the acute respiratory distress syndrome (ARDS) is characterized by rapid-onset, severe lung inflammation with massive influx of polymorphonuclear neutrophils (PMNs) into the lungs and injury to the alveolar capillary barrier and is associated with a high mortality rate. There is mounting evidence that matrix metalloproteinases (MMPs) contribute to the pathogenesis of ARDS. Most MMPs implicated in the pathogenesis of ARDS promote lung inflammation and/or injury to the alveolar capillary barrier in animal models of acute lung injury (ALI). The study of Yamakawa et al. (23) in this issue of the journal is noteworthy because it has implicated two MMPs (MMP-3 and -13), which hitherto have not been well studied in ALI or ARDS, in protecting the lung from developing ALI by cleaving a novel substrate: the receptor for advanced glycation end products (RAGE).
MMPs and ARDS pathogenesis.
MMPs are a family of zinc-dependent proteinases with a multidomain structure. There are 23 members of this family in humans. MMPs are expressed by all cell types relevant to ARDS pathogenesis including alveolar epithelial cells, PMNs, macrophages, and fibroblasts. MMPs have been implicated in the pathogenesis of ARDS because lung levels of several MMPs are increased in patients with ALI and ARDS and lung levels of some MMPs correlate positively with adverse clinical outcomes in ARDS patients. For example, bronchoalveolar lavage (BAL) fluid (BALF) levels of collagenases (MMP-1, -8, and -13), gelatinases (MMP-2 and -9), and stromelysin-1 (MMP-3) are elevated in patients with ALI and ARDS (9). MMP-1 and -3 may promote disease progression in ARDS since BALF levels of MMP-1 and -3 (but not MMP-2, -8, or -9) correlate positively with lung injury severity and also with multiorgan failure and mortality rates in ARDS patients (9). Although degradation of extracellular matrix proteins was believed to be the main function of MMPs for years after MMPs were first identified, more recent studies of MMP-null mice in models of ALI have shown that MMPs generally promote ALI by cleaving cytokines and chemokines to regulate the biological activities of these mediators (Table 1). Until now, only two MMPs have been shown to possess anti-inflammatory activities during ALI: 1) MMP-8, which protects mice from bacterial lipopolysaccharide (LPS)- and hyperoxia-induced ALI by degrading macrophage inflammatory protein-1α (18), and 2) MMP-13, which limits lung inflammation during hyperoxia-induced ALI by inactivating monocyte chemoattractant protein-1 (19). The study of Yamakawa et al. (23) is noteworthy because it not only adds MMP-3 to this short list of MMPs having protective activities during ALI but also identifies RAGE as a potentially novel substrate for MMPs in the setting of ALI and ARDS.
Table 1.
MMPs and their activities in ALI
| MMP | ALI Model and Species | Activity of the MMP | Mechanism |
|---|---|---|---|
| MMP-3 | IT-MIP2 (mouse) | ↑ PMN accumulation | Not known (21) |
| Immune complex –mediated ALI (mouse) | ↑ Lung injury | ||
| MMP-7 | Bleomycin-mediated ALI (mouse) | ↑Transepithelial efflux of PMNs | MMP7 cleaves syndecan-1 from epithelial cell surfaces to release and activate KC bound to syndecan-1 (13) |
| ↑ Lung fibrotic responses to lung injury | |||
| MMP-8 | High pressure VILI (mouse) | ↑ Lung edema | ↑ Lung levels of CXCL5, MIP-2, and IFN-γ |
| ↑ Lung injury | ↓ Lung levels of IL-4 and IL-10 (2) | ||
| ↓ Gas exchange | |||
| ↑ PMN accumulation | |||
| IT LPS and hyperoxia (mouse) | ↓Lung PMNs and macrophages | Proteolytic inactivation of MIP-1α (18) | |
| ↓Lung injury | |||
| Low-tidal-volume VILI | ↓Lung injury | Not known (8) | |
| MMP-9 | Hyperoxia (mouse) | ↑ Mortality | Not known (14) |
| VILI with MMP9 inhibition (rat) | ↑ PMN accumulation | Not known (12) | |
| ↑ Lung injury | |||
| VILI (MMP9−/− mice) | ↓ Lung PMN accumulation | ↓ Lung levels of IL-1β and IL-4 (3) | |
| ↓ Lung injury | |||
| Immune complex (mouse) | ↑ Lung injury | Not known (21) | |
| MMP-12 | IT instillation of hrMMP-12 (mouse) | ↑ Lung PMN and macrophage counts | ↑ Lung levels of IL-6, TNF-α, MIP1α, MCP-1, KC (16) |
| Immune complex (mouse) | ↑ PMN accumulation | Not known (22) | |
| ↑ Lung injury | |||
| MMP-13 | Hyperoxia (mouse) | ↓ Lung macrophage counts | Proteolytic inactivation of MCP-1 (19) |
ALI, acute lung injury; hrMMP-12, human recombinant matrix metalloproteinase (MMP)12; IFN-γ, interferon-γ; IL, interleukin; IT, intratracheal; KC, keratinocyte-derived chemokine; MCP-1, monocyte chemotactic protein-1; MIP-1α, macrophage inflammatory protein-1α; PMN, polymorphonuclear neutrophil; VILI, ventilator-induced lung injury; ↑, increased; ↓, decreased.
RAGE biology and activities during ALI.
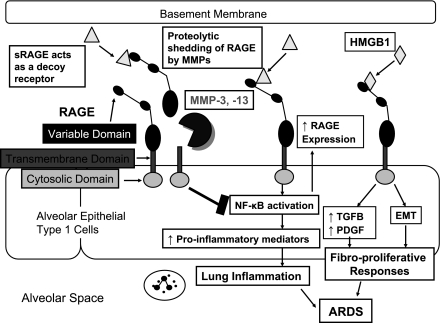
RAGE is a 35-kDa transmembrane receptor belonging to the immunoglobulin superfamily. RAGE has five domains including 1) a cytosolic domain that is responsible for signal transduction; 2) a transmembrane domain that anchors the receptor in the cell membrane; 3) a variable (V-type) domain that binds ligands; and 4) two constant (C-type) immunoglobulin-like regions domains (Fig. 1). RAGE is expressed in many tissues and cells including lung, kidney, and endothelial cells of large vessels. However, RAGE is expressed most abundantly in the lung, where it is localized exclusively to the basolateral membrane of alveolar epithelial type I (AT1) cells (Fig. 1). RAGE binds several ligands including advanced glycation end products (AGEs), high-mobility group protein B1 (HMGB1), amyloid β-peptide, and members of the S100/calgranulin family of proinflammatory proteins. The binding of AGE, HBGB1, and amyloid β-peptide to the ectodomain of RAGE initiates intracellular signaling leading to activation of nuclear factor-κB (NF-κB) and induction of proinflammatory gene expression (Fig. 1). Interestingly, RAGE itself is also upregulated by activation of NF-κB (Fig. 1). This may lead to a positive feedback cycle that amplifies tissue inflammation and injury in diseases in which levels of RAGE ligands are increased. One RAGE ligand in particular, HMGB1, is a potent mediator of ALI since it drives PMN accumulation, edema formation, and production of proinflammatory mediators in the lung (1). HMGB1 is also a mediator of lethality during endotoxemia and sepsis in mice. In humans with severe trauma, plasma HMGB1 levels correlate positively with severity of injury and progression to ALI (7). RAGE−/− mice are protected from systemic inflammatory response during septic shock (15). Thus HMBG1-RAGE signaling likely promotes progression to and/or increases the severity of the acute-exudative phase of ARDS. RAGE also has the potential to promote progression to the fibroproliferative phase of ARDS since RAGE−/− mice are protected from bleomycin-induced lung fibrosis (11). The profibrotic activities of RAGE are due to HMGB1-RAGE signaling leading to increased production of profibrotic growth factors and induction of epithelial-mesenchymal transition (Fig. 1).
Fig 1.
Potential impact of matrix metalloproteinase (MMP)-mediated proteolytic shedding of receptor for advanced glycation end products (RAGE) on the pathogenesis of acute respiratory distress syndrome (ARDS). Transmembrane RAGE is expressed by alveolar epithelial type I cells, and the variable domain of this receptor binds various ligands including high-mobility group box 1 (HMGB1). Binding of ligands to the ectodomain of RAGE can promote 1) lung inflammation in the acute-exudative phase of ARDS by activating NF-κB to increase the expression of proinflammatory genes and 2) fibroproliferative responses of the lung in the subacute phase of ARDS by increasing lung production of profibrotic growth factors including transforming growth factor-β (TGF-β) and platelet-derived growth factor (PDGF) and inducing epithelial-mesenchymal transition (EMT). MMP-3 and -13-mediated shedding of RAGE ectodomain releases soluble RAGE (sRAGE) forms. The latter may block pathological processes in the lungs of ARDS patients by acting as decoy receptors that bind RAGE ligands to prevent them from inducing intracellular signaling via the transmembrane form of RAGE.
sRAGE and ALI/ARDS.
Although RAGE is thought to function as a signaling transmembrane protein, soluble forms of RAGE (sRAGE) are generated in the lung and plasma during ALI/ARDS. In rodents with ALI, lung levels of sRAGE are increased and correlate positively with the severity of ALI (20). In human ALI patients ventilated with high tidal volumes, higher baseline plasma levels of sRAGE correlate with severity of lung injury and increased mortality rates (6). sRAGE levels in alveolar fluid from isolated and perfused human lungs also correlate inversely with alveolar fluid clearance (5). sRAGE forms have been used as a biomarker of AT1 cellular injury, which is a cardinal feature of ARDS (4).
Most sRAGE isoforms bind the same ligands as transmembrane RAGE to act as decoy receptors by preventing RAGE ligands from signaling through transmembrane-form RAGE (Fig. 1). Although sRAGE levels are increased in animals and humans with ALI and correlate positively with ALI severity and mortality, sRAGE forms have anti-inflammatory activities in the lung. This concept is supported by studies showing that delivering sRAGE to the lungs of mice attenuates LPS-induced lung inflammation and lung injury (24).
Five sRAGE isoforms lacking the transmembrane domain have been identified. Although sRAGE forms were thought to be generated by alternative splicing of the primary transcript of the human RAGE pre-mRNA, one study reported that sRAGE can be produced by carboxy terminal truncation, suggesting that proteolytic cleavage might be involved (10). The study of Yamakawa et al. (23) builds on this prior literature by confirming that MMP-3 and -13 proteolytically shed RAGE ectodomain from AT1 surfaces to generate sRAGE. The authors treated alveolar epithelial cells with LPS in vitro and showed that this increased cellular expression of MMP-3 and -13 and induced the release of sRAGE from AT1 cells without altering RAGE steady-state mRNA levels. Moreover, release of sRAGE by activated AT1 cells was markedly reduced by coculturing the cells with nonselective metalloproteinase inhibitors, implicating metalloproteinases in proteolytic shedding of RAGE. The authors demonstrated that these in vitro findings might be relevant to events occurring during ALI since delivering MMP-3 and -13 to the lungs of rats resulted in increased BAL levels of sRAGE, which was inhibited by delivering metalloproteinase inhibitors to the lungs of the animals. Translational studies of lung samples from ARDS patients demonstrated that the BALF levels of MMP-3 and -13 correlate positively with BALF sRAGE concentrations. These data suggest that during ALI and ARDS there is increased production of MMP-3 and -13 by AT1 cells that contributes to sRAGE generation by the MMPs cleaving the ectodomain of RAGE from AT1 cell surfaces.
Despite these new findings, there are still gaps in our knowledge about RAGE activities during ALI. Yamakawa et al. (23) did not quantify the contributions of MMP-3 and 13 endogenously expressed in the lung to sRAGE generation during ALI by comparing sRAGE levels in BAL samples from MMP-3−/− and MMP-13−/− mice with ALI vs. wild-type mice with ALI. It is possible that proteinases other than MMP-3 and -13 are significant RAGE sheddases in vivo. Future studies should also determine all of the contributions of MMP-3 and -13 to the pathogenesis of ARDS especially since MMP-3 has proinflammatory activities in some animal models of ALI (Table 1). Nevertheless, the study of Yamakawa et al. raises the possibility that MMP-mediated RAGE shedding might limit the severity of the acute-exudative phase of ARDS by reducing proinflammatory gene expression in the lung via the decoy receptor activities of sRAGE (Fig. 1). MMP-mediated shedding of RAGE might also reduce progression to, or severity of, the fibroproliferative phase of ARDS by reducing lung production of profibrotic growth factors and/or epithelial-mesenchymal transition (Fig. 1).
Although the studies of Yamakawa et al. (23) suggest that RAGE and sRAGE might be new therapeutic targets for ARDS, there are several caveats. First, there may be species differences in the biology of sRAGE. It has been suggested that alternative splicing of RAGE pre-mRNA is the main pathway for generating sRAGE in humans whereas proteolytic shedding of RAGE may predominate in rodents (10). Thus the contribution of MMPs and other classes of proteinases to RAGE shedding in the human lung needs to be addressed. Second, sRAGE decreases delayed hypersensitivity reactions in the footpads of RAGE−/− mice (15), indicating that sRAGE forms have anti-inflammatory activities distinct from their effects on transmembrane RAGE signaling that have yet to be characterized. Third, although sRAGE forms have potent anti-inflammatory activities during ALI in mice, sRAGE levels correlate positively with worse clinical outcomes in human ARDS patients (6). In this respect it is noteworthy that sRAGE forms also have proinflammatory activities. When mice with inflammatory arthritis were given intraperitoneal injections of sRAGE, joint inflammation was reduced; this was due to sRAGE redirecting the inflammatory response to the peritoneal cavity (17). In this model, sRAGE bound avidly to Mac-1 (CD11b/CD18) integrin on leukocytes to trigger NF-κB activation and secretion of proinflammatory mediators (17). sRAGE also directly stimulated PMN chemotaxis in vitro (17). Whether this proinflammatory sRAGE signaling also occurs in the lungs of ARDS patients needs to be addressed. Thus before RAGE or sRAGE shedding can be targeted as new therapeutic strategies for ARDS patients more studies addressing the biology and activities of RAGE and sRAGE are needed.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL063137 and HL086814.
DISCLOSURES
C. Owen received research funding for a Chronic Obstructive Pulmonary Disease project from Pulmatrix Inc. This project was not active when this editorial was written.
REFERENCES
- 1.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol 165: 2950–2954, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Albaiceta GM, Gutierrez-Fernandez A, Garcia-Prieto E, Puente XS, Parra D, Astudillo A, Campestre C, Cabrera S, Gonzalez-Lopez A, Fueyo A, Taboada F, Lopez-Otin C. Absence or inhibition of matrix metalloproteinase-8 decreases ventilator-induced lung injury. Am J Respir Cell Mol Biol 43: 555–563, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Albaiceta GM, Gutierrez-Fernandez A, Parra D, Astudillo A, Garcia-Prieto E, Taboada F, Fueyo A. Lack of matrix metalloproteinase-9 worsens ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 294: L535–L543, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Bastarache JA, Fremont RD, Kropski JA, Bossert FR, Ware LB. Procoagulant alveolar microparticles in the lungs of patients with acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 297: L1035–L1041, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briot R, Frank JA, Uchida T, Lee JW, Calfee CS, Matthay MA. Elevated levels of the receptor for advanced glycation end products, a marker of alveolar epithelial type I cell injury, predict impaired alveolar fluid clearance in isolated perfused human lungs. Chest 135: 269–275, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calfee CS, Ware LB, Eisner MD, Parsons PE, Thompson BT, Wickersham N, Matthay MA. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax 63: 1083–1089, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen MJ, Brohi K, Calfee CS, Rahn P, Chesebro BB, Christiaans SC, Carles M, Howard M, Pittet JF. Early release of high mobility group box nuclear protein 1 after severe trauma in humans: role of injury severity and tissue hypoperfusion. Crit Care 13: R174, 2009. doi: 1186/cc8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolinay T, Wu W, Kaminski N, Ifedigbo E, Kaynar AM, Szilasi M, Watkins SC, Ryter SW, Hoetzel A, Choi AM. Mitogen-activated protein kinases regulate susceptibility to ventilator-induced lung injury. PLoS ONE 3: e1601, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fligiel SE, Standiford T, Fligiel HM, Tashkin D, Strieter RM, Warner RL, Johnson KJ, Varani J. Matrix metalloproteinases and matrix metalloproteinase inhibitors in acute lung injury. Hum Pathol 37: 422–430, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Hanford LE, Enghild JJ, Valnickova Z, Petersen SV, Schaefer LM, Schaefer TM, Reinhart TA, Oury TD. Purification and characterization of mouse soluble receptor for advanced glycation end products (sRAGE). J Biol Chem 279: 50019–50024, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He M, Kubo H, Ishizawa K, Hegab AE, Yamamoto Y, Yamamoto H, Yamaya M. The role of the receptor for advanced glycation end-products in lung fibrosis. Am J Physiol Lung Cell Mol Physiol 293: L1427–L1436, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Suk MH, Yoon DW, Lee SH, Hur GY, Jung KH, Jeong HC, Lee SY, Lee SY, Suh IB, Shin C, Shim JJ, In KH, Yoo SH, Kang KH. Inhibition of matrix metalloproteinase-9 prevents neutrophilic inflammation in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 291: L580–L587, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 111: 635–646, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Lian X, Qin Y, Hossain SA, Yang L, White A, Xu H, Shipley JM, Li T, Senior RM, Du H, Yan C. Overexpression of Stat3C in pulmonary epithelium protects against hyperoxic lung injury. J Immunol 174: 7250–7256, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, Plachky J, Grone HJ, Kurschus FC, Schmidt AM, Yan SD, Martin E, Schleicher E, Stern DM, Hammerling GG, Nawroth PP, Arnold B. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest 113: 1641–1650, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nenan S, Planquois JM, Berna P, De MI, Hitier S, Shapiro SD, Boichot E, Lagente V, Bertrand CP. Analysis of the inflammatory response induced by rhMMP-12 catalytic domain instilled in mouse airways. Int Immunopharmacol 5: 511–524, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Pullerits R, Brisslert M, Jonsson IM, Tarkowski A. Soluble receptor for advanced glycation end products triggers a proinflammatory cytokine cascade via beta2 integrin Mac-1. Arthritis Rheum 54: 3898–3907, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Quintero PA, Knolle MD, Cala LF, Zhuang Y, Owen CA. Matrix metalloproteinase-8 inactivates macrophage inflammatory protein-1 alpha to reduce acute lung inflammation and injury in mice. J Immunol 184: 1575–1588, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sen AI, Shiomi T, Okada Y, D'Armiento JM. Deficiency of matrix metalloproteinase-13 increases inflammation after acute lung injury. Exp Lung Res 36: 615–624, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med 173: 1008–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warner RL, Beltran L, Younkin EM, Lewis CS, Weiss SJ, Varani J, Johnson KJ. Role of stromelysin 1 and gelatinase B in experimental acute lung injury. Am J Respir Cell Mol Biol 24: 537–544, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Warner RL, Lewis CS, Beltran L, Younkin EM, Varani J, Johnson KJ. The role of metalloelastase in immune complex-induced acute lung injury. Am J Pathol 158: 2139–2144, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamakawa N, Uchida T, Matthay M, Makita K. Proteolytic release of the receptor for advanced glycation end-products from in vitro and in situ alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol (January 21, 2011). doi:10.1152/ajplung.00118.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H, Tasaka S, Shiraishi Y, Fukunaga K, Yamada W, Seki H, Ogawa Y, Miyamoto K, Nakano Y, Hasegawa N, Miyasho T, Maruyama I, Ishizaka A. Role of soluble receptor for advanced glycation end products on endotoxin-induced lung injury. Am J Respir Crit Care Med 178: 356–362, 2008 [DOI] [PubMed] [Google Scholar]