Abstract
Inducible nitric oxide (NO) synthase (iNOS) is a stress response protein upregulated in inflammatory conditions, and NO may suppress cellular proliferation. We hypothesized that preventing l-arginine (l-arg) uptake in endothelial cells would prevent lipopolysaccharide/tumor necrosis factor-α (LPS/TNF)-induced, NO-mediated suppression of cellular proliferation. Bovine pulmonary arterial endothelial cells (bPAEC) were treated with LPS/TNF or vehicle (control), and either 10 mM l-leucine [l-leu; a competitive inhibitor of l-arg uptake by the cationic amino acid transporter (CAT)] or its vehicle. In parallel experiments, iNOS or arginase II were overexpressed in bPAEC using an adenoviral vector (AdiNOS or AdArgII, respectively). LPS/TNF treatment increased the expression of iNOS, arginase II, CAT-1, and CAT-2 mRNA in bPAEC, resulting in greater NO and urea production than in control bPAEC, which was prevented by l-leu. LPS/TNF treatment resulted in fewer viable cells than in controls, and LPS/TNF-stimulated bPAEC treated with l-leu had more viable cells than LPS/TNF treatment alone. LPS/TNF treatment resulted in cleaved caspase-3 and cleaved poly(ADP-ribose) polymerase expression, which was attenuated by l-leu. AdiNOS reduced viable cell number, and treatment of AdiNOS transfected bPAEC with l-leu preserved cell number. AdArgII increased viable cell number, and treatment of AdArgII transfected bPAEC with l-leu prevented the increase in cell number. These data demonstrate that iNOS expression in pulmonary endothelial cells leads to decreased cellular proliferation, which can be attenuated by preventing cellular l-arg uptake. We speculate that CAT activity may represent a novel therapeutic target in inflammatory lung diseases characterized by NO overproduction.
Keywords: l-arginine uptake, inducible nitric oxide synthase, arginase, ornithine
arginine is the substrate for many metabolic pathways in endothelial cells. These pathways include the nitric oxide (NO) synthases (NOS), arginase, arginine deiminase, arginine decarboxylase, arginine-glycine aminotransferase, as well as protein synthesis (19). The NOS and arginase metabolic pathways are perhaps the largest contributors to arginine metabolism in endothelial cells (20, 27). Inducible NOS (iNOS) is a stress response protein upregulated in response to inflammatory stimuli in endothelial cells. The relatively large amounts of NO produced by iNOS are vital in immune responses and increasing blood flow to inflamed areas. However, overproduction of NO by iNOS can lead to cytotoxicity and shock. Arginase metabolizes l-arginine to l-ornithine and urea, and endothelial cells express two isoforms: arginase I and arginase II (15, 20). The arginases are the first step in the generation of proline and polyamines, which are crucial for cell proliferation, and thus the induction of arginase is vital for tissue repair after injury (10, 31). Stimuli that lead to lung injury have been shown to upregulate arginase. For example, hyperoxia led to increased expression of arginase I and arginase II (24). We have demonstrated that treating bovine pulmonary arterial endothelial cells (bPAEC) with lipopolysaccharide and tumor necrosis factor-α (LPS/TNF) results in increased protein expression of both arginase I and arginase II (7).
Arginase and iNOS share a common substrate, l-arginine. It has been suggested that the coinduction of iNOS and arginase is a mechanism to limit NO production in macrophages, to avoid NO overproduction (5, 21). Our laboratory has recently found that LPS/TNF triggered the upregulation of iNOS and arginase protein levels in bPAEC, and that inhibiting iNOS increased urea production, while inhibiting arginase increased NO production (7). These data demonstrate that iNOS and arginase compete for their common substrate in cytokine-stimulated endothelial cells. l-Arginine is taken up by pulmonary endothelial cells via a family of proteins called the cationic amino acid transporters (CAT), of which CAT-1 and CAT-2 are expressed in endothelial cells and are encoded by two separate genes, SLC7A1 and SLC7A2, respectively (16). Our laboratory has previously shown in bPAEC that LPS/TNF treatment resulted in an increase in CAT-2 mRNA expression and an increase in NO production, and that exogenous l-arginine had a greater effect on NO and urea production in LPS/TNF-treated cells (20). It has also been shown that treatment of bPAEC with LPS results in increased apoptosis via increased caspase-3 activity (9). Furthermore, inflammation-induced caspase-3 activation in bPAEC has been found to be significantly attenuated by treatment with the NOS inhibitor, Nω-nitro-l-arginine methyl ester (28). Our laboratory has previously shown that overexpression of iNOS in bPAEC decreases cellular proliferation (27). Taken together, these data suggest that LPS leads to an increase in iNOS-derived NO production, which activates proapoptotic pathways, which would result in a decrease in viable cell numbers.
Thus understanding the regulation of l-arginine bioavailability to iNOS and arginase in inflammation is imperative in understanding the pathogenesis, as well as the development of novel therapeutic targets, for inflammatory lung diseases. We hypothesized that inflammatory stimuli would lead to an increase in both iNOS and CAT expression in endothelial cells, and that the suppression of cellular proliferation caused by iNOS-derived NO would be attenuated by inhibiting l-arginine uptake. We tested this hypothesis in bPAEC stimulated with LPS/TNF or vehicle. We utilized l-leucine as a competitive inhibitor of l-arginine uptake. To isolate iNOS-induced effects independent of LPS/TNF, we also studied cells in which iNOS was overexpressed using adenoviral vectors containing the human iNOS gene (AdiNOS). To further examine the role of l-arginine bioavailability to iNOS and arginase, we also overexpressed arginase II in bPAEC using adenoviral vector containing the arginase II gene (AdArgII).
METHODS
bPAEC culture.
bPAEC were cultured as previously described (7, 20, 27). Briefly, bPAEC were obtained from Lonza (Allendale, NJ). On arrival, bPAEC were placed in T25 flasks with 5 ml of endothelial growth media (EGM; Lonza). When the bPAEC were 80–90% confluent, the bPAEC were passaged using trypsin/EDTA, followed by trypsin neutralizing solution. The bPAEC were centrifuged at 1,200 g for 5 min, and the bPAEC pellet was resuspended in EGM. Nine milliliters of EGM were placed in a T75 flask, and then 1 ml of the resuspended bPAEC pellet was added, and the T75 flask was returned to the incubator at 37°C in 5% CO2, balance air. bPAEC between passages 3 and 8 were used for these studies.
On the day of study, the bPAEC were washed three times with 4 ml of HEPES balanced salt solution (HBSS; Lonza). Then 4 ml of EGM were placed on the cells (control), and the bPAEC were returned to the incubator at 37°C in 5% CO2, balance air for 24 h. In the LPS/TNF-treated bPAEC, 1.5 μg/ml LPS and 1.5 ng/ml TNF-α (both from Sigma Chemical, St. Louis, MO) were included in the EGM, as previously described (7, 20). After 24 h, the media was removed and stored at −80°C. The bPAEC were washed three times with 4 ml HBSS and lysed to either extract proteins or purify total RNA using Trizol (Life Technologies, Carlsbad, CA).
Protein isolation.
Protein was isolated from the bPAEC, as previously described (7, 20, 27). Briefly, cells were washed with HBSS, and lysis buffer (0.2 M NaOH, 0.2% SDS) was added. Thirty minutes before use, the following protease inhibitors were added to each milliliter of lysis buffer: 0.2 μl aprotinin (10 mg/ml double-distilled H2O), 0.5 μl leupeptin (10 mg/ml double-distilled H2O), 0.14 μl pepstatin A (5 mg/ml methanol), and 5 μl of phenylmethylsulfonyl fluoride (34.8 mg/ml methanol). The cells were scraped and placed in sterile centrifuge tubes on ice. The supernatant was stored in 1 ml tubes at −80°C for Western blot analysis. Total protein concentration was determined by the Bradford method using a commercially available assay (BioRad, Hercules, CA).
RNA isolation.
RNA was isolated from bPAEC, as previously described (5, 7). Briefly, Trizol (Life Technologies) was added to the cells and incubated for 5 min at room temperature. Chloroform (0.2 ml) was added, and the tubes were shaken for 15 s and then incubated at room temperature for 3 min. The mixture was centrifuged at 12,000 g for 15 min at 4°C. The supernatant was transferred to a fresh tube. Isopropyl alcohol (0.5 ml) was added, and the mixture incubated at room temperature for 10 min, then centrifuged at 12,000 g for 15 min at 4°C. The supernatant was discarded, and the pellet was washed with 75% ethanol and centrifuged at 7,500 g for 5 min at 4°C. The supernatant was discarded, and the pellet partially dried, dissolved in RNase free water, and stored at −80°C.
Nitrite assay.
The samples of medium were assayed in duplicate for nitrite (NO2−) using a chemiluminescence NO analyzer (model 280i, Sievers Instruments, Boulder, CO), as previously described (21, 27). Briefly, 100 μl of sample were placed in a reaction chamber containing a mixture of NaI in glacial acetic acid to reduce NO2− to NO. The NO gas was carried into the NO analyzer using a constant flow of helium gas. The analyzer was calibrated using a NaNO2 standard curve.
Urea assay.
The samples of medium were assayed in duplicate for urea colorimetrically as previously described (21, 27). Briefly, 100 μl of sample were added to 3 ml of chromogenic reagent [5 mg thiosemicarbazide, 250 mg diacetyl monoxime, 37.5 mg FeCl3 in 150 ml 25% (vol/vol) H2SO4, 20% (vol/vol) H3PO4], or the same reagents with 0.5 units urease were added. After 1 h at 37°C, the mixtures were vortexed and then boiled at 100°C for 5 min. The mixtures were cooled to room temperature, and the difference in absorbance (530 nm) with and without urease was determined and compared with a urea standard curve.
Western blotting.
Cell lysates were assayed for arginase I, arginase II, cleaved caspase-3, cleaved poly(ADP-ribose) polymerase (PARP), or iNOS protein using Western blot analysis, as previously described (5, 7, 27). Aliquots of cell lysate were diluted 1:1 with SDS sample buffer, heated to 80°C for 15 min and then centrifuged at 10,000 g at room temperature for 2 min. Aliquots of the supernatant were used for SDS-polyacrylamide gel electrophoresis. The proteins were transferred to polyvinylidene difluoride membranes, and blocked overnight in phosphate-buffered saline with 0.1% Tween (PBS-T) containing 5% nonfat dried milk and 3% albumin. Blots were incubated for 4 h at room temperature with an antibody against arginase I (1:1,000, Transduction Laboratories), arginase II (1:500; Santa Cruz Biotechnology, Santa Cruz, CA), cleaved caspase-3 (1:1,000, Cell Signaling Technology, Danvers, MA), cleaved PARP (1:1,000, Cell Signaling Technology), or iNOS (1:1,000 BD Transduction Laboratories, San Diego, CA). The blots were then washed with PBS-T with 1% nonfat dried milk. The membranes were then incubated with the biotinylated IgG secondary antibody (1:5,000; Vector Laboratories, Burlingame, CA) for 1 h, washed, and then incubated with streptavidin-horseradish peroxidase conjugate (1:1,500; BioRad) for 30 min. The bands of interest were visualized using enhanced chemiluminescence (Amersham, Piscataway, NJ) and quantified using densitometry (Sigma Gel, Jandel Scientific, San Rafael, CA). To control for protein loading, the blots were then stripped using a stripping buffer (each 100 ml contained 6.25 ml 1 M Tris·HCl, pH 6.8, 20 ml 10% SDS, 0.7 ml 2-β-mercaptoethanol, and 73 ml double-distilled H2O). The blots were reprobed for β-actin (1:10,000; Abcam, Cambridge, MA).
Reverse transcription-polymerase chain reaction.
Reverse transcription-polymerase chain reaction (RT-PCR) was performed as previously described (5,20). Briefly, 2 μg of total RNA were reversed transcribed in 2.5 μM dT16 (Applied Biosystems, Foster City, CA), 20 units avian myeloblastosis virus-reverse transcriptase, 1 mM dNTP, 1× buffer (Promega, Madison, WI), and balance RNase-free water, total volume 40 μl. The samples were incubated in a PCR-iCycler (BioRad) at 42°C for 60 min, 95°C for 5 min, and stored at −20°C. PCR reactions (total volume 50 μl) contained 5 μl of RT product, 1 mM MgCl2, 1.25 units AmpliTaqGold (Applied Biosystems, Foster City, CA), 0.2 mM dNTP (Promega, Madison, WI), and 15 μM forward and 15 μM reverse primer. iNOS was amplified using forward primer (5′-TCCAGAAGCAGAATGTGACC-3′) and reverse primer (5′-GGACCAGCCAAATCCAGT-3′). GAPDH was amplified using forward primer (5′-GAAGACTGTGGATGGCCCCTCC-3′) and reverse primer (5′-GTTGAGGGCAATGCCAGCCCC-3′). The mixed samples were heated to 94°C for 4 min and then cycled as follows: 94°C for 1 min, 53°C for 1 min, and 72°C for 2 min for 35 cycles for iNOS, and 27 cycles for GAPDH. The PCR products were sized by electrophoresis using 2.0% agarose gel electrophoresis and poststained with Syber Gold (Molecular Probes, Eugene, OR) for 30 min. The gels were scanned and densitized using a MultiGenius Bio Imaging System (Syngene, Frederick, MD).
Real-time PCR (quantitative PCR).
Quantitative real-time PCR (qPCR) was performed using the SYBR Green Jumpstart Taq Ready Mix (Bio-Rad, Hercules, CA) with the Chromo 4 Real-time PCR Detection System (Bio-Rad) with 40 cycles of real-time data collection using 95°C for 15 s, 55°C for 30 s, and 72°C for 30 s, followed by melting curve analysis to verify the presence of a single product. For each reaction, negative controls containing reaction mixture and primers without cDNA were performed to verify that primers and reaction mixtures were free of template contamination. Relative iNOS, endothelial NOS, arginase I, arginase II, CAT-1, and CAT-2 amounts were normalized to 18S expression using the ΔΔCT (threshold cycle) method. All samples were analyzed in duplicate. Data are shown as fold change relative to vehicle-treated bPAEC controls.
Proliferation assay.
The proliferation of bPAEC was determined in six-well plates, as previously described (27). Cultured bPAEC, 5 × 104 cells, were plated into each well of six-well plates. The appropriate treatments were included in the media, and the cells were incubated for a period of 24 or 48 h. At the end of the experimental protocol, the cells were removed from the incubator, and plates were washed three times with HBSS. After the final wash, 1 ml of trypsin was added to each well. The plates were incubated for ∼3 min, followed by the addition of 2 ml trypsin neutralizing solution. The cells from each well were placed in 15-ml conical tubes. The cells were centrifuged for 5 min at 1,220 g at 4°C. The supernatant was discarded, and the cells were resuspended in 1 ml of EGM. The cells were mixed 1:1 with Trypan blue, and viable cells were counted using a hemocytometer.
Adenoviral transfection.
Adenovirus, serotype 5, containing either the cDNA for Escherichia coli β-galactosidase and a CMV promoter (Adβ-gal), the cDNA for murine iNOS and a CMV promoter (AdiNOS), or the cDNA for human arginase II and a CMV promoter (AdArgII) were derived and prepared as previously described (27). bPAEC were transfected with either AdiNOS, AdArgII, or Adβ-gal; the latter was used as a control for nonspecific gene transfer effects. A cell count was performed on a single flask of bPAEC before the transfection. The amount of virus placed on each flask was determined by multiplying the number of bPAEC per T-75 flask by the multiplicity of infection (MOI), divided by the number of plaque-forming units per milliliter virus. For each flask, the required amount of viral stock was added to 4 ml EGM. bPAEC were incubated at 37°C in 5% CO2/95% air for 4 h. Then, the medium was removed, and the bPAEC were washed three times with HBSS. Four milliliters of the virus-containing medium to be used in the specific experimental protocols were placed on the bPAEC, and the bPAEC were incubated at 37°C in 5% CO2/95% air. After a 24-h incubation period, the medium was removed and frozen at −80°C in 1-ml aliquots. The bPAEC were harvested in cell lysis buffer for protein isolation.
Statistical analysis.
Values are expressed as means ± SE. One-way ANOVA was used to compare the densitometry data, the effect of the additives on either NO2− or urea production, or cellular proliferation, and significant differences were identified using a Neuman-Keuls post hoc test (SigmaStat, Jandel Scientific, Carlsbad, CA). Differences were considered significant when P < 0.05.
RESULTS
Effects of LPS/TNF on NOS and arginase.
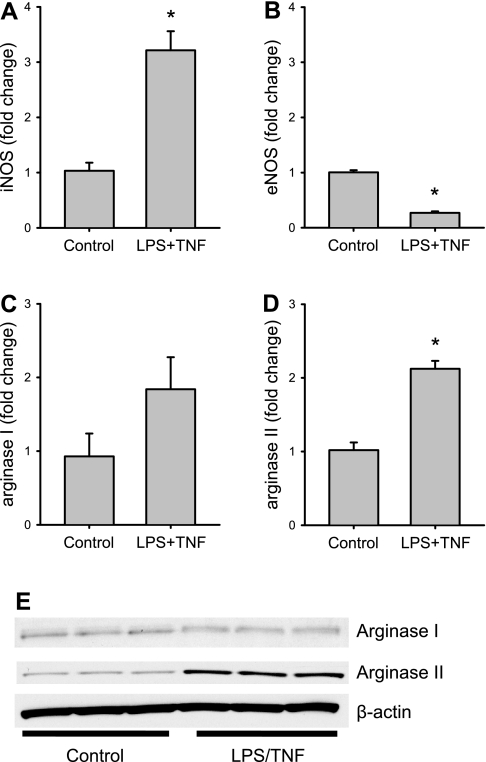
To determine the effects of LPS/TNF treatment on the expression of NOS and arginase, bPAEC were incubated for 24 h in the presence of either vehicle (control) or LPS/TNF. After 24 h, the cells were harvested for mRNA. Under the conditions of our study and our qPCR, treatment with LPS/TNF resulted in approximately threefold greater iNOS mRNA levels than that in control cells (Fig. 1A). LPS/TNF treatment of bPAEC caused lower endothelial NOS mRNA levels than in control cells (Fig. 1B). There was variability in arginase I mRNA levels in bPAEC, and LPS treatment had a variable effect on arginase I mRNA levels; the net effect was that there was no statistical difference in arginase I mRNA levels between control and LPS/TNF-treated bPAEC (Fig. 1C). Treatment of bPAEC resulted in a twofold greater arginase II mRNA expression than in control cells (Fig. 1D). Since there was a great deal of variability in the arginase I mRNA levels in bPAEC, we also measured protein levels of arginase I and arginase II using Western blotting on cell protein lysates from a separate set of experiments in bPAEC treated for 24 h with either vehicle (control) or LPS/TNF. Arginase I protein levels were not different between control and LPS/TNF-treated bPAEC (Fig. 1E). However, mirroring the arginase II mRNA results, arginase II protein expression was substantially greater in LPS/TNF-treated bPAEC than in control bPAEC (Fig. 1E).
Fig. 1.
Lipopolysaccharide/tumor necrosis factor-α (LPS/TNF) treatment induces inducible nitric oxide synthase (iNOS) and arginase II in bovine pulmonary arterial endothelial cells (bPAEC). bPAEC were incubated for 24 h in the presence of either vehicle or LPS/TNF. After 24 h, the cells were harvested for mRNA. A: under the conditions of our study, treatment with LPS/TNF resulted in a threefold increase in mRNA for iNOS. B: LPS/TNF treatment resulted in a decrease in endothelial nitric oxide synthase (eNOS) mRNA expression. n = 4 in each group. C: LPS/TNF treatment had no significant effect on arginase I mRNA expression. D: LPS/TNF treatment resulted in significantly greater arginase II mRNA levels. Values are means ± SE; n = 4 in each group. *LPS/TNF different from control, P < 0.005. E: Since there was a large variation in arginase I mRNA expression in these bPAEC, we performed Western blotting on cell protein lysates from a separate set of experiments in bPAEC treated for 24 h with either vehicle (control) or LPS/TNF. Mirroring the mRNA results, arginase I protein levels were not different between control and LPS/TNF-treated bPAEC, while arginase II protein expression was substantially greater in LPS/TNF-treated bPAEC than in control bPAEC.
Effects of LPS/TNF on CAT expression.
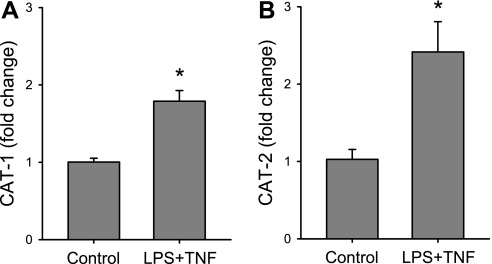
To determine whether LPS/TNF treatment also affected the expression of CAT-1 and/or CAT-2 in bPAEC, cells were incubated for 24 h in the presence of vehicle or LPS/TNF. The mRNA from the cells was harvested at 24 h for qPCR for CAT-1, CAT-2, and 18S rRNA. Treatment of bPAEC with LPS/TNF resulted in greater CAT-1 mRNA levels than those in control cells (Fig. 2A). Treatment with LPS/TNF resulted in substantially greater CAT-2 mRNA levels than those in vehicle-treated cells (Fig. 2B).
Fig. 2.
LPS/TNF treatment resulted in the induction of both cationic amino acid transporter (CAT)-1 and CAT-2 mRNA in bPAEC. The cells were incubated for 24 h in the presence of vehicle or LPS/TNF. The mRNA from the cells was harvested at 24 h for quantitative PCR for CAT-1, CAT-2, and 18S rRNA. A: CAT-1 mRNA levels in the LPS/TNF-treated bPAEC were approximately twofold greater than CAT-1 mRNA levels in control cells. *LPS/TNF different from control, P < 0.01. B: CAT-2 mRNA levels were significantly greater in LPS/TNF-treated bPAEC than in vehicle-treated cells (control). *LPS/TNF different from control, P < 0.05. Values are means ± SE; n = 4–5 in each group.
Effects of inhibiting l-arginine uptake on LPS/TNF-α-induced NO and urea production.
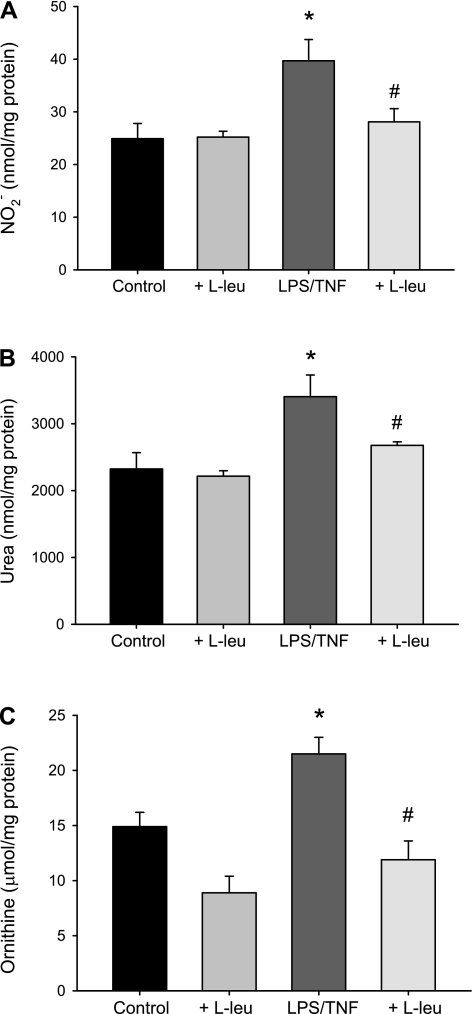
To determine the effect of competitive inhibition of l-arginine uptake (using l-leucine) on NO production, LPS/TNF-treated bPAEC were incubated with 10 mM l-leucine or vehicle. The media for these experiments also contained 1 mM l-arginine. Therefore, given that l-leucine is a competitive inhibitor of CAT-mediated l-arginine uptake, we used a 10-fold greater concentration. This concentration of l-leucine used here is pharmacological, and in humans plasma l-arginine and l-leucine levels are similar and affected by dietary intake. The fasting levels of arginine and leucine range from 15 to 150 μM. The 24-h production of NO2− was measured in the medium and normalized to the bPAEC protein concentration. Addition of l-leucine to the vehicle cells had little effect on NO2− (Fig. 3A). Treatment with LPS/TNF resulted in an approximately twofold greater NO production than in vehicle-treated bPAEC (Fig. 3A). l-Leucine added to the media prevented the LPS/TNF-induced increase in NO production in bPAEC (Fig. 3A). This suggests that LPS/TNF-stimulated NO production requires extracellular l-arginine.
Fig. 3.
Inhibiting l-arginine uptake prevented the LPS/TNF-induced increases in nitric oxide, urea, and ornithine production in bPAEC. bPAEC were treated with vehicle or LPS/TNF, and either no added l-leucine (l-leu) or 10 mM l-leu for 24 h, and the media was collected. A: LPS/TNF treatment resulted in significantly greater nitrite (NO2−) production than in the vehicle-treated control bPAEC, and the addition of l-leu prevented the LPS/TNF-increase in NO2− production. Treatment of vehicle control bPAEC with l-leu had little effect on NO2−. B: LPS/TNF treatment resulted in significantly greater urea production than in the vehicle-treated control bPAEC, and the addition of l-leu prevented the LPS/TNF-increase in urea production. Treatment of vehicle control bPAEC with l-leu had little effect on urea production. C: LPS/TNF treatment resulted in significantly greater ornithine production than in the vehicle-treated control bPAEC, and the addition of l-leu prevented the LPS/TNF increase in ornithine production. Values are means ± SE; n = 5–10 in each group. *LPS/TNF different from control, P < 0.01. #LPS/TNF + l-leu different from LPS/TNF alone, P < 0.05.
To examine the effect of inhibiting l-arginine uptake on LPS/TNF-induced arginase activity, the following study was done. The bPAEC were treated with vehicle or LPS/TNF and either no added l-leucine or 10 mM l-leucine for 24 h, and the media was collected for urea and ornithine determinations. Again, addition of l-leucine to the vehicle-treated bPAEC had little effect on urea or ornithine production (Fig. 3, B and C). LPS/TNF treatment resulted in significantly greater urea and ornithine production than in the vehicle-treated control bPAEC (Fig. 3, B and C). Inhibiting l-arginine uptake using l-leucine prevented the LPS/TNF increase in urea and ornithine (Fig. 3, B and C). Similar to the results with NO production, these results suggest that LPS/TNF-stimulated arginase activity requires extracellular l-arginine.
Effects of inhibiting l-arginine uptake on cell viability.
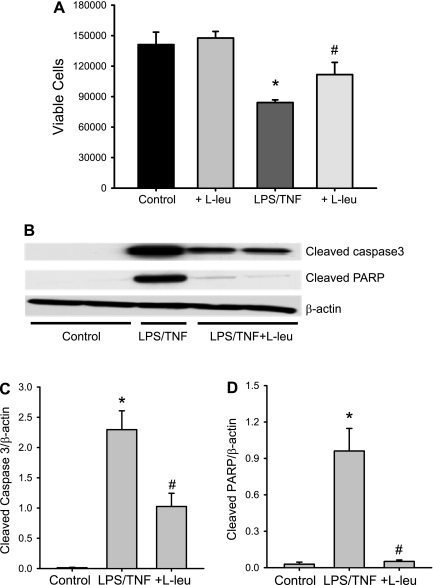
bPAEC were treated with vehicle or LPS/TNF and either no added l-leucine or 10 mM l-leucine for 24 h. The bPAEC were harvested, and viable cells were counted using Trypan blue exclusion. LPS/TNF treatment resulted in fewer viable bPAEC than in vehicle-treated controls (Fig. 4). The addition of 10 mM l-leucine to vehicle-treated bPAEC had no apparent effect on viable cell numbers (Fig. 4). However, the addition of 10 mM l-leucine to the LPS/TNF-treated bPAEC resulted in significantly more viable cells than in bPAEC treated with LPS/TNF alone (Fig. 4).
Fig. 4.
LPS/TNF treatment suppressed proliferation in bPAEC, and inhibiting l-arginine uptake attenuated the LPS/TNF-induced suppression of proliferation. A: bPAEC were treated with vehicle (control) or LPS/TNF and either no added l-leu or 10 mM l-leu for 24 h. The bPAEC were harvested, and viable cells were counted using Trypan blue exclusion. LPS/TNF treatment resulted in fewer viable bPAEC than in vehicle-treated bPAEC (control). The addition of 10 mM l-leu to vehicle-treated bPAEC had no apparent effect on viable cell numbers. However, the addition of 10 mM l-leu to the LPS/TNF-treated bPAEC resulted in significantly more viable cells than in bPAEC treated with LPS/TNF alone. Values are means ± SE; n = 5–6 in each group. *LPS/TNF different from control, P < 0.005. #LPS/TNF + l-leu different from TNF/LPS alone, P = 0.049. B: LPS/TNF treatment decreased viable cell number in part by increasing apoptosis. Cells were treated with vehicle (control), LPS/TNF, or LPS/TNF + l-leu (10 mM) for 24 h, and then protein was harvested for detection of cleaved poly(ADP-ribose) polymerase (PARP) and cleaved caspase-3 by Western blotting. A representative blot is shown in B. The first two lanes are from control with little cleaved PARP or caspase-3 expression, the middle lane is from LPS/TNF with substantial bands for both cleaved PARP and caspase-3, and the last two lanes are from LPS/TNF + l-leu-treated cells, and there is a substantial attenuation of both the cleaved PARP and cleaved caspase-3 bands. The β-actin is a protein loading control and demonstrates equal protein loading of the gels. C: the densitometry results for the cleaved caspase-3 Western blots normalized to β-actin. Values are means ± SE; n = 3–4 in each group. *LPS/TNF different from control, P < 0.001. #LPS/TNF + l-leu different from LPS/TNF alone, P < 0.005. D: the densitometry results for cleaved PARP Western blots normalized to β-actin. Values are means ± SE; n = 3–4 in each group. *LPS/TNF different from control, P < 0.05. #LPS/TNF + l-leu different from LPS/TNF alone, P < 0.05.
To determine the role for apoptosis in the LPS/TNF-induced decrease in viable cell numbers, the following experiment was done. The bPAEC were again treated with vehicle or LPS/TNF, and the LPS/TNF-treated cells had either no added l-leucine or 10 mM l-leucine added for the 24-h incubation period. The bPAEC were then harvested for protein, and Western blotting was done for the markers of apoptosis, cleaved caspase-3, and cleaved PARP. Under the conditions of our experiment and Western blotting, there was little if any detectable cleaved caspase-3 or cleaved PARP in the vehicle-treated bPAEC (Fig. 4B). Treatment of bPAEC with LPS/TNF resulted in readily detectable bands for cleaved caspase-3, while adding 10 mM l-leucine to the bPAEC significantly attenuated the cleaved caspase-3 protein expression (Fig. 4C). LPS/TNF treatment of bPAEC resulted in significantly greater cleaved PARP protein levels than in control cells, and addition of 10 mM l-leucine to the LPS/TNF treatment prevented the LPS/TNF-induced increase in cleaved PARP protein expression (Fig. 4D).
The role of arginine uptake in AdiNOS-induced alterations in viable cell number.
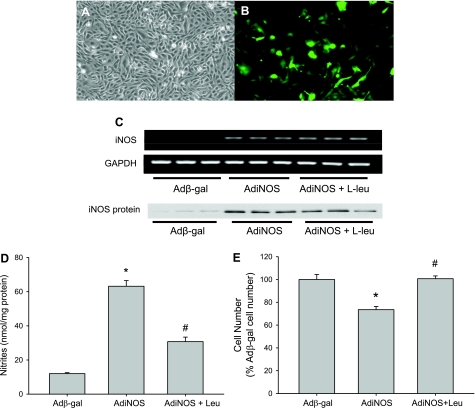
To isolate effects of iNOS alone, without cytokine-induced effects on CAT or arginase, we studied bPAEC wherein iNOS was overexpressed using AdiNOS. The adenoviral vector also contained the gene for green fluorescent protein (Fig. 5, A and B). The transfection efficiency in the bPAEC was dependent on the concentration of AdiNOS added to the media, as evidenced by an increasing numbers of green cells with increasing MOI (with an MOI of 0.1, there were 3 ± 0.2% green cells; with an MOI of 1, there were 26 ± 4%; and with an MOI of 10, there were 57 ± 6%). An MOI of 10 was used for subsequent studies. Transfection of bPAEC with a control Adβ-gal did not result in detectable iNOS mRNA bands on RT-PCR, while transfection of bPAEC with AdiNOS resulted in easily detectable iNOS mRNA bands (Fig. 5C). When l-leucine was added to the media, there was no effect on the AdiNOS-induced iNOS mRNA expression (Fig. 5C). Similarly, Adβ-gal-transfected cells had very low levels of iNOS protein, whereas AdiNOS-transfected cells had substantial iNOS protein expression, which was unaffected by adding l-leucine to the media (Fig. 5C). Transfection of bPAEC with AdiNOS resulted in a significantly greater NO production than in Adβ-gal-transfected bPAEC (Fig. 5D). The addition of 10 mM l-leucine to the media significantly attenuated NO production in AdiNOS-transfected bPAEC (Fig. 5D). Transfection of bPAEC with AdiNOS resulted in fewer viable cells than in Adβ-gal-transfected cells (Fig. 5E). Inhibiting l-arginine uptake with l-leucine resulted in viable cell numbers in AdiNOS-transfected bPAEC that were not different from the Adβ-gal-transfected cells (Fig. 5E).
Fig. 5.
AdiNOS (adenoviral vectors containing the human iNOS gene)-induced iNOS overexpression in bPAEC and arginine uptake was necessary for AdiNOS-induced nitric oxide production and changes in cellular proliferation. To isolate effects of iNOS alone, without cytokine-induced effects on CAT or arginase, we studied bPAEC, wherein iNOS was overexpressed using AdiNOS. The adenoviral vector also contained the gene for green fluorescent protein. The transfection efficiency in the bPAEC was good, as can be seen in the two examples: A is phase contrast, and B is fluorescence. C: transfection of bPAEC with Adβ-gal did not result in detectable iNOS mRNA bands on RT-PCR, while transfection of bPAEC with AdiNOS resulted in easily detectable iNOS mRNA bands. When 10 mM l-leu was added to the media, there was no effect on the AdiNOS-induced iNOS mRNA expression. Transfection with AdiNOS resulted in readily detectable iNOS protein bands on Western blotting, and l-leu had no effect on AdiNOS-mediated iNOS protein expression. D: transfection of bPAEC with AdiNOS resulted in a significantly greater NO production than in Adβ-gal-transfected bPAEC. The addition of 10 mM l-leu (Leu) to the media significantly attenuated NO production in AdiNOS-transfected bPAEC. Values are means ± SE; n = 6 for each group. *AdiNOS different from Adβ-gal, P < 0.01. #AdiNOS + Leu different from AdiNOS, P < 0.01. E: transfection of bPAEC with AdiNOS reduced viable cell numbers compared with transfection with Adβ-gal. Inhibiting l-arginine uptake with l-leu returned cellular numbers in AdiNOS-transfected bPAEC to those seen in Adβ-gal-transfected bPAEC. The number of viable bPAEC were counted after a 48-h incubation. Values are means ± SE; n = 4–6 for each group. *AdiNOS viable cell number different from Adβ-gal viable cell number, P < 0.01. #AdiNOS + Leu different from AdiNOS alone, P < 0.01.
The role of arginine uptake in AdArgII-induced alterations in viable cell number.
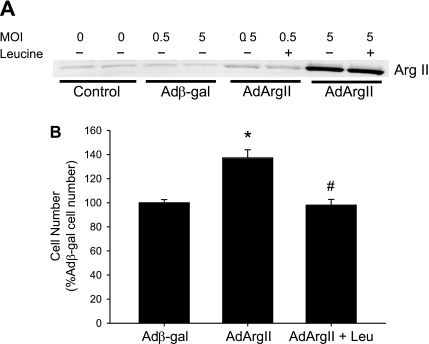
To isolate effects of arginase alone, without cytokine-induced effects on CAT or iNOS, we studied bPAEC, wherein arginase II was overexpressed using AdArgII. Expression of arginase II in transfected bPAEC was dependent on the concentration of AdArgII added to the media, as evidenced by an increasing arginase II protein band on Western blotting (Fig. 6A). An MOI of 5 was used for subsequent studies. Transfection with Adβ-gal or addition of l-leucine to the media had no effect on arginase II protein levels (Fig. 6A). Transfection of bPAEC with AdArgII resulted in more viable cells than in Adβ-gal-transfected bPAEC (Fig. 6B). Inhibiting l-arginine uptake with l-leucine resulted in viable cell numbers in AdArgII-transfected bPAEC that were not different from the Adβ-gal-transfected cells (Fig. 6B).
Fig. 6.
Transfection of bPAEC with an adenoviral vector containing the arginase II gene (AdArgII) resulted in more viable cells after a 24-h incubation. A: transfection with AdArgII resulted in a dose-dependent increase in arginase II protein expression in bPAEC. The cells were either not transfected, or transfected with Adβ-gal or AdArgII at either 0.5 or 5 multiplicity of infection (MOI), and 24-h later the protein was harvested for Western blotting for arginase II. AdArgII transfection resulted in a dose-dependent increase in arginase II protein expression, and l-leu had no effect on arginase II protein expression following AdArgII transfection. B: transfection with AdArgII resulted in more viable cells after a 24-h incubation than in Adβ-gal-transfected bPAEC. Inhibiting arginine uptake using pharmacological doses of l-leu prevented the AdArgII-induced increase in viable cell number. Values are means ± SE; n = 4–6 in each group. *AdArgII transfected different from Adβ-gal transfected, P < 0.001. #AdArgII + Leu different from AdArgII, P < 0.001.
DISCUSSION
The main findings of this study in bPAEC were that: 1) LPS/TNF treatment increased iNOS, arginase II, CAT-1, and CAT-2 expression; 2) LPS/TNF treatment resulted in greater NO and urea production; 3) inhibiting l-arginine uptake attenuated LPS/TNF-induced NO and urea production; 4) inhibiting l-arginine uptake improved viable cell numbers in LPS/TNF-treated bPAEC; 5) LPS/TNF resulted in substantial expression of cleaved caspase-3 and cleaved PARP protein expression that was inhibited by inhibiting l-arginine uptake; 6) inhibiting l-arginine uptake in bPAEC overexpressing iNOS decreased NO production and improved viable cell numbers; and 7) inhibiting l-arginine uptake in bPAEC overexpressing arginase II decreased viable cell numbers. These results support our hypothesis that the suppression of cellular proliferation caused by iNOS-derived NO in bPAEC can be attenuated by inhibiting l-arginine uptake.
We found that treating bPAEC with LPS/TNF resulted in iNOS, arginase II, CAT-1, and CAT-2 expression and activity. This suggests that the inflammatory pathways activated by LPS/TNF result in induction of genes necessary for upregulating and maintaining arginine metabolism in bPAEC. Similar findings have been described in saphenous vein endothelial cells (29) and in the glomerulus (26). The inflammatory pathways involved are not entirely clear. We have recently demonstrated a role for the Src family tyrosine kinases in the LPS/TNF-induced upregulation of iNOS and arginase II mRNA, as well as NO and urea production in bPAEC (5). Taken together, these findings suggest that upregulation of arginine metabolism by NOS and arginase following LPS and/or cytokines is associated with upregulation of the CAT, which likely represents a mechanism to maintain l-arginine bioavailability to iNOS and arginase in a variety of endothelial cells from various organs.
We found that the LPS/TNF treatment resulted in a decrease in viable cell number in bPAEC. This finding is consistent with studies in PAEC from fetal lambs (25). The exact mechanisms leading to decreased viable cell number following cytokine stimulation are probably multiple; however, iNOS-derived NO production is likely involved (1, 18, 30). Consistent with this concept, it has been found in cell culture experiments that inflammatory stimuli result in iNOS expression, increased NO production, and decreased cellular proliferation, and that all of these effects can be prevented by pharmacological inhibition of iNOS (11–13). Our laboratory (27) and others (8) have previously found that gene transfer of iNOS into endothelial cells leads to decreased cellular proliferation (27). Our laboratory also previously reported that gene transfer with iNOS leads to decreased arginase activity due to competition between overexpressed iNOS and endogenous arginase for their common substrate l-arginine (27). Consistent with this concept was our finding that overexpression of arginase II in bPAEC resulted in greater numbers of viable cells. Interestingly, Ignarro et al. (11) found that, in rat aortic smooth muscle cells, NO donors inhibited ornithine decarboxylase, the enzyme that catalyzes the conversion of ornithine to putrescine, the first step in polyamine synthesis, and that the effects of NO donors on cell proliferation could be prevented by adding putrescine, but not ornithine, to the media. It should also be pointed out that, in smooth muscle cells treated with NO donors, the NO-induced decrease in cellular proliferation was associated with extracellular-regulated kinase-1/2-mediated induction and nuclear localization of the cyclin-dependent kinase inhibitor, p21waf1/cip1, which is known to regulate cell cycle progression (2, 14, 18, 30). Thus it may be that iNOS-derived NO production suppresses cellular proliferation through three distinct mechanisms: 1) competition with arginase for substrate-limiting ornithine production; 2) NO directly inhibiting ornithine decarboxylase activity and thereby limiting polyamine production; and 3) activation of extracellular-regulated kinase-1/2, resulting in increased expression of p21waf1/cip1 and inhibiting progression through the cell cycle.
We have found that the competitive inhibition of l-arginine uptake prevented the LPS/TNF-induced increases in NO, urea, and ornithine production by bPAEC. This strongly suggests that cytokine-induced NOS and arginase activity depends on the uptake of extracellular l-arginine. Our laboratory has previously found that the extracellular concentration of l-arginine affects NO and urea production in cytokine-stimulated bPAEC (20). Furthermore, in isolated, perfused lungs from LPS-treated neonatal pigs, we found that competitive inhibition of l-arginine uptake decreased lung NO production (3). Interferon-γ/LPS-stimulated macrophages (22) or astrocytes (17) from CAT-2-deficient mice had ∼95% less l-arginine uptake and ∼90% less NO production than similarly stimulated cells from wild-type mice. The key finding of our study was that inhibiting l-arginine uptake using l-leucine prevented the LPS/TNF-induced increase in NO and urea production and improved viable cell numbers in LPS/TNF-treated endothelial cells. The improvement in viable cell numbers in LPS/TNF-stimulated cells treated with l-leucine is at least in part due to inhibition of iNOS-mediated NO production. Furthermore, LPS/TNF resulted in substantial expression of cleaved caspase-3 and cleaved PARP in bPAEC. Thus LPS/TNF not only suppressed proliferation, but affected viable cell numbers by increasing apoptosis. LPS treatment of endothelial cells has also been shown to cause apoptosis through upregulation of caspase-3 via activation of the MAPKs, particularly c-Jun NH2-terminal kinase (6). Thus there are likely iNOS-derived, NO-mediated effects on cell viability, as well as other direct effects of LPS signaling on the apoptotic pathways that underlie the suppression of cellular proliferation caused by LPS/TNF treatment of bPAEC. Of note, inhibiting l-arginine uptake significantly attenuated the LPS/TNF-induced increase in cleaved caspase-3 and completely prevented the LPS/TNF-induced increase in cleaved PARP. Recently, Xia et al. (32) found that TNF-α-induced apoptosis was increased in human umbilical vein endothelial cells by l-arginine supplementation. These results support the conclusion that l-arginine uptake by the CAT is necessary for LPS/TNF-induced apoptosis in bPAEC.
In conclusion, we found that LPS/TNF treatment of bPAEC results in suppressed cellular proliferation. The LPS/TNF treatment also resulted in induction of iNOS, arginase II, CAT-1, and CAT-2, leading to increased NO and urea production that depended on the uptake of extracellular l-arginine. Inhibiting l-arginine uptake attenuated the LPS/TNF-induced decrease in viable cell numbers. At least a part of the suppression of cellular proliferation caused by LPS/TNF was due to activation of apoptotic pathways. Overexpressing iNOS in bPAEC resulted in decreased viable cell numbers, while overexpression of arginase II in bPAEC resulted in greater viable cell numbers, and importantly both effects depended on the uptake of extracellular l-arginine. We speculate that CAT-mediated l-arginine uptake may be a novel therapeutic target to manipulate endothelial cell proliferation and/or apoptosis in the lung.
GRANTS
This study was supported by Grant R01HL075261 from the National Heart, Lung, and Blood Institute.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
REFERENCES
- 1. Ambalavanan N, Mariani G, Bulger A, Philips JB., III Role of nitric oxide in regulating neonatal porcine pulmonary artery smooth muscle cell proliferation. Biol Neonate 76: 291–300, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Bauer PM, Buga GM, Ignarro LJ. Role of p42/p44 mitogen-activated protein kinase and p21waf1/cip1 in the regulation of vascular smooth muscle cell proliferation by nitric oxide. Proc Natl Acad Sci USA 98: 12802–12807, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carter BW, Chicoine LG, Nelin LD. l-Lysine decreases nitric oxide production and increases vascular resistance in lungs isolated from lipopolysaccharide treated neonatal pigs. Pediatr Res 55: 979–987, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Chang IC, Zoghi B, Liao JC, Kuo L. The involvement of tyrosine kinases, cyclic AMP/protein kinase A, and p38 mitogen-activated protein kinase in IL-13-mediated arginase I induction in macrophages: its implications in IL-13-inhibited nitric oxide production. J Immunol 165: 2134–2141, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Chang R, Chicoine LG, Cui H, Kanagy NL, Walker BR, Liu Y, English BK, Nelin LD. Cytokine-induced arginase activity in pulmonary endothelial cells is dependent on Src family tyrosine kinase activity. Am J Physiol Lung Cell Mol Physiol 295: L688–L697, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaudhury H, Zakkar M, Boyle J, Cuhlmann S, van der Heiden K, Luong LA, Davis J, Platt A, Mason JC, Krams R, Haskard DO, Clark AR, Evans PC. c-jun N-terminal kinase primes endothelial cells at atheroprone sites for apoptosis. Arterioscler Thromb Vasc Biol 30: 546–553, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Chicoine LG, Paffett ML, Young TL, Nelin LD. Arginase inhibition increases nitric oxide production in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 287: L60–L68, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Cooney R, Hynes SO, Duffy AM, Sharif F, O′Brien T. Adenoviral-mediated gene transfer of nitric oxide synthase isoforms and vascular cell proliferation. J Vasc Res 43: 462–472, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Dhanasedaran A, Bodiga S, Gruenloh S, Gao Y, Dunn L, Falck JR, Buonaccorsi JN, Medhora M, Jacobs ER. 20-HETE increases survival and decreases apoptosis in pulmonary arteries and pulmonary artery endothelial cells. Am J Physiol Heart Circ Physiol 296: H777–H786, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoet PHM, Nemery B. Polyamines in the lung: polyamine uptake and polyamine-linked pathological and toxicological conditions. Am J Physiol Lung Cell Mol Physiol 278: L417–L433, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Ignarro LJ, Buga GM, Wei LH, Bauer PM, Wu G, del Soldato P. Role of the arginine-nitric oxide pathway in the regulation of vascular smooth muscle cell proliferation. Proc Natl Acad Sci USA 98: 4202–4208, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jin H, Liu Q, Cao X, Wu Z, Zhang G, Zhang M, Sha Z. Dysfunction of microvascular endothelial cells induced by tumor necrosis factor: cellular and molecular mechanisms. Clin Hemorheol Microcirc 23: 109–112, 2000 [PubMed] [Google Scholar]
- 13. Jourdan KB, Evans TW, Lamb NJ, Goldstraw P, Mitchell JA. Autocrine function of inducible nitric oxide synthase and cyclooxygenase-2 in proliferation of human and rat pulmonary artery smooth-muscle cells: species variation. Am J Respir Cell Mol Biol 21: 105–110, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Kibbe MR, Li J, Nie S, Watkins SC, Lizonova A, Kovesdi I, Simmons RL, Billiar TR, Tzeng E. Inducible nitric oxide synthase expression upregulates p21 and inhibits vascular smooth muscle cell proliferation through p42/p44 mitogen-activated protein kinase activation and independent of p53 and cyclic guanosine monophosphate. J Vasc Surg 31: 1214–1228, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Li H, Meininger CJ, Hawker JR, Haynes TE, Kepka-Lenhart D, Mistry SK, Morris SM, Wu G. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab 280: E75–E82, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Mann GE, Yudilevich DL, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev 83: 183–252, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Manner CK, Nicholson B, MacLeod CL. CAT2 arginine transporter deficiency significantly reduces iNOS-mediated NO production in astrocytes. J Neurochem 85: 476–482, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Mizuno S, Kadowaki M, Demura Y, Ameshima S, Miyamori I, Ishizaki T. p42/44 Mitogen-activated protein kinase regulated by p53 and nitric oxide in human pulmonary arterial smooth muscle cells. Am J Respir Cell Mol Biol 31: 184–192, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Morris SM. Enzymes of arginine metabolism. J Nutr 134: 2743S–2747S, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Nelin LD, Nash HE, Chicoine LG. Cytokine treatment increases arginine metabolism and uptake in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 281: L1232–L1239, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Nelin LD, Wang X, Zhao Q, Chicoine LG, Young TL, Hatch DM, English BK, Liu Y. MKP-1 switches arginine metabolism from nitric oxide synthase to arginase following endotoxin challenge. Am J Physiol Cell Physiol 293: C632–C640, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Nicholson B, Manner CK, Kleeman J, MacLeod CL. Sustained nitric oxide production in macrophages requires the arginine transporter CAT2. J Biol Chem 276: 15881–15885, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Nicholson B, Manner CK, MacLeod CL. Cat2 l-arginine transporter-deficient fibroblasts can sustain nitric oxide production. Nitric Oxide 7: 236–243, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Que LG, Kantrow SP, Jenkinson CP, Piantadosi CA, Huang YC. Induction of arginase isoforms in the lung during hyperoxia. Am J Physiol Lung Cell Mol Physiol 275: L96–L102, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Sampath V, Radish AC, Eis AL, Broniowska K, Hogg N, Konduri GG. Attenuation of lipopolysaccharide-induced oxidative stress and apoptosis in fetal pulmonary artery endothelial cells by hypoxia. Free Radic Biol Med 46: 663–671, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schwartz D, Schwartz IF, Gnessin E, Wollman Y, Chernichovsky T, Blum M, Iaina A. Differential regulation of glomerular arginine transporters (CAT-1 and CAT-2) in lipopolysaccharide-treated rats. Am J Physiol Renal Physiol 284: F788–F795, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Stanley KP, Chicoine LG, Young TL, Reber KM, Lyons CR, Liu Y, Nelin LD. Gene transfer with inducible nitric oxide synthase decreases production of urea by arginase in pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 290: L298–L306, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Sylte MJ, Inzana TJ, Czuprynski CJ. Reactive oxygen and nitrogen intermediates contribute to Haemophilus somnus lipooligosaccharide-mediated apoptosis of bovine endothelial cells. Vet Immunol Immunopathol 97: 207–217, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Visigalli R, Bussolati O, Sala R, Barilli A, Rotoli BM, Parolari A, Alamanni F, Gazzola GC, Dall'Asta V. The stimulation of arginine transport by TNF alpha in human endothelial cells depends on NF-kappa B activation. Biochim Biophys Acta 1664: 45–52, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Wedgwood S, Black SM. Molecular mechanisms of nitric oxide-induced growth arrest and apoptosis in fetal pulmonary arterial smooth muscle cells. Nitric Oxide 9: 201–210, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Witte MB, Barbul A. Arginine physiology and its implications for wound healing. Wound Repair Regen 11: 419–423, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Xia Z, Luo T, Liu H, Wang F, Xia Z, Irwin M, Vanhoutte P. l-Arginine enhances nitrative stress and exacerabates tumor necrosis factor-α toxicity to human endothelial cells in culture: Prevention by propofol. J Cardiovasc Pharmacol 55: 358–367, 2010 [DOI] [PubMed] [Google Scholar]