Abstract
Lung volume reduction surgery (LVRS) improves lung function, respiratory symptoms, and exercise tolerance in selected patients with chronic obstructive pulmonary disease, who have heterogeneous emphysema. However, the reported effects of LVRS on gas exchange are variable, even when lung function is improved. To clarify how LVRS affects gas exchange in chronic obstructive pulmonary disease, 23 patients were studied before LVRS, 14 of whom were again studied afterwards. We performed measurements of lung mechanics, pulmonary hemodynamics, and ventilation-perfusion (V̇a/Q̇) inequality using the multiple inert-gas elimination technique. LVRS improved arterial Po2 (PaO2) by a mean of 6 Torr (P = 0.04), with no significant effect on arterial Pco2 (PaCO2), but with great variability in both. Lung mechanical properties improved considerably more than did gas exchange. Post-LVRS PaO2 depended mostly on its pre-LVRS value, whereas improvement in PaO2 was explained mostly by improved V̇a/Q̇ inequality, with lesser contributions from both increased ventilation and higher mixed venous Po2. However, no index of lung mechanical properties correlated with PaO2. Conversely, post-LVRS PaCO2 bore no relationship to its pre-LVRS value, whereas changes in PaCO2 were tightly related (r2 = 0.96) to variables, reflecting decrease in static lung hyperinflation (intrinsic positive end-expiratory pressure and residual volume/total lung capacity) and increase in airflow potential (tidal volume and maximal inspiratory pressure), but not to V̇a/Q̇ distribution changes. Individual gas exchange responses to LVRS vary greatly, but can be explained by changes in combinations of determining variables that are different for oxygen and carbon dioxide.
Keywords: chronic obstructive lung disease, multiple inert elimination technique
lung volume reduction surgery (LVRS) improves lung function, respiratory symptoms, and exercise tolerance in selected patients with chronic obstructive pulmonary disease (COPD) who have heterogeneous emphysema (16). The underlying mechanisms of improvement have not been fully elucidated, although increased elastic recoil (30), reduction in dynamic hyperinflation (20), and augmented force exerted by the diaphragm (10, 32) have been suggested to explain the improvement in forced expiratory volume in 1 s (FEV1).
The reported effects of LVRS on gas exchange are variable. Initial small observational trials have reported improvement (31), no change (22), or a deterioration in gas exchange (1). Randomized studies were similarly variable in outcome, reporting either no change (11, 17, 21) or small improvements after surgery (18). Furthermore, in most studies, great variability in the individual responses has been observed (34). The large National Emphysema Treatment Trial (NETT) with over 1,000 individuals confirmed a slight improvement after surgery (33). This powerful study demonstrated the importance of less severe disease and the predominance of upper lobe emphysema in improved gas exchange after LVRS. None of these studies, however, was designed to determine the specific and detailed mechanisms of change in gas exchange following surgery.
Structure-function correlation studies conducted in patients with moderate COPD have shown that emphysema results in ventilation-perfusion (V̇a/Q̇) mismatching, with a wide spectrum of V̇a/Q̇ heterogeneity, including areas of low V̇a/Q̇ ratio, probably due to reduced ventilation because of airway narrowing and distortion (6). There is a steady progression of V̇a/Q̇ imbalance from mild to very severe COPD stages (6, 28, 35).
LVRS could lead to improved pulmonary gas exchange in several possible ways. First, if before surgery, excised tissue exhibited very abnormal V̇a/Q̇ relationships, its removal could per se leave the remaining lung more homogeneous. Second, changes in lung mechanical properties caused by LVRS could alter the distribution of ventilation, perfusion, or both in such a way as to improve gas exchange. Third, if LVRS enables greater alveolar ventilation, arterial Po2 (PaO2) could improve, even if the V̇a/Q̇ distributions are per se unaffected. Fourth, if LVRS results in an increase in mixed venous Po2 (Pv̄O2), due to either increases in cardiac output (Q̇t), or decreased oxygen consumption (V̇o2), or both, this alone would contribute to an increase in PaO2, with other factors unchanged. Most of these mechanisms can be individually assessed by combining the multiple inert-gas elimination technique (MIGET) (27) with respiratory blood-gas analysis using arterial and mixed venous sampling and at the same time assessing changes in lung mechanics. Accordingly, we undertook the present study to 1) assess the mechanisms of pulmonary gas exchange impairment in patients with upper lobe, predominant heterogenous emphysema eligible for LVRS and well characterized by lung function, imaging, and pathology; 2) investigate the effects of LVRS on gas exchange by using the MIGET; and 3) analyze whether changes in gas exchange after LVRS can be related to altered V̇a/Q̇ distribution, alveolar ventilation, and/or V̇o2 and/or Q̇t. We also asked whether lung mechanical changes produced by LVRS could account for the gas exchange alterations.
METHODS
Subjects
Twenty-three patients undergoing evaluation for LVRS in S. Maugeri Foundation, Veruno, Italy (n = 12), and Hospital Clínic, Barcelona, Spain (n = 11), consented to the study approved by the Ethical Committees of both institutions. Candidate selection followed the National Heart, Lung, and Blood Institute (36) and the NETT guidelines (3). Indications for surgery were disabling dyspnea, severe airway obstruction with lung hyperinflation, despite maximal medical therapy, and predominantly upper-lobe involvement. Assessment for suitability involved clinical history and physical examination, lung function testing, 6-min walk test, high-resolution computed tomography scan, echocardiography, and V̇a/Q̇ scans. On acceptance for surgery, patients underwent lung mechanics, right heart catheterization, and gas exchange studies. LVRS was performed 7–10 days after assessment. The type of procedure was decided by the surgeons purely on clinical grounds, but, in all cases, involved surgical reduction of the upper lobe. Thoracic computed tomography and perfusion scans were used to decide the site and extent of tissue to be removed.
Pulmonary Function Testing
Lung function testing was performed on all patients and included spirometry, body plethysmography, and single-breath gas transfer for carbon monoxide (MasterScreen, E. Jaeger, Wuerzburg, Germany; and Vmax 2200D, Sensor Medics, Yorba Linda CA). European Respiratory Society reference values were used (9, 24, 26).
Assessment of Pulmonary Mechanics
Pulmonary mechanics were assessed in seated patients during stable breathing in ambient air measuring simultaneous flow and esophageal and gastric pressures (23, 29). Dynamic pulmonary compliance (Cdyn), inspiratory airway resistance (Raw) and dynamic intrinsic positive end-expiratory pressure (PEEPi) corrected for expiratory muscle activity were measured from the signals reported above with standard methods previously published (4). Only eight patients consented to the necessary balloon catheters, of whom six were also studied postsurgery. Minute ventilation (V̇e), breathing frequency, respiratory cycle duration (Ttot), tidal volume (Vt), inspiratory (Ti) and expiratory times (Te), and flows (Vt/Ti and Vt/Te, respectively) were calculated from the flow and pressure records.
Hemodynamic Measurements
Intravascular pressures were continuously recorded (M1166A, Hewlett-Packard, Boeblingen, Germany) by means of balloon-tipped 7F Swan-Ganz pulmonary artery (Edwards Laboratories, Santa Ana, CA; and Arrow International) and radial artery catheters (Seldicath 3F, Plastimed; and Arterial Line Kit, Arrow). Measurements of pulmonary vascular pressures were taken at the end of expiration. Q̇t was determined by thermodilution (M1012A, Hewlett-Packard) as the mean of three measurements. Pulmonary (PVR) and systemic vascular resistances were calculated using standard formulas.
Gas Exchange Assessment
The MIGET technique was used to assess V̇a/Q̇ distribution (27). Patients were studied in a semirecumbent position while breathing ambient air. V̇o2 and CO2 production were calculated from mixed expired air (CPX System, Medical Graphics, St. Paul, MN). PaO2, Pv̄O2, PaCO2, and pH were analyzed in duplicate using standard electrodes (860 Gas Analyzer, CIBA-Corning; Medfield, MA; and ABL300, Radiometer, Copenhagen, Denmark); the alveolar-arterial Po2 gradient [(A-a)Po2] was calculated according to the standard formula measuring the respiratory gas exchange ratio.
Statistical Analysis
Data are expressed as means ± SD. Comparisons between patients or pre- and post-LVRS were performed using Student's t-test or Wilcoxon signed-rank test, as appropriate. Correlation between changes in PaO2 and PaCO2 and other variables was investigated by simple regression. Stepwise multiple linear regression (MLR) identified the most parsimonious model using adjusted R2 values and controlling for colinearity. The contribution of each variable to the model was assessed by standardized β-coefficients. Reported P values are for two-tailed tests, considered significant when P was <0.05.
RESULTS
Preoperative Findings (23 Patients)
Patients had severe emphysema with marked airflow obstruction (FEV1 = 0.84 liter; 33% predicted), static lung hyperinflation, pronounced air trapping, and substantial reduction in transfer factor for carbon monoxide (DlCO) (Table 1). The only significant differences between patients studied in Veruno and in Barcelona, in terms of general characteristics, consisted of a 5% lower FEV1-to-forced vital capacity (FVC) ratio (attributable to a slightly larger FVC) and a 15% lower Q̇t in the Barcelona group. There were small differences in specific gas exchange parameters derived from MIGET, but, taken altogether, these differences were insufficient to cause group differences in PaO2, PaCO2, or (A-a)Po2. On average, patients showed moderate hypoxemia, normocapnia, and increased (A-a)Po2. While all patients exhibited severe airflow obstruction, there was wide variation in arterial blood-gas values: PaO2 ranged between 48 and 91 Torr, PaCO2 between 31 and 60 Torr, and (A-a)Po2 between 19 and 60 Torr.
Table 1.
Dynamic and static lung volumes, pulmonary hemodynamics, and gas exchange before LVRS
| Variable | |
|---|---|
| N | 23 |
| Age, yr | 62 ± 8 |
| Sex (men/women) | 21/2 |
| Height, cm | 170 ± 10 |
| Weight, kg | 65 ± 14 |
| FVC, %predicted | 60 ± 16 |
| FEV1, liters | 0.84 ± 0.33 |
| FEV1, %predicted | 26 ± 10 |
| FEV1/FVC ratio | 0.33 ± 0.06 |
| TLC, %predicted | 127 ± 18 |
| RV, %predicted | 237 ± 54 |
| IC, liters | 1.57 ± 0.34 |
| DlCO, %predicted | 30 ± 9 |
| PaO2, Torr | 65 ± 11 |
| PaCO2, Torr | 41 ± 7 |
| (A-a)Po2, Torr | 37 ± 10 |
| PV̄O2, Torr | 33.6 ± 4.1 |
| V̇e, l/min | 8.7 ± 2.3 |
| V̇o2, ml/min | 251 ± 87 |
| PAP, mmHg | 18 ± 5 |
| Q̇t, l/min | 4.99 ± 1.12 |
| CI, l · min−1 · m−2 | 2.76 ± 0.50 |
| PVR, dyn · s · cm−5 | 192 ± 51 |
| V̇a/Q̇ inequality | |
| RSS | 4.4 ± 3.2 |
| DISP R-E* | 13.6 ± 5.5 |
| Shunt, %Q̇t | 4.1 ± 2.8 |
| Low, V̇a/Q̇, %Q̇t | 0.01 ± 0.02 |
| Mean Q̇ | 0.79 ± 0.29 |
| Log SDQ̇ | 0.77 ± 0.23 |
| High, V̇a/Q̇, %V̇e | 2.58 ± 3.71 |
| Mean V̇ | 2.00 ± 0.54 |
| Log SDV̇ | 1.13 ± 0.40 |
| Dead space, %V̇e | 33.3 ± 10.2 |
| Predicted-observed PaO2, Torr | −0.5 ± 4.6 |
| Observed-predicted (A-a)Po2, Torr | 2.6 ± 10.3 |
Values are means ± SD; N, no. of subjects. Spirometric data are prebronchodilator values. FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; TLC, total lung capacity; RV, residual volume; IC, inspiratory capacity; DlCO, transfer factor for carbon monoxide; PaO2, partial pressure of oxygen in arterial blood; PaCO2, partial pressure of carbon dioxide in arterial blood; (A-a)Po2, alveolar-arterial oxygen partial pressure gradient; PV̄O2, mixed venous partial pressure of oxygen; V̇e, minute ventilation; V̇o2, oxygen uptake; PAP, mean pulmonary arterial pressure; Q̇t, cardiac output; CI, cardiac index; PVR, pulmonary vascular resistance; V̇a/Q̇, ventilation-perfusion; RSS, residual sum of squares of the best fit to the excretion and retention data; DISP R-E*, dispersion of retention minus excretion of inert gases corrected for dead space, an overall index of V̇a/Q̇ heterogeneity; shunt, percentage of blood flow to very low V̇a/Q̇ units (<0.005); low, V̇a/Q̇, %Q̇t: blood flow to units with V̇a/Q̇ ratios between 0.005 and 0.1; mean Q̇, mean V̇a/Q̇ ratio of the blood flow distribution; log SDQ̇, the second moment blood flow distribution; high, V̇a/Q̇, %V̇e: ventilation to units with V̇a/Q̇ ratios between 10 and 100; mean V̇, mean V̇a/Q̇ ratio of the ventilation distribution; log SDV̇, the second moments of the ventilation distribution; dead space, fraction of ventilation to dead space.
In agreement with these findings, there was also wide variation in the degree of V̇a/Q̇ inequality. All patients but one had increased V̇a/Q̇ mismatching, as shown by an abnormal value of the dispersion of retention minus excretion of inert gases corrected for dead space (DISP R-E*), which ranged between 2.0 (one patient) and 33.0. Characteristics of V̇a/Q̇ distributions were comparable to other series involving patients with severe COPD (14, 35), with all but one patient showing abnormal log SDV̇ (the second moment of the ventilation distribution; >0.65) (8). The distribution of ventilation was broad and unimodal in 11 patients and bimodal in 12. Inert dead space varied widely, from 16 to 55% of V̇e. Most patients also showed areas with high V̇a/Q̇ ratio, which ranged between 0.4 and 13.1% of alveolar ventilation. The log SDQ̇ (the second moment blood flow distribution) was abnormal (>0.6) (8) in 17 patients, with a broad and unimodal distribution in 11 patients and bimodal in 6. Seven patients showed a shunt of >5.0% of Q̇t, although, on average, shunt was discrete and similar to that in other reported series of COPD patients (Table 1). Perfusion to lung units with low V̇a/Q̇ ratios (between 0.1 and 0.005) was minimal (<1% Q̇t) in all patients.
Mean baseline DlCO (27% of normal) was in the range of overt diffusion limitation. MIGET allows for an indirect assessment of diffusion limitation at any inspired fraction of O2 by means of the comparison between PaO2 predicted by MIGET (i.e., ignoring diffusion limitation) against observed PaO2. The implication is that, if predicted PaO2 is systematically higher than the observed values, diffusion limitation should be present. Overall predicted-observed PaO2 was −0.5 ± 4.6 Torr, with 6 out of 23 subjects showing positive differences (range 1.2–13 Torr). Similarly, mean observed-predicted (A-a)Po2 was 2.6 ± 10.3 Torr, which was not significantly different from zero (P = 0.4), indicating that, even accounting for changes in alveolar ventilation, diffusion limitation was not significant at rest while breathing ambient air. No correlation was observed between mean observed-predicted (A-a)Po2 and DlCO (R2 = 0.02).
Pulmonary hemodynamics were normal or only mildly impaired in the majority of patients (15). Only six patients showed a mean pulmonary artery pressure > 20 Torr, with the highest value being 27 Torr, similar to that found in the NETT study (13). The cardiac index was >2 l·min−1·m−2 in all but one patient. PVR ranged between 119 and 353 dyn·s·cm−5.
From a lung mechanical viewpoint, Cdyn, Raw, and PEEPi were moderately increased, whereas inspiratory-to-total cycle time ratio (Ti/Ttot) and maximal inspiratory pressure (MIP) were reduced.
Regression analysis showed that PaO2 correlated only with inspiratory capacity (r = 0.48, P = 0.031) and DISP R-E* (r = −0.45, P = 0.03), with both variables together accounting for, and contributing equally to, ∼50% of the total variance in PaO2 (r2 = 0.49, P = 0.001).
Changes After LVRS (14 Patients)
Surgical procedure.
LVRS was performed by video-assisted thoracoscopy (Veruno, n = 8; Barcelona, n = 1), median sternotomy (Veruno, n = 3; Barcelona, n = 8), or thoracotomy (Veruno, n = 1; Barcelona, n = 2). The procedure was bilateral in 17 cases (Veruno, n = 9; Barcelona, n = 8) and unilateral in 6 cases (Veruno, n = 3; Barcelona, n = 3). Of the original 23 patients, 14 (Veruno, n = 9; Barcelona, n = 5) were reassessed in stable clinical conditions and lung function at around 6 mo after surgery. In the remaining 6 patients in Spain and 3 in Italy, postoperative studies were not conducted because of death (n = 2), substantial postoperative pleural changes impacting on lung function (n = 3), or loss to follow-up (n = 4). While it was not possible to quantify the amount of tissue removed, there was no correlation between type of procedure (surgical approach and uni- or bilateral resection) and change in functional variables.
Key variables are compared before and after surgery in Table 2. LVRS led to a significant (P < 0.004) and relatively substantial increase in FEV1 of 0.50 ± 0.54 liter (that is, by a mean of 55%). FVC also increased significantly after surgery, such that the FEV1-to-FVC ratio did not change significantly (Table 2). Total lung capacity (TLC) decreased by 1.27 ± 2.02 liter (i.e., −14% from 127 ± 17 to 107 ± 18% predicted, P = 0.04) and residual volume (RV) by 1.68 ± 2.06 liter (i.e., −28%, RV from 237 ± 53 to 168 ± 75%, P = 0.001) after LVRS, accompanied by a fall in RV/TLC (11%, P = 0.01) and an increase in inspiratory capacity (by 0.65 ± 0.75 liter, +24%, P = 0.02). There were, in addition, significant reductions in Cdyn (14 ± 13 ml/cmH2O, −25%, P = 0.04), Raw (2.5 ± 2.7 cmH2O·l−1·s, −25%, P = 0.04), and PEEPi (1.9 ± 1.6 cmH2O, −54%, P = 0.02), and increases in Ti/Ttot (0.05 ± 0.04, +16%, P = 0.02) and MIP (16 ± 19 cmH2O, +63%, P = 0.03).
Table 2.
Changes in dynamic and static lung volumes, lung mechanics, and pulmonary hemodynamics and gas exchange after LVRS
| Variable | N | Before LVRS | After LVRS | P Value |
|---|---|---|---|---|
| FVC, liters | 14 | 2.41 ± 0.78 | 3.36 ± 0.99 | <0.001 |
| FEV1, liters | 14 | 0.83 ± 0.35 | 1.33 ± 0.82 | 0.004 |
| FEV1, %predicted | 14 | 25 ± 10 | 40 ± 24 | 0.003 |
| FEV1/FVC ratio | 14 | 0.33 ± 0.05 | 0.38 ± 0.15 | 0.24 |
| TLC, liters | 14 | 8.54 ± 1.33 | 7.27 ± 1.70 | 0.04 |
| RV, liters | 14 | 5.54 ± 1.68 | 3.86 ± 1.95 | 0.01 |
| IC, liters | 14 | 1.54 ± 0.40 | 2.04 ± 0.75 | 0.02 |
| DlCO, %predicted | 14 | 27 ± 6 | 37 ± 12 | 0.08 |
| V̇e, l/min | 14 | 8.8 ± 2.3 | 9.0 ± 2.3 | 0.72 |
| Vt, liters | 9 | 0.59 ± 0.09 | 0.61 ± 0.09 | 0.51 |
| Ti, s | 9 | 1.28 ± 0.27 | 1.61 ± 0.32 | 0.01 |
| Te, s | 9 | 3.01 ± 1.92 | 2.80 ± 1.28 | 0.46 |
| Ti/Ttot | 9 | 0.33 ± 0.07 | 0.38 ± 0.05 | 0.003 |
| BF, min−1 | 9 | 16 ± 5 | 15 ± 4 | 0.29 |
| PEEPi, cmH2O | 7 | 3.3 ± 2.4 | 1.7 ± 1.6 | 0.11 |
| MIP, cmH2O | 9 | 53 ± 20 | 69 ± 26 | 0.04 |
| MEP, cmH2O | 9 | 83 ± 24 | 81 ± 26 | 0.82 |
| Cdyn, l/cmH2O | 6 | 0.48 ± 0.17 | 0.34 ± 0.10 | 0.05 |
| Raw, cmH2O · l−1 · s | 6 | 3.34 ± 1.26 | 4.87 ± 2.15 | 0.01 |
| PTPdi, cmH2O/s | 6 | 205 ± 51 | 231 ± 41 | 0.29 |
| TTdi | 6 | 0.06 ± 0.03 | 0.03 ± 0.01 | 0.06 |
| P0.1, cmH2O | 8 | 2.27 ± 1.12 | 1.47 ± 0.44 | 0.04 |
| PAP, mmHg | 13 | 19 ± 5 | 18 ± 4 | 0.10 |
| Q̇t, l/min | 13 | 6.0 ± 2.6 | 5.8 ± 1.6 | 0.79 |
| PVR, dyn · s · cm−5 | 13 | 204 ± 59 | 180 ± 50 | 0.13 |
| PaO2, Torr | 14 | 65 ± 11 | 71 ± 15 | 0.04 |
| PaCO2, Torr | 14 | 42 ± 8 | 38 ± 6 | 0.11 |
| (A-a)Po2, Torr | 14 | 36 ± 10 | 33 ± 17 | 0.41 |
| PV̄O2, Torr | 14 | 35 ± 4 | 36 ± 4 | 0.63 |
| V̇o2, ml/min | 14 | 250 ± 102 | 270 ± 72 | 0.48 |
| Hb, g/dl | 14 | 14.1 ± 1.7 | 14.2 ± 1.7 | 0.90 |
| pH | 14 | 7.41 ± 0.04 | 7.43 ± 0.03 | 0.30 |
| P50 | 14 | 25.7 ± 0.8 | 26.0 ± 0.6 | 0.30 |
| DISP R-E* | 14 | 14.4 ± 6.3 | 13.1 ± 8.2 | 0.59 |
| Shunt, %Q̇t | 14 | 5.0 ± 3.3 | 3.6 ± 2.6 | 0.05 |
| Log SDQ̇ | 14 | 0.79 ± 0.21 | 0.65 ± 0.16 | 0.03 |
| Log SDV̇ | 14 | 1.13 ± 0.30 | 0.99 ± 0.42 | 0.21 |
| Dead space, %V̇e | 14 | 33.7 ± 10.7 | 35.6 ± 6.1 | 0.45 |
| Observed-predicted (A-a)Po2, Torr | 14 | 2.5 ± 10.5 | 4.3 ± 15.8 | 0.65 |
Values are means ± SD; N, no. of subjects. VT, tidal volume; Ti, inspiratory time; Te, expiratory time; Ti/Ttot, inspiratory duty cycle; BF, breathing frequency; PEEPi, intrinsic positive end-expiratory pressure; MIP, maximal inspiratory pressure; MEP, maximal expiratory pressure; Cdyn, dynamic pulmonary compliance; Raw, airway resistance; PTPdi, pressure-time product for the diaphragm; TTdi, tension-time index for the diaphragm; P0.1, inspiratory occlusion pressure; P50, O2 concentration required for half-maximal reduction. P values correspond to significance difference between variables pre- and post-LVRS estimated by paired t-test. Values in bold are ???.
On average, PaO2 increased significantly, but only modestly, by 6 ± 10 Torr (P = 0.04) (Table 2), but with remarkable individual variations; the median change was similar (5 Torr, a 9% increase). The change in PaCO2 after LVRS was also very variable. On average, the value of PaCO2 was 3.5 ± 7.7 Torr lower after surgery, but this apparent fall was not significant (P = 0.1). On average, (A-a)Po2 remained unaltered after LVRS, as did V̇e, V̇o2, and Pv̄O2 (Table 2).
LVRS resulted in a moderate improvement of V̇a/Q̇ mismatching (Table 2). The mean log SDQ̇ improved significantly, falling from 0.79 to 0.65 (P = 0.03). The amount of intrapulmonary shunt decreased after surgery, from 5.0 to 3.6% (P = 0.05). By contrast, there were no significant changes in either the log SDV̇ or in the amount of dead space. Changes in the pattern of the ventilation distribution, from a bimodal to a unimodal pattern, were observed in five patients and in that of the blood flow distribution in three; in the other patients, the ventilation and distribution patterns were unchanged. Overall V̇a/Q̇ dispersion estimated by DISP RE* worsened in four subjects, increasing by a mean of 61% in these patients compared with a mean percent decrease of 54% in the patients who improved V̇a/Q̇ dispersion post-LVRS.
Following LVRS mean observed-predicted (A-a)Po2 showed a slight and nonsignificant increase from 2.5 ± 10.5 to 4.3 ± 15.8 Torr (P = 0.5, Table 2), which was also not significantly different from zero (P = 0.3). No correlation between change in observed-predicted (A-a)Po2 and change in PaO2 was found (R2 = 0.01).
Mean hemoglobin, pH, and P50 (O2 concentration required for half-maximal reduction) values were not significantly different following LVRS (Table 2).
While mean pulmonary hemodynamic variables were unchanged (Table 2), there was substantial individual variability here as well. After surgery, pulmonary artery pressure decreased in seven patients, increased in two, and remained equal in four. Similar variation occurred for PVR and for Q̇t (this increasing in 7 patients and decreasing in 6).
Gas exchange determinants of PaO2 and PaCO2 after surgery.
As the gas exchange responses to LVRS varied widely, analysis of mean changes was of limited value in understanding the responses to LVRS. Therefore, regression analysis is now reported. Figure 1, top, shows, that pre-LVRS, PaO2 was the single best indicator of the absolute value of post-LVRS PaO2. However, the regression line is essentially parallel to that of identity, and its offset by 6 Torr represents the average effect of LVRS on PaO2, which is independent of pre-LVRS values. In the case of PaCO2, post-LVRS PaCO2 had no relationship to pre-LVRS PaCO2 values, with r2 = 0.17 (Fig. 1, middle). As for (A-a)Po2, only 28% of the post-LVRS value could be explained by the value before surgery; in addition, LVRS had no significant effect on (A-a)Po2 (Fig. 1, bottom).
Fig. 1.
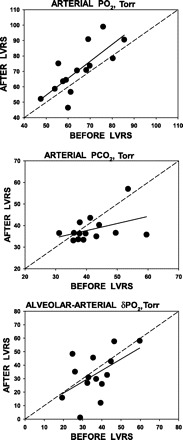
Linear regressions of arterial Po2 (PaO2; top), arterial Pco2 (PaCO2; middle), and alveolar-arterial Po2 gradient [(A-a)Po2; bottom] pre- and post-lung volume reduction surgery (LVRS). Top: almost 60% of the post-LVRS PaO2 is accounted for by the pre-LVRS value, leaving ∼40% explained by the effects of LVRS itself. Note that, in all except three patients, PaO2 improved after LVRS, and the mean increase (δ) over all patients was 6.0 Torr. The regression equation is PaO2 (post-LVRS) = −0.2 + 1.10 × PaO2 (pre-LVRS), r2 = 0.58, P = 0.04. Middle: post-LVRS PaCO2 is poorly predicted by the pre-LVRS value (r2 = 0.17). In 4 patients, PaCO2 rose, while in 2 it was unchanged, and in 8 it fell. There was no significant group change after LVRS. The regression equation is PaCO2 (post-LVRS) = 24.5 + 0.33 × PaCO2 (pre-LVRS), r2 = 0.17, P = nonsignificant (NS), δ over all patients was −3.5 Torr. Bottom: only in 30% of the post-LVRS patients is (A-a)Po2 accounted for by the pre-LVRS value, leaving ∼70% explained by the effects of LVRS itself. Note that, in 4 patients, (A-a)Po2 worsened after LVRS. The mean numerical decrease over all patients was only 3.0 Torr (NS). The regression equation is (A-a)Po2 (post-LVRS) = 2.5 + 0.84 × (A-a)Po2 (pre-LVRS), r2 = 0.28, P = NS.
Figure 2 shows similar relationships for the three major indexes of V̇a/Q̇ inequality: log SDQ̇ (top), log SDV̇ (middle), and DISP R-E* (bottom). This figure shows that values of these indexes after LVRS were each poorly related to their pre-LVRS values. The changes in PaO2 due to LVRS can be explained in terms of the classical contributors to hypoxemia. These factors are as follows: 1) total alveolar ventilation; 2) pulmonary gas exchange defects (V̇a/Q̇ inequality and shunt); 3) diffusion limitation; and 4) extrapulmonary factors (Q̇t and V̇o2) that together directly affect Pv̄O2. The magnitude of (A-a)Po2 per se is not useful in deciding on its mechanism, and the fact that observed-predicted (A-a)Po2 was not different from zero indicates that it is unlikely that diffusion limitation was present in the subjects at rest breathing ambient air. The lack of change in predicted-observed PaO2 and observed-predicted (A-a)Po2 following LVRS, as well as the lack of correlation of these variables with change in PaO2 and change in DlCO, would suggest that diffusion limitation does not appear to be a major determinant of resting PaO2 after LVRS.
Fig. 2.
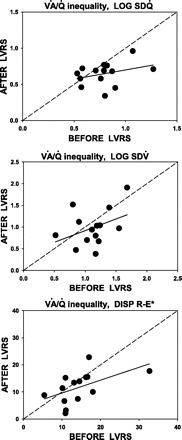
Linear regression of indexes of ventilation-perfusion (V̇a/Q̇) distribution [top: second moment of blood flow distribution (log SDQ̇); middle: second moment of ventilation distribution (log SDV̇); and bottom: dispersion of retention minus excretion of inert gases corrected for dead space (DISP R-E*)] pre- and post-LVRS. Top: almost none of the post-LVRS value of log SDQ̇ is accounted for by the pre-LVRS value. The mean decrease over all patients was significant at 0.14 units. The regression equation is log SDQ̇ (post-LVRS) = 0.45 + 0.25 × log SDQ̇ (pre-LVRS), r2 = 0.11, P = 0.03. Middle: almost none of the post-LVRS value of log SDV̇ is accounted for by the pre-LVRS value. The mean decrease over all patients was insignificant at 0.14 units. The regression equation is log SDV̇ (post-LVRS) = 0.38 + 0.54 × log SDV̇ (pre-LVRS), r2 = 0.15, P = NS. Bottom: almost none of the post-LVRS value of DISP R-E* is accounted for by the pre-LVRS value. The mean decrease over all patients was insignificant at 1.3 units. The regression equation is DISP R-E* (post-LVRS) = 4.9 + 0.47 × DISP R-E* (pre-LVRS), r2 = 0.27, P = NS.
Examined individually, the relationships between the changes in PaO2 and those in the other three variable clusters were not strong. The relationship between PaO2 and PaCO2 (the latter reflecting ventilation) yielded r2 = 0.02 (Fig. 3, top); that between PaO2 and Pv̄O2 (the latter reflecting Q̇t and V̇o2) yielded r2 = 0.54 (Fig. 3, middle); and that between PaO2 and V̇a/Q̇ inequality yielded r2 = 0.28 (Fig. 3, bottom). However, because all three variable clusters are expected to contribute independently to PaO2, their role in determining PaO2 changes was examined by MLR after first confirming their lack of covariance. Figure 4 shows the relationship between the measured changes in PaO2 induced by LVRS on the abscissa and those predicted by MLR using the above variable clusters on the ordinate. As can be seen, there was good predictability with 71% of the variance in the PaO2 change after LVRS accounted for by a linear combination of the three variables PaCO2 (reflecting ventilation), Pv̄O2 (reflecting combined cardiac and metabolic influences), and DISP-RE* (representing overall V̇a/Q̇ inequality and shunt) (P = 0.01). Moreover, the remaining 29% of the variance that is not explained by these three variables is presumably due to cumulative measurement errors, a reasonable finding, since 30% of the overall average change in PaO2 (of 6 Torr) was just 2 Torr. The regression equation provided in Fig. 4 also shows that 45% of the change in PaO2 resulted from improved V̇a/Q̇ mismatch, while the remainder was split approximately equally between changes in ventilation (24%) and changes in Pv̄O2 (31%). MLR analysis of changes in PaCO2 caused by LVRS revealed that no combination of these determining gas exchange variables could explain the PaCO2 changes.
Fig. 3.
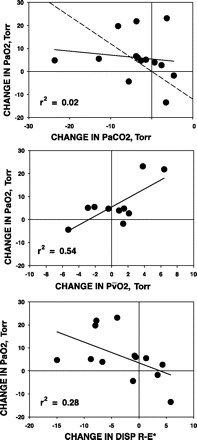
Regression of changes post-LVRS in PaO2 and changes in PaCO2 (top), changes in mixed venous Po2 (Pv̄O2; middle), and changes in DISP R-E* (bottom). Top: changes in PaCO2 (reflecting ventilation) do not explain changes in PaO2 (r2 = 0.02). Dashed line is expected relationship, if PaCO2 were the complete explanation. In 4 patients, PaO2 improved less than expected from change in PaCO2; in 7 there were small improvements in PaO2, about as expected from PaCO2; while, in 3, PaO2 improved more than expected from changes in PaCO2. Middle: changes in Pv̄O2 (reflecting changes in cardiac output and/or O2 uptake) explain ∼50% of the changes in PaO2 after LVRS (r2 = 0.54). Bottom: changes in DISP R-E* (reflecting changes in V̇a/Q̇ inequality and shunt) explain only ∼28% of the changes in PaO2 after LVRS (r2 = 0.28).
Fig. 4.
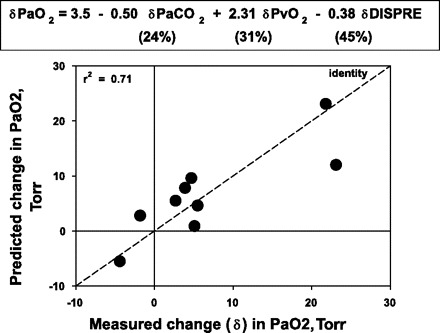
Plot of measured vs. predicted LVRS-induced changes in PaO2 obtained from a multiple linear regression model using ventilation (reflected by PaCO2), extrapulmonary factors (Pv̄O2), and V̇a/Q̇ inequality (i.e., DISP R-E*) as independent variables. About 70% of the changes are accounted for by these three factors, with V̇a/Q̇ inequality about twice as important as the other two, which are about equal in importance. The average total PaO2 change being just 6 Torr, 30% of the change, or 2 Torr, is not explained by these variables and is presumed due to measurement error.
Mechanical determinants of PaO2 and PaCO2 after surgery.
Table 2 shows that several indexes of lung mechanical properties were improved after LVRS when the group mean data were analyzed. These included indexes of lung hyperinflation, airflow, and pressure generation. The question that arises is whether the changes in gas exchange can be related to any of the changes in lung mechanical properties.
For PaO2, MLR analysis revealed that no combination of mechanical property changes was significantly related to PaO2 changes after LVRS. However, changes in indexes of V̇a/Q̇ distribution were mainly related to changes in indexes related to hyperinflation. Regression analysis showed that 80% (P < 0.001) of the variance in change in log SDQ̇ before and after LVRS was explained by changes in FEV1 (47%), TLC (27%), and PVR (26%); 76% (P < 0.001) of the variance in change in shunt was explained by changes in FEV1 (66%) and RV/TLC (34%); and 64% (P = 0.014) of the changes in DISP-RE* was explained by changes in FEV1 (34%), RV/TLC (49%), and PVR (17%).
For PaCO2, the outcome was very different. MLR showed that 96% of the variance in changes in PaCO2 from pre- to post-LVRS could be accounted for (P < 0.001) by a model that included four non-covariant mechanical property variables: PEEPi (accounting for 36% of the PaCO2 change), RV-to-TLC ratio (29%), Vt (22%), and MIP (14%). This is shown in Fig. 5.
Fig. 5.
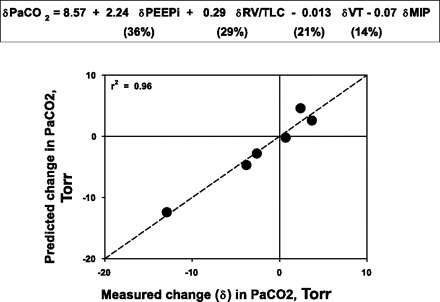
Plot of measured vs. predicted LVRS-induced changes in PaCO2 obtained from a multiple linear regression model using variables dependent on lung hyperinflation [intrinsic positive end-expiratory pressure (PEEPi), ratio of residual volume to total lung capacity (RV/TLC)], tidal volume (Vt), and inspiratory muscle strength (MIP). Essentially all of the post-LVRS change in PaCO2 can be explained by lung mechanical property changes reflecting these key mechanical properties.
DISCUSSION
This study confirms that, in patients with severe emphysema, LVRS results in relatively substantial and uniform improvements in respiratory mechanical properties, but in only modest improvements in gas exchange. A major finding was considerable individual variability in the gas exchange response, reducing the interpretability of group mean data. Another was that changes in PaO2 appear to depend on different sets of determining factors than those affecting PaCO2. What this study adds that is novel is a better understanding of the several possible and simultaneous contributions to altered gas exchange following LVRS.
Pulmonary Function and Gas Exchange Before LVRS
At baseline, there was wide variation in gas exchange status despite the fairly strict inclusion criteria deriving from the NETT study, which were used to select the candidates (2). In agreement with the variability in PaO2, there was also wide variation in the degree of V̇a/Q̇ inequality. The dispersion of blood flow and/or ventilation distributions was abnormal in almost all patients, reflecting a wide range of V̇a/Q̇ inequality with high log SDV̇ and log SDQ̇ in agreement with other MIGET studies in COPD patients (5).
Previous V̇a/Q̇ studies in patients with COPD-emphysema have not dealt with patients so fully characterized by imaging, pathology, and lung function. Recently, our laboratory (28) documented a steady progression of V̇a/Q̇ mismatching from Global Obstructive Lung Disease (GOLD) initiative stages 1–4 (25). Compared with these findings, the patients in the present study showed similar degrees of overall V̇a/Q̇ dispersion to those in GOLD stages 3 and 4, consistent with their clinical severity. Increased overall V̇a/Q̇ dispersion was due mainly to increases in log SDV̇, which was similar to that in GOLD stage 4. In contrast, the distribution of perfusion was dissimilar from that of patients with comparable spirometry with log SDQ̇ values closer to those seen in stages 1 and 2. That said, shunt was twofold higher than in those reported by Rodriguez-Roisin et al. (28). This pattern of modest abnormalities in log SDQ̇ with slightly greater shunt and a wide scatter in log SDV̇ probably reflects the markedly heterogeneous distribution of emphysema in our LVRS candidates, who, by selection, have lung regions that are relatively well preserved, along with others showing severe emphysema. As noted previously (28), the progression of V̇a/Q̇ imbalance with increasing airflow limitation is modest, suggesting that areas with advanced emphysema may contribute little to gas exchange, possibly because they receive not only little blood flow, but also little ventilation. This may be analogous to what occurs in noncommunicating bullous spaces that are apparently neither perfused nor ventilated (19). Nevertheless, even in these severe and highly selected subset of patients, PaO2 was closely dependent on V̇a/Q̇ dispersion, confirming V̇a/Q̇ mismatch as a major cause of arterial hypoxemia in COPD.
Changes in Lung Mechanical Properties After LVRS
LVRS resulted in substantially improved airflow rates and reduction in hyperinflation, consistent with previous findings (7). The similar improvements in FVC, FEV1, RV, and RV/TLC, as well as the strong correlation between Raw and RV/TLC (0.96, P = 0.01) support suggestions that the improvement in airflow rates resulted from a decrease in static hyperinflation and an increase in elastic recoil, resulting in greater radial traction on peripheral airways. Thus LVRS appears to convey benefit to mechanical lung function in several ways.
Changes in V̇a/Q̇ Ratio Distribution After LVRS
In contrast to the large changes in lung mechanical properties, changes in V̇a/Q̇ distribution and shunt were less impressive. The modest average changes are consistent with the correspondingly small improvements in mean PaO2 and lack of significant mean reduction in PaCO2.
There are several possible mechanisms of improvement in the V̇a/Q̇ distribution. First, lung regions compressed by hyperinflated emphysematous areas before LVRS may have been less compressed after removal of emphysematous tissue, therefore improving V̇a/Q̇ matching. Second, improvement in airflow rates associated with increased radial traction could lead to better ventilation after LVRS, especially in those patients previously subject to the greatest dynamic compression. Third, reduced hyperinflation and PEEPi might lead to lower PVR and improved blood flow distribution. A fourth possibility is that, by removing the most abnormal lung (in terms of V̇a/Q̇ inequality), what is left is, by implication, less abnormal. The results of the present study suggest that improvement in V̇a/Q̇ distribution is mainly related to the decrease in hyperinflation, as estimated by improvement in FEV1 and a decrease in RV/TLC and PVR. While not formally assessed, almost all of the patients indicated a reduction of dyspnea independently of the changes in gas exchange parameters. The uniform improvement observed in spirometry and plethysmography would indicate that dyspnea was more affected by the decrease in hyperinflation than by improvement in PaO2 or PaCO2.
Determinants of Post-LVRS PaO2 and PaCO2
The small average increase in PaO2 was similar to that reported in NETT (33). Both PaCO2 and (A-a)Po2 decreased by ∼7% to account for this, and, while neither change reached statistical significance individually, (A-a)Po2 correlated well with the increase in PaO2. Essentially the same observations were made by Albert et al. (1), but others found no significant change in PaO2 and (A-a)Po2 at rest or on exercise (22), although with wide individual scatter. The NETT study showed that patients with upper lobe predominant emphysema and milder disease (as assessed by lower TLC and as well as by higher baseline PaO2 and DlCO levels) had a greater chance of improving their PaO2 after LVRS. However, as the study included only routine lung function testing, no inference could be made regarding the mechanisms underlying the change in PaO2. In a randomized controlled study, Criner et al. (11) could not show an improvement in PaO2, but reported a significant amelioration in V̇o2 at 3 mo post-LVRS, mainly due to an improvement in V̇e.
In the present study, in accordance to the results of the NETT study (33), PaO2 after LVRS was primarily (60%) a function of pre-LVRS values, and was only secondarily (40%) affected by LVRS itself. Considering that the subjects for this study were chosen using the fairly strict NETT criteria, the similarities of our results with those of the NETT study are perhaps unsurprising.
The relatively small (6 Torr) mean increase in PaO2 after LVRS was accounted for by a combination of the factors that together are known to determine PaO2 in nonhomogenous lungs (Fig. 6): the level of ventilation; the amount of intrapulmonary gas exchange inefficiency caused by V̇a/Q̇ mismatching, intrapulmonary shunt, when present, diffusion limitation; and the value of Pv̄O2 (reflecting the balance between Q̇t and metabolic rate). Also, diffusion/perfusion inequality would contribute to diffusion limitation, even if total diffusion were normal. It is important to note that, while likely linked physiologically (see below), these factors were not linked mathematically in the present study, demonstrating essentially no colinearity, and thus making possible the assessment of their independent contributions to changes in PaO2. The quantitative aspect of this partitioning, described as roughly 50% from improved V̇a/Q̇ relationships, and the rest roughly equally split between effects of ventilation and of extrapulmonary factors affecting Pv̄O2 should be interpreted with some caution because of the low number of subjects who provided data, both before and after LVRS for all variables required for the analysis. However, it does seem reasonable to conclude that the changes in PaO2 (both positive and negative) are multifactorial, with identifiable contributions from all three sources.
Fig. 6.
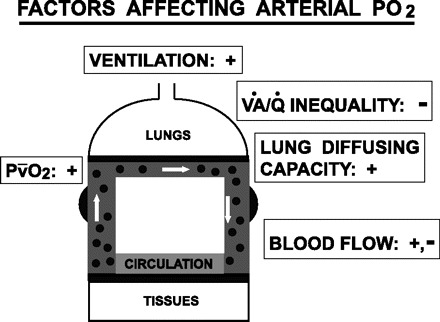
Diagram showing how PaO2 depends on the integrated effects of extrapulmonary factors (ventilation, blood flow, Pv̄O2) and intrapulmonary factors [V̇a/Q̇ inequality (which includes shunting) and diffusion limitation]. For each factor, the sign indicates the direction in which PaO2 will move as the indicated factor increases in value (i.e., the positive or negative effect on PaO2 of an increase in the factor). Actual PaO2 thus represents the integrated effects of the individual factors. An increase in blood flow raises PaO2 by increasing Pv̄O2, but may at the same time act to lower it by 1) shortening red cell transit time in the lung, worsening diffusion limitation, and 2) causing more V̇a/Q̇ inequality.
The multivariate analysis described in Fig. 4 partitions the importance of these factors, but does not address reasons why each contributed in any mechanistic sense. It is likely that improved mechanical function of the respiratory system would have contributed to all three sources of improved PaO2. Thus reduced hyperinflation allowing greater Vt and reduced airways resistance together could enhance ventilation. Reduction in lung hyperinflation and in PEEPi could enhance venous return and Q̇t, while decreasing work of breathing, would tend to reduce V̇o2. While changes in Q̇t and V̇o2 were not evident by group statistics, their combined effects on Pv̄O2 led to its significant contribution to improved PaO2 when multiple linear correlation analysis was performed. V̇a/Q̇ inequality could have improved by several mechanisms. MLR analysis of the relationship between changes in PaO2 and changes in the several indexes of mechanical function (listed in Table 2) revealed no combination of indexes capable of accounting for the effects of LVRS on PaO2.
Thus we suggest that, while individual change in PaO2 can be understood physiologically on the basis of separate contributions from ventilation, V̇a/Q̇ inequality, shunt, and extrapulmonary factors, the mechanistic basis of how these three factors are affected by LVRS remains speculative. While reduced hyperinflation following LVRS does not appear to directly affect PaO2, it has a major impact on the improvement in V̇a/Q̇ distribution, itself one of the main determinants of PaO2. The relatively small number of subjects studied may have been a major limitation to the study. On the other hand, this study clearly demonstrated homogenous baseline functional and morphological characteristics of the patients and a uniform pattern of change in lung mechanics. This uniformity was belied by the peculiar and variable distribution of baseline V̇a/Q̇ distribution, as well as it is inconsistent behavior after LVRS that explains, at least in part, the variability of the changes in PaO2. It is possible that other distributions of emphysema (e.g., homogenous) could have a different pattern of V̇a/Q̇ mismatch and may well have responded differently to LVRS.
As described, there was no effect of LVRS on average group PaCO2, but there was great variability among patients in their response, with some patients reducing PaCO2 substantially, some increasing PaCO2 by a few Torr, and others essentially not changing. In contrast to the findings for PaO2, no combination of the gas exchange variables in Table 2 accounted for changes in PaCO2, but a group of mechanical properties was identified that explained almost all (96%) of the variance in post-LVRS PaCO2 changes. These variables, also shown not to have significant colinearity, included markers of lung hyperinflation (PEEPi, RV-to-TLC ratio), Vt, and chest wall muscle function (MIP). Altogether PEEPi and RV/TLC jointly accounted for about two-thirds of the changes in PaCO2, while Vt and MIP jointly contributed roughly one-third (Fig. 5). Although this relationship must be interpreted with caution because it comes from only six patients, it is so tight (r2 = 0.96) that it would appear to have some validity. It also makes physiological sense in that PaCO2 is widely known to be very sensitive to ventilation, and, in turn, the changes in mechanical properties would all directly affect the ability to breathe. In 33 moderately hypercapnic subjects studied before and 3–6 mo after LVRS, Shade et al. (31) also reported a significant correlation between improvement in FEV1, MIP, and RV-to-TLC ratio, and decrease in PaCO2, albeit with a large intersubject variability. They also observed that those patients with higher baseline values of PaCO2 had the greatest reduction in PaCO2 post-LVRS. Thus the present study suggests that individual changes in PaCO2 can be predicted and understood on the basis of individual changes in these mechanical properties and not to changes in V̇a/Q̇ distribution. We do acknowledge that, while it is tempting to speculate that mitigation of hyperinflation is the major cause of the improved ventilatory ability, linear regression at best finds associations between variables and cannot identify cause-and-effect relationships.
Conclusions
This study has made a number of novel observations. First, patients all having very severe COPD, satisfying the strict selection criteria for LVRS, show a very wide range of physiological abnormality in terms of pulmonary gas exchange. Second, post-LVRS PaO2 was found to reflect mostly its pre-LVRS value, with changes after surgery explained mostly by V̇a/Q̇ distribution improvement, with lesser contributions from changes in ventilation and in Pv̄O2, and no direct contribution from changes in lung mechanical properties. Third, improvement in V̇a/Q̇ distribution is mainly due to a reduction in hyperinflation after LVRS. Fourth, the changes in PaCO2 were closely related to changes in lung mechanical properties and not to the factors that affected PaO2.
While improvement in gas exchange has never been the main outcome in LVRS, long-term oxygen therapy is burdensome to the patient and expensive. Attempting to optimize the effects of LVRS on arterial oxygenation is important and certainly impacts on the acceptance of the procedure by both patients and health service providers. With the recent advent of bronchoscopic lung volume reduction, where mechanical improvement appears to be more modest (12), gas exchange analysis may prove useful in evaluating clinical outcomes.
GRANTS
Grant support was from Fondo de Investigación Sanitaria (PI00/0922 and PI04/1424) and National Institutes of Health (P01 HL091830 and R01 HL084281). T. Melgosa was the recipient of a Research Training Fellowship from Hospital Clínic, Barcelona.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Albert RK, Benditt JO, Hildebrandt J, Wood DE, Hlastala MP. Lung volume reduction surgery has variable effects on blood gases in patients with emphysema. Am J Respir Crit Care Med 158: 71–76, 1998 [DOI] [PubMed] [Google Scholar]
- 2. [Anon]. Rationale and design of the National Emphysema Treatment Trial (NETT). A prospective randomized trial of lung volume reduction surgery. J Thorac Cardiovasc Surg 118: 518–528, 1999 [DOI] [PubMed] [Google Scholar]
- 3. [Anon]. Rationale and design of The National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. The National Emphysema Treatment Trial Research Group. Chest 116: 1750–1761, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Appendini L, Patessio A, Zanaboni S, Carone M, Gukov B, Donner CF, Rossi A. Physiologic effects of positive end-expiratory pressure in patients with chronic obstructive pulmonary disease during acute exacerbations and non-invasive ventilatory assistance. Am J Respir Crit Care Med 149: 1069–1076, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Barbera JA. Chronic obstructive pulmonary disease. In: Pulmonary and Peripheral Gas Exchange in Health and Disease, edited by Roca J, Rodriguez-Roisin R, Wagner PD. New York: Dekker, 2000, p. 229–261 [Google Scholar]
- 6. Barbera JA, Ramirez J, Roca J, Wagner PD, Sanchez-Lloret J, Rodriguez-Roisin R. Lung structure and gas exchange in mild chronic obstructive pulmonary disease. Am Rev Respir Dis 141: 895–901, 1990 [DOI] [PubMed] [Google Scholar]
- 7. Berger RL, Wood KA, Cabral HJ, Goodnight-White S, Ingenito EP, Gray A, Miller J, Springmeyer SC. Lung volume reduction surgery: a meta-analysis of randomized clinical trials. Treat Respir Med 4: 201–209, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Cardus J, Burgos F, Diaz O, Roca J, Barbera JA, Marrades RM, Rodriguez-Roisin R, Wagner PD. Increase in pulmonary ventilation-perfusion inequality with age in healthy individuals. Am J Respir Crit Care Med 156: 648–653, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Cotes JE, Chinn DJ, Quanjer PH, Roca J, Yernault JC. Standardization of the measurement of transfer factor (diffusing capacity). Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 16: 41–52, 1993 [PubMed] [Google Scholar]
- 10. Criner G, Cordova FC, Leyenson V, Roy B, Travaline J, Sudarshan S, O'Brien G, Kuzma AM, Furukawa S. Effect of lung volume reduction surgery on diaphragm strength. Am J Respir Crit Care Med 157: 1578–1585, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Criner GJ, Cordova FC, Furukawa S, Kuzma AM, Travaline JM, Leyenson V, O'Brien GM. Prospective randomized trial comparing bilateral lung volume reduction surgery to pulmonary rehabilitation in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 160: 2018–2027, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Criner GJ, Pinto-Plata V, Strange C, Dransfield M, Gottfried M, Leeds W, McLennan G, Refaely Y, Tewari S, Krasna MJ, Celli BR. Biologic lung volume reduction in advanced upper lobe emphysema: phase 2 results. Am J Respir Crit Care Med 179: 791–798, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Criner GJ, Scharf SM, Falk JA, Gaughan JP, Sternberg AL, Patel NB, Fessler HE, Minai OA, Fishman AP. Effect of lung volume reduction surgery on resting pulmonary hemodynamics in severe emphysema. Am J Respir Crit Care Med 176: 253–260, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dantzker DR, D'Alonzo GE. The effect of exercise on pulmonary gas exchange in patients with severe chronic obstructive pulmonary disease. Am Rev Respir Dis 134: 1135–1139, 1986 [DOI] [PubMed] [Google Scholar]
- 15. Fishman A. Disorders of the pulmonary circulation. In: Pulmonary Diseases and Disorders, edited by Fishman A. New York: McGraw Hill, 2008, p. 1331–1358 [Google Scholar]
- 16. Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood DE. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 348: 2059–2073, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Geddes D, Davies M, Koyama H, Hansell D, Pastorino U, Pepper J, Agent P, Cullinan P, MacNeill SJ, Goldstraw P. Effect of lung-volume-reduction surgery in patients with severe emphysema. N Engl J Med 343: 239–245, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Lim E, Ali A, Cartwright N, Sousa I, Chetwynd A, Polkey M, Geddes D, Pepper J, Diggle P, Goldstraw P. Effect and duration of lung volume reduction surgery: mid-term results of the Brompton trial. Thorac Cardiovasc Surg 54: 188–192, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Macklem PT, Eidelman D. Reexamination of the elastic properties of emphysematous lungs. Respiration 57: 187–192, 1990 [DOI] [PubMed] [Google Scholar]
- 20. Martinez FJ, de Oca MM, Whyte RI, Stetz J, Gay SE, Celli BR. Lung-volume reduction improves dyspnea, dynamic hyperinflation, and respiratory muscle function. Am J Respir Crit Care Med 155: 1984–1990, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Miller JD, Malthaner RA, Goldsmith CH, Goeree R, Higgins D, Cox PG, Tan L, Road JD. A randomized clinical trial of lung volume reduction surgery versus best medical care for patients with advanced emphysema: a two-year study from Canada. Ann Thorac Surg 81: 314–320, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Oswald-Mammosser M, Kessler R, Massard G, Wihlm JM, Weitzenblum E, Lonsdorfer J. Effect of lung volume reduction surgery on gas exchange and pulmonary hemodynamics at rest and during exercise. Am J Respir Crit Care Med 158: 1020–1025, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Purro A, Appendini L, Polillo C, Musso G, Taliano C, Mecca F, Colombo R, Carbone G. Mechanical determinants of early acute ventilatory failure in COPD patients: a physiologic study. Intensive Care Med 35: 639–647, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 16: 5–40, 1993 [PubMed] [Google Scholar]
- 25. Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 176: 532–555, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Roca J, Burgos F, Sunyer J, Saez M, Chinn S, Anto JM, Rodriguez-Roisin R, Quanjer PH, Nowak D, Burney P. References values for forced spirometry. Group of the European Community Respiratory Health Survey. Eur Respir J 11: 1354–1362, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Roca J, Wagner PD. Contribution of multiple inert gas elimination technique to pulmonary medicine. 1. Principles and information content of the multiple inert gas elimination technique. Thorax 49: 815–824, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodriguez-Roisin R, Drakulovic M, Rodriguez DA, Roca J, Barbera JA, Wagner PD. Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J Appl Physiol 106: 1902–1908, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Rossi A, Polese G, Brandi G, Conti G. Intrinsic positive end-expiratory pressure (PEEPi). Intensive Care Med 21: 522–536, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Sciurba FC. Early and long-term functional outcomes following lung volume reduction surgery. Clin Chest Med 18: 259–276, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Shade D, Jr, Cordova F, Lando Y, Travaline JM, Furukawa S, Kuzma AM, Criner GJ. Relationship between resting hypercapnia and physiologic parameters before and after lung volume reduction surgery in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 159: 1405–1411, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Suga K, Tsukuda T, Awaya H, Matsunaga N, Sugi K, Esato K. Interactions of regional respiratory mechanics and pulmonary ventilatory impairment in pulmonary emphysema: assessment with dynamic MRI and xenon-133 single-photon emission CT. Chest 117: 1646–1655, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Synder M, Goss CH, Neradilek B, Polissar NL, Mosenifar Z, Wise R, Fishman AP, Benditt JO. Changes in arterial oxygenation and self-reported oxygen use after lung volume reduction surgery. Am J Respir Crit Care Med 178: 339–345, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wagner PD. Functional consequences of lung volume reduction surgery for COPD. Am J Respir Crit Care Med 158: 1017–1019, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Wagner PD, Dantzker DR, Dueck R, Clausen JL, West JB. Ventilation-perfusion inequality in chronic obstructive pulmonary disease. J Clin Invest 59: 203–216, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weinmann GG, Hyatt R. Evaluation and research in lung volume reduction surgery. Am J Respir Crit Care Med 154: 1913–1918, 1996 [DOI] [PubMed] [Google Scholar]


