. However, adenoviruses increased Gt significantly (P = 0.0155) compared with control (no adenovirus) or ebselen (Supplemental Fig. S3, A and B). In addition, an empty adenoviral vector did not prevent the hypoxia-induced occludin reduction at the plasma membrane in AEC (Supplemental Fig. S3C).
Fig. 4.
Overexpression of catalase and superoxide dismutase (SOD) 1 prevent the hypoxia-induced Gt increase and occludin reduction in AEC. A: polarized AEC were pretreated for 30 min with ebselen (20 μM), followed by exposure to normoxia or hypoxia for 60 min. Gt was measured at 30 and 60 min. In addition, surface biotinylation was performed on AEC exposed to normoxia or hypoxia for 60 min and plasma membrane occludin abundance was determined by Western blot (WB) analysis (n = 8 independent experiments in triplicate). B–D: polarized AEC were infected with adenovirus vectors containing catalase, SOD1, and SOD2 24 h before treatments. Cells were then exposed to normoxia or hypoxia for 30 min. Surface biotinylation was performed and occludin abundance was determined by WB analysis. E-cadherin was used as loading control for each experiment. Shown are representative blots and quantitative analysis. Graph represents means ± SE; means with dots represent individual experiments (n = 3 independent experiments in triplicate). **P < 0.01 and ***P < 0.001 compared with control.
Blocking PKC-ζ prevents hypoxia-induced Gt increase and occludin reduction in AEC.
It has been reported that hypoxia-generated ROS activates PKC-ζ in AEC (5, 10). To determine whether PKC-ζ is involved in the hypoxia-induced Gt increase and occludin reduction at the plasma membrane, we pretreated AEC with BIS, which inhibits all PKCs including PKC-ζ at a 10 μM concentration. As shown in Fig. 5, A and B, when AEC were pretreated with BIS, 10 μM, for 30 min and then exposed to normoxia or hypoxia for 60 min, they were protected against the hypoxia-induced Gt increase(P = 0.0024) and occludin reduction at the plasma membrane (P = 0.0035). In addition, we used a specific PKC-ζ inhibitor (myristoylated pseudo-substrate) that binds to the pseudo-substrate sequence, which is specific for each PKC isozyme and keeps the enzyme inactive with no interference from ATP binding (13). As shown in Fig. 5, C and D, 30 min pretreatment with PKC-ζ inhibitor (0.1 μM), followed by exposure of AEC to normoxia or hypoxia for 60 min, prevented the hypoxia-induced Gt increase (P = 0.001) and occludin reduction at the plasma membrane in AEC when analyzed by WB (P = 0.0002). Furthermore, experiments were complemented by microscopy analysis. AEC were fixed in 4% PFA and processed for IF by standard procedures. As shown in Fig. 5E, AEC exposed to normoxia had a PKC-ζ perinuclear staining. After 30 and 60 min, PKC-ζ perinuclear staining decreased and shifted to the plasma membrane. This staining increased over time, being more pronounced at 60 min. In addition, polarized AEC exposed to hypoxia for 30 and 60 min had an increase in occludin and PKC-ζ colocalization, with evidence of intracellular vesicles suggestive of hypoxia-induced endocytosis. As shown in Fig. 5F, when cells were pretreated with PKC-ζ inhibitor (0.1 μM) 30 min prior to exposure to normoxia or hypoxia for 30 and 60 min, it resulted in almost no colocalization of occludin and PKC-ζ. Occludin was preserved at the plasma membrane and almost no intracellular vesicles were observed. In addition, PKC-ζ staining was minimally present at the membrane, suggestive of inactivation of this enzyme. We also decided to determine whether BIS and PKC-ζ inhibitors had any effect under basal conditions (control). Polarized AEC were treated with BIS (10 μM) and PKC-ζ (0.1 μM) for 30 min, followed by Gt measurements performed at 30 and 60 min. Also, polarized AEC were treated with above inhibitors for a total of 90 min, followed by labeling with biotin and analyzing results by WB. As shown in Supplemental Fig. S4, neither Gt nor occludin abundance at the plasma membrane in AEC were significantly different compared with control condition (no treatments).
Fig. 5.
Blocking PKC-ζ prevents hypoxia-induced Gt increase and occludin reduction in AEC. A: polarized AEC were pretreated for 30 min with bisindolylmaleimide (BIS) (10 μM), followed by exposure to normoxia or hypoxia for 60 min. Gt was measured at 30 and 60 min (n = 9 independent experiments in triplicate). B: polarized AEC were pretreated for 30 min with BIS (10 μM), followed by exposure to normoxia or hypoxia for 30 min. Surface biotinylation was performed and occludin abundance was determined by WB analysis. C: polarized AEC were pretreated for 30 min with PKC-ζ pseudosubstrate (0.1 μM), followed by exposure to normoxia or hypoxia for 60 min. Gt was measured at 30 and 60 min (n = 9 independent experiments in triplicate). D: polarized AEC were pretreated for 30 min with PKC-ζ pseudosubstrate (0.1 μM), followed by exposure to normoxia or hypoxia for 30 min. Surface biotinylation was performed and occludin abundance was determined by WB analysis. Shown are representative blots and quantitative analysis. E: polarized AEC were exposed to normoxia or hypoxia for 30 and 60 min. Occludin and PKC-ζ abundance was determined via IF. Panels show x, y, and z projections. F: polarized AEC were pretreated for 30 min with PKC-ζ pseudosubstrate (0.1 μM), followed by exposure to normoxia or hypoxia for 30 and 60 min. Occludin and PKC-ζ abundance was determined via IF. Panels show x, y, and z projections. Graph represents means ± SE; means with dots represent individual experiments (n = 3 independent experiments in triplicate). *P < 0.05; **P < 0.01; ***P < 0.001 compared with control.
PP2A is involved in hypoxia-induced Gt increase and occludin reduction in AEC.
Since hypoxia increases ROS and H2O2 disrupts TJ in Caco-2 cell monolayers by increasing the association of PP2A with occludin (29), we decided to determine whether PP2A activity is increased in AEC exposed to hypoxia. As shown in Fig. 6A, PP2A activity was measured in polarized AEC exposed to hypoxia for 15, 30, and 60 min compared with normoxia. PP2A activity increased by twofold after 15 min of hypoxia exposure (P < 0.0001) and remained at the same level for the rest of the time. We then set out to determine whether PP2A is involved in the hypoxia-induced Gt increase and occludin reduction at the plasma membrane in AEC. Polarized AEC were preincubated for 15 min with 5 nM okadaic acid (OA), which inhibits PP2A. AEC were exposed to normoxia or hypoxia for 60 min. As shown in Fig. 6B, OA prevented the hypoxia-induced Gt increase (P = 0.0491). Also, AEC were treated with above inhibitor for 15 min, followed by exposure to normoxia or hypoxia for 60 min. Cells were then labeled with biotin and results were analyzed by WB. As shown in Fig. 6C, pretreatment of polarized AEC with OA prevented the hypoxia-induced occludin reduction at the plasma membrane. In addition, this inhibitor alone did not exhibit any difference in Gt or occludin abundance at the plasma membrane in AEC compared with control conditions (Supplemental Fig. S4). To further confirm that PP2A is involved, we proceeded to infect AEC with adenovirus that carried small-t antigen or empty vector with only CMV promoter. Small-t is a simian virus 40 (SV40) protein that has been shown to bind and inhibit PP2A by displacing lower affinity regulatory B subunits of the multimeric PP2A enzyme, resulting in PP2A complexes with low phosphatase activity and altered intracellular locations (30). Twenty-four hours after infection, polarized AEC were exposed to normoxia or hypoxia for 60 min. As shown in Fig. 6, D and E, infection of AEC with adenovirus vector expressing small-t prevented the hypoxia-induced occludin reduction at the plasma membrane whereas infection with empty vector did not prevent it (P = 0.0008). Expression of small-t was confirmed by WB analysis (Fig. 6F). These results suggest that PP2A is involved in the hypoxia-induced Gt increase and occludin reduction at the plasma membrane in AEC.
Fig. 6.
Protein phosphatase 2A (PP2A) is involved in hypoxia-induced Gt increase and occludin reduction in AEC. A: polarized AEC were exposed to normoxia or hypoxia for 60 min. PP2A activity was measured at different time intervals (15, 30, and 60 min) (n = 6 independent experiments in triplicate). B: polarized AEC were pretreated for 30 min with okadaic acid (OA) (5 nM), followed by exposure to normoxia or hypoxia for 60 min. Gt was measured at 30 min and 60 min (n = 8 independent experiments in triplicate). C: polarized AEC were pretreated for 30 min with OA (5 nM), followed by exposure to normoxia or hypoxia for 30 min. Surface biotinylation was performed and occludin abundance was determined by WB analysis. D and E: polarized AEC were infected 24 h before normoxia or hypoxia exposures, with adenovirus vectors expressing empty vector with only cytomegalovirus (CMV) promoter (D) or small-t (St; E). Cells were then exposed to normoxia or hypoxia for 30 min. Surface biotinylation was performed and occludin abundance was determined by WB analysis. E-cadherin was used as loading control. F: representative blot of overexpression of small-t protein. Shown are representative blots and quantitative analysis. Graph represents means ± SE; means with dots represent individual experiments (n = 3 independent experiments in triplicate). *P < 0.05; ***P < 0.001 compared with control.
DISCUSSION
Epithelial tissues, such as the lung, not only provide a physical barrier between biological compartments but mediate vectorial and selective transport of ions, water, and macromolecules. One of the elements that provide integrity to the barrier is the TJ, which are comprised of a group of integral proteins, specifically occludin, claudins, and JAM. Rat primary type II alveolar epithelial cells in culture express claudins 3, 4, and 5 and have the typical transepithelial electrical resistances of > 500 Ω × cm2, or Gt of 1–2 mS/cm2, consistent with the formation of TJ (35).
The development of ALI is the result of the disruption of the alveolar epithelial barrier integrity, causing pulmonary edema and respiratory failure. Resolution of this edema depends on repair of the epithelial barrier and AFR via active sodium transport mechanisms (22). The alveolar epithelium is normally well oxygenated, with partial pressures of oxygen of ∼100 mmHg. However, alveolar hypoxia is present in the fetal lung and in high-altitude pulmonary edema. In addition, alveolar edema, which is present in patients with congestive heart failure and acute respiratory distress syndrome, can create a hypoxic environment, since there is impairment of oxygenation of the alveolar space and the blood flow into this area will decrease as a result of hypoxic vasoconstriction (17). Hypoxia in the alveolar epithelium causes a decrease in AFR and disruption of the cytoskeleton and TJ (2, 38). Although AFR is required for the resolution of pulmonary edema, as long as the alveolar epithelial barrier is disrupted, the continuous flooding of the alveolar space will interfere with the ability of the lung to resolve the pulmonary edema.
Bouvry et al. (2) reported that AEC exposed to 3 and 0.5% oxygen for 18 h no longer had occludin localized to the TJ but scattered in the cell interior. When they looked at the total levels of occludin by WB, they did not find any change, whereas total protein levels of ZO-1 were reduced. Our results show that mild hypoxia (∼7%) produces a leakier barrier within 30 min, as evidenced by an increase in alveolar epithelial Gt and increased lanthanum staining on the basolateral side of AEC. Conversely, hypoxia does not increase permeability to large molecules such as 4-kDa FITC-dextran (data not shown) nor disrupts the AEC monolayer architecture (Fig. 1C). This implies that hypoxia causes a more subtle dysfunction of the barrier and that this is a regulated phenomenon, since it only affects the abundance of occludin at the plasma membrane in AEC, with no alteration in the abundance of claudins and ZO-1. In addition, the abundance of E-cadherin at the plasma membrane is not affected by hypoxia, suggesting that the development of a dysfunctional alveolar epithelial barrier is not a consequence of disruption of adhesion molecules. The other interesting finding is that exposing AEC to hypoxia for 30 min did not altered the total protein abundance of occludin in the cell lysate, whereas after 60 min there is ∼30% decrease. This is likely due to increased degradation of probable endocytosed occludin. Our results are different than those of Bouvry et al. This could be explained by the fact that AEC in our model are exposed to very mild hypoxia (∼7% O2) for 60 min, whereas Bouvry et al. exposed AEC to severe (0.5% O2) and moderate hypoxia (3% O2) for 18 h. Therefore, the sensing and signaling mechanisms are likely to be very different in these two models.
Previous studies demonstrated that severe hypoxia increases the generation of mitochondrial reactive oxygen species (H2O2 and superoxide) in vitro and in vivo (4). However, our model is different since AEC exposed to mild hypoxia did not increase H2O2 levels but only superoxide. Our results, where SOD1 overexpression prevented the hypoxia-induced occludin reduction in AEC is consistent with results shown in Fig. 3. In addition, the lack of protection of SOD2 suggests that mitochondrial ROS, specifically from the matrix, are not generated in this model. Conversely, despite not detecting an increase in H2O2 in AEC exposed to hypoxia, overexpression of catalase and pretreatment with ebselen (glutathione peroxidase mimetic) prevents the hypoxia-induced occludin reduction and Gt increase in AEC. The possible explanations for this observation includes lack of sensitivity of our methods to detect very small increases in H2O2 concentrations or local production of H2O2 in the cell microenvironment that cannot be detected with the methods used. Another possibility is nonspecific effects of catalase overexpression and ebselen, which prevent the hypoxia-induced occludin reduction in AEC.
During severe hypoxia the mitochondria are the source of ROS. Our results in this very mild hypoxic model raise the hypothesis that the source of ROS is probably different than the mitochondria, although the fact that overexpression of SOD1 prevented the hypoxia-induced dysfunction of the alveolar epithelium raises the possibility of the mitochondrial intermembrane space as a potential source of ROS (9). Since the source of ROS in our model is unclear, it leaves us to explore other sources such as the NADPH system. It has been reported that type II pneumocytes release ROS, and their generation has been attributed in part to the NADPH oxidase like enzyme system [reviewed by Bedard et al. (1)]. Also, the dual oxidase isoforms of the novel NADPH oxidase family are expressed and function at the apical membrane of both airway and alveolar epithelial cells (14). Multiple authors have reported different oxygen sensors in diverse organs. One of these potential sensors is the NADPH oxidase system, which plays a role in the oxygen sensing at the level of the carotid body. This organ is exposed to higher partial pressures of oxygen than most organs, except for the lung, cornea, and skin. Since small changes in oxygen tension require early responses by the carotid body, it is conceivable that the regulation by the alveolar epithelium shares some of the same mechanisms.
It has been reported that hypoxia activates PKC-ζ in AEC (10). Our results show that PKC-ζ translocates to the plasma membrane within 30 min of exposure to mild hypoxia and associated with occludin. At the TJ region, PKC-ζ and PP2A have been identified (33). It has been reported that PKCs could be upstream in the signaling pathway, regulating epithelial barrier function and causing either inactivation of a serine/threonine kinase or activation of a phosphatase associated with occludin (6). Not only is PKC-ζ activated during hypoxia, but it has been reported that rodents exposed to hypoxia have an increase in the amount of PP2A and its activity (18, 21). We show that PP2A activity in AEC increased by twofold after 15 min of exposure to hypoxia. Seth et al. (28) reported that PP2A and PP1 interact with occludin and negatively regulate the assembly of TJ in Caco cells. The same group of investigators reported that H2O2 increased the association of PP2A with occludin and induced TJ disruption in Caco-2 cell monolayer (29). In addition, PP2A associates with and regulates atypical PKCs, including PKC-ζ (25). Our data show that inhibition of PP2A or PKC-ζ prevents the hypoxia-induced Gt increase and occludin reduction at the plasma membrane in AEC. The mechanism by which these two enzymes maintain the alveolar epithelial barrier integrity is unknown. Since these enzymes are localized at the TJ complex (25), it provides a spatial relation that makes it possible to consider their biological role.
In summary, our model postulates that hypoxia induces a leakier alveolar epithelial barrier by generation of ROS. Mild hypoxia in AEC modestly increases the amount of superoxide and also increases the activity of PKC-ζ and PP2A. Blocking PKC-ζ and PP2A prevents hypoxia-induced increase in Gt and occludin reduction at the plasma membrane. This model of mild hypoxia is more representative of the possible effects that occur in vivo, providing the opportunity to study this effect in humans and different animal models.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant K01HL080966.
DISCLOSURES
All authors declare they have no actual or potential competing financial interest.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Joseph Zabner for advice and discussions. Also, we thank Shubha Murthy for providing technical advise in the measurement of different ROS.
REFERENCES
- 1.Bedard K, Lardy B, Krause KH. NOX family NADPH oxidases: not just in mammals. Biochimie 89: 1107–1112, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Bouvry D, Planes C, Malbert-Colas L, Escabasse V, Clerici C. Hypoxia-induced cytoskeleton disruption in alveolar epithelial cells. Am J Respir Cell Mol Biol 35: 519–527, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bush KT, Keller SH, Nigam SK. Genesis and reversal of the ischemic phenotype in epithelial cells. J Clin Invest 106: 621–626, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA 95: 11715–11720, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z, Krmar RT, Dada L, Efendiev R, Leibiger IB, Pedemonte CH, Katz AI, Sznajder JI, Bertorello AM. Phosphorylation of adaptor protein-2 mu2 is essential for Na+,K+-ATPase endocytosis in response to either G protein-coupled receptor or reactive oxygen species. Am J Respir Cell Mol Biol 35: 127–132, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke H, Soler AP, Mullin JM. Protein kinase C activation leads to dephosphorylation of occludin and tight junction permeability increase in LLC-PK1 epithelial cell sheets. J Cell Sci 113: 3187–3196, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Clayburgh DR, Barrett TA, Tang Y, Meddings JB, VanEldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest 115: 2702–2715, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clerici C, Planes C. Gene regulation in the adaptive process to hypoxia in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 296: L267–L274, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Comellas AP, Dada LA, Lecuona E, Pesce LM, Chandel NS, Quesada N, Budinger GR, Strous GJ, Ciechanover A, Sznajder JI. Hypoxia-mediated degradation of Na,K-ATPase via mitochondrial reactive oxygen species and the ubiquitin-conjugating system. Circ Res 98: 1314–1322, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Dada LA, Chandel NS, Ridge KM, Pedemonte C, Bertorello AM, Sznajder JI. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J Clin Invest 111: 1057–1064, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dada LA, Sznajder JI. Hypoxic inhibition of alveolar fluid reabsorption. Adv Exp Med Biol 618: 159–168, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Dobbs LG, Gonzalez R, Williams MC. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis 134: 141–145, 1986 [DOI] [PubMed] [Google Scholar]
- 13.Eichholtz T, deBont DB, deWidt J, Liskamp RM, Ploegh HL. A myristoylated pseudosubstrate peptide, a novel protein kinase C inhibitor. J Biol Chem 268: 1982–1986, 1993 [PubMed] [Google Scholar]
- 14.Fischer H. Mechanisms and function of DUOX in epithelia of the lung. Antioxid Redox Signal 11: 2453–2465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn AN, Itani OA, Moninger TO, Welsh MJ. Acute regulation of tight junction ion selectivity in human airway epithelia. Proc Natl Acad Sci USA 106: 3591–3596, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol 81: 1–44, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Guidot DM, Folkesson HG, Jain L, Sznajder JI, Pittet JF, Matthay MA. Integrating acute lung injury and regulation of alveolar fluid clearance. Am J Physiol Lung Cell Mol Physiol 291: L301–L306, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Hoshikawa Y, Nana-Sinkam P, Moore MD, Sotto-Santiago S, Phang T, Keith RL, Morris KG, Kondo T, Tuder RM, Voelkel NF, Geraci MW. Hypoxia induces different genes in the lungs of rats compared with mice. Physiol Genomics 12: 209–219, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Howe AK, Gaillard S, Bennett JS, Rundell K. Cell cycle progression in monkey cells expressing simian virus 40 small t antigen from adenovirus vectors. J Virol 72: 9637–9644, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam EW, Zwacka R, Seftor EA, Nieva DR, Davidson BL, Engelhardt JF, Hendrix MJ, Oberley LW. Effects of antioxidant enzyme overexpression on the invasive phenotype of hamster cheek pouch carcinoma cells. Free Radic Biol Med 27: 572–579, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Larsen KO, Lygren B, Sjaastad I, Krobert KA, Arnkvaern K, Florholmen G, Larsen AK, Levy FO, Tasken K, Skjonsberg OH, Christensen G. Diastolic dysfunction in alveolar hypoxia: a role for interleukin-18-mediated increase in protein phosphatase 2A. Cardiovasc Res 80: 47–54, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 82: 569–600, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Minkenberg I, Ferber E. Lucigenin-dependent chemiluminescence as a new assay for NAD(P)H-oxidase activity in particulate fractions of human polymorphonuclear leukocytes. J Immunol Methods 71: 61–67, 1984 [DOI] [PubMed] [Google Scholar]
- 24.Morgan L, Shah B, Rivers LE, Barden L, Groom AJ, Chung R, Higazi D, Desmond H, Smith T, Staddon JM. Inflammation and dephosphorylation of the tight junction protein occludin in an experimental model of multiple sclerosis. Neuroscience 147: 664–673, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Nunbhakdi-Craig V, Machleidt T, Ogris E, Bellotto D, White CL, 3rd, Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol 158: 967–978, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Planes C, Friedlander G, Loiseau A, Amiel C, Clerici C. Inhibition of Na-K-ATPase activity after prolonged hypoxia in an alveolar epithelial cell line. Am J Physiol Lung Cell Mol Physiol 271: L70–L78, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Ridge KM, Rutschman DH, Factor P, Katz AI, Bertorello AM, Sznajder JL. Differential expression of Na-K-ATPase isoforms in rat alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 273: L246–L255, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Seth A, Sheth P, Elias BC, Rao R. Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J Biol Chem 282: 11487–11498, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Sheth P, Samak G, Shull JA, Seth A, Rao R. Protein phosphatase 2A plays a role in hydrogen peroxide-induced disruption of tight junctions in Caco-2 cell monolayers. Biochem J 421: 59–70, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skoczylas C, Fahrbach KM, Rundell K. Cellular targets of the SV40 small-t antigen in human cell transformation. Cell Cycle 3: 606–610, 2004 [PubMed] [Google Scholar]
- 31.Snyder CM, Chandel NS. Mitochondrial regulation of cell survival and death during low-oxygen conditions. Antioxid Redox Signal 11: 2673–2683, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sohn JH, Han KL, Lee SH, Hwang JK. Protective effects of panduratin A against oxidative damage of tert-butylhydroperoxide in human HepG2 cells. Biol Pharm Bull 28: 1083–1086, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Suzuki A, Ishiyama C, Hashiba K, Shimizu M, Ebnet K, Ohno S. aPKC kinase activity is required for the asymmetric differentiation of the premature junctional complex during epithelial cell polarization. J Cell Sci 115: 3565–3573, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Tephly LA, Carter AB. Asbestos-induced MKP-3 expression augments TNF-alpha gene expression in human monocytes. Am J Respir Cell Mol Biol 39: 113–123, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang F, Daugherty B, Keise LL, Wei Z, Foley JP, Savani RC, Koval M. Heterogeneity of claudin expression by alveolar epithelial cells. Am J Respir Cell Mol Biol 29: 62–70, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 163: 1376–1383, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Wodopia R, Ko HS, Billian J, Wiesner R, Bartsch P, Mairbaurl H. Hypoxia decreases proteins involved in epithelial electrolyte transport in A549 cells and rat lung. Am J Physiol Lung Cell Mol Physiol 279: L1110–L1119, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Zhou G, Dada LA, Sznajder JI. Regulation of alveolar epithelial function by hypoxia. Eur Respir J 31: 1107–1113, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Zwacka RM, Dudus L, Epperly MW, Greenberger JS, Engelhardt JF. Redox gene therapy protects human IB-3 lung epithelial cells against ionizing radiation-induced apoptosis. Hum Gene Ther 9: 1381–1386, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Zwacka RM, Zhou W, Zhang Y, Darby CJ, Dudus L, Halldorson J, Oberley L, Engelhardt JF. Redox gene therapy for ischemia/reperfusion injury of the liver reduces AP1 and NF-kappaB activation. Nat Med 4: 698–704, 1998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.