Abstract
Doxorubicin (Dox) is a potent antitumor agent used in cancer treatment. Unfortunately, Dox is myotoxic and results in significant reductions in skeletal muscle mass and function. Complete knowledge of the mechanism(s) by which Dox induces toxicity in skeletal muscle is incomplete, but it is established that Dox-induced toxicity is associated with increased generation of reactive oxygen species and oxidative damage within muscle fibers. Since muscular exercise promotes the expression of numerous cytoprotective proteins (e.g., antioxidant enzymes, heat shock protein 72), we hypothesized that muscular exercise will attenuate Dox-induced damage in exercise-trained muscle fibers. To test this postulate, Sprague-Dawley rats were randomly assigned to the following groups: sedentary, exercise, sedentary with Dox, or exercise with Dox. Our results show increased oxidative stress and activation of cellular proteases (calpain and caspase-3) in skeletal muscle of animals treated with Dox. Importantly, our findings reveal that exercise can prevent the Dox-induced oxidative damage and protease activation in the trained muscle. This exercise-induced protection against Dox-induced toxicity may be due, at least in part, to an exercise-induced increase in muscle levels of antioxidant enzymes and heat shock protein 72. Together, these novel results demonstrate that muscular exercise is a useful countermeasure that can protect skeletal muscle against Dox treatment-induced oxidative stress and protease activation in skeletal muscles.
Keywords: antioxidants, reactive oxygen species, myocytes
doxorubicin (Dox) is a highly effective antitumor agent widely used in the treatment of solid tumors and hematologic malignancies (3, 6, 11). However, Dox has been shown to induce deleterious effects in several tissues and organs, including skeletal and cardiac muscle (11, 44, 47). For example, cardiac failure as a result of Dox administration can lead to progressive skeletal muscle dysfunction, which can be characterized by diminished exercise capacity and increased fatigability. This dysfunction is attributed to increased reactive oxygen species (ROS) production by the mitochondria. Specifically, it has been proposed that Dox stimulates ROS through mitochondrial NADH dehydrogenase, leading to the generation of a free radical cascade with a potent oxidizing potential (18). Therefore, the clinical use of Dox is limited because of drug-induced toxicity.
Numerous studies have attempted to identify the molecular mechanisms responsible for Dox toxicity. Nevertheless, a complete understanding of the mechanisms responsible for Dox-mediated muscle toxicity remains elusive. One mechanism of Dox toxicity is its ability to generate ROS in cells (39, 42). Specifically, ring B of Dox's four-ring anthracycline structure is an unsubstituted quinone that can readily form a redox cycle with appropriate electron donors and promote superoxide radical production at complex I within the electron transport chain (2, 28). It follows that Dox's generation of ROS could lead to cellular oxidative damage and activation of proteases, leading to catabolic processes.
Disturbed redox signaling in cells has been shown to activate several proteolytic pathways (36). For example, ROS can act as a signaling molecule and contribute to the activation of calpain and caspase-3 (17, 20, 35, 36, 38). Calpain is a calcium-dependent cysteine protease that promotes muscle atrophy by cleaving structural proteins. Increased ROS can lead to elevated levels of cytosolic calcium, which leads to calpain activation (13). Although Dox has been shown to induce calpain activation in cardiomyocytes, its effect on skeletal muscle remains unclear (23).
Furthermore, caspase-3 can also play an important role in muscle protein degradation during a variety of wasting conditions (7). Importantly, abundant evidence suggests that caspase-3 can also be activated by ROS, and several reports reveal that Dox administration promotes caspase-3 activation in cardiomyocytes (4, 15, 42). The impact of Dox administration on caspase-3 activity in skeletal muscle remains unknown.
In addition to Dox-induced activation of calpain and caspase-3, Dox-mediated ROS production in skeletal muscle fibers can potentially accelerate proteolysis via oxidative modification of muscle proteins. Our group has demonstrated that oxidative modification of myofibrillar proteins increases their susceptibility to degradation via calpain and caspase-3. Indeed, myosin, actin, troponin I, and α-actinin were shown to be preferentially degraded by increased levels of oxidation (40).
Endurance exercise has been shown to protect skeletal muscle against a variety of stressors, including oxidative stress (30, 34). In this context, it is established that exercise results in elevated levels of several cytoprotective proteins. For example, exercise has been shown to upregulate antioxidant enzymes and to induce the expression of heat shock protein 72 (HSP72) in skeletal muscle (14, 24, 25, 32, 33, 46). Antioxidant enzymes and HSP72 can protect skeletal muscle against ROS-mediated damage (30, 34, 46). Nonetheless, it is unknown whether exercise training will protect skeletal muscle from the deleterious effects associated with Dox administration, and this forms the basis for the current experiments. On the basis of prior work and preliminary experiments, we hypothesized that exercise training would retard Dox-mediated damage to skeletal muscle by preventing Dox-induced oxidative stress and protease activation. Our results support this prediction, as exercise training increased skeletal muscle antioxidant enzymes and HSP72 and protected against protease activation in Dox-treated animals. These novel findings reveal that endurance exercise is an effective means of preventing Dox-induced skeletal muscle toxicity and suggest that regular exercise could improve patient tolerance to Dox treatment.
METHODS
Experimental Design
Adult 6-mo-old male Sprague-Dawley rats were used in these experiments, which were approved by the Animal Care and Use Committee of the University of Florida. The animals were housed at the University of Florida Animal Care Facility according to guidelines set forth by the Institutional Animal Care and Use Committee. They were were maintained on a 12:12-h reverse light-dark cycle, with food (AIN93 diet) and water provided ad libitum throughout the experimental period. Rats were randomly assigned to one of four experimental groups: 1) sedentary control (Sed, n = 7); 2) exercise-trained, no drug treatment (ExTr, n = 6); 3) sedentary, Dox-treated (SedDox, n = 5); and 4) exercise-trained, Dox-treated (ExTrDox, n = 6).
Animals assigned to exercise groups were habituated to running by increasing durations of treadmill exercise for 5 days (10, 20, 30, 40, 50 min/day on days 1–5). After 2 days of rest, animals performed 5 consecutive days of treadmill exercise for 60 min/day at 30 m/min, 0% grade. This work rate represents an estimated 70% of maximum O2 consumption (19). The ExTrDox animals received Dox hydrochloride (20 mg/kg body wt ip) immediately after the final exercise bout and were killed 24 h later. The SedDox animals received Dox hydrochloride (20 mg/kg body wt ip) 24 h prior to death. Saline was used as the vehicle and the placebo treatment. These doses of Dox are human clinical doses that are pharmacologically scaled for use in rats (4, 16, 48).
At the conclusion of the experimental period, animals in each group were acutely anesthetized with pentobarbital sodium (60 mg/kg ip). After the animals reached a surgical plane of anesthesia, the soleus muscle was removed and immediately frozen in liquid nitrogen and stored at −80°C for subsequent analyses. The animals were subsequently euthanized by removal of the heart. The soleus muscle was used, because the effects of Dox have been shown to be more pronounced in the soleus than in other hindlimb skeletal muscles (9).
Biochemical Analyses
Western blot analysis.
Soleus muscle was homogenized 1:10 (wt/vol) in 5 mM Tris (pH 7.5) and 5 mM EDTA (pH 8.0) with a protease inhibitor cocktail (Sigma) and centrifuged at 1,500 g for 10 min at 4°C. The resulting supernatant (cytosolic) and pellet (noncytosolic) were collected. The pellet was resuspended 1:10 in the homogenization buffer. Soleus protein content was assessed by the method of Bradford (Sigma). The cytosolic fraction was used for the following measurements. Proteins (40 μg) were then separated by PAGE via 4–20% gradient polyacrylamide gels containing 0.1% SDS for ∼1 h at 200 V. After electrophoresis, the proteins were transferred to nitrocellulose membranes via the Criterion system for 90 min at 65 V. To control for protein loading and transfer differences, membranes were stained with Ponceau S (Fig. 1). Ponceau S-stained membranes were scanned and the lanes were quantified (440CF imaging system, Kodak, New Haven, CT) to normalize Western blots to protein loading. Nonspecific sites were blocked for 2 h at room temperature in PBS solution containing 0.05% Tween and 5% nonfat milk. Membranes were then incubated overnight at 4°C with primary antibodies directed against the proteins of interest (see below). After incubation with primary antibodies, membranes were washed extensively with PBS-Tween and then incubated with secondary antibodies (Amersham Biosciences). After the membranes were washed, a chemiluminescence system (GE Healthcare) was used to detect labeled proteins. Membranes were developed using autoradiography film, and images of the film were captured and analyzed using the Kodak 440CF imaging system.
Fig. 1.
Representative Ponceau S-stained membrane was used to control for equal protein loading and transfer differences. Membrane was scanned, and lanes were quantified to normalize Western blots to protein loading. Lane 1, sedentary control (Sed); lane 2, exercise-trained, no treatment (ExTr); lane 3, sedentary, doxorubicin-treated (SedDox); lane 4, exercise-trained, doxorubicin-treated (ExTrDox). MW, molecular weight.
Assessment of protein oxidation via reactive carbonyl derivatives.
Levels of reactive carbonyl derivatives in the myofibrillar protein samples were assessed as an index of the magnitude of protein modification. This was accomplished using an oxidized protein detection kit (Oxyblot, Chemicon, Temecula, CA) as described by the manufacturer. Briefly, the carbonyl groups in the protein side chains of myofibrillar proteins were derivatized to 2,4-dinitrophenylhydrazone (DNP). These DNP-derivatized proteins were separated via PAGE, transferred to a nitrocellulose membrane, and incubated with primary antibody against DNP to detect the presence of myofibrillar oxidation in the samples, as described previously (49).
Oxidative modification of soleus proteins.
Oxidative modification of proteins obtained from soleus muscles was determined by measurement of cellular lipid peroxidation [e.g., total 4-hydroxy-2-nonenal (4-HNE)]. Lipid peroxidation occurs as a response to oxidative stress and produces several biologically active aldehydes. 4-HNE is an unsaturated α,β-hydroxy alkenal generated during lipid peroxidation and reacts with muscle proteins to form a 4-HNE-protein adduct. 4-HNE was probed using a 1:200 dilution of a monoclonal primary antibody (Abcam).
Antioxidant enzyme protein content in soleus muscles.
To assess the effect of exercise and Dox treatment on soleus antioxidant capacity, protein levels of CuZn-superoxide dismutase [SOD1 (∼23 kDa); located in the cytosol and mitochondrial intermembrane space], Mn-superoxide dismutase [SOD2 (∼25 kDa); located in the mitochondrial matrix], glutathione peroxidase [GPX1 (∼23 kDa); mitochondrial-specific], and catalase (∼60 kDa) were determined using Western blots. SOD1 and SOD2 were probed using 1:1,000 dilutions of polyclonal primary antibodies (Santa Cruz Biotechnology). GPX1 was probed using a 1:500 dilution of a polyclonal primary antibody (Santa Cruz Biotechnology), and catalase was probed using a 1:500 dilution of a polyclonal antibody (Abcam).
HSP content in soleus muscle.
To determine the effects of exercise and Dox administration on skeletal muscle HSP content, we measured the content of HSP72 (∼72 kDa) in the soleus. HSP72 was probed using a 1:500 dilution of a monoclonal antibody (Calbiochem).
Proteolytic system activation in skeletal muscle.
Calpain activity in the soleus muscle was determined by assessment of the calpain-to-calpastatin ratio. Calpain (∼80 kDa) protein content was assayed using a 1:500 dilution of a polyclonal primary antibody (Cell Signaling Technology), and calpastatin (∼126 kDa) was assayed using a 1:500 dilution of a polyclonal primary antibody (Santa Cruz Biotechnology). In addition, cleaved (i.e., active) caspase-3 (∼19 kDa) protein content in the soleus muscle was determined using a 1:500 dilution of monoclonal primary antibodies (Cell Signaling Technology). In addition, α-II spectrin (Santa Cruz Biotechnology) calpain-specific cleavage (∼145-kDa cleavage product) and caspase-3-specific cleavage (∼120-kDa cleavage product) were determined using a 1:200 dilution of monoclonal primary antibody to obtain an additional measurement of calpain-1 and caspase-3 activity.
Actin degradation in soleus muscle.
Overall proteolysis in the soleus muscle was determined by measurement of the protein abundance of actin. Actin (∼40 kDa) was probed using a 1:1,000 dilution of a polyclonal primary antibody (Santa Cruz Biotechnology).
Myofibrillar protein isolation and release.
Soleus myofibrillar protein isolation and easily releasable myofilament protein were assayed with a previously described protocol (26, 43). Briefly, soleus muscle was homogenized in buffer A (10 mM Tris-maleate, 2 mM MgCl2, 2 mM EGTA, and 1 mM DTT, with 0.1 M KCl and 1% Triton X-100, pH 7.0). Homogenates were centrifuged (10 min) at 1,500 g and 4°C. The resulting pellet was resuspended in an additional 9 ml of homogenizing buffer and filtered through two layers of gauze for 20 min at 4°C. The resulting supernatants were centrifuged (10 min) at 1,500 g and 4°C, and the pellet was washed twice in buffer B (10 mM Tris-maleate, 2 mM MgCl2, and 2 mM EGTA, with 0.1 M KCl, pH 7.0). Released myofilaments were extracted from the myofibrillar protein fraction by 10 passages through a Pasteur pipette in 1.5 ml of buffer C (10 mM Tris-maleate, 2 mM MgCl2, 2 mM EGTA, mM 5.0 ATP, and 1.0 mM DTT, with 0.1 M KCl, pH 7.0). Samples were then layered over 0.75 ml of buffer D (10 mM Tris-maleate, 2 mM MgCl2, 2 mM EGTA, and 1.0 mM DTT, with 0.1 M KCl and 20% glycerol, pH 7.0) in a conical tube and subsequently centrifuged (10 min) at 1,500 g and 4°C. The final supernatant containing the released myofilaments was collected, and the pellet containing the residual myofibrillar protein fraction was resuspended in buffer D. Proteins from both fractions were then assayed by the Bradford method (Sigma). Myofibrillar protein (released myofibrillar protein + intact myofibrillar protein) was corrected for muscle weight and expressed as myofibrillar protein concentration (μg myofibrillar protein/mg diaphragm muscle). Released myofilaments were expressed as a percentage of the combined amount of protein in the two fractions.
Data Analyses
Data are presented as means ± SE. Comparisons between groups for each dependent variable were made by a one-way ANOVA; when appropriate, Tukey's honestly significantly difference tests were performed post hoc. Significance was established at P < 0.05.
RESULTS
All animals in the exercise groups completed the exercise protocol without incident, with no noticeable differences in exercise performance and no apparent complications.
Dox Administration Increases Oxidative Modification of Muscle Proteins
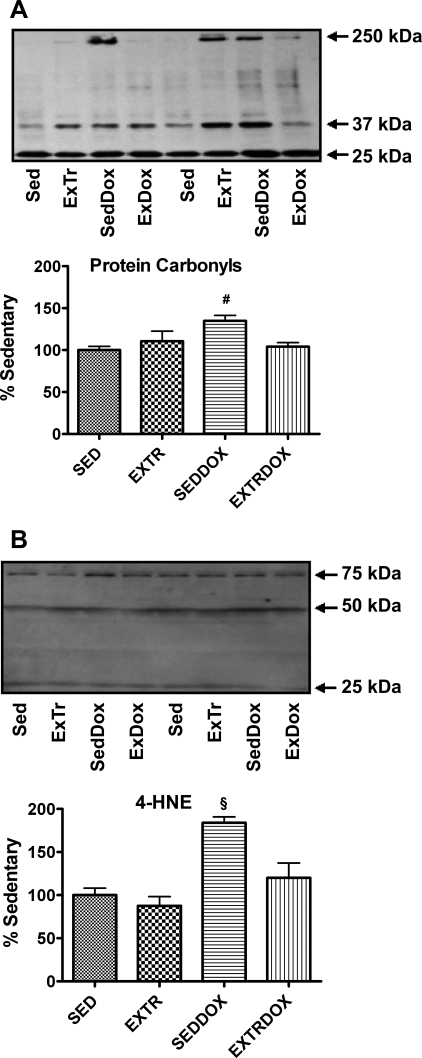
Oxidative modification of cellular proteins can occur when the rate of ROS production exceeds the antioxidant capacity. The levels of reactive carbonyl derivatives in myofibrillar protein samples were assessed as an index of protein modification, and the conjugation of 4-HNE to proteins was used as a second biomarker of oxidative damage. Our results indicate significantly higher levels of soleus muscle protein carbonyls in SedDox than Sed and ExTrDox animals (Fig. 2A) and significantly higher levels of soleus muscle 4-HNE protein conjugates in the SedDox group than all other treatment groups (Fig. 2B). However, there were no differences in 4-HNE accumulation in the soleus muscle between the Sed, ExTr, and ExTrDox groups. Together, these results indicate that although Dox administration promotes oxidative damage to the soleus muscle, several bouts of endurance exercise result in a protective phenotype in muscle that resists Dox-induced oxidative damage.
Fig. 2.
Protein carbonyls (A) and 4 hydroxy-2-nonenal (4-HNE; B) in soleus muscle were analyzed as indicators of oxidative damage. Representative Western blots are shown above graphs. Values (means ± SE) represent percent change. #Significantly higher than Sed and ExTrDox. §Significantly higher than Sed, ExTr, and ExTrDox.
Exercise Training Promotes an Increase in Antioxidant Enzyme Activity in Skeletal Muscle
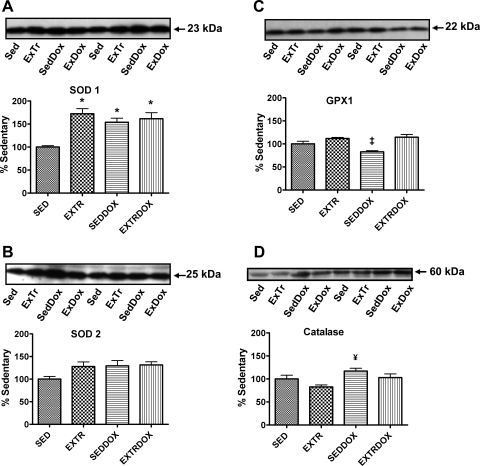
Mammalian cells contain a network of enzymatic and nonenzymatic antioxidants that serve as a protective barrier against ROS-induced damage. Primary cellular enzymatic antioxidants include SOD, GPX, and catalase (34), and each of these enzymes was assessed to determine the effect of exercise and Dox treatment on the antioxidant capacity of the soleus muscle. Protein levels of soleus SOD1 were significantly higher in all experimental groups than in Sed animals (Fig. 3A). Although the levels of SOD2 tended to be higher in all experimental groups than in Sed animals, these levels did not reach significance (Fig. 3B). Furthermore, the GPX1 protein abundance in the soleus was significantly higher in ExTr and ExTrDox than SedDox animals (Fig. 3C). Finally, catalase levels in the soleus muscle were significantly greater in SedDox than ExTr animals (Fig. 3D).
Fig. 3.
Protein expression of primary antioxidants in soleus muscle. Representative Western blots are shown above graphs. Values (means ± SE) represent percent change. A: protein levels of CuZn-SOD (SOD1). *Significantly higher than Sed. B: protein levels of Mn-SOD (SOD2). C: protein levels of glutathione peroxidase 1 (GPX1). ‡Significantly lower than ExTr and ExTrDox. D: protein levels of catalase. #Significantly higher than ExTr.
HSP72 Is Reduced Following Dox Administration
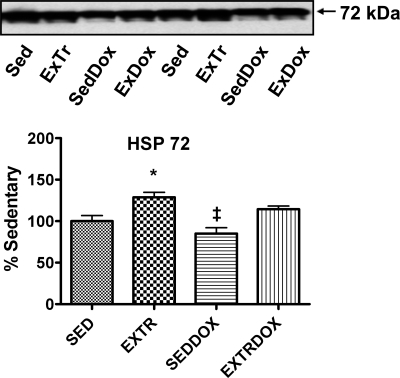
Previous work indicates that endurance exercise promotes HSP72 expression in skeletal muscles (29), and our results verify that exercise resulted in increased HSP72 in the soleus muscle. Specifically, HSP72 protein levels in the soleus muscle were higher in ExTr than Sed and SedDox animals. Finally, soleus levels of HSP72 were elevated (P < 0.05) in the ExTrDox group compared with the SedDox group (Fig. 4).
Fig. 4.
Heat shock protein 72 (HSP72) content in soleus muscle. Representative Western blots are shown above graphs. Values (means ± SE) represent percent change. ‡Significantly lower than ExTr and ExTrDox. *Significantly higher than Sed.
Proteolytic Systems Are Activated in Soleus Muscle Following Dox Administration
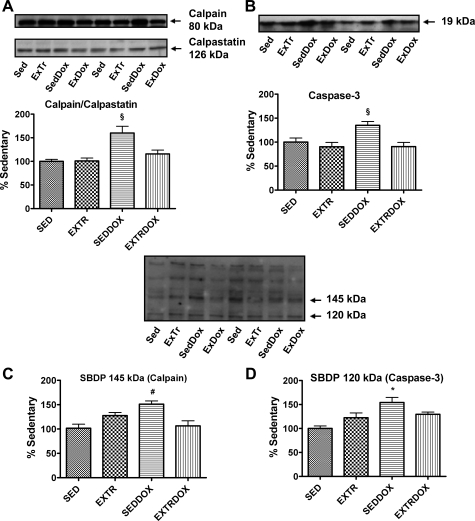
To determine the effects of Dox administration on protease activation in the soleus muscle, we employed Western blotting to determine the activity of calpain and caspase-3. In regard to calpain, the calpain-to-calpastatin ratio compares the expression of the protease calpain with its endogenous inhibitor calpastatin. In general, an increase in the calpain-to-calpastatin ratio indicates that calpain is active in cells (8). Our data revealed that the calpain-to-calpastatin ratio in the soleus muscle was higher in the SedDox group than all other groups (Fig. 5A). Furthermore, active (i.e., cleaved) caspase-3 was significantly higher in the soleus muscle of the SedDox group than all other groups (Fig. 5B). In addition, assessment of calpain and caspase-3 activity via spectrin-specific cleavage indicated that calpain and caspase-3 activity was increased in the SedDox animals (Fig. 5, C and D).
Fig. 5.
Calpain and caspase-3 activation in soleus muscle. Representative Western blots are shown above graphs. Values (means ± SE) represent percent change. A: calpain-to-calpastatin ratio as an indicator of calpain activity. §Significantly higher than Sed, ExTr, and ExTrDox. B: cleaved caspase-3. §Significantly higher than Sed, ExTr, and ExTrDox. C: α-II spectrin calpain-specific cleavage (SBDP, 145 kDa). #Significantly higher than Sed and ExTrDox. D: α-II spectrin caspase-3-specific cleavage (SBDP, 120 kDa). *Significantly higher than Sed.
Exercise Protects Against Dox-Induced Proteolysis in Soleus Muscle
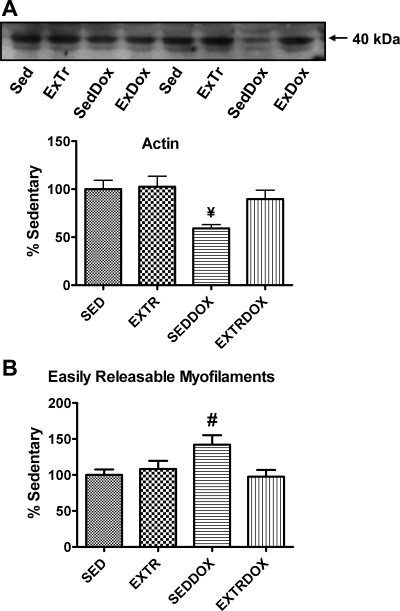
During skeletal muscle wasting, the loss of sarcomeric actin occurs at a rapid rate; therefore, actin disappearance can be used as a general marker of proteolysis in skeletal muscle (22). Hence, after demonstrating that Dox increases the activity of calpain and caspase-3 in soleus muscle, we measured the relative abundance of the contractile protein actin as an index of muscle proteolysis. Our results reveal that actin levels were significantly lower in soleus muscles from SedDox than Sed and ExTr animals. Importantly, exercise training attenuated this Dox-induced degradation of actin in the soleus muscle (Fig. 6A). In addition to use of actin as a marker of proteolysis, the percentage of released myofilament protein is a biomarker of myofilament release and, therefore, can be viewed as an index of proteolysis (35, 36). Our results reveal that compared with Sed animals, the percentage of easily released myofilament protein increased in the SedDox group and that this increase was attenuated by exercise (Fig. 6B).
Fig. 6.
Protein abundance of actin (A) and easily releasable myofilaments (B) as indicators of soleus muscle proteolysis. Representative Western blots for actin are shown above graph. Values (means ± SE) represent percent change. #Significantly lower than ExTr. #Significantly higher than ExTr and ExTrDox.
DISCUSSION
Overview of Principal Findings
These experiments provide new and important information regarding the effects of exercise training on the prevention of Dox-induced cytotoxicity in skeletal muscle. We hypothesized that exercise training would have cytoprotective effects on skeletal muscle and reduce the toxic side effects of Dox. Our results support this prediction, as exercise promoted an increase in muscle levels of antioxidants and HSP72 and, importantly, attenuated Dox-induced oxidative damage and protease activation in the trained skeletal muscle.
Exercise Protects Against Dox-Induced Oxidative Damage
Human and animal experiments demonstrate that Dox administration can promote skeletal muscle weakness. For example, patients treated with Dox experience muscle weakness, as indicated by reduced maximal handgrip strength and a faster rate of handgrip fatigue (41). A recent report indicates that systemic Dox administration at clinical doses depresses specific force production of rat skeletal muscle (12).
Dox-induced cellular dysfunction is proposed to result from the oxidation of cellular components via elevated ROS production. Dox causes cytotoxicity by generating ROS through redox cycling within the mitochondrial electron transport chain (45). To determine if exercise training can attenuate Dox-induced oxidative damage in skeletal muscle, we measured 4-HNE protein conjugates in the soleus muscle of our experimental groups. Our results clearly indicate that Dox administration increases oxidative damage in skeletal muscle of sedentary animals and that exercise training is a practical intervention to attenuate this problem.
Although our findings show that exercise is highly protective against Dox-induced oxidative damage in skeletal muscle, our results do not identify the specific mechanism behind this protection. Nonetheless, it seems likely that the exercise-mediated protection against Dox-induced oxidative damage in skeletal muscle could occur for the following reasons: 1) lower mitochondrial ROS production in trained muscle fibers, 2) increased detoxification/removal of ROS, or 3) some combination of reasons 1 and 2. In reference to the removal of ROS in muscle fibers, the first line of defense against superoxide radicals is SOD (34). In mammalian cells, SOD exists in two isoforms (i.e., SOD1 and SOD2). Both isoforms offer defense against ROS, as each promotes the dismutation of the superoxide radical to form hydrogen peroxide and oxygen. Importantly, research shows that exercise can induce SOD expression in skeletal muscle, which can provide cellular protection against ROS (5, 21, 32, 33). Our data suggest that exercise-induced increases in SOD1 could be a contributory factor in retarding Dox-induced myocyte injury. In reference to increases in muscle levels of SOD in the SedDox group, it appears likely that Dox treatment and the associated increase in cellular ROS production act as an upstream signal to increase the expression of SOD as a defense mechanism to protect against the increase in cellular superoxide production.
GPX is another important cellular antioxidant enzyme that catalyzes the reduction of hydrogen peroxide or hydroperoxide to water and alcohol. Previous studies have demonstrated that endurance exercise training promotes an increase in GPX activity in skeletal muscle, and our results are consistent with these findings (5, 21, 32, 33). In the present study, we measured a mitochondrial-specific isoform of GPX (i.e., GPX1) and found that GPX1 levels increased in the soleus muscle of both groups of exercised animals (i.e., ExTr and ExTrDox). Hence, it is feasible that exercise-induced increases in GPX1 in the soleus muscle may also contribute to exercise-induced protection against Dox-induced oxidative stress in skeletal muscles.
Exercise Increases HSP72 Levels in Skeletal Muscle
Numerous studies reveal cellular upregulation of HSP72 in skeletal muscle in response to a variety of stresses (10, 29, 31, 46). This is an important physiological adaptation, because HSP72 plays a key role in the correct folding of proteins and in the repair of damaged proteins (37). It is well documented that exercise can increase HSP72 expression in skeletal muscle, and our results indicate that our exercise training protocol was sufficient to increase HSP72 expression in the soleus muscle of our experimental animals. It follows that the exercise-induced upregulation of HSP72 may play a role in protecting the soleus muscle from Dox toxicity. Specifically, upregulation of HSP72 can contribute to the preservation of mitochondrial integrity, protection against apoptosis, prevention against lipid peroxidation, and maintenance of calcium handling (1). Finally, our data also reveal that Dox administration reduces the levels of HSP72 in the soleus muscle. This Dox-induced decline in muscle levels of HSP72 could impair the ability of the fibers to defend against Dox-mediated toxicity.
Exercise Protects Against Dox-Induced Protease Activation in Skeletal Muscle
Administration of Dox has been reported to reduce muscle cross-sectional area (27). This Dox-induced skeletal muscle atrophy is likely due, in part, to increased protease activity. Indeed, skeletal muscle fibers contain a network of proteases, including calpain and caspase-3 (35). Therefore, we investigated the possible role of calpain and caspase-3 in skeletal muscle following Dox treatment. Our findings reveal that Dox administration increases the activity of calpain and caspase-3 in the soleus muscle. Importantly, our findings also reveal that exercise training can attenuate the Dox-induced activation of calpain and caspase-3 in skeletal muscle. On the basis of our finding that exercise protects muscle fibers against Dox-induced oxidative damage, it seems likely that exercise training attenuates Dox-induced skeletal muscle protease activation by preserving redox homeostasis.
Conclusions
In summary, our study provides the first evidence that exercise training is an effective intervention against Dox-induced oxidative injury and protease activation in skeletal muscle. The mechanism(s) to explain this protection against Dox-induced muscle damage is likely linked to the exercise-induced increase in muscle levels of HSP72 and antioxidants that can protect muscle fibers against oxidative damage and protease activation. The present work using an animal model provides the experimental rationale for future translational studies to determine if regular aerobic exercise can protect human skeletal muscle against Dox-induced muscle wasting.
GRANTS
This work was supported by an award from the American Heart Association (A. N. Kavazis) and National Institutes of Health Grant R01 HL-067855 (S. K. Powers).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Ascensao A, Magalhaes J, Soares JM, Ferreira R, Neuparth MJ, Marques F, Oliveira PJ, Duarte JA. Moderate endurance training prevents doxorubicin-induced in vivo mitochondriopathy and reduces the development of cardiac apoptosis. Am J Physiol Heart Circ Physiol 289: H722–H731, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29: 222–230, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Carter SK. Adriamycin—a review. J Natl Cancer Inst 55: 1265–1274, 1975 [DOI] [PubMed] [Google Scholar]
- 4. Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome c release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res 62: 4592–4598, 2002 [PubMed] [Google Scholar]
- 5. Criswell D, Powers S, Dodd S, Lawler J, Edwards W, Renshler K, Grinton S. High intensity training-induced changes in skeletal muscle antioxidant enzyme activity. Med Sci Sports Exerc 25: 1135–1140, 1993 [PubMed] [Google Scholar]
- 6. Doroshow JH, Tallent C, Schechter JE. Ultrastructural features of adriamycin-induced skeletal and cardiac muscle toxicity. Am J Pathol 118: 288–297, 1985 [PMC free article] [PubMed] [Google Scholar]
- 7. Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, Price SR, Mitch WE. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest 113: 115–123, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Enns D, Karmazyn M, Mair J, Lercher A, Kountchev J, Belcastro A. Calpain, calpastatin activities and ratios during myocardial ischemia-reperfusion. Mol Cell Biochem 241: 29–35, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Ertunc M, Sara Y, Korkusuz P, Onur R. Differential contractile impairment of fast- and slow-twitch skeletal muscles in a rat model of doxorubicin-induced congestive heart failure. Pharmacology 84: 240–248, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Febbraio MA, Steensberg A, Walsh R, Koukoulas I, van Hall G, Saltin B, Pedersen BK. Reduced glycogen availability is associated with an elevation in HSP72 in contracting human skeletal muscle. J Physiol 538: 911–917, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferrans VJ. Overview of cardiac pathology in relation to anthracycline cardiotoxicity. Cancer Treat Rep 62: 955–961, 1978 [PubMed] [Google Scholar]
- 12. Gilliam LA, Ferreira LF, Bruton JD, Moylan JS, Westerblad H, St, Clair DK, Reid MB. Doxorubicin acts through tumor necrosis factor receptor subtype 1 to cause dysfunction of murine skeletal muscle. J Appl Physiol 107: 1935–1942, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev 83: 731–801, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Jackson MJ. Redox regulation of skeletal muscle. IUBMB Life 60: 497–501, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Jang YM, Kendaiah S, Drew B, Phillips T, Selman C, Julian D, Leeuwenburgh C. Doxorubicin treatment in vivo activates caspase-12 mediated cardiac apoptosis in both male and female rats. FEBS Lett 577: 483–490, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Kang YJ, Chen Y, Yu A, Voss-McCowan M, Epstein PN. Overexpression of metallothionein in the heart of transgenic mice suppresses doxorubicin cardiotoxicity. J Clin Invest 100: 1501–1506, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol Cell Physiol 275: C1–C24, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Kowaltowski AJ, Vercesi AE. Mitochondrial damage induced by conditions of oxidative stress. Free Radic Biol Med 26: 463–471, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Lawler JM, Powers SK, Hammeren J, Martin AD. Oxygen cost of treadmill running in 24-mo-old Fischer-344 rats. Med Sci Sports Exerc 25: 1259–1264, 1993 [PubMed] [Google Scholar]
- 20. Leeuwenburgh C. Role of apoptosis in sarcopenia. J Gerontol A Biol Sci Med Sci 58: 999–1001, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Leeuwenburgh C, Hollander J, Leichtweis S, Griffiths M, Gore M, Ji LL. Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am J Physiol Regul Integr Comp Physiol 272: R363–R369, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Li W, Moylan JS, Chambers MA, Smith J, Reid MB. Interleukin-1 stimulates catabolism in C2C12 myotubes. Am J Physiol Cell Physiol 297: C706–C714, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lim CC, Zuppinger C, Guo X, Kuster GM, Helmes M, Eppenberger HM, Suter TM, Liao R, Sawyer DB. Anthracyclines induce calpain-dependent titin proteolysis and necrosis in cardiomyocytes. J Biol Chem 279: 8290–8299, 2004 [DOI] [PubMed] [Google Scholar]
- 24. McArdle A, Jackson MJ. Exercise, oxidative stress and ageing. J Anat 197: 539–541, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McArdle A, Pattwell D, Vasilaki A, Griffiths RD, Jackson MJ. Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am J Physiol Cell Physiol 280: C621–C627, 2001 [DOI] [PubMed] [Google Scholar]
- 26. McClung JM, Whidden MA, Kavazis AN, Falk DJ, Deruisseau KC, Powers SK. Redox regulation of diaphragm proteolysis during mechanical ventilation. Am J Physiol Regul Integr Comp Physiol 294: R1608–R1617, 2008 [DOI] [PubMed] [Google Scholar]
- 27. McLoon LK, Falkenberg JH, Dykstra D, Iaizzo PA. Doxorubicin chemomyectomy as a treatment for cervical dystonia: histological assessment after direct injection into the sternocleidomastoid muscle. Muscle Nerve 21: 1457–1464, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Mukhopadhyay P, Rajesh M, Yoshihiro K, Hasko G, Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun 358: 203–208, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Naito H, Powers SK, Demirel HA, Aoki J. Exercise training increases heat shock protein in skeletal muscles of old rats. Med Sci Sports Exerc 33: 729–734, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Noble EG, Milne KJ, Melling CW. Heat shock proteins and exercise: a primer. Appl Physiol Nutr Metab 33: 1050–1065, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Poso AR, Eklund-Uusitalo S, Hyyppa S, Pirila E. Induction of heat shock protein 72 mRNA in skeletal muscle by exercise and training. Equine Vet J Suppl September: 214–218, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Powers SK, Criswell D, Lawler J, Ji LL, Martin D, Herb RA, Dudley G. Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol 266: R375–R380, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Powers SK, Criswell D, Lawler J, Martin D, Ji LL, Herb RA, Dudley G. Regional training-induced alterations in diaphragmatic oxidative and antioxidant enzymes. Respir Physiol 95: 227–237, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88: 1243–1276, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol 288: R337–R344, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J Appl Physiol 102: 2389–2397, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Powers SK, M LA, Demirel HA. Exercise, heat shock proteins, and myocardial protection from I-R injury. Med Sci Sports Exerc 33: 386–392, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Primeau AJ, Adhihetty PJ, Hood DA. Apoptosis in heart and skeletal muscle. Can J Appl Physiol 27: 349–395, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Singal PK, Li T, Kumar D, Danelisen I, Iliskovic N. Adriamycin-induced heart failure: mechanism and modulation. Mol Cell Biochem 207: 77–86, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Smuder AJ, Kavazis AN, Hudson MB, Nelson WB, Powers SK. Oxidation enhances myofibrillar protein degradation via calpain and caspase-3. Free Radic Biol Med 49: 1152–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stone P, Hardy J, Broadley K, Tookman AJ, Kurowska A, A'Hern R. Fatigue in advanced cancer: a prospective controlled cross-sectional study. Br J Cancer 79: 1479–1486, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis 49: 330–352, 2007 [DOI] [PubMed] [Google Scholar]
- 43. van der Westhuyzen DR, Matsumoto K, Etlinger JD. Easily releasable myofilaments from skeletal and cardiac muscles maintained in vitro. Role in myofibrillar assembly and turnover. J Biol Chem 256: 11791–11797, 1981 [PubMed] [Google Scholar]
- 44. van Norren K, van Helvoort A, Argiles JM, van Tuijl S, Arts K, Gorselink M, Laviano A, Kegler D, Haagsman HP, van der Beek EM. Direct effects of doxorubicin on skeletal muscle contribute to fatigue. Br J Cancer 100: 311–314, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wallace KB. Adriamycin-induced interference with cardiac mitochondrial calcium homeostasis. Cardiovasc Toxicol 7: 101–107, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Yamada P, Amorim F, Moseley P, Schneider S. Heat shock protein 72 response to exercise in humans. Sports Med 38: 715–733, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Yamamoto Y, Hoshino Y, Ito T, Nariai T, Mohri T, Obana M, Hayata N, Uozumi Y, Maeda M, Fujio Y, Azuma J. Atrogin-1 ubiquitin ligase is upregulated by doxorubicin via p38-MAP kinase in cardiac myocytes. Cardiovasc Res 79: 89–96, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Yen HC, Oberley TD, Vichitbandha S, Ho YS, St. Clair DK. The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J Clin Invest 98: 1253–1260, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zergeroglu MA, McKenzie MJ, Shanely RA, Van Gammeren D, DeRuisseau KC, Powers SK. Mechanical ventilation-induced oxidative stress in the diaphragm. J Appl Physiol 95: 1116–1124, 2003 [DOI] [PubMed] [Google Scholar]