Abstract
The average time myosin cross bridges remain bound to actin (ton) can be measured by sinusoidal length perturbations (sinusoidal analysis) of striated muscle fibers using recently developed analytic methods. This approach allows measurements of ton in preparations possessing a physiologically relevant myofilament lattice. In this study, we developed an approach to measure ton in 5–10% of the time required for sinusoidal analysis by using stochastic length perturbations (white noise analysis). To compare these methods, we measured the influence of MgATP concentration ([MgATP]) on ton in demembranated myocardial strips from mice, sampling muscle behavior from 0.125 to 200 Hz with a 20-s burst of white noise vs. a 300-s series of sinusoids. Both methods detected a similar >300% increase in ton as [MgATP] decreased from 5 to 0.25 mM, differing by only 3–14% at any [MgATP]. Additional experiments with Drosophila indirect flight muscle fibers demonstrated that faster cross-bridge cycling kinetics permit further reducing of the perturbation time required to measure ton. This reduced sampling time allowed strain-dependent measurements of ton in flight muscle fibers by combining 10-s bursts of white noise during periods of linear shortening and lengthening. Analyses revealed longer ton values during shortening and shorter ton values during lengthening. This asymmetry may provide a mechanism that contributes to oscillatory energy transfer between the flight muscles and thoracic cuticle to power flight. This study demonstrates that white noise analysis can detect underlying molecular processes associated with dynamic muscle contraction comparable to sinusoidal analysis, but in a fraction of the time.
Keywords: muscle mechanics, cross-bridge kinetics, cardiac and insect flight muscle, biological systems identification, motor proteins
viscoelastic behavior in activated muscle tissue arises from cyclical binding of the motor protein myosin with actin to create a force producing cross bridge. Traditional system analysis techniques for analyzing cross-bridge kinetics examine the relationship between muscle stress (force divided by cross-sectional area) and muscle strain [the change in muscle length (ML) divided by the original length]. These techniques employ either a step change in ML (4, 5, 11, 18), a series of sinusoidal strains (19, 20, 26, 27, 44, 46), or a variety of stochastic strains (3, 14, 42), each of which embodies benefits and limitations for investigating linear vs. nonlinear muscle responses. The likelihood of observing nonlinearities in the force response increases with length changes greater than 0.5–1% ML (26); thus small amplitude length changes maintain system linearity and enhance the capacity of mathematical models for describing cross-bridge kinetics that underlie the measured stress-strain relationship (1, 5, 8, 20, 23, 35, 46).
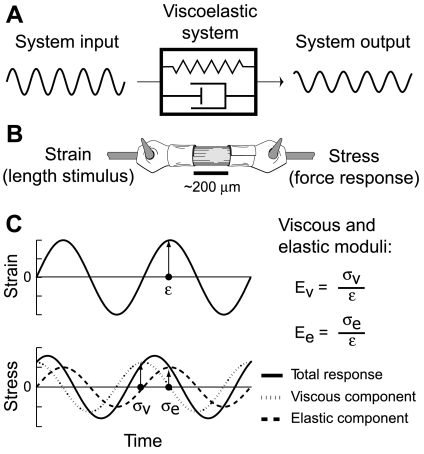
Sinusoidal analysis of activated muscle fibers is based on a series of small-amplitude sinusoidal length perturbations applied at several discrete frequencies, thus characterizing the complex modulus as a complex sum: Y(ω) = Ee(ω) + iEv(ω) (see Eq. 1), where Ee is elastic modulus, Ev is viscous modulus, and ω is the angular frequency defined as 2π × frequency (Fig. 1). Frequency-dependent characteristics of the complex modulus reflect the enzymatic cross-bridge behavior measured during force-generating portions of the cross-bridge cycle. In particular, the frequency parameter of the historically defined C-process, c in Eq. 1 (20), provides an estimate of mean myosin cross-bridge attachment duration as ton = (2πc)−1 (35), making sinusoidal analysis a powerful technique for examining cross-bridge kinetics underlying force production in muscle preparations retaining myofilament lattice structure.
Fig. 1.
A: a common engineering approach for mechanical system identification applies a sine-wave stimulus (system input) to probe characteristics of a viscoelastic system, which acts as a filter on the stimulus to produce the measured response (system output). B: applying similar analysis to muscle fibers, the length stimulus is normalized to muscle length (ML; strain), and the measured force response from the muscle is normalized via fiber cross-sectional area (stress σ). C: these normalizations facilitate calculating the elastic moduli (Ee) and viscous (Ev), by separating the total stress response into an in-phase component that aligns temporally with the measured strain (ε) and an out-of-phase component shifted π/2 radians compared with the measured strain. The ratios of this stress-to-strain amplitude (arrows in C) represent the Ee and Ev. As frequency of the length stimulus changes, the viscoelastic muscle response changes, characterizing enzymatic behavior of actin-myosin cross bridges in activated muscle fibers. σv, Viscous stress; σe, elastic stress.
Although white noise analysis in physiological systems encompasses a history of linear and nonlinear analysis (28), we focused on linear aspects of muscle mechanics for comparison with the intrinsically linear systems analysis method of sinusoidal analysis. Prior studies have assessed the viscoelastic dynamics of intact and skinned muscle fibers through random changes in ML (14, 15, 17, 33, 42, 43), but none have linked measured system responses to varied kinetics at specific portions of the cross-bridge cycle, as described here for shifts in ton with MgATP concentration ([MgATP]) and load.
A potent advantage of white noise analysis over sinusoidal analysis is the ability to simultaneously sample a desired frequency range of the muscle response in a fraction of the time required to complete the multiple frequency sweeps of sinusoidal analysis (Fig. 2). This feature elevates the measured information content to facilitate extracting ton from the “noisy” system response often associated with these measurements. The benefit of simultaneously sampling a specified frequency range enables layering the white noise analysis upon linear shortening and lengthening transients to probe the effect of strain, or load, on actomyosin cross-bridge cycling kinetics. The following study not only confirms that similar values for ton can be obtained from sinusoidal and white noise analysis, but clearly demonstrates that white noise analysis is a robust and useful method for probing strain-dependent molecular processes underlying muscle contraction.
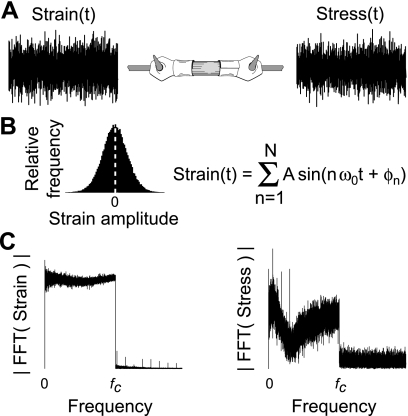
Fig. 2.
A: system analysis similar to the description in Fig. 1 can be applied to muscle fibers using a white noise stimulus. B: the strain signal represents band-limited Gaussian white noise, constructed by summing a series of sine waves of the same amplitude but of random phase, and covering a frequency range up to a prescribed cutoff frequency (fc). Theoretically, this creates a strain signal with a Gaussian amplitude distribution and a flat power spectrum. See materials and methods for further detail. C: representative Fourier transforms of the measured strain stimulus and stress response illustrate the measured behavior from a demembranated fruit fly dorsolongitudinal muscle fiber. FFT, fast Fourier transform; ω0, angular frequency; ϕn, random phase.
MATERIALS AND METHODS
Solutions.
Solutions were prepared according to a computer program that solves the ionic equilibria (13). Unless listed otherwise, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO), and concentrations are expressed in millimoles per liter (mM). For experiments using mouse myocardium, relaxing solution (pCa 8.0, where pCa = −log10[Ca2+]) consisted of (in mM) 20 N,N-bis[2-hydroxyethyl]-2-aminoethanesulfonic acid (BES), 35 creatine phosphate (CP), 300 U/mL creatine phosphokinase, 1 dithiothreitol, 5 EGTA, 1 Mg2+, and 5 MgATP, at an ionic strength of 200 meq adjusted with sodium methane sulfate, and pH 7.0. Activating solution was similar to relaxing solution, but at pCa 4.0. Skinning solution for the mouse myocardium experiments was similar to the relaxing solution described above, except for having an ionic strength of 190 meq, 30 mM 2,3-butanedione monoxime, 50% (wt/vol) glycerol, and 1% (wt/vol) Triton X-100. Mouse storage solution was similar to skinning solution with 10 μg/ml leupeptin and no Triton.
Skinning and storage solutions for insect flight muscle experiments were described previously (29). Relaxing solution consisted of (in mM) pCa 8, 20 BES, 20 creatine phosphate, 450 U/ml creatine phosphokinase, 1 dithiothreitol, 5 EGTA, 1 Mg2+, 12 MgATP, 2 Pi, ionic strength of 200 meq adjusted with sodium methane sulfate, and pH 7.0. Activating solution was similar to relaxing solution, but at pCa 4.0.
Mouse cardiac strips.
Mouse cardiac muscle is well described by Eq. 1 (34, 35, 36), which is used to estimate ton from sinusoidal and white noise analysis measurements. The response of mouse cardiac muscle to varying MgATP is well characterized by Eq. 1, in contrast with other striated muscles, such as slow-twitch skeletal muscle (31). For these reasons, we chose mouse cardiac muscle as the most appropriate tissue for applying and assessing the white noise technique to calculate the response of ton to varying [MgATP].
All procedures were reviewed and approved by the Institutional Animal Care and Use Committees of University of Vermont College of Medicine and complied with the Guide for the Use and Care of Laboratory Animals published by the National Institutes of Health. Hearts were removed from mice after cervical dislocation and placed into Krebs solution containing 30 mM 2,3-butanedione monoxime, bubbled with 95% O2-5% CO2 at room temperature. Papillary muscle strips were prepared as previously described (35), and demembranated (skinned) strips were stored at −20°C for no more than 4 days.
Myocardial strips were dissected to 120- to 180-μm diameter. At the time of study, aluminum T-clips were attached to the ends of a strip ∼300 μm apart. The strip was mounted between a piezoelectric motor (model P-841.40, Physik Instrumente, Auburn, MA) driven by a custom-built amplifier and a strain gauge (model AE801, SensorNor, Horten, Norway), lowered into a 30-μl droplet of relaxing solution at pCa 8, maintained at 27°C. Strips were adjusted to 2.2-μm sarcomere length, as determined by digital fast Fourier transform (FFT) analysis (IonOptix, Milton, MA). Strips (n = 2, from a single papillary muscle) were calcium activated to pCa 4.8, and MgATP concentration was varied from 5 to 0.25 mM. At each MgATP condition, sinusoidal length perturbations of amplitude 0.125% ML were applied at 0.125–200 Hz, as previously described (38, 39). Gaussian white noise length perturbation was also applied as described below. Data were sampled at 5 kHz.
Insect flight muscle fibers.
Insect flight muscle is stiffer and possesses faster myosin kinetics than cardiac muscle. These attributes enhance the signal-to-noise ratio for measures of Ee and Ev using white noise analysis, contributing to a more precise calculation of ton in insect flight muscle than in cardiac muscle (Table 1) and thus enabled better detection of small changes in the frequency characteristics of the muscle response. For these reasons and our familiarity with fruit fly flight muscle mechanics (7, 29, 30, 45), we used this preparation to detect ton during muscle shortening and lengthening.
Table 1.
Coefficients of variation among curve fits to Eq. 1 from repeated measurements within a single fiber
| Mouse |
Fruit Fly |
|||
|---|---|---|---|---|
| Sinusoid | White noise | Sinusoid | White noise | |
| A | 5.5 | 1.5 | 2.0 | 2.9 |
| k | 2.2 | 0.5 | 1.0 | 2.0 |
| B | 1.3 | 4.9 | 10.2 | 6.7 |
| b | 3.8 | 2.4 | 1.0 | 1.7 |
| C | 5.2 | 15.5 | 10.4 | 7.9 |
| c | 3.5 | 5.6 | 1.3 | 1.6 |
| ton | 3.5 | 5.5 | 1.2 | 1.6 |
Values are in percent (n = 5). ton, mean myosin cross-bridge attachment time. See text for definition of other terms.
Newly eclosed female Drosophila melanogaster (Oregon-R strain) were placed in 35-ml plastic vials containing standard cornmeal fly food and maintained at 25°C, 70% humidity, and a 12:12-h light-dark cycle. At 2 to 3 days old, flies were anesthetized with CO2, and single dorsolongitudinal muscle fibers were isolated from half-thoraces. Fibers were then split lengthwise to ∼100-μm diameter to reduce the cross-sectional area. The fibers were demembranated in skinning solution for 1 h at 4°C and clipped with aluminum T-clips at both ends ∼300 μm apart. Where fibers were not used immediately, they were transferred to storage solution at −20°C and used within 2 days of dissection. Fibers were mounted between a piezoelectric motor (model P-841.10, Physik Instrumente) driven by an amplifier (model E662, Physik Instrumente) and a strain gauge (model AE801, SensorNor). As previously described (7, 29, 45), fibers (n = 9, from six flies) were then lowered into 30 μl of relaxing solution maintained at 15°C, then stretched 5% from just taut, and activated to pCa 4.5, from which followed additional stretches in 2% increments until oscillatory work production reached a stable maximum. Additional mechanical analyses were then performed using white noise length perturbations, where data were sampled at 8 kHz.
Mechanical analysis.
A digital FFT was applied to the strain and stress signals, and the complex modulus [Y(ω), where ω is angular frequency] is calculated from the quotient of these FFTs (stress/strain, as depicted in Fig. 1). Ee and Ev are defined as the in-phase (real part) and out-of-phase (imaginary part) portions of complex modulus, respectively. Fitting Eq. 1 to these data provides an estimate of mean myosin attachment time (ton), where ton = (2πc)−1 (35).
| (1) |
Mechanical measurements of Y(ω) arise from passive structure elements of the muscle cell and force-producing cross bridges. The A-process reflects the passive elements, where length perturbations produce force responses of constant relative phase, independent of frequency, as indicated by straight line in plots of Ee vs. Ev (35). In our interpretation, the parameter A represents the combined mechanical stiffness of the filaments and the number of strongly bound cross bridges (34), and k describes the degree of the viscoelastic response as elastic (k → 0) vs. viscous (k → 1). The power-law frequency response described in the A-process provides a good description of almost all viscoelastic materials, including living cells, relaxed muscle (9), and the passive elements parallel to, and in series with, sarcomeres in activated muscle.
The B- and C-processes reflect enzymatic cross-bridge behavior in activated muscle and are sensitive to concentrations of MgATP, MgADP, and Pi (21, 22), as well as to temperature with a Q10 ∼ 2–3 (35). It should be noted that using 2πc to estimate the mean ton requires that a single-exponential function reasonably describes the probability distribution of ton (35). A single-exponential distribution is reported from single myosin molecule experiments in the laser trap under low (<0.1 mM) MgATP conditions (2, 10). To demonstrate the flexibility of the single-exponential assumption under high MgATP conditions, Palmer et al. (35) successfully used computational simulations to validate the technique using a double-exponential distribution, which could represent a relatively long ADP state convolved with a short Pi state. These studies (2, 10, 35) and current measurements herein suggest a valid use of 2πc to calculate mean ton in demembranated muscle fibers under low- and high-MgATP conditions. Our current methods do not, however, provide a description of time detached, toff.
Sinusoidal analysis provides the complex modulus at several discrete frequencies, each calculated separately. In contrast, one can combine multiple sinusoids covering a specified frequency range to create a particular type of stochastic signal termed band-limited Gaussian noise (Fig. 2). In principle, this noise analysis should describe identical system behavior as sinusoidal analysis when applied to muscle fibers (Fig. 2A). In practice, band-limited Gaussian noise is created a priori by summing a series of individual sinusoidal length perturbations (28). The “band-limited” adjective refers to the frequency content represented by the signal, which includes sinusoids with frequencies corresponding to integer values of the fundamental frequency (ω0 = 2π/T) up to a prescribed cutoff frequency (fc). As an example, for the fruit fly measurements, the stimulus period (T) was 10 s, and fc was 1,000 Hz, thereby comprising sinusoids with frequencies from 0.1 to 1,000 Hz at every 0.1 Hz. The “Gaussian” adjective refers to the amplitude distribution taking on a Gaussian profile (Fig. 2B) as a consequence of the central limit theorem, created by each individual sinusoid having a constant amplitude (A) with a random phase (ϕn, drawn from a uniform distribution between −π to π). The “white noise” adjective describes a flat power spectrum for the stochastic stimulus across the frequency range ω0 to fc (Fig. 2C), indicating that the stimulus contains equal power across the specified bandwidth.
For our white noise analysis, the standard deviation of the strain amplitude distribution was 0.1 and 0.075% ML, with T of 20 and 10 s for the myocardial strip and insect flight muscle fiber measurements, respectively. Insect flight muscle experiments combined this white noise stimulus with linear lengthening and shortening transients (of strain rate 0.02% ML/s) to probe strain dependence of ton. Similar to sinusoidal analysis, Ee and Ev were calculated from the quotient of the FFTs for stress and strain (example magnitudes for these FFTs are depicted in Fig. 2C). The mean slope across T was subtracted from the stress and strain profiles before Fourier analysis. For each strain stimulus (T seconds long), we wrapped the final and initial 2.5 s of the stimulus onto the front and back ends, respectively, creating a stimulus (T+5 s long) to mitigate discontinuities at the edges of the intermediate T seconds of data analyzed to calculate the complex modulus. This approach also decreased mechanical heterogeneities in the measured stress response due to the abrupt length transitions at the start and end of the white noise stimulus. To increase visual clarity, the Ee and Ev plotted in Figs. 3 and 5 were downsampled every 10 data points, although the complex moduli were calculated using complete data sets. Curve-fitting of these data (using complete data sets) allowed us to estimate ton using white noise analysis techniques, similar to the sinusoidal analysis techniques described above.
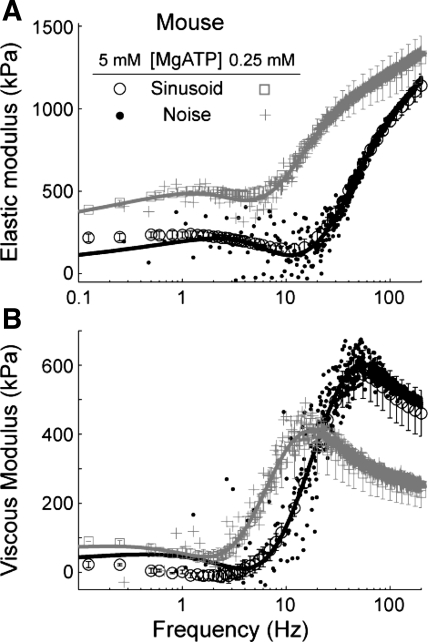
Fig. 3.
Direct comparisons of sinusoidal and white noise analyses illustrate consistent shifts among measured system behaviors as MgATP concentration ([MgATP]) varied for demembranated myocardial strips from mice. Ee (A) and Ev (B) are plotted against frequency for measurements at 27°C. Lines represent the average curve fits to Eq. 1 for the white noise analysis data at each condition. Just as each sinusoidal data point is an average among strips (with error bars representing SE), each white noise point represents the average among strips with error bars omitted (where SE at any frequency is comparable to that depicted by the sinusoidal analysis data).
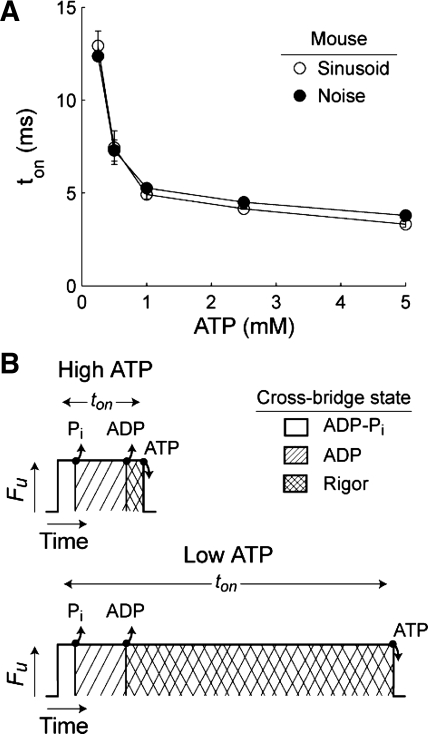
Fig. 5.
A: estimates of actin-myosin attachment time (ton) from sinusoidal and white noise analysis are shown (means ± SE) as a function of [MgATP] for the mouse myocardial measurements from Fig. 3, representing the expected increases in ton with decreasing [MgATP]. B: schematics of cross-bridge attachment events illustrating a longer rigor state as [MgATP] decreases from 5 to 0.25 mM (labeled High MgATP and Low MgATP, respectively), showing unitary force production (Fu) vs. time throughout a single cross-bridge cycle.
Statistical analysis.
All values are means ± SE. Statistical analyses were performed using SPSS (version 14.0; SPSS, Chicago, IL) and Matlab (version 7.9.0, The Mathworks, Natick, MA). Nonlinear least-squares fitting of Eq. 1 to recorded complex moduli was performed using a Levenberg-Marquardt routine (IDL 7.0, ITT Visual Information Solutions, Boulder, CO). Most data were examined using a one-way ANOVA, followed by a multiple comparison of the means to access significance. To explore whether there were population-specific trends for model parameters from Eq. 1 or whether ton varied with [MgATP] or muscle shortening and lengthening transients, data were initially examined using a repeated-measures ANOVA, followed with a one-way ANOVA and multiple comparisons of the means to evaluate significance. For all analysis, data were significant for P < 0.05.
RESULTS
Comparing sinusoidal and white noise analyses for detecting ton.
We compared sinusoidal and white noise analyses using mouse myocardial strips under maximal Ca2+ activation (pCa 4.8) and varied [MgATP] conditions (Fig. 3). These experiments demonstrated that sinusoidal and white noise analyses measure similar shifts in mechanical behavior, as cross-bridge kinetics varied with [MgATP]. Leftward or rightward shifts in the plots of moduli vs. frequency represent slower and faster kinetics, respectively. Thus the leftward shift as [MgATP] decreased from 5 to 0.25 mM (Fig. 3) indicates slower cross-bridge cycling rates, as shown by previous measurements using sinusoidal analysis (21, 47).
While both methods described similar biomechanical phenomena, it is important to note that white noise analysis took only 20 s, while the sinusoidal analysis took ∼300 s to complete. However, the discrete period (T) for white noise analysis presents a trade off in measurement duration vs. the ability to resolve strain and stress amplitudes at the smallest frequencies, due to fewer cycles as ω approaches 2π/T. Thus there appears more variability between adjacent moduli values at the lowest frequencies (particularly for the semilog plots in Fig. 3), although standard error in moduli values was similar for both analysis methods.
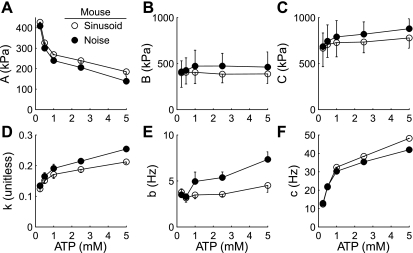
Fitting Eq. 1 to the moduli data also illustrated similarities between the two techniques, where average fits for the noise data (lines in Fig. 3) closely resembled sinusoidal analysis. These fits show similar responses among all model parameters for both analysis methods (Fig. 4), and that [MgATP] significantly affected the trend observed for each parameter value, except for B (Fig. 4B), which was not sensitive to [MgATP]. Importantly, this comparison further demonstrates that both analysis methods are sampling and describing similar biomechanical phenomena, although systematic differences in the average parameter values returned by each method are evident.
Fig. 4.
A–F: the effect of [MgATP] on estimates of model parameters (A, B, C, k, b, c, respectively) from fits of Eq. 1 to the Ee and Ev from mouse cardiac muscle (example data shown in Fig. 3). Data are plotted as means ± SE, with error bars extending downward for sinusoidal analysis and upward for white noise analysis. See text for definition of terms.
Model parameters A, k, and c (Fig. 4, A, D, and F, respectively) were most sensitive to [MgATP]. As [MgATP] decreased, the increased A values and decreased k values demonstrate an increasingly elastic response due to the augmented population of strongly bound cross-bridges. The nearly fourfold change in A and c suggests a fourfold increase in cross-bridge duty cycle as [MgATP] decreases, given the minimal change in the cross-bridge recruitment rate indicated by relatively small shifts in the B-process parameters with [MgATP] (Fig. 4, B and E). These results show that [MgATP] only modestly affects work-producing portions of the cross-bridge cycle, while decreasing [MgATP] significantly increases ton (Fig. 5A).
Decreasing [MgATP] produced a nonlinear increase in ton, with only a 4–13% divergence in estimated ton values between both methods (correlation coefficient = 0.99, Fig. 5A). As MgATP decreased from 5 and 0.25 mM, ton ranged from 3.3 ± 0.1 to 12.9 ± 0.8 ms for sinusoidal analysis and from 3.8 ± 0.1 to 12.4 ± 0.2 ms for white noise analysis, suggesting less MgATP prolongs the rigor state of the cross-bridge cycle (Fig. 5B) (12). This small divergence across a broad range of values further supports the premise that these two analysis techniques describe similar physiological processes.
Strain dependence of ton detected by white noise analysis.
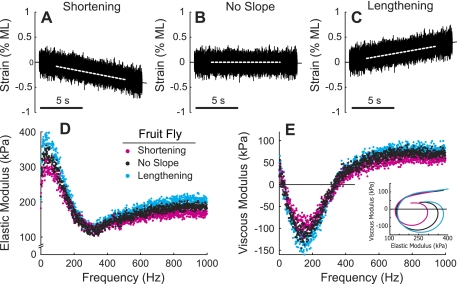
White noise analysis was applied to maximally activated (pCa 4.5) indirect flight muscle (IFM) fibers from fruit flies (Fig. 6). The super-fast actomyosin kinetics underlying IFM contraction helped to reduce white noise analysis to 10 s (Fig. 6B) vs. ∼200 s for sinusoidal analysis. We probed strain-dependent behavior by applying white noise stimuli during periods of muscle shortening and lengthening (Fig. 6, A and C), capitalizing on white noise analysis simultaneously sampling the entire system response. Mean strain rates (0.02% ML/s) were kept small during shortening and lengthening transients to maintain system linearity.
Fig. 6.
Condensed temporal requirements of white noise analysis facilitated combining normal stimuli (B) with linear shortening (A) and lengthening (C) transients to probe the effect of load on cross-bridge behavior using fruit fly dorsolongitudinal muscle fibers. Dashed lines show the mean length stimulus during the 10-s duration used to calculate the Ee (D) and Ev (E), plotted against frequency. Each point represents the average among fibers at a single frequency. E, inset: solid lines show average curve fits to Eq. 1, depicting Nyquist behavior for each condition. Temperature, 15°C.
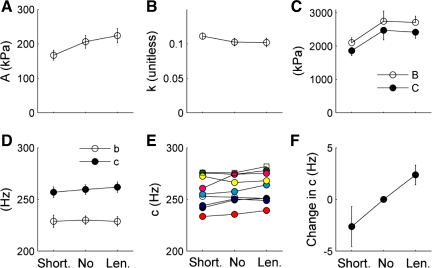
Viscous and elastic moduli values from the no-slope (mean strain = 0) measurements consistently fell between moduli values measured during shortening and lengthening. Although variability in the average moduli values made it difficult to resolve differences at individual frequencies, Nyquist plots depicting average fits to Eq. 1 (lines inset in Fig. 6E, with data and fits shown in Supplemental Fig. 1; the online version of this article contains supplemental data) demonstrate strain-dependent shifts in the moduli. The effects of shortening and lengthening transients on mechanics and cross-bridge cycling kinetics were evaluated statistically by fitting Eq. 1 to the data (Fig. 7) and comparing parameter values. Analysis of the estimated model parameters using a repeated-measures ANOVA only showed significant (P < 0.05) strain-sensitive effects for A, k, and c (Fig. 7, A, B, and D, respectively). The population response among all fibers for the entire set of c values (Fig. 7E) showed a strain-dependent shift in c for most fibers, averaging an ∼3 Hz decrease or increase with shortening and lengthening, respectively. Even though the resolution of c is 1.5% (Table 1), the repeated-measures analysis highlights the strength of the white noise analysis for resolving very small kinetic changes within a single fiber.
Fig. 7.
A–D: the effect of shortening (Short), no-slope (No), and lengthening (Len) on estimates of model parameters from fits of Eq. 1 to the Ee and Ev from insect flight muscle. The effect of strain on c for the entire population of fibers indicates a significant trend (E), better illustrated by the change in c from the no-slope value (F). Except for E, data are plotted as means ± SE.
The fits of Eq. 1 to the data demonstrated strain-dependent shifts in ton (Table 2), showing ton increased with shortening and decreased with lengthening. The minimum Ev during shortening, no-slope, and lengthening conditions (Table 3) are directly proportional to the maximum oscillatory work produced within a single oscillatory cycle [work = −EvπA2 (29)]. The lower Ev at ∼135 Hz during lengthening (Fig. 6) may reflect reduced energy loss as a consequence of shorter ton (i.e., less frictional resistance to lengthening). Likewise, the higher Ev at ∼135 Hz during shortening (Fig. 6) may reflect increased energy loss as a consequence of the longer ton.
Table 2.
ton varies with strain in flight muscle fibers from fruit flies
| Shortening | No Slope | Lengthening | |
|---|---|---|---|
| ton, ms† | 0.62 ± 0.01 | 0.61 ± 0.01 | 0.60 ± 0.01 |
| Change in ton, %† | 1.1 ± 0.8‡ | 0 | −0.9 ± 0.4‡ |
Repeated-measures ANOVA indicates significant strain dependency represented by the three conditions (P < 0.05).
Normalized measurements within each fiber to the No Slope value indicate significant differences for the relative change in ton due to lengthening or shortening (P < 0.05).
Table 3.
Minimum viscous modulus varied with strain in flight muscle fibers from fruit flies
| Shortening | No Slope | Lengthening | |
|---|---|---|---|
| Magnitude, kPa† | 94.0 ± 8.6‡ | 119.6 ± 11.3 | 133.5 ± 13.0‡ |
| Frequency, Hz | 138 ± 3 | 134 ± 3 | 133 ± 3 |
Repeated-measures ANOVA indicates significant strain dependency represented by the three conditions (P < 0.05).
Different from the No Slope value (P < 0.05).
DISCUSSION
This study describes similarities and differences between sinusoidal and white noise analysis techniques for measuring mechanical characteristics of mouse myocardial strips. Both methods yield similar values for ton in Ca2+-activated muscle fibers exposed to various [MgATP]. Sinusoidal analysis individually samples the muscle response at one applied frequency, requiring a sweep across multiple frequencies to reconstruct a composite muscle response over a desired frequency range. White noise analysis, on the other hand, has a distinct advantage in sampling the muscle response simultaneously over a desired frequency range. Thus the data-acquisition time required for estimating ton can be significantly reduced using white noise analysis (10–20 vs. 200–300 s for comparable sinusoidal analysis measurements), which also minimizes possible errors due to altered muscle behavior over the duration of an experiment. Because muscle fiber integrity and function often deteriorate with activation duration throughout an experiment, the reduced time required for white noise analysis represents an important benefit to experimental protocols.
The fixed duration of a white noise stimulus, on the other hand, represents a significant limitation in some applications because the relationship between strain and stress becomes most difficult to resolve for the lowest frequency data. White noise stimuli contain more periodic cycles for the highest frequency data (near fc) and one cycle for the lowest frequency data (f0; Fig. 2). Thus the signal-to-noise ratio for resolving Ee and Ev increases with the number of cycles sampled, illustrated by diminished scatter in moduli values from f0 to fc (Fig. 3). Although more obvious for mouse myocardium measurements due to the log-scaling of abscissa (Fig. 3), this phenomenon can be seen in the insect flight muscle measurements as well (Fig. 6). Consistently, variability in the estimate of ton decreases as cross-bridge cycling rates increase, shown by the lower variability in ton for insect flight muscle (Table 1) and the relatively higher variability in ton as its value increases with reduced [MgATP] in mouse myocardium (Fig. 5A). Thus white noise analysis and sinusoidal analysis impart different systematic errors that arise from inherent differences in frequency content and differences in signal-to-noise ratio at any given frequency. These different systematic errors underlie the small differences in absolute values for the estimated model parameters from Eq. 1 shown in Fig. 4. Nevertheless, these analytic methods clearly describe and track the same MgATP-dependent biochemical phenomena in activated muscle fibers (Figs. 3–5). One can further optimize white noise analysis by varying the measurement period to appropriately sample kinetics associated with faster and slower muscles (Figs. 3 and 6).
Applying white noise during periods of shortening and lengthening in Ca2+-activated fruit fly flight muscle allowed us to take advantage of its high-strain sensitivities and super-fast cross-bridge kinetics (6, 32, 40, 41, 45) to detect strain-dependent shifts in ton. We found that ton increased during shortening and decreased during lengthening (Table 2), which may be the fundamental mechanism leading to positive work production throughout an oscillatory cycle. As posited by Palmer et al. (37), our reported strain dependence in ton would increase duty ratio during shortening and decrease duty ratio during lengthening (given no changes in myosin off time), leading to the force response lagging the length perturbation to generate positive work. This asymmetric molecular behavior may underlie viscoelastic energy transfer between the muscles and thoracic cuticle throughout a wing beat. In contrast with more traditional force-velocity protocols that link macroscopic measurements with coarse changes in cross-bridge cycling rate (16), white noise analysis estimates kinetic changes of the cross-bridge cycle when myosin is bound to actin with high resolution (e.g., changes in ton of the order of 1%). Strain rates during lengthening and shortening translate into ∼0.3 nm·half-sarcomere−1·s−1 (assuming sarcomere length 3.2 μm), well within the ∼7-nm power stroke for flight muscle (24). Such lengthening and shortening transients would strain an attached myosin head an average of ∼0.2 pm, given a mean ton of roughly 0.6 ms (Table 2). Although small, this strain appears sufficient to measurably bias myosin cross-bridge kinetics in Drosophila (Fig. 7).
Cross-bridge behavior in Drosophila IFM is highly sensitive to variations in [MgATP], with exceptionally fast release of MgADP (45). Under our experimental conditions (12 mM [MgATP] and 2 mM [Pi]), Pi release becomes the rate-limiting step of the cross-bridge cycle (45), likely due to cross bridges rebinding Pi to reverse the power stroke. Single-molecule studies of ton for myosin II also show that a very short-lived population of events develops for [Pi] = 40 mM, which is not present in the absence of Pi (2). Therefore, the elevated Pi sensitivity in insect flight muscle could shorten ton via Pi rebinding and reduce the likelihood of myosin completing a full MgATP-dependent cycle. However, strain dependencies in ton (Table 2 and Fig. 7) may also reflect kinetic differences in Pi release and rebinding that vary with load.
Linear system analysis methods enable us to estimate ton in a muscle strip or fiber, where cross-bridge kinetics are influenced by the organized structure of the myofilament lattice. Over the past few years, we have validated the use of sinusoidal length perturbation analysis to estimate mean ton by computational modeling and empirical assessments of ton due to the known effects of varying myosin heavy chain isoform and temperature (35), and now [MgATP]. These tests have provided a consistent linear systems interpretation of the C-process of Eq. 1 expected from enzymatic cross-bridge cycling behavior (12, 25). The present study shows that white noise analysis can likewise robustly measure ton in muscle strips and illustrates the benefits and limitations of the white noise vs. sinusoidal analysis to probe mechanical and kinetic behavior in muscle.
GRANTS
B. C. W. Tanner was supported by National Heart, Lung, and Blood Institute (NHLBI) postdoctoral fellowship (T32-HL007647) and National Science Foundation Postdoctoral Fellowship in Biology DBI-0905830. This work was also supported by NHLBI Grants P01-HL59408 (D. W. Maughan, B. M. Palmer, Y. Wang) and R01-HL086902 (B. M. Palmer).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Mark S. Miller for suggestions on previous versions of this manuscript, and also Dr. Jim O. Vigoreaux and Panagiotis Lekkas for supplying the fruit flies.
REFERENCES
- 1. Abbott RH. An interpretation of the effects of fiber length and calcium on the mechanical properties of insect flight muscle. Cold Spring Harb Symp Quant Biol 37: 647–654, 1973 [Google Scholar]
- 2. Baker JE, Brosseau C, Joel PB, Warshaw DM. The biochemical kinetics underlying actin movement generated by one and many skeletal muscle myosin molecules. Biophys J 82: 2134–2147, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calancie B, Stein RB. Measurement of rate constants for the contractile cycle of intact mammalian muscle fibers. Biophys J 51: 149–159, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Civan MM, Podolsky RJ. Contraction kinetics of striated muscle fibres following quick changes in load. J Physiol 184: 511–534, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis JS, Epstein ND. Kinetic effects of fiber type on the two subcomponents of the Huxley-Simmons phase 2 in muscle. Biophys J 85: 390–401, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dickinson MH, Farman G, Frey M, Beckyarova T, Gore D, Maughan DW, Irving T. Molecular dynamics of cyclically contracting insect flight muscle in vivo. Nature 433: 330–334, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Dickinson MH, Hyatt C, Lehmann F, Moore J, Reedy M, Simcox A, Tohtong R, Vigoreaux J, Yamashita H, Maughan D. Phosphorylation-dependent power output of transgenic flies: an integrated study. Biophys J 73: 3122–3134, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eisenberg E, Hill TL, Chen Y. Cross-bridge model of muscle contraction: quantitative analysis. Biophys J 29: 195–227, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Fredberg JJ. Scaling the microrheology of living cells. Phys Rev Lett 87: 148102, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Finer JT, Simmons RM, Spudich JA. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature 368: 113–119, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Ford L, Huxley AF, Simmons RM. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol 269: 441–515, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Geeves MA, Holmes KC. Structural mechanism of muscle contraction. Annu Rev Biochem 68: 687–728, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Godt RE, Lindley BD. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol 80: 279–297, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Halpern W, Alpert NR. A stochastic signal method for measuring dynamic mechanical properties of muscle. J Appl Physiol 31: 913–925, 1971 [DOI] [PubMed] [Google Scholar]
- 15. Halpern W, Moss RL. Elastic modulus and stress relationships in stretched and shortened frog sartorii. Am J Physiol 230: 205–210, 1976 [DOI] [PubMed] [Google Scholar]
- 16. Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond B Biol Sci 126: 136–195, 1938 [DOI] [PubMed] [Google Scholar]
- 17. Hoh J, Rossmanith GH, Kwan LJ, Hamilton AM. Adrenaline increases the rate of cycling of crossbridges in rat cardiac muscle as measured by pseudo-random binary noise-modulated perturbation analysis. Circ Res 62: 452–461, 1988 [DOI] [PubMed] [Google Scholar]
- 18. Huxley AF, Simmons RM. Proposed mechanism of force generation in striated muscle. Nature 233: 533–538, 1971 [DOI] [PubMed] [Google Scholar]
- 19. Jewell BR, Ruegg JC. Oscillatory contraction of insect fibrillar muscle after glycerol extraction. Proc R Soc Lond B Biol Sci 164: 428–459, 1964 [Google Scholar]
- 20. Kawai M, Brandt P. Sinusoidal analysis: a high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscles of rabbit, frog and crayfish. J Muscle Res Cell Motil 1: 279–303, 1980 [DOI] [PubMed] [Google Scholar]
- 21. Kawai M, Halvorson H. Role of MgATP and MgADP in the cross-bridge kinetics in chemically skinned rabbit psoas fibers. Study of a fast exponential process (C). Biophys J 55: 595–603, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kawai M, Halvorson H. Two step mechanism of phosphate release and the mechanisms of force generation in chemically skinned fibers of rabbit psoas muscle. Biophys J 59: 329–342, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawai M, Halvorson H. Force transients and minimum cross-bridge models in muscle contraction. J Muscle Res Cell Motil 28: 371–395, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Littlefield KP, Swank DM, Sanchez BM, Knowles AF, Warshaw DM, Bernstein SI. The converter domain modulates kinetic properties of Drosophila myosin. Am J Physiol Cell Physiol 284: C1031–C1038, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Lymn RW, Taylor EW. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry 10: 4617–4624, 1971 [DOI] [PubMed] [Google Scholar]
- 26. Machin KE. Feedback theory and its application to biological systems. Symp Soc Exp Biol 18: 421–445, 1964 [PubMed] [Google Scholar]
- 27. Machin KE, Pringle JWS. The physiology of insect fibrillar muscle. III. The effect of sinusoidal changes of length on a beetle flight muscle. Proc R Soc Lond B Biol Sci 151: 311–330, 1960 [DOI] [PubMed] [Google Scholar]
- 28. Marmarelis P, Marmarelis V. Analysis of Physiological Systems: the White-Noise Approach. New York: Plenum, 1978 [Google Scholar]
- 29. Miller MS, Dambacher CM, Knowles AF, Braddock JM, Farman GP, Irving TC, Swank DM, Bernstein SI, Maughan DW. Alternative S2 hinge regions of the myosin rod affect myofibrillar structure and myosin kinetics. Biophys J 96: 4132–4143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miller MS, Lekkas P, Braddock JM, Farman G, Ballif BA, Irving TC, Maughan DW, Vigoreaux JO. Aging enhances indirect flight muscle fiber performance yet decreases flight ability in Drosophila. Biophys J 95: 2391–2401, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miller MS, VanBuren P, LeWinter MM, Braddock JM, Ades PA, Maughan DW, Palmer BM, Toth MJ. Chronic heart failure decreases cross-bridge kinetics in single skeletal muscle fibres from humans. J Physiol 588: 4039–4053, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Molloy JE, Kyrtatas V, Sparrow JC, White DCS. Kinetics of flight muscle from insects with different wingbeat frequencies. Nature 328: 449–451, 1987 [Google Scholar]
- 33. Moss RL, Halpern W. Elastic and viscous properties of resting frog skeletal muscle. Biophys J 17: 213–228, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mulieri LA, Barnes WD, Leavett BJ, Ittleman F, LeWinter MM, Alpert NR, Maughan DW. Alterations of myocardial dynamic stiffness implicating abnormal crossbridge function in human mitral regurgitation heart failure. Circ Res 90: 66–72, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Palmer B, Suzuki T, Wang Y, Barnes W, Miller M, Maughan D. Two-state model of acto-myosin attachment-detachment predicts C-process of sinusoidal analysis. Biophys J 93: 760–769, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Palmer B, Wang Y, Teekakirikul P, Hinson J, Fatkin D, Strouse S, Vanburen P, Seidman C, Seidman J, Maughan D. Myofilament mechanical performance is enhanced by R403Q myosin in mouse myocardium independent of sex. Am J Physiol Heart Circ Physiol 294: H1939–H1947, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Palmer BM. A strain-dependency of myosin off-rate must be sensitive to frequency to predict the B-process of sinusoidal analysis. Adv Exp Med Biol 682: 57–75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Palmer BM, Georgakopoulos D, Janssen PM, Wang Y, Alpert NR, Belardi DF, Harris SP, Moss RL, Burgon PG, Seidman CE, Seidman JG, Maughan DW, Kass DA. Role of cardiac myosin binding protein C in sustaining left ventricular systolic stiffening. Circ Res 94: 1249–1255, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Palmer BM, Noguchi T, Wang Y, Heim JR, Alpert NR, Burgon PG, Seidman JG, Seidman CE, Maughan DW, LeWinter MM. Effect of cardiac myosin binding protein-C on mechanoenergetics in mouse myocardium. Circ Res 94: 1615–1622, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Peckham M, Molloy JE, Sparrow J, White D. Physiological properties of the dorsal longitudinal flight muscle and the tergal depressor of the trochanter muscle of drosophila melanogaster. J Muscle Res Cell Motil 11: 203–215, 1990 [DOI] [PubMed] [Google Scholar]
- 41. Pringle JWS. Stretch activation of muscle: function and mechanism. Proc R Soc Lond B Biol Sci 201: 107–130, 1978 [DOI] [PubMed] [Google Scholar]
- 42. Rossmanith GH. Tension responses of muscle to n-step pseudo-random length reversals: a frequency domain representation. J Muscle Res Cell Motil 7: 299–306, 1986 [DOI] [PubMed] [Google Scholar]
- 43. Rossmanith GH, Hoh J, Kirman A, Kwan LJ. Influence of V1 and V3 isomyosins on the mechanical behaviour of rat papillary muscle as studied by pseudo-random binary noise modulated length perturbations. J Muscle Res Cell Motil 7: 307–319, 1986 [DOI] [PubMed] [Google Scholar]
- 44. Steiger GJ, Ruegg JC. Energetics and “efficiency” in the isolated contractile machinery of an insect fibrillar muscle at various frequencies of oscillation. Pflügers Arch 307: 1–21, 1969 [DOI] [PubMed] [Google Scholar]
- 45. Swank D, Vishnudas V, Maughan D. An exceptionally fast actomyosin reaction powers insect flight muscle. Proc Natl Acad Sci U S A 103: 17543–17547, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thorson J, White DC. Distributed representations for actin-myosin interaction in the oscillatory contraction of muscle. Biophys J 9: 360–390, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang G, Kawai M. Effects of MgATP and MgADP on the cross-bridge kinetics of rabbit soleus slow-twitch muscle fibers. Biophys J 71: 1450–1461, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.