Abstract
The objective of this study was to evaluate the influence of calorie restriction (CR) on free-living physical activity levels among humans. Data were from three CALERIE phase I site-specific protocols. Participants were nonobese (body mass index = 23.5–29.9 kg/m2) adults randomly assigned to 25% CR, low-calorie diet (LCD, 890 kcal/day supplement diet until 15% weight loss, then weight maintenance), or control at Pennington Biomedical Research Center (PBRC); 30% or 10% CR at Tufts University; and 20% CR or control at Washington University School of Medicine (WUSM). Activity was measured at months 0, 3, and 6 (PBRC) and at months 0, 3, 6, 9, and 12 (WUSM and Tufts). Total daily energy expenditure (TEE) by doubly labeled water and resting metabolic rate (RMR) were used to compute activity energy expenditure: AEE = TEE − RMR − 0.1 * TEE. Accelerometry and 7-day recall categorized activities by intensity. At Tufts, the 10% and 30% CR groups experienced significant decreases in AEE at months 6, 9, and 12. At month 6, a larger decrease in AEE was observed in the CR than the control group at WUSM. At months 3 and 6, larger decreases in AEE were observed in the CR and LCD groups than the control group at PBRC. Accelerometry and 7-day PAR did not consistently detect changes in activity categories. CR-associated changes in AEE were variable but, generally, reduced the energy deficit, which would reduce the expected rate of weight loss. Accelerometry and recall did not consistently explain reduced AEE, suggesting that increased muscle efficiency and/or decreased fidgeting accounted for decreased AEE. Inaccuracy of accelerometry and recall also likely negatively affected sensitivity.
Keywords: accelerometry, exercise, activity energy expenditure
energy expended during physical activity is the most variable component of energy expenditure (39) and is influenced by the amount of physical activity performed and muscle work efficiency. During semistarvation in controlled experimental conditions, physical activity energy expenditure (AEE) is reduced in humans and energy is conserved (13), but the effect of less severe nutritionally adequate calorie restriction (CR) on physical activity levels and AEE is unclear. CR has been shown to extend lifespan in a variety of species (37), and the ongoing “CALERIE” (Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy) study is testing the effect of CR on biomarkers of longevity in nonobese humans. The beneficial health effects of CR and weight loss among overweight and obese individuals are well documented. Understanding the effect of CR on physical activity is important, since decreased AEE would conserve energy, slow the rate of weight loss, contribute to weight regain or the inability to maintain weight loss, and potentially confound the effects of CR on health outcomes.
Studies conducted in the confined quarters of respiratory chambers most frequently find that spontaneous physical activity (SPA) does not change during calorie restriction. SPA refers to the energy cost of fidgeting and changing posture and is reliably measured in respiratory chambers with radar motion detectors (25). Most (15–17, 24), but not all (6), studies indicate that SPA does not change in response to CR, which is consistent with the theory that SPA is biologically regulated (16, 40). Nevertheless, the confined quarters of respiratory chambers, where SPA is measured, can limit physical activity and reduce the ability to detect changes. Indeed, levels of physical activity are lower in respiratory chambers than in free-living conditions (28), demonstrating the need to measure activity levels in free-living conditions during CR.
Relatively few studies have investigated the effect of CR on free-living physical activity levels and AEE because of the cost and burden of measuring physical activity accurately in participants' natural environment. Furthermore, many of the findings are contradictory. No changes in posture allocation (time spent lying or sitting vs. standing or ambulating) were observed when obese people lost weight (16), and other studies only found trends toward decreases in physical activity among nonobese weight-reduced men (12, 35). In contrast, other groups found a decrease in nonresting energy expenditure after weight loss (27) or a decrease in physical activity during a CR diet, with inclusion of exercise training during CR preventing the decline in AEE (20, 26).
The purpose of this analysis within the CALERIE phase I randomized controlled trials was to test the effect of CR on physical activity measured in a free-living state. Data were collected at the three sites [Pennington Biomedical Research Center (PBRC), Tufts University, and Washington University School of Medicine (WUSM)] conducting the trials. Results of the primary end points have been reported previously (4, 11, 23). In this analysis, we define physical activity using two complementary approaches by analyzing measures that rely on energy expenditure, as well as measures that rely on accelerometry and self-report (34). These physical activity definitions allow us to analyze two types of end points: 1) the energy (kcal) expended in activity and 2) the time spent engaging in different categories of activity. This is one of the first studies to test the effect of CR on the physical activity of nonobese participants using measures of energy expenditure and to attempt to quantify with accelerometry and a standardized physical activity recall (PAR) interview the aspects of activity that change in response to CR. We hypothesized that the duration and intensity of physical activity would decrease in response to CR and that accelerometers and the PAR interview would identify the types (intensities) of physical activity that changed in response to CR.
METHODS
Participants
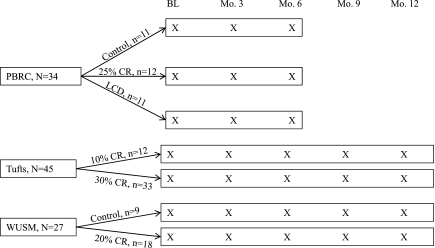
The study designs at each site are illustrated in Fig. 1. Participants from all sites were nonsmoking adults who were free of chronic disease, with body mass index between 25.0 and 29.9 kg/m2 at PBRC and Tufts and between 23.5 and 29.9 kg/m2 at WUSM. Age range was 25–49 yr (men) and 25–44 yr (women) at PBRC, 24–42 yr at Tufts, and 50–60 yr at WUSM. Participants who, at baseline, exercised ≥2 day/wk (PBRC and WUSM) or reported exercising ≥12 h/wk (Tufts) were excluded. Study protocols were approved by the sites' respective Institutional Review Boards, and participants provided written informed consent.
Fig. 1.
Design of the CALERIE studies at Pennington Biomedical Research Center (PBRC), Tufts University (Tufts), and Washington University School of Medicine (WUSM). Activity was measured at the time points denoted with X. BL, baseline; CR, calorie restriction; LCD, low-calorie diet.
Interventions
Data from participants randomly assigned to CR and control groups are reported here. Although the studies at PBRC and WUSM also included exercise interventions, data from these groups are not included in the present report. At all sites, the level of CR (e.g., 25%) for each participant was calculated from baseline energy requirements, which were determined by measuring total daily energy expenditure for two consecutive 14-day periods (28 total days of measurement) with doubly labeled water (DLW) during weight stability.
Data were collected from March 2002 to August 2004 at PBRC. Participants assigned to the CR (n = 12) group were prescribed a 25% CR diet based on baseline energy requirements; the low-calorie diet (LCD, n = 11) group was prescribed an 890 kcal/day liquid supplement diet until 15% weight loss was achieved, followed by a weight-maintenance diet. The control group (n = 11) was prescribed a healthy weight-maintenance diet. Food was provided to participants during the first 12 wk and the last 2 wk of the intervention (weeks 22–24). Participants ate a self-selected diet during weeks 13–22. A detailed meal plan and calorie target were provided, and the participants attended weekly group sessions to foster adherence during the self-selected diet phase.
Data were collected from November 2002 to January 2005 at Tufts University. Participants were randomly assigned to a 30% CR (n = 33) high-glycemic load (HG) or low-glycemic load (LG) diet or a 10% CR (n = 12) HG or LG diet. The HG diet consisted of 60% carbohydrate, 20% protein, and 20% fat, and the LG diet consisted of 40% carbohydrate, 30% protein, and 30% fat. Adherence to the calorie prescription and diets was fostered by providing food to participants for the first 6 mo of the study and with weekly individual or group meetings. As previously reported (4), weight loss, hunger, and satiety did not differ significantly between the HG and LG diets; therefore, data from these two groups were combined for this report.
Data were collected from February 2003 to February 2005 at WUSM. Participants assigned to the CR group (n = 18) were prescribed a 16% CR diet for the first 3 mo of the intervention and then a 20% CR diet throughout the remaining 9 mo. The 16% and 20% CR diets were calculated from baseline energy requirements. Food was provided for 5 consecutive days at months 1 and 3, and the participants attended sessions led by a registered dietitian and behavioral psychologist throughout the study to foster adherence to the calorie target. Participants assigned to the healthy-lifestyle/control group (n = 9) were offered general health information and yoga classes but did not receive diet prescriptions.
Measures
No single measure of physical activity adequately quantifies all aspects of activity; therefore, multiple and complementary methods should be used (34). In this study, two complementary approaches were used to define and quantify physical activity, measures based on energy expenditure and measures that rely on accelerometry and self-report (34), resulting in two types of end points: 1) the energy (kcal) expended in activity and 2) the time spent in different categories of activity (activity categories were based on activity intensity). The procedures for quantifying physical activity using this framework are provided here.
Resting metabolic rate.
At all three sites, resting metabolic rate (RMR) was measured in a thermoneutral condition after participants fasted overnight (participants resided in an inpatient unit during this time). At PBRC and WUSM, RMR was measured by indirect calorimetry using a metabolic cart (Deltatrac II, Datex-Ohmeda, Helsinki, Finland) over a 60-min period at the time points noted in Fig. 1. After 20 min of quiet rest, a transparent plastic hood connected to the device was placed over the head of the participant. O2 consumption and CO2 production were calculated from continuous measurements of O2 and CO2 concentrations in inspired and expired air diluted with a constant airflow (40 l/min) generated by the metabolic cart. Participants remained motionless and awake during the test, and the last 30 min of the measurement were used to calculate RMR.
At Tufts University, RMR was measured by indirect calorimetry using a portable metabolic cart (Deltatrac, Sensor Medics, Yorba Linda, CA). While the participants rested in a supine position, O2 consumption and CO2 production were measured for 40 min, and the last 30 min of data were used to calculate RMR using Weir's equation (38).
Total daily energy expenditure.
As described previously (4, 11, 23), total daily energy expenditure (TEE) was measured with DLW for two consecutive 2-wk periods at baseline to measure baseline energy requirements and for 2-wk periods at months 3 and 6 (PBRC) and at months 3, 6, 9, and 12 (WUSM and Tufts University). Briefly, participants provided urine samples before being dosed with 2.0 g of 10% enriched H218O and 0.12 g of 99.9% enriched 2H2O per kilogram of estimated total body water. Urine samples were also collected at 6 h, 7 days, and 14 days after dosing. Each sample was analyzed for 18O and 2H abundance by isotope ratio mass spectrometry using automated devices for 2H (H/Device, Finnigan) and 18O (GasBench, Finnigan) in the Mass Spectrometry Laboratory at PBRC (7). We assumed a food quotient of 0.86, although additional TEE values were generated using food quotients that were calculated from food records and adjusted for change in body composition (see below). The isotopic enrichments of the postdose urine samples compared with the predose samples were used to calculate isotope elimination rates using linear regression. CO2 production was calculated using the equations of Schoeller (32), later modified by Racette et al. (21). TEE was calculated by multiplying the rate of CO2 production by the energy equivalent of CO2 based on the estimated food quotient of the diet (0.86).
The energy expended in activity, or AEE, was calculated with the following formula: AEE = TEE − RMR − 0.1 * TEE, where TEE was measured by DLW, RMR was measured by indirect calorimetry, and the thermic effect of food is estimated as 10% of TEE (34). This method was considered the “gold standard,” and these AEE values were analyzed using inferential statistics. Levels of TEE are influenced by the food quotient of the diet; therefore, TEE was also calculated at the group level using food quotients from food records, which were adjusted for change in body composition measured with dual-energy X-ray absorptiometry immediately before and after the DLW period.1 AEE values were then calculated at the group level, which relied on these TEE values and values for the thermic effect of food, which were obtained from energy intake measured with food records. Physical activity level (PAL) was calculated with the following formula: PAL = TEE/RMR. PAL was not considered a primary end point, however, because of problems inherent in using a ratio with a nonzero intercept (1, 3) and problems associated with RMR being present in the numerator and denominator and RMR changing during CR and body mass [fat-free mass (FFM) and fat mass (FM)] change. One participant from WUSM had an outlying RMR value; therefore, the equation of Mifflin et al. (19) was used to calculate RMR, and this value was used to determine this participant's AEE and PAL. Another participant's DLW data from WUSM were unusable because of low enrichment; hence, this participant's data were excluded.
Accelerometry.
The accelerometry methods used at each site are reported in detail elsewhere (22; unpublished observations). Briefly, ActiGraph accelerometers (model 7164, ActiGraph, Fort Walton Beach, FL) were worn at PBRC and Tufts. RT3 Tri-axial Research Tracker accelerometers (Stayhealthy, Monrovia CA), which were programmed in the 1-min vector magnitude mode (14), were worn at WUSM. The accelerometer was worn on the participant's waist during the DLW periods, and data were recorded every minute.
Data from the accelerometers were processed at the coordinating center for the CALERIE study (Duke Clinical Research Institute) using the manufacturers' software and a program that was written in SAS code and is available from the authors on request. Data from the RT3 accelerometer were converted to minute-by-minute energy expenditure using the manufacturers' software (version 1.0.7). Minute-by-minute energy expenditure data from the ActiGraph accelerometer were calculated using our SAS program and the combination of the work-energy theorem and the equation of Freedson et al. (9). On data files containing 1,440 min records per day, categories of activity for each minute were created based on intensity using the following cut points: basal was intensity less than the RMR, low was defined as ≥RMR and <3 times RMR, moderate was defined as ≥3 times RMR and <5 times RMR, and hard/very hard was defined as ≥5 times RMR. The minute-by-minute data were converted to one record per day, with the total number of hours engaged in each activity category being represented. Then the average daily number of hours spent at each activity level was calculated for each visit (i.e., baseline, month 3, month 6).
7-Day PAR questionnaire.
Physical activity was assessed for each of the 2-wk DLW periods with the 7-day PAR questionnaire (30, 31). The participant reported the amount of time spent sleeping and engaging in moderate, hard, or very hard activities (time spent in light activity was calculated by difference).
Body weight and composition.
At each assessment point, body weight was measured on a clinic scale. Body composition was measured using dual-energy X-ray absorptiometry at PBRC (QDA 4500A, Hologic, Bedford, MA) and WUSM (4500-W, Hologic). At Tufts, air displacement plethysmography (BOD POD, Life Measurement, Concord, CA) was used to measure body density in duplicate at baseline and months 6 and 12 of CR.
Statistical Analyses
The primary aim of the statistical analyses was to determine if CR affected physical AEE compared with control. Mixed-model analyses were performed to account for within-subject correlation using SAS (version 8) software. The primary dependent variable was change in AEE from baseline to follow-up months 3, 6, 9, and 12. Because of the shorter study duration at PBRC (6 mo) than at Tufts and WUSM (12 mo), separate mixed models were conducted for data through month 6 and data through month 12 (WUSM and Tufts only). All the mixed models included treatment arm, month, and interaction of treatment arm and time point. Since the interventions differed among sites, each of the seven treatment arms across the three sites was treated as a distinct arm. Following a conservative modified intent-to-treat approach, participants who had data at baseline and at least one follow-up time point (e.g., month) were included in the analyses. Because the amount of energy expended during a given amount of activity decreases as body mass is lost, the analyses for AEE included FM and FFM as covariates (34). Sex was also included in the model as a covariate to determine if change in the outcome variables varied by sex,2 and the effect of age was examined using the same approach. Within each model, tests were performed to assess whether the end point (AEE) changed significantly from baseline to each follow-up time point separately within each treatment arm. For PBRC and WUSM, tests were also conducted to compare the changes in AEE at each time point between the CR and control arms separately at each site. Because a modified intent-to-treat approach, which relies on all participants' data even if they miss an assessment point, was utilized, raw change scores on the outcome variables were not reported, rather, difference between groups (mean ± SE) is reported after adjustment for covariates (means were also calculated without adjustment, and they did not meaningfully differ from adjusted means). Although the ratio of TEE to RMR (PAL) normalizes for body size, there is a large amount of variability in the relationship between TEE and RMR when the intercept is not zero (1, 3); therefore, PAL was analyzed as a secondary end point using statistical techniques identical to the analyses for AEE (e.g., FM and FFM were included as covariates in the PAL analyses).2
Accelerometry and 7-day PAR data were used in a series of secondary analyses to identify the aspects of physical activity that changed in response to CR. Percent change in time spent in sleep or light activity, moderate-intensity activity, and hard or very hard activity was quantified with accelerometers and the 7-day PAR. For the PBRC and WUSM data, analyses were conducted to determine if change in the percentage of time in each category differed between the CR and control groups. For the Tufts data, within-subject analyses were conducted to determine if the percentage of time in these activities changed significantly during the 12-mo intervention period. Unlike the AEE analyses, FM and FFM were not entered as covariates, and the results and means from unadjusted models are reported. To determine if age and sex affected activity change, one additional model was conducted that covaried age and sex, and the significance of these covariates was examined.
Correlation analysis was used to determine if prescribed percent CR was associated with change in activity or if change in weight was associated with change in AEE. Lastly, the proportion of TEE accounted for by AEE was calculated at baseline and during CR. Change in this ratio was correlated with percent CR. All tests of significance were performed at the α = 0.05 level of significance.
RESULTS
Baseline Characteristics and Body Weight Loss
The baseline descriptive characteristics of the study sample for this analysis are included in Table 1. Complete descriptions of the studies are reported elsewhere (4, 5, 11, 23). Table 2 includes baseline body weight, FM, and FFM, as well as change in these values for the study sample included in this analysis.
Table 1.
Participant characteristics by site
| PBRC (n = 34) | Tufts University (n = 45) | WUSM (n = 26) | |
|---|---|---|---|
| Sex | |||
| Male | 15 (44%) | 12 (27%) | 11 (42%) |
| Female | 19 (56%) | 33 (73%) | 15 (58%) |
| Race | |||
| Caucasian | 22 (65%) | 38 (84%) | 23 (88%) |
| African-American | 11 (32%) | 2 (5%) | 1 (4%) |
| Other | 1 (3%) | 5 (11%) | 2 (8%) |
| Age, yr | 39.2 (6.6) | 34.7 (4.9) | 55.2 (3.1) |
| Weight, kg | 81.8 (10.2) | 80.3 (10.6) | 80.5 (10.3) |
| BMI, kg/m2 | 27.9 (1.7) | 27.9 (1.5) | 27.4 (2.2) |
Continuous variables are means (SD); categorical variables are number and percentage. PBRC, Pennington Biomedical Research Center; WUSM, Washington University School of Medicine; BMI, body mass index.
Table 2.
Baseline values and change in body weight, FM, and FFM during interventions
| PBRC |
Tufts University |
WUSM |
|||||
|---|---|---|---|---|---|---|---|
| Control | 25% CR | LCD | 10% CR | 30% CR | Control | 20% CR | |
| Weight, kg | |||||||
| Baseline | 83.15 ± 2.08 | 81.14 ± 2.09 | 80.03 ± 2.26 | 84.66 ± 1.43 | 78.68 ± 0.94 | 81.72 ± 2.01 | 79.01 ± 1.10 |
| Month 3 | −0.53 ± 0.63* | −5.57 ± 0.49† | −11.29 ± 0.55† | −4.44 ± 0.58 | −5.35 ± 0.43 | −0.96 ± 0.46* | −5.53 ± 0.48† |
| Month 6 | −0.35 ± 0.75* | −8.07 ± 0.88† | −10.67 ± 0.56† | −5.39 ± 1.50 | −7.43 ± 0.73 | −2.69 ± 1.01* | −7.42 ± 1.07† |
| Month 9 | −3.56 ± 2.30 | −7.73 ± 0.71 | −1.73 ± 0.68* | −7.76 ± 1.11† | |||
| Month 12 | −3.48 ± 2.67 | −6.58 ± 0.70 | −1.09 ± 0.64* | −8.21 ± 1.10† | |||
| FM, kg | |||||||
| Baseline | 25.80 ± 0.91 | 24.93 ± 1.36 | 26.35 ± 1.37 | 30.89 ± 1.00 | 27.31 ± 0.58 | 26.99 ± 0.49 | 26.50 ± 0.63 |
| Month 3 | −0.43 ± 0.35* | −3.60 ± 0.44† | −7.29 ± 0.26† | −4.34 ± 0.51 | −4.45 ± 0.32 | −0.98 ± 0.34* | −3.87 ± 0.35† |
| Month 6 | −0.15 ± 0.67 | −5.68 ± 0.74† | −7.86 ± 0.46† | −5.51 ± 1.29 | −6.72 ± 0.64 | −1.68 ± 0.74* | −5.53 ± 0.80† |
| Month 9 | −3.69 ± 1.95 | −6.83 ± 0.68 | −0.89 ± 0.41* | −6.04 ± 0.81† | |||
| Month 12 | −3.51 ± 2.30 | −6.03 ± 0.62 | −0.17 ± 0.49* | −6.30 ± 0.89† | |||
| FFM, kg | |||||||
| Baseline | 57.35 ± 2.26 | 56.21 ± 2.31 | 53.68 ± 2.41 | 53.77 ± 1.18 | 51.37 ± 0.95 | 54.73 ± 2.20 | 52.51 ± 1.29 |
| Month 3 | −0.09 ± 0.39* | −1.97 ± 0.29† | −4.00 ± 0.50† | −0.11 ± 0.39 | −0.91 ± 0.22 | 0.02 ± 0.58* | −1.66 ± 0.28† |
| Month 6 | −0.20 ± 0.34* | −2.38 ± 0.42† | −2.81 ± 0.46† | 0.12 ± 0.48 | −0.71 ± 0.21 | −1.01 ± 0.40* | −1.89 ± 0.38* |
| Month 9 | 0.13 ± 0.75 | −0.91 ± 0.25 | −0.84 ± 0.33* | −1.72 ± 0.41* | |||
| Month 12 | 0.03 ± 0.66 | −0.55 ± 0.24 | −0.93 ± 0.45* | −1.91 ± 0.35* | |||
Values are means ± SE. CR, calorie-restricted; FM, fat mass; FFM, fat-free mass; LCD, low-calorie diet. Means with different symbols (*, †) indicate that change scores at that time point differed significantly (P < 0.05) from control group at PBRC and WUSM. Within-group comparisons were conducted for change scores for Tufts University, and all were nonsignificant (P > 0.05).
AEE and PAL
PBRC.
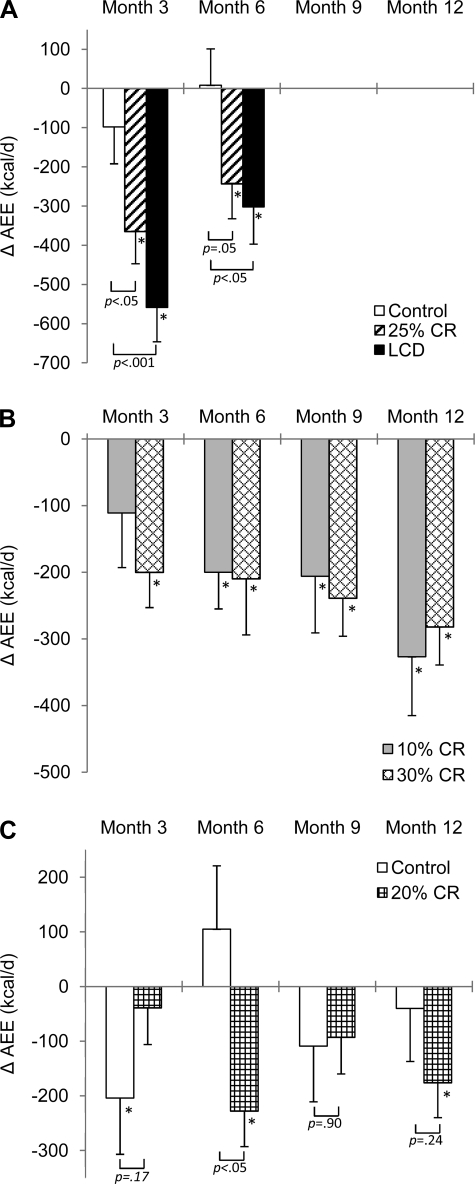
AEE (mean ± SE) at baseline in the control, CR, and LCD groups was 973 ± 78, 1,025 ± 96, and 1,025 ± 94 kcal/day, respectively. A significant decrease in AEE was observed in the CR and LCD groups at months 3 and 6. The differences in AEE change between the CR and control groups and between the LCD and control groups were significant at months 3 and 6 (Fig. 2).
Fig. 2.
Mean change in physical activity energy expenditure (AEE) over time by site and group. Error bars, SE. A: data from PBRC. B: data from Tufts University. C: data from WUSM. *Significantly different (P < 0.05) within groups. Brackets report P values comparing change in control vs. CR and LCD groups at PBRC and control and CR groups at WUSM. Only within-group change was evaluated from Tufts University data.
A similar pattern was observed with the PAL results. A significant decrease in PAL (adjusted means ± SE) was observed in the CR (−0.23 ± 0.07, P < 0.01) and LCD (−0.42 ± 0.08, P < 0.001) groups at month 3. The decrease in PAL was significantly larger in the LCD than the control group at month 3 (adjusted mean difference between the LCD and control group, −0.36 ± 0.11, P < 0.01). No significant change was observed in PAL (P > 0.06) in the control and CR groups at month 6, but a significant decrease in PAL (−0.18 ± 0.08, P < 0.05) was observed in the LCD group. PAL change in the CR and LCD groups did not differ significantly from the control group (P > 0.10) at month 6.
Tufts University.
AEE (means ± SE) at baseline in the 10% and 30% CR groups was 913 ± 73 and 932 ± 50 kcal/day, respectively. A significant decrease in AEE was observed in the 10% and 30% CR groups at months 6, 9, and 12. A significant decrease in AEE was also observed in the 30% CR group at month 3 (Fig. 2).
The decrease in PAL was significant at months 3, 6, 9, and 12 for the 30% CR group (−0.20 to −0.13, P < 0.05), but only at month 12 for the 10% CR group (−0.21, P < 0.01).
WUSM.
AEE (means ± SE) at baseline in the control and CR groups was 938 ± 95 and 990 ± 66 kcal/day, respectively. A significant decrease in AEE was observed in the control group at month 3 and in the CR group at months 6 and 12 (Fig. 2). Change in AEE differed between groups at month 6, with a significant decrease in the CR group and no significant change in the control group.
The PAL data support the AEE findings. There was a significant decrease from baseline in PAL in the control group at month 3 (−0.18 ± 0.09, P < 0.05) and in the CR group at months 6 (−0.15 ± 0.06, P < 0.05) and 12 (−0.16 ± 0.06, P < 0.01). The control and CR groups differed significantly on PAL change at month 6 (larger decrease in the CR than control group, with adjusted mean difference between the control and CR group of −0.25 ± 0.12, P < 0.05). All other comparisons were nonsignificant (P > 0.07).
Accelerometry and 7-Day PAR
The accelerometry results are summarized in the Supplemental Table in Supplemental Material for this article, available online at the Journal website. Results from the 7-day PAR are summarized here but not provided in a table.
PBRC.
Percent change in time spent in sleep or light and moderate activity did not differ at month 3 or 6 between the control and CR group or between the control and LCD group when measured objectively with accelerometers. At month 3, the LCD and control groups differed significantly on change in hard or very hard activity, with a significant increase in the control group and a nonsignificant decrease in the LCD group (see Supplemental Table).
When measured with the 7-day PAR, percent change in time spent in sleep or light activity from baseline to month 6 differed between the control and LCD group, with a significant increase in the LCD group and no significant change in the control group. No other comparisons were significant.
Tufts University.
The within-group comparisons for Tufts University data demonstrated that the percentage of time spent in moderate-intensity activity measured with accelerometers increased significantly in the 30% CR group at month 6. At month 9, a significant decrease in hard or very hard activity was observed in the 10% CR group (P < 0.05).
When measured with the 7-day PAR, there was a significant increase in percent time spent in hard/very hard activity in the 30% CR group at month 9. No other within-group comparisons were significant.
WUSM.
Percent change in time spent in sleep or light, moderate, and hard or very hard activity measured with accelerometry did not differ significantly at months 3, 6, 9, and 12 between the control and CR group.
When percent change in time spent in the activity categories was evaluated with the 7-day PAR, the control and CR groups differed on change in sleep or light activity at month 9: the control group reported a significant decrease in sleep or light activity, and the CR group reported no significant change. The control and CR groups also differed on change in hard/very hard activity at month 9, with the control group reporting a significant increase and the CR group reporting no significant change.
Association Between Change in Activity and Age, Sex, and Prescribed CR
Percent CR did not correlate significantly with change in AEE (r = −0.15 to 0.02, P > 0.16) or change in time spent in activity categories measured with accelerometry (r = −0.21 to 0.21, P > 0.09). Self-reported change in time spent in hard/very hard activity, measured with the 7-day PAR, correlated significantly with prescribed CR only at month 3 (r = −0.26, P < 0.05), with greater CR being associated with larger reported reductions in hard/very hard activity. Change in body weight correlated significantly with change in AEE (r = 0.30, P < 0.01). The ratio of change in AEE to TEE did not correlate with percent CR.
A larger reduction in AEE was observed in men than women (>100 kcal/day, P < 0.05), but this effect was nonsignificant after adjustment for FM and FFM. Change in AEE was not significantly associated with age. Change in the percentage of time in activities of different intensity measured with accelerometry was not associated with age or sex. Women reported a larger reduction in percentage of time spent in intense activity measured with the 7-day PAR. No other sex effects were significant. Age was not significantly associated with change in 7-day PAR data.
DISCUSSION
This is one of the first studies to 1) test the effect of CR on the AEE of nonobese participants using DLW and indirect calorimetry and 2) attempt to quantify with accelerometry and a standardized PAR interview the aspects of activity that change in response to CR in nonobese participants. When measured with the gold standard, AEE was reduced during CR, with some variation across study samples and with the duration of CR. On the basis of data from the two sites that included a control group, the decrease among the CR groups was ≥175 kcal/day larger than the change in the control groups at month 6. The association analyses indicated that change in body weight accounted for 9% of the variance in change in AEE, and the direction of the relation was as expected: greater weight loss was associated with larger decreases in AEE. The ratio of change in AEE to TEE did not correlate with percent CR.
The PAL data support the AEE findings, even though PAL is calculated by dividing TEE by RMR and represents the relation between TEE and RMR and is, therefore, not necessarily an accurate measure of the energy expended in activity (3). When expressed in metabolic equivalent (MET) hours per day, the CR groups experienced decreases of >3.1 MET h/day by month 6. Change in MET hours per day in the CR compared with control groups plateau by month 6 at −3.8 to −6.0 MET h/day, and this information, in addition to the kilocalorie values (see above), allows clinicians and researchers to describe change in activity using commonly used metrics.
The AEE results support earlier studies that report decreased AEE with CR and weight loss among humans (12, 27, 35). The results also build on the finding that semistarvation results in a marked decrease in AEE (13) by demonstrating that AEE is also decreased during nutritionally adequate CR. Animal studies, however, indicate that CR results in higher levels of activity (8, 36) due to increased food-seeking behavior (36). It is possible that the effect of CR on humans is environment- or context-specific, and, under certain conditions, humans might mimic the behavior of CR animals. For example, during periods of famine when food is scarce, humans might increase physical activity secondary to seeking a food supply, similar to behavior observed in animal studies. Alternatively, in an environment rich with food where people are more likely to restrict energy intake in an attempt to lose weight, they might experience decreased energy expenditure from activity. In such an environment, the decrease in energy expenditure could preserve energy and body mass and limit weight loss. In our study, CR was associated with a mean control group-corrected savings of ≥175 kcal/day at month 6. Such energy savings would slow the rate of weight loss. To counter this effect, energy (food) intake must be further decreased or activity levels volitionally increased. Volitionally increasing activity, particularly the energy expended during activity, may be especially difficult. Our results suggest that the energy expended in activity is reduced beyond values expected from change in body mass, and this is likely due, at least in part, to increased muscle work efficiency (29).
Accelerometers provide an objective measure of activity, and 7-day PAR provides participants' perceived activity levels; yet the accuracy of both methods is variable. At PBRC, the accelerometry and 7-day PAR data suggest that participants decreased time spent in higher-intensity activity in favor of lower-intensity activity, a conclusion that is supported by the 7-day PAR data from WUSM. The within-group accelerometry and 7-day PAR comparisons from Tufts University suggest that the time spent in activities of different intensity fluctuated during the trial.
The accelerometry and PAR results have important implications. First, according to previous research, CR and a 10% reduction of body weight increase muscle work efficiency by up to 27%, with change in efficiency accounting for 10–35% change in the energy expenditure from activity (29). These findings suggest that 65–90% of the change in energy expended during activity in our study remains unexplained. Although the accelerometry and PAR results suggest a decrease in higher-intensity activity among some CR groups, the results are inconsistent, and it is concluded that these data do not fully support the AEE results. This suggests that 1) the validity of accelerometry and PAR methods negatively affects their sensitivity at detecting activity change, 2) changes in behaviors that cost energy (fidgeting, changing posture, upper body movements) but are not adequately quantified by these methods (see Ref. 34 for review) account for a sizable proportion of the observed decrease in energy expended during activity, or 3) other changes, biological or behavioral, not captured in this study affect change in energy expended during activity.
Change in body weight was associated with change in AEE, suggesting that the degree of energy deficit was associated with decreased AEE. When activity was self-reported with the 7-day PAR, women reported a larger reduction in percentage of time spent in intense activity, and change in time spent in hard/very hard activity was negatively associated with prescribed CR. Nevertheless, these findings were not supported by objective measures such as AEE and accelerometry. Reductions in AEE were larger in men than women, but this effect was not significant when the model was adjusted for FM and FFM. This result is not surprising, since the energy expended during activity is affected by body mass, and men and women differ in body mass and composition. Age did not correlate significantly with change in objectively measured activity.
The present study has a number of strengths, such as the use of DLW and measured RMR for the main outcomes, inclusion of control groups at PBRC and WUSM, and inclusion of nonobese, overweight participants. In addition, the CR groups lost a significant amount of weight, verifying that an energy deficit was achieved (4, 11, 23). Possible limitations of the study include different study samples, protocols, and interventions among sites, but these features allowed us to examine change in activity in response to different levels of CR, and the range of samples tested improves generalizability of the results. The use of different brands of accelerometers at the study sites could also be considered a limitation. Because all tests of significance were performed at the α = 0.05 level of significance, they are vulnerable to type I error; hence, the results need to be replicated in other independent studies.
In summary, CR resulted in reduced AEE, and the extent of the decrease varied among study samples. Age and sex were not found to be associated with the effect of CR on change in activity. Objective accelerometry data did not reliably identify a shift away from higher-intensity activity in favor of lower-intensity activity, and subjective reports of change in activity from the 7-day PAR provided mixed results. The inability of accelerometry to account for activity change suggests that change in the energy expended during activity is secondary to increased muscle work efficiency (29) and possibly other factors that are not accurately assessed with accelerometry and recall, such as fidgeting or upper body movements (34). The validity of accelerometry also negatively affects sensitivity and their ability to detect differences in change among groups. The findings have important implications for researchers and clinicians who wish to quantify and detect changes in activity levels in response to an intervention. The results provide evidence that energy is conserved during CR, which may reduce the rate of weight loss.
GRANTS
This work was supported by National Institutes of Health Grants K23 DK-068052 (C. K. Martin), PBRC Clinical Nutrition Research Unit Grant 1P30 DK-072476 entitled “Nutritional Programming: Environmental and Molecular Interactions,” U01 AG-20478, U01 AG-020487, U01 AG-020480, and U01 AG-022132; Boston Obesity Nutrition Research Center Grant H150001; National Institutes of Health General Clinical Research Center Grant RR-00036 and AG-00078 (E. P. Weiss); and the US Department of Agriculture under Agreement 58-1950-4-401.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health or US Department of Agriculture.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We are profoundly grateful to all the volunteers who spent so much time participating in this demanding research study. We thank the members of the CALERIE Study Team who contributed to this research: Leonie Heilbronn, Lilian de Jonge, Madlyn Frisard, Enette Larson-Meyer, Jennifer Rood, Tuong Nguyen, Julia Volaufova, Marlene Most, Frank Greenway, Steven Smith, Donald Williamson, Walter Deutsch, Eric Ravussin, Steven Anton, Emily York-Crowe, Catherine Champagne, Paula Geiselman, Michael Lefevre, Jennifer Howard, Jana Ihrig, Brenda Dahmer, Anthony Alfonso, Darlene Marquis, Connie Murla, Aimee Stewart, Amanda Broussard, and Vanessa Tarver (PBRC); Dennis Villareal, Christopher Koenig, Morgan Schram, Karen Steger-May, Kenneth Schechtman, and John Holloszy (WUSM) and the nurses of the Washington University Intensive Research Unit; Cheryl Gilhooly, Julie Golden, Paul Fuss, Stephanie Tyler, Edward Saltzman, and Susan Roberts (Tufts University); James Topping and Manjushiri Bhapkar (Duke Clinical Research Institute). We also thank Health and Nutrition Technology (Carmel, CA) for providing the HealthOne formula used in the study.
Present address of J. P. DeLany: Department of Medicine, University of Pittsburgh, Pittsburgh, PA.
Footnotes
AEE was also calculated using TEE values that were obtained from the food quotients of the diets (food quotients were determined from food records and were adjusted for change in body composition). These AEE values were calculated at the group level and were not analyzed with inferential statistics. With few exceptions, these AEE values indicated that decreases in AEE were larger than those shown in Fig. 2 for all groups, including control groups at PBRC and WUSM (AEE values in Fig. 2 were obtained with a respiratory quotient of 0.86). Therefore, differences in AEE change between control and CR groups were similar to those shown in Fig. 2. Because of the similarity of the results, we report AEE values that were obtained with a respiratory quotient of 0.86 and the thermic effect of food calculated as 10% of TEE.
AEE and PAL analyses were also conducted without adjustment for covariates. Results did not meaningfully differ; therefore, only the adjusted model is reported.
REFERENCES
- 1. Allison DB, Paultre F, Goran MI, Poehlman ET, Heymsfield SB. Statistical considerations regarding the use of ratios to adjust data. Int J Obes Relat Metab Disord 19: 644–652, 1995 [PubMed] [Google Scholar]
- 2. Bandini LG, Schoeller DA, Cyr HN, Dietz WH. Validity of reported energy intake in obese and nonobese adolescents. Am J Clin Nutr 52: 421–425, 1990 [DOI] [PubMed] [Google Scholar]
- 3. Carpenter WH, Poehlman ET, O'Connell M, Goran MI. Influence of body composition and resting metabolic rate on variation in total energy expenditure: a meta-analysis. Am J Clin Nutr 61: 4–10, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, Lichtenstein AH, Dallal GE, Dutta C, Bhapkar MV, Delany JP, Saltzman E, Roberts SB. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr 85: 1023–1030, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Das SK, Saltzman E, Gilhooly CH, Delany JP, Golden JK, Pittas AG, Dallal GE, Bhapkar MV, Fuss PJ, Dutta C, McCrory MA, Roberts SB. Low or moderate dietary energy restriction for long-term weight loss: what works best? Obesity (Silver Spring) 17: 2019–2024, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Groot LC, van Es AJ, van Raaij JM, Vogt JE, Hautvast JG. Adaptation of energy metabolism of overweight women to alternating and continuous low energy intake. Am J Clin Nutr 50: 1314–1323, 1989 [DOI] [PubMed] [Google Scholar]
- 7. DeLany JP, Schoeller DA, Hoyt RW, Askew EW, Sharp MA. Field use of D218O to measure energy expenditure of soldiers at different energy intakes. J Appl Physiol 67: 1922–1929, 1989 [DOI] [PubMed] [Google Scholar]
- 8. Duffy PH, Feuers RJ, Hart RW. Effect of chronic caloric restriction on the circadian regulation of physiological and behavioral variables in old male B6C3F1 mice. Chronobiol Int 7: 291–303, 1990 [DOI] [PubMed] [Google Scholar]
- 9. Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc., accelerometer. Med Sci Sports Exerc 30: 777–781, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Goris AH, Westerterp-Plantenga MS, Westerterp KR. Undereating and underrecording of habitual food intake in obese men: selective underreporting of fat intake. Am J Clin Nutr 71: 130–134, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 295: 1539–1548, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heyman MB, Young VR, Fuss P, Tsay R, Joseph L, Roberts SB. Underfeeding and body weight regulation in normal-weight young men. Am J Physiol Regul Integr Comp Physiol 263: R250–R257, 1992 [DOI] [PubMed] [Google Scholar]
- 13. Keys A, Brozek J, Henschel A, Mickelsen F, Taylor HL. The Biology of Human Starvation. Minneapolis: University of Minnesota Press, 1950 [Google Scholar]
- 14. Leenders NY, Sherman WM, Nagaraja HN. Energy expenditure estimated by accelerometry and doubly labeled water: do they agree? Med Sci Sports Exerc 38: 2165–2172, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 332: 621–628, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, Jensen MD, Clark MM. Interindividual variation in posture allocation: possible role in human obesity. Science 307: 584–586, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Martin CK, Heilbronn LK, de Jonge L, DeLany JP, Volaufova J, Anton SD, Redman LM, Smith SR, Ravussin E. for the Pennington CALERIE Team Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity (Silver Spring) 15: 2964–2973, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Mifflin MD, St, Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 51: 241–247, 1990 [DOI] [PubMed] [Google Scholar]
- 20. Racette SB, Schoeller DA, Kushner RF, Neil KM, Herling-Iaffaldano K. Effects of aerobic exercise and dietary carbohydrate on energy expenditure and body composition during weight reduction in obese women. Am J Clin Nutr 61: 486–494, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF. Relative dilution spaces of 2H- and 18O-labeled water in humans. Am J Physiol Endocrinol Metab 267: E585–E590, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Racette SB, Weiss EP, Schechtman KB, Steger-May K, Villareal DT, Obert KA, Holloszy JO. Influence of weekend lifestyle patterns on body weight. Obesity (Silver Spring) 16: 1826–1830, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, Fontana L, Klein S, Holloszy JO. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci 61: 943–950, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ravussin E, Burnand B, Schutz Y, Jequier E. Energy expenditure before and during energy restriction in obese patients. Am J Clin Nutr 41: 753–759, 1985 [DOI] [PubMed] [Google Scholar]
- 25. Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest 78: 1568–1578, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Redman LM, Heilbronn LK, Martin CK, de Jonge L, Williamson DA, DeLany JP, Ravussin E. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLos One 4: e4377, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr 88: 906–912, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Rosenbaum M, Ravussin E, Matthews DE, Gilker C, Ferraro R, Heymsfield SB, Hirsch J, Leibel RL. A comparative study of different means of assessing long-term energy expenditure in humans. Am J Physiol Regul Integr Comp Physiol 270: R496–R504, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau JA, Heymsfield S, Joanisse DR, Hirsch J, Murphy E, Matthews D, Segal KR, Leibel RL. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol 285: R183–R192, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, Paffenbarger RS., Jr Physical activity assessment methodology in the Five-City Project. Am J Epidemiol 121: 91–106, 1985 [DOI] [PubMed] [Google Scholar]
- 31. Sarkin JA, Campbell L, Gross L, et al. Project GRAD Seven-Day Physical Activity Recall Interviewer's Manual. Med Sci Sports Exerc 29 Suppl 6: S91–S102, 1997 [Google Scholar]
- 32. Schoeller DA. Measurement of energy expenditure in free-living humans by using doubly labeled water. J Nutr 118: 1278–1289, 1988 [DOI] [PubMed] [Google Scholar]
- 33. Schoeller DA, Bandini LG, Dietz WH. Inaccuracies in self-reported intake identified by comparison with the doubly labelled water method. Can J Physiol Pharmacol 68: 941–949, 1990 [DOI] [PubMed] [Google Scholar]
- 34. Schutz Y, Weinsier RL, Hunter GR. Assessment of free-living physical activity in humans: an overview of currently available and proposed new measures. Obes Res 9: 368–379, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Velthuis-te Wierik EJ, Westerterp KR, van den Berg H. Impact of a moderately energy-restricted diet on energy metabolism and body composition in non-obese men. Int J Obes Relat Metab Disord 19: 318–324, 1995 [PubMed] [Google Scholar]
- 36. Weed JL, Lane MA, Roth GS, Speer DL, Ingram DK. Activity measures in rhesus monkeys on long-term calorie restriction. Physiol Behav 62: 97–103, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: Thomas, 1988 [Google Scholar]
- 38. Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Westerterp KR, Kester AD. Physical activity in confined conditions as an indicator of free-living physical activity. Obes Res 11: 865–868, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Zurlo F, Ferraro RT, Fontvielle AM, Rising R, Bogardus C, Ravussin E. Spontaneous physical activity and obesity: cross-sectional and longitudinal studies in Pima Indians. Am J Physiol Endocrinol Metab 263: E296–E300, 1992 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.