Abstract
Resistance and aerobic exercise is recommended for cardiovascular health and disease prevention. However, the accompanying increase in arterial pressure during resistance exercise may be detrimental to vascular health. This study tests the vascular benefits of aerobic compared with resistance exercise on preventing impaired vascular function induced by a single weight lifting session that is associated with acute hypertension. Healthy, lean sedentary (SED) subjects, weight lifters, runners (>15 miles/wk), and cross trainers (chronic aerobic and resistance exercisers), underwent a single progressive leg press weight lifting session with blood pressure measurements. Brachial artery flow-mediated vasodilation (FMD; an index of arterial endothelial function) was determined using ultrasonography immediately before and after weight lifting. Sublingual nitroglycerin (0.4 mg) was used to determine endothelium-independent dilation after weight lifting. All subjects were normotensive with similar blood pressure responses during exercise. Baseline FMD was lower in runners (5.4 ± 0.5%; n = 13) and cross trainers (4.44 ± 0.3%; n = 13) vs. SED (8.5 ± 0.8%; n = 13; P = 0.037). Brachial FMD improved in conditioned weight lifters (to 8.8 ± 0.9%; P = 0.007) and runners (to 7.6 ± 0.6%; P < 0.001) but not cross trainers (to 5.3 ± 0.6%; P = NS) after acute hypertension. FMD was decreased in SED (to 5.7 ± 0.4%; P = 0.019). Dilation to nitroglycerin was similar among groups. These data suggest that endothelial responses are maintained after exposure to a single bout of weight lifting in resistance and aerobic athletes. Resistance and aerobic exercise may confer similar protection against acute vascular insults such as exertional hypertension.
Keywords: hypertension, endothelium, inflammation, blood flow
it is well recognized that exercise protects against the development of cardiovascular disease (40). While aerobic exercise clearly improves cardiovascular function (46), the benefits of resistance exercise are less certain. Participants in resistance training programs are known to suffer fewer postexertion cardiac events (29), and resistance exercise is commonly recommended for cardiovascular health and disease prevention (39). The mechanism whereby chronic exercise protects against increased risk of cardiovascular events after acute exercise is unknown.
The vascular endothelium is critical in the regulation of vascular health, likely through release of endothelial-derived factors such as nitric oxide (NO), which confer anti-proliferative, anti-inflammatory, and anti-thrombotic properties in addition to vasodilation (46a). Abnormal endothelial function marked by reduced dilation to an increase in flow (endothelium-dependent flow-mediated dilation; FMD) is an early hallmark of cardiovascular disease and a strong prognostic factor for future cardiovascular events (10, 15, 22, 25, 37, 49). Exercise training has been shown to improve peripheral vascular endothelial function in patients with cardiovascular disease (7). However, weight lifting can induce large, transient increases in arterial pressure (26, 31) and an associated pro-inflammatory response involving oxidant stress and circulating cytokines (27) known to impair endothelial function.
In previous studies, we observed impaired vascular endothelial function following a single weight lifting session in sedentary subjects but not conditioned weight lifters (26). Despite similar increases in arterial pressure to exercise, sedentary subjects demonstrated impaired FMD vs. conditioned weight lifters following exercise. The effects of transient elevations in arterial pressure on impaired endothelial function can last at least 2.5 h (28), making it important to understand the relationships between chronic exercise and the protection against the risk of reduced vascular endothelial function after acute exercise. Therefore, the purpose of this study was to test the hypothesis that endurance runners, weight lifters, and athletes participating in both running and weight lifting are protected from endothelial dysfunction after weight lifting associated with acute hypertension.
METHODS
Study participants and group assignment.
All protocols were approved by the Institutional Review Boards of the Medical College of Wisconsin and the University of Illinois at Chicago. Fifty-three male subjects without known cardiovascular risk factors were recruited for this study. Of these, 13 subjects were conditioned weight lifters (CWL), performing at least 1 h of lower extremity weight lifting at least three times per week for the 6 mo prior to the study. An additional 27 subjects were runners (RUN; n = 13) or cross trainers (CT; n = 14) running at least 15 miles/wk alone (RUN) or in combination with weight lifting (CT) at least three 45-min sessions per week for the 6 mo before the study. Finally, a group of 13 sedentary control subjects (SED) was recruited as inactive controls who did not engage in any exercise programs for 13 mo prior to study. Subjects were excluded if they had hypertension, hypercholesterolemia, diabetes, smoking, pregnancy, pituitary tumor, thyroid disorder, chromosomal abnormalities, known adverse reaction to nitroglycerin, known hypercoagulable state, anorexia nervosa, bulimia nervosa, or tobacco 6 mo prior to enrollment.
After subjects gave written informed consent, they provided a medical history and underwent physical examination at least 12 h after their last exercise training session (athletic groups) and 24 h after taking vitamin supplements at enrollment. A Block brief food frequency questionnaire was used to determine dietary intake and nutritional content (8). This study recruited only men to exclude the confounding effects of endothelial dysfunction observed in women runners with athletic amenorrhea (50) and to avoid fluctuations in FMD observed during the menstrual cycle (23). In separate studies, physical activity was estimated by an accelerometer (ActiGraph GT1M) worn for 24 h for 5 consecutive days. Activity counts were recorded during this period, analyzed in 1-min epochs, and then categorized into one of four activity levels: sedentary [441 counts (<1.5 METs)], light [441–2260 counts (1.5–3 METs)], moderate [2,260–5,896 counts (3–6 METs)], and vigorous [>5,896 counts (>6 METs)]. Data were reported as minutes of physical activity (light, moderate, and vigorous) as previously described (14, 43).
Brachial artery measurements of flow- and nitroglycerin-mediated dilation.
Brachial artery flow-mediated vasodilation was assessed in the fasting state (at least 12 h) in the morning (8:00 to 10:00 AM), prior to and within 30 min following leg press exercise as previously described (26). Ultrasound imaging (GE Logiq 500) of the brachial artery was performed in a longitudinal plane at a site 1–3 cm proximal to the antecubital fossa, with the arm abducted ∼80° from the body and the forearm supinated. The ultrasound probe (11 MHz) was positioned to visualize the anterior and posterior lumen-intima interfaces to measure diameter or central flow velocity (pulsed Doppler). The probe site was marked for accurate repositioning after exercise. After baseline images were recorded, a BP cuff on the forearm was inflated to 200 mmHg for 5 min. To assess FMD, images of the brachial artery in longitudinal cross section were recorded continuously throughout the entire study onto a VHS tape. From the recorded images, 100 frames (10 frames/s for 10 s) were captured and digitized using edge detection software at baseline and 1, 2, and 3 min after cuff deflation (12). Baseline brachial flow velocity and peak velocity after cuff release were recorded using central velocity measures described previously (30). This process was repeated immediately after resistance exercise with the same transducer placement. Ten minutes after brachial flow and diameter returned to baseline, sublingual nitroglycerin (NTG; 0.4 mg) was administered and brachial images were recorded at 2, 3, 4, and 5 min after administration. NTG was given after exercise and the second FMD to 1) avoid any residual effects of NTG during the post-exercise measurement and 2) because the duration of the effects of acute weight lifting exercise on vascular function are unknown, making it difficult to predict an appropriate wash-out period for acute exercise.
Images were digitally recorded using Brachial Imager (Medical Imaging, Iowa City, IA) and analyzed as previously described (38). Percent FMD and the response to NTG were calculated using the averaged minimum mean brachial artery diameter at baseline compared with the largest mean values obtained after release of the forearm occlusion or administration of NTG. Shear rate was calculated as blood velocity (cm/s) divided by vessel diameter (cm; Ref. 9).
Exercise protocol.
A free-weight leg press machine was used for this study as previously described (26). Subjects sat in the machine with legs superior at an ∼30° angle. Weight was added using standard steel plates. Subjects familiar with the machine performed a warm-up with minimal resistance, one or two sets of 8–12 repetitions each, and then attempted near maximal exertion for two or three more sets of six to eight repetitions each. Subjects not familiar with the machine (SED and RUN) each started with minimum resistance (∼90 pounds) and weight was added as tolerated. The maximum weight lifted was determined by the subject but each subject was encouraged to continue to add resistance until systolic blood pressure reached >170 mmHg.
Blood pressure.
Baseline blood pressures were recorded with subjects resting supine using a DynaMap automated blood pressure machine. Systolic blood pressures during exercise were taken using the right arm by brachial auscultation and palpation of the radial artery (using the higher value) with a mercury sphygmomanometer. Exercise blood pressures were obtained when subjects were at near maximal effort during a momentary hold of the final repetition.
Laboratory analysis.
Blood samples were drawn before and immediately after each weight lifting session. Plasma glucose concentration was measured using the glucose oxidase procedure (Beckman II autoanalyzer). Triglycerides (Stanbio), total cholesterol, and HDL and direct LDL cholesterol (Roche Diagnostics) were measured with spectrophotometric assays. High sensitivity C-reactive protein was measured with an enzyme immunoassay (MP Biomedicals). The pro-inflammatory HDL assay was measured based on previously described methods (36). Briefly, 1 μg of HDL cholesterol was incubated with CuCl2 (5 mM, final concentration) at 37°C for 1 h using a 384-well plate (Corning, NY) in duplicate. After incubation, 2 μg of 2′7′-dichlorofluorescein diacetate (DCFH-DA) was added to HDL-CuCl2 mixture in each well in a total volume of 50 μl. Fluorescence intensity is measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm on the microplate reader (SpectraMax Gemini EM, Molecular Devices).
Power analysis.
Sample size was estimated for comparison of differences in change in brachial artery dilation before and after a single weight lifting session between the groups. The sample size (n = 13/group) achieves 80% power to detect a difference of at least 50% with an α of 0.05.
Statistical analysis.
Subjects served as their own controls. Comparisons of FMD between groups were made using repeated-measures ANOVA. Where repeated-measures ANOVA showed a statistically significant defect for FMD, t-tests were performed for individual differences between time points (paired) or between groups at a single time point (unpaired). Comparisons of brachial artery diameter, percent brachial artery FMD, normalized FMD, BP, and blood markers of inflammation were made using repeated-measures ANOVA. Tukey's post hoc test was used for multiple comparisons. Baseline physiological characteristics (age, cholesterol, LDL, glucose, SBP) were compared using ANOVA. For covariate testing, a random effect model with a group-by-time interaction in the model was used to compare FMD change over time between groups. The same random effect model with adding additional covariates (CRP, lipids, baseline BP, maximum BP during exercise, amount of weight lifted, brachial artery diameter, and shear rate) in the model was used for testing if the covariates impact the group effects on the FMD change over time. Means ± SE were reported and P < 0.05 was considered significant.
RESULTS
Patient demographics and baseline physiology.
The mean age of all subjects was 27 ± 1 years (range 18–39 years). One subject in each of the groups had previously undetected hypercholesterolemia (total cholesterol > 200 mg/dl), all others had serum lipid measurements within the normal range. One subject in the CT group had resting systolic hypertension (BP 145/80 mmHg) while the remaining subjects had normal resting blood pressures. These individuals did not demonstrate reduced FMD at baseline and were not excluded from the final analysis. Subjects in each group demonstrated similar ages, %body fat, body mass index, waist circumference, and percentage body fat measurements (Table 1). Physical activity patterns were similar between athletic groups and total physical activity was higher than the sedentary group (Table 1). In addition, the subjects demonstrated similar diet intake patterns of macronutrients (fat, carbohydrate, and protein). While caloric intake tended to be higher in the athletic groups than sedentary subjects, this difference was not statistically significant (see Table 3).
Table 1.
Physical characteristics
| Group | Sedentary | CWL | Runners | Cross Trainers | P Value |
|---|---|---|---|---|---|
| Number, n | 13 | 13 | 13 | 14 | NA |
| Age, yr | 26 ± 3 | 25 ± 1 | 29 ± 2 | 28 ± 2 | 0.554 |
| BMI, kg/m2 | 24.1 ± 1.2 | 25.6 ± 0.8 | 24.3 ± 1.1 | 25.6 ± 0.8 | 0.511 |
| %Body fat | 19.4 ± 3.4 | 18.4 ± 1.8 | 15.9 ± 1.7 | 18.8 ± 2.1 | 0.742 |
| Waist circumference, cm | 87 ± 6 | 82 ± 2 | 81 ± 2 | 83 ± 2 | 0.652 |
| Total cholesterol, mg/dl | 163 ± 14 | 168 ± 11 | 178 ± 18 | 161 ± 11 | 0.910 |
| LDL, mg/dl | 103 ± 9 | 86 ± 10 | 102 ± 7 | 96 ± 6 | 0.471 |
| HDL, mg/dl | 51 ± 4 | 48 ± 5 | 46 ± 3 | 54 ± 3 | 0.496 |
| Glucose, mg/dl | 94 ± 2 | 90 ± 2 | 93 ± 3 | 93 ± 2 | 0.608 |
| Maximum SBP, mmHg | 187 ± 6 | 184 ± 8 | 180 ± 9 | 191 ± 8 | 0.644 |
| c-Reactive protein | 1.8 ± 0.8 | 0.6 ± 0.4 | 2.5 ± 0.8* | 0.5 ± 0.1 | 0.048 |
| Maximum weight lifted, lb | 229 ± 15 | 300 ± 15 | 260 ± 25 | 377 ± 44*† | 0.003 |
| Minutes of total physical activity/wk | 694 ± 104 | 1,061 ± 70* | 1,336 ± 81* | 1,149 ± 104* | <0.001 |
Data are presented as means ± SE. BMI, body mass index; DBP, diastolic blood pressure; CWL, conditioned weight lifters; HR, heart rate; HDL, high density lipoprotein; LDL, low density lipoprotein; SBP, systolic blood pressure; SED, sedentary.
Significant difference observed vs. SED (P < 0.05).
Significant difference vs. Runners.
Table 3.
Nutritional characteristics
| Sedentary | CWL | Runners | Cross Trainers | P Value | |
|---|---|---|---|---|---|
| Energy, kcal/day | 2700 ± 399 | 3207 ± 312 | 2932 ± 258 | 2945 ± 224 | 0.789 |
| Total fat, g | 92 ± 12 | 129 ± 15 | 113 ± 12 | 108 ± 9 | 0.210 |
| Total carbohydrate, g | 365 ± 63 | 387 ± 34 | 366 ± 44 | 349 ± 42 | 0.968 |
| Total protein, g | 104 ± 9 | 123 ± 15 | 117 ± 10 | 108 ± 9 | 0.654 |
| Vitamin C, mg | 141 ± 56 | 203 ± 58 | 161 ± 80 | 177 ± 41 | 0.949 |
| Vitamin E, mg | 8 ± 2 | 42 ± 27 | 17 ± 4 | 33 ± 16 | 0.373 |
Data are presented as means ± SE.
Blood pressure response to exercise.
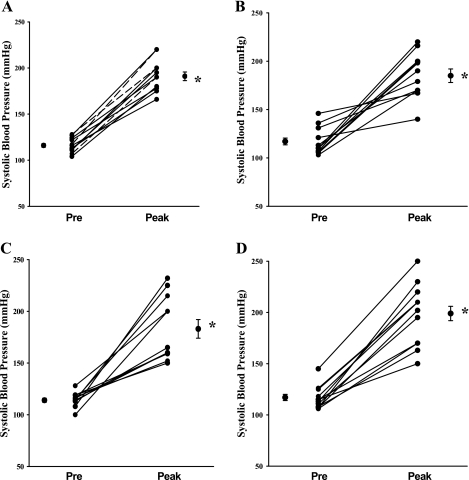
All subjects exhibited an increase in SBP from 117 ± 2 mmHg (at rest) to 188 ± 4 mmHg during heavy lifting (P < 0.001). The increase in SBP during weight lifting in these subjects ranged from 63 to 93 (SED), 28 to 110 (CWL), 31 to 124 (RUN), and 35 to 123 mmHg (CT). The mean increase was similar among groups (Table 1).
Brachial artery reactivity to FMD and NTG.
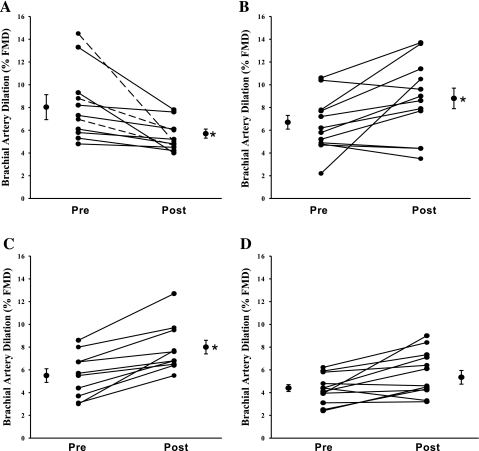
Basal brachial artery diameters were similar among groups and were not altered after the weight lifting episode (Table 2). The baseline brachial artery FMD was similar in SED (8.5 ± 0.8%) and CWL (6.7 ± 0.7%). Consistent with previous studies (26), FMD was impaired in SED but not CWL (Table 2) following weight lifting. Similar to CWL subjects, in the runners and CT group FMD responses were not impaired following a single weight lifting episode (Fig. 1). However, baseline FMD was reduced compared with SED subjects in runners (5.4 ± 0.5%; P < 0.001 vs. SED) and CT (4.4 ± 0.3; P < 0.001 vs. SED). The differences in baseline FMD between groups between before and after exercise within groups were similar when normalized for shear stress (Table 2) since peak shear rates were similar between groups (Table 2). Endothelium-independent dilation to NTG and peak hyperemic flow velocity responses were similar among groups (Table 2). The peak change in flow velocity during reperfusion was similar before and after weight lifting in all groups (Table 2). The effect of exercise group on FMD was maintained with covariate adjustment for brachial diameter, cholesterol, BP, CRP, baseline glucose or insulin, maximum systolic BP, change in BP during exercise, oxidized HDL, or maximal weight lifted.
Table 2.
Hemodynamic characteristics before and after exercise
| SED |
CWL |
Runners |
Cross Trainers |
|||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Before | After | Before | After | Before | After | Before | After |
| Resting HR, beats/min | 59 ± 2 | 72 ± 4* | 55 ± 3 | 65 ± 3 | 63 ± 3 | 60 ± 3 | 57 ± 4 | 65 ± 3.5 |
| SBP, mmHg | 116 ± 2 | 119 ± 2.7 | 119 ± 4 | 118 ± 3 | 115 ± 1 | 115 ± 3 | 117 ± 3 | 119 ± 3 |
| DBP, mmHg | 66 ± 3 | 65 ± 3 | 60 ± 4 | 58 ± 3 | 63 ± 3 | 64 ± 3 | 64 ± 2 | 63 ± 2 |
| Baseline artery diameter, mm | 4.6 ± 0.2 | 4.6 ± 0.2 | 4.6 ± 0.2 | 4.6 ± 0.2 | 4.8 ± 0.3 | 4.7 ± 0.2 | 4.4 ± 0.1 | 4.4 ± 0.1 |
| Brachial artery FMD, % | 8.0 ± 1.0 | 5.7 ± 0.4* | 6.7 ± 0.9 | 8.8 ± 1.0* | 5.4 ± 1.0† | 8.0 ± 0.1* | 4.4 ± 0.3† | 5.4 ± 0.6 |
| Max NTG diameter, mm | 5.52 ± 0.4 | 5.4 ± 0.3 | 5.65 ± 0.2 | 5.2 ± 0.1 | ||||
| NTG dilation, % | 20.6 ± 1.7 | 18.0 ± 1.2 | 19.4 ± 1.3 | 19.53 ± 1.4 | ||||
| Peak velocity, cm/s | 107 ± 3 | 122 ± 8 | 105 ± 7 | 118 ± 6 | 108 ± 8 | 117 ± 10 | 97 ± 4 | 109 ± 5 |
| Peak shear rate, s | 262 ± 12 | 297 ± 22 | 243 ± 26 | 273 ± 28 | 235 ± 16 | 260 ± 21 | 255 ± 16 | 272 ± 10 |
| Normalized FMD, FMD/shear rate | 0.031 ± 0.004 | 0.020 ± 0.003* | 0.033 ± 0.005 | 0.037 ± 0.006 | 0.024 ± 0.003† | 0.0329 ± 0.004* | 0.018 ± 0.001† | 0.020 ± 0.002 |
Data are presented as means ± SE. NTG, nitroglycerin.
Significant difference observed vs. before exertion (P < 0.05).
Significant difference vs. before exercise in SED.
Fig. 1.
Individual subject percent flow-mediated dilation (FMD) responses at pre- and postexercise time points in sedentary (A), conditioned weight lifters (B), runners (C), and cross trainers (D) groups. Mean pre- and post-FMD are represented as means ± SE in each group. *Significant change in responses pre-and post-acute hypertension induced by weight lifting.
Lipids, glucose, and blood markers of inflammation.
There were no differences in total cholesterol and HDL and LDL cholesterol within or among groups before and after weight lifting. Blood glucose was similar between groups (Table 1). In addition, there was no effect of weight lifting on hsCRP a predictive marker of systemic inflammation (mean CRP: SED: 1.8 ± 0.7 mg/dl; CWL: 0.6 ± 0.2 mg/dl; runners: 2.5 ± 0.8 mg/dl; cross-trainers: 0.5 ± 0.1 mg/dl) or pro-inflammatory HDL (pre-WL = 2.0 ± 0.2 vs. post-WL = 2.2 ± 0.2), an index of lipid hydroperoxides (LOOH) in HDL (48) in any group. There was no correlation between CRP or oxidized HDL and FMD.
DISCUSSION
The primary findings of this study are that 1) similar to weight lifters, subjects engaged in regular aerobic exercise (running) are protected against the impaired endothelium-dependent vasodilation that is observed in sedentary subjects following an acute episode of hypertension associated with brief weight lifting; 2) there is no additive protective benefit of weight lifting and running in the cross training group after exertional hypertension; 3) baseline brachial artery FMD is reduced in runners and cross trainers compared with sedentary subjects. These data suggest that elevations in blood pressure during a single resistance exercise session acutely impair endothelial function in sedentary subjects.
The effects of acute exercise on FMD appear to depend on the disease status of the individual and the type of exercise administered. Similar to previous published data (26), SED subjects not performing regular exercise programs demonstrated impaired brachial FMD following acute hypertension induced by a relatively short weight lifting session. This is consistent with other studies that showed reduced FMD after acute aerobic exercise in overweight and obese inactive subjects but increased FMD in active obese subjects (21). In contrast, other studies have shown reduced endothelial function in brachial arteries of hypertensive patients (33) and in radial arteries of healthy subjects (16) immediately following acute hand grip exercise. Regardless, physical inactivity is related to several confounders that are inversely associated to endothelial dysfunction such as adiposity, hypertension, smoking, and elevated cholesterol (4). All of the subjects enrolled in this study were lean with normal BMI and waist circumference measurements (Table 1), reducing the likelihood that abdominal adiposity and hypertension confounded the effects of lower extremity weight lifting on endothelial dysfunction in this group (6). Since our sedentary population included healthy nonsmokers, we were able to carefully control for confounding and complex interactions that may exist between acute hypertension and endothelial function that normally require larger sample sizes. Finally, the exercise mode used (lower extremity weight lifting) involved muscle contractions at a site distant to the area of measure (forearm) allowing us to control for local metabolic effects of exercise on blood flow and arterial diameter (33).
There is a delicate balance between pro- and antioxidant capacity in the vasculature during health and disease. Previous studies implicate C-reactive protein and circulating reactive oxygen species in the inflammatory response to exercise (27, 32). While CRP is known to be detrimental to endothelial function (42) and can be elevated with exercise (45), there was no acute increase in CRP levels after a single bout of resistance exercise in any of the groups (Table 1). The lack in responsiveness of CRP to acute exercise may be related to the relatively short duration of exercise compared with other studies involving longer bouts of athletic competition. Similar to CRP, there was no effect of exercise on pro-inflammatory HDL in SED or exercise groups (36). Interestingly, baseline FMD was lower in RUN and CT groups (Fig. 1), suggesting that reduced NO bioavailability in populations that run >15 miles/wk. In a previous study by Hoch et al. (50), brachial FMD was reduced in elite women runners with amenorrhea compared with control subjects. Since other longitudinal exercise training studies demonstrate improved FMD (7, 33), the cross-sectional design of this study cannot replace the strength of conclusions drawn from longitudinal training studies showing the benefits of exercise training on endothelial function in subjects with disease or differing physiological profiles.
Other studies indicated that there are increased markers of oxidant stress after high-intensity exercise (17, 32). While the specific link between exercise and NO in male athletes is unknown, it is possible that lower baseline FMD is related to the chronic performance of more vigorous bouts of exercise in the groups of CT and runners. Chronic exercise is associated with increased cardiac output and blood flow. Since increased shear has the capacity to upregulate expression of antioxidant (5, 24)- and pro-oxidant (34)-generating enzyme systems in the endothelium, it is also possible that NO bioavailability is reduced after a single weight lifting session. However, shear rates were not different between groups during hyperemia and there was no association between CRP, oxidized HDL, and FMD, arguing against this possible conclusion.
The magnitude of hypertension generated in our studies is sufficient to reduce endothelial function for sustained periods of time (28, 35); however, the duration of reduced FMD after acute weight lifting was not tested in this study. There was no decrease in FMD following exercise in any of our athletic populations (RUN, CT, and CWL; Table 2) arguing against a hypertension-induced endothelial dysfunction in physically active populations. Although the specific mechanism responsible for the protective effect of chronic exercise on endothelial function is not known, it appears that running and weight lifting afford a similar protection against endothelial dysfunction during acute hypertension with physical exertion (Fig. 2). The protective effects of chronic exercise have been ascribed to increases in NO bioavailability since chronic aerobic exercise training improves FMD (47) while reducing NADPH oxidase enzyme expression (1) in patients with coronary disease. Other studies indicate that exercise training improves eNOS (19) and superoxide dismutase (SOD) enzyme expression (44), both of which would improve NO release. In our study, FMD after weight lifting remains significantly lower in CT compared with post weight lifting FMD in CWL and RUN groups (Table 2), suggesting that the capacity to restore endothelial function may be reduced in populations exposed to higher intensities of physical activity. Consistent with the results of our studies, Goto et al. (18) reported less of an increase in endothelial function after high intensity (75% V̇o2max) compared with moderate intensity (50% V̇o2max) exercise training (18). Resistance and endurance training increases ROS generation more than endurance training alone (13). Therefore, future studies should examine the mode and intensity effects of acute exercise on NO-dependent vasodilation in patients with cardiovascular risk factors (i.e., hypertension, hyperlipidemia) known to increase ROS and impair endothelial function.
Fig. 2.
Individual subject systolic blood pressure at pre-and peak exercise time points in sedentary (A), conditioned weight lifters (B), runners (C), and cross trainers (D) groups. Mean pre- and peak SBP are represented as means ± SE in each group. *Indicates significant increase from baseline (P < 0.05).
Study limitations.
There are several limitations of this study. First, we eliminated many of the risk factors with known detrimental effects on endothelium such as high blood pressure and hypercholesterolemia. Since exercise prescriptions are commonly recommended for patients with these cardiovascular profiles, it is difficult to generalize our findings to clinical populations. Second, to eliminate the confounding effects of amenorrhea on endothelium in women athletes, we limited our recruitment to men (50). Future studies should determine if the conditioning effects against acute hypertension induced endothelial dysfunction are similar in women. Third, the cross sectional design of this study prevents us from accounting for the training differences between populations using maximum exercise capacity (V̇o2max) between groups. However, the total exercise time per week (total minutes of physical activity) was greater in the exercise training groups than the SED group (Table 1), suggesting similar levels of time spent participating in physical activity among athletic populations. Fourth, the time course including duration of the training effect of exercise on endothelial protection against acute exertional hypertension is unknown. Fifth, we were unable to test endothelium independent dilation to nitroglycerin before the weight lifting session because of the residual vasodilator effects of this compound on blood pressure during weight lifting. However, all subjects were healthy and absent of cardiovascular risk factors, lowering the risk of impaired vascular function at baseline time points. Sixth, only peak shear rate was assessed. Other studies have shown that alterations of shear rate within a subject may limit and under-represent the normalized FMD compared with area under the curve (AUC) measurements taken until peak diameter (41). Although AUC between cuff release and peak diameter was not measured, results shown in Table 2 suggest that the magnitude change between pre- and postexercise using either normalized FMD or %FMD were similar. Similar to shear, brachial diameter was not calculated continuously throughout the cuff release period. Consistent with other studies (9), the diameter calculations were made at 30 s, 1, 2, and 3 min post-cuff release. However, continuous measurement to identify peak diameter, independent of timing, may provide a more robust measure of FMD (11, 20).
In conclusion, the results reported here suggest that acute hypertension during exertion, induced by heavy weight lifting, impairs endothelial function in sedentary men. The protective effect of chronic exercise against impaired flow-mediated dilation induced by acute weight lifting does not appear to be mode specific, indicating that chronic resistance and aerobic exercise may afford similar protection of the endothelium against exposure to acute stressors (i.e., exertional hypertension) that impair vascular health in sedentary subjects. These results may help to explain the exercise paradox whereby chronic exercise is associated with improved cardiovascular health, whereas acute exercise can be associated with increased risk of cardiovascular events (2). In addition, it is possible that maintained endothelium-dependent vasodilation in conditioned individuals could protect against altered vascular and hemodynamic responses to other physiological perturbations such as acute hypertension, systemic hypoxemia, and hyperglycemia.
GRANTS
This study was supported by the National Institutes of Health General Clinical Research Center (MO1-RR00058); the Cardiovascular Center of the Medical College of Wisconsin; and National Heart, Lung, and Blood Institute Grant K23-HL-85614 (to S. A. Phillips). This project was also supported by the University of Illinois at Chicago Center for Clinical and Translational Science (CCTS), Award Number UL1RR029879 from the National Center For Research Resources (to S. A. Phillips).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Ms. Joan Pleuss (Medical College of Wisconsin), David Marquez [University of Illinois-Chicago (UIC)], Melissa Goslawski (UIC), and Jessica Pipal (UIC) for their expert technical assistance and Weihua Gao (UIC) for statistical assistance.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
REFERENCES
- 1. Adams V, Linke A, Krankel N, Erbs S, Gielen S, Mobius-Winkler S, Gummert JF, Mohr FW, Schuler G, Hambrecht R. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation 111: 555–562, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Bacon SL, Pelletier R, Lavoie KL. The impact of acute and chronic exercise on thrombosis in cardiovascular disease. Thromb Haemost 101: 452–459, 2009 [PubMed] [Google Scholar]
- 4. Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF, Jr, Lehman BT, Fan S, Osypiuk E, Vita JA. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart. Study Circ 109: 613–619, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, Jo H. Shear stress stimulates phosphorylation of eNOS at Ser(635) by a protein kinase A-dependent mechanism. Am J Physiol Heart Circ Physiol 283: H1819–H1828, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Brook RD, Bard RL, Rubenfire M, Ridker PM, Rajagopalan S. Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am J Cardiol 88: 1264–1269, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Clarkson P, Montgomery HE, Mullen MJ, Donald AE, Powe AJ, Bull T, Jubb M, World M, Deanfield JE. Exercise training enhances endothelial function in young men. J Am Coll Cardiol 33: 1379–1385, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Coates RJ, Eley JW, Block G, Gunter EW, Sowell AL, Grossman C, Greenberg RS. An evaluation of a food frequency questionnaire for assessing dietary intake of specific carotenoids and vitamin E among low-income black women. Am J Epidemiol 134: 658–671, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002 [DOI] [PubMed] [Google Scholar]
- 10. De Jongh S, Lilien MR, Bakker HD, Hutten BA, Kastelein JJ, Stroes ES. Family history of cardiovascular events and endothelial dysfunction in children with familial hypercholesterolemia. Atherosclerosis 163: 193–197, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Donald AE, Charakida M, Cole TJ, Friberg P, Chowienczyk PJ, Millasseau SC, Deanfield JE, Halcox JP. Non-invasive assessment of endothelial function: which technique? J Am Coll Cardiol 48: 1846–1850, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Donald AE, Halcox JP, Charakida M, Storry C, Wallace SM, Cole TJ, Friberg P, Deanfield JE. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. J Am Coll Cardiol 51: 1959–1964, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Franzoni F, Ghiadoni L, Galetta F, Plantinga Y, Lubrano V, Huang Y, Salvetti G, Regoli F, Taddei S, Santoro G, Salvetti A. Physical activity, plasma antioxidant capacity, and endothelium-dependent vasodilation in young and older men. Am J Hypertens 18: 510–516, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 30: 777–781, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation 105: 1567–1572, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Gori T, Grotti S, Dragoni S, Lisi M, di Stolfo G, Sonnati S, Fineschi M, Parker JD. Assessment of vascular function: flow-mediated constriction complements the information of flow-mediated dilatation. Heart 96: 141–147, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, Kawamura M, Chayama K, Yoshizumi M, Nara I. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation 108: 530–535, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, Kawamura M, Chayama K, Yoshizumi M, Nara I. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. C R Seances Soc Biol Fil 108: 530–535, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 107: 3152–3158, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris RA, Padilla J, Hanlon KP, Rink LD, Wallace JP. The flow-mediated dilation response to acute exercise in overweight active and inactive men. Obesity (Silver Spring) 16: 578–584, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Harrison DG, Freiman PC, Armstrong ML, Marcus ML, Heistad DD. Alterations of vascular reactivity in atherosclerosis. Circ Res 61: II74–II80, 1987 [PubMed] [Google Scholar]
- 23. Hashimoto M, Akishita M, Eto M, Ishikawa M, Kozaki K, Toba K, Sagara Y, Taketani Y, Orimo H, Ouchi Y. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation 92: 3431–3435, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Inoue N, Ramasamy S, Fukai T, Nerem RM, Harrison DG. Shear stress modulates expression of Cu/Zn superoxide dismutase in human aortic endothelial cells. Circ Res 79: 32–37, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Jambrik Z, Venneri L, Varga A, Rigo F, Borges A, Picano E. Peripheral vascular endothelial function testing for the diagnosis of coronary artery disease. Am Heart J 148: 684–689, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Jurva JW, Phillips SA, Syed AQ, Syed AY, Pitt S, Weaver A, Gutterman DD. The effect of exertional hypertension evoked by weight lifting on vascular endothelial function. J Am Coll Cardiol 48: 588–589, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol 45: 1563–1569, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Lamping KG, Dole WP. Acute hypertension selectively potentiates constrictor responses of large coronary arteries to serotonin by altering endothelial function in vivo. Circ Res 61: 904–913, 1987 [DOI] [PubMed] [Google Scholar]
- 29. Leon AS, Franklin BA, Costa F, Balady GJ, Berra KA, Stewart KJ, Thompson PD, Williams MA, Lauer MS. Cardiac rehabilitation and secondary prevention of coronary heart disease: an American Heart Association scientific statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in collaboration with the American association of Cardiovascular and Pulmonary Rehabilitation. Circulation 111: 369–376, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Li S, Hoskins PR, Anderson T, McDicken WN. Measurement of mean velocity during pulsatile flow using time-averaged maximum frequency of Doppler ultrasound waveforms. Ultrasound Med Biol 19: 105–113, 1993 [DOI] [PubMed] [Google Scholar]
- 31. MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol 58: 785–790, 1985 [DOI] [PubMed] [Google Scholar]
- 32. Mastaloudis A, Morrow JD, Hopkins DW, Devaraj S, Traber MG. Antioxidant supplementation prevents exercise-induced lipid peroxidation, but not inflammation, in ultramarathon runners. Free Radic Biol Med 36: 1329–1341, 2004 [DOI] [PubMed] [Google Scholar]
- 33. McGowan CL, Levy AS, Millar PJ, Guzman JC, Morillo CA, McCartney N, MacDonald MJ. Acute vascular responses to isometric handgrip exercise and effects of training in persons medicated for hypertension. Am J Physiol Heart Circ Physiol 291: H1797–H1802, 2006 [DOI] [PubMed] [Google Scholar]
- 34. McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol 285: H2290–H2297, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Millgard J, Lind L. Acute hypertension impairs endothelium-dependent vasodilation. Clin Sci (Lond) 94: 601–607, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N, Berliner JA, Lusis AJ, Fogelman AM. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res 45: 993–1007, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Patti G, Pasceri V, Melfi R, Goffredo C, Chello M, D'Ambrosio A, Montesanti R, Di SG. Impaired flow-mediated dilation and risk of restenosis in patients undergoing coronary stent implantation. Circulation 111: 70–75, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Phillips SA, Jurva JW, Syed AQ, Syed AQ, Kulinski JP, Pleuss J, Hoffmann RG, Gutterman DD. Benefit of low-fat over low-carbohydrate diet on endothelial health in obesity. Hypertension 51: 376–382, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pollock ML, Franklin BA, Balady GJ, Chaitman BL, Fleg JL, Fletcher B, Limacher M, Pina IL, Stein RA, Williams M, Bazzarre T. AHA Science Advisory. Resistance exercise in individuals with and without cardiovascular disease: benefits, rationale, safety, and prescription: An advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association; Position paper endorsed by the American College of Sports Medicine. Circulation 101: 828–833, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Powell KE, Thompson PD, Caspersen CJ, Kendrick JS. Physical activity and the incidence of coronary heart disease. Annu Rev Public Health 8: 253–287, 1987 [DOI] [PubMed] [Google Scholar]
- 41. Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol 102: 1510–1519, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Qamirani E, Ren Y, Kuo L, Hein TW. C-reactive protein inhibits endothelium-dependent NO-mediated dilation in coronary arterioles by activating p38 kinase and NAD(P)H oxidase. Arterioscler Thromb Vasc Biol 25: 995–1001, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Rothney MP, Schaefer EV, Neumann MM, Choi L, Chen KY. Validity of physical activity intensity predictions by ActiGraph, Actical, and RT3 accelerometers. Obesity (Silver Spring) 16: 1946–1952, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rush JW, Turk JR, Laughlin MH. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. Am J Physiol Heart Circ Physiol 284: H1378–H1387, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Siegel AJ, Stec JJ, Lipinska I, Van Cott EM, Lewandrowski KB, Ridker PM, Tofler GH. Effect of marathon running on inflammatory and hemostatic markers. Am J Cardiol 88: 918–920, A9, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Thompson PD, Funk EJ, Carleton RA, Sturner WQ. Incidence of death during jogging in Rhode Island from 1975 through 1980. JAMA 247: 2535–2538, 1982 [PubMed] [Google Scholar]
- 46a. Vanhoutte P.(editor). Endothelium-Derived Hyperpolarizing Factor. Amsterdam: Harwood Academic, 1996 [Google Scholar]
- 47. Walsh JH, Bilsborough W, Maiorana A, Best M, O'Driscoll GJ, Taylor RR, Green DJ. Exercise training improves conduit vessel function in patients with coronary artery disease. J Appl Physiol 95: 20–25, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Weihrauch D, Xu H, Shi Y, Wang J, Brien J, Jones DW, Kaul S, Komorowski RA, Csuka ME, Oldham KT, Pritchard KA. Effects of d-4F on vasodilation, oxidative stress, angiostatin, myocardial inflammation, and angiogenic potential in tight-skin mice. Am J Physiol Heart Circ Physiol 293: H1432–H1441, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Yoshida T, Kawano H, Miyamoto S, Motoyama T, Fukushima H, Hirai N, Ogawa H. Prognostic value of flow-mediated dilation of the brachial artery in patients with cardiovascular disease. Intern Med 45: 575–579, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Zeni HA, Dempsey RL, Carrera GF, Wilson CR, Chen EH, Barnabei VM, Sandford PR, Ryan TA, Gutterman DD. Is there an association between athletic amenorrhea and endothelial cell dysfunction? Med Sci Sports Exerc 35: 377–383, 2003 [DOI] [PubMed] [Google Scholar]