Abstract
We reported previously that although there is disruption of coordinated cardiac hypertrophy and angiogenesis in transition to heart failure, matrix metalloproteinase (MMP)-9 induced antiangiogenic factors play a vital role in this process. Previous studies have shown the cardioprotective role of hydrogen sulfide (H2S) in various cardiac diseases, but its role during transition from compensatory hypertrophy to heart failure is yet to be unveiled. We hypothesize that H2S induces MMP-2 activation and inhibits MMP-9 activation, thus promoting angiogenesis, and mitigates transition from compensatory cardiac hypertrophy to heart failure. To verify this, aortic banding (AB) was created to mimic pressure overload in wild-type (WT) mice, which were treated with sodium hydrosulfide (NaHS, H2S donor) in drinking water and compared with untreated control mice. Mice were studied at 3 and 8 wk. In the NaHS-treated AB 8 wk group, the expression of MMP-2, CD31, and VEGF was increased while the expression of MMP-9, endostatin, angiostatin, and tissue inhibitor of matrix metalloproteinase (TIMP)-3 was decreased compared with untreated control mice. There was significant reduction in fibrosis in NaHS-treated groups. Echocardiograph and pressure-volume data revealed improvement of cardiac function in NaHS-treated groups over untreated controls. These results show that H2S by inducing MMP-2 promotes VEGF synthesis and angiogenesis while it suppresses MMP-9 and TIMP-3 levels, inhibits antiangiogenic factors, reduces intracardiac fibrosis, and mitigates transition from compensatory hypertrophy to heart failure.
Keywords: matrix metalloproteinase-2, matrix metalloproteinase-9, tissue inhibitor of matrix metalloproteinase-3, endostatin, angiostatin, vascular endothelial growth factor, aortic banding
cardiovascular disease (CVD) is the leading cause of death for both men and women. Hypertension is the major risk factor of CVD (23). Although initial pressure overload during hypertension is compensated by cardiac hypertrophy, sustained overload results in progression toward heart failure due to an imbalance in myocyte-to-capillary ratio (14, 32). In previous studies we demonstrated that during the compensatory hypertrophy phase, matrix metalloproteinase (MMP)-2 is induced and leads to the production of angiogenic growth factors like vascular endothelial growth factor (VEGF) and promotes angiogenesis. In later stages of compensatory hypertrophy, MMP-9 and tissue inhibitor of matrix metalloproteinase (TIMP)-3 are induced and play a significant role in the production of antiangiogenic growth factors like endostatin and angiostatin. This leads to apoptosis and causes transition from compensatory cardiac hypertrophy to decompensatory heart failure (15). Recent studies report that the endogenous gaseous transmitter hydrogen sulfide (H2S) plays an important role in cardiac protection during ischemia, infarction, and heart failure (10, 13, 26, 28). H2S is endogenously generated by pyridoxal-5′-phosphate-dependent enzymes cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), using l-cysteine as a substrate (16). More recently, it has been reported that H2S is also generated by 3-mercaptopyruvate sulfur transferase (3-MST), which is expressed in vascular endothelium (39). CBS is highly expressed in the brain, whereas CSE is mostly found in vasculature (1, 20). H2S promotes vascular relaxation and also has a negative inotropic effect on cardiac muscle. Both effects are attributed to the ability of H2S to open ATP-sensitive potassium (KATP) channels, which are widely distributed in coronary vasculature and myocardium (48). Studies quantify the expression of CSE in the heart and correlate it with levels of H2S. The results suggest that the heart is one of the major sources of endogenous H2S (13). Mutant mice lacking CSE exhibit pronounced hypertension and diminished endothelium-dependent vasorelaxation (46). This provides evidence that H2S is a physiological vasodilator and regulator of blood pressure. Exogenous supplementation of H2S decreases medial thickening of intracardiac coronary vessels and reduces interstitial fibrosis in spontaneously hypertensive rats, suggesting that H2S increases the coronary reserve in hypertrophied hearts (38). Hypertrophied hearts are protected against severe ischemia by promotion of angiogenesis (12). In vitro, VEGF causes an increase in H2S release in endothelial cells while pharmacological inhibition of H2S production or KATP channels attenuates VEGF signaling and endothelial cell migration (30). The AKT and MAPK signaling pathways are involved as mediators of protection by H2S against myocardial ischemia-reperfusion (30, 47). We suggest that these signaling aspects are also involved in the proangiogenic effect of H2S.
MMPs and their natural endogenous inhibitors, TIMPs, are predominantly present in the heart, and their expression is upregulated under pathological conditions (44). MMP-2 and MMP-9 are the two important gelatinases that play an important role in the development and remodeling of the heart and vasculature (7). Previous studies report that MMP-2 plays a significant role in angiogenesis and heart valve development (2, 6). Knockout mice deficient in MMP-2 have various anomalies of the heart during embryogenesis (11, 18, 31, 42). Brooks and colleagues (4, 5) reported that an angiogenic stimulus induces vascular remodeling through activation of MMP-2 and integrin (αvβ3). Although previous studies have reported that MMP-2 expression and activity were significantly elevated in VEGF-treated hypertrophied hearts (11), only a few studies have examined the role of endostatin and angiostatin in heart failure (15, 40). We previously reported (15) that MMP-9 induces endostatin and angiostatin production, which contribute to transition toward the decompensatory phase of heart failure. Studies in MMP-9 knockout mice have shown attenuation of ventricular enlargement, collagen deposition, and facilitation of angiogenesis following experimental myocardial infarction (9, 22, 33). There is evidence that TIMP-3 plays an important role in inducing apoptosis as well as inhibiting angiogenesis (3, 15, 21). The purpose of this study was to determine whether H2S could mitigate the transition from compensatory cardiac hypertrophy to heart failure. We hypothesize that H2S induces MMP-2 expression and inhibits MMP-9/TIMP-3 expression, thereby promoting angiogenesis that mitigates the transition from compensatory cardiac hypertrophy to heart failure.
MATERIALS AND METHODS
Animals.
Wild-type (WT) mice (C57BL6/J) aged 8 wk were obtained from Jackson Laboratories (Bar Harbor, ME) and housed in the animal care facility at the University of Louisville with access to standard chow and water. At the age of 12 wk with an approximate weight of 23–25 g, animals underwent pressure-overload aortic banding (AB) surgery and were divided into two groups: one group received 30 μmol/l of sodium hydrogen sulfide (NaHS) in the drinking water, and the other received plain water (untreated control). Each group was further subdivided into a sham-operated group and 3 wk (AB 3 wk) and 8 wk (AB 8 wk) post-AB surgery groups, with respect to the time points at which they attained hypertrophy and heart failure. Six animals per group were used (n = 6). After the study period, animals were euthanized in accordance with National Institutes of Health guidelines for animal research. The protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Louisville.
Pressure-overload animal model.
Animals were anesthetized with pentobarbital sodium, intubated, and ventilated with a Harvard miniventilator. Body temperature was maintained with a heating pad (TR 200, Fine Science Tools, Foster City, CA). Under sterile conditions the thorax was opened by left parasternal thoracotomy, and the ascending aorta was dissected and separated from adjacent structures. Care was taken to maintain proper homeostasis. To create cardiac pressure overload, several surgical procedures such as transthoracic and abdominal AB are routinely used (15, 19). We performed transthoracic AB by placing a 26-gauge needle on the ascending aorta and ligating around the vessel with 6-0 silk for optimum constriction in all mice. The needle was quickly removed to keep the constricted aorta patent. The wound was closed in layers with 6-0 Vicryl for subcutaneous tissues and 5-0 silk for the skin (8). The mortality rate was <20% under these conditions. All animals received intraperitoneal injection of Ketofen (5 mg/kg body wt) for postoperative analgesia. Animals in the sham-operated subgroups underwent similar procedures except for AB.
Hydrogen sulfide treatment.
Animals were given NaHS (H2S donor; Sigma), which in aqueous solution releases H2S, in drinking water for 6 wk. Although there are differences in the physiological range of H2S and some reports say that it is much lower than the micromolar level (25, 27), a 30 μmol/l concentration was supplemented to keep the plasma H2S in the physiological range, which is widely variable from 5 to 300 μmol/l (25). Diffusion of H2S into room air is minimal since its density is 18% higher than that of air. Furthermore, the drinking water was changed daily with fresh NaHS solution to provide adequate levels of H2S to the mice (36). To estimate daily intake of NaHS, previous studies from our lab reported that there was no difference in the consumption of water among the treated and untreated groups (37) and also that there was an increase in plasma H2S concentration with exogenous supplementation (26, 36). It was reported that oxidation of H2S due to air is minimal and yields a yellow color to solutions (17). As we changed the NaHS-containing water every 24 h without change in color, we assume that we prevented the consumption of oxidized NaHS to some extent.
Antibodies and reagents.
The following primary antibodies were used: rabbit polyclonal anti-angiostatin, mouse monoclonal anti-endostatin, rabbit polyclonal MMP-2 and rabbit polyclonal MMP-9, and rabbit polyclonal antibody against TIMP-3 (Abcam, Cambridge, MA); anti-mouse VEGF antibody (R&D Systems, Minneapolis, MN); and mouse monoclonal anti-GAPDH (Sigma-Aldrich, St. Louis, MO). Fluorescent secondary antibodies for immunohistochemistry (IHC) were ordered from Invitrogen (Carlsbad, CA): Texas red raised in mouse and Alexa Fluor 488 and 594 raised in rabbit.
Western blot analysis.
Heart tissue from experimental animals and control animals was harvested, washed thoroughly in PBS, and snap-frozen in liquid nitrogen. Protein extraction was done with 1× RIPA buffer (in mM: 50 Tris·HCl pH 7.4, 150 NaCl, 1 EDTA, 1 PMSF, 1 Na3VO4, and 1 NaF, with 1% NP-40, 0.25% Na-deoxycholate, and 1 μg/ml protease inhibitor cocktail). Estimation of protein was done by the bicinchoninic acid (BCA) method (Thermo Fisher, Pittsburgh, PA). Fifty to seventy-five micrograms of protein was fractionated by SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membrane (Bio-Rad Laboratories, Hercules, CA) by the wet transfer method. The transferred proteins were processed for immunodetection of specific antigens. Briefly, nonspecific sites were blocked with 5% nonfat dry milk in TBS-T (50 mM Tris·HCl, 150 mM NaCl, 0.1% Tween 20, pH 7.4) for 1 h at room temperature. The blot was then incubated with appropriate primary antibody in blocking solution according to the supplier's specific instructions. The blots were washed with TBS-T (3 times, 10 min each) and incubated with appropriate horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. After washing, ECL Plus substrate (Amersham Biosciences, Pittsburgh, PA) was applied to the blot for 1 min. The blot was developed with Bio-Rad ChemiDoc XRS+ (Bio-Rad). The blots were stripped and reprobed with GAPDH. The immunoreactive bands were scanned and densitometry analyzed with Un-Scan-It software (Silk Scientific, Orem, UT).
Echocardiography.
Transthoracic echocardiography was performed on mice to achieve two-dimensional left ventricle (LV) images from an apical view with a SONOS 5500 or 2500 (Hewlett-Packard) and a 12.5-MHz transducer. The procedure was performed as previously published (15) with tribromoethanol (TBE) anesthesia to minimize the cardiodepressing actions produced by other anesthetics (29). First, mice were anesthetized with intraperitoneal injection of TBE (240 mg/kg body wt dose), depilated with hair removal cream (Nair), and placed on a heating pad to maintain body temperature. The functional status of the heart was assessed by LV internal dimension in diastole (LVIDd) and systole (LVIDs), LV posterior wall dimension (LVPWD), and percent fractional shortening (%FS). In murine echocardiography %FS is the most common method to evaluate LV function (34).
Cryosectioning.
After mice were euthanized, hearts were harvested, thoroughly washed in PBS, preserved in Peel-A-Way disposable plastic tissue embedding molds (Polysciences, Warrington, PA) filled with tissue freezing medium (Triangle Biomedical Sciences, Durham, NC), and stored at 70°C until analysis. Tissue sections 5 μm in thickness were made with a Leica CM 1850 Cryocut (Bannockburn, IL). Sections were placed on Superfrost Plus glass slides, air-dried, and processed for histological and IHC staining.
Immunohistochemistry.
IHC was performed on 5-μm-thick frozen tissue sections according to a standard IHC protocol (Abcam). Primary antibodies applied overnight included anti-endostatin, anti-TIMP-3 (Abcam), CD31 (BD Pharmingen), and VEGF (R&D Systems) antibodies. A secondary antibody labeled with Texas red and Alexa Fluor 594, 647 (Invitrogen) was applied for immunodetection of these proteins. Similarly, primary anti-MMP-2 and anti-MMP-9 antibodies (Abcam) were applied overnight and secondarily conjugated with Alexa Fluor 488 (Invitrogen) to detect expression of these MMPs. Stained slides were analyzed for fluorescence with a laser scanning confocal microscope (Olympus FluoView1000) set at the appropriate filter settings.
Masson's trichrome staining.
Collagen expression in tissue sections was assessed by Masson's trichrome staining according to the manufacturer's instructions (Richard-Allan Scientific, Kalamazoo, MI). Collagen was identified as blue staining.
Image Proplus software.
Images from IHC and Masson's trichrome staining were analyzed with Image Proplus software.
Pressure-volume loop study.
To assess LV function, a pressure-volume (P-V) study was done with the Millar Pressure-Volume system (Millar Instruments, Houston, TX). According to the standard Millar protocol, steady-state P-V loops were recorded, followed by saline bolus and cuvette calibration for the conversion of relative volume units (RVU) to microliters. Hemodynamic variables obtained were analyzed by P-V analysis (PVAN) software. The results were used to substantiate echocardiography findings. Percent ejection fraction (%EF) was used to assess the functional status of the LV.
Statistical analysis.
All data are expressed as means ± SE. Data were analyzed with one-way analysis of variance (ANOVA) to test for treatment effects, and differences between groups were determined with Tukey's post hoc test. A P value <0.05 was considered to be significant.
RESULTS
Effect of H2S on ventricular function of the heart.
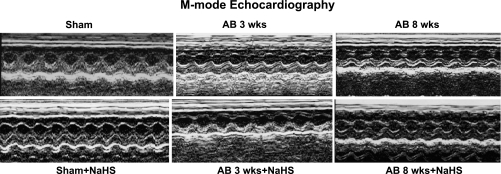
LV function was evaluated to determine the effects of H2S on minimizing the remodeling of the heart in a pressure-overload mouse model. Echocardiography data showed a decrease in LV chamber diameter in NaHS-treated AB 3 wk and AB 8 wk groups compared with untreated control mice. These findings appear to suggest that dilatation of the heart decreases with NaHS treatment (Fig. 1). The hemodynamic parameters obtained from echocardiography and P-V loop studies also showed improvement in LV functional status (Table 1).
Fig. 1.
Changes in left ventricular (LV) function following aortic banding (AB) and the effects of treatment with sodium hydrogen sulfide (NaHS), a hydrogen sulfide (H2S) donor. Top: representative M-mode echocardiography images from sham-operated (Sham), 3 wk post-AB (AB 3 wk), and 8 wk post-AB (AB 8 wk) groups. Bottom: effect of NaHS on LV function.
Table 1.
Hemodynamic data
| Sham | AB 3 wk | AB 8 wk | Sham + H2S | AB 3 wk + H2S | AB 8 wk + H2S | |
|---|---|---|---|---|---|---|
| FS% | 65.7 ± 1.8 | 45.75 ± 1.3 | 32.4 ± 2.2* | 65.7 ± 1.3 | 54.3 ± 1.5 | 48.9 ± 0.8† |
| LVIDd, mm | 2.27 ± 0.2 | 2.85 ± 0.2* | 2.99 ± 0.02 | 2.45 ± 0.1 | 2.41 ± 0.03 | 2.51 ± 0.2† |
| LVIDs, mm | 0.89 ± 0.1 | 1.55 ± 0.1* | 2.03 ± 0.1 | 1.02 ± 0.01 | 1.2 ± 0.1 | 1.27 ± 0.2† |
| LVPWD, mm | 1.84 ± 0.2 | 2.37 ± 0.1* | 1.98 ± 0.01 | 1.91 ± 0.02 | 2.01 ± 0.02 | 2.23 ± 0.03† |
| EF, % | 62.4 ± 1.6 | 50.2 ± 0.3 | 32.6 ± 0.2* | 67.48 ± 1.6 | 72.8 ± 1.2 | 59.13 ± 0.5† |
| EDP, mmHg | 5.6 ± 0.9 | 7.6 ± 0.7 | 15.4 ± 0.6* | 5.3 ± 0.5 | 6.1 ± 0.6 | 11.7 ± 0.8† |
| ESP, mmHg | 91 ± 8.7 | 119 ± 9.2 | 132 ± 9.6 | 93 ± 7.3 | 107 ± 7.2 | 114 ± 6.1 |
| dP/dtmax, mmHg/s | 7,887 ± 344 | 6,131 ± 278 | 3,896 ± 314* | 9,090 ± 184 | 6,938 ± 388 | 5,503 ± 267† |
| SV, μl | 15.5 ± 1.9 | 11.9 ± 0.8 | 8.8 ± 0.8* | 15.9 ± 0.7 | 15.1 ± 0.9 | 13.9 ± 0.6† |
Values are means ± SE. Measurements of left ventricular (LV) chamber dimension and anterior and posterior wall thickness during systole and diastole and echocardiography and pressure-volume (P-V) loop studies were performed. Sham, sham operated; AB, aortic banding; FS, fractional shortening; LVIDd, LV internal dimension in diastole; LVIDs, LV internal dimension in systole; LVPWD, LV posterior wall dimension; EF, ejection fraction; EDP, end-diastolic pressure; ESP, end-systolic pressure; dP/dtmax, maximum change in pressure with respect to time; SV, stroke volume.
P < 0.05 vs. Sham;
P < 0.05 vs. AB 8 wk.
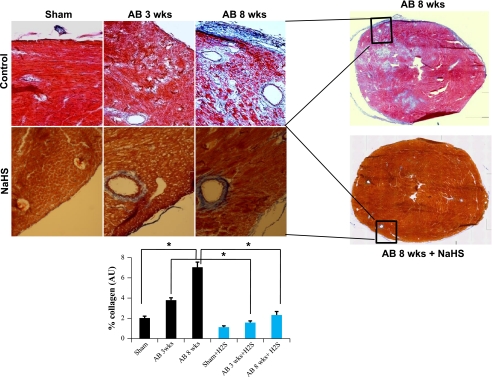
Role of H2S in perivascular and cardiac parenchymal tissue collagen deposition.
Tissue sections were analyzed for changes in fibrosis content after AB. Interstitial and perivascular collagen deposition analysis demonstrated an increase in fibrosis after 8 wk of AB compared with sham-operated control animals and animals 3 wk after AB. Collagen deposition was significantly reduced in NaHS-treated animals at 3 and 8 wk after AB compared with untreated control animals (Fig. 2). These findings suggest that H2S appears to decrease vascular resistance and reduces the effect of pressure overload on heart.
Fig. 2.
Masson's trichrome blue staining of collagen depicting perivascular fibrosis. Top: heart sections from untreated sham-operated, AB 3 wk, and AB 8 wk groups and from corresponding groups treated with H2S. Bottom: quantification of collagen (blue staining) was done with the help of Image Proplus software. AU, arbitrary units. Data represent means ± SE from n = 6/group. *Significant difference (P < 0.05).
Proangiogenic role of H2S.
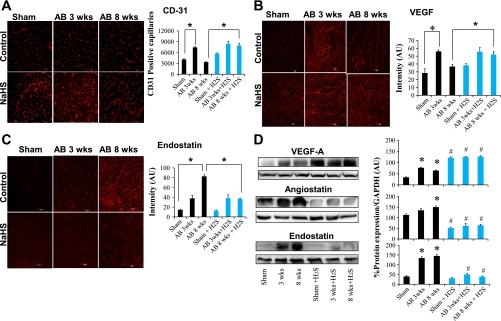
As H2S was shown to be proangiogenic, expression of the most potent angiogenic factor, VEGF, was measured in the heart tissue sections. VEGF expression was significantly increased in the NaHS-treated AB 8 wk group compared with the untreated AB 8 wk group (Fig. 3, B and D), suggesting increased angiogenesis. We measured CD31 expression, which is an endothelial cell marker, along with VEGF expression to measure the proangiogenic role of H2S. CD31 staining (Fig. 3A) showed significant increase in the NaHS-treated AB 8 wk group compared with its corresponding control. Increased angiogenesis results in replacing the capillary density-to-cardiomyocyte ratio and impedes the transition to decompensatory heart failure.
Fig. 3.
A: CD31 immunohistochemical (IHC) staining of heart (secondarily stained with Alexa Fluor 647) in sham-operated, AB 3 wk, and AB 8 wk groups (top) and corresponding H2S-treated groups (bottom). The expression of CD31 is seen as red fluorescence intensity. Data represent means ± SE from n = 6/group. *Significant difference (P < 0.05). B: vascular endothelial growth factor (VEGF) IHC staining of heart sections (secondarily stained with Alexa Fluor 594) in sham-operated, AB 3 wk, and AB 8 wk groups (top) and corresponding H2S-treated groups (bottom). The expression of VEGF is seen as red fluorescence intensity. Data represent means ± SE from n = 6/group. *Significant (P < 0.05). C: endostatin IHC staining of heart sections with endostatin (secondarily stained with Texas red fluorescent antibody) in sham-operated, AB 3 wk, and AB 8 wk groups (top) and corresponding H2S-treated groups (bottom). The expression of endostatin is seen as red fluorescence intensity. Data represent means ± SE from n = 6/group. *Significant difference (P < 0.05). Although all images used similar fixation procedures, because the immunostaining images were taken with a ×20 objective, myocyte contour may not be clear. Representative immunostaining is shown in image panels. Mean ± SE scanned intensity values from n = 6 are presented in bar graphs. D, left: Western blot analysis of VEGF, angiostatin, and endostatin expression in sham-operated, AB 3 wk, and AB 8 wk groups in control (first 3 lanes) and H2S-treated (last 3 lanes) groups. Right: densitometry analysis of protein expression [in arbitrary units (AU)]. Each bar represents mean ± SE from n = 6/ group. *P < 0.05 compared with untreated sham operated; #P < 0.05 compared with corresponding untreated controls. All images were exposed for the same duration. An equal amount of total protein was loaded onto each lane in the gel before Western blot analysis.
H2S inhibits antiangiogenic factors.
To assess the effect of H2S on the transition from compensatory to decompensatory phases of heart failure, antiangiogenic factor levels were quantified. Endostatin and angiostatin levels were increased in untreated control animals 8 wk after AB compared with sham-operated animals and in animals 3 wk after AB. Increasing levels of these factors suggest that post-AB angiogenesis at 8 wk was decreased and led to decompensatory heart failure. NaHS treatment significantly reduced expression of endostatin and angiostatin in AB 8 wk animals compared with untreated control animals at 8 wk. These results suggest the inhibitory action of H2S on antiangiogenic factors endostatin and angiostatin (Fig. 3, C and D).
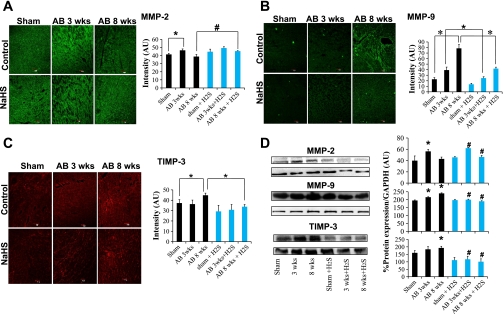
Role of hydrogen sulfide in MMP/TIMP axis.
To assess the effect of H2S on the MMP/TIMP axis in heart failure, expression of MMP-2, MMP-9, and TIMP-3 was quantified. MMP-2 expression was increased in untreated mice 3 wk after AB compared with sham-operated mice and AB 8 wk mice. Expression of MMP-9 and TIMP-3 was increased in untreated mice 8 wk after AB compared with sham-operated mice and AB 3 wk mice. NaHS treatment increased expression of MMP-2 8 wk after AB compared with untreated control animals of the same group (Fig. 4, A and D). In contrast, NaHS treatment decreased expression of both MMP-9 and TIMP-3 at 8 wk after AB compared with untreated control animals at 8 wk (Fig. 4, B–D). These findings suggest that the beneficial effect of H2S is associated with the inhibition of antiangiogenic factors controlled by the MMP/TIMP axis.
Fig. 4.
A: Matrix metalloproteinase (MMP)-2 IHC staining of heart sections with MMP-2 antibody (secondarily stained with Alexa Fluor 488 fluorescent antibody) in sham-operated, AB 3 wk, and AB 8 wk groups (top) and corresponding H2S-treated groups (bottom). The expression of MMP-2 is seen as green fluorescence intensity. Images were quantified with Image Proplus software, and the data are represented in the bar diagram. Data represent means ± SE from n = 6/group. *Significant difference (P < 0.05). B: MMP-9 IHC staining of heart sections with MMP-9 antibody (secondarily stained with Alexa Fluor 488 fluorescent antibody) in sham-operated, AB 3 wk, and AB 8 wk groups (top) and corresponding H2S-treated groups (bottom) The expression of MMP-9 is seen as green fluorescence intensity. Data represent means ± SE from n = 6/group. *Significant difference (P < 0.05). C: tissue inhibitor of matrix metalloproteinase (TIMP)-3 IHC staining of heart sections with TIMP-3 antibody (secondarily stained with Texas red fluorescent antibody) in sham-operated, AB 3 wk, and AB 8 wk groups (top) and corresponding H2S-treated groups (bottom). The expression of TIMP-3 is seen as red fluorescence intensity. Data represent means ± SE from n = 6/group. *Significant difference (P < 0.05). Although all images used similar fixation procedures, because the immunostaining images were taken with a ×20 objective, myocyte contour may not be clear. Representative immunostaining is shown in image panels. Mean ± SE scanned intensity values from n = 6 are presented in bar graphs. D: Western blot analysis of MMP-2, MMP-9, and TIMP-3 in sham-operated, AB 3 wk, and AB 8 wk groups in control (first 3 lanes) and H2S-treated (last 3 lanes) groups. Densitometry analysis of protein expression [in arbitrary units (AU)] is depicted in bar diagram. Each bar represents mean ± SE from n = 6.*P < 0.05 compared with untreated sham operated; #P < 0.05 compared with corresponding untreated controls. All images were exposed for the same duration. An equal amount of total protein was loaded onto each lane in the gel before Western blot analysis.
DISCUSSION
We reported previously that during compensatory hypertrophy there was increase in the expression of MMP-2, which promotes the production of VEGF. Subsequently, we have shown that in the decompensatory phase MMP-9 expression supersedes MMP-2 expression and induces the production of antiangiogenic factors endostatin and angiostatin. This causes the release of TIMP-3, resulting in decreased angiogenesis and progression to heart failure. The purpose of this study was to determine the effects of H2S on the development of heart failure induced by pressure overload in a mouse AB model. The rationale and hypothesis of this study were that H2S increased MMP-2 expression, reduced MMP-9 and TIMP-3 expression, reduced cardiac fibrosis, and mitigated transition from compensatory hypertrophy to heart failure. We designed the study with most appropriate experimental approach and statistical analysis. It was demonstrated that H2S treatment increased MMP-2 and VEGF expression 8 wk after banding and reduced the expression of MMP-9, endostatin, angiostatin, and TIMP-3. These effects were accompanied by significant reduction in myocardial fibrosis. In addition, H2S improved LV function as assessed by echocardiography and P-V studies.
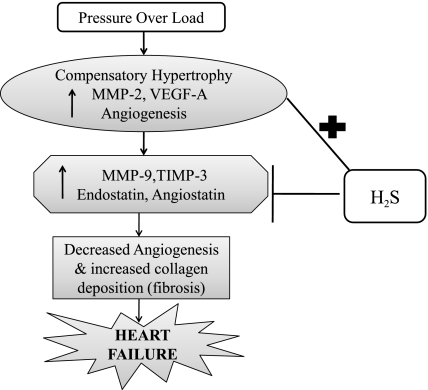
The effect of the significant LV hypertrophy after the transverse aortic constriction (TAC) surgery would be to push the capillaries apart and dilute the capillary proliferation effect, but this was effectively counterbalanced by the increase in angiogenic factors especially during the AB 3 wk period. We suggest that H2S promotes angiogenesis by inducing MMP-2 production and by inhibiting antiangiogenic factors endostatin and angiostatin. H2S also decreases the level of MMP-9 and TIMP-3, thereby arresting the conversion from compensatory cardiac hypertrophy to heart failure (Fig. 5). Our results showed that treatment of pressure-overload mice with NaHS (H2S) increases the production of angiogenic factors like VEGF. This promotes the increase in angiogenesis and thereby increases the capillary-to-myocyte ratio. Similarly, MMP-2 expression was also increased in the NaHS-treated groups, especially in the AB 8 wk group, supporting the hypothesis that MMP-2 as an angiogenic promoter induced VEGF production. Interestingly, we observed that MMP-9 expression decreased in the H2S-treated groups along with the expression of antiangiogenic factors like angiostatin, endostatin, and TIMP-3. Since we previously reported that MMP-9 promotes antiangiogenic factor production and TIMP-3 instigates apoptosis, these results suggest the role of H2S in inhibiting both antiangiogenic factor production and apoptosis by regulating the MMP/TIMP axis.
Fig. 5.
Hypothetical representation of altered MMP/TIMP axis regulating the generation of angiogenic and antiangiogenic factors during pressure-overload condition on the heart and the therapeutic role of H2S in stimulating angiogenesis and inhibiting antiangiogenesis.
Previous studies have shown that TIMP-3 has strong antiangiogenic activity that can be seen in all stages of angiogenesis (41). MMP-9 is shown to be induced in heart failure and generates antiangiogenic factors endostatin and angiostatin (15, 40, 43). Researchers report that MMP-2 expression and activity are significantly elevated in VEGF-treated hypertrophied hearts (11). This unequivocally suggests that MMP-9 is an antiangiogenic and MMP-2 a proangiogenic factor. Treatment with H2S promotes angiogenesis and thereby inhibits the transition from compensatory cardiac hypertrophy to heart failure. Functional data from echocardiography and P-V studies also showed improvement in LV function after treatment with H2S compared with untreated control groups.
Heart local renin production is important in the development of hypertrophy in hypertension (45). Interestingly, a recent study reported that H2S may decrease renin release (24). Therefore, it is possible that the protective effect of H2S may be mediated by the inhibitory effect of NaHS on the heart local renin-angiotensin system (RAS). This suggests that the cardioprotective role of H2S could also be due to its inhibitory action on local renin production in the heart, which needs further investigation.
Clinically, it is well known that heart disease is the leading cause of mortality not only in the United States but also in the rest of the world. Hypertension is one of the main risk factors leading to heart disease. Our research provides insight into the mechanism of transition from compensatory cardiac hypertrophy to heart failure. The beneficial effects of H2S in impeding the transition will help in clinical outcomes of heart failure.
Conclusions.
On the basis of our results, we conclude that H2S plays a role in 1) ameliorating LV dysfunction following pressure overload, 2) minimizing accumulation of collagen deposition in perivascular and intracardiac parenchymal tissue, 3) altering expression of MMPs and TIMPs and thus regulating ECM remodeling, 4) promoting angiogenesis by stimulating the production of VEGF, and 5) inhibiting antiangiogenic factors like endostatin and angiostatin.
Although a uniform cardiac fibrosis and high content of collagen would make a “stone” heart, the cardiac fibrosis was not uniform in this model. To determine the perivascular fibrosis we analyzed the collagen around the vessels, and to determine the interstitial fibrosis we analyzed the intracardiac areas (Fig. 2). As collagen deposition was not uniformly deposited, collagen content (fibrotic area) was calculated in relation to the entire LV myocardium, excluding the pericardium and tips of papillary muscles. Although it may appear that the myocyte color is different between H2S and control groups, the collagen concentration is within the physiological range in the sham-operated group. Interestingly, it was increased in the AB 8 wk group. Although both MMP-2 and -9 degrade collagen and elastin, the turnover of elastin is slower than that of collagen (35); therefore, elastin is replaced with collagen and over time to oxidized collagen. Furthermore, since TIMP-3 causes apoptosis of smooth muscle cells (3), this may contributes to perivascular fibrosis. Increased collagen deposition (fibrosis) along with decreased angiogenesis leads to heart failure (Fig. 5).
Limitations.
NaHS may undergo spontaneous oxidation in aqueous solutions (17). We did not make any attempt to inhibit NaHS oxidation or to estimate the fraction of oxidized NaHS; however, as we changed the NaHS-containing water every 24 h without change in color, we prevented the consumption of oxidized NaHS to some extent. Although we did not measure H2S concentration in the drinking water at 0 and 24 h, previously we measured absorbed H2S levels and reported that there is an increase in plasma H2S concentration following exogenous supplementation (37). Also, the density of H2S is ∼18% higher than air; therefore the evaporation rate of H2S from the solutions is normally low (17, 36). Moreover, the water bottle had a drinking tube with a small aperture, which minimized evaporation. We dissolved the NaHS in drinking water. Since this is a long-term treatment, it is important to record the volume of water each mouse drank every day and calculate how much H2S was absorbed. Previously, we reported that there was no difference in consumption of water between treated and untreated groups and also that there was an increase in plasma H2S concentration after exogenous supplementation (26, 37).
GRANTS
A part of this study was supported by National Heart, Lung, and Blood Institute Grants HL-71010, HL-74185, and HL-88012.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16: 1066–1071, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SM, Jackson KJ, Bushnell KM, McGuire PG. Spatial and temporal expression of the 72-kDa type IV collagenase (MMP-2) correlates with development and differentiation of valves in the embryonic avian heart. Dev Dyn 209: 261–268, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Baker AH, Zaltsman AB, George SJ, Newby AC. Divergent effects of tissue inhibitor of metalloproteinase-1, -2, or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Invest 101: 1478–1487, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brooks PC, Silletti S, von Schalscha TL, Friedlander M, Cheresh DA. Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell 92: 391–400, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell 85: 683–693, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Cai W, Vosschulte R, Afsah-Hedjri A, Koltai S, Kocsis E, Scholz D, Kostin S, Schaper W, Schaper J. Altered balance between extracellular proteolysis and antiproteolysis is associated with adaptive coronary arteriogenesis. J Mol Cell Cardiol 32: 997–1011, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Chow AK, Cena J, Schulz R. Acute actions and novel targets of matrix metalloproteinases in the heart and vasculature. Br J Pharmacol 152: 189–205, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ding B, Price RL, Borg TK, Weinberg EO, Halloran PF, Lorell BH. Pressure overload induces severe hypertrophy in mice treated with cyclosporine, an inhibitor of calcineurin. Circ Res 84: 729–734, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest 106: 55–62, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA 104: 15560–15565, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Friehs I, Margossian RE, Moran AM, Cao-Danh H, Moses MA, del Nido PJ. Vascular endothelial growth factor delays onset of failure in pressure-overload hypertrophy through matrix metalloproteinase activation and angiogenesis. Basic Res Cardiol 101: 204–213, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Friehs I, Moran AM, Stamm C, Choi YH, Cowan DB, McGowan FX, del Nido PJ. Promoting angiogenesis protects severely hypertrophied hearts from ischemic injury. Ann Thorac Surg 77: 2004–2010, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Geng B, Yang J, Qi Y, Zhao J, Pang Y, Du J, Tang C. H2S generated by heart in rat and its effects on cardiac function. Biochem Biophys Res Commun 313: 362–368, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Gerdes AM, Kellerman SE, Moore JA, Muffly KE, Clark LC, Reaves PY, Malec KB, McKeown PP, Schocken DD. Structural remodeling of cardiac myocytes in patients with ischemic cardiomyopathy. Circulation 86: 426–430, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Givvimani S, Tyagi N, Sen U, Mishra PK, Qipshidze N, Munjal C, Vacek JC, Abe OA, Tyagi SC. MMP-2/TIMP-2/TIMP-4 versus MMP-9/TIMP-3 in transition from compensatory hypertrophy and angiogenesis to decompensatory heart failure. Arch Physiol Biochem 116: 63–72, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527–531, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Hughes MN, Centelles MN, Moore KP. Making and working with hydrogen sulfide: the chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic Biol Med 47: 1346–1353, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem 272: 22389–22392, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Jiang Y, Reynolds C, Xiao C, Feng W, Zhou Z, Rodriguez W, Tyagi SC, Eaton JW, Saari JT, Kang YJ. Dietary copper supplementation reverses hypertrophic cardiomyopathy induced by chronic pressure overload in mice. J Exp Med 204: 657–666, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino Acids 26: 243–254, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Kang JA, Kim JT, Song HS, Bae MK, Yi EY, Kim KW, Kim YJ. Anti-angiogenic and anti-tumor invasive activities of tissue inhibitor of metalloproteinase-3 from shark, Scyliorhinus torazame. Biochim Biophys Acta 1620: 59–64, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, McClister DM, Jr, Su H, Gannon J, MacGillivray C, Lee RT, Sinusas AJ, Spinale FG. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol 290: H232–H239, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 121: e46–e215, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Lu M, Liu YH, Goh HS, Wang JJ, Yong QC, Wang R, Bian JS. Hydrogen sulfide inhibits plasma renin activity. J Am Soc Nephrol 21: 993–1002, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mancardi D, Penna C, Merlino A, Del Soldato P, Wink DA, Pagliaro P. Physiological and pharmacological features of the novel gasotransmitter: hydrogen sulfide. Biochim Biophys Acta 1787: 864–872, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mishra PK, Tyagi N, Sen U, Givvimani S, Tyagi SC. H2S ameliorates oxidative and proteolytic stresses and protects the heart against adverse remodeling in chronic heart failure. Am J Physiol Heart Circ Physiol 298: H451–H456, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olson KR. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim Biophys Acta 1787: 856–863, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Pan TT, Feng ZN, Lee SW, Moore PK, Bian JS. Endogenous hydrogen sulfide contributes to the cardioprotection by metabolic inhibition preconditioning in the rat ventricular myocytes. J Mol Cell Cardiol 40: 119–130, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Papaioannou VE, Fox JG. Efficacy of tribromoethanol anesthesia in mice. Lab Anim Sci 43: 189–192, 1993 [PubMed] [Google Scholar]
- 30. Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci USA 106: 21972–21977, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robbins JR, McGuire PG, Wehrle-Haller B, Rogers SL. Diminished matrix metalloproteinase 2 (MMP-2) in ectomesenchyme-derived tissues of the Patch mutant mouse: regulation of MMP-2 by PDGF and effects on mesenchymal cell migration. Dev Biol 212: 255–263, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Roberts JT, Wearn JT. Quantitative changes in the capillary-muscle relationship in human hearts during normal growth and hypertrophy. Am Heart J 21: 617–633, 1941 [Google Scholar]
- 33. Romanic AM, Harrison SM, Bao W, Burns-Kurtis CL, Pickering S, Gu J, Grau E, Mao J, Sathe GM, Ohlstein EH, Yue TL. Myocardial protection from ischemia/reperfusion injury by targeted deletion of matrix metalloproteinase-9. Cardiovasc Res 54: 549–558, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Rottman JN, Ni G, Brown M. Echocardiographic evaluation of ventricular function in mice. Echocardiography 24: 83–89, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Rucklidge GJ, Milne G, McGaw BA, Milne E, Robins SP. Turnover rates of different collagen types measured by isotope ratio mass spectrometry. Biochim Biophys Acta 1156: 57–61, 1992 [DOI] [PubMed] [Google Scholar]
- 36. Sen U, Basu P, Abe OA, Givvimani S, Tyagi N, Metreveli N, Shah KS, Passmore JC, Tyagi SC. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am J Physiol Renal Physiol 297: F410–F419, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sen U, Munjal C, Qipshidze N, Abe O, Gargoum R, Tyagi SC. Hydrogen sulfide regulates homocysteine-mediated glomerulosclerosis. Am J Nephrol 31: 442–455, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shi YX, Chen Y, Zhu YZ, Huang GY, Moore PK, Huang SH, Yao T, Zhu YC. Chronic sodium hydrosulfide treatment decreases medial thickening of intramyocardial coronary arterioles, interstitial fibrosis, and ROS production in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 293: H2093–H2100, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem (Tokyo) 146: 623–626, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Sodha NR, Clements RT, Boodhwani M, Xu SH, Laham RJ, Bianchi C, Sellke FW. Endostatin and angiostatin are increased in diabetic patients with coronary artery disease and associated with impaired coronary collateral formation. Am J Physiol Heart Circ Physiol 296: H428–H434, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Takigawa M, Nishida Y, Suzuki F, Kishi J, Yamashita K, Hayakawa T. Induction of angiogenesis in chick yolk-sac membrane by polyamines and its inhibition by tissue inhibitors of metalloproteinases (TIMP and TIMP-2). Biochem Biophys Res Commun 171: 1264–1271, 1990 [DOI] [PubMed] [Google Scholar]
- 42. Tyagi SC. Proteinases and myocardial extracellular matrix turnover. Mol Cell Biochem 168: 1–12, 1997 [DOI] [PubMed] [Google Scholar]
- 43. Tyagi SC, Kumar SG, Haas SJ, Reddy HK, Voelker DJ, Hayden MR, Demmy TL, Schmaltz RA, Curtis JJ. Post-transcriptional regulation of extracellular matrix metalloproteinase in human heart end-stage failure secondary to ischemic cardiomyopathy. J Mol Cell Cardiol 28: 1415–1428, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Tyagi SC, Matsubara L, Weber KT. Direct extraction and estimation of collagenase(s) activity by zymography in microquantities of rat myocardium and uterus. Clin Biochem 26: 191–198, 1993 [DOI] [PubMed] [Google Scholar]
- 45. Xu J, Carretero OA, Liao TD, Peng H, Shesely EG, Liu TS, Yang JJ, Reudelhuber TL, Yang XP. Local angiotensin II aggravates cardiac remodeling in hypertension. Am J Physiol Heart Circ Physiol 299: H1328–H1338, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang GD, Wu LY, Jiang B, Yang W, Qi JS, Cao K, Meng QH, Mustafa AK, Mu WT, Zhang SM, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322: 587–590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yong QC, Lee SW, Foo CS, Neo KL, Chen X, Bian JS. Endogenous hydrogen sulphide mediates the cardioprotection induced by ischemic postconditioning. Am J Physiol Heart Circ Physiol 295: H1330–H1340, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Zhang Z, Huang H, Liu P, Tang C, Wang J. Hydrogen sulfide contributes to cardioprotection during ischemia-reperfusion injury by opening KATP channels. Can J Physiol Pharmacol 85: 1248–1253, 2007 [DOI] [PubMed] [Google Scholar]