Abstract
Background
Variation in TLR2 gene (TLR2/−16934) is associated with allergic diseases amongst farmers' children, but not amongst children not living on farms.
Objective
To test the hypothesisis that the same genetic variant which confers protection in the farming environment is associated with reduced risk of developing allergic phenotypes amongst urban children attending day-care in early life.
Methods
In two population-based birth cohorts (Manchester, UK-MAAS and Tucson, USA-IIS) participants were recruited prenatally and followed prospectively (MAAS: 3, 5, 8 and 11 years; IIS: 1, 2, 3 and 5 years). We assessed allergic sensitization and atopic wheezing at each follow-up.
Results
727 children participated in Manchester and 263 in Tucson. We found no significant associations between TLR2/−16934 and sensitization and atopic wheeze in either cohort. However different pattern emerged when we explored the interaction between TLR2/−16934 and day-care attendance on these outcomes. We found a significant interaction between day-care and TLR2/-16934 on the development of sensitization in the longitudinal model in MAAS, in that children carrying T allele who attended day-care were less likely to be sensitized than those who did not attend day-care, whilst amongst AA homozygotes the association tended to be in the opposite direction. In a longitudinal model in IIS, we found a significant interaction between day-care attendance and TLR2/-16934 on the development atopic wheezing. Significant interactions between TLR2/-16934 and day-care were maintained when adjusting for socioeconomic status
Conclusions
The effect of day-care on sensitization and atopic wheezing may differ among children with different variants of theTLR2 gene.
Keywords: gene*environment interactions, asthma, allergic sensitisation, birth cohorts, TLR2
INTRODUCTION
Asthma and allergies are the most common chronic diseases in childhood in western societies1. Although evidence from twin studies suggests a strong genetic component2, there has been little replication among genetic studies3. The fundamental role of the environment in the development of these conditions is emphasised by the rapid increase in prevalence which occurred in the last 4–5 decades1, a time frame too short to be attributable to genetic factors alone. Various environmental exposures have been associated with the development of asthma and allergies. However, as with genetics, the dataon the role of environment are often inconsistent, with the same environmental exposure (e.g.day -care attendance) in different studies conferring an increase in risk4, protection5–9 or no effect 10, 11. The conflicting evidence on the effect of genetic variants and environmental exposures on allergic phenotypes may be in part due to the fact that they have largely been studied separately. We propose that the development of sensitization and/orasthma is likely a consequence of environmental factors actin g upon genetically susceptible individuals through gene-environment interactions. Thus, to understand the role of either genes or environment, it is essential to study both.
The hygiene hypothesis proposes that relative reduction in immune stimulation by microbial exposure consequent to increasedhygiene may result in a slower post -natal maturation of the immune system, resulting in higher prevalence of allergies12, 13. The most convincing evidence for the role of suchexposure comes from studies amongst farmers in central Europe, with lower prevalence of allergic diseases amongst farmers' children compared to those not living on farms 14, 15. A recent study in this setting reported that variation in toll-like receptor 2 gene (TLR2/−16934, rs4696480)is strongly associated with the frequency of allergies, and farmers children carrying a T allele were significantly less likely to have asthma, sensitisation and hay-fever compared to children with genotype AA16. No such association was found amongst children not living on farms. Similarly, variations in TLR2 were shown to modify the associations between country living in childhood and adult asthma in France17. In contrast, results from Japan indicated that polymorphisms in TLRs are not associated with the development of atopy-related phenotypes18.
Children who attend day-care may be exposed to a higher microbialload than those cared for at home19–21, and consequently have a lower risk of developing allergic phenotypes. However, similar to many other environmental exposures, studies investigating the associations between day-care attendance and allergic disease have produced conflicting results4–11.
We hypothesized that the same genetic variant which confers a reduction in risk of allergic phenotypes in the farming environment may be associated with a reduction in risk amongst urban children attending day-care in early life. To test this hypothesis, we used data collected prospectively in two separate population-based birth cohorts.
METHODS
Study design, setting and participants
Two population samples were studied (Manchester and Tucson). Both studies were approved by local research ethics committees. Informed consent was obtained from all parents, and children gave their assent if appropriate.
The Manchester Asthma and Allergy Study (MAAS)22–26 and Tucson Infant Immune Study (IIS)27–29 are unselected birth cohort studies (for detailed description see Online supplement). Participants were recruited prenatally, and followed prospectively attending review clinics at ages 3, 5, 8 and 11 years (MAAS) and 1, 2, 3 and 5 years (IIS). .
Definitions of variables
Day-care attendance
In MAAS, Day-care included children who regularly attended day-care at any time during the first two years of life and No day-care included children who were looked after at home or by a child-minder9. In IIS, Day-care included children who were regularly cared for outside of the home at any time during the first nine months of life27.
Sensitization
In MAAS we carried out skin prick testing (SPT) to common allergens at age 3, 5, 8 and 11 years and defined sensitization as a wheal diameter 3mm greater than negative control to at least one allergen. In addition, we measured specific IgEs at age s3, 5 and 8 years. In IIS , we measured specific IgEs at age s1, 2 3 , and 5 years and defined sensitization as sIgE>0.35 kUA/L to at least one allergen.,
Atopic wheeze
Questionnaires were administered to collect information on parentally-reported symptoms. Current wheeze was defined as wheeze in the last 12 months, and atopic wheeze as current wheeze in the presence of sensitization at corresponding age.
Airway reactivity (MAAS)
Assessed by Eucapnic Voluntary Hyper-ventilation (EVH) challenge at age 5 years (Online supplement). Bronchial hyper responsiveness (BHR) was defined as a change in lung function after challenge greater than the ninetieth percentile for the reference subjects (skin test-negative, never-wheezing at age 5)30.
Genotyping
Genotyping was performed using the Single Base Extension method (Sequenom, Hamburg, Germany; MAAS) and 5 -exonuclease assays (Taqman, Applied Biosystems; IIS); see Online supplement. For all analyses, AT and TT genotypes were combined to assess our a priori hypothesis that the association between day-care and sensitization would be evident for children carrying a T-allele.
Statistical methods
We used Stata 11.1 and SPSS 15.0 for all analyses. In order to minimize false positive results due to multiple testing and capitalize on the longitudinal nature of the collected data, we made a priori decision to use longitudinal rather than cross-sectional analyses of the two phenotypes of interest (sensitization and atopic wheeze) as the primary outcomes. For completeness, the data on a secondary outcome (current wheezing) are presented in the Online supplement.
Longitudinal analyses were performed by Generalized Estimating Equations (GEE) using the exchangeable correlation structure and the logit link function. We investigated other covariates which might influence clinical outcomes of interest (socioeconomic status, number of siblings and position of sib-ship), and models were adjusted as appropriate. For airway reactivity in MAAS at age 5 years, the categorical associations were assessed using logistic regression models. Only children of European ancestry were included in the analysis.
RESULTS
Participants
In Manchester we reviewed 1025 children at age 8 years; of those, 122 were randomized to an environmental intervention31 and excluded from this analysis. Samples for genotyping were provided by 727C aucasian children, of whom 504 attended day-care. Of the total IIS population (n=482), the analyzed sample included 263 Caucasian children with data on genotype, day -care and at least one outcome. Genotype frequencies were consistent with other populations (AA 22.0% and 26.6%, AT 50.9% and 48.7%, TT 27.1% and 24.7%, MAAS and IIS respectively); no deviation from Hardy-Weinberg equilibrium could be detected.
Descriptive data
Tables 1 and E1 summarize gender, day-care attendance and clinical outcomes overall and by TLR2/−16934 in the two cohorts. There were no significant associations between TLR2/−16934 and any clinical outcomes in either cohort. Data ongender, and clinical outcomes by day -care attendance are presented in Table 2 and E2. In MAAS, day-care was significantly associated with reduced atopic wheeze at age 8(Tables 2) and reduced wheeze at ages 5 and 8 (E-Table 2). In IIS, day-care was significantly associated with increased wheeze at age 1 and reduced sensitization at age 2(Table 2 and E-Table 2). These findings are consistent with our previously reported data9, 27 . In the MAAS cohort, we found that socioeconomic status was significantly associated with day-care attendance and some of the outcomes (e.g. children from a higher socioeconomic class were more likely to attend day-care; additionally, these children were less likely to develop wheeze in early life, but more likely to develop sensitization). In IIS, we found no association between socioeconomic status and day-care. There was no significant association between the number of siblings and position of sib-ship with exposure of interest and clinical outcomes in either cohort,; therefore these have not been included in the longitudinal models.
Table 1.
Gender, day-care attendance and outcomes by TLR2/−16934 genotype.
| Variable | Whole group Frequency (%) | AA Frequency (%) | AT+TT Frequency (%) | p-value* |
|---|---|---|---|---|
| MAAS | ||||
| Population (n=727) | 160 (22.0) | 567 (78.0) | ||
| Male | 386/727 (53.1) | 78/160 (48.8) | 308/567 (54.3) | 0.21 |
| Attended day-care | 504/727 (69.3) | 109/160 (68.1) | 395/567 (69.7) | 0.71 |
| Sensitization (IgE) | ||||
| Age 3 years | 29/129 (22.5) | 5/27 (18.5) | 24/101 (23.5) | 0.58 |
| Age 5 years | 116/416 (27.9) | 30/96 (31.3) | 86/320 (26.9) | 0.40 |
| Age 8 years | 167/414 (40.3) | 40/88 (45.5) | 127/326 (39.0) | 0.27 |
| Sensitization (SPT) | ||||
| Age 3 years | 135/646 (20.9) | 33/145 (22.8) | 93/501 (20.4) | 0.53 |
| Age 5 years | 165/648 (25.5) | 34/142 (23.9) | 131/506 (25.9) | 0.64 |
| Age 8 years | 192/657 (29.2) | 34/144 (23.6) | 158/513 (30.8) | 0.09 |
| Age 11 years | 177/563 (31.4) | 38/122 (31.2) | 139/441 (31.5) | 0.94 |
| Atopic wheeze (IgE) | ||||
| Age 3 years | 11/128 (8.6) | 2/27 (7.4) | 9/101 (8.9) | 1.00 |
| Age 5 years | 31/412 (7.5) | 9/96 (9.4) | 22/316 (7.0) | 0.51 |
| Age 8 years | 53/412 (12.9) | 12/87 (13.8) | 41/325 (12.6) | 0.72 |
| Atopic wheeze (SPT) | ||||
| Age 3 years | 38/642 (5.9) | 12/145 (8.3) | 26/497 (5.2) | 0.17 |
| Age 5 years | 48/647 (7.4) | 12/142 (8.5) | 32/505 (7.1) | 0.60 |
| Age 8 years | 69/657 (10.5) | 12/144 (8.3) | 57/513 (11.1) | 0.34 |
| Age 11 years | 57/563 (10.1) | 11/122 (9.0) | 46/441 (10.4) | 0.65 |
| EVH airway hyperreactivity | ||||
| Age 5 years | 73/473 (15.4) | 14/105 (13.3) | 59/368 (16) | 0.50 |
| IIS | ||||
| Population (n = 263) | 70/263 (26.6) | 193/263 (73.4) | ||
| Male | 120/263 (45.6) | 32/70 (45.7) | 88/193 (45.6) | 1.00 |
| Attended day-care | 130/263 (49.4) | 34/70 (48.6) | 96/193 (49.7) | 0.89 |
| Sensitization (IgE) | ||||
| Age 1 year | 26/209 (12.4) | 7/57 (12.3) | 19/152 (12.5) | 1.00 |
| Age 2 years | 39/187 (20.9) | 13/54 (24.1) | 26/133 (19.6) | 0.55 |
| Age 3 years | 57/178 (32.0) | 18/50 (36.0) | 39/128 (30.5) | 0.48 |
| Age 5 years | 62/153 (40.5) | 15/44 (34.1) | 47/109 (43.1) | 0.36 |
| Atopic wheeze (IgE) | ||||
| Age 1 year | 6/205 (2.9) | 2/56 (3.6) | 4/149 (2.7) | 0.67 |
| Age 2 years | 10/181 (5.5) | 3/52 (5.8) | 7/129 (5.4) | 1.00 |
| Age 3 years | 17/175 (9.7) | 5/49 (10.2) | 12/126 (9.5) | 1.00 |
| Age 5 years | 16/146 (11.0) | 4/42 (9.5) | 12/104 (11.5) | 1.00 |
Chi-squared test
Table 2.
Gender and outcomes by day-care attendance.
| No Day-care Frequency (%) | Day-care Frequency (%) | p-value* | |
|---|---|---|---|
|
MAAS | |||
| Population | 223/727 (30.7) | 504/727 (69.3) | |
| Male | 112/223 (50.2) | 274/504 (54.4) | 0.30 |
| Sensitization (SPT) | |||
| Age 3 years | 47/196 (24.0) | 88/450 (19.6) | 0.20 |
| Age 5 years | 51/200 (25.5) | 114/448 (25.5) | 0.99 |
| Age 8 years | 62/200 (31.0) | 130/457 (28.5) | 0.51 |
| Age 11 years | 52/166 (31.3) | 125/397 (31.5) | 0.97 |
| Sensitization (IgE) | |||
| Age 3 years | 13/45 (28.9) | 16/84 (19.1) | 0.20 |
| Age 5 years | 33/134 (24.6) | 83/282 (29.4) | 0.31 |
| Age 8 years | 55/127 (43.3) | 112/287 (39.0) | 0.41 |
| Atopic wheeze (SPT) | |||
| Age 3 years | 12/194 (6.2) | 26/448 (5.8) | 0.85 |
| Age 5 years | 15/200 (7.5) | 33/447 (7.4) | 0.96 |
| Age 8 years | 28/200 (14.0) | 41/457 (8.9) | 0.05 |
| Age 11 years | 19/166 (11.5) | 38/397 (9.6) | 0.50 |
| Atopic wheeze (IgE) | |||
| Age 3 years | 5/45 (11.1) | 7/84 (8.3) | 0.61 |
| Age 5 years | 10/134 (7.5 ) | 22/282 (7.8) | 0.90 |
| Age 8 years | 25/127 (19.7) | 28/285 (9.8) | 0.01 |
| IIS | |||
| Population | 133/263 (50.6) | 130/263 (49.4) | |
| Male | 64/133 (48.1) | 56/130 (43.1) | 0.46 |
| Sensitization (IgE) | |||
| Age 1 year | 16/110 (14.5) | 10/99 (10.1) | 0.40 |
| Age 2 years | 26/94 (27.7) | 13/93 (14.0) | 0.03 |
| Age 3 years | 32/88 (36.4) | 25/90 (27.8) | 0.26 |
| Age 5 years | 35/82 (42.7) | 27/71 (38.0) | 0.62 |
| Atopic wheeze (IgE) | |||
| Age 1 year | 3/109 (2.8) | 3/96 (3.1) | 1.00 |
| Age 2 years | 7/91 (7.7) | 3/90 (3.3) | 0.33 |
| Age 3 years | 8/86 (9.3) | 9/89 (10.1) | 1.00 |
| Age 5 years | 9/78 (11.5) | 7/68 (10.3) | 1.00 |
Chi-squared test
Interaction between TLR2/−16934 and day-care
When we explored the interaction between TLR2/−16934 and day-care attendance on clinical outcomes, genotype-specific patterns emerged that were similar in the two populations. All estimates for odds ratios and confidence intervals from longitudinal models for allergic sensitization and atopic wheeze are presented in Table 3 . Significant interactions between TLR2/-16934 and day-care were maintained when adjusting for socioeconomic status; it is of note that adjusting for socioeconomic status did not materially change the odds ratios inthese models.
Table 3.
Odds ratios and confidence intervals for the effect of day-care on clinical outcomes in longitudinal models, stratified by TLR2/-16934 (using no day care as a reference group)
| TLR2 = AA | TLR2 = AT/TT | Interaction p-value | |||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Sensitization (SPT) | |||||
| MAAS, age 3-5-8-11 years | 1.8 (0.9, 3.6) | 0.110 | 0.8 (0.6, 1.2) | 0.278 | 0.05 |
| Sensitization (IgE) | |||||
| MAAS, age 3-5-8 years | 2.1 (0.9, 4.6) | 0.078 | 0.9 (0.6, 1.3) | 0.435 | 0.06 |
| IIS, age 1-2-3-5 years | 1.3 (0.5, 3.1) | 0.609 | 0.5 (0.3, 0.9) | 0.027 | 0.10 |
| Atopic wheeze | |||||
| MAAS, age 3-5-8-11 years (SPT) | 1.2 (0.5–2.8) | 0.671 | 0.7 (0.5–1.2) | 0.170 | 0.34 |
| MAAS, age 3-5-8 years (IgE) | 1.2 (0.5, 5.2) | 0.484 | 0.5 (0.3, 0.9) | 0.016 | 0.10 |
| IIS, age 1-2-3-5 years (IgE) | 5.8 (1.1, 30.5) | 0.038 | 0.5 (0.2, 1.2) | 0.137 | 0.01 |
SPT-Skin prick tests
Sensitization
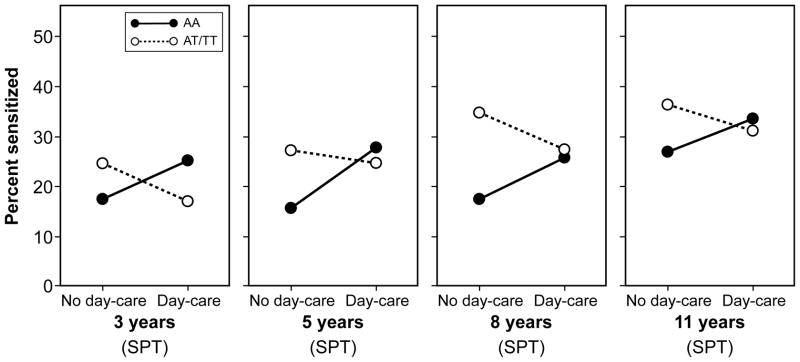
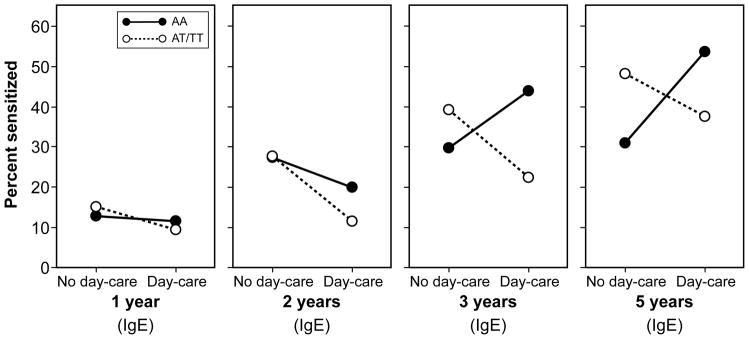
In both cohorts the effect of day-care on sensitization differed by TLR2/−16934 genotype . In MAAS, in a longitudinal model including skin prick tests from all 4 time-points (3, 5, 8 and 11 years), we found a significant interaction between day-care and TLR2/-16934 on the development of sensitization(p=0.0 5, Table 3). Results did not materially change when sensitization was defined by IgE (Table 3, E-Figure 1). For either measure of atopic sensitization and at each time point, children carrying a T allele who attended day-care tended to have lower risk of sensitization than those who did not attend day-care (Figure 1a, E-Figure 1). In contrast, among children with AA genotype, day-care attendance appeared to increase the risk of sensitization (Table 3, Figure 1a, E-Figure 1). Similarly, in IIS, in a longitudinal model including data from all 4 time-points, children carrying a T allele were significantly less likely to develop sensitization if they attended day-care (p=0.03), though the interaction between day-care and TLR2/-16934 was not statistically significant (p=0.10, Table 3). Inspection of the patterns suggested that among children withan AA genotype, day -care did not appear to have an effect on sensitization at ages 1 and 2 years, but there was a trend towards increase at ages 3 and 5 years amongst children who attended day-care (Figure 1b).
Figure 1.
Percentage of children with allergic sensitization (assessed by skin prick testing [SPT] or specific IgE measurement [IgE]) by TLR2/-16934 genotype and day-care attendance in early childhood
a) Manchester Asthma and Allergy Study (MAAS)
b) Tucson Infant Immune Study (IIS)
Data on current wheeze are presented in E-Tables 1–3 and E-Figures 2 and 3.
Atopic wheeze
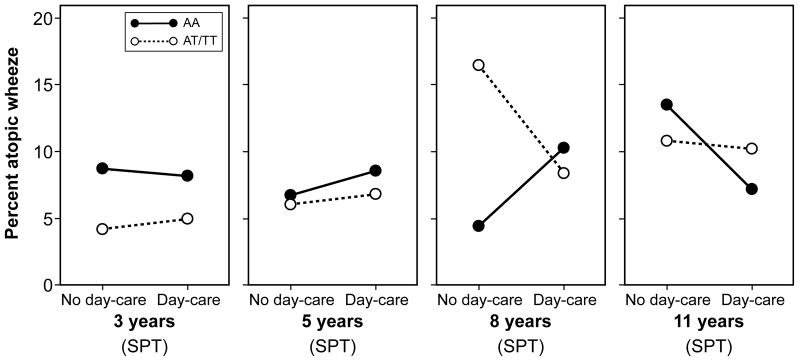
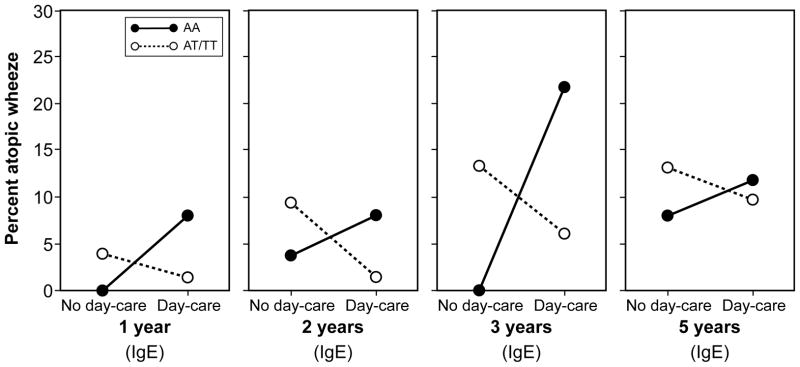
In a longitudinal model in IIS including data from ages 1, 2, 3 and 5 years, we found a significant interaction between day-care attendance and TLR2/-16934 on the development atopic wheezing (p=0.01; Table 3), in that children with AA genotype who attended day-care had higher risk of atopic wheezing than those who did not attend day-care, whilst among T-allele carriers day-care attendance appeared protective. Although we observed a similar pattern in MAAS, the interaction between day-care and genotype failed to reach statistical significance (Table 3). In a longitudinal model of atopic wheezing (IgE) in the UK cohort, day-care was associated with protection only amongst T-allele carriers (p=0.017), whilst the direction of the association in children with AA genotype appeared to be in the opposite direction (Table 3, E-Figure 4). Inspection of the patterns (Figure 2a) suggested that the interaction between day-care attendance and TLR2/-16934 was not evident prior to age 8 years (consistent with the finding that atopic wheeze at age 8 years was less common amongst children who attended day-care, Table 2).
Figure 2.
Percentage of children with atopic wheeze by TLR2/-16934 genotype and day-care attendance in early childhood; atopy was assessed by skin prick testing (SPT) or specific IgE measurement (IgE)
a) Manchester Asthma and Allergy Study (MAAS)
b) Tucson Infant Immune Study (IIS)
Bronchial hyperresponsiveness (MAAS)
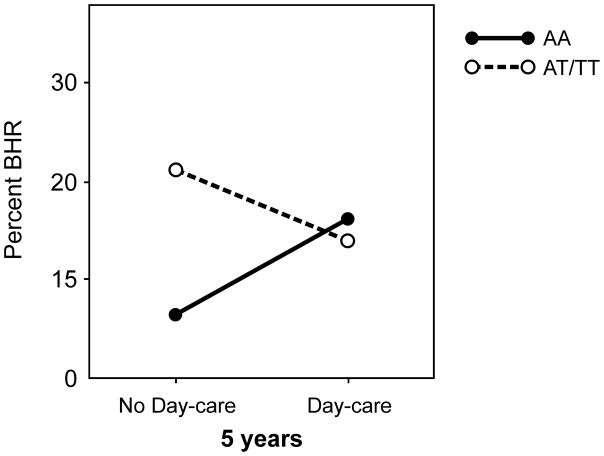
For the whole population there was no association between day-care and BHR at age 5 years. However, in children with T allele, day-care was associated with less BHR, whereas among AA homozygotes day-care was associated with more BHR (Figure 3). The interaction between TLR2/−16934 and day-care was statistically significant (adjusted for baseline lung function: p=0.04).
Figure 3.
Percentage of children with bronchial hyper-responsiveness (BHR) to Eucapnic Voluntary Hyper-ventilation (EVH) challenge at age 5 years by TLR2/-16934 genotype and day-care attendance in the Manchester Asthma and Allergy Study (MAAS)
DISCUSSION
Key results
In two independent unselected birth cohorts from distinct geographic areas, we demonstrated that the association between day care attendance with sensitization and atopic wheezing appears dependent on a genetic variant in TLR2. Day-care was protective, but only amongst children carrying the T allele for TLR2/−16934, whilst among AA homozygotes there was no association between day-care attendance and outcomes of interest, or the association tended to be in the opposite direction. In the MAAS cohort, socioeconomic status was significantly associated with day-care attendance and some of the outcomes, but the significant interactions between day-care and TLR2/−16934 were maintained after adjusting for this factors with no material changes in the odds ratios for these models. These results were further strengthened by the similar findings for physiological measures strongly related to childhood asthma (dry air bronchial hyperreactivity) in one of the cohorts. Our results are consistent with those in children raised in a farming environment, where T allele carriers were less likely to have asthma and sensitisation compared to AA homozygotes16. We postulate that attending day-care and being raised on farm are markers of increased exposure to microbial products, which may have different or even opposite effects on asthma and allergies amongst carriers of different TLR2/−16934 genotype.
Limitations and Strengths
We did not directly measure exposure to microbial agents, but used day-care attendance as a proxy. Several reports have shown that children attending day-care centers experience more infections than children cared for at home19, 20, and exposure to endotoxin (a component of the cell wall of gram-negative bacteria) is markedly higher in day-care centers than in homes32. The precise nature of the exposures in either the farming or day care environments nonetheless remain to be identified. Another limitation is that we reliedon parental reports of wheezing, which may beunreliable as many parents have little understanding of what physicians mean by the term “wheeze”33.
We made every effort to minimize false positive results due to multiple testing, but we acknowledge that we cannot fully eliminate the possible impact of multiple testing on the degree to which conclusions related to the statistical interactions can be considered reliable. The analysis was hypothesis-driven and limited to one genotype comparison in two carefully defined phenotypes. We minimized the number of phenotypes tested by capitalizing on the longitudinal nature of data collection, and used longitudinal rather than a series of cross-sectional analyses. We used slightly different definitions of “day-care” attendance in the two populations. This is an inevitable consequence of the different provisions for maternity leave in the two countries, which influenced the age of entry to day-care. In contrast to the USA, in the UK, paid maternity leave is provided for at least 9 months, and children are usually looked after by their mothers at home during this time (consequently, only 30 children in Manchester started nursery within the first six months of life and we could not use more similar definitions of day-care). It is worth noting that we used similar definitions of day-care to those used in our previous studies which demonstrated that in the whole populations, early day-care exposure reduced IgE levels (IIS)27 and reduced risk of wheezing (MAAS)9. We therefore believe that our definitions of day-care exposure in the two cohorts are appropriate for the distinct geographical areas and represent reasonable proxy measures of the exposure to infectious agents .
We acknowledge that the findings in two cohorts are not identical, and that the interaction terms are either not significant or are only marginally below the conventional 0.05 level. For example, the interaction between day-care attendance and TLR2/-16934 was significant for sensitization in MAAS and atopic wheeze in IIS, but failed to reach statistical significance for atopic wheeze in MAAS and sensitization in IIS. Clearly, our conclusions would be stronger if the p-values for interaction were all significant and if all were in the 0.001 range or below. However, even when the interaction did not reach statistical significance, all trends across different phenotypes in two populations were in the same direction. How does this compare with “replication” in studies of asthma and other complex diseases? Despite more than a decade of intensive work using a range of approaches from family based linkage and candidate gene-based association studies through to whole genome association studies, genetic studies have produced heterogeneous results with little replication3. It should be noted that in this context replication refers to the finding of any association between the gene and any asthma or allergy phenotype. The gene is usually considered as the unit of replication, reflecting the fact that not only is it frequently a different SNP within the gene that is a risk for disease, but sometimes even the opposite allele of the same SNP that is the risk allele in different populations3. This phenomenon has been noted in most complex diseases; precise replication (i.e. the same association of the same SNP with the same phenotype) is very rare34. Thus, whilst we recognize that the findings in our two cohorts are not identical, it is reassuring that the direction of the interaction between the same SNP and similar environmental exposure across phenotypes in the two different populations was very similar. Finally, we do not have the functional explanation for our findings. The TLR2/−16934 polymorphism is a marker for a group of highly linked TLR2 SNPs, and any of these SNPs may be responsible for the interaction described. We chose TLR2/−16934 for these studies because it had been previously associated with asthma and allergies infarming environment 16. TLR2 expression is increased in blood cells from children of farmers compared with children not raised on farms, suggesting that the innate immune system may respond to the microbial products present in the farming environment and may modulate the development of allergic disease35. Whether similar changes in TLR2 are present in children attending day-care is unknown. TLR2 is the innate immune receptor for molecular patterns present on the surface of many microbial agents36. It is likely that expression of TLR2 on the cell surfaces is in part genetically determined, and this differential expression by genotype could modulate susceptibility to the effects of ligands present in microbial products.
A major strength of our studies is careful longitudinal phenotyping from birth in two unselected populations in distinct geographical areas. T he phenotypic expression of asthma and allergic diseases start early in life and these phenotypes are unstable and may progress or remit over time. Thus, the optimal study design is a birth cohort, as it overcomes problems of recall bias and permits longitudinal phenotyping and contemporaneous measurement of environmental exposures. This approach is crucial for the assessment of gene-environment interactions.
Interpretation
Published studies investigating the effect of day-care on the development of allergic disease are inconsistent, with some showing increased risk4, and others decreased risk5–8 or no effect10, 11. Similarly, polymorphisms in TLRs have been associated with allergic diseases in some16, but not all studies18. These inconsistencies may be in part consequent to the differences in study designs, definitions of exposures and outcomes or sample size. However, they may also reflect the fundamentally different nature of the relationship between genetic polymorphisms, environmental exposures and phenotype in complex diseases compared to diseases determined predominantly by genetic factors. The relationship between genotype and phenotype in complex diseases may not be linear or unidirectional37, but modulated by a number of environmental factors (for example, we have recently reported that cat ownership substantially increases the risk of early-life eczema in children with filaggrin loss-of-function variants, but not amongst those without)38. Thus, the true associations between genetic variants and phenotype expression may be lost in studies in which study participants are exposed to a wide range of unmeasured environmental factors37. It is important to note that we found no association between TLR2 genotype and clinical outcomes before we explored its interaction with day-care attendance. The true significance of the genetic variant was only uncovered when the relevant environmental exposure was taken into account. Similarly, when we carried out the analysis in the whole population, day-care appeared to be associated with a significant protection from atopic wheezing. However, this concealed the fact that amongst AA homozygotes, day-care was not associated with protection, but actually tended to increase the risk of atopic wheezing. The apparent protective effect in the whole population was consequent to the fact that children with a T allele (in whom day-care was associated with less atopic wheezing) outnumbered AA homozygotes (in whom day-care was associated with more atopic wheezing) by a factor of 3:1. Recent studies in mouse models have strongly suggested that gene-environment interaction plays a crucial role in determining complex phenotypes. Valdar et al39 reported the heritability of 88 complex traits that included models of human disease such as asthma and immunological, biochemical and hematological phenotypes. They found that environmental covariates were involved in a large number of significant interactions with genetic background. Moreover, the effects of gene-environment interactions were more frequent and larger than the main effects: half of the interactions explained more than 20% of the variance of the complex phenotypes studied. It is thus plausible to surmise that the type of gene-environment interactions we have observed are not limited to the phenotypes we studied, but may be crucial determinants of many other complex human phenotypes.
Generalizability
Our results suggest that in complex diseases such as asthma and allergies, genetic predisposition may need to be taken into account when assessing the effect of environmental exposures, and vice-versa, relevant environmental exposures may need to be factored into the genetic association studies. Furthermore, we often use epidemiological data to identify potentially modifiable risk factors to help devise primary prevention strategies. If we extrapolate our data to the context of primary prevention, the results suggest that only individuals with particular genotypes may benefit from a specific intervention, whilst the same intervention amongst individuals with different susceptibility may cause harm.
Conclusions
Our data indicate that the effects of day-care on allergic phenotypes may differ among children with different variants of the TLR2 gene. Children with T allele for TLR2/−16934 may benefit from attending day-care, whereas for those who are AA homozygotes being cared for at home may prove beneficial. However, we emphasize that a caution is needed when interpreting our results, due to marginal p-values of the interaction terms and the fact that we cannot fully eliminate the multiple testing problem.
Clinical Implications.
Extrapolation of our data to the context of primary prevention suggests that only individuals with particular genotypes may benefit from a specific intervention, whilst the same intervention amongst individuals with different susceptibility may cause harm.
Supplementary Material
Acknowledgments
MAAS was supported by Asthma UK Grant No 04/014 and Moulton Charitable Trust , and is currently supported by MRC Grant G0601361; IIS is funded by the National Institutes of Health (AI 42268 and AI61811).
Abbreviations
- TLR2
toll-like receptor 2
- MAAS
Manchester Asthma and Allergy Study
- IIS
Tucson Infant Immune Study
- SPT
skin prick test
- EVH
Eucapnic Voluntary Hyper-ventilation
- BHR
Bronchial hyperresponsiveness
- GEE
Generalized Estimating Equations
- SNP
Single nucleotide polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–43. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 2.Duffy DL. A population-based study of bronchial asthma in adult twin pairs. Chest. 1992;102:654. doi: 10.1378/chest.102.2.654. [DOI] [PubMed] [Google Scholar]
- 3.Ober C, Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun. 2006;7:95–100. doi: 10.1038/sj.gene.6364284. [DOI] [PubMed] [Google Scholar]
- 4.Hagerhed-Engman L, Bornehag CG, Sundell J, Aberg N. Day-care attendance and increased risk for respiratory and allergic symptoms in preschool age. Allergy. 2006;61:447–53. doi: 10.1111/j.1398-9995.2006.01031.x. [DOI] [PubMed] [Google Scholar]
- 5.Ball TM, Castro-Rodriguez JA, Griffith KA, Holberg CJ, Martinez FD, Wright AL. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med. 2000;343:538–43. doi: 10.1056/NEJM200008243430803. [DOI] [PubMed] [Google Scholar]
- 6.Celedon JC, Wright RJ, Litonjua AA, Sredl D, Ryan L, Weiss ST, et al. Day care attendance in early life, maternal history of asthma, and asthma at the age of 6 years. Am J Respir Crit Care Med. 2003;167:1239–43. doi: 10.1164/rccm.200209-1063OC. [DOI] [PubMed] [Google Scholar]
- 7.Illi S, von Mutius E, Lau S, Bergmann R, Niggemann B, Sommerfeld C, et al. Early childhood infectious diseases and the development of asthma up to school age: a birth cohort study. Bmj. 2001;322:390–5. doi: 10.1136/bmj.322.7283.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Infante-Rivard C, Amre D, Gautrin D, Malo JL. Family size, day-care attendance, and breastfeeding in relation to the incidence of childhood asthma. Am J Epidemiol. 2001;153:653–8. doi: 10.1093/aje/153.7.653. [DOI] [PubMed] [Google Scholar]
- 9.Nicolaou NC, Simpson A, Lowe LA, Murray CS, Woodcock A, Custovic A. Day-care attendance, position in sibship, and early childhood wheezing: a population-based birth cohort study. J Allergy Clin Immunol. 2008;122:500–6. e5. doi: 10.1016/j.jaci.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 10.Nafstad P, Brunekreef B, Skrondal A, Nystad W. Early respiratory infections, asthma, and allergy: 10-year follow-up of the Oslo Birth Cohort. Pediatrics. 2005;116:e255–62. doi: 10.1542/peds.2004-2785. [DOI] [PubMed] [Google Scholar]
- 11.Salam MT, Li YF, Langholz B, Gilliland FD. Early-life environmental risk factors for asthma: findings from the Children's Health Study. Environ Health Perspect. 2004;112:760–5. doi: 10.1289/ehp.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu AH, Murphy JR. Hygiene hypothesis: fact or fiction? J Allergy Clin Immunol. 2003;111:471–8. doi: 10.1067/mai.2003.172. [DOI] [PubMed] [Google Scholar]
- 13.von Mutius E. Of attraction and rejection--asthma and the microbial world. N Engl J Med. 2007;357:1545–7. doi: 10.1056/NEJMe078119. [DOI] [PubMed] [Google Scholar]
- 14.Riedler J, Braun-Fahrlander C, Eder W, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358:1129–33. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 15.Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–77. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 16.Eder W, Klimecki W, Yu L, von Mutius E, Riedler J, Braun-Fahrlander C, et al. Toll-like receptor 2 as a major gene for asthma in children of European farmers. J Allergy Clin Immunol. 2004;113:482–8. doi: 10.1016/j.jaci.2003.12.374. [DOI] [PubMed] [Google Scholar]
- 17.Smit LA, Siroux V, Bouzigon E, Oryszczyn MP, Lathrop M, Demenais F, et al. CD14 and toll-like receptor gene polymorphisms, country living, and asthma in adults. Am J Respir Crit Care Med. 2009;179:363–8. doi: 10.1164/rccm.200810-1533OC. [DOI] [PubMed] [Google Scholar]
- 18.Noguchi E, Nishimura F, Fukai H, Kim J, Ichikawa K, Shibasaki M, et al. An association study of asthma and total serum immunoglobin E levels for Toll-like receptor polymorphisms in a Japanese population. Clin Exp Allergy. 2004;34:177–83. doi: 10.1111/j.1365-2222.2004.01839.x. [DOI] [PubMed] [Google Scholar]
- 19.Brady MT. Infectious disease in pediatric out-of-home child care. Am J Infect Control. 2005;33:276–85. doi: 10.1016/j.ajic.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Denny FW, Collier AM, Henderson FW. Acute respiratory infections in day care. Rev Infect Dis. 1986;8:527–32. doi: 10.1093/clinids/8.4.524. [DOI] [PubMed] [Google Scholar]
- 21.Lu N, Samuels ME, Shi L, Baker SL, Glover SH, Sanders JM. Child day care risks of common infectious diseases revisited. Child Care Health Dev. 2004;30:361–8. doi: 10.1111/j.1365-2214.2004.00411.x. [DOI] [PubMed] [Google Scholar]
- 22.Custovic A, Simpson BM, Murray CS, Lowe L, Woodcock A. The National Asthma Campaign Manchester Asthma and Allergy Study. Pediatr Allergy Immunol. 2002;13 (Suppl 15):32–7. doi: 10.1034/j.1399-3038.13.s.15.3.x. [DOI] [PubMed] [Google Scholar]
- 23.Murray CS, Woodcock A, Smillie FI, Cain G, Kissen P, Custovic A. Tobacco smoke exposure, wheeze, and atopy. Pediatr Pulmonol. 2004;37:492–8. doi: 10.1002/ppul.20019. [DOI] [PubMed] [Google Scholar]
- 24.Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125:191–7. e1–13. doi: 10.1016/j.jaci.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Lowe LA, Simpson A, Woodcock A, Morris J, Murray CS, Custovic A. Wheeze phenotypes and lung function in preschool children. Am J Respir Crit Care Med. 2005;171:231–7. doi: 10.1164/rccm.200406-695OC. [DOI] [PubMed] [Google Scholar]
- 26.Lowe LA, Woodcock A, Murray CS, Morris J, Simpson A, Custovic A. Lung function at age 3 years: effect of pet ownership and exposure to indoor allergens. Arch Pediatr Adolesc Med. 2004;158:996–1001. doi: 10.1001/archpedi.158.10.996. [DOI] [PubMed] [Google Scholar]
- 27.Rothers J, Stern DA, Spangenberg A, Lohman IC, Halonen M, Wright AL. Influence of early day-care exposure on total IgE levels through age 3 years. J Allergy Clin Immunol. 2007;120:1201–7. doi: 10.1016/j.jaci.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 28.Stern DA, Guerra S, Halonen M, Wright AL, Martinez FD. Low IFN-gamma production in the first year of life as a predictor of wheeze during childhood. J Allergy Clin Immunol. 2007;120:835–41. doi: 10.1016/j.jaci.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 29.Su Y, Rothers J, Stern DA, Halonen M, Wright AL. Relation of early antibiotic use to childhood asthma: confounding by indication? Clin Exp Allergy. 2010;40:1222–9. doi: 10.1111/j.1365-2222.2010.03539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lombardi E, Morgan WJ, Wright AL, Stein RT, Holberg CJ, Martinez FD. Cold air challenge at age 6 and subsequent incidence of asthma. A longitudinal study. Am J Respir Crit Care Med. 1997;156:1863–9. doi: 10.1164/ajrccm.156.6.9612066. [DOI] [PubMed] [Google Scholar]
- 31.Custovic A, Simpson BM, Simpson A, Kissen P, Woodcock A. Effect of environmental manipulation in pregnancy and early life on respiratory symptoms and atopy during first year of life: a randomised trial. Lancet. 2001;358:188–93. doi: 10.1016/S0140-6736(01)05406-X. [DOI] [PubMed] [Google Scholar]
- 32.Rizzo MC, Naspitz CK, Fernandez-Caldas E, Lockey RF, Mimica I, Sole D. Endotoxin exposure and symptoms in asthmatic children. Pediatr Allergy Immunol. 1997;8:121–6. doi: 10.1111/j.1399-3038.1997.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 33.Lowe L, Murray CS, Martin L, Deas J, Cashin E, Poletti G, et al. Reported versus confirmed wheeze and lung function in early life. Arch Dis Child. 2004;89:540–3. doi: 10.1136/adc.2003.038539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K. A comprehensive review of genetic association studies. Genet Med. 2002;4:45–61. doi: 10.1097/00125817-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Lauener RP, Birchler T, Adamski J, Braun-Fahrlander C, Bufe A, Herz U, et al. Expression of CD14 and Toll-like receptor 2 in farmers' and non-farmers' children. Lancet. 2002;360:465–6. doi: 10.1016/S0140-6736(02)09641-1. [DOI] [PubMed] [Google Scholar]
- 36.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 37.Vercelli D, Martinez FD. The Faustian bargain of genetic association studies: bigger might not be better, or at least it might not be good enough. J Allergy Clin Immunol. 2006;117:1303–5. doi: 10.1016/j.jaci.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 38.Bisgaard H, Simpson A, Palmer CN, Bonnelykke K, McLean I, Mukhopadhyay S, et al. Gene-environment interaction in the onset of eczema in infancy: filaggrin loss-of-function mutations enhanced by neonatal cat exposure. PLoS Med. 2008;5:e131. doi: 10.1371/journal.pmed.0050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valdar W, Solberg LC, Gauguier D, Cookson WO, Rawlins JN, Mott R, et al. Genetic and environmental effects on complex traits in mice. Genetics. 2006;174:959–84. doi: 10.1534/genetics.106.060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.