Abstract
Induction of tolerance in memory T cells has profound implications in the treatment of autoimmune diseases and transplant rejection. Previously, we reported that the presentation of low densities of agonist peptide/MHC class II complexes induced anergy in memory CD4+ T cells. In the present study, we address the specific interaction of different types of APCs with memory CD4+ T cells. A novel ex vivo anergy assay first suggested that B cells induce anergy in memory T cells, and an in vivo cell transfer assay further confirmed those observations. We demonstrated that B cells pulsed with defined doses of Ag anergize memory CD4 cells in vivo. We established that CD11c+ dendritic cells do not contribute to anergy induction to CD4 memory T cells, because diphtheria toxin receptor-transgenic mice that were conditionally depleted of dendritic cells optimally induced anergy in memory CD4+ T cells. Moreover, B cell-deficient muMT mice did not induce anergy in memory T cells. We showed that B2 follicular B cells are the specific subpopulation of B cells that render memory T cells anergic. Furthermore, we present data showing that anergy in this system is mediated by CTLA-4 up-regulation on T cells. This is the first study to demonstrate formally that B cells are the APCs that induce anergy in memory CD4+ T cells.
Memory T cells are a small subpopulation of a pool of Ag-experienced lymphocytes that have undergone proliferation and morphological changes and survived Ag-induced cell death (1–4). Memory T cells are believed to play crucial roles in mounting fast and efficient recall responses against Ags (5, 6). For naive T cells residing in lymph nodes (LNs),6 it is documented that dendritic cells (DCs) that have captured Ag from the peripheral tissues are the primary APCs for initiation of the immune responses. There is also evidence that DCs can tolerize Ag-specific naive T cells (7). Although recent reports document Ag presentation as a contributing factor to the development of memory T cells (8, 9), evidence for specific interaction of different types of APCs with memory CD4+ T cells remains elusive. It is not clear whether DCs are also potent activators of memory T cells. Even less information is available on the types of APCs that interact with memory T cells and render them tolerant. In the present study, we address this specific question using our well-characterized system. By studying human T cell clones, we reported that the presentation of 1–10 peptide/MHC II complexes per APC induce anergy (10). Extending these observations to in vivo systems, we found that memory, but not activated or naïve, T cells were anergized upon the presentation of low densities of specific Ags (11). Induction of anergy in these reports demonstrated that unresponsiveness was not due to deletion or ignorance and that IL-2 plus peptide could reverse the state of unresponsiveness. In another report, we examined differences in the levels of activation of TCR-mediated signaling molecules triggered by the presentation of stimulatory vs anergy-inducing ligands to T cells (12). Those studies provided clear evidence that stimulation of T cells with ligands of different avidity dictated the nature of T cell response: a high avidity stimulation induced T cell activation, whereas a low avidity stimulation by the same agonist peptide caused T cell anergy. TCR engagement below the tolerogenic threshold had no inhibitory effects on recall responses. These observations highlight the significance of APCs that encounter T cells. Because the level of expression of MHC II and other molecules that contribute to T cell-APC membrane interaction can vary on different subpopulations of APCs, it is important to investigate whether certain APCs preferably interact with memory T cells.
Induction of tolerance in memory T cells has profound implications for the treatment of autoimmune diseases and for controlling transplant rejection. It would be of clinical benefit to identify APCs that can present Ag to memory T cells in a tolerogenic fashion. Mature DCs express high levels of MHC II on their surface (13), making them potential candidates for capture and presentation of low amounts of the peptide Ags necessary for the induction of anergy in our system. However, B cells bearing specific receptors for Ag might be suitable APCs to anergize memory T cells when the antigenic load becomes sparse at the end of an infection and a contraction of the T cell response is desired.
To test different populations of APCs for their abilities to trigger anergy ex vivo, we designed an assay that incorporates the coincubation of memory DO11.10 transgenic (Tg) T cells (chicken OVA (cOVA)323–339/I-Ad specific) with sets of APCs devoid of T cells isolated from groups of mice that had previously received different doses of cOVA323–339. We demonstrate that anergy is induced in memory T cells ex vivo when B cells presented the Ag at a certain range of low peptide doses. Further, we validated this finding in vivo by demonstrating that transferring resting B cells pulsed with low doses of peptide to mice bearing memory CD4+ T cells induced anergy. We ruled out a role for CD11c+ or other DCs in the induction of anergy in memory T cells. Consistent with the role for resting B cells expressing low levels of B7-1 in the induction of anergy, we showed that anergy in this system is regulated by CTLA-4.
Materials and Methods
Mice
TCR Tg mice (DO11.10) that express α/β TCR recognizing an I-Ad-restricted cOVA323–339 on BALB/c background were used as the source of T cells. Non-Tg female BALB/c mice and C57BL/6 (B6) mice at 5–6 wk of age, Tg female DO11.10 and OTII mice, and B cell-deficient muMT mice were all purchased from The Jackson Laboratory. Diphtheria toxin receptor (DTR) Tg mice on BALB/c background were bred with non-Tg BALB/c mice, and the heterozygous offspring for DTR were used for the study. All mice were housed in the Johns Hopkins University animal facilities (Baltimore, MD) under virus-free conditions. All experiments were performed in accordance with protocols approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine.
Peptides and Abs
The peptide cOVA323–339 (ISQAVHAAHAEINEAGR) was synthesized by Global-Peptide Services. The peptide was >90% pure as analyzed by reverse-phase HPLC. Fluorescently labeled Abs to mouse CD4, CD25, CD44, CD45RB, CD62L, CD69, CD45R (B220), CD19, CD11c, IL-2, IFN-γ, anti-mouse CTLA-4, CD80 (B7.1), and CD86 (B7.2) were purchased from BD Pharmingen; the Ab to clonotypic TCR (KJ1.26) specific for DO11.10 CD4+ T cells was purchased from Caltag Laboratories; and anti-mouse CTLA-4 Ab was purified from the culture supernatant of hybridoma (UC10-4F10-11; American Type Culture Collection (ATCC)).
Adoptive transfer and immunizations
KJ1.26+ Tg CD4+ T cells (2.0 × 105 cells per mouse), prepared from pooled LNs and spleens of DO11.10 mice, were resuspended in 100 µl of sterile PBS (Invitrogen) and transferred i.v. into BALB/c recipients. Two days later, mice were immunized s.c. (at the base of the tail) with 15 nmol of cOVA323–339 peptide emulsified in CFA.
Induction of anergy in cOVA323–339-specific memory CD4+ T cells in vivo by administration of low doses of peptide in IFA
Five weeks postimmunization, to allow for the generation of memory T cells (verified by staining for memory T cell markers; data not shown), mice were injected s.c. with increasing concentrations of cOVA323–339 peptide (0.005–50,000 pmol) mixed with IFA. Ten days later, inguinal LNs were removed and T cells were challenged in vitro with cOVA323–339 peptide. Proliferation was measured by CFSE dilution assay. Briefly, LN cells were labeled with 1 µM CFSE in PBS for 80 s at room temperature and washed twice with RPMI 1640 containing 10% FCS. Cells were cultured with or without peptide at 37°C for 72 h. Following the culture, T cells were stained with fluorescently labeled anti-mouse CD4 Ab and KJ1.26 anticlonotypic TCR Ab and analyzed by flow cytometry. T cell division in response to in vitro peptide challenge was calculated as described (14) using the following formula: number of events (x) at each cell division n divided by 2n. The percentage of total dividing cells = (total number of cohorts undergoing 1–6 divisions/total number of cohorts undergoing 0–6 divisions) × 100.
Isolation of T cells
Spleens from 5-wk postimmunized mice were removed and a single-cell suspension was made after lysis of RBCs and used as the source of T cells. The mice sacrificed for this purpose had received a single immunization after adoptive transfer of T cells. By using an R&D Systems CD3 enrichment column, T cells were isolated from splenocytes. T cell purity was >95%.
Isolation of in vivo pulsed APCs
Forty-eight hours after s.c. injection of peptide emulsified in IFA, mice were sacrificed and inguinal LNs were removed. To separate whole APCs, LN cells were depleted of T cells by using either Dynal-Thy1.2 beads or MACS-Thy1.2 microbeads (Miltenyi Biotec) (~95% depletion). To isolate B cells, a MACS B220 microbead was used (~95% purity). To obtain APCs devoid of B cells, LN cells were depleted of T and B cells by using a combination of Thy1.2 and B220 microbeads (MACS). MACS CD43 microbeads were used to purify B2 B cells. To obtain APCs devoid of B2 B cells, LN cells were depleted of T cells by Thy1.2 microbeads followed by positive selection with CD4 microbeads (15). These APCs are termed in vivo pulsed APCs in this study.
Induction of anergy in memory CD4+ T cells ex vivo by in vivo pulsed APCs
To induce anergy in memory CD4+ T cells, enriched T cells (1 × 105/well) were incubated with in vivo pulsed APCs (irradiated, 2000 rad) at a ratio of 1:2 for 48 h in a 96-well plate. To test for anergy, splenocytes from normal mice were pulsed in vitro with cOVA323–339 peptide, irradiated, and added (2 × 105 per well) to triplicate culture wells and incubated further for 72 h before adding [3H]thymidine or for 10 h before assaying for intracellular cytokine synthesis. To determine the percentage of cells making IL-2 or IFN-γ, cells were stained with Abs to CD4, clonotypic TCR (KJ1.26), and IL-2 or IFN-γ and analyzed by flow cytometry. During data analysis, the quadrants were drawn based on the matched isotype Ab controls. The percentage of DO11.10 cells making IL-2 or IFN-γ was calculated after subtracting the percentage of DO11.10 cells stained positive for the matched isotypes.
Induction of anergy in memory CD4+ T cells ex vivo by in vitro pulsed B cells, DCs, or activated B cells
We followed the same anergy protocol as above except that B cells or DCs were purified from the spleens of unimmunized mice by using MACs-B220 for B cells (purity >95%) or MACs-CD11c microbeads for DCs (purity >80%) and pulsed in vitro for 3 h at 37°C with the cOVA323–339 peptide before incubating with memory T cells. For activated B cells, purified B cells were stimulated with CpG (6 µg/ml) for 24 h (16) and washed twice before pulsing with peptide. Note that the amount of peptide for pulsing APCs in vitro was calculated based on molar peptide concentration, whereas the peptides given to mice were in mole units.
Induction of anergy in memory T cells in vivo in the absence of CD11c+ DCs
BALB/c mice expressing DTR under the CD11c promoter (17) were adoptively transferred with DO11.10 Tg T cells. Two days later, mice were immunized s.c. with cOVA323–339 peptide in CFA. After 5 wk, DCs were depleted by multiple injections of diphtheria toxin (DT) (Sigma-Aldrich) during the induction of anergy in vivo in these mice. Briefly, mice were injected i.p. with 100 ng of DT per mouse 12 h before the injection of peptide emulsified in IFA. Then, mice were injected with the same dose of DT twice more, at 10 and 36 h after Ag challenge. Sampling of spleen and LN tissues during the treatment in a parallel set of mice confirmed the depletion of DCs in DT-injected mice as detected by selection on CD11c and GFP (expressed under the CD11c promoter), indicating <0.2% DCs in the LNs of those mice. On day 3 (60 h after Ag injection), mice were sacrificed and cells harvested from the draining LNs were challenged ex vivo with cOVA323–339 peptide for 72 h. Induction of anergy in Ag-specific CD4+ T cells was determined by CFSE dilution assay. The percentage of Ag-specific (DO11.10) CD4+ T cell divisions in response to in vitro peptide challenge was calculated as described above.
Induction of anergy in vivo in memory T cells by in vitro pulsed B cells
B cells were purified from the spleens of unimmunized mice and pulsed in vitro with increasing amounts of cOVA323–339 peptide (0.00001–1000 nM) for 3 h at 37°C. Following incubation, B cells (not irradiated) were washed twice to remove excess peptide and 107 cells/mouse were i.v. transferred to BALB/c mice bearing cOVA323–339-specific memory CD4+ T cells. Ten days later, mice were sacrificed, and cells from draining LNs and spleens were harvested. Induction of anergy in Ag-specific T cells was determined by the CFSE dilution assay after in vitro challenge with the cOVA323–339 peptide for 72 h. The percentage of Ag-specific (DO11.10) CD4+ T cell division in response to in vitro peptide challenge was calculated as described above.
Induction of anergy in memory CD4+ T cells in vitro by in vivo pulsed B2 B cells
All B cells, B2 B cells, or APCs devoid of B2 B cells were obtained from the draining LNs of mice immunized 48 h earlier with peptide in IFA and incubated with memory CD4+ T cells for 48 h. Induction of anergy was tested by restimulating T cells with cOVA323–339 peptide-pulsed splenocytes for 72 h, followed by [3H]thymidine incorporation.
Induction of anergy in memory CD4+ T cells in B cell-deficient muMT mice
Because muMT mice are on B6 background, only for this experiment B6 mice were used as recipients and OT-II Tg CD4 T cells specific for cOVA323–339/I-Ab were used for adoptive transfer and the generation of memory T cells. Recipient mice were immunized with cOVA323–339 in CFA for the induction of memory T cells a day after T cell transfer as described previously. In the experiments shown, memory cells were isolated 4–6 mo after immunization. CD4 T cells (7 × 106 to include ~4.2 × 104 OT-II T cells) were then purified and transferred to muMT mice before the injection of Ag for anergy induction. B6 mice bearing memory OT-II cells were called B cell-sufficient mice. All groups of mice were immunized s.c. with cOVA323–339 in IFA. Nine days later, cells from draining LNs were harvested and assayed for anergy by their ability to proliferate and to make IL-2 and IFN-γ using thymidine incorporation and intracellular cytokine assays. For thymidine incorporation, cells were stimulated with peptide for 72 h followed by the addition of [3H]thymidine and incubated for 18 h before harvest. For intracellular cytokine synthesis, cells were stimulated with peptide for 5.5 h in the presence of GolgiStop. OT-II cells were then stained with Abs to CD4, Vα2, Vβ5.1, Vβ5.2, and IL-2 or IFN-γ. During data analysis, the quadrants were drawn based on the matched isotype Ab controls. The percentage of OT-II cells making IL-2 or IFN-γ was calculated after subtracting the percentage of OT-II cells stained positive for the matched isotypes.
Expression of CTLA-4 in memory CD4+ T cells
Memory CD4+ T cells were incubated with in vivo pulsed APCs (B cells or APCs depleted of B cells) for 48 h. Surface expression of CTLA-4 on CD4+ T cells was determined by flow cytometry. The level of CTLA-4 was expressed as CTLA-4 index: mean fluorescence intensity × fraction of cells positive for CTLA-4.
Reversal of anergy in memory CD4+ T cells by anti-CTLA-4
Memory CD4+ T cells were incubated with in vivo pulsed B cells for 48 h in the presence of 10 µg/ml anti-mouse CTLA-4 Ab (UC10-4F10-11; ATCC). Anergy or reversal was tested by restimulating T cells with cOVA323–339 peptide-pulsed splenocytes for 72 h, followed by [3H] thymidine incorporation.
Statistical analyses
Level of significance (p value) was determined by using unpaired Student’s t test. p < 0.02 was considered significant.
Results
cOVA323–339 specific CD4+ T cells acquire memory phenotype 35 days postpriming
We have previously shown the induction of anergy in memory CD4 T cells in response to low dose antigenic challenge in two antigenic systems (11). To extend those findings to the DO11.10 Tg system (cOVA323–339/IAd specific), DO11.10 Tg T cells were transferred into wild-type BALB/c mice (18) and 2 days later, which allowed the homing of transferred cells to the lymphoid organs, recipients were immunized with 15 nmol of cOVA323–339 in CFA. Thirty-five days after immunization, the time required for the generation of memory T cells (11, 19), splenocytes were harvested and DO11.10 CD4+ T cells were stained for memory markers. Levels of CD25, CD44, CD45RB, CD62L, and CD69 expression were analyzed on CD4+ and KJ1.26+ double positive cells and compared with the levels on naive T cells from control mice treated with IFA or CFA alone. By day 35 postimmunization, >80% of the CD4+/KJ1–26+ T cells acquired the memory phenotype as evidenced by an increased level of CD44 and decreased levels of CD45RB and CD62L expression on their cell surfaces (20).
In vivo administration of low doses of peptide in IFA induces anergy in cOVA323–339-specific memory CD4+ T cells
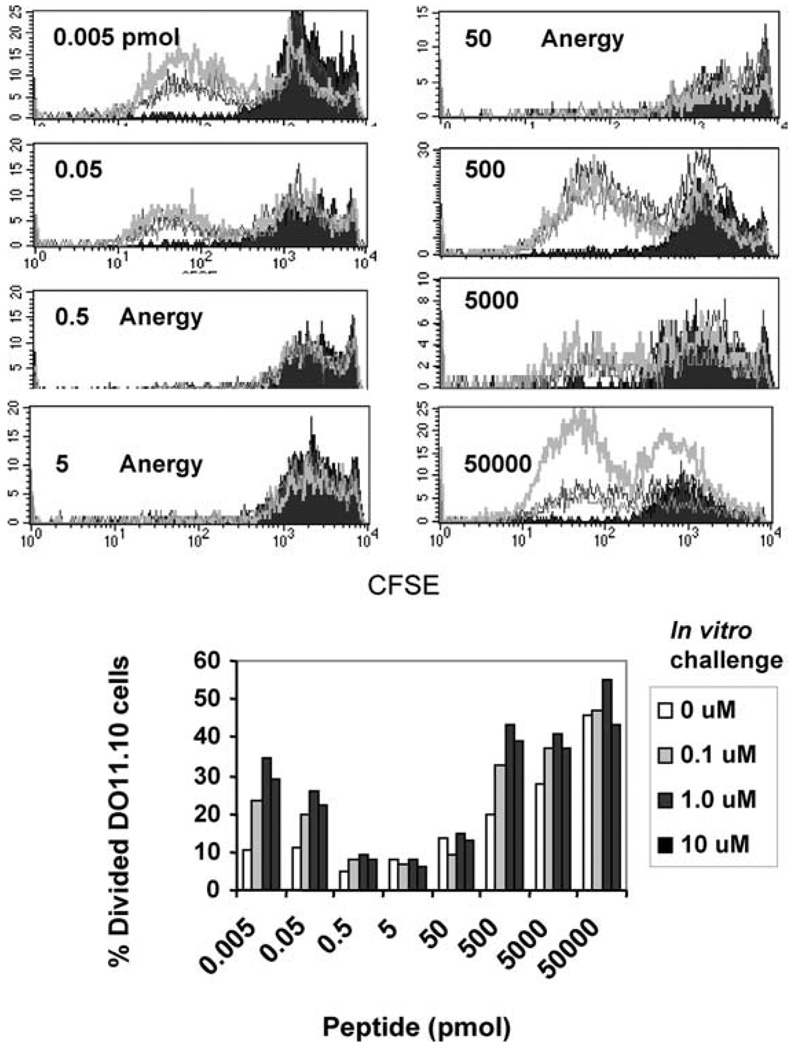
Mice previously immunized with cOVA323–339 for the generation of memory T cells were administered different amounts of peptide in IFA. After 10 days, cells from draining LNs were labeled with CFSE and CD4+ T cells were stimulated in vitro by fresh APCs pulsed with increasing doses of peptide for 72 h. To determine Ag-specific cell proliferation, CFSE dilution analysis was done on CD4+/KJ1–26+ double positive cells by flow cytometry. We found that Ag-specific T cells that had encountered 0.5–50 pmol of peptide in vivo remained undivided, whereas T cells from the mice immunized with doses below or above that range divided several cycles (Fig. 1). The starting numbers of CD4+KJ1.26+ T cells were comparable in all of the groups of mice immunized with peptide in IFA, indicating that T cells are not deleted but rather are anergized. These results extend the applicability of our previous findings to an entirely new antigenic system in which memory CD4+ T cells can be anergized by a range of suboptimal doses of Ags (11, 12).
FIGURE 1.
Administration of suboptimal doses of cOVA323–339 peptide in IFA induces anergy in specific memory CD4+ T cells in vivo. Eight groups of mice (three mice per group) bearing memory CD4+ T cells were challenged with increasing doses (0.005–50,000 pmol) of cOVA323–339 peptide in IFA. Ten days later, cells from draining LNs were harvested and anergy was assayed by peptide rechallenge for 72 h in vitro. T cell proliferation was measured by CFSE dilution assay of Ag-specific KJ1–26+ CD4+ T cells. The doses for the in vitro challenge with cOVA323–339 peptide are represented as follows: filled black histogram, 0 µM; thin gray line, 0.1 µM; black line, 1 µM; thick gray line, 10 µM. Data shown represent one of three independent experiments. The lower panel represents the percentage of Ag-specific cells (DO11.10) divided in response to in vitro challenge.
Ex vivo anergy assay to test roles for different APCs in the induction of anergy in memory CD4+ T cells
Determining which APCs uniquely interact with memory T cells in vivo is experimentally challenging. To simplify the problem, an ex vivo assay was designed to evaluate the contribution of different APCs to the induction of anergy in CD4+ memory T cells. We hypothesized that APCs loaded in vivo with low doses of peptide could anergize memory T cells ex vivo in a similar manner as they do in vivo. To test this, memory T cells were isolated from mice 35 days postimmunization and coincubated with APCs from the draining LNs of mice that had been immunized with increasing doses of peptide (range 0–5,000 pmol) in IFA 48 h earlier. Cells were cultured for 48 h and then tested for anergy induction upon stimulation with splenocytes pulsed with 0, 0.1, 1, and 10 µM peptide. Fig. 2A is a control proliferation experiment indicating that anergy is established within 48 h in vivo. Fig. 2, B–D, depict results of the ex vivo assay indicating the proliferation of T cells exposed to APCs from mice injected with different doses of peptide in IFA. These experiments clearly show that during the 48-h exposure to APCs, memory T cells became anergic in response to whole APCs (LN cells free of T cells) loaded with tolerogenic doses of peptide, while APCs loaded with nontolerogenic doses of peptide did not prevent the subsequent proliferation of T cells (Fig. 2B). The proliferation of memory T cells encountering tolerogenic doses of Ag, as accessed by [3H]thymidine incorporation, was significantly reduced ( p < 0.005) compared with the proliferation of T cells encountering nontolerogenic doses of Ag, although not completely blocked. This background proliferation was likely because of nonspecific thymidine uptake by bystander lymphocytes. This might be due to the distribution of the MHC II molecules/MHC II-peptide complexes on APCs that most likely follow the Poisson distribution and, thus, those APCs with higher numbers of MHC II-peptide complexes induce activation in T cells rather than anergy.
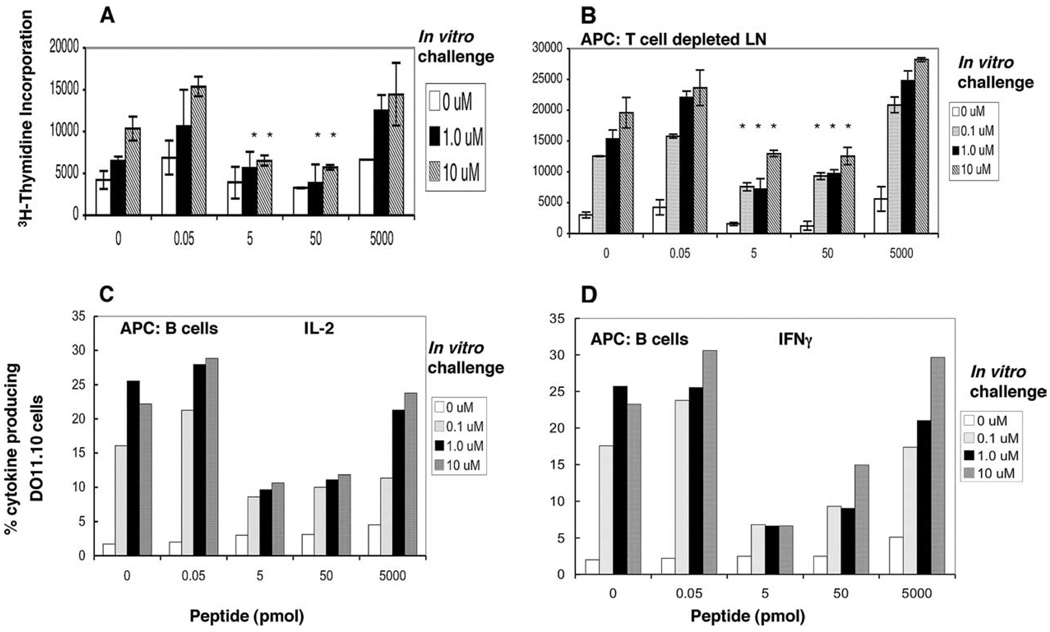
FIGURE 2.
B cells loaded with low doses of Ag induce anergy in memory CD4+ T cells ex vivo. A, Anergy was induced in vivo (as described in Materials and Methods). Mice bearing memory CD4+T cells were given cOVA323–339 in IFA for the induction of anergy and 48 h later draining LNs were harvested and tested for anergy in vitro using [3H]thymidine incorporation. B–D, Anergy was induced ex vivo. Memory T cells isolated (APC-depleted T cells) from mice immunized 5 wk or longer were incubated with APC populations (whole LN cells depleted of T cells (B) or enriched for B cells (C and D)) isolated from BALB/c mice given cOVA323–339 in IFA 48 h before the cell harvest. Isolated APCs (depleted of T cells by Thy1.2 microbeads) were pulsed with different doses of peptide as shown on the x-axis. Memory T cells and pulsed APCs were incubated for 48 h. Cultured cells were assayed for the induction of anergy during the first 48 h of ex vivo culture by the addition of freshly pulsed splenocytes at 0, 0.1, 1.0, and 10 µM cOVA323–339 peptide in vitro and cell proliferation (B) and IL-2 (C) and IFN-γ production (D) were measured. The data shown represent one of three independent experiments. Bars represent mean ± SD of triplicate cultures. *, p < 0.005, 5, or 50 pmol vs 0.05 or 5000 pmol of peptide doses (Student’s t test). There were five mice per group and cells from triplicate wells were pooled before measuring IL-2 and IFN-γ synthesis. Cytokine synthesis was analyzed on KJ1.26+IL-2+ or KJ1.26+IFN-γ+ cells gated on CD4+ T cells.
B cells as the APCs that induce anergy in memory T cells
Next, we tested the ability of purified B220+-enriched B cells (to be called purified B cells throughout the article) loaded with peptide in vivo to induce anergy in Ag-specific memory T cells ex vivo (Fig. 2, C and D) by measuring IL-2 and IFN-γ synthesis. We found that B cells exposed to 5–50 pmol of peptide in vivo anergized memory T cells as assessed by their reduced ability to produce intracellular IL-2 and IFN-γ. Encouraged by these results, we investigated whether B cells pulsed with Ag in vitro could induce anergy ex vivo. Purified splenic B cells from unimmunized mice were pulsed in vitro with different amounts of peptide and tested for the induction of anergy in memory T cells ex vivo. We found that splenic B cells pulsed with 0.1–1 nM peptide in vitro induced anergy (Fig. 3A). Note that we have used mole (mol) units for absolute amounts of peptide injected per mouse and concentrations of peptide used to pulse APCs ex vivo are in molar (M) units.
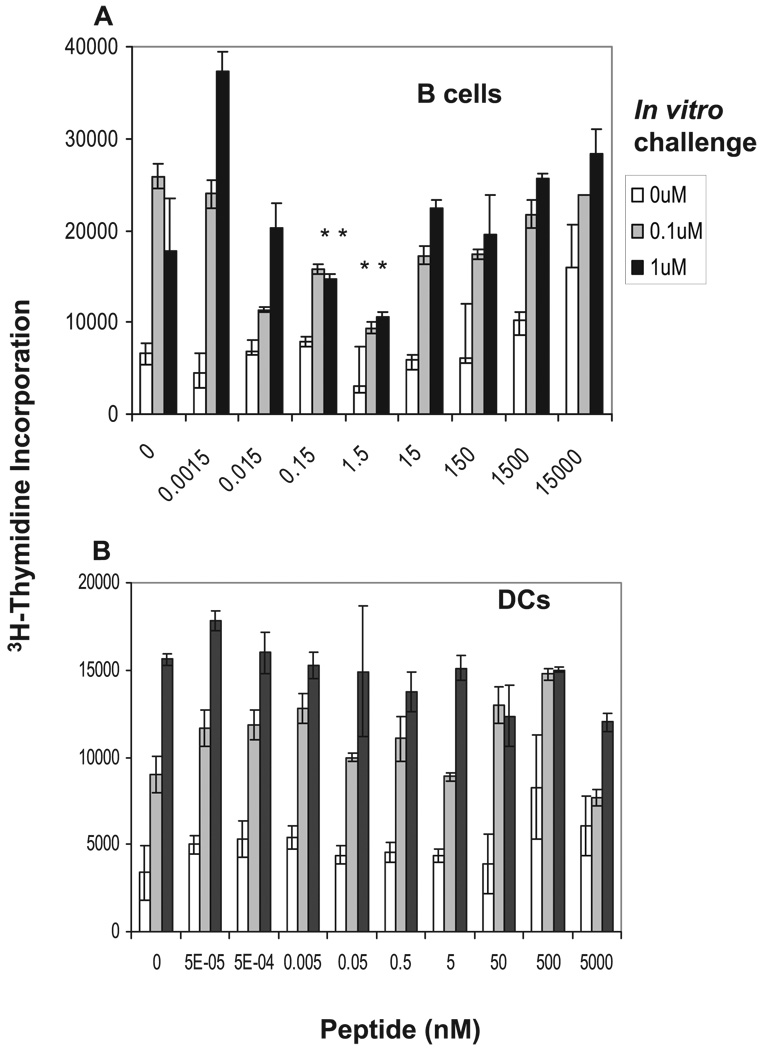
FIGURE 3.
In vitro pulsed B cells induce anergy in memory CD4+ T cells ex vivo. Enriched B220+ B cells (A) or CD11c+ enriched DCs (B) from spleens of five normal BALB/c mice were pulsed with the indicated doses of cOVA323–339 peptide for 3 h, incubated with memory CD4+ T cells for 48 h, and tested for anergy as described in the legend of Fig. 2 using [3H]thymidine incorporation. Data shown represent one of four independent experiments. Bars represent mean ± SD of triplicate cultures. For A, *, p < 0.001, 0.15, or 1.5 nM vs 0.0015 or 15,000 nM peptide (Student’s t test).
DCs do not induce anergy in memory CD4+ T cells ex vivo
We next examined the contribution of DCs to the induction of anergy. Purified DCs from the splenocytes of unimmunized BALB/c mice were pulsed with different concentrations of cOVA323–339 peptide and incubated with memory T cells. Because the levels of MHC II expression on DCs were three times higher than those on B cells, the concentration of peptide used for pulsing was adjusted 3-fold to keep the absolute numbers of peptide/MHC complexes between B and DCs the same. Fig. 3B depicts one representative experiment of four showing that DCs did not anergize memory CD4+ T cells at any of the peptide concentrations tested.
DC depletion does not affect the induction of anergy in vivo
Although we found that B cells but not DCs induce anergy, because of the broad immunological significance of DCs, it is possible that our in vitro conditions were not ideal and that DCs might contribute to anergy induction in memory T cells in vivo. To address this issue, we used DTR Tg mice as DO11.10 Tg T cell recipients and generated memory T cells by OVA peptide in CFA immunization as described above. DTR Tg mice express a fusion protein of DTR and enhanced green fluorescence protein (EGFP) under the control of the CD11c promoter (17). This promoter is constitutively active in nearly all conventional DC subsets. Mice do not possess a native DTR and their cells are thus highly resistant to DT. Transgenic DCs express sufficient amounts of DTR-EGFP and thus are depleted following an injection of DT. Because DCs are replenished from precursors, depletion persists only for 24 h after treatment and new DCs are generated 48 h after DT injection (17). Of note, anergy in our system was optimally developed during the first 48 h of Ag encounter in vivo (11). To avoid the presence of DCs during anergy induction, three injections of DT were given: once 12 h before, and twice at 10 h, and + 36 h after peptide injection in IFA. The percentage of DCs, as judged by GFP fluorescence, did not exceed 0.15% of the total leukocytes in draining LNs, i.e., ~85–90% of DCs remained depleted 24 h after the last DT injection.
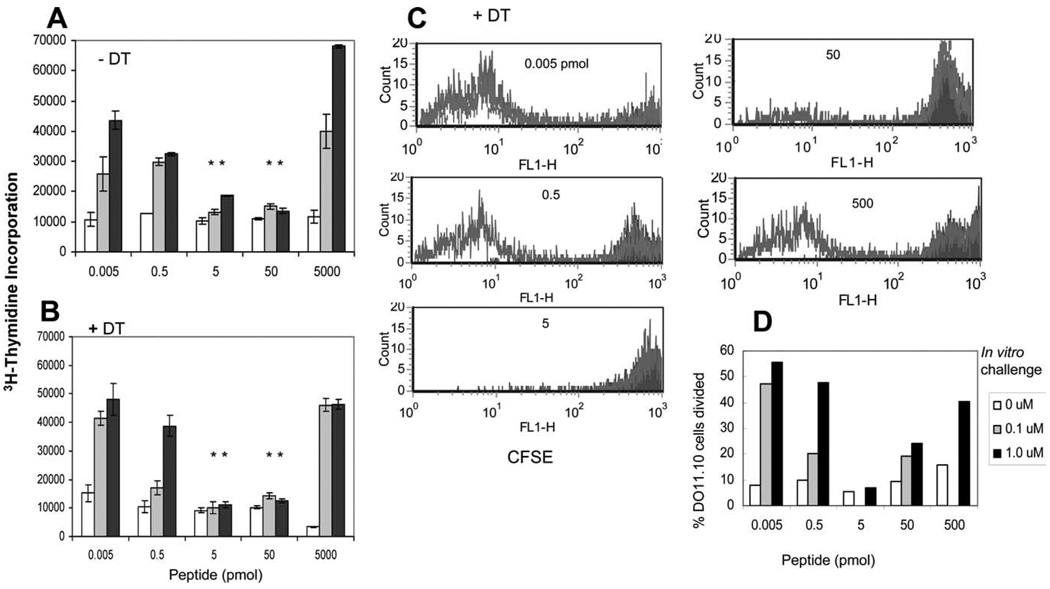
Fig. 4 shows results from two sets of experiments. In Fig, 4A, experiments were done in the presence of CD11c+ DCs, i.e., DTR Tg mice without DT injection, and in B, with multiple DT injections. We observed that T cells from the mice injected with 5–50 pmol of peptide were anergized in the absence of DCs (Fig. 4, A and B) as tested by [3H]thymidine uptake. Similar results were obtained in another set of experiments in Fig. 4C by CSFE dilution assay, suggesting that the contribution of DCs to the induction of anergy in memory T cells is not essential.
FIGURE 4.
Absence of CD11c+ DCs does not affect the induction of anergy in memory CD4+ T cells in vivo. DTR Tg mice expressing DTR under the CD11c promoter (BALB/c background) were used as the source of memory CD4+ T cells. Mice were given daily i.p. injections of DT for 3 days starting 12 h before Ag challenge in IFA. Mice were sacrificed 60 h after Ag challenge, and cells from draining LNs were harvested. Induction of anergy was measured by [3H]thymidine incorporation (A and B) as well as by the CFSE dilution assay (C). For thymidine incorporation, cells were challenged ex vivo with cOVA323–339 peptide for 54 h and pulsed with [3H]thymidine for 18 h. For CFSE dilution assay, cells were labeled with CFSE and similarly challenged ex vivo with cOVA323–339 peptide for 72 h. Ag-specific T cell proliferation was measured by CFSE dilution assay using flow cytometry on CD4+ KJ1.26+ double positive cells. Doses for in vitro challenge with cOVA323–339 peptide are represented as follows: filled black histogram, 0 µM; black line, 0.1 µM; gray line, 1 µM. Data shown represent one of three independent experiments. Data in C are summarized as the percentage of dividing cells and shown in D. There were three mice per group. Bars represent mean ± SD of triplicate cultures. *, p < 0.001, 5, or 50 pmol vs 0.005 or 5000 pmol of peptide doses (Student’s t test).
In vitro pulsed B cells induce anergy in memory CD4+ T cells in vivo
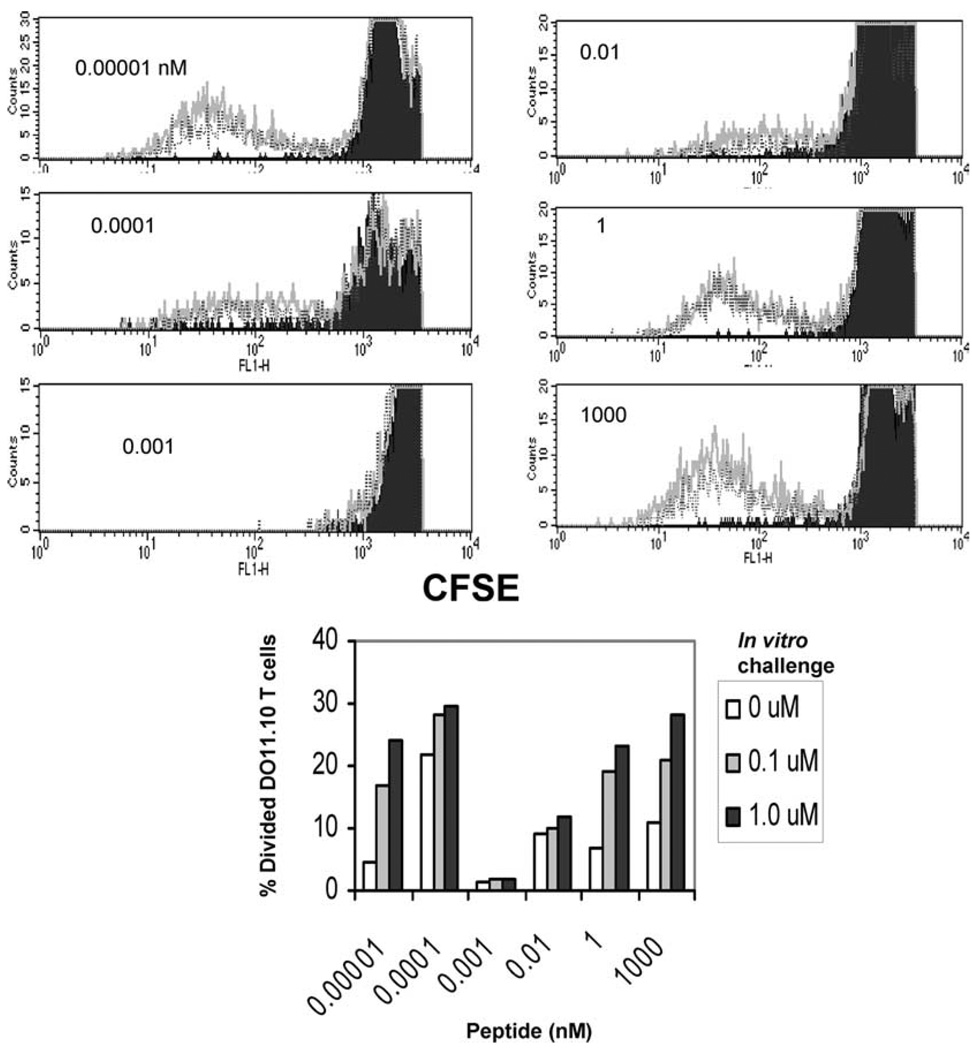
To clarify further the role of B cells in the induction of anergy, we investigated whether memory T cells could be rendered anergic by in vitro pulsed B cells in vivo. To test this, we transferred 107 B cells per mouse (pulsed in vitro with peptide concentrations ranging 0.00001–1000 nM for 3 h) into different groups of mice bearing memory T cells. Ten days later, we used a CFSE dilution assay to determine whether anergy was induced in those memory T cells. We found that Ag-specific T cells ceased to divide beyond 1–2 divisions in groups of mice that were injected with purified B cells loaded with 0.001– 0.01 nM peptide, whereas T cells in mice that received B cells pulsed with peptide below or above those concentrations proliferated multiple times (Fig. 5).
FIGURE 5.
Induction of anergy in memory CD4+ T cells in vivo by in vitro pulsed B cells. Splenic B cells were pulsed in vitro with doses of cOVA323–339 peptide (as shown on the x-axis) for 3 h, transferred to mice bearing memory T cells, and 10 days later the mice were sacrificed. T cell proliferation was measured in draining LNs by the CFSE dilution assay after in vitro challenge with cOVA323–339 peptide. Doses for in vitro challenge with cOVA323–339 peptide are represented as follows: filled black histogram, 0 mM; black line, 0.1 µM; gray line, 1 µM. Data shown represent one of two independent experiments. There were three mice per group. Lower panel represents the percentage of Ag-specific cells (DO11.10) divided in response to in vitro challenge.
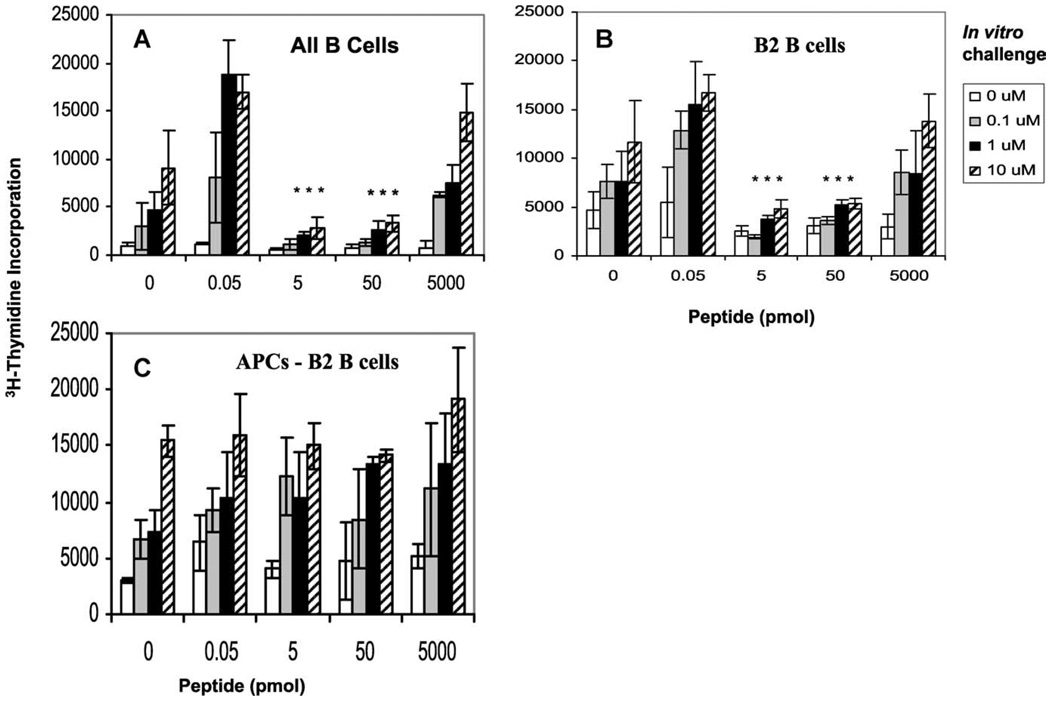
B2 B cells are major contributors for induction of anergy in memory CD4+ T cells
To dissect the population of B cells that induce anergy in memory CD4+ T cells, we purified B2 B cells (conventional B cells residing in lymphoid follicles; Refs. 21 and 22) from draining LNs of mice immunized with peptide in IFA 48 h earlier, and incubated them with CD4+ T cells for the induction of anergy ex vivo. In a parallel experiment, we also used all APCs depleted of B2 B cells and tested their ability to induce anergy. We found that both purified B cells and B2 B cells induced anergy in memory T cells whereas APCs devoid of B2 B cells failed to do so, indicating that B2 B cells are the primary B cells that induce anergy in memory T cells (Fig. 6).
FIGURE 6.
B2 B cells loaded with low doses of Ag induce anergy in memory CD4+ T cells ex vivo. Draining LN cells were harvested from the mice that had been immunized 48 h earlier with cOVA323–339 peptide emulsified in IFA. All B cells (A) were purified by B220 microbeads, B2 B cells (B) were purified by CD43 microbeads, and APCs devoid of B2 B cells (C) were obtained by depleting T cells from LN cells by Thy1.2 microbeads followed by positive selection with CD43 microbeads. All three different types of APCs were incubated with memory T cells for 48 h. Induction of anergy was tested by restimulating the T cells with cOVA323–339 peptide-pulsed splenocytes at 0, 0.1, 1, and 10 µM followed by [3H]thymidine incorporation. The data shown represent one of three independent experiments. Bars represent mean ± SD of triplicate cultures. *, p < 0.005, 5, or 50 pmol vs 0.05 or 5000 pmol of peptide doses (Student’s t test).
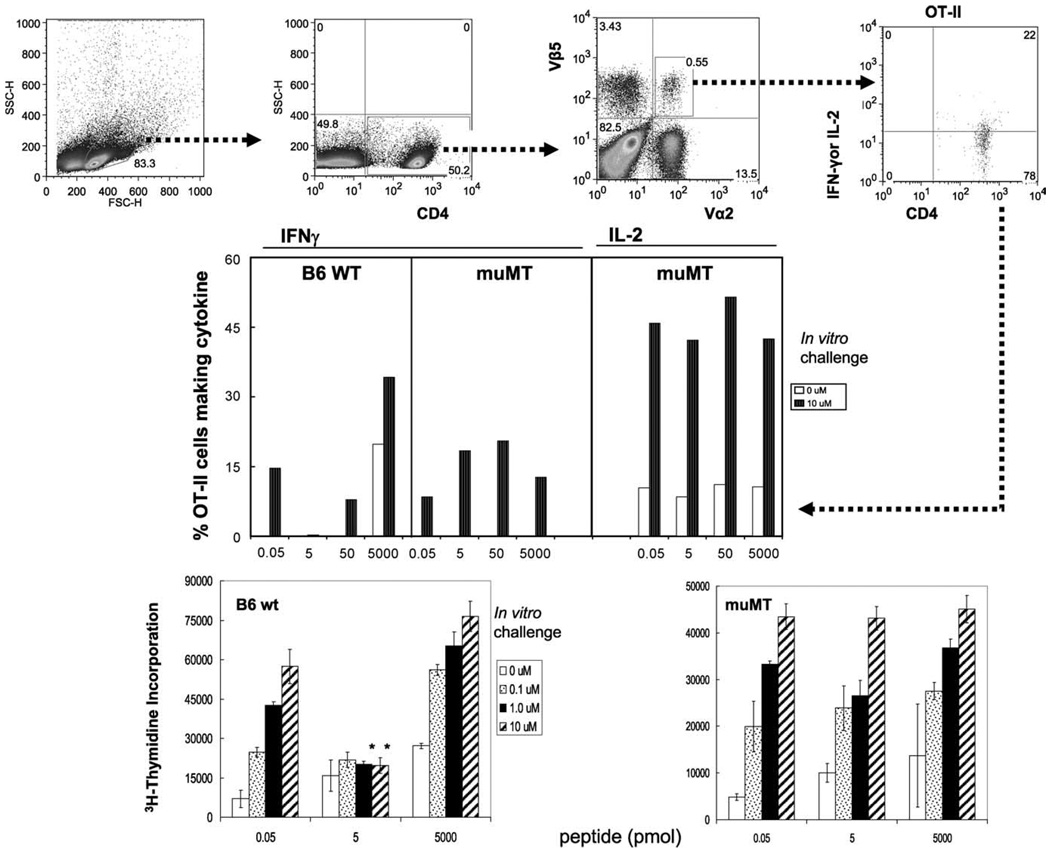
B cell-deficient mice do not support memory T cell anergy
To establish that only B cells and not any other APCs induce anergy in memory T cells in vivo, we used B cell-deficient muMT mice (23). Because muMT mice do not support efficient development of CD4 memory T cells (24), we first generated memory T cells using OT II Tg cells specific for OVA323–339/I-Ab (25, 26) by transferring OT II Tg cells to B6 recipients and immunizing them. Memory cells were then transferred to muMT mice. All groups of mice were given different doses of peptide in IFA as before and assayed for anergy 9 days later. We found that while 5 or 50 pmol of peptide induced anergy in B cell-sufficient (B6 wild type) mice, memory OT-II cells from B cell-deficient muMT mice did not show signs of anergy as evidenced by IFN-γ and IL-2 production (Fig. 7). This experiment confirms that B cells are the critical APCs for inducing anergy in memory CD4+ T cells.
FIGURE 7.
B cell-deficient mice do not support memory T cell anergy. Groups of B6 and B cell-deficient muMT mice bearing memory OT-II T cells (18 h after adoptive transfer of purified CD4+ T cells containing memory T cells) were challenged with increasing doses (0.05–5,000 pmol) of cOVA323–339 peptide in IFA. Nine days later, cells from draining LNs were harvested and tested for anergy in vitro. T cell responses were measured by intracellular IFN-γ and IL-2 production (upper and middle panels) and [3H]thymidine incorporation (lower panels). The quadrants for IL-2- or IFN-γ-producing cells (upper panels) were drawn based on the matched isotype controls. There were three mice per group and were assayed individually with similar results. The data shown represent one of two independent experiments. Bars represent mean ± SD of triplicate cultures. *, p < 0.001 or 5 pmol vs 0.05 or 5000 pmol of peptide doses (Student’s t test).
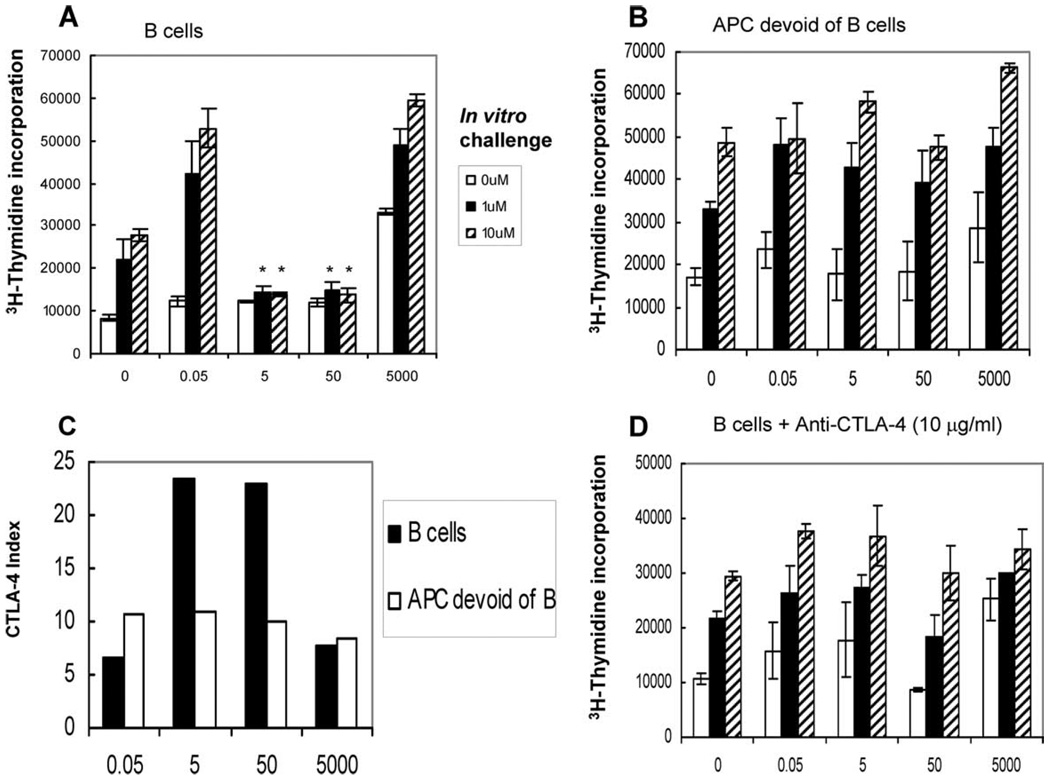
Induction of anergy in memory T cells by B cells is mediated by CTLA-4
CTLA-4 is a potent negative regulator of T cell activation. To test whether CTLA-4 was mediating B cell-induced anergy in memory CD4+ T cells, we incubated memory CD4+ T cells for 48 h with APCs (B cells or APCs without B cells) pulsed in vivo and measured CTLA-4 expression on the T cell surface (Fig. 8, A and B). We found a direct correlation between the induction of anergy and the up-regulation of CTLA-4 (Fig. 8C). T cells interacting with B cells pulsed with anergizing doses of Ag expressed higher (>2-fold) levels of CTLA-4 compared with T cells interacting with B cells pulsed with nonanergizing doses of peptide. APCs devoid of B cells pulsed with any dose of Ag did not induce anergy and the levels of CTLA-4 remained unchanged. In one further study of the role of CTLA-4, we included an anti-mouse CTLA-4 blocking Ab during ex vivo anergy assay and found that the presence of anti-CTLA-4 Ab prevented the induction of anergy by B cells (Fig. 8D).
FIGURE 8.
Induction of anergy in memory T cells by B cells is CTLA-4 mediated. Four groups of mice were immunized with increasing doses (0.05–5000 pmol) of cOVA323–339 peptide in IFA. After 48 h, mice were sacrificed; B cells and APCs devoid of B cells were purified from the draining LNs and incubated with memory CD4+ T cells for 48 h. Ex vivo induction of anergy by B cells (A) or APCs devoid of B cells (B) was tested by restimulating T cells with cOVA323–339 peptide-pulsed splenocytes (at concentrations shown) for 72 h followed by [3H]thymidine incorporation. CTLA-4 expression on Ag-specific (CD4+ KJ1.26+) T cells was determined by flow-cytometry (C). Reversal of anergy by B cells was tested similarly, except that anti-mouse CTLA-4 Ab was added to the culture in the beginning of the coincubation of B cells and memory CD4+ T cells (D). There were five mice per group. Data shown in A and D represent one of two independent experiments; those in B and C have been repeated four times. Bars represent mean ± SD of triplicate cultures. *, p < 0.001, 5, or 50 pmol vs 0.05 or 5000 pmol of peptide doses (Student’s t test).
Discussion
Induction of T cell tolerance is critical in protection against self-destructive immune responses. Because memory T cells are key players in autoimmune pathogenesis (27–29), it is important to silence them to prevent autoimmunity. Although tolerance is well documented in naive T cells, it is poorly understood in memory T cells. Our early studies showed that low avidity engagement of the TCR by agonist peptide-MHC II complexes induced anergy in memory CD4+ T cells (10–12). Anergy induced in those studies was long lasting and met the definition of anergy offered by Schwartz and colleagues as “a state of T cell unresponsiveness accompanied by a lack of response to proliferation signals including IL-2 production, and its reversibility by exogenous IL-2 (30–32).” In the present study, we have taken a step further in understanding how anergy is induced and asked which APCs trigger anergy in memory CD4+ T cells.
The ability to manipulate memory T cell responses has high potential for providing new therapeutic avenues. To accomplish this, a novel ex vivo approach was designed in which memory T cells were exposed to different purified APC populations pulsed with different doses of antigenic peptide. We found that resting, but not activated, B cells are the primary APCs that induce anergy in memory CD4+ T cells. Our experiments also strongly suggested that CD11c+ DCs did not play a significant role in the induction of anergy in memory T cells. This was confirmed in both ex vivo and in vivo settings. Several reports indicate that resting B cells may be the predominant APCs that induce tolerance. The tolerogenic nature of resting B cells may be due to the low abundance of costimulatory molecules such as B7, which is highly expressed in DCs and activated B cells (33–35). A seemingly contrary study reports that resting B cells failed to induce anergy in memory CD8+ T cells specific for HY Ag (36). Failure to induce anergy in that study might be because of one or more of the following reasons: 1) the ligand density presented by B cells is not regulated; 2) Ag presentation by MHC-I to CD8+ T cells might be different from the presentation by MHC-II to CD4+ T cells; and 3) the activation status of CD8+ T cells in response to low density of ligand is likely different from that of CD4+ T cells.
Our study reveals the dual relationship of B cells with memory T cells: B cells were tolerogenic when presenting limited numbers of peptide/MHC complexes and stimulatory when presenting higher numbers of complexes. The latter is consistent with the report that all APCs, including resting B cells, can activate memory T cells, which may be due to a less stringent requirement of memory T cells for costimulatory molecules (34, 37). The data presented here provide strong evidence in favor of the former, in that B cells induce anergy in memory CD4+ T cells. In this study, memory CD4+ T cells were anergized both ex vivo by B cells that had captured peptide in vivo, and in vivo by B cells that were loaded with peptide in vitro.
Dendritic cells are potent initiators of immune responses, although they are also reported to be involved in the induction of tolerance in vivo (38–40). When the availability of peptide Ag is limited, as was the case here, mature DCs with high MHC class II expression on their surfaces might be the likely APCs to capture low levels of Ag and render T cells anergic. Nevertheless, we clearly demonstrate that DCs were not responsible for inducing anergy in memory CD4+ T cells by using several separate experimental designs. In one, APCs depleted of B cells or enriched for DCs failed to induce anergy in our ex vivo anergy assay. In the other, anergy was induced by APCs in the absence of DCs in vivo. Moreover, B cells that were loaded with peptide in vitro successfully induced anergy in memory CD4+ T cells when transferred in vivo. Finally, B cell-deficient mice, while presumably sufficient in all other APC types, failed to induce anergy in CD4 memory T cells. Therefore, we feel convinced that B cells are the critical APCs to induce anergy in memory CD4+ T cells.
A role for CTLA-4 in the negative regulation of T cell activation is well documented (41–44). Interestingly, we found that B cells presenting tolerogenic doses of peptide induced higher levels of CTLA-4 expression on T cells. Upon the addition of anti-CTLA-4 Ab to the culture, anergy was prevented, strongly suggesting that anergy induced by B cells is mediated by CTLA-4. The expression of low levels of B7.1 on B cells, which does not change at different doses of antigenic stimulation (data not shown), is in agreement with the notion that B cells are the APCs involved in the induction of anergy in a system that is regulated by CTLA-4. Because CTLA-4/B7.1 avidity is higher than CD28/B7.1 interaction, it is certainly feasible that under limiting B7.1 expression the inhibitory effects of CTLA-4 will be dominant (45). In agreement with this notion, CpG-stimulated B cells failed to induce anergy in memory T cells in all concentrations of the peptide (0–10,000 nM) tested, whereas the control B cells pulsed with 0.1–1.0 nM peptide were successful in inducing anergy (data not shown).
Recent reports have indicated that programmed death (PD)-1/PD-L1, and not CTLA-4/B7, interactions dictate the state of exhaustion generated in CD8+ T cells in chronic viral infections (46, 47). Based on our data, we suggest that anergy might be regulated differently in CD4+ vs CD8+ T cells. Indeed, it is reported that anergy in CD8+ T cells was induced in the absence of CTLA-4/B7 interaction (48). Thus, our study opens new avenues for investigating differences in molecular mechanisms underlying CD4 vs CD8 anergy.
Because of the involvement of CTLA-4 in the induction of anergy in memory CD4+ T cells and in light of expression of CTLA-4 and CD25 on regulatory T cells (Tregs), one possible explanation for our observations might be that the hyporesponsiveness shown here is induced by T cells that have Treg function. Interestingly, a recent report documented that Ag presentation by B cells caused an increase in FoxP3 expression in Treg cells (49). Despite those findings, in our system, Tregs do not seem to be the inducer of the observed hyporesponsiveness. We had previously reported that induction of anergy is not due to regulatory T cells (11). In those experiments, cells from in vivo anergized groups were mixed with the nonanergized responding groups at different ratios to find out whether potential Tregs present in the anergized groups would render the responding cells anergic ex vivo. The results indicated no reduction in response in the cell-mixed culture wells. Additionally, a microarray gene chip assay comparing in vivo anergized cells to nonanergized cells demonstrated no significant increase of FoxP3 in anergized T cells. In the same experiment, the levels of expression of CD25, a marker of Tregs, remained lower in the anergized cells compared with the nonanergized cells (data not shown). We find these data to argue against a role for Tregs in the induction of anergy in our system.
One might argue that the induction of anergy in memory T cells might not be beneficial to the maintenance of a healthy immune response. We propose that a reduced level of Ag/MHC expression on the surface of B cells might be a mechanism that has evolved to signal memory T cells of the “end of an infection” so that cells would stop proliferating and secreting inflammatory cytokines (50). Undoubtedly, excess cytokine secretion is harmful and not needed once the infection is terminated. Following the termination of infection, Ag load is gradually diminished with inflammation. B cells bearing specific high affinity Ag receptors, such as B2 B cells, at a certain threshold of Ag load would preferentially capture Ag and present it to memory CD4+ T cells. Our study, therefore, suggests a new role for B cells as the APCs of choice when Ag falls to nonthreatening low levels (51). Thus, it is logical to hypothesize that B cells signal memory T cells to undergo anergy. In contrast, at the beginning of an infection, when the Ag load might also be low, the induction of anergy in memory T cells would not be desirable. A feature of anergy as described in this article is its reversibility upon an encounter with IL-2 and Ag, a condition that is met during the onset of an infection when a surge of inflammatory responses from the innate immune system coincides with the release of multiple cytokines including IL-2 (52). In support of this hypothesis are our previous reports showing that an encounter with low densities of Ag in the presence of IL-2 does not lead to the induction of anergy and that anergized cells, when incubated with IL-2 and Ag, are no longer anergized (10–12).
The points described above suggest that the induction of anergy in memory T cells might be an evolutionarily beneficial mechanism. A better understanding of this phenomenon could help in revealing the underlying mechanisms for viral and tumor surveillance. Many viruses and several tumors are known to decrease the expression of cell surface MHC class II (53). Also, some tumor-associated peptides bind poorly to the MHC molecules (54). The reduced surface expression of MHC and/or low affinity peptide/MHC complexes leads to the presentation of low densities of specific peptide/MHC, which would induce anergy to the viral or tumor-derived Ags. Thus, our findings suggest new strategies for overcoming viral infections and treating tumors. Rather than repeated immunizations, it might be more productive to focus on the reversal of anergy. In the case of autoimmunity, self-reactive memory T cells might be kept under check from effector function by encountering low densities of peptide-MHC presented by B cells, which could be an important process in the prevention of organ-specific autoimmune diseases. Thus, memory T cells in autoimmune conditions could be targeted specifically for induction of anergy by transferring syngeneic B cells pulsed ex vivo with the specific peptides. Controversial observations have been reported on the roles that B cells might play in autoimmune disease models (55–57). The identification of follicular B cells (B2 B cells) as the critical APCs for induction of anergy in memory CD4+ T cells leads the way to further studies on the unique characteristics of the membrane of memory CD4+ T cells and/or molecules expressed on the surface of memory T cells and B cells that contribute specifically to the induction of T cell anergy.
Acknowledgments
We especially thank Drs. David Scott, Ron Germain, and Ron Schwartz for insightful discussions, Abdel Hamad, Chung Dang, and Kedar Narayan for discussion and critical reading of the manuscript, and Stanislav Khoruzhenko for FACS data analysis and preparation of high resolution figures.
Footnotes
This work was supported by National Institutes of Health Grants R01GM53549 and R01AI063764 to S.S.-N.
Portions of this work were presented (poster and oral) by S. K. Dalai at “Experimental (EB) Biology 2005,” the American Association of Immunologists (AAI) 2005 Annual Meeting, April 2–6, 2005, San Diego, CA and at “Immunology 2007,” 94th Annual Meeting of the AAI, May 18–22, 2007, Miami Beach, FL.
Abbreviations used in this paper: LN, lymph node; B6, C57BL6; cOVA, chicken OVA; DC, dendritic cell; DT, diphtheria toxin; DTR, diphtheria toxin receptor; Tg, transgenic; Treg, regulatory T cell.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Moulton VR, Farber DL. Committed to memory: lineage choices for activated T cells. Trends Immunol. 2006;27:261–267. doi: 10.1016/j.it.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Seder RA, Sacks DL. Memory may not need reminding. Nat. Med. 2004;10:1045–1047. doi: 10.1038/nm1004-1045. [DOI] [PubMed] [Google Scholar]
- 3.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 4.McKinstry KK, Golech S, Lee WH, Huston G, Weng NP, Swain SL. Rapid default transition of CD4 T cell effectors to functional memory cells. J. Exp. Med. 2007;204:2199–2211. doi: 10.1084/jem.20070041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandok MR, Okoye FI, Ndejembi MP, Farber DL. A biochemical signature for rapid recall of memory CD4 T cells. J. Immunol. 2007;179:3689–3698. doi: 10.4049/jimmunol.179.6.3689. [DOI] [PubMed] [Google Scholar]
- 6.Watson AR, Lee WT. Differences in signaling molecule organization between naive and memory CD4+ T lymphocytes. J. Immunol. 2004;173:33–41. doi: 10.4049/jimmunol.173.1.33. [DOI] [PubMed] [Google Scholar]
- 7.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 8.Blair DA, Lefrancois L. Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proc. Natl. Acad. Sci. USA. 2007;104:15045–15050. doi: 10.1073/pnas.0703767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patke DS, Farber DL. Modulation of memory CD4 T cell function and survival potential by altering the strength of the recall stimulus. J. Immunol. 2005;174:5433–5443. doi: 10.4049/jimmunol.174.9.5433. [DOI] [PubMed] [Google Scholar]
- 10.Korb LC, Mirshahidi S, Ramyar K, Sadighi Akha AA, Sadegh-Nasseri S. Induction of T cell anergy by low numbers of agonist ligands. J. Immunol. 1999;162:6401–6409. [PubMed] [Google Scholar]
- 11.Mirshahidi S, Huang CT, Sadegh-Nasseri S. Anergy in peripheral memory CD4+ T cells induced by low avidity engagement of T cell receptor. J. Exp. Med. 2001;194:719–731. doi: 10.1084/jem.194.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirshahidi S, Ferris LC, Sadegh-Nasseri S. The magnitude of TCR engagement is a critical predictor of T cell anergy or activation. J. Immunol. 2004;172:5346–5355. doi: 10.4049/jimmunol.172.9.5346. [DOI] [PubMed] [Google Scholar]
- 13.Wilson NS, Villadangos JA. Regulation of antigen presentation and cross-presentation in the dendritic cell network: facts, hypothesis, and immunological implications. Adv. Immunol. 2005;86:241–305. doi: 10.1016/S0065-2776(04)86007-3. [DOI] [PubMed] [Google Scholar]
- 14.Lyons A. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J. Immunol. Methods. 2000;243:147–154. doi: 10.1016/s0022-1759(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 15.de Vinuesa CG, Cook MC, Ball J, Drew M, Sunners Y, Cascalho M, Wabl M, Klaus GG, MacLennan IC. Germinal centers without T cells. J. Exp. Med. 2000;191:485–494. doi: 10.1084/jem.191.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Yu Y, Shao C, Zhang M, Wang W, Zhang L, Cao X. Enhancement of antigen-presenting ability of B lymphoma cells by immunostimulatory CpG-oligonucleotides and anti-CD40 antibody. Immunol. Lett. 2001;77:17–23. doi: 10.1016/s0165-2478(01)00189-4. [DOI] [PubMed] [Google Scholar]
- 17.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 19.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J. Exp. Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu. Rev. Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 21.Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire: marginal zone and B1 B cells as part of a “natural immune memory.”. Immunol. Rev. 2000;175:70–79. [PubMed] [Google Scholar]
- 22.Hardy RR. B-1 B cells: development, selection, natural autoantibody, and leukemia. Curr. Opin. Immunol. 2006;18:547–555. doi: 10.1016/j.coi.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 24.Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J. Immunol. 2000;165:5558–5565. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- 25.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 26.Robertson JM, Jensen PE, Evavold BD. DO11.10 and OT-II T cells recognize a C-terminal ovalbumin 323–339 epitope. J. Immunol. 2000;164:4706–4712. doi: 10.4049/jimmunol.164.9.4706. [DOI] [PubMed] [Google Scholar]
- 27.Ponchel F, Morgan AW, Bingham SJ, Quinn M, Buch M, Verburg RJ, Henwood J, Douglas SH, Masurel A, Conaghan P, et al. Dysregulated lymphocyte proliferation and differentiation in patients with rheumatoid arthritis. Blood. 2002;100:4550–4556. doi: 10.1182/blood-2002-03-0671. [DOI] [PubMed] [Google Scholar]
- 28.Davis LS, Cush JJ, Schulze-Koops H, Lipsky PE. Rheumatoid synovial CD4+ T cells exhibit a reduced capacity to differentiate into IL-4-producing T-helper-2 effector cells. Arthritis Res. 2001;3:54–64. doi: 10.1186/ar140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skapenko A, Wendler J, Lipsky PE, Kalden JR, Schulze-Koops H. Altered memory T cell differentiation in patients with early rheumatoid arthritis. J. Immunol. 1999;163:491–499. [PubMed] [Google Scholar]
- 30.Schwartz RH. Models of T cell anergy: is there a common molecular mechanism? J. Exp. Med. 1996;184:1–8. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quill H, Schwartz RH. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J. Immunol. 1987;138:3704–3712. [PubMed] [Google Scholar]
- 32.Jenkins MK, Pardoll DM, Mizuguchi J, Quill H, Schwartz RH. T-cell unresponsiveness in vivo and in vitro: fine specificity of induction and molecular characterization of the unresponsive state. Immunol. Rev. 1987;95:113–135. doi: 10.1111/j.1600-065x.1987.tb00502.x. [DOI] [PubMed] [Google Scholar]
- 33.Eynon EE, Parker DC. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J. Exp. Med. 1992;175:131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ronchese F, Hausmann B. B lymphocytes in vivo fail to prime naive T cells but can stimulate antigen-experienced T lymphocytes. J. Exp. Med. 1993;177:679–690. doi: 10.1084/jem.177.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuschenkoff VN, Sethna MP, Freeman GJ, Parker DC. Co-expression of B7-1 and antigen blocks tolerance induction to antigen presented by resting B cells. J. Immunol. 1996;157:1987–1995. [PubMed] [Google Scholar]
- 36.Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 37.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen: memory cells are less dependent on accessory cell co-stimulation and can respond to many antigen-presenting cell types including resting B cells. J. Immunol. 1994;152:2675–2685. [PubMed] [Google Scholar]
- 38.Steinman R, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J. Exp. Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svensson M, Maroof A, Ato M, Kaye PM. Stromal cells direct local differentiation of regulatory dendritic cells. Immunity. 2004;21:805–816. doi: 10.1016/j.immuni.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 40.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 41.Greenwald RJ, Boussiotis VA, Lorsbach R, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–155. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 42.Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 43.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 44.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 45.Chambers CA, Kuhns MS, Egen JG, Allison JP. Ctla-4-mediated inhibition in regulation of t cell responses: mechanisms and manipulation in tumor immunotherapy. Annu. Rev. Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 46.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 47.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 48.Frauwirth KA, Alegre ML, Thompson CB. CTLA-4 is not required for induction of CD8+ T cell anergy in vivo. J. Immunol. 2001;167:4936–4941. doi: 10.4049/jimmunol.167.9.4936. [DOI] [PubMed] [Google Scholar]
- 49.Chen X, Jensen PE. Primary B lymphocytes preferentially expand allogeneic FoxP3+ CD4 T cells. J. Immunol. 2007;179:2046–2050. doi: 10.4049/jimmunol.179.4.2046. [DOI] [PubMed] [Google Scholar]
- 50.Marsden VS, Kappler JW, Marrack PC. Homeostasis of the memory T cell pool. Int. Arch. Allergy Immunol. 2006;139:63–74. doi: 10.1159/000090000. [DOI] [PubMed] [Google Scholar]
- 51.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 52.Granucci F, Feau S, Angeli V, Trottein F, Ricciardi-Castagnoli P. Early IL-2 production by mouse dendritic cells is the result of microbial-induced priming. J. Immunol. 2003;170:5075–5081. doi: 10.4049/jimmunol.170.10.5075. [DOI] [PubMed] [Google Scholar]
- 53.Brodsky FM, Lem L, Solache A, Bennett EM. Human pathogen subversion of antigen presentation. Immunol. Rev. 1999;168:199–215. doi: 10.1111/j.1600-065x.1999.tb01294.x. [DOI] [PubMed] [Google Scholar]
- 54.Topalian SL, Gonzales MI, Parkhurst M, Li YF, Southwood S, Sette A, Rosenberg SA, Robbins PF. Melanoma-specific CD4+ T cells recognize nonmutated HLA-DR-restricted tyrosinase epitopes. J. Exp. Med. 1996;183:1965–1971. doi: 10.1084/jem.183.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen CC, Rivera A, Dougherty JP, Ron Y. Complete protection from relapsing experimental autoimmune encephalomyelitis induced by syngeneic B cells expressing the autoantigen. Blood. 2004;103:4616–4618. doi: 10.1182/blood-2004-01-0091. [DOI] [PubMed] [Google Scholar]
- 56.Litzinger MT, Su Y, Lei TC, Soukhareva N, Scott DW. Mechanisms of gene therapy for tolerance: B7 signaling is required for peptide-IgG gene-transferred tolerance induction. J. Immunol. 2005;175:780–787. doi: 10.4049/jimmunol.175.2.780. [DOI] [PubMed] [Google Scholar]
- 57.Melo ME, Qian J, El-Amine M, Agarwal RK, Soukhareva N, Kang Y, Scott DW. Gene transfer of Ig-fusion proteins into B cells prevents and treats autoimmune diseases. J. Immunol. 2002;168:4788–4795. doi: 10.4049/jimmunol.168.9.4788. [DOI] [PubMed] [Google Scholar]