Abstract
Decreased Toll-like receptor 2 (TLR2) expression has been reported in patients with chronic obstructive pulmonary disease and in a murine asthma model, which may predispose the hosts to bacterial infections, leading to disease exacerbations. Since airway epithelial cells serve as the first line of respiratory mucosal defense, the present study aimed to reveal the role of airway epithelial TLR2 signaling to lung bacterial [i.e., Mycoplasma pneumoniae (Mp)] clearance. In vivo TLR2 gene transfer via intranasal inoculation of adenoviral vector was performed to reconstitute TLR2 expression in airway epithelium of TLR2−/− BALB/c mice, with or without ensuing Mp infection. TLR2 and lactotransferrin (LTF) expression in airway epithelial cells and lung Mp load were assessed. Adenovirus-mediated TLR2 gene transfer to airway epithelial cells of TLR2−/− mice reconstituted 30–40% TLR2 expression compared with TLR2+/+ cells. Such airway epithelial TLR2 reconstitution in TLR2−/− mice significantly reduced lung Mp load (an appropriate 45% reduction), coupled with elevated LTF expression. LTF expression in mice was shown to be mainly dependent on TLR2 signaling in response to Mp infection. Exogenous human LTF protein dose-dependently decreased lung bacterial load in Mp-infected TLR2−/− mice. In addition, human LTF protein directly dose-dependently decreased Mp levels in vitro. These data indicate that reconstitution of airway epithelial TLR2 signaling in TLR2−/− mice significantly restores lung defense against bacteria (e.g., Mp) via increased lung antimicrobial protein LTF production. Our findings may offer a deliverable approach to attenuate bacterial infections in airways of asthma or chronic obstructive pulmonary disease patients with impaired TLR2 function.
Keywords: Toll-like receptor 2, airway epithelial cells, bacterial clearance
airway bacterial infections have been linked to the development and progression of chronic obstructive pulmonary disease (COPD) and asthma (7, 19). Mycoplasma pneumoniae (Mp) is one of the common strains of bacteria identified in COPD and asthmatic airways and is relevant to disease exacerbations (20, 31). To reveal the mechanisms underlying Mp persistence in allergic airways, we recently focused on the role of Toll-like receptor 2 (TLR2) signaling in host defense against bacteria. We have demonstrated that ovalbumin-induced airway allergic inflammation impairs TLR2 expression and IL-6 production of lung cells [e.g., dendritic cells (DCs)], which consequently dampens lung Mp clearance in mice (37). Moreover, lung Mp levels in TLR2-deficient (TLR2−/−) C57BL/6 mice were shown to be significantly higher than those in wild-type mice (38). In addition, decreased TLR2 expression has been reported on alveolar macrophages in healthy cigarette smokers and COPD patients compared with healthy nonsmokers, which may be associated with impaired host defense in COPD airways (8).
Previous studies using the TLR2−/− mouse models have indicated an important role of TLR2 signaling pathway in host defense against a broad spectrum of pathogens, including gram-negative bacteria (e.g., Legionella pneumophila and Porphyromonas gingivalis), gram-positive bacteria (e.g., Pseudomonas aeruginosa), and atypical bacteria (e.g., Chlamydia pneumoniae and Mp) (2, 11, 14, 16, 22, 26, 38). Airway epithelial cells represent the very front line of respiratory tract mucosal defense to eliminate inhaled pathogens from the lung via various mechanisms, including TLR signaling (13). Compared with the professional inflammatory cells (e.g., macrophages), human airway epithelial cells express less TLR2 protein. Even such a low level of TLR2 expression enables airway epithelial cells to generate a robust response to TLR2 agonists in vitro (17, 25). However, the in vivo role of airway epithelial TLR2 signaling in lung defense against bacterial infections (e.g., Mp) has not been directly demonstrated.
Activation of airway TLRs not only induces production of proinflammatory cytokines, but also antimicrobial substances that directly enhance bacterial clearance (3). Lactotransferrin (LTF) is a multifunctional antimicrobial protein found in airway epithelial lining fluid, milk, and other secretions in most mammalian species (34, 35). Human airway epithelial cells and submucosal gland serous cells produce LTF (9, 10). In normal human subjects, LTF levels in bronchial epithelial lining fluid are 122 ± 29 μg/ml (29). Whole LTF protein and its NH2-terminal peptides exhibit antimicrobial activity against a broad spectrum of pathogens (18). Additionally, LPS provokes a significant increase of LTF protein in bovine tracheal epithelial cells (1). These results suggest that LTF may participate in airway mucosal immunity against infections, but whether TLR2 signaling contributes to LTF production following an infection remains unclear.
In the present study, we hypothesized that airway epithelial TLR2 signaling is sufficient to prime host defense against respiratory bacterial infection. We performed in vivo airway epithelial TLR2 gene transfer in TLR2−/− mice via intranasal inoculation of adenoviral vector to determine the contribution of airway epithelial TLR2 signaling to lung bacterial (e.g., Mp) clearance. Furthermore, we investigated the role of TLR2 in LTF production and the function of LTF in Mp clearance. We found that a partial reconstitution of TLR2 in airway epithelium of TLR2−/− mice improves lung Mp clearance via increased lung LTF production.
METHODS
Mice.
All experimental animals used in this study were covered by a protocol approved by our Institutional Animal Care and Use Committee. TLR2−/− BALB/c mice were provided by Dr. S. Akira (Osaka University, Osaka, Japan) and were inbred in our biological resource center under pathogen-free housing condition. Age- and sex-matched wild-type BALB/c mice (8–12 wk) were obtained from Jackson Laboratory (Bar Harbor, ME). All of the mice were quarantined to establish that they were virus and M. pulmonis free.
Mp preparation.
Mp (strain FH; American Type Culture Collection 15531, Manassas, VA) was grown and prepared, as previously described (4).
Generation of recombinant adenovirus containing murine TLR2 cDNA.
The murine TLR2 (mTLR2) full-length cDNA in pCMV-SPORT6 plasmid was obtained from the Open Biosystems (Huntsville, AL) and further confirmed by DNA sequencing. A replication-defective recombinant human type 5 adenoviral vector expressing mTLR2 cDNA or empty control vector was generated using AdEasy Adenoviral Vector System (Stratagene, La Jolla, CA) per manufacturer's instruction. Recombinant adenovirus containing mTLR2 cDNA (mTLR2-Adv) or control virus (control Adv) was produced in AD-293 packaging cells. Adenovirus was then purified using an Adeno-X maxi purification kit (Clontech, Mountain View, CA), and eluted into sterile 1× formation buffer (20 mM Tris·HCl pH 8.0, 25 mM NaCl, 2.5% glycerol; Sigma-Aldrich, St. Louis, MO) by buffer change. The viral titers were determined using the Adeno-X Rapid Titration Kit (Clontech).
Mouse models of intranasal inoculation with recombinant adenovirus, Mp, or LTF.
In our preliminary time course experiments, TLR2−/− mice were pretreated with control Adv or mTLR2-Adv for 2, 3, or 4 days, followed by Mp infection for an additional 24 h. Significant airway epithelial TLR2 reconstitution and improved Mp clearance in TLR2−/− mice were observed similarly on both days 2 and 3 after adenoviral inoculation. Since an earlier time point is more specific to study lung innate immunity, we chose 2 days of adenoviral pretreatment as the current mouse model to investigate the in vivo role of airway epithelial TLR2 signaling in priming host innate response to Mp.
Mice were anesthetized intraperitoneally with 0.25 g/kg body wt of Avertine (2,2,2-Tribromoethanol dissolved in liquid tert-Amyl alcohol at 0.025 g/ml, Sigma-Aldrich). TLR2−/− mice were inoculated intranasally with 30 μl of control Adv or mTLR2-Adv at 108 plaque-forming unit (pfu)/mouse, as previously described (40). Wild-type (TLR2+/+) mice were instilled intranasally with 30 μl of 1× formation buffer as a vehicle control. Two days later, mice were inoculated intranasally with 50 μl of saline or Mp at 108 colony-forming unit (cfu)/mouse and were killed after 24 h. To obtain sufficient airway epithelial cells for detecting TLR2 protein in airway epithelial cells of TLR2−/− mice with TLR2 gene transfer, four to five mice from each group were killed without bronchoalveolar lavage (BAL). Specifically, mouse tracheas were harvested to isolate primary tracheal epithelial cells for TLR2 Western blot analysis, and whole lung tissues were processed for TLR2 immunofluorescence staining. The remaining mice (n = 6–7) in each group were then processed with BAL. Briefly, the lung was lavaged with 1 ml of sterile saline. For Mp-infected mice, 10 μl of BAL or left lung homogenate in PBS were plated on pleuropneumonia-like organis agar plates (Remel, Lenexa, KS) and incubated at 37°C with 5% CO2 for 7 days to quantify lung Mp load. The right lung was processed for laser capture microdissection (LCM) to determine TLR2 and LTF mRNA expression in airway epithelial cells. Cell-free BAL fluid was saved at −80°C and used for examining LTF protein levels by Western blot analysis.
To determine whether LTF expression is dependent on TLR2 signaling, TLR2−/− and wild-type mice (n = 6/group) were intranasally inoculated with 50 μl of saline or Mp at 108 cfu/mouse and killed 24 h later to examine lung LTF expression. Moreover, to test the role of LTF in promoting lung Mp clearance, purified human milk LTF protein (0, 125, 250, or 500 μg/50 μl saline; Sigma-Aldrich) or bovine serum albumin (BSA) (an irrelevant protein control, 500 μg/50 μl saline) was intranasally inoculated 30 min after Mp (108 cfu/mouse) infection in TLR2−/− mice. Mice were killed 24 h later to examine lung Mp load. The doses of purified human LTF were selected according to the previous studies in humans (29) and were also consistent with several mouse studies (15, 32).
Mouse primary tracheal epithelial cell isolation.
To confirm whether TLR2 gene transfer by adenoviral vectors results in epithelial TLR2 protein expression, mouse primary tracheal epithelial cells were isolated from TLR2−/− mice with or without TLR2 gene transfer and wild-type mice (38). Briefly, mouse tracheas were cut longitudinally and pooled from the same group for digestion in ice-cold DMEM media (GIBCO, Grand Island, NY) supplemented with 0.1% protease solution (Sigma-Aldrich) at 4°C for 6 h. After vigorous flushing wash, the released cells were lysed in 50 μl of RIPA buffer (Pierce, Rockford, IL) supplemented with 1% halt protease and phosphatase inhibitor cocktail (Pierce) for TLR2 Western blot. The purity of epithelial cells was >95%, as evaluated under a light microscope.
Mouse primary alveolar macrophages isolation and culture.
To confirm if adenovirus-mediated TLR2 reconstitution is relatively specific in airway epithelial cells, mouse primary alveolar macrophages (pAMs) were isolated from BAL cells of TLR2−/− mice, with or without TLR2 gene transfer and wild-type mice (36). Specially, isolated pAMs were pooled from the same group, seeded in triplicate in 48-well tissue culture plates, and treated with 10 ng/ml of Pam3CSK4 (a TLR2 agonist; InvivoGen, San Diego, CA) or PBS for 24 h. The culture supernatants were collected for IL-6 measurement by ELISA (R&D Systems, Minneapolis, MN) to indicate TLR2 activation.
LCM.
LCM was performed in frozen lung sections, as previously described (4), to isolate mouse bronchial epithelial cells for examining TLR2 and LTF mRNA expression.
TLR2 immunofluorescence staining.
Mouse lung tissues were embedded in Tissue-Tek optimal cutting temperature compound (Sakura FineTek USA, Torrance, CA) and stored at −80°C until use. Frozen sections were cut at 6-μm thickness and fixed in ice-cold acetone/methanol (1:1). They were then blocked with 10% normal goat serum (Vector Laboratories, Burlingame, CA), probed with 2 μg/ml of rabbit anti-mouse TLR2 (Santa Cruz Biotechnology, Santa Cruz, CA) or control rabbit Ig (Vector Laboratories) at 4°C overnight. The slides were incubated with FITC-conjugated goat anti-rabbit Ig at room temperature for 1 h (Vector Laboratories), and then with 4′,6-diamidino-2-phenylindole to counterstain the nuclei. After being mounted with DAKO fluorescent mount medium (Dako, Glostrup, Denmark), the slides were examined under a fluorescent microscope.
Quantitative real-time RT-PCR.
Quantitative real-time RT-PCR was performed on the ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA). 18S rRNA was evaluated as an internal positive control. The comparative cycle of threshold (ΔΔCt) method was used to demonstrate the relative mRNA levels of target genes (4). The following primers and probe of mTLR2 cDNA (Genbank accession number, BC014693) were used: forward, 5′-CCATCGAAAAGAG-CCACA-3′; reverse, 5′-CAGCAAAACAAGGATGGC-3′; probe, 5′-CCGTACGAAGTTCTC-AGAAAGCACGA-3′. TaqMan Gene Expression Assay for murine LTF (Mm00434787_m1) was obtained from Applied Biosystems.
Western blot analysis.
Protein (50 μg) from isolated mouse primary tracheal epithelial cells was used for TLR2 Western blot analysis and normalized with GAPDH (loading control). Mouse BAL fluid (10 μl/mouse) was used for LTF Western blot analysis. The blotting membranes of BAL fluid were stained with Ponceau S to confirm equal protein loading by comparing the 68-kDa protein (corresponding to albumin) levels (4). The primary antibodies were rabbit anti-mouse TLR2 (Santa Cruz Biotechnology) and rabbit anti-mouse LTF (Millipore, Billerica, MA).
Anti-Mp activity of purified human LTF protein.
Mp (4 × 104 cfu/ml) was incubated with purified human LTF protein from milk at various concentrations (62.5, 125, 250, and 500 μg/ml) or BSA (an irrelevant protein control) in a 96-well tissue culture plate for 2 h. The LTF doses were justified based on previous studies (12, 15, 29, 32). The supernatants were plated on pleuropneumonia-like organis agar plates (Remel) and incubated at 37°C with 5% CO2 for 7 days to qualify Mp.
Statistical analysis.
Normally distributed data are presented as means ± SE. One-way ANOVA was used for multiple comparisons, and a Turkey's post hoc test was applied where appropriate. Student's t-test was used when only two groups were compared. Nonnormally distributed data are expressed as medians with interquartile (25–75%) ranges and compared using the Wilcoxon rank-sum test. P < 0.05 was considered significant.
RESULTS
Adenovirus-mediated gene transfer reconstitutes TLR2 expression in mouse airway epithelial cells.
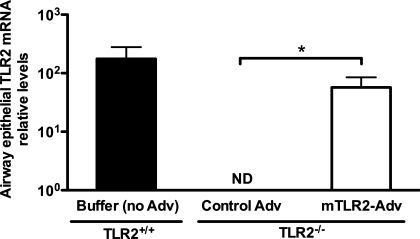
As shown in Fig. 1, intranasal inoculation of mTLR2-Adv, but not control Adv, in TLR2−/− mice reconstituted TLR2 mRNA expression in bronchial epithelial cells obtained through laser microdissection. This basal TLR2 mRNA level was ∼32% of that in airway epithelial cells of wild-type (TLR2+/+) mice.
Fig. 1.
Adenovirus-mediated gene transfer reconstitutes Toll-like receptor 2 (TLR2) mRNA expression in airway epithelial cells of TLR2−/− mice. TLR2−/− mice were intranasally inoculated with recombinant adenovirus containing murine TLR2 gene (mTLR2-Adv) or control virus (control Adv). Wild-type (TLR2+/+) mice were instilled intranasally with 1× formation buffer (no Adv) as a vehicle control. Two days later, all of the mice were intranasally treated with saline and killed 24 h later. TLR2 mRNA expression was measured using real-time RT-PCR in mouse bronchial epithelial cells isolated by laser capture microdissection. Relative levels of mRNA expression were normalized to 18S rRNA levels and calculated via comparative cycle of threshold (ΔΔCt) method. Values are presented as means ± SE; n = 6–7/group. Results shown are from one representative experiment of two independent experiments. ND, not detectable. *P < 0.05, compared with control Adv-treated mice.
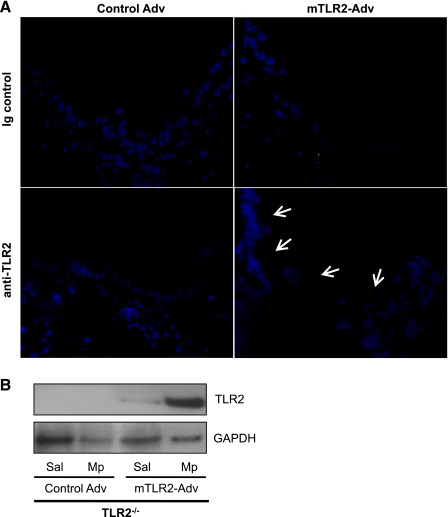
Bronchial epithelial localization of TLR2 protein in mTLR2-Adv-treated TLR2−/− mice was confirmed by immunofluorescence staining, despite weak TLR2 signals in all saline-treated TLR2−/− mice after TLR2 gene transfer. However, with Mp infection, TLR2 protein was clearly localized on the apical surface of bronchial epithelium of TLR2−/− mice with mTLR2-Adv-mediated gene transfer (Fig. 2A). No TLR2 staining was found in the lungs from mice treated with control Adv and Mp.
Fig. 2.
Mycoplasma pneumoniae (Mp) enhances adenovirus-mediated basal TLR2 protein reconstitution in airway epithelial cells of TLR2−/− mice. TLR2−/− mice were intranasally inoculated with recombinant mTLR2-Adv or control Adv. Two days later, mice were intranasally treated with saline (Sal) or Mp and killed 24 h later. A: representative photomicrographs of TLR2 immunofluorescence staining (indicated by white arrows) in frozen lung tissue sections from mice with adenoviral treatment and Mp infection (original magnification ×400). B: TLR2 protein was examined in isolated mouse primary tracheal epithelial cells (pooled from the same group) by Western blot. GAPDH protein served as a loading control. Values are means ± SE; n = 4–5/group. Results shown are from one representative experiment of two independent experiments.
Furthermore, we isolated tracheal epithelial cells from TLR2−/− mice with mTLR2-Adv-mediated gene transfer to further confirm TLR2 protein reconstitution by Western blot analysis. As shown in Fig. 2B, the basal (saline treatment) TLR2 protein expression was detected in tracheal epithelial cells of TLR2−/− mice with mTLR2-Adv-mediated gene transfer, which was ∼50% of that in wild-type epithelial cells (data not shown). Such a basal TLR2 protein expression was further enhanced by an ensuing in vivo Mp infection.
Previous studies have reported that an in vivo adenovirus-mediated gene transfer via intranasal delivery is localized mainly to bronchial epithelial cells, but to a lesser degree to alveolar macrophages (21). To confirm if TLR2 reconstitution is relatively specific in airway epithelial cells, but not in alveolar macrophages in our mouse model, pAMs isolated from TLR2−/− mice with or without TLR2 gene transfer and wild-type mice were stimulated with a TLR2 agonist Pam3CSK4 or PBS in vitro. Pam3CSK4 treatment in pAMs from mice treated with mTLR2-Adv did not significantly induce IL-6 production compared with PBS control [42 (0–83) pg/ml vs. undetectable, P = 0.69]. The IL-6 production level in isolated pAMs from mTLR2-Adv treated TLR2−/− mice was <2% of that in wild-type (TLR2+/+) pAMs [42 (0–83) vs. 3,012 (2,951–3,369) pg/ml, P < 0.0001].
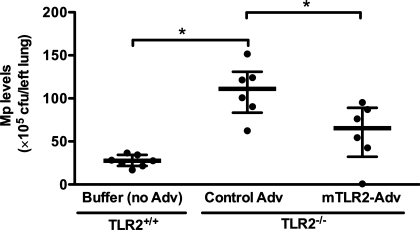
TLR2 reconstitution in airway epithelial cells improves mouse lung Mp clearance.
As shown in Fig. 3, TLR2−/− mice had significantly higher levels of lung tissue Mp burden (4-fold) than wild-type (TLR2+/+) mice. Adenovirus-mediated airway epithelial TLR2 reconstitution in TLR2−/− mice significantly reduced lung Mp load (an appropriate 45% reduction) compared with control Adv-treated mice.
Fig. 3.
TLR2 reconstitution in airway epithelial cells of TLR2−/− mice improves lung Mp clearance. TLR2−/− mice were intranasally inoculated with recombinant mTLR2-Adv or control Adv. Wild-type (TLR2+/+) mice were instilled intranasally with 1× formation buffer (no Adv) as a vehicle control. Two days later, all of the mice were intranasally treated with Mp and killed 24 h later. Lung Mp levels were quantified by culture. Data are presented as medians with interquartile (25–75%) ranges; n = 6–7/group. The thick horizontal line indicates the median; the vertical axis extends from 25 to 75% range. Results shown are from one representative experiment of two independent experiments. *P < 0.05.
In accordance with lung Mp load data, airway epithelial TLR2 reconstitution in TLR2−/− mice also resulted in a significant reduction of BAL Mp load compared with control Adv-pretreated mice [81 (57–97) vs. 123 (93–178) × 104 cfu/ml, P = 0.025].
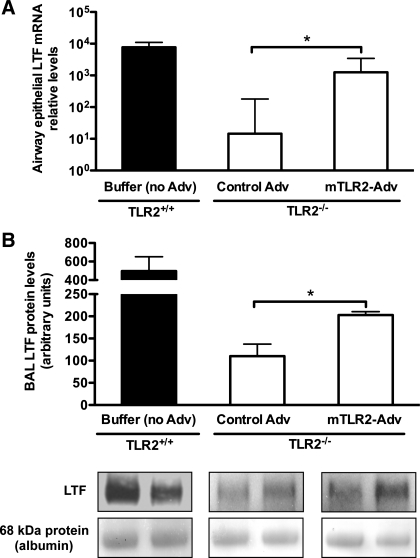
Mp increases LTF expression in TLR2−/− mice with airway epithelial TLR2 reconstitution.
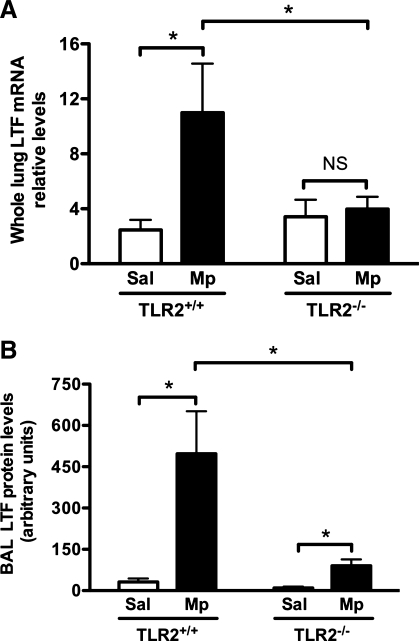
To determine the potential mechanisms of improved lung bacterial clearance in Mp-infected TLR2−/− mice with airway epithelial TLR2 reconstitution, LTF mRNA and protein levels were examined in microdissected bronchial epithelial cells and BAL fluid, respectively. As shown in Fig. 4A, Mp infection significantly increased epithelial LTF mRNA expression (∼90-fold) in mTLR2-Adv-treated TLR2−/− mice compared with that of control Adv-treated TLR2−/− mice. The LTF mRNA levels in Mp-infected mTLR2-Adv-treated TLR2−/− mice were ∼20% of those in Mp-infected wild-type mice.
Fig. 4.
Mp increases lactotransferrin (LTF) expression in TLR2−/− mice with airway epithelial TLR2 reconstitution. TLR2−/− mice were intranasally inoculated with recombinant mTLR2-Adv or control Adv. Wild-type (TLR2+/+) mice were instilled intranasally with 1× formation buffer (no Adv) as a vehicle control. Two days later, all of the mice were intranasally treated with Mp and killed 24 h later. A: LTF mRNA expression in mouse bronchial epithelial cells isolated by laser capture microdissection was measured by real-time RT-PCR. Relative levels of mRNA expression were normalized to 18S rRNA levels and calculated via ΔΔCt method. B, top: densitometric analysis of LTF protein levels in bronchoalveolar lavage (BAL) fluid examined by Western blot. Bottom: a representative graph of LTF protein in BAL fluid by Western blot. The blot membranes were stained with Ponceau S, and the intensity of 68-kDa protein (corresponding to albumin) was used to confirm equal protein loading. Values are means ± SE; n = 6–7/group. *P < 0.05, compared with control Adv-treated mice.
Furthermore, we found that, after Mp infection, BAL LTF protein levels were significantly higher in mTLR2-Adv-treated mice than those in control Adv-treated mice (Fig. 4B). The LTF protein levels in Mp-infected mTLR2-Adv-treated TLR2−/− mice were ∼40% of those in Mp-infected wild-type mice.
TLR2 dependency of LTF induction following Mp infection.
The observation of improved LTF expression coupled with reconstituted airway epithelial TLR2 expression after Mp infection led us to hypothesize that LTF production is dependent on TLR2 signaling. To test this hypothesis, we measured LTF expression in TLR2−/− and wild-type mice. As shown in Fig. 5, Mp infection in wild-type mice significantly increased LTF expression at both mRNA and protein levels compared with saline controls. However, in TLR2−/− mice, Mp infection did not significantly induce LTF mRNA expression in the lung compared with saline controls (Fig. 5A). Although LTF protein was upregulated by Mp infection in TLR2−/− mice compared with saline controls, the induction levels of LTF were much lower (80% less) than those in Mp-infected wild-type mice (Fig. 5B).
Fig. 5.
LTF induction is mainly TLR2 dependent. TLR2−/− and wild-type (TLR2+/+) mice were intranasally treated with Sal or Mp and killed 24 h later to examine lung LTF expression. A: whole lung LTF mRNA levels were measured by real-time RT-PCR. Relative levels of mRNA expression were normalized to 18S rRNA levels and calculated via ΔΔCt method. B: densitometric analysis of BAL LTF protein levels examined by Western blot. Values are means ± SE; n = 6/group. NS, not significant (P > 0.05). *P < 0.05.
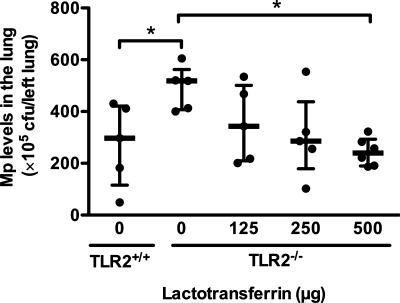
Human LTF protein dose-dependently reduces lung bacterial load in Mp-infected TLR2−/− mice.
To test if LTF promotes lung Mp clearance in vivo, we determined lung Mp load in Mp-infected TLR2−/− mice with or without human LTF protein inoculation. As shown in Fig. 6, lung Mp load was significantly higher in Mp-infected TLR2−/− BALB/c mice than Mp-infected wild-type (TLR2+/+) mice. This set of new data was similar to our laboratory's previous results in TLR2−/− C57BL/6 mice (38), and further confirmed our data shown in Fig. 3. Intranasal administration of human LTF protein dose-dependently reduced lung Mp load in TLR2−/− mice compared with BSA control.
Fig. 6.
Human LTF protein dose-dependently reduces lung bacterial load in Mp-infected TLR2−/− mice. TLR2−/− mice were intranasally inoculated with purified LTF protein from human milk or bovine serum albumin (BSA) 30 min after Mp infection. Mice were killed 24 h later to examine lung Mp load. Lung Mp levels were quantified by culture. Values are presented as medians with interquartile (25–75%) ranges; n = 6/group. The thick horizontal line indicates the median; the vertical axis extends from 25 to 75% range. *P < 0.05.
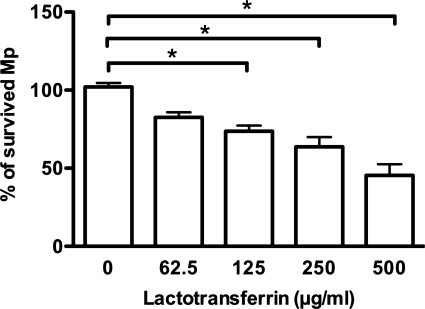
Human LTF protein directly inhibits Mp growth in vitro.
Since mouse and human LTF shares ∼70% of identity in amino acids (28), it has been predicted that LTF proteins from the two species may have similar functions. Mp was incubated with different doses of purified human LTF protein to examine its antimicrobial activity in vitro. As shown in Fig. 7, compared with BSA control, human LTF protein directly decreased Mp levels in a dose-dependent manner. The LTF concentration that gave a significant reduction of Mp levels was 125 μg/ml, which was close to the normal LTF protein levels in human bronchial epithelial lining fluid (122 ± 29 μg/ml) (29).
Fig. 7.
Human LTF protein directly inhibits Mp growth in vitro. Mp was incubated with purified human LTF protein from milk at various concentrations or BSA in a 96-well tissue culture plate for 2 h. Mp levels in the supernatants were quantified by culture. Values are means ± SE. Results shown are from one representative experiment (6 replicate wells/condition) of two independent experiments. *P < 0.05.
DISCUSSION
Understanding the in vivo function of airway epithelial cell TLR signaling is critical to improve mucosal immunization and therapy to fight against a broad spectrum of invading bacteria or viruses. However, the relative contribution of airway epithelial TLR2 function to in vivo lung host defense against bacterial infections has not been revealed. For the first time, we demonstrated that reconstitution of airway epithelial TLR2 signaling in TLR2-deficient mice via adenovirus-mediated in vivo gene transfer significantly restored lung host defense against Mp infection, which is partly through the induction of antimicrobial protein LTF.
Previous studies have suggested that the efficiency of adenovirus-mediated gene transfer to mouse trachea and intrapulmonary airways via the luminal surface of polarized epithelial cells is low due to the paucity of apical receptors required for viral entry (24, 33). Thus the doses of adenoviral vectors for in vivo gene transfer in airway epithelial cells usually are high (1–2 × 109 pfu/mouse) (23, 27). As a high dose of adenovirus may cause side effects such as excessive lung inflammation, it will limit its therapeutic implication. Therefore, a low dose of adenovirus containing the target gene would be preferred to attain therapeutic efficacy. In the present study, we utilized a relatively low single dose (108 pfu/mouse) of adenovirus containing mouse TLR2 cDNA and achieved a significant improvement of bacterial (i.e., Mp) clearance from Mp-infected TLR2−/− mouse lungs. Such an improvement of lung bacterial clearance is associated with specific TLR2 reconstitution at both mRNA and protein levels in large-airway (e.g., tracheal and bronchial) epithelial cells, but not in the more distal airways and alveolar tissue including alveolar macrophages. Our findings provide a valuable strategy to improve airway mucosal innate immunity in patients with dampened TLR2 signaling due to chronic lung diseases (e.g., COPD and asthma) by administrating a low dose of replication-deficient adenovirus containing a TLR2 gene. However, the airway epithelial cell TLR2 reconstitution only partially restored lung bacterial clearance from Mp-infected TLR2−/− mice. Although the exact mechanisms for such incomplete restoration of Mp clearance remain unclear, the continued TLR2 deficiency in immune cells of Mp-infected TLR2−/− mice could be one of the factors. In our laboratory's previous report, we have clearly shown that decreased TLR2 expression and IL-6 production in lung DCs contribute to the dampened lung Mp clearance in allergic mice (37). The chimeric TLR2−/− mice reconstituted with bone marrow from wild-type mice could be used to clearly define the relative role of hematopoietic immune cells (e.g., DCs) vs. nonhematopoietic cells (e.g., epithelial cells) to lung mycoplasma clearance.
The major advantage for administrating a low dose of replication-deficient adenovirus is that, while the virus transiently restores airway mucosal immunity (e.g., epithelial TLR2 function), it may not pose any significant safety concern based on previous studies. First, in healthy human subjects, intranasal administration of a replication-deficient recombinant adenovirus-based influenza vaccine expressing hemaglutinin is found to be safe and more effective than cutaneous patch immunization (30). Second, Damjanovic and colleagues (5) have recently reported that intranasal delivery of replication-defective recombinant adenoviral vector results in gene transfer predominantly in the respiratory system, but not in other organs, including the brain. Third, the expression of a gene in airway epithelial cells after intranasal gene transfer by adenoviral vector only lasts for 10–12 days (21). This suggests that a single dose of adenoviral vector may not be suitable for long-term correction of chronic disorders, but should be more appropriate for therapeutic strategies that require effective and transient gene expression, especially for enhancement of a normal gene rather than correction or replacement of a defective gene. For example, the duration of acute disease exacerbations due to airway bacterial infections usually lasts for ∼1 wk in patients with chronic lung diseases. Such a short-term TLR2 reconstitution would be sufficient to restore airway epithelial host defense function, but could avoid unpredictable side effects from the prolonged TLR2 expression in airway epithelium. Lastly, in our preliminary experiments, a low dose (108 pfu/mouse) of control or mTLR2 adenovirus alone resulted in very minimal lung inflammation (e.g., BAL inflammatory cells and IL-6 production) in TLR2−/− mice on day 2 postinoculation compared with buffer controls. Together, these data suggest that a narrow window of TLR2 reconstitution in airway epithelial cells following a low dose of replication-deficient adenovirus would be sufficient to promote bacterial clearance from the lungs with a dampened TLR2 function, but does not cause excessive inflammation.
Airway mucosa not only serves as a physical barrier but also activates immune defense mechanisms such as induction of antimicrobial molecules including LTF. With TLR2 reconstitution in airway epithelial cells by mTLR2-Adv pretreatment in TLR2−/− mice, LTF expression in bronchial epithelial cells and BAL fluid was significantly elevated after Mp infection. However, BAL LTF protein levels did not have any significant correlation with lung inflammatory markers, such as BAL neutrophils (r = −0.36, P = 0.96), suggesting that LTF more likely exhibits its direct anti-bacterial activity to promote lung Mp clearance in the present mouse model. Furthermore, by comparing lung LTF levels in TLR2−/− and TLR2+/+ mice, we reveal, for the first time, that LTF induction is mainly dependent on TLR2 signaling following Mp infection. However, Mp infection is still able to induce LTF production in TLR2−/− mice compared with saline controls, although the LTF levels are only ∼20% of those in Mp-infected wild-type (TLR2+/+) mice. These findings suggest that additional signaling pathways may contribute to LTF production in our current mouse model. One of the possible pathways to induce LTF expression by Mp infection in TLR2−/− mice could be TLR9 signaling since Mp has unmethylated CpG motifs in its DNA sequence (39). This hypothesis needs to be tested in future experiments by infecting TLR9−/− and/or TLR2−/− mice with Mp in the absence or presence of TLR2 gene transfer.
Although LTF has antimicrobial activity against a broad range of pathogens, its efficacy varies among different strains of bacteria. For example, human LTF exerts in vitro bactericidal effects on Micrococcus luteus, but not on other Micrococcus species (M. radiophilus, M. roseus, and M. varians) (6). For the first time, we have clearly demonstrated that LTF production contributes to the clearance of Mp from the airways. First, the impaired Mp clearance in TLR2−/− mice was restored by intranasal administration of purified human LTF protein. We realize that supplementation of LTF may not explicitly demonstrate its direct role in promoting lung bacterial clearance of Mp-infected TLR2−/− mice. An alternative approach to reveal the relative importance of LTF in improving lung Mp clearance is to deplete mouse LTF protein or block its function in vivo by using a neutralizing antibody. However, due to the lack of a commercial LTF neutralizing antibody, we cannot perform the LTF neutralization experiment in the present study. Second, human LTF protein directly inhibited Mp growth in vitro in a dose-dependent manner. However, the molecular mechanisms underlying the antimicrobial function of LTF against Mp remain unclear and is worthy of being investigated.
In summary, we have demonstrated that reconstitution of airway epithelial TLR2 signaling in TLR2−/− mice via adenovirus-mediated in vivo gene transfer significantly restored lung defense against Mp infection partly through induction of antimicrobial LTF protein. Our findings emphasize the importance of airway epithelial TLR2-mediated antimicrobial mechanisms and may offer a deliverable approach to attenuate bacterial infections in airways of asthma or COPD patients with impaired TLR2 function. Although adenoviral vectors provide more viable and efficient gene transfer compared with other gene delivery approaches for human gene therapy, the translation of our mouse work into a clinical setting still could be technically challenging.
GRANTS
This work was supported by National Institutes of Health Grants RO1AI070175 and RO1HL088264 to H. W. Chu.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
REFERENCES
- 1.Al-Haddawi M, Mitchell GB, Clark ME, Wood RD, Caswell JL. Impairment of innate immune responses of airway epithelium by infection with bovine viral diarrhea virus. Vet Immunol Immunopathol 116: 153–162, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Archer KA, Roy CR. MyD88-dependent responses involving toll-like receptor 2 are important for protection and clearance of Legionella pneumophila in a mouse model of Legionnaires' disease. Infect Immun 74: 3325–3333, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutler B, Hoebe K, Du X, Ulevitch RJ. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J Leukoc Biol 74: 479–485, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Chu HW, Thaikoottathil J, Rino JG, Zhang G, Wu Q, Moss T, Refaeli Y, Bowler R, Wenzel SE, Chen Z, Zdunek J, Breed R, Young R, Allaire E, Martin RJ. Function and regulation of SPLUNC1 protein in Mycoplasma infection and allergic inflammation. J Immunol 179: 3995–4002, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Damjanovic D, Zhang X, Mu J, Fe Medina M, Xing Z. Organ distribution of transgene expression following intranasal mucosal delivery of recombinant replication-defective adenovirus gene transfer vector. Genet Vaccines Ther 6: 5, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lillo A, Quiros LM, Fierro JF. Relationship between antibacterial activity and cell surface binding of lactoferrin in species of genus Micrococcus. FEMS Microbiol Lett 150: 89–94, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Diederen BM, van der Valk PD, Kluytmans JA, Peeters MF, Hendrix R. The role of atypical respiratory pathogens in exacerbations of chronic obstructive pulmonary disease. Eur Respir J 30: 240–244, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Droemann D, Goldmann T, Tiedje T, Zabel P, Dalhoff K, Schaaf B. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir Res 6: 68, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkbeiner WE, Carrier SD, Teresi CE. Reverse transcription-polymerase chain reaction (RT-PCR) phenotypic analysis of cell cultures of human tracheal epithelium, tracheobronchial glands, and lung carcinomas. Am J Respir Cell Mol Biol 9: 547–556, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Fischer AJ, Goss KL, Scheetz TE, Wohlford-Lenane CL, Snyder JM, McCray PB., Jr Differential gene expression in human conducting airway surface epithelia and submucosal glands. Am J Respir Cell Mol Biol 40: 189–199, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuse ET, Tateda K, Kikuchi Y, Matsumoto T, Gondaira F, Azuma A, Kudoh S, Standiford TJ, Yamaguchi K. Role of Toll-like receptor 2 in recognition of Legionella pneumophila in a murine pneumonia model. J Med Microbiol 56: 305–312, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Gomez HF, Ochoa TJ, Carlin LG, Cleary TG. Human lactoferrin impairs virulence of Shigella flexneri. J Infect Dis 187: 87–95, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Greene CM, McElvaney NG. Toll-like receptor expression and function in airway epithelial cells. Arch Immunol Ther Exp (Warsz) 53: 418–427, 2005 [PubMed] [Google Scholar]
- 14.Hajishengallis G, Wang M, Bagby GJ, Nelson S. Importance of TLR2 in early innate immune response to acute pulmonary infection with Porphyromonas gingivalis in mice. J Immunol 181: 4141–4149, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haversen LA, Engberg I, Baltzer L, Dolphin G, Hanson LA, Mattsby-Baltzer I. Human lactoferrin and peptides derived from a surface-exposed helical region reduce experimental Escherichia coli urinary tract infection in mice. Infect Immun 68: 5816–5823, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawn TR, Smith KD, Aderem A, Skerrett SJ. Myeloid differentiation primary response gene (88)- and toll-like receptor 2-deficient mice are susceptible to infection with aerosolized Legionella pneumophila. J Infect Dis 193: 1693–1702, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Hertz CJ, Wu Q, Porter EM, Zhang YJ, Weismuller KH, Godowski PJ, Ganz T, Randell SH, Modlin RL. Activation of Toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin-2. J Immunol 171: 6820–6826, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Jenssen H, Hancock RE. Antimicrobial properties of lactoferrin. Biochimie 91: 19–29, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Kraft M. The role of bacterial infections in asthma. Clin Chest Med 21: 301–313, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Kraft M, Cassell GH, Henson JE, Watson H, Williamson J, Marmion BP, Gaydos CA, Martin RJ. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med 158: 998–1001, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Lei XF, Ohkawara Y, Stampfli MR, Gauldie J, Croitoru K, Jordana M, Xing Z. Compartmentalized transgene expression of granulocyte-macrophage colony-stimulating factor (GM-CSF) in mouse lung enhances allergic airways inflammation. Clin Exp Immunol 113: 157–165, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller M, Postius S, Thimm JG, Gueinzius K, Muehldorfer I, Hermann C. Toll-like receptors 2 and 4 do not contribute to clearance of Chlamydophila pneumoniae in mice, but are necessary for the release of monokines. Immunobiology 209: 599–608, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Parsons DW, Grubb BR, Johnson LG, Boucher RC. Enhanced in vivo airway gene transfer via transient modification of host barrier properties with a surface-active agent. Hum Gene Ther 9: 2661–2672, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Pickles RJ, McCarty D, Matsui H, Hart PJ, Randell SH, Boucher RC. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J Virol 72: 6014–6023, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regueiro V, Moranta D, Campos MA, Margareto J, Garmendia J, Bengoechea JA. Klebsiella pneumoniae increases the levels of Toll-like receptors 2 and 4 in human airway epithelial cells. Infect Immun 77: 714–724, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skerrett SJ, Wilson CB, Liggitt HD, Hajjar AM. Redundant Toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 292: L312–L322, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Stonebraker JR, Wagner D, Lefensty RW, Burns K, Gendler SJ, Bergelson JM, Boucher RC, O'Neal WK, Pickles RJ. Glycocalyx restricts adenoviral vector access to apical receptors expressed on respiratory epithelium in vitro and in vivo: role for tethered mucins as barriers to lumenal infection. J Virol 78: 13755–13768, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teng CT. Lactoferrin gene expression and regulation: an overview. Biochem Cell Biol 80: 7–16, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Thompson AB, Bohling T, Payvandi F, Rennard SI. Lower respiratory tract lactoferrin and lysozyme arise primarily in the airways and are elevated in association with chronic bronchitis. J Lab Clin Med 115: 148–158, 1990 [PubMed] [Google Scholar]
- 30.Van Kampen KR, Shi Z, Gao P, Zhang J, Foster KW, Chen DT, Marks D, Elmets CA, Tang DC. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine 23: 1029–1036, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Varma-Basil M, Dwivedi SK, Kumar K, Pathak R, Rastogi R, Thukral SS, Shariff M, Vijayan VK, Chhabra SK, Chaudhary R. Role of Mycoplasma pneumoniae infection in acute exacerbations of chronic obstructive pulmonary disease. J Med Microbiol 58: 322–326, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Viejo-Diaz M, Andres MT, Fierro JF. Modulation of in vitro fungicidal activity of human lactoferrin against Candida albicans by extracellular cation concentration and target cell metabolic activity. Antimicrob Agents Chemother 48: 1242–1248, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walters RW, Grunst T, Bergelson JM, Finberg RW, Welsh MJ, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem 274: 10219–10226, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Ward PP, Paz E, Conneely OM. Multifunctional roles of lactoferrin: a critical overview. Cell Mol Life Sci 62: 2540–2548, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Widdicombe J. Relationships among the composition of mucus, epithelial lining liquid, and adhesion of microorganisms. Am J Respir Crit Care Med 151: 2088–2092; discussion 2092–2083, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Wu Q, Martin RJ, LaFasto S, Chu HW. A low dose of Mycoplasma pneumoniae infection enhances an established allergic inflammation in mice: the role of the prostaglandin E2 pathway. Clin Exp Allergy 39: 1754–1763, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Q, Martin RJ, Lafasto S, Efaw BJ, Rino JG, Harbeck RJ, Chu HW. Toll-like receptor 2 down-regulation in established mouse allergic lungs contributes to decreased mycoplasma clearance. Am J Respir Crit Care Med 177: 720–729, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Wu Q, Martin RJ, Rino JG, Jeyaseelan S, Breed R, Chu HW. A deficient TLR2 signaling promotes airway mucin production in Mycoplasma pneumoniae-infected allergic mice. Am J Physiol Lung Cell Mol Physiol 292: L1064–L1072, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Xia X. DNA methylation and Mycoplasma genomes. J Mol Evol 57, Suppl 1: S21–S28, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Xing Z, Zganiacz A, Wang J, Sharma SK. Enhanced protection against fatal mycobacterial infection in SCID beige mice by reshaping innate immunity with IFN-gamma transgene. J Immunol 167: 375–383, 2001 [DOI] [PubMed] [Google Scholar]