Abstract
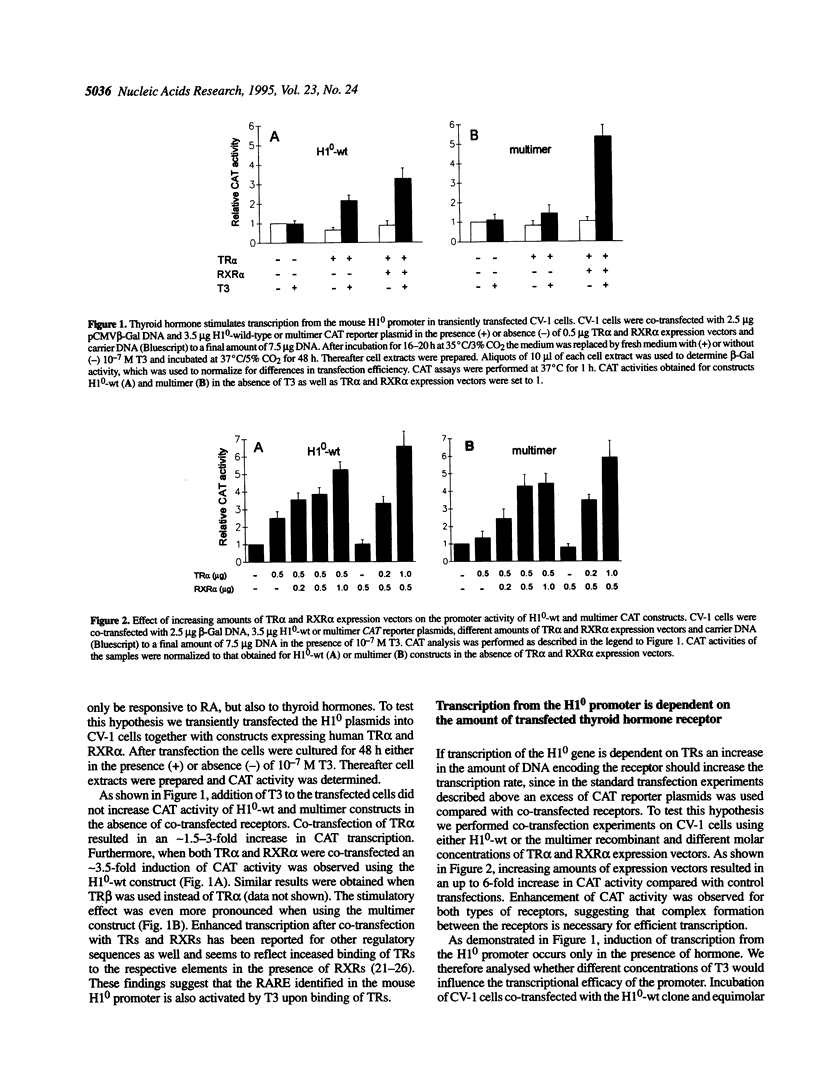
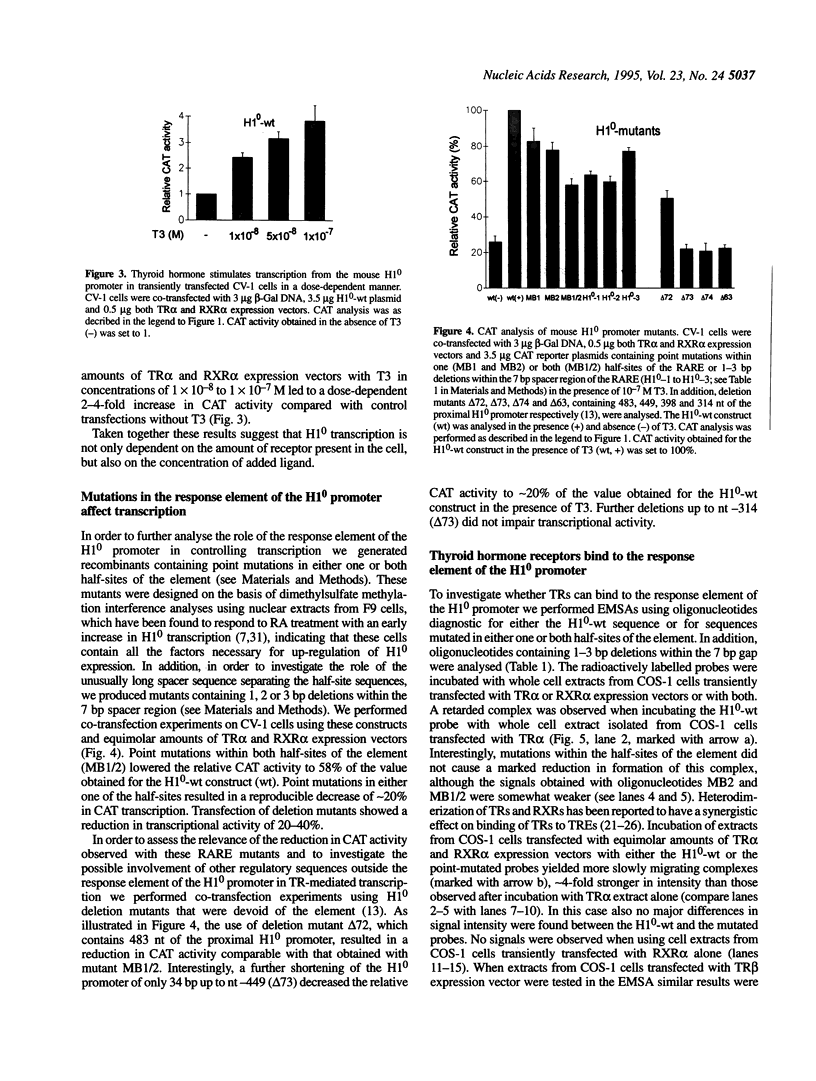
It has been shown that the mouse histone H10 promoter contains a DNA element, composed of a direct repeat of the sequence GGTGACC separated by 7 nt, which is able to bind retinoic acid receptors and to modulate transcription of reporter genes following treatment with retinoic acid. We have now investigated whether this DNA motif is also responsive to thyroid hormone. We co-transfected CV-1 monkey kidney cells with chloramphenicol acetyltransferase (CAT) expression plasmids containing either 740 bp of the H10 wild-type promoter or five copies of the repeat element cloned in front of the thymidine kinase promoter and expression vectors for human thyroid hormone receptors (TRs) alpha or beta and retinoid X receptor alpha (RXR alpha). Treatment of transfected cells with triiodothyronine led to a dose-dependent increase in CAT activity. Transfection experiments with increasing amounts of expression vectors for either TR alpha or RXR alpha resulted in up to 6-fold enhancement of CAT transcription. Furthermore, point mutations within the half-sites of the response element of the H10 promoter, as well as deletions within the interspace region, lowered CAT activity to 60-80% of that of the wild-type control. Electrophoretic mobility shift assays showed that the repeat element was able to form retarded complexes with TR alpha homodimers, as well as with TR alpha-RXR alpha heterodimers. Our results suggest that thyroid hormone receptors are involved in the regulation of mouse histone H10 expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso A., Breuer B., Bouterfa H., Doenecke D. Early increase in histone H1(0) mRNA during differentiation of F9 cells to parietal endoderm. EMBO J. 1988 Oct;7(10):3003–3008. doi: 10.1002/j.1460-2075.1988.tb03163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A., Breuer B., Steuer B., Fischer J. The F9-EC cell line as a model for the analysis of differentiation. Int J Dev Biol. 1991 Dec;35(4):389–397. [PubMed] [Google Scholar]
- Brent G. A., Harney J. W., Chen Y., Warne R. L., Moore D. D., Larsen P. R. Mutations of the rat growth hormone promoter which increase and decrease response to thyroid hormone define a consensus thyroid hormone response element. Mol Endocrinol. 1989 Dec;3(12):1996–2004. doi: 10.1210/mend-3-12-1996. [DOI] [PubMed] [Google Scholar]
- Breuer B., Steuer B., Alonso A. Basal level transcription of the histone H1(0) gene is mediated by a 80 bp promoter fragment. Nucleic Acids Res. 1993 Feb 25;21(4):927–934. doi: 10.1093/nar/21.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugge T. H., Pohl J., Lonnoy O., Stunnenberg H. G. RXR alpha, a promiscuous partner of retinoic acid and thyroid hormone receptors. EMBO J. 1992 Apr;11(4):1409–1418. doi: 10.1002/j.1460-2075.1992.tb05186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croston G. E., Kerrigan L. A., Lira L. M., Marshak D. R., Kadonaga J. T. Sequence-specific antirepression of histone H1-mediated inhibition of basal RNA polymerase II transcription. Science. 1991 Feb 8;251(4994):643–649. doi: 10.1126/science.1899487. [DOI] [PubMed] [Google Scholar]
- García-Iglesias M. J., Ramirez A., Monzo M., Steuer B., Martínez J. M., Jorcano J. L., Alonso A. Specific expression in adult mice and post-implantation embryos of a transgene carrying the histone H1(0) regulatory region. Differentiation. 1993 Dec;55(1):27–35. doi: 10.1111/j.1432-0436.1993.tb00030.x. [DOI] [PubMed] [Google Scholar]
- Gjerset R., Gorka C., Hasthorpe S., Lawrence J. J., Eisen H. Developmental and hormonal regulation of protein H1 degrees in rodents. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2333–2337. doi: 10.1073/pnas.79.7.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass C. K., Lipkin S. M., Devary O. V., Rosenfeld M. G. Positive and negative regulation of gene transcription by a retinoic acid-thyroid hormone receptor heterodimer. Cell. 1989 Nov 17;59(4):697–708. doi: 10.1016/0092-8674(89)90016-0. [DOI] [PubMed] [Google Scholar]
- Graupner G., Wills K. N., Tzukerman M., Zhang X. K., Pfahl M. Dual regulatory role for thyroid-hormone receptors allows control of retinoic-acid receptor activity. Nature. 1989 Aug 24;340(6235):653–656. doi: 10.1038/340653a0. [DOI] [PubMed] [Google Scholar]
- Ikeda M., Rhee M., Chin W. W. Thyroid hormone receptor monomer, homodimer, and heterodimer (with retinoid-X receptor) contact different nucleotide sequences in thyroid hormone response elements. Endocrinology. 1994 Oct;135(4):1628–1638. doi: 10.1210/endo.135.4.7925126. [DOI] [PubMed] [Google Scholar]
- Katz R. W., Subauste J. S., Koenig R. J. The interplay of half-site sequence and spacing on the activity of direct repeat thyroid hormone response elements. J Biol Chem. 1995 Mar 10;270(10):5238–5242. doi: 10.1074/jbc.270.10.5238. [DOI] [PubMed] [Google Scholar]
- Khochbin S., Wolffe A. P. Developmentally regulated expression of linker-histone variants in vertebrates. Eur J Biochem. 1994 Oct 15;225(2):501–510. doi: 10.1111/j.1432-1033.1994.00501.x. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992 Jan 30;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Leng X., Blanco J., Tsai S. Y., Ozato K., O'Malley B. W., Tsai M. J. Mechanisms for synergistic activation of thyroid hormone receptor and retinoid X receptor on different response elements. J Biol Chem. 1994 Dec 16;269(50):31436–31442. [PubMed] [Google Scholar]
- Mader S., Thiele K., Breuer B., Alonso A. The promoter of the H1zero histone gene contains a DNA element bound by retinoic acid receptors. J Mol Biol. 1994 Sep 9;242(1):37–44. doi: 10.1006/jmbi.1994.1555. [DOI] [PubMed] [Google Scholar]
- Mellström B., Naranjo J. R., Santos A., Gonzalez A. M., Bernal J. Independent expression of the alpha and beta c-erbA genes in developing rat brain. Mol Endocrinol. 1991 Sep;5(9):1339–1350. doi: 10.1210/mend-5-9-1339. [DOI] [PubMed] [Google Scholar]
- Pfahl M. Vertebrate receptors: molecular biology, dimerization and response elements. Semin Cell Biol. 1994 Apr;5(2):95–103. doi: 10.1006/scel.1994.1013. [DOI] [PubMed] [Google Scholar]
- Roche J., Gorka C., Goeltz P., Lawrence J. J. Association of histone H1(0) with a gene repressed during liver development. Nature. 1985 Mar 14;314(6007):197–198. doi: 10.1038/314197a0. [DOI] [PubMed] [Google Scholar]
- Steuer B., Breuer B., Alonso A. Multiple cis-acting elements of the proximal promoter region are required for basal level transcription of the H1(0) histone gene. Biochem Biophys Res Commun. 1992 Nov 16;188(3):1153–1160. doi: 10.1016/0006-291x(92)91352-q. [DOI] [PubMed] [Google Scholar]
- Tsai M. J., O'Malley B. W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Umesono K., Giguere V., Glass C. K., Rosenfeld M. G., Evans R. M. Retinoic acid and thyroid hormone induce gene expression through a common responsive element. Nature. 1988 Nov 17;336(6196):262–265. doi: 10.1038/336262a0. [DOI] [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. R., Harney J. W., Moore D. D., Larsen P. R., Brent G. A. Differential capacity of wild type promoter elements for binding and trans-activation by retinoic acid and thyroid hormone receptors. Mol Endocrinol. 1992 Oct;6(10):1527–1537. doi: 10.1210/mend.6.10.1333048. [DOI] [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Hoffmann B., Tran P. B., Graupner G., Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992 Jan 30;355(6359):441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]
- Zlatanova J., Doenecke D. Histone H1 zero: a major player in cell differentiation? FASEB J. 1994 Dec;8(15):1260–1268. doi: 10.1096/fasebj.8.15.8001738. [DOI] [PubMed] [Google Scholar]
- Zlatanova J., Yaneva J. Histone H1-DNA interactions and their relation to chromatin structure and function. DNA Cell Biol. 1991 May;10(4):239–248. doi: 10.1089/dna.1991.10.239. [DOI] [PubMed] [Google Scholar]
- van Hemert F. J., Jonk L. J., Destrée O. H. Histone H1(0) mRNA and protein accumulate early during retinoic acid induced differentiation of synchronized embryonal carcinoma cells. Mol Biol Rep. 1992 Feb;16(1):33–38. doi: 10.1007/BF00788751. [DOI] [PubMed] [Google Scholar]