Abstract
Sedentary aging leads to increased cardiovascular stiffening, which can be ameliorated by sufficient amounts of lifelong exercise training. An even more extreme form of cardiovascular stiffening can be seen in heart failure with preserved ejection fraction (HFpEF), which comprises ∼40∼50% of elderly patients diagnosed with congestive heart failure. There are two major interrelated hypotheses proposed to explain heart failure in these patients: 1) increased left ventricular (LV) diastolic stiffness and 2) increased arterial stiffening. The beat-to-beat dynamic Starling mechanism, which is impaired with healthy human aging, reflects the interaction between ventricular and arterial stiffness and thus may provide a link between these two mechanisms underlying HFpEF. Spectral transfer function analysis was applied between beat-to-beat changes in LV end-diastolic pressure (LVEDP; estimated from pulmonary artery diastolic pressure with a right heart catheter) and stroke volume (SV) index. The dynamic Starling mechanism (transfer function gain between LVEDP and the SV index) was impaired in HFpEF patients (n = 10) compared with healthy age-matched controls (n = 12) (HFpEF: 0.23 ± 0.10 ml·m−2·mmHg−1 and control: 0.37 ± 0.11 ml·m−2·mmHg−1, means ± SD, P = 0.008). There was also a markedly increased (3-fold) fluctuation of LV filling pressures (power spectral density of LVEDP) in HFpEF patients, which may predispose to pulmonary edema due to intermittent exposure to higher pulmonary capillary pressure (HFpEF: 12.2 ± 10.4 mmHg2 and control: 3.8 ± 2.9 mmHg2, P = 0.014). An impaired dynamic Starling mechanism, even more extreme than that observed with healthy aging, is associated with marked breath-by-breath LVEDP variability and may reflect advanced ventricular and arterial stiffness in HFpEF, possibly contributing to reduced forward output and pulmonary congestion.
Keywords: cardiovascular variability
healthy, sedentary aging leads to increased cardiovascular stiffening, which can be ameliorated by sufficient amounts of lifelong exercise training (1, 35). An even more extreme form of cardiovascular stiffening associated with aging can be seen in the syndrome of heart failure with preserved ejection fraction (HFpEF) (28). These patients have elevated filling pressures, impaired exercise tolerance, and comprise ∼40∼50% of all elderly patients diagnosed with congestive heart failure (4, 26, 27, 41). Although studies addressing the pathophysiology of HFpEF are increasing in number with the recognition of the syndrome, its underlying mechanisms are still controversial (2, 19, 39, 43, 44). To date, there are two major interrelated hypotheses to explain the pathophysiology of HFpEF: increased left ventricular (LV) diastolic stiffness (17, 42) and increased arterial stiffening (13, 16).
Despite the fact that epidemiological studies (26, 27, 37) have unequivocally demonstrated that this syndrome is uniquely age related, the specific effects of aging on the pathogenesis of HFpEF are not well understood. We speculated that the pathophysiological hallmark of HFpEF might be an extreme form of the age-related deterioration in ventricular-arterial coupling and that comparing HFpEF patients with healthy seniors and master athletes could describe a continuum of age- and activity-related cardiovascular stiffening in humans.
We (31) proposed a novel approach to quantify the beat-to-beat dynamic Starling mechanism, that is, the beat-to-beat dynamic modulation of stroke volume (SV) due to breath-to-breath changes in LV filling pressures. Conceptually, the beat-to-beat dynamic Starling mechanism reflects both LV end-diastolic and end-systolic pressure-volume relationships (EDPVR and ESPVR) and, thus, the interaction between ventricular and arterial stiffness. The dynamic Starling mechanism is impaired with healthy but sedentary human aging, whereas this impairment and, by extension, ventricular-arterial stiffening was minimized in the highly trained elderly (31). However, it is unknown whether the dynamic Starling mechanism is more impaired in patients with HFpEF compared with sedentary age-matched controls.
The primary aim of the present study was to assess the beat-to-beat dynamic Starling mechanism in patients with HFpEF. We hypothesized that the dynamic Starling mechanism would be more impaired in HFpEF patients than expected from sedentary but otherwise healthy aging subjects.
METHODS
HFpEF Patients
Subjects were defined as having HFpEF by the following criteria: 1) an index hospitalization for documented congestive heart failure, 2) meeting Framingham criteria for congestive heart failure during the event (21), and 3) ejection fraction measured by echocardiography of >50% at the time of index hospitalization. These criteria were confirmed by chart review, patient history, and a review of medical records with the individual treating physicians. Subjects were enrolled into the study after they were clinically stable and discharged; therefore, all subjects were studied when stable and well compensated.
Exclusion criteria for the study included the following: 1) significant obstructive coronary artery disease as determined by provocable ischemia during exercise electrocardiogram and echocardiogram, 2) significant valvular heart disease by echocardiogram, 3) renal dysfunction (serum creatinine > 2.5 mg/dl), 4) previous coronary artery bypass surgery, 5) arrhythmias such as atrial fibrillation/flutter and left bundle branch block, 6) lung disease (pulmonary hypertension or chronic obstructive pulmonary disease), 7) untreated thyroid disorders, and 8) warfarin use.
The most recent epidemiological studies (4, 26, 27, 37, 41) have shown that the prototypical patient with HFpEF is elderly, female, obese, hypertensive, and often diabetic. Since the primary purpose of the present study was to address this population, we did not exclude patients with diabetes, hypertension, or body mass index (BMI) >30 for the recruitment of HFpEF patients. We recruited 4 male patients and 6 female patients with HFpEF, and all of them were older than 65 yr. The detailed patient characteristics, recruitment process, and demographics along with static Starling and pressure-volume curves for these patients have been reported previously (28). The physiological variables from the patients with HFpEF were compared with those of age- and gender-matched controls and young healthy individuals as a reference (31).
Previous work from our group focused on the effects of aging and physical activity on cardiovascular function using both cross-sectional and longitudinal approaches (25). Briefly, our laboratory conducted 1 yr of endurance exercise training in sedentary but otherwise healthy elderly subjects (>65 yr old), in which subjects gradually increased their exercise level and finally achieved weekly exercise training up to 4–6 h at the end of the year, including long distance and maximal steady-state and interval training. The results of the beat-to-beat dynamic Starling mechanism from this previous study (31) were published and are reproduced, in part, in Fig. 3. Of note, the exclusion criteria for this study were the same as in the present study except for the fact that we also excluded 1) obesity (BMI > 30), 2) diabetes, and 3) hypertension in these healthy volunteers. Moreover, we applied the same protocol to quantify the physiological variables, so that they could serve as appropriate controls without exposing additional volunteers to the risk of right heart catheterization.
Fig. 3.
Means ± SE of transfer function gain between PAD and SVi (gain PAD-SVi) at 0.18–0.22 Hz for CHF-pEF patients, sedentary elderly (elderly unfit) subjects, sedentary elderly subjects after exercise training (elderly post-Ex), master athletes (elderly fit), and sedentary young individuals (young unfit) from the present study and a previous study (31).
The experimental procedures were explained to all subjects with informed consent obtained as approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center (Dallas, TX) and Presbyterian Hospital (Dallas, TX).
Peak O2 Uptake
A modified Astrand-Saltin incremental treadmill protocol was used to determine the peak exercise capacity as previously described (3, 25). β-Blockers were stopped at least 48 h before testing to avoid chronotropic effects of β-blockade limiting exercise performance. Other medications, including diuretics, angiotensin-converting enzyme (ACE) inhibitor, angiotensin receptor blocker (ARB), and insulin, were not stopped to avoid severe hypertension and/or hyperglycemia during exercise.
Dynamic and Static Hemodynamics
Cardiac preload.
A 6-Fr balloon-tipped, fluid-filled catheter (Swan-Ganz, Baxter) was placed through an antecubital vein into the pulmonary artery under fluoroscopic guidance. Pulmonary artery pressure (PAP) and right atrial pressure (RAP) were referenced to atmospheric pressure with the pressure transducer (Transpac IV, Abbott) zero reading set at 5 cm below the sternal angle. The static mean pulmonary capillary wedge pressure (PCWP) and RAP were determined visually at end expiration.
Beat-to-beat pulmonary artery diastolic pressure (PAD) was used as an index of beat-to-beat LV end-diastolic pressure (LVEDP) to avoid the risks of prolonged balloon inflation (12). Mean RAP (mRAP) of one cardiac cycle was used as an index of pericardial pressure. LV preload pressure, responsible for the Starling mechanism, was estimated by two ways to consider the effects of ventricular interdependence and intrathoracic pressure on the dynamic Starling mechanism: 1) LVEDP estimated by PAD and 2) LV transmural pressure estimated by PAD minus mRAP (34).
To confirm that changes in PAD would track changes in PCWP in this patient population, we compared the relationship between PAD and PCWP at end expiration in sedentary elderly individuals and patients with HFpEF across multiple preload conditions using lower body negative pressure (−15 and −30 mmHg) and normal saline infusion (10–15 and 20–30 ml/kg), during which PAD and PCWP ranged from ∼2.0 to 24 mmHg (28). There was a strong linear relationship between PCWP versus PAD with slopes approximating unity in both sedentary elderly individuals (R2: 0.93 ± 0.19, slope: 0.92 ± 0.13) and patients with HFpEF (R2: 0.99 ± 0.01 and slope: 0.97 ± 0.10).
SV.
Cardiac output was measured with the C2H2 rebreathing method (15), and SV was calculated from the cardiac output and corresponding heart rate. Cuff blood pressure was measured at the brachial artery (Suntech, SunTech Medical) during cardiac output measurements.
Photoplethysmography (Portapres, FMS, Amsterdam, The Netherlands) was used to measure finger arterial blood pressure continuously. Beat-to-beat changes in SV were calculated from the finger arterial pressure waveform reconstructed to a central arterial waveform using pulse contour analysis with the Modelflow method (BeatScope, FMS) (36). The Modelflow method has been well validated to estimate SV changes even in patients with coronary heart disease when the baseline SV was calibrated (5, 14, 40). Patients with coronary heart disease are likely to show similar comorbidities as patients with HFpEF, such as hypertension and diabetes, supporting the validity of the Modelflow method for HFpEF. Each Modelflow SV computed from the finger arterial pressure waveform was calibrated by a constant, the ratio of mean Modelflow SV to baseline SV with the C2H2 rebreathing method (5, 36, 40). SV index (SV divided by body surface area; Mosteller's equation) (38) was used to minimize the effects of differences in body size between individuals.
Experimental protocol.
All experiments were performed in the morning at least 2 h after a light breakfast in a quiet environmentally controlled laboratory with an ambient temperature of 25°C. Subjects were asked to refrain from heavy exercise and caffeinated or alcoholic beverages for at least 24 h before the tests. Patients with HFpEF recruited for this study were taking cardiovascular medications such as diuretics, β-blockers, Ca2+ channel blockers, ACE inhibitors, ARBs, and 3-hydroxy-3-methyl-glutaryl (HMG)-CoA reductase inhibitors, whereas none of control subjects were. β-Blockers were withdrawn 48 h before the study to avoid their direct effects on diastolic function. In addition, diuretics were withheld the morning of the study, although they were given as soon as the study was over. Since virtually all of these patients had hypertension, we chose to keep them on drugs such as ARBs, ACE inhibitors, and/or Ca2+ channel blockers for two reasons: 1) patient safety and 2) we felt that the direct effects of the drugs on ventricular-arterial coupling would be less than the effects of acute hypertension in the laboratory. Hemodynamic measurements were made as previously described (31, 32). Briefly, after confirmation of hemodynamic stability and steady-state hemodynamic measurements, subjects were asked to breathe at a controlled frequency (0.2 Hz, 12 breaths/min) for 8 min to collect beat-to-beat SV index, mRAP, and PAD. The last 6 min of data were used for the transfer functional and power spectral analysis.
Data analysis for dynamic hemodynamics.
Spectral transfer function analysis between PAD versus SV index and transmural pressure versus SV index were applied to obtain gain, phase, and coherence as previously described (31, 32). Mean values of gain, phase, and coherence were calculated in the respiratory range (0.18–0.22 Hz) and averaged for all subjects in each group. The phase ranges from −π to +π and yields a negative value when changes in the input precede changes in the output at each frequency. Phase zero indicates complete synchrony between the input and output. The gain, similar conceptually to the slope of a linear regression, reflects changes in the output against a unit change of the input at each frequency. Thus, the gain between PAD and SV index was used as an index of the dynamic Starling mechanism that reflects a function of beat-to-beat modulations of SV against changes in LV preload pressures. The reliability of the linear transfer function estimation was evaluated by estimates of coherence function, which ranges between 0 and 1. A value of unity indicates a perfectly linear relationship between the input and output at each frequency,conceptually similar to the r2 value of a linear regression. The spectral power of PAD, transmural pressure (PAD − RAP), and SV index was also calculated in the respiratory frequency range (0.18–0.22 Hz) by integrating the corresponding autospectra (31, 32).
Statistic Analysis
Numerical data are presented as means ± SD except for graphics, in which means ± SE is used. A Student's t-test was performed between HFpEF and age-matched controls.
RESULTS
Subject Characteristics
All patients had hypertension and were being treated with antihypertensive medications; seven patients had diabetes, six patients were being treated with medication and one patients with diet therapy; five patients had a BMI > 30. Patients with HFpEF had lower maximal O2 consumption and higher body weight and BMI compared with age-matched controls (Table 1).
Table 1.
Subject characteristics
| HFpEF Patients | Controls | P Value | |
|---|---|---|---|
| Men/Women | 4/6 | 6/6 | |
| Age, yr | 73 ± 7 | 70 ± 3 | 0.177 |
| Height, cm | 163 ± 10 | 168 ± 10 | 0.236 |
| Weight, kg | 89 ± 22* | 73 ± 11 | 0.042 |
| Body mass index, kg/m2 | 33.3 ± 7.0* | 25.8 ± 2.4 | 0.002 |
| Body surface area, m2 | 2.00 ± 0.29 | 1.85 ± 0.18 | 0.153 |
| Maximal O2 consumption, ml · min−1 · kg−1 | 13.7 ± 3.5* | 21.9 ± 3.6 | <0.001 |
Values are means ± SD. HF-pEF patients, patients with congestive heart failure with preserved ejection fraction; controls, age-matched control subjects.
P < 0.05 vs. controls.
Steady-State Hemodynamics
Patients with HFpEF had higher pulse pressure and lower diastolic blood pressure than age-matched controls. Patients also had higher PCWP, PAD, and transmural pressure (PCWP − RAP) than age-matched controls. SV as well as SV index were comparable between HFpEF and age-matched controls. Time series SDs of beat-to-beat PAD as well as beat-to-beat transmural pressure (PAD − mRAP) were higher in patients with HFpEF than age-matched controls (Table 2).
Table 2.
Hemodynamic variables
| HFpEF Patients | Controls | P Value | |
|---|---|---|---|
| HR, beats/min | 74 ± 22 | 67 ± 9 | 0.356 |
| SBP, mmHg | 145 ± 12 | 138 ± 15 | 0.227 |
| DBP, mmHg | 70 ± 11* | 79 ± 8 | 0.039 |
| MBP, mmHg | 95 ± 10 | 98 ± 10 | 0.426 |
| PP, mmHg | 75 ± 10* | 59 ± 9 | <0.001 |
| PCWP, mmHg | 16 ± 5* | 12 ± 2 | 0.012 |
| PCWP − RAP, mmHg | 6.0 ± 2.9* | 3.4 ± 1.5 | 0.011 |
| PAD mean, mmHg | 13.7 ± 4.0* | 7.6 ± 2.0 | <0.001 |
| PAD SD, mmHg | 4.1 ± 1.3* | 2.2 ± 0.7 | <0.001 |
| Trans-P mean, mmHg | 6.0 ± 1.7* | 2.6 ± 1.7 | <0.001 |
| Trans-P SD, mmHg | 2.8 ± 0.7* | 1.2 ± 0.6 | <0.001 |
| SV mean, ml | 87 ± 22 | 73 ± 19 | 0.129 |
| SV SD, ml | 4 ± 2 | 3 ± 1 | 0.108 |
| SVi mean, ml/m2 | 45 ± 15 | 40 ± 8 | 0.310 |
| SVi SD, ml/m2 | 2 ± 1 | 2 ± 1 | 0.300 |
| PSD SVi | 0.88 ± 0.91 | 0.66 ± 0.53 | 0.499 |
| Coherence PAD-SVi | 0.78 ± 0.18 | 0.74 ± 0.09 | 0.492 |
| Phase PAD-SVi | 0.15 ± 0.93 | −0.15 ± 0.77 | 0.409 |
| Coherence Trans-P-SVi | 0.71 ± 0.21 | 0.66 ± 0.11 | 0.529 |
| Phase Trans-P-SVi | −0.16 ± 1.1 | −0.43 ± 0.48 | 0.476 |
Values are means ± SD. HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; PP, pulse pressure; PCWP, pulmonary capillary wedge pressure measured at end expiration; RAP, right atrial pressure measured at end expiration; PAD mean, mean of beat-to-beat PAD during fixed breathing at 0.2 Hz; PAD SD, time series SD of PAD; Trans-P mean, mean of beat-to-beat PAD-RAP; Trans-P SD, time series SD of Trans-P; SV mean, mean of beat-to-beat stroke volume; SV SD, time series SD of SV; SVi mean, mean of beat-to-beat SV index, SVi SD, time series SD of SVi; PSD SVi, power spectral density of SVi at the respiratory frequency; coherence PAD-SVi, coherence function between PAD and SVi at respiratory frequency; phase PAD-SVi, transfer function phase between PAD and SVi at respiratory frequency; coherence Trans-P-SVi, coherence function between transmural pressure (Trans-P) and SVi at respiratory frequency; phase Trans-P-SVi, transfer function phase between Trans-P and SVi at respiratory frequency.
P < 0.05 vs. controls.
Dynamic Hemodynamics
The input variable of the dynamic Starling mechanism (spectral power of PAD and transmural pressure variabilities at the respiratory frequency) was substantially higher in patients with HFpEF than age-matched controls (Fig. 1). The output variable of the dynamic Starling mechanism (namely, spectral power of SV index variability at the respiratory frequency) was comparable between patients and controls (Table 2). The dynamic Starling mechanism (transfer function gain between PAD vs. SV index) was lower in patients than controls (Fig. 2A). Even when controlling for respiratory-induced changes in intrathoracic pressure using transmural pressure as the input variable to the transfer function, the gain remained much lower in HFpEF patients (Fig. 2B).
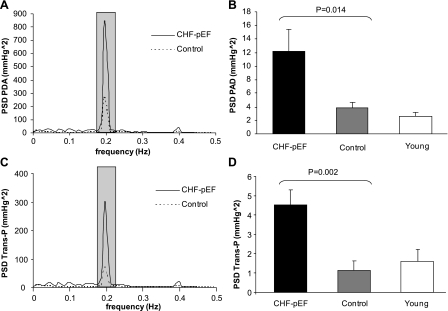
Fig. 1.
A: mean autospectra of pulmonary artery diastolic pressure (PAD) for congestive heart failure (CHF) with preserved ejection fraction (CHF-pEF) and controls. The shaded bar shows enhanced 0.18–0.22 Hz. B: means ± SE of power spectral density of PAD (PSD PAD) at 0.18–0.22 Hz. The PSD PAD of young individuals is shown as a reference. C: mean autospectra of transmural pressure (Trans-P) for CHF-pEF patients and controls. D: means ± SE of PSD Trans-P at 0.18–0.22 Hz. The PSD trans-P of young individuals is shown as a reference.
Fig. 2.
A,a: mean coherence function between PAD and stroke volume (SV) index (SVi) for CHF-pEF patients and controls. The shaded bar shows enhanced 0.18–0.22 Hz. A,b: mean transfer function gain (gain PAD-SVi) at 0.18–0.22 Hz for CHF-pEF patients and controls. A,c: means ± SE of gain PAD-SVi at 0.18–0.22 Hz for CHF-pEF patients and controls. The gain PAD-SVi of young individuals is shown as a reference. B,a: mean coherence function between Trans-P and SVi for CHF-pEF patients and controls. The shaded bar shows enhanced 0.18–0.22 Hz. B,b: mean transfer function gain (gain Trans-P-SVi) at 0.18–0.22 Hz for CHF-pEF patients and controls. B,c: means ± SE of gain Trans-P-SVi at 0.18–0.22 Hz for CHF-pEF patients and controls. The gain Trans-P-SVi of young individuals is shown as a reference.
Although the numbers of men and women were small, we observed no clear differences in any key parameter between male and female HFpEF patients [gain: PAD − SV index, 0.19 ± 0.13 ml·m−2·mmHg−1 in men and 0.26 ± 0.10 ml·m−2·mmHg−1 in women, P = 0.402; HFpEF PAD, 12.4 ± 12.4 mmHg2 in men and 12.1 ± 10.1 mmHg2 in women, P = 0.971; HFpEF SV index, 1.12 ± 1.48 (ml/m2)2 in men and 0.72 ± 0.32 (ml/m2)2 in women, P = 0.522.
DISCUSSION
The dynamic Starling mechanism is impaired with healthy sedentary aging (31). The key new finding from the present study was that the dynamic Starling mechanism was even more impaired in patients with HFpEF than sedentary but healthy age-matched controls. This finding suggests that an impaired dynamic Starling mechanism reflects the essential pathophysiology of HFpEF, i.e., enhanced LV and arterial stiffness, and provides a rationale as to how sedentary cardiovascular aging may contribute to the pathogenesis of HFpEF.
Dynamic Starling Mechanism in HFpEF
There are two major interrelated hypotheses that have been advanced to explain HFpEF (6, 8, 16, 42). Both of these suggest static functional impairments in either LV diastolic compliance or end-systolic elastance (EDPVR and ESPVR in Fig. 4) (20). Zile et al. (42–44) argued that patients with HFpEF have abnormal LV relaxation and increased LV passive stiffness. Therefore, this syndrome conventionally has been called “diastolic heart failure.” Conversely, Kawaguchi et al. (16) showed that increased LV end-systolic elastance leads to the appearance of diastolic dysfunction, that is, a steeper ESPVR results in a steeper EDPVR in patients with HFpEF. Conversely, a steeper EDPVR results in a steeper ESPVR (2, 45). Since EDPVR and ESPVR interact via a beat-to-beat LV pressure-volume relationship, it is difficult to distinguish the primary changes of either EDPVR or ESPVR from the secondary changes that occur from their interdependence via LV pressure-volume loops (16, 20, 45). Intriguingly, it is recognized by both major hypotheses that both EDPVR and ESPVR become steeper in patients with HFpEF regardless of the specific mechanisms. Since the beat-to-beat dynamic Starling mechanism reflects time-varying ventricular-arterial stiffness, this novel index may quantify the integrated feature of HFpEF by unifying the previous two interrelated hypotheses (Fig. 4).
Fig. 4.
Effects of aging, exercise training, and CHF-pEF on the dynamic Starling mechanism. Each loop represents the left ventricular (LV) pressure-volume relationship during one cardiac cycle. The end-systolic pressure-volume relationship (ESPVR; LV end-systolic pressure-LV end-systolic volume relationship) reflects LV end-systolic stiffness and thus arterial stiffness, whereas the end-diastolic pressure-volume relationship (EDPVR; LV end-diastolic pressure-LV end-diastolic volume relationship) reflects LV end-diastolic stiffness or compliance.
Our previous report (28) using many of these same patients showed that the static Starling mechanism was not different between patients with HFpEF and healthy age-matched controls. Although the mechanisms underlying this discrepancy between static versus dynamic LV function are not clear in the present study, this is not so surprising since the dynamic nature of LV as well as vascular compliance/stiffness are different from static behavior. This fact emphasizes the importance of the evaluation of the dynamic nature of the Starling mechanism in HFpEF.
Increased central arterial stiffness and LV load have been observed in patients with congestive heart failure (10, 23, 24) and those with HFpEF (11), as evidenced by augmented reconstructed central pulse pressure, aortic characteristic impedance, and central pulse wave velocity consistent with the steeper ESPVR in patients with HFpEF (6, 16). The calculation of SV using the ModelFlow method in this study estimates the central aortic pressure waveform similar in many respects to this previous work. Moreover, the extent of beat-to-beat changes in SV in response to changes in LV preload and contractile work is primarily determined by the relationship between aortic flow and pressure. Thus, the impaired dynamic Starling mechanism conceptually reflects increased pulsatile load as characterized by aortic impedance. Therefore, our work is a natural extension of previous work by further addressing the integrated dynamic feature of ventricular-arterial coupling accompanied with an invasive measurement of changes in LV preload.
Prominent Fluctuation of LV Filling Pressure in HFpEF
The input variable of the dynamic Starling mechanism, that is, spectral power of LVEDP at the respiratory frequency, was prominently higher in patients with HFpEF than controls (Fig. 1). The Starling mechanism functions to modulate cardiac output depending on LV preload. One outcome of the failure of the Starling mechanism is higher LVEDP and PCWP and pulmonary edema, which is one of the common features for patients with congestive heart failure, as observed in patients with HFpEF in the present study. As a consequence, failure of the dynamic Starling mechanism may result in a marked increase in LVEDP variability at the respiratory frequency since the beat-to-beat dynamic Starling mechanism modulates beat-to-beat fluctuations of LV preload. Importantly, the higher LVEDP variability in HFpEF implies that patients with HFpEF are intermittently exposed to higher cardiac filling pressure during the respiratory cycle as blood flows into a noncompliant LV. Moreover, when given a transient volume challenge or increased cardiac output, such as during exercise, these patients experience a larger rise in filling pressures (7). We speculate that not only higher mean PCWP but also these dynamic mechanisms may predispose patients with HFpEF to pulmonary edema, although, of course, both the dynamic and static mechanisms are interrelated with each other.
Effects of Aging and Physical Activity on HFpEF
Intriguingly, the difference of the dynamic Starling mechanism between young and healthy but sedentary elderly subjects (∼75%) was much larger than that between HFpEF patients and their age-matched controls (∼38%; Fig. 2A). These results emphasize the important impact of aging on HFpEF, consistent with epidemiological findings that patients with HFpEF are more common in the elderly population than younger individuals (26, 27, 37). Moreover, age-related cardiovascular stiffening may be one of the key mechanisms for the occurrence of HFpEF. Ventricular-arterial stiffening with human aging is accelerated by obesity, diabetes, hypertension, and changes in sex hormones by promoting the accumulation of cross-linked collagen in cardiovascular tissues (18, 22, 29, 33). Therefore, these findings provide one explanation as to why the prototypical patient with HFpEF is elderly, female, obese, hypertensive, and more often diabetic (4, 26, 27, 37, 41).
Masters athletes functioned with a dynamic Starling mechanism much greater than that of the sedentary elderly population. Even though the dynamic Starling mechanism increased after 1 yr of exercise training in previously sedentary elderly subjects, this value remained substantially lower than that of Masters athletes (Fig. 3) (31). These findings suggest that lifelong exercise training prevents the impairment of the dynamic Starling mechanism with aging, whereas the effect is somewhat limited when training is started later in life (31). Together, these observations suggest a continuum of cardiovascular stiffening ranging from the highly trained elderly subject to patients with HFpEF (31). One implication of this continuum is that daily exercise training begun in youth or middle age is a possible strategy to fundamentally ameliorate the substrate for HFpEF, that is, ventricular-arterial stiffening with aging, although this hypothesis would have to be tested prospectively.
The classic Starling mechanism is, of course, widely appreciated, and its failure contributes substantially to all forms of heart failure (particularly systolic heart failure). However, the dynamic nature of the Starling mechanism as it adjusts output of the heart to the ebb and flow of cardiac filling on a breath-by-breath basis is less well appreciated and rarely considered clinically. Since patients are continuously exposed to these dynamic changes during daily life, the recognition that filling pressure fluctuates widely in HFpEF patients even during quiet respiration is novel and clinically relevant. That this dynamic function is so severely impaired in patients with HFpEF provides new insights into the underlying mechanism for HFpEF, and the presentation in the context of a broad range of healthy individuals of varying ages and fitness levels further emphasizes the importance of aging and physical activity on ventricular-arterial coupling.
Technical Considerations and Limitations
The coherence function was higher than 0.9 at respiratory frequencies and transfer function phase was negative and close to zero in patients with HFpEF as well as their age-matched controls. These results imply that 1) >90% of changes in SV are correlated with fluctuation of PAD and 2) higher (or lower) LV preload pressure precedes higher (or lower) SV with zero or one beat delays. Therefore, the majority of SV variability due to respiration is most likely caused by the dynamically operating Starling mechanism and fluctuation of LV preload. However, ventricular interdependence and/or changes in intrapleural pressure due to respiration may confound the relationship between LV preload and SV. To account for this possibility, we also estimated the transmural pressure as an input variable for the dynamic Starling mechanism (34). The dynamic Starling mechanism estimated by the transmural pressure was also impaired with aging and even more impaired in patients with HFpEF than in their peers, similar to that estimated by the LV preload pressure referenced to the atmosphere. Thus, the effects of ventricular interdependence and intrathoracic pressure due to respiration on the beat-to-beat dynamic Starling mechanism are, if any, relatively small, although we cannot exclude the possibility that the impaired dynamic Starling mechanism is affected by changes in ventricular interdependence in patients with HFpEF.
The absolute value of PAD may not be a robust surrogate for left atrial pressure in patients with HFpEF. However, it is important to emphasize that the present study focused on the changes in PAD as a measure of changes in PCWP rather than the absolute values. Most importantly, to address this concern, we compared the relationship between PAD and PCWP directly and found a strong linear relationship with a slope approaching unity. These data strongly support our contention that there is a close 1:1 relationship between changes in PAD and changes in PCWP over a broad physiological range of preload and defend our strategy in this experiment in patients with HFpEF.
We applied an indirect approach for the measurement of SV. However, this approach has been validated and widely accepted in either clinical settings or physiological studies (5, 36, 40). The validity of SV changes with the Modelflow method was first demonstrated in patients with coronary disease, who often have diabetes and/or hypertension, supporting the validity of the Modelflow method for our patient population (40). Direct measurements of beat-to-beat SV and LVEDP, such as left heart catheterization and direct aortic flow measurement, may be needed to confirm these findings, although they are too invasive for outpatients with HFpEF and healthy individuals. Our approaches without left heart catheterization enabled us to compare the prototypical HFpEF patients with healthy age-matched controls using the same methodologies without any potential complication or referral bias.
Subject Recruitment
We excluded patients with atrial fibrillation and/or coronary artery disease, although epidemiological studies (27, 37) have shown that ∼50% of HFpEF have coronary artery disease and that ∼40% of HFpEF have atrial fibrillation. Since coronary artery disease is likely to impair the dynamic Staling mechanism via an impairment of diastolic function (9), and atrial fibrillation impairs the beat-by-beat dynamic relationship itself, especially at short R-R intervals, the dynamic Starling mechanism may be underestimated in subjects with coronary artery disease and/or atrial fibrillation. Therefore, it was essential to exclude patients with these comorbidities to test our hypothesis. Moreover, this recruitment strategy ensured a clear diagnosis of HFpEF since rapidly conducted atrial fibrillation may elevate left atrial pressure even though cardiac function is normal, and intermittent ischemia is known to cause large and rapid increases in left atrial pressure (30).
We did not exclude patients with BMI > 30 because obesity is one of the common characteristics in patients with HFpEF. There were no significant differences in dynamic Starling gain between patients with normal BMI (n = 5) versus patients with higher BMI (n = 5) (gain LVEDP − SV index, 0.20 ± 0.08 vs. 0.27 ± 0.12 ml·m−2·mmHg−1, P = 0.335). Also, even if we selectively compared patients with normal BMI versus age-matched controls, a clear difference was observed in the dynamic Starling gain (gain LVEDP − SV index, 0.20 ± 0.08 vs. 0.37 ± 0.11 ml·m−2·mmHg−1, P = 0.006), indicating that the effects of different body size on the findings were, if any, negligible. Nevertheless, we cannot exclude the possibility that the impaired dynamic Starling mechanism in patients with HFpEF is influenced at least in part by the cardiovascular effects of obesity.
The small number of patients with HFpEF recruited could be considered one limitation of the present study to apply this concept to larger population. However, the fact that we found a significant difference in the dynamic Starling mechanism despite the small number of patients recruited rather strengthens our hypothesis. Our thorough inclusion criteria may have increased the statistical power by reducing confounding factors and limited the number of patients undergoing the risks of right heart catheterization. Some of the key studies by other researchers in this field also recruited the same (13, 16) or even smaller numbers (17) of patients with HFpEF to address pathophysiology of HFpEF.
As previously reported (28), the patients with HFpEF recruited for this study were taking cardiovascular medications such as diuretics, β-blockers, Ca2+ channel blockers, ACE inhibitors, ARBs, and HMG-CoA reductase inhibitors. Since ACE inhibitors and ARBs were not held during the study to avoid uncontrolled hypertension, and these drugs by themselves may improve ventricular and/or arterial stiffness, the impairment of the dynamic Starling mechanism in patients with HFpEF might have been underestimated due to drugs in the present study.
Conclusions
An impaired dynamic Starling mechanism associated with marked breath-by-breath LVEDP variability in elderly patients with HFpEF is likely to be explained by advanced ventricular and arterial stiffening, consistent with two previous major hypotheses. Our results also suggest that ventricular-arterial stiffening with sedentary aging contributes to the prevalence of HFpEF in the elderly population.
GRANTS
This study was supported by National Institute on Aging Grant AG17479-02 and National Space Biomedical Research Institute Postdoctoral Fellowship Grant PR01101 through NASA NCC 9-58.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Colin Connor and Daniel Creson for the nursing care of the patients and healthy subjects during the study, Diane Bedenkop for invaluable efforts in recruiting subjects, Cyrus Oufi and Murugappan Ramanathan for engineering support, and finally Robin Shook and Tiffany VanGundy for assistance in performing the experiments.
REFERENCES
- 1. Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation 110: 1799–1805, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Aurigemma GP, Zile MR, Gaasch WH. Contractile behavior of the left ventricle in diastolic heart failure: with emphasis on regional systolic function. Circulation 113: 296–304, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Balke B, Nagle FJ, Daniels J. Altitude and maximum performance in work and sports activity. JAMA 194: 646–649, 1965 [PubMed] [Google Scholar]
- 4. Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 355: 260–269, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol 90: 437–446, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol 54: 410–418, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 3: 588–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol 56: 845–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carroll JD, Carroll EP. Diastolic function in coronary artery disease. Herz 16: 1–12, 1991 [PubMed] [Google Scholar]
- 10. Chae CU, Pfeffer MA, Glynn RJ, Mitchell GF, Taylor JO, Hennekens CH. Increased pulse pressure and risk of heart failure in the elderly. Jama 281: 634–639, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Desai AS, Mitchell GF, Fang JC, Creager MA. Central aortic stiffness is increased in patients with heart failure and preserved ejection fraction. J Cardiac Fail 15: 658–664, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Fisher ML, De Felice CE, Parisi AF. Assessing left ventricular filling pressure with flow-directed (Swan-Ganz) catheters. Detection of sudden changes in patients with left ventricular dysfunction. Chest 68: 542–547, 1975 [DOI] [PubMed] [Google Scholar]
- 13. Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Link KM, Little WC. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol 38: 796–802, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Jansen JR, Schreuder JJ, Mulier JP, Smith NT, Settels JJ, Wesseling KH. A comparison of cardiac output derived from the arterial pressure wave against thermodilution in cardiac surgery patients. Br J Anaesth 87: 212–222, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Jarvis SS, Levine BD, Prisk GK, Shykoff BE, Elliott AR, Rosow E, Blomqvist CG, Pawelczyk JA. Simultaneous determination of the accuracy and precision of closed-circuit cardiac output rebreathing techniques. J Appl Physiol 103: 867–874, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation 107: 714–720, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol 17: 1065–1072, 1991 [DOI] [PubMed] [Google Scholar]
- 18. Legedz L, Bricca G, Lantelme P, Rial MO, Champomier P, Vincent M, Milon H. Insulin resistance and plasma triglyceride level are differently related to cardiac hypertrophy and arterial stiffening in hypertensive subjects. Vasc Health Risk Manag 2: 485–490, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maeder MT, Kaye DM. Heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol 53: 905–918, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Maurer MS, Kronzon I, Burkhoff D. Ventricular pump function in heart failure with normal ejection fraction: insights from pressure-volume measurements. Prog Cardiovasc Dis 49: 182–195, 2006 [DOI] [PubMed] [Google Scholar]
- 21. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 285: 1441–1446, 1971 [DOI] [PubMed] [Google Scholar]
- 22. Mitchell GF, Guo CY, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Cross-sectional correlates of increased aortic stiffness in the community: the Framingham Heart Study. Circulation 115: 2628–2636, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121: 505–511, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mitchell GF, Tardif JC, Arnold JM, Marchiori G, O'Brien TX, Dunlap ME, Pfeffer MA. Pulsatile hemodynamics in congestive heart failure. Hypertension 38: 1433–1439, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Okazaki K, Iwasaki K, Prasad A, Palmer MD, Martini ER, Fu Q, Arbab-Zadeh A, Zhang R, Levine BD. Dose-response relationship of endurance training for autonomic circulatory control in healthy seniors. J Appl Physiol 99: 1041–1049, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355: 251–259, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis 47: 320–332, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Prasad A, Hastings J, Shibata S, Popovic ZB, Arbab-Zadeh A, Bhella PS, Okazaki K, Berk M, Palmer D, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. Characterization of static and dynamic left ventricular diastolic function in patients with heart failure and a preserved ejection fraction. Circ Heart Fail 3: 617–626, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Regitz-Zagrosek V, Brokat S, Tschope C. Role of gender in heart failure with normal left ventricular ejection fraction. Prog Cardiovasc Dis 49: 241–251, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Ritzema-Carter JL, Smyth D, Troughton RW, Crozier IG, Melton IC, Richards AM, Eigler N, Whiting J, Kar S, Krum H, Abraham WT. Images in cardiovascular medicine. Dynamic myocardial ischemia caused by circumflex artery stenosis detected by a new implantable left atrial pressure monitoring device. Circulation 113: e705–e706, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Shibata S, Hastings JL, Prasad A, Fu Q, Okazaki K, Palmer MD, Zhang R, Levine BD. The “dynamic” starling mechanism: effects of ageing and physical fitness on ventricular-arterial coupling. J Physiol 586: 1951–1962, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shibata S, Zhang R, Hastings J, Fu Q, Okazaki K, Iwasaki K, Levine BD. Cascade model of ventricular-arterial coupling and arterial-cardiac baroreflex function for cardiovascular variability in humans. Am J Physiol Heart Circ Physiol 291: H2142–H2151, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Tomiyama H, Hashimoto H, Hirayama Y, Yambe M, Yamada J, Koji Y, Shiina K, Yamamoto Y, Yamashina A. Synergistic acceleration of arterial stiffening in the presence of raised blood pressure and raised plasma glucose. Hypertension 47: 180–188, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Tyberg JV, Taichman GC, Smith ER, Douglas NW, Smiseth OA, Keon WJ. The relationship between pericardial pressure and right atrial pressure: an intraoperative study. Circulation 73: 428–432, 1986 [DOI] [PubMed] [Google Scholar]
- 35. Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 88: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 36. van Lieshout JJ, Toska K, van Lieshout EJ, Eriksen M, Walloe L, Wesseling KH. Beat-to-beat noninvasive stroke volume from arterial pressure and Doppler ultrasound. Eur J Appl Physiol 90: 131–137, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Vasan RS, Benjamin EJ, Levy D. Prevalence, clinical features and prognosis of diastolic heart failure: an epidemiologic perspective. J Am Coll Cardiol 26: 1565–1574, 1995 [DOI] [PubMed] [Google Scholar]
- 38. Verbraecken J, Van de Heyning P, De Backer W, Van Gaal L. Body surface area in normal-weight, overweight, and obese adults. A comparison study. Metabolism 55: 515–524, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Wang J, Nagueh SF. Current perspectives on cardiac function in patients with diastolic heart failure. Circulation 119: 1146–1157, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol 74: 2566–2573, 1993 [DOI] [PubMed] [Google Scholar]
- 41. Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol 47: 76–84, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure–abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 350: 1953–1959, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: part I: diagnosis, prognosis, and measurements of diastolic function. Circulation 105: 1387–1393, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: part II: causal mechanisms and treatment. Circulation 105: 1503–1508, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Zile MR, Izzi G, Gaasch WH. Left ventricular diastolic dysfunction limits use of maximum systolic elastance as an index of contractile function. Circulation 83: 674–680, 1991 [DOI] [PubMed] [Google Scholar]