Abstract
Fibroblast growth factor-23 (FGF23) is a hormone that modulates circulating phosphate (Pi) levels by controlling Pi reabsorption from the kidneys. When FGF23 levels are deficient, as in tumoral calcinosis patients, hyperphosphatemia ensues. We show here in a murine model that Fgf23 ablation disrupted morphology and protein expression within the dentoalveolar complex. Ectopic matrix formation in pulp chambers, odontoblast layer disruption, narrowing of periodontal ligament space, and alteration of cementum structure were observed in histological and electron microscopy sections. Because serum Pi levels are dramatically elevated in Fgf23−−, we assayed for apoptosis and expression of members from the small integrin-binding ligand, N-linked glycoprotein (SIBLING) family, both of which are sensitive to elevated Pi in vitro. Unlike X-linked hypophosphatemic (Hyp) and wild-type (WT) specimens, numerous apoptotic osteocytes and osteoblasts were detected in Fgf23−/− specimens. Further, in comparison to Hyp and WT samples, decreased bone sialoprotein and elevated dentin matrix protein-1 protein levels were observed in cementum of Fgf23−/− mice. Additional dentin-associated proteins, such as dentin sialoprotein and dentin phosphoprotein, exhibited altered localization in both Fgf23−/− and Hyp samples. Based on these results, we propose that FGF23 and (Pi) homeostasis play a significant role in maintenance of the dentoalveolar complex.
INTRODUCTION
Disruptions in serum phosphate (Pi) levels lead to skeletal and tooth abnormalities, which are evident in autosomal recessive hypophosphatemic rickets (ARHR) and X linked hypophosphatemic rickets (XLH) patients (Feng et al., 2006; Hardy et al., 1989; Pereira et al., 2004). Along with low Pi levels, these patients and murine homologues exhibit osteomalacia, expanded alveolar bone, increased predentin/dentin ratio, interglobular dentin, and enlarged pulp chambers (Abe et al., 1989; Feng et al., 2006; Liu et al., 2006; Murayama et al., 2000; Ye et al., 2004). The skeletal and tooth abnormalities associated with low Pi have been associated with an excess of serum fibroblast growth factor 23 (FGF23), a hormone discovered in patients with tumor induced osteomalacia (TIO), another Pi wasting disorder (ADHR_Consortium, 2000; Liu et al., 2006; Liu et al., 2008; Lorenz-Depiereux et al., 2006; Shimada et al., 2001; Sitara et al., 2004). FGF23 production has been localized predominantly to osteocytes, with lower levels noted in osteoblasts and cementoblasts (Quarles, 2003; Riminucci et al., 2003; Yoshiko et al., 2007).
FGF23 is also referred to as a phosphatonin because it decreases circulating Pi, joining ranks with classical hormonal Pi and calcium regulators 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) and parathyroid hormone (PTH). FGF23 reduces Pi by decreasing intestinal absorption via the sodium Pi transporter Npt2b, decreasing renal reabsorption via sodium Pi transporters Npt2a and Npt2c, as well as inhibition of 25-hydroxyvitamin D-1-α-hydroxylase (Jurutka et al., 2007; Larsson et al., 2004). 25-hydroxyvitamin D-1-α-hydroxylase is necessary for 1,25(OH)2D3 activation, which, when suppressed, indirectly promotes Npt2a expression as FGF23 levels are lowered and PTH levels are increased(Sitara et al., 2006). Posttranslational modification is required for secretion of full-length FGF23. The enzyme uridine diphosphate-N-acetyl-α–D-galactosamine: polypeptide N-acetylgalactosaminyltransferase 3 (GALNT3) O-glycosylates FGF23, protecting it from proteolytic degradation (Ichikawa et al., 2007a; Ichikawa et al., 2009; Topaz et al., 2004). Further, in order for FGF23 to function, there is evidence that Klotho, a transmembrane protein expressed in the kidney, parathyroid, pituitary gland, and choroid plexus (Liu and Quarles, 2007; Xiao et al., 2004) is required to interact with cognate FGF receptors on cell surfaces and exert bioactivity on specific tissues (Memon et al., 2008; Nakatani et al., 2008). In situations where FGF23 function is diminished or ablated, hyperphosphatemia ensues as a consequence of excess Pi reabsorption. For example, one family of hyperphosphatemic disorders is hyperphosphatemic familial tumoral calcinosis (TC), which is subdivided into three types (Bergwitz and Juppner, 2009). Type I patients have deficient levels of full-length FGF23as a result of GALNT3 mutations (Garringer et al., 2007; Ichikawa et al., 2007a; Ichikawa et al., 2009; Topaz et al., 2004). Type II TC patients also have low full-length FGF23 levels, but these are attributed to mutations in FGF23 independent of GALNT3 function (Benet-Pages et al., 2005; Ichikawa et al., 2005; Masi et al., 2009). Type III TC patients have high levels of FGF23, but Klotho function is ablated, preventing FGF23 from functioning normally (Ichikawa et al., 2007b).
Research directed at elucidating the effects of systemic Pi dysregulation on mineralized tissues have been greatly aided by the use of homologous murine models associated with hypophosphatemia, i.e. ARHR (Dmp1-null mice), XLH (Hyp mouse), and hyperphosphatemia, i.e. TC (Fgf23−/−, Klotho−/−, and GALNT3 mutant mice) (Abe et al., 1989; Ichikawa et al., 2009; Kuro-o et al., 1997). Mutations in dentin matrix protein-1 (Dmp1)and phosphate-regulating gene with homologies to endopeptidases on the X- chromosome (Phex), factors which may indirectly influence systemic Pi levels, cause ARHR and XLH in humans, respectively (Drezner, 2000; Farrow et al., 2007). Corresponding to human case reports, studies of hypophosphatemic Dmp1−/− and Hyp (Phex mutant) mice have identified dental defects primarily in the dentin, with minor changes in the cementum (Fong et al., 2009; Ye et al., 2008). In our studies characterizing tooth development in Hyp mice, dentin defects were detected by histology, whereas the more subtle aberrant cementum phenotype required electron microscopy to be detected (Fong et al., 2009). In contrast, the teeth of hyperphosphatemic TC patients or mouse models (Fgf23−/−, Klotho−/−, and GALNT3 mutant mice) have not been studied extensively. Limited case reports of TC skeletal and tooth abnormalities noted calcification around major joints, hyperostosis, and ectopic calcification of the pulp chambers (Naikmasur et al., 2008; Witcher et al., 1989). Further, examination of incisors from Klotho−/− revealed dentin abnormalities (Suzuki et al., 2008). Because of the emphasis on dentin and pulp anomalies as a result of systemic Pi dysregulation in case studies, we hypothesized that hyperphosphatemia would lead to dramatic dentin and pulp abnormalities and minor, if any cementum alterations.
In order to put into context the effects of FGF23-mediated systemic Pi regulation on the dentoalveolar complex, we characterized the tooth phenotype of the hyperphosphatemic Fgf23−/− mouse, a homologue for TC Type II, using histological staining and electron microscopic analysis. To investigate mechanisms by which systemic Pi impacts mineralization, we used immunohistochemistry to determine expression patterns for selective bone/tooth markers in Fgf23−/− and Hyp mice. The Hyp mouse, while not a perfect inverse control of the Fgf23−/− mouse does feature high levels of circulating Fgf23 and is hypophosphatemic, providing a useful comparison regarding the roles of Fgf23 and Pi in tooth development. We report dramatic alterations in morphology, mineralization, and protein distribution in teeth and supporting structures as a consequence of Fgf23 ablation.
MATERIALS AND METHODS
Animal maintenance and genotyping
Fgf23 heterozygote mouse breeding pairs were used to generate Fgf23−/− and wild type (WT) littermates for histological studies. Generation of these mice has been described previously (Sitara et al., 2004). Mice were housed in a specific pathogen free facility in 12 hr light-dark cycles and fed a standard rodent diet with access to water ad libitum. All animal studies were approved by the Institutional Animal Care and Use Committee, University of Washington (Seattle, WA, USA). Animals were genotyped with PCR amplified DNA extracted from tail snips using a RedExtract-N-Amp For Tissue kit (Sigma). The following PCR primers were used: Fgf23 (5′ AGT GGA CGC TGG AGA ATG GCT ATG 3′ and 5′ CTG GGA AAG GGG CGA CAC C 3′, specific to Exon 3 of the wild-type); Neo (5′ AAG GTG AGA TGA CAG GAG ATC 3′ and 5′ GAT CGG CCA TTG AAC AAG ATG 3′, specific to neomycin of the mutant allele construct. The wild-type Fgf23 product was 397 bp, while the mutant product was 310 bp. PCR cycling conditions used were: 94° C for 2 min; 37 cycles at 94° C for 40 sec, 60° C for 1 min, 72° C for 40 sec; 72° C for 10 min. Agarose gels were used to visualize PCR products.
Histology
Fgf23−/− and WT littermates were sacrificed at 23, 27, 33, 45, 61, and 75 days post-coital (dpc), where date of birth is about 19dpc. At least three samples from each time point and genotype were obtained. Sample heads were fixed in Bouin’s overnight, and mandibles were dissected. Samples from 27dpc and later were demineralized (10% acetic acid, neutral buffered formalin, and sodium chloride), processed, and embedded for paraffin sectioning. 5μm buccolingual sections of the mandibular first molar were H&E stained. Images were obtained using a Nikon Eclipse E400 microscope camera system. In addition, 45dpc Hyp sections were prepared for comparison. Hyp samples were prepared as previously reported (Fong et al., 2009).
Electron Microscopy (EM)
Scanning electron microscopy (SEM) analyses were performed on lower right mandibles from 45dpcmice. Mandibles were sequentially dehydrated in 5%, 10%, 25%, 50%, 75%, and 100% aqueous ethanol solutions for 30 min each and mounted in room-temperature-cure epoxy (Allied High Tech Inc, Rancho Dominguez, CA). Subsequent preparation of epoxy-mounted specimens involved cutting the erupted incisor using a precision wafering saw (Buehler Ltd, Lake Bluff, IL) to expose the mesial surface of the first molar and the cross-section of the unerupted incisor. The cut surface was then ground further distally to expose the interior of the first molar using 600 then 1500 grit SiC papers, followed by smoothening via ultramicrotoming with a 45° angle diamond knife (Diatome, Inc., Hatfield, PA) fitted onto a MT 6000-XL ultra-microtome (Bal-Tec RMC, Inc., Tucson, AZ). All specimens were then mounted on SEM stubs, sputter coated with 5 nm of Pt for electron conductivity (SPI Supplies Inc, West Chester, PA), and imaged by an JSM7000F (JEOL-USA, Inc., Peabody, MA) SEM operating at 15kV in backscattering mode.
For transmission electron microscopy (TEM) analyses, mandibular molars still attached to alveolar bone were dehydrated and mounted following the same procedure as that for SEM. Mounted specimens were then ground to reveal the first molar interior. Without demineralizing, ultra-sections were prepared using a 45° angle diamond knife (Diatome, Inc., Hatfield, PA) fitted onto a MT 6000-XL ultra-microtome (Bal-Tec RMC, Inc., Tucson, AZ) and collected onto lacey-carbon coated Cu grids. TEM characterization was performed on a Philips EM420(FEI, Inc., Hillsborough, OR)microscope with a tungsten filament at 100keV.
Apoptosis assay
Apoptosis was detected using the TACS TdT Kit for terminal deoxynucleotidyl transferase-mediated deoxyuridinetriphosphate nick end-labeling (TUNEL) per kit instructions and a rabbit anti-mouse antibody for Caspase-3 (using the procedures described in the following immunohistochemistry section).
Immunohistochemistry (IHC)
Tissues from 45dpc Fgf23−/−, Hyp, and WT mice were selected for IHC. Antibodies against mouse proteins included: BSP, dentin phosphoprotein (DPP), dentin sialoprotein (DSP), and DMP1. The BSP antibody was a gift from Dr. Renny Franceschi (University of Michigan), the DPP antibody was a gift from Dr. Arthur Veis (Northwestern University), the DSP (LF-153) antibody was a gift from Dr. Larry Fisher (NIH), and the DMP1 antibodies were gifts from Dr. Chunlin Qin (Baylor College of Dentistry) and purchased from Takara. Positive reactions were detected with AEC (3-amino-9-ethylcarbazole) solution. Sections were counterstained with hematoxylin. Antibodies were evaluated with n≥3 Fgf23−/−, Hyp, and WT samples.
RESULTS
Alveolar bone, pulp, and PDL are dramatically altered in Fgf23−/− molar teeth (Figure 1)
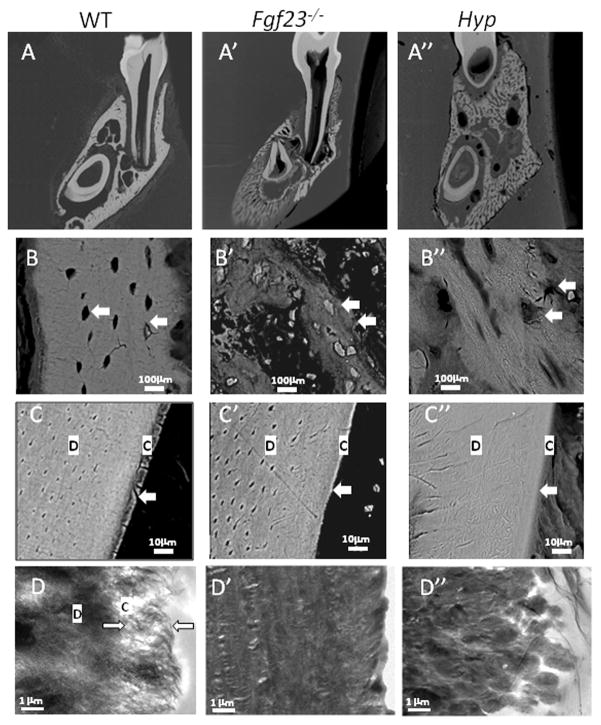
Figure 1. Mandibular molars of Fgf23−/− mice exhibit disruption of odontoblast layer and ectopic matrix deposition in pulp chambers.
Representative H&E stained histological sections from mouse molars at 33, 45, and 61dpc (at least three samples from each age and genotype were evaluated). P=pulp, oD=odontoblasts, D=dentin, C=cementum, PDL=periodontal ligament, B=bone
(A) Low magnifcation of WT buccolingual section. L and B signify lingual and buccal (labial), respectively. (A′) Low magnification of Fgf23−/− buccolingual section. Note increase in volume of the alveolar bone region in the Fgf23−/− mouse (A′) vs. WT. (A″) Low magnification of Hyp mouse buccolingual section. Note increase in volume of alveolar bone region compared to WT.(B) Buccal aspect of WT mandibular first molar at 33dpc. (B′) Buccal aspect of Fgf23−/− mandibular first molar at 33dpc. Fgf23−/− mice exhibited increased volume in the alveolar bone region. No clear differences were noted in the odontoblasts, predentin, dentin, cementum, and PDL in Fgf23−/− vs. WT. (C, C′) Lingual aspect of WT (1C) and Fgf23−/− (1C′) mandibular first molar at 45dpc. Compared to WT, the odontoblast layer in the Fgf23−/− mouse has lost its polarized nature (arrows), PDL width is reduced and fibers are slightly disorganized. (D, D′) Coronal region of Fgf23−/− mandibular first molar at 61dpc (1D′) compared to WT (1D). Note loss of polarization in the Fgf23−/− odontoblast layer compared to WT. Arrows indicate areas of ectopic matrix in the pulp chamber. (E, E′) Lingual aspect of WT (1E) and Fgf23−/− (1E′) mandibular first molars at 61dpc. Note near ankylosis (arrow) in the Fgf23−/− mouse. Also, many empty lacunae are visible in the bone of the Fgf23−/− mouse (arrowhead).
As a first step, to characterize the effects of Fgf23 ablation on the dentoalveolar complex, we conducted a histological developmental time course of the mandibular first molar. Days were selected to capture developmental time points of interest, i.e. before root formation (23dpc), initiation of root/cementum formation (27dpc), during root formation and tooth eruption (33 and 45dpc), and following closure of the apex (61 through 96dpc). Disruptions in the dentoalveolar complex of the hyperphosphatemic Fgf23−\− mouse were first noted at 33dpc, and by 45dpc, marked disturbances were present in the periodontium (Fig. 1A′).
At 33dpc and subsequent time points in Fgf23−/− mice, mandibular bone volume surrounding the incisor and molar was greatly expanded compared to WT, a trend particularly evident at 45dpc (Figs. 1A vs. A′, B vs. B′). The buccal aspect of the alveolar bone proper of Fgf23−/− mice was more than twice the width compared to that of WT controls (Figs. 1A vs. A′, B vs. B′). The expansion of the alveolar bone is reminiscent of reports from Hyp mice (Fong et al., 2009), and a mandibular first molar section from the hypophosphatemic Hyp mouse at 45dpc is provided for comparison (1A″). Further, the alveolar bone of Fgf23−/− mice appeared to be composed predominantly of woven bone with a higher density of osteocytes when compared with WT tissue sections (Fig. 1B vs. B′). By 61dpc, numerous empty lacunae were visible in alveolar bone of Fgf23−/− tissues (arrowhead, Fig. 1E′).
By 45dpc, disruption of the odontoblast layer characterized by a loss of cell polarity was observed in molars of Fgf23−/− mice, while the cementum, dentin, and predentin of the mandibular first molars appeared comparable in Fgf23−/− vs. WT tissues (Fig. 1C vs. C′, arrow). This directly contrasts with the marked dentin and predentin abnormalities observed in Hyp teeth (Fig. A″). Additionally, an ectopic matrix had developed in pulp chambers of Fgf23−/− mice at 45dpc and became more apparent by 61dpc (Fig. 1D′, arrows).
Alterations were present in the periodontal ligament (PDL) region by 45dpc, most consistently at the lingual aspect of the mandibular first molar in the coronal third of the root. Furthermore, PDL width was markedly reduced compared to the WT, with disorientation and compression of the PDL fibers (Fig. 1C vs. 1C′). At 61dpc, the PDL space was narrowed, and areas of near ankylosis were observed between bone and cementum at the site of PDL disruption. Few fibers were noted in the scant space between bone and cementum (Fig. 1E′, arrow).
Fgf23−/− mice exhibit marked mineralization defects, including a cementum phenotype (Figure 2)
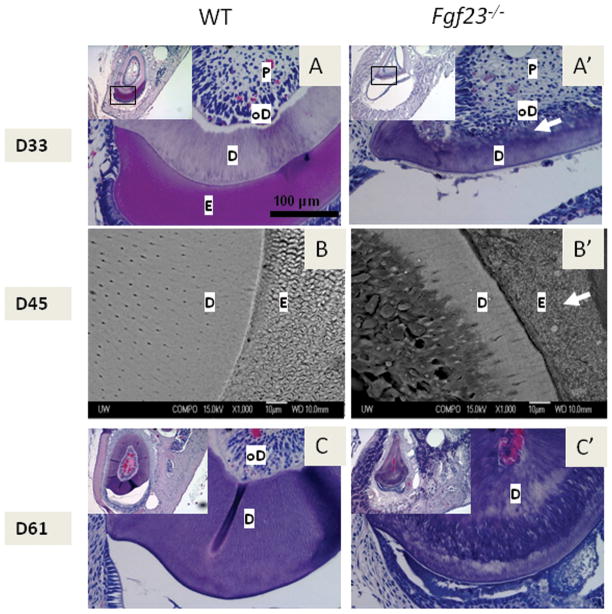
Figure 2. Fgf23−/− mice exhibit marked mineralization defects, including a cementum phenotype.
Representative images from SEM and TEM analysis of 45dpc WT, Fgf23−/−, and Hyp mandibular first molars and surrounding alveolar bone (At least three samples from each age and genotype were evaluated). D=dentin, C=cementum
(A, A′, A″) Cross section of Fgf23−/− (2A′) compared to WT (2A) and (2A″). (B, B′, B″) High magnification of alveolar bone region of Fgf23−/− (2B′) mice compared to WT (2B) and Hyp (2B″). Note inconsistent mineralization and abnormal osteocytes (arrow) in the Fgf23−/− bone (2B′). (C, C′, C″) Root surface of Fgf23−/− (2C′) mice compared to WT (2C) and Hyp (2C″). Note lack of a clear demarcation between dentin and cementum in the Fgf23−/− (2C′) and the Hyp (2C″) mice. (D, D′, D″) TEM analysis of root surface of Fgf23−/− (2D′) mice compared to WT (2D) and Hyp (2D″). Note lack of fibrillar structure in the Fgf23−\− mice and a lack of a clear demarcation between dentin and cementum in the Fgf23−/− (2D′) and the Hyp (2D″) mice even under high magnification.
In order to assess the mineral content of the hyperphosphatemic Fgf23−/− compared to the WT specimens, backscatter scanning electron microscopy (SEM) analysis was used. In these 45dpc un-demineralized sections, image brightness serves as an indicator of high mineral content. Consistent with reports of a lower mineral density of long bones in Fgf23−/− mice (Sitara et al., 2004; Sitara et al., 2006), the alveolar bone in the Fgf23−/− was significantly darker compared to WT, likely reflecting a composition of primarily osteoid(Figs. 2A vs 2A′). The mineral content was so severely reduced in Fgf23−/− mice that alveolar bone was difficult to visualize by SEM; thus, expansion of bone width was not as apparent in these images as in comparable histological sections. This accumulation of osteoid is similar to the Hyp mouse bone (Fig. 2A″), in which mineralization defects in the bone, dentin, and cementum have been documented (Abe et al., 1989; Abe et al., 1992; Fong et al., 2009). Representative images of Hyp dentin and cementum are also shown here for comparison.
In support of the histological findings (Fig. 1), the alveolar bone of Fgf23−/− mice appeared less organized, i.e. characteristic lamellar structure as noted in the WT specimens was not observed. Further, compared to WT bone, osteocytes in Fgf23−/− bone were present in greater numbers and exhibited abnormal morphology (Figs. 2B vs. B, arrows). In contrast, in Hyp sections, osteocytes appeared more comparable to WT in morphology, although they inhabited irregular, large lacunae (Figs. 2B vs. B″, arrows).
Confirming histological observations, the dentin of Fgf23−/− mice was similar to that of WT mice and different from the Hyp mouse, which exhibited disruption of the tubules (Figs. 2C, C′ vs. C″). However, Fgf23−/− molar roots did not have the clear separation noted between cementum and dentin in WT specimens (Fig. 2C′, D′ vs. C, D). Transmission electron microscopy analysis suggested alteration of cementum structure in Fgf23−/− mice compared to WT (Fig. 2D′ vs. 2D). However, the deviation from normal cementum in Fgf23−/− teeth was not the same as reported for Hyp mice, although Hyp specimens did not exhibit a clear demarcation between dentin and cementum as well (Figs. 2C″, D″).
Incisors of Fgf23−/− mice displaydentin and enamel abnormalities (Figure 3)
Figure 3. Mandibular incisors of Fgf23−/− mice exhibit dentin and enamel abnormalities.
Representative H&E stained histological sections from mouse molars at 33 and 61dpc and SEM images from 45dpc incisors (At least three samples from each age and genotype were evaluated). P=pulp, oD=odontoblasts, D=dentin, E=enamel
(A, A′) Cross section of Fgf23−/− mouse incisor (3A) compared to the WT (3A′) at 33dpc. Inset shows cross-sections of the entire mandible. Note large cyst-like structure in the Fgf23−/− section (inset), and cells embedded in the dentin of Fgf23−/− mouse incisors (white arrows). Absence of enamel matrix (stains pink in the WT) in the Fgf23−/− mouse suggests dysfunctional amelogenesis. (B, B′) SEM analysis of 45dpc WT and Fgf23−/− mandibular first molars and incisors. (B) WT incisor dentin with normal tubular structure and interwoven enamel rods. (B′) Fgf23−/− incisor dentin was hypomineralized on the labial aspect and multiple embedded cells could be seen, and enamel rod structure is lacking (arrow). (C, C′) Cross section of Fgf23−/− mouse incisor (3C′) compared to WT (3C) at 61dpc. Note almost complete obliteration of pulp chamber in the Fgf23−/− mouse incisor and lack of the cyst-like structure (observed in 33 and 45dpc tissues).
Although the murine incisor does not have a human counterpart, examining the continuously erupting murine incisors allows for the visualization of long-term sustained Fgf23 loss on tooth development. At 23 and 27dpc, no obvious differences were detected in mandibular incisors of Fgf23−/− vs. WT mice (data not shown). By 33dpc, incisors of Fgf23−/− mice exhibited morphological changes, including distorted shape, i.e. triangular cross-section vs. elliptical in WT, as well as development of a cyst-like structure in the region normally occupied by enamel (Fig. 3A vs. A′). Additionally, by 33dpc, a mineralized tissue-like matrix originating from the labial dentin (“crown analogue”) was observed extending into the incisor pulp chamber, with cells entrapped within this matrix (Figs. 3A′). SEM analysis revealed defective mineralization with an abnormal rod patterning in enamel of Fgf23−/− mice (Fig. 3B vs. B′, arrow). By 61dpc, the cyst-like structure at the labial aspect was no longer present, and the enamel space was reduced (Fig. 3C vs. C′). The mineral-like tissue present in the pulp at 33 and 45dpc increased markedly to the point of nearly obliterating the pulp chamber (Fig. 3C′). The odontoblast layer was completely disrupted and cells did not form the characteristic discrete, polarized layer on the border of the dentin matrix (C vs. C′, inset). In contrast, high levels of circulating FGF 23 characteristic of Hyp mice did not result in any unique pattern for incisors vs. molars during development.
Fgf23−/− mice exhibit increased apoptotic cells in the mandible compared to WT (Figure 4)
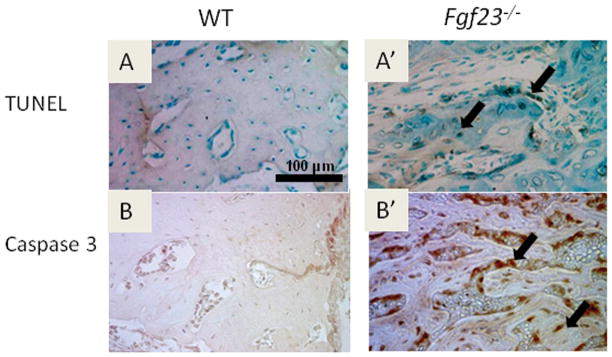
Figure 4. Fgf23−/− mice exhibit increased apoptotic cells in the mandible compared to WT.
Histological sections from 45dpc mice (at least three of each genotype were used) were used to stain for (A, A′) TUNEL (dark brown stain) and (B, B′) Caspase 3 (red stain) to detect apoptotic cells. (A′, B′) Both stains indicated increased incidence of apoptosis in osteocytes and osteoblasts (arrows).
We observed numerous empty lacunae in mandibular bone and abnormal morphology of Fgf23−/− osteocytes. Because increased apoptosis has been reported in skeletal cells stimulated by elevated Pi levels in vitro (Adams et al., 2001), we assayed for apoptosis. Numerous positive TUNEL (Fig. 4A′) and caspase-3 reactions (Fig. 4B′) were observed in the marrow spaces, osteoblasts, and osteocytes of the alveolar bone in the Fgf23−/− mice. In addition, positive reactions were observed in the cells entrapped in the ectopic matrix of the incisor (data not shown). In contrast, very few apoptotic cells were detected in the WT tissues (Figs. 4A, B). Hyp tissues also exhibited very few apoptotic cells (data not shown).
Loss of Fgf23 and mutations in Phex cause altered expression of extracellular matrix proteins (Figure 5, Table 1)
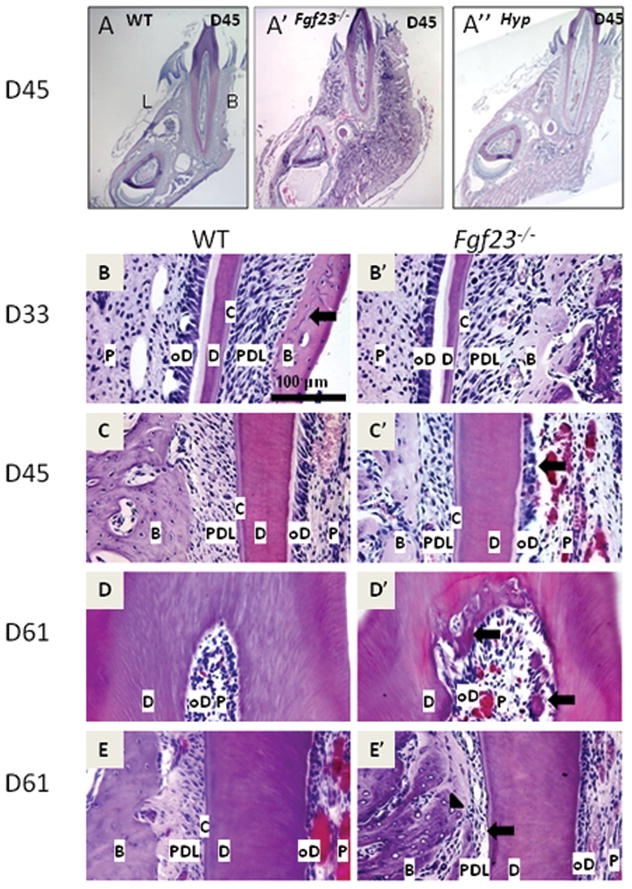
Figure 5. Fgf23 ablation or Phex mutations cause altered expression of extracellular matrix genes and proteins of the oral mineralized tissues.
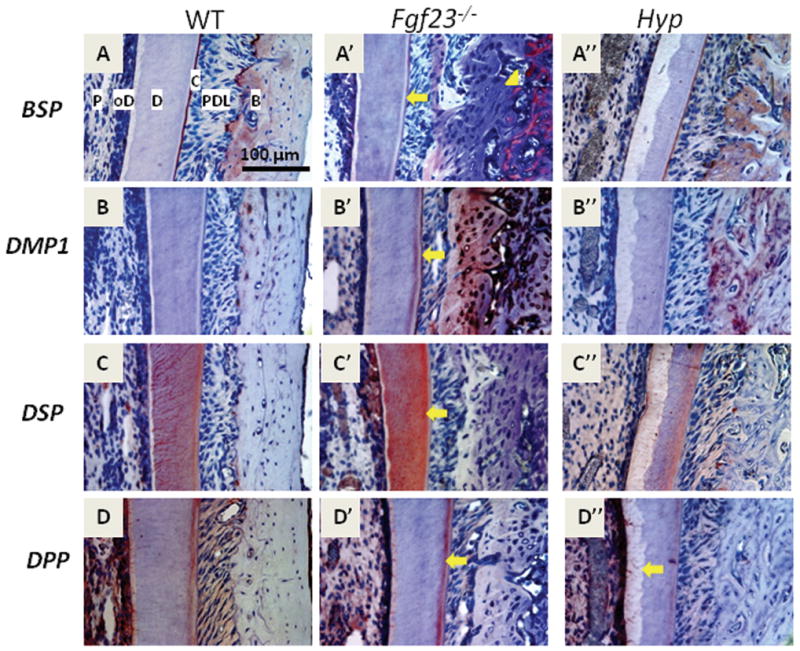
Representative immunohistochemical images from the buccal side of the mandibular first molar at 45dpc (all antibodies were evaluated with at least three Fgf23−/−, Hyp, and WT samples). Labels are provided in 5A for reference: P=pulp, oD=odontoblasts, D=dentin, C=cementum, PDL=periodontal ligament, B=bone
(A, A′, A″) BSP was localized to cementum and alveolar bone in WT and Hyp sections, whereas BSP was seemingly absent in Fgf23−/− alveolar bone directly adjacent to the PDL, strongly localized in the region associated with open osteocyte lacunae (5A′, arrowhead), and stained weakly in cementum (arrow) (B, B′, B″) DMP1 staining was absent in cementum of WT and Hyp tissues, whereas DMP1 was increased in mantle dentin, cementum (arrow), and bone in Fgf23 −/− mouse tissues. DMP1 was also increased in bone in Hyp mouse tissues (C′). (C, C′, C″) DSP was detected in the dentin tubules and mantle dentin of WT specimens (5C). DSP staining in Fgf23 −/− dentin tubules was diffuse, but intensely localized to mantle dentin (5C′, arrow). DSP staining in Hyp dentin appeared lower, i.e. no clear staining in dentin tubules and sparse staining in the mantle dentin (5C″). (D, D′, D″) DPP staining detected at the predentin/dentin mineralization front, in odontoblasts and in mantle dentin of WT samples, and similarly localized in Fgf23−/− sections, but staining was stronger in the mantle dentin (5D′, arrow). DPP staining was weak in the mantle dentin of Hyp mice compared to WT, with no staining at the mineralization front (5D″, arrow).
Table 1.
Summary of immunohistochemical staining of SIBLING members: bone sialoprotein, BSP; DMP1, dentin sialoprotein, DSP; dentin phosphoprotein; DPP
| WT | Fgf23−/− | Hyp | |
|---|---|---|---|
| BSP | Acellular cementum and extracellular matrix (ECM) of bone | Decreased staining in acellular cementum, some areas of strong, localized staining in ECM of bone | Similar to WT, acellular cementum staining width thinner in Hyp |
| DMP1 | Osteocytes and perilacunar bone | Increased staining detected in acellular cementum, mantle dentin, osteocytes, and bone ECM | Staining detected in osteocytes, perilacunar bone, and more widely in bone ECM |
| DSP | Staining in dentin confined to dentin tubules and mantle dentin | Strong, diffuse staining in tubules and mantle dentin | No apparent staining in dentin tubules, some staining in mantle dentin |
| DPP | Staining detected in odontoblasts, mineralization front between dentin and predentin, and mantle dentin | Strong staining in mantle dentin, weak staining in odontoblasts and predentin/dentin mineralization front | Staining in odontoblasts, weak staining in mantle dentin, no staining at dentin/predentin mineralization front |
Members of the small integrin-binding ligand, N-linked glycoprotein (SIBLING) family of extracellular matrix proteins (Fisher and Fedarko, 2003)have been reported to influence systemic Pi levels as well as being responsive to calcium/Pi regulating hormones, e.g. DMP1 loss results in excess FGF23 and hypophosphatemia (Chaussain-Miller et al., 2007; Feng et al., 2006; Fisher and Fedarko, 2003). To investigate possible mechanisms resulting in the dentoalveolar phenotype in Fgf 23 −/− mice, described above, immunohistochemistry was used to assay expression for selected SIBLING proteins in Fgf23−/− vs. WT samples. Further, we contrasted ECM protein expression of Fgf23−/− mice with comparable Hyp tissues (data not published previously). A descriptive summary of the results are provided in Table 1.
Bone sialoprotein (BSP)
BSP, a positive regulator of mineral formation (Malaval et al., 2008; Qin et al., 2004), was localized to cementum and alveolar bone in WT and Hyp sections (Figs. 5A vs. A″). In corresponding Fgf23−/− tissues, cementum stained very weakly and sporadically for BSP (Fig. 5A′, arrow), suggesting an altered cementum composition and mineralization consistent with changes noted in SEM and TEM analysis. Further, BSP was apparently absent in Fgf23−/− alveolar bone adjacent to the PDL, contrasting with the strong localized BSP in the region associated with open osteocyte lacunae (Fig. 5A′, arrowhead).
Dentin Matrix Protein-1 (DMP1)
DMP1 is an important factor in maturation of osteoblasts into osteocytes, and is a regulator of osteocyte behavior (Qin et al., 2007; Rios et al., 2005). In WT, positive staining for DMP1 was primarily localized around osteocytes in alveolar bone, as previously reported (Fig. 5B) (Toyosawa et al., 2001). Alveolar bone of Hyp mice exhibited intense DMP1 staining in perilacunar regions, but in contrast, in Fgf23−/− bone, large regions of bone adjacent to PDL were intensely positive for DMP1 (Figs. 5B′, B″). Further, DMP1 staining was present in high levels in mantle dentin and a cellular cementum of Fgf23−/− samples, but was not detected in comparable sections from either Hyp or WT mice. These results were verified using multiple DMP1 antibodies (sections shown in Fig. 5 were stained with a commercially available DMP1 antibody targeted towards the N-terminal region).
Dentin sialoprotein (DSP) and Dentin phosphoprotein (DPP)
Immunopositive reactions for DSP and DPP, both protein products of Dspp mRNA transcript (MacDougall et al., 1997), indicated a different pattern of deposition in Fgf23−/− vs. Hyp vs. WT tissues. In WT sections, DSP was detected in dentinal tubules and mantle dentin, whereas in Fgf23−/− sections, DSP staining was strong but dispersed in dentin, with a higher intensity in the mantle dentin region (Figs. 5C vs. C′, arrow). In Hyp tissues, significant DSP staining was not apparent in the dentinal tubules, and staining in the mantle dentin appeared weaker than in WT and Fgf23−/− tissues(Figs. 5C″ vs. 5C, 5C′). In WT tissues, DPP was present in the predentin/dentin mineralization front, mantle dentin, and in odontoblasts, with low levels in pulp (Fig. 5D). DPP was similarly localized in Fgf23−/− sections, robust staining in the mantle dentin compared to WT. Hyp mouse molars featured overall weak DPP staining, including staining in the mantle dentin and predentin (Figs. 5D vs. 5D′, 5D″).
DISCUSSION
We sought to delineate the influence of hyperphosphatemia on development and mineralization of the dentoalveolar complex using Fgf23−/− mice and contrasting with hypophosphatemic Hyp and WT (normal Pi levels) controls. Based on previous studies, we hypothesized that systemic Pi dysregulation would lead to dramatic dentin and pulp abnormalities and less marked cementum abnormalities. Unexpectedly, our findings only partially correlated with our hypothesized results. Although both Fgf23−/− and Hyp mice exhibit abnormal systemic Pi regulation, dentin was dramatically altered only in the hypophosphatemic Hyp mice, whereas cementum was altered in both Hyp and Fgf23−/− mice. Extracellular matrix protein composition was altered in Fgf23−/− and Hyp dentin, cementum, and bone vs. WT. In addition to expanded and hypomineralized alveolar bone in Fgf23−/− mice, increased and widespread osteocyte apoptosis was apparent. Altogether, these results indicate that development and maintenance of the dentoalveolar complex is sensitive to systemic Pi dysregulation.
Although loss of Fgf23 seemingly results in widespread detrimental effects, i.e. multiple organ atrophy (Sitara et al., 2004), the results here indicate that Fgf23 has specific effects on each mineralized tissue type. The oral cavity presents a unique opportunity to simultaneously observe four different types of mineralized tissue: bone, cementum, dentin, and enamel. In the dentoalveolar complex at the level of the mandibular first molar, dentin and bone defects were the most conspicuous alterations (i.e. readily detected with H&E) in Hyp mice, whereas bone expansion coupled with PDL narrowing were the most apparent in Fgf23−/− mice (Fig. 1). In both Hyp and Fgf23−/− specimens, cementum aberrations were not detectable by histology, but alterations in mineral density were observed using high magnification SEM and TEM (Fig. 2). Our observations here, as well as the globular cementum phenotype observed in Hyp mice, suggest that processes such as PDL maintenance and cementogenesis may be altered subsequent to Pi dysregulation, though the mechanism remains unknown. Additionally, studies on mice with loss of progressive ankylosis protein (ANK), ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1), or tissue non-specific alkaline phosphatase (TNAP) function have demonstrated sensitivity of cementum to dysregulation of Pi/PPi homeostasis (Beertsen et al., 1999; Groeneveld et al., 1995; Millan et al., 2008; Nociti et al., 2002; van den Bos et al., 2005). Ank and Enpp1 mutant mice exhibit a marked hypercementosis phenotype, whereas Tnap mutants have cementum aplasia/hypoplasia resulting in exfoliation of teeth. Although these cementum alterations are a likely consequence of a microenvironmental Pi/PPi ratio imbalance, these studies, along with the results presented here on systemic Pi dysregulation, indicate the importance of proper Pi regulation for formation of a normal dentoalveolar complex.
Alterations in protein expression described here suggest further roles for Pi and/or regulators of Pi metabolism on mineralized tissues of the oral cavity (summarized in Table 1). SIBLING proteins, extracellular matrix proteins found in dentin, cementum, and bone (D’Errico et al., 1997; Fisher et al., 2001; Fisher and Fedarko, 2003; Hunter and Goldberg, 1994; Hunter et al., 1996; Papagerakis et al., 2002; Qin et al., 2004; Rosen, 2008), exhibited altered expression in hypophosphatemic Hyp and hyperphosphatemic Fgf23−/− samples. BSP is a marker of bone and cementum, involved in osteoblast and cementoblast differentiation, and has roles in matrix mineralization (Bianco et al., 1993; Chen et al., 1992; D’Errico et al., 1997; Hunter and Goldberg, 1994; Hunter et al., 1996; Malaval et al., 2008). Notably, in cementoblasts, BSP mRNA transcription has been demonstrated to be down regulated in response to exogenous Pi in vitro, which parallels the sparse staining in the cementum of hyperphosphatemic Fgf23−/− mice (Foster et al., 2006; Rutherford et al., 2006). Moreover, BSP in Fgf23−/− alveolar bone region was characterized by areas of relatively heavy staining and areas with no apparent BSP present. Reductions in mineral markers such as BSP may reflect disrupted cell function or unregulated matrix synthesis by osteoblasts.
Interestingly, whereas BSP was down regulated in Fgf23−/− cementum and bone, DMP1 was up regulated. Further, regarding location of staining in the alveolar bone, DMP1 and BSP staining appeared to be inverses of each other in the hyperphosphatemic Fgf23−/− mouse. There are no studies demonstrating a feedback relationship between BSP and DMP1, and thus while speculative, the findings reported here suggest a common regulatory factor. Increased DMP1 expression in Fgf23−/− samples correlated with in vitro studies on cementoblasts demonstrating increased mRNA transcripts for DMP1 in response to elevated Pi, suggesting that the elevated levels of DMP1 seen in Fgf23−/− tissues may be related directly to or regulated by high Pi levels (Foster et al., 2006; Rutherford et al., 2006). In bone, DMP1 was localized around osteocytes in WT samples, but was found in the extracellular matrix of Fgf23−/− and Hyp tissues. The increase in DMP1 staining in Hyp mice suggests local effects occurring independent of serum Pi and via mechanisms not completely understood. For example, studies have shown that osteocytes respond to force by synthesizing DMP1, so the unexpected DMP1 expression increase in the hypophosphatemic Hyp mice may be a consequence of amplified force transmission caused by a hypomineralized environment (Harris et al., 2007).
DMP1 has been reported to act as a nucleator of hydroxyapatite and is known to be required for skeletal development as well as dentinogenesis, as indicated by a reduced rate of dentin apposition and disruption in organization of dentinal tubules in Dmp1−/− mice (He et al., 2003a; b; Lu et al., 2007; Tartaix et al., 2004; Ye et al., 2004). Dmp1−/− mice exhibit a tooth and bone phenotype remarkably similar to Hyp mice, including increased circulating levels of FGF23. A feedback loop between DMP1 and FGF23 has been proposed (Liu et al., 2008; Strom and Juppner, 2008). In light of this, increased DMP1 protein observed here in Fgf23−/− mice may in part contribute to the notable increase in alveolar bone volume and in ectopic extracellular matrix formation in the pulp region. Further, DMP1 regulates Dspp mRNA expression (Narayanan et al., 2006), which gives rise to DSP and DPP proteins (MacDougall et al., 1997). DSP and DPP exhibited altered localization in Hyp and Fgf23−/− mice. DSP and DPP have important roles in dentin mineralization and possibly in mineralization of other matrices (Baba et al., 2004; Hao et al., 2004; Qin et al., 2002; Qin et al., 2003a; Qin et al., 2003b; Sreenath et al., 2003). Thus, changes in localization and expression of DMP1, DSP and DPP possibly contribute to the ectopic matrix formation in Fgf23−/− mice, dentin mineralization abnormalities in Hyp mice, and the accumulation of osteoid in both Hyp and Fgf23−/− mice.
The alterations noted in the dentoalveolar complex of Fgf23−/− are consistent with case studies of TC patients and other hyperphosphatemic animal models. Reports of tumoral calcinosis patients, who have deficient FGF23 and elevated phosphate levels, include dystrophic pulp calcification, root dilacerations, and thistle shaped pulps (Dumitrescu et al., 2009; Naikmasur et al., 2008; Witcher et al., 1989). Further, Klotho−/− mice exhibit a nearly identical physical and biochemical phenotype to Fgf23−/− mice (e.g. elevated Pi and 1,25(OH)2D3 levels) although Fgf23 levels in Klotho−/− mice are elevated (Kuro-o et al., 1997; Kuro-o, 2006; Memon et al., 2008). The dental aberrations noted in Fgf23−/− mice were similar to reports of increased apoptotic reactions and marked disturbances in odontoblasts, predentin, and dentin of incisors in Klotho−/− mice (Suzuki et al., 2005). In addition to Pi (Adams and Shapiro, 2003), apoptosis is induced by 1,25(OH)2D3 (Medici et al., 2008), suggesting that at least some of the bone alterations in Fgf23−/− mice may be attributed to both elevated Pi and 1,25(OH)2D3. Hyp/Klotho−/− and Hyp/Fgf23−/− compound mutants are also hyperphosphatemic with elevated 1,25(OH)2D3 levels, demonstrating the importance of Fgf23 and Klotho in the pathology of hypophosphatemic rickets (Liu et al., 2006; Nakatani et al., 2009; Sitara et al., 2004). Our results, when considered alongside the similar tooth phenotype of the Klotho−/− mice, support the concept that klotho-FGF23 interactions are required for FGF23 to activate downstream events linked to controlling Pi metabolism in order to maintain a healthy dentoalveolar complex.
Considering data from this and other studies designed to define the key factors controlling Pi metabolism, it is important to recognize the intimate linkage of the hormones associated with the parathyroid-kidney-bone and tooth axis and when one of the three major hormones, i.e. PTH, 1,25(OH)2D3, or FGF23is perturbed, all of these hormones and the downstream products they regulate are impacted. For example, in addition to being hyperphosphatemic, the Fgf23−/− mouse exhibits, hypercalcemia, hypoparathyrodisim, and hypervitamatosis D (Liu et al., 2006; Sitara et al., 2004). When either loss of 1-α hydroxylase or the 1,25(OH)2D3 receptor is superimposed on loss of FGF23, hyperphosphatemia is reversed to hypophosphatemia and soft tissue calcifications are not observed (Hesse et al., 2007; Sitara et al., 2006). This outcome suggests elevated 1,25(OH)2D3 levels play an important role in the mineralization defects characterizing Fgf23−/− mice (Shimada et al., 2004; Sitara et al., 2004). Additionally, the tooth phenotype in humans and animals lacking functional 1,25(OH)2D3 included mineralization defects consistent with histological descriptions of the tooth phenotype in Hyp mice and XLH patients. Mice lacking 1,25(OH)2D3 receptor expression displayed thin incisor dentin, enlarged pulp chambers, as well as widened and irregular predentin (Zhang et al., 2007). Thus, possible downstream effects of 1,25(OH)2D3 in conjunction with FGF23 and Pi alterations will need to be investigated.
In the hypophosphatemic Hyp and the hyperphosphatemic Fgf23−/− mice, mineralization defects were first observed in 33dpc samples, suggesting a stage-specific role for FGF23. At early stages of development, Fgf23 expression is normally low, indicating a minimal role in early developing tissues (Yoshiko et al., 2007). This is supported by lack of bone and teeth phenotypes at embryonic and early developmental ages, but an alternate explanation would be that the “normal” hormonal milieu that Fgf23−/− pups experience in utero protects against the inborn Fgf23 deficiency. With development of the mouse mandibular first molar completed by 45dpc, any detected changes in molar tissues from Fgf23−/− mice would likely be more homeostatic than developmental. In contrast, the mouse incisor is in a state of continuous development and eruption. Although the murine incisor does not have a human counterpart, examining murine incisors allows for the visualization of developmental processes completed by the molars prior to significant FGF23 expression. The presence of various stages of tooth development simultaneously is one possible explanation for the prominent incisor phenotype, including enamel defects, not seen in the molars. Further, we reported an improvement in Hyp dentin with time, suggesting a decreased role for FGF23 with age (Fong et al., 2009). Corroborating evidence includes findings that FGF23 expression may be suppressed in older tissues (Liu et al., 2007). Because the lifespan of Fgf23−/− mice is severely reduced (Sitara et al., 2004), we were unable to determine the effects of Fgf23 ablation on older ages. Nonetheless, the marked alterations in the dentoalveolar complex illustrate profound effects of Fgf23 loss, indicating that FGF23 has a significant impact on development and maintenance of healthy teeth and supporting structures.
CONCLUDING REMARKS
Fgf23 ablation dramatically altered morphology and matrix composition of dentin, bone, and cementum. Current data, including our studies described here, highlight the complexity of positive and negative feedback interactions among these homeostatic factors, e.g. in addition to the influence of FGF23 and PHEX, Pi and 1,25(OH)2D3 are regulated by other factors including calcium levels and PTH. Although direct and indirect effects on development of mineralized tissues are difficult to separate, the importance of further investigation to define regulators of Pi homeostasis is clear. The significant similarities between the Fgf 23 −/− in mice and humans in terms of bone pathology, coupled with the added knowledge from our studies may lead to more accurate diagnosis of phosphate metabolism disorders. Understanding the roles (s) for FGF23 in control of the dentoalveolar complex may lead to new approaches for developing more effective treatments for disorders in phosphate metabolism than those used at present.
Acknowledgments
The authors would like to thank the following for their contributions to this work: Dr. Ayu Murakami in the Somerman Lab, and support from the Departments of Periodontics and Oral Biology at the University of Washington School of Dentistry. This work was supported by NIH/NIDCR DE15109 (MJS), T32DE07132, and NIH/NIDDK DK073944 (BL).
Funding sources: NIH/NIDCR DE15109 (MJS), T32DE07132, NIH/NIDDK DK073944(BL)
Contributor Information
Ms. Emily Y. Chu, Email: eychu@u.washington.edu.
Dr. Hanson Fong, Email: hfong@u.washington.edu.
Dr. Fleur Blethen, Email: fblethen@gmail.com.
Dr. Kevin A. Tompkins, Email: kevin0318@yahoo.com.
Mr. Brian L. Foster, Email: blfoster@u.washington.edu.
Dr. Kuang-Dah Yeh, Email: yehkd592@u.washington.edu.
Dr. Kanako J. Nagatomo, Email: nagakana@u.washington.edu.
Ms. Daisy Matsa-Dunn, Email: daisy@dunns.info.
Dr. Despina Sitara, Email: despina_sitara@hsdm.harvard.edu.
Dr. Beate Lanske, Email: beate_lanske@hsdm.harvard.edu.
Dr. R. Bruce Rutherford, Email: rbruth@u.washington.edu.
Dr. Martha J. Somerman, Email: somerman@u.washington.edu.
References
- Abe K, Ooshima T, Masatomi Y, Sobue S, Moriwaki Y. Microscopic and crystallographic examinations of the teeth of the X-linked hypophosphatemic mouse. J Dent Res. 1989;68(11):1519–24. doi: 10.1177/00220345890680111001. [DOI] [PubMed] [Google Scholar]
- Abe K, Masatomi Y, Nakajima Y, Shintani S, Moriwaki Y, Sobue S, et al. The occurrence of interglobular dentin in incisors of hypophosphatemic mice fed a high-calcium and high-phosphate diet. J Dent Res. 1992;71(3):478–83. doi: 10.1177/00220345920710031101. [DOI] [PubMed] [Google Scholar]
- Adams CS, Mansfield K, Perlot RL, Shapiro IM. Matrix regulation of skeletal cell apoptosis. Role of calcium and phosphate ions. J Biol Chem. 2001;276(23):20316–22. doi: 10.1074/jbc.M006492200. [DOI] [PubMed] [Google Scholar]
- Adams CS, Shapiro IM. Mechanisms by which extracellular matrix components induce osteoblast apoptosis. Connect Tissue Res. 2003;44(Suppl 1):230–9. [PubMed] [Google Scholar]
- ADHR_Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26(3):345–8. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- Baba O, Qin C, Brunn JC, Jones JE, Wygant JN, McIntyre BW, et al. Detection of dentin sialoprotein in rat periodontium. Eur J Oral Sci. 2004;112(2):163–70. doi: 10.1111/j.0909-8836.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- Beertsen W, VandenBos T, Everts V. Root development in mice lacking functional tissue non-specific alkaline phosphatase gene: inhibition of acellular cementum formation [In Process Citation] J Dent Res. 1999;78(6):1221–9. doi: 10.1177/00220345990780060501. [DOI] [PubMed] [Google Scholar]
- Benet-Pages A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14(3):385–90. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- Bergwitz C, Juppner H. Disorders of Phosphate Homeostasis and Tissue Mineralisation. Endocr Dev. 2009;16:133–156. doi: 10.1159/000223693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Bonucci E, Termine JD, Robey PG. Bone sialoprotein (BSP) secretion and osteoblast differentiation: relationship to bromodeoxyuridine incorporation, alkaline phosphatase, and matrix deposition. J Histochem Cytochem. 1993;41(2):183–91. doi: 10.1177/41.2.8419458. [DOI] [PubMed] [Google Scholar]
- Chaussain-Miller C, Sinding C, Septier D, Wolikow M, Goldberg M, Garabedian M. Dentin structure in familial hypophosphatemic rickets: benefits of vitamin D and phosphate treatment. Oral Dis. 2007;13(5):482–9. doi: 10.1111/j.1601-0825.2006.01326.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Shapiro HS, Sodek J. Developmental expression of bone sialoprotein mRNA in rat mineralized connective tissues. J Bone Miner Res. 1992;7(8):987–97. doi: 10.1002/jbmr.5650070816. [DOI] [PubMed] [Google Scholar]
- D’Errico JA, MacNeil RL, Takata T, Berry J, Strayhorn C, Somerman MJ. Expression of bone associated markers by tooth root lining cells, in situ and in vitro. Bone. 1997;20(2):117–26. doi: 10.1016/s8756-3282(96)00348-1. [DOI] [PubMed] [Google Scholar]
- Drezner MK. PHEX gene and hypophosphatemia. Kidney Int. 2000;57(1):9–18. doi: 10.1046/j.1523-1755.2000.00807.x. [DOI] [PubMed] [Google Scholar]
- Dumitrescu CE, Kelly MH, Khosravi A, Hart TC, Brahim J, White KE, et al. A case of familial tumoral calcinosis/hyperostosis-hyperphosphatemia syndrome due to a compound heterozygous mutation in GALNT3 demonstrating new phenotypic features. Osteoporos Int. 2009;20(7):1273–8. doi: 10.1007/s00198-008-0775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow EG, Davis SI, Ward LM, White KE. The role of DMP1 in autosomal recessive hypophosphatemic rickets. J Musculoskelet Neuronal Interact. 2007;7(4):310–2. [PubMed] [Google Scholar]
- Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006 doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun. 2001;280(2):460–5. doi: 10.1006/bbrc.2000.4146. [DOI] [PubMed] [Google Scholar]
- Fisher LW, Fedarko NS. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res. 2003;44(Suppl 1):33–40. [PubMed] [Google Scholar]
- Fong H, Chu EY, Tompkins KA, Foster BL, Sitara D, Lanske B, et al. Aberrant cementum phenotype associated with the hypophosphatemic hyp mouse. J Periodontol. 2009;80(8):1348–54. doi: 10.1902/jop.2009.090129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BL, Nociti FH, Jr, Swanson EC, Matsa-Dunn D, Berry JE, Cupp CJ, et al. Regulation of cementoblast gene expression by inorganic phosphate in vitro. Calcif Tissue Int. 2006;78(2):103–12. doi: 10.1007/s00223-005-0184-7. [DOI] [PubMed] [Google Scholar]
- Garringer HJ, Mortazavi SM, Esteghamat F, Malekpour M, Boztepe H, Tanakol R, et al. Two novel GALNT3 mutations in familial tumoral calcinosis. Am J Med Genet A. 2007;143A(20):2390–6. doi: 10.1002/ajmg.a.31947. [DOI] [PubMed] [Google Scholar]
- Groeneveld MC, Everts V, Beertsen W. Alkaline phosphatase activity in the periodontal ligament and gingiva of the rat molar: its relation to cementum formation. J Dent Res. 1995;74(7):1374–81. doi: 10.1177/00220345950740070901. [DOI] [PubMed] [Google Scholar]
- Hao J, Zou B, Narayanan K, George A. Differential expression patterns of the dentin matrix proteins during mineralized tissue formation. Bone. 2004;34(6):921–32. doi: 10.1016/j.bone.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Hardy DC, Murphy WA, Siegel BA, Reid IR, Whyte MP. X-linked hypophosphatemia in adults: prevalence of skeletal radiographic and scintigraphic features. Radiology. 1989;171(2):403–14. doi: 10.1148/radiology.171.2.2539609. [DOI] [PubMed] [Google Scholar]
- Harris SE, Gluhak-Heinrich J, Harris MA, Yang W, Bonewald LF, Riha D, et al. DMP1 and MEPE expression are elevated in osteocytes after mechanical loading in vivo: theoretical role in controlling mineral quality in the perilacunar matrix. J Musculoskelet Neuronal Interact. 2007;7(4):313–5. [PMC free article] [PubMed] [Google Scholar]
- He G, Dahl T, Veis A, George A. Dentin matrix protein 1 initiates hydroxyapatite formation in vitro. Connect Tissue Res. 2003a;44(Suppl 1):240–5. [PubMed] [Google Scholar]
- He G, Dahl T, Veis A, George A. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat Mater. 2003b;2(8):552–8. doi: 10.1038/nmat945. [DOI] [PubMed] [Google Scholar]
- Hesse M, Frohlich LF, Zeitz U, Lanske B, Erben RG. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol. 2007;26(2):75–84. doi: 10.1016/j.matbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Hunter GK, Goldberg HA. Modulation of crystal formation by bone phosphoproteins: role of glutamic acid-rich sequences in the nucleation of hydroxyapatite by bone sialoprotein. Biochem J. 1994;302(Pt 1):175–9. doi: 10.1042/bj3020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter GK, Hauschka PV, Poole AR, Rosenberg LC, Goldberg HA. Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. Biochem J. 1996;317(Pt 1):59–64. doi: 10.1042/bj3170059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa S, Lyles KW, Econs MJ. A novel GALNT3 mutation in a pseudoautosomal dominant form of tumoral calcinosis: evidence that the disorder is autosomal recessive. J Clin Endocrinol Metab. 2005;90(4):2420–3. doi: 10.1210/jc.2004-2302. [DOI] [PubMed] [Google Scholar]
- Ichikawa S, Guigonis V, Imel EA, Courouble M, Heissat S, Henley JD, et al. Novel GALNT3 mutations causing hyperostosis-hyperphosphatemia syndrome resultin low intact fibroblast growth factor 23 concentrations. J Clin Endocrinol Metab. 2007a;92(5):1943–7. doi: 10.1210/jc.2006-1825. [DOI] [PubMed] [Google Scholar]
- Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007b;117(9):2684–91. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa S, Sorenson AH, Austin AM, Mackenzie DS, Fritz TA, Moh A, et al. Ablation of the Galnt3 gene leads to low-circulating intact fibroblast growth factor 23 (Fgf23) concentrations and hyperphosphatemia despite increased Fgf23 expression. Endocrinology. 2009;150(6):2543–50. doi: 10.1210/en.2008-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurutka PW, Bartik L, Whitfield GK, Mathern DR, Barthel TK, Gurevich M, et al. Vitamin D receptor: key roles in bone mineral pathophysiology, molecular mechanism of action, and novel nutritional ligands. J Bone Miner Res. 2007;22(Suppl 2):V2–10. doi: 10.1359/jbmr.07s216. [DOI] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Kuro-o M. Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens. 2006;15(4):437–41. doi: 10.1097/01.mnh.0000232885.81142.83. [DOI] [PubMed] [Google Scholar]
- Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, et al. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145(7):3087–94. doi: 10.1210/en.2003-1768. [DOI] [PubMed] [Google Scholar]
- Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD. Pathogenic Role of FGF23 in Hyp Mice. Am J Physiol Endocrinol Metab. 2006 doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18(6):1637–47. doi: 10.1681/ASN.2007010068. [DOI] [PubMed] [Google Scholar]
- Liu S, Tang W, Zhou J, Vierthaler L, Quarles LD. Distinct roles for intrinsic osteocyte abnormalities and systemic factors in regulation of FGF23 and bone mineralization in Hyp mice. Am J Physiol Endocrinol Metab. 2007;293(6):E1636–44. doi: 10.1152/ajpendo.00396.2007. [DOI] [PubMed] [Google Scholar]
- Liu S, Zhou J, Tang W, Menard R, Feng JQ, Quarles LD. Pathogenic role of Fgf23 in Dmp1-null mice. Am J Physiol Endocrinol Metab. 2008;295(2):E254–61. doi: 10.1152/ajpendo.90201.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz-Depiereux B, Bastepe M, Benet-Pages A, Amyere M, Wagenstaller J, Muller-Barth U, et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006;38(11):1248–50. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Ye L, Yu S, Zhang S, Xie Y, McKee MD, et al. Rescue of odontogenesis in Dmp1-deficient mice by targeted re-expression of DMP1 reveals roles for DMP1 in early odontogenesis and dentin apposition in vivo. Dev Biol. 2007;303(1):191–201. doi: 10.1016/j.ydbio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu TT. Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. Dentin phosphoprotein DNA sequence determination. J Biol Chem. 1997;272(2):835–42. doi: 10.1074/jbc.272.2.835. [DOI] [PubMed] [Google Scholar]
- Malaval L, Wade-Gueye NM, Boudiffa M, Fei J, Zirngibl R, Chen F, et al. Bone sialoprotein plays a functional role in bone formation and osteoclastogenesis. J Exp Med. 2008;205(5):1145–53. doi: 10.1084/jem.20071294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi L, Gozzini A, Franchi A, Campanacci D, Amedei A, Falchetti A, et al. A novel recessive mutation of fibroblast growth factor-23 in tumoral calcinosis. J Bone Joint Surg Am. 2009;91(5):1190–8. doi: 10.2106/JBJS.H.00783. [DOI] [PubMed] [Google Scholar]
- Medici D, Razzaque MS, Deluca S, Rector TL, Hou B, Kang K, et al. FGF-23-Klotho signaling stimulates proliferation and prevents vitamin D-induced apoptosis. J Cell Biol. 2008;182(3):459–65. doi: 10.1083/jcb.200803024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memon F, El-Abbadi M, Nakatani T, Taguchi T, Lanske B, Razzaque MS. Does Fgf23-klotho activity influence vascular and soft tissue calcification through regulating mineral ion metabolism? Kidney Int. 2008 doi: 10.1038/ki.2008.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan JL, Narisawa S, Lemire I, Loisel TP, Boileau G, Leonard P, et al. Enzyme replacement therapy for murine hypophosphatasia. J Bone Miner Res. 2008;23(6):777–87. doi: 10.1359/JBMR.071213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama T, Iwatsubo R, Akiyama S, Amano A, Morisaki I. Familial hypophosphatemic vitamin D-resistant rickets: dental findings and histologic study of teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(3):310–6. doi: 10.1067/moe.2000.107522. [DOI] [PubMed] [Google Scholar]
- Naikmasur V, Guttal K, Bhargava P, Burde K, Sattur A, Nandimath K. Tumoral calcinosis with dental manifestations--a case report. Dent Update. 2008;35(2):134–6. 138. doi: 10.12968/denu.2008.35.2.134. [DOI] [PubMed] [Google Scholar]
- Nakatani T, Sarraj B, Ohnishi M, Densmore MJ, Taguchi T, Goetz R, et al. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23) -mediated regulation of systemic phosphate homeostasis. Faseb J. 2008 doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani T, Ohnishi M, Razzaque MS. Inactivation of klotho function induces hyperphosphatemia even in presence of high serum fibroblast growth factor23 levels in a genetically engineered hypophosphatemic (Hyp) mouse model. Faseb J. 2009 doi: 10.1096/fj.08-123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan K, Gajjeraman S, Ramachandran A, Hao J, George A. Dentin matrix protein 1 regulates dentin sialophosphoprotein gene transcription during early odontoblast differentiation. J Biol Chem. 2006;281(28):19064–71. doi: 10.1074/jbc.M600714200. [DOI] [PubMed] [Google Scholar]
- Nociti FH, Jr, Berry JE, Foster BL, Gurley KA, Kingsley DM, Takata T, et al. Cementum: a phosphate-sensitive tissue. J Dent Res. 2002;81(12):817–21. doi: 10.1177/154405910208101204. [DOI] [PubMed] [Google Scholar]
- Papagerakis P, Berdal A, Mesbah M, Peuchmaur M, Malaval L, Nydegger J, et al. Investigation of osteocalcin, osteonectin, and dentin sialophosphoprotein in developing human teeth. Bone. 2002;30(2):377–85. doi: 10.1016/s8756-3282(01)00683-4. [DOI] [PubMed] [Google Scholar]
- Pereira CM, de Andrade CR, Vargas PA, Coletta RD, de Almeida OP, Lopes MA. Dental alterations associated with X-linked hypophosphatemic rickets. J Endod. 2004;30(4):241–5. doi: 10.1097/00004770-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Cadena E, Ridall A, Tsujigiwa H, Nagatsuka H, et al. The expression of dentin sialophosphoprotein gene in bone. J Dent Res. 2002;81(6):392–4. doi: 10.1177/154405910208100607. [DOI] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Baba O, Wygant JN, McIntyre BW, Butler WT. Dentin sialoprotein isoforms: detection and characterization of a high molecular weight dentin sialoprotein. Eur J Oral Sci. 2003a;111(3):235–42. doi: 10.1034/j.1600-0722.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Cadena E, Ridall A, Butler WT. Dentin sialoprotein in bone and dentin sialophosphoprotein gene expressed by osteoblasts. Connect Tissue Res. 2003b;44(Suppl 1):179–83. [PubMed] [Google Scholar]
- Qin C, Baba O, Butler WT. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med. 2004;15(3):126–36. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- Qin C, D’Souza R, Feng JQ. Dentin matrix protein 1 (DMP1): new and important roles for biomineralization and phosphate homeostasis. J Dent Res. 2007;86(12):1134–41. doi: 10.1177/154405910708601202. [DOI] [PubMed] [Google Scholar]
- Quarles LD. FGF23, PHEX, and MEPE regulation of phosphate homeostasis and skeletal mineralization. Am J Physiol Endocrinol Metab. 2003;285(1):E1–9. doi: 10.1152/ajpendo.00016.2003. [DOI] [PubMed] [Google Scholar]
- Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112(5):683–92. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios HF, Ye L, Dusevich V, Eick D, Bonewald LF, Feng JQ. DMP1 is essential for osteocyte formation and function. J Musculoskelet Neuronal Interact. 2005;5(4):325–7. [PubMed] [Google Scholar]
- Rosen CJ. Bone remodeling, energy metabolism, and the molecular clock. Cell Metab. 2008;7(1):7–10. doi: 10.1016/j.cmet.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Rutherford RB, Foster BL, Bammler T, Beyer RP, Sato S, Somerman MJ. Extracellular phosphate alters cementoblast gene expression. J Dent Res. 2006;85(6):505–9. doi: 10.1177/154405910608500605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98(11):6500–5. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113(4):561–8. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, et al. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23(7):421–32. doi: 10.1016/j.matbio.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitara D, Razzaque MS, St-Arnaud R, Huang W, Taguchi T, Erben RG, et al. Genetic ablation of vitamin D activation pathway reverses biochemical and skeletal anomalies in Fgf-23-null animals. Am J Pathol. 2006;169(6):2161–70. doi: 10.2353/ajpath.2006.060329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenath T, Thyagarajan T, Hall B, Longenecker G, D’Souza R, Hong S, et al. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 2003;278(27):24874–80. doi: 10.1074/jbc.M303908200. [DOI] [PubMed] [Google Scholar]
- Strom TM, Juppner H. PHEX, FGF23, DMP1 and beyond. Curr Opin Nephrol Hypertens. 2008;17(4):357–62. doi: 10.1097/MNH.0b013e3282fd6e5b. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Amizuka N, Oda K, Li M, Yoshie H, Ohshima H, et al. Histological evidence of the altered distribution of osteocytes and bone matrix synthesis in klotho-deficient mice. Arch Histol Cytol. 2005;68(5):371–81. doi: 10.1679/aohc.68.371. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Amizuka N, Oda K, Noda M, Ohshima H, Maeda T. Involvement of the klotho protein in dentin formation and mineralization. Anat Rec (Hoboken) 2008;291(2):183–90. doi: 10.1002/ar.20630. [DOI] [PubMed] [Google Scholar]
- Tartaix PH, Doulaverakis M, George A, Fisher LW, Butler WT, Qin C, et al. In vitro effects of dentin matrix protein-1 on hydroxyapatite formation provide insights into in vivo functions. J Biol Chem. 2004;279(18):18115–20. doi: 10.1074/jbc.M314114200. [DOI] [PubMed] [Google Scholar]
- Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36(6):579–81. doi: 10.1038/ng1358. [DOI] [PubMed] [Google Scholar]
- Toyosawa S, Shintani S, Fujiwara T, Ooshima T, Sato A, Ijuhin N, et al. Dentin matrix protein 1 is predominantly expressed in chicken and rat osteocytes but not in osteoblasts. J Bone Miner Res. 2001;16(11):2017–26. doi: 10.1359/jbmr.2001.16.11.2017. [DOI] [PubMed] [Google Scholar]
- van den Bos T, Handoko G, Niehof A, Ryan LM, Coburn SP, Whyte MP, et al. Cementum and dentin in hypophosphatasia. J Dent Res. 2005;84(11):1021–5. doi: 10.1177/154405910508401110. [DOI] [PubMed] [Google Scholar]
- Witcher SL, Jr, Drinkard DW, Shapiro RD, Schow CE., Jr Tumoral calcinosis with unusual dental radiographic findings. Oral Surg Oral Med Oral Pathol. 1989;68(1):104–7. doi: 10.1016/0030-4220(89)90123-0. [DOI] [PubMed] [Google Scholar]
- Xiao NM, Zhang YM, Zheng Q, Gu J. Klotho is a serum factor related to human aging. Chin Med J (Engl) 2004;117(5):742–7. [PubMed] [Google Scholar]
- Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, et al. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem. 2004;279(18):19141–8. doi: 10.1074/jbc.M400490200. [DOI] [PubMed] [Google Scholar]
- Ye L, Zhang S, Ke H, Bonewald LF, Feng J. Periodontal breakdown in the dmp1 null mouse model of hypophosphatemic rickets. J Dent Res. 2008;87(7):624–9. doi: 10.1177/154405910808700708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiko Y, Wang H, Minamizaki T, Ijuin C, Yamamoto R, Suemune S, et al. Mineralized tissue cells are a principal source of FGF23. Bone. 2007;40(6):1565–73. doi: 10.1016/j.bone.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Zhang X, Rahemtulla FG, MacDougall MJ, Thomas HF. Vitamin D receptor deficiency affects dentin maturation in mice. Arch Oral Biol. 2007;52(12):1172–9. doi: 10.1016/j.archoralbio.2007.06.010. [DOI] [PubMed] [Google Scholar]