Abstract
Indexes for arterial stiffness are, by their nature, influenced by the ambient blood pressure due to the curvilinear nature of arterial compliance. We developed a new concept of the “Modelflow aortic age,” which is, theoretically, not influenced by the ambient blood pressure and provides an easily understood context (biological vs. chronological age) for measures of arterial stiffness. The purpose of the present study was to validate this pressure-independent index for aortic stiffness in humans. Twelve sedentary elderly (65–77 yr), 11 Masters athletes (65–73 yr), and 12 sedentary young individuals (20–42 yr) were studied. Modelflow aortic ages were comparable with chronological ages in both sedentary groups, indicating that healthy sedentary individuals have age-appropriate aortas. In contrast, Masters athletes showed younger Modelflow aortic ages than their chronological ages. The coefficient of variation of sedentary subjects was three times smaller with the Modelflow aortic age (21%) than with other indexes, such as static systemic arterial stiffness (61%), central pulse wave velocity (61%), or carotid β-stiffness index (58%). The typical error was very small and two times smaller in the Modelflow aortic age (<7%) than in static systemic arterial stiffness (>13%) during cardiac unloading by lower body negative pressure. The Modelflow aortic age can more precisely and reliably estimate aortic stiffening with aging and modifiers, such as life-long exercise training compared with the pressure-dependent index of static systemic arterial stiffness, and provides a physiologically relevant and clinically compelling context for such measurements.
Keywords: arterial stiffness, aging, Modelflow
arteriosclerosis with aging leads to large-vessel arterial stiffening in humans. This process is characterized primarily by the development of fibrosis and degeneration of elastin, which result in structural changes in the arterial wall (28, 29). Indeed, work by Langewouters et al. (30, 31) and Wesseling et al. (49) showed that structural properties of the thoracic and abdominal aortas are altered linearly with age in humans and are clinically relevant. Large arterial stiffness is an independent predictor of mortality and morbidity from cardiovascular disease in the elderly population (3, 4, 7, 11, 33, 37, 39, 44, 50).
Central aortic stiffness can be evaluated by several methods, such as pulse-wave velocity (PWV) of the central aorta, systemic arterial compliance (ratio of stroke volume to pulse pressure), or aortic impedance (dynamic relationship between aortic flow and pressure) (34, 41). However, all of these approaches are, by their nature, influenced by the ambient blood pressure due to the curvilinear nature of arterial compliance (arterial pressure-area relationship) (38). For example, higher blood pressures shift the operational point of the arterial compliance curve to the flatter portion, resulting in functionally stiffer arteries on the same arterial compliance curve. Therefore, it is virtually impossible to distinguish between conditions in which high blood pressure causes functionally stiffer arteries by changing the operational point on the same compliance curve, from one in which mechanically stiff arteries (changes in the compliance curve itself) lead to high blood pressure due to impaired buffering of the arterial pressure waveform. Therefore, an index for aortic stiffness that is not affected by the ambient blood pressure is warranted. At present, among commonly used indexes for arterial stiffness, only the β-stiffness index considers the curvilinear relationship of arterial compliance curve using a simple exponential correction (20).
The Modelflow method has been utilized in clinical settings and/or physiological studies to estimate aortic flow and thus stroke volume from the arterial blood pressure waveform via aortic input impedance (5). Since the aortic input impedance used in the Modelflow calculation is generated from the known age-related changes in the aortic pressure-area relationship, the Modelflow system requires both the arterial pressure waveform and subject chronological age as input variables to estimate stroke volume (49). We have recently developed a new concept of the “Modelflow aortic age” by inversely applying the original Modelflow method, in which the arterial pressure waveform and stroke volume are required as input variables (see Supplemental Appendix; the online version of this article contains supplemental data). Importantly, the Modelflow aortic age, which reflects age-related changes in the aortic pressure-area relationship, is theoretically not influenced by the ambient blood pressure, because it considers the entire aortic compliance curve in its calculations. Although this new index is a strong candidate for an aortic stiffness index independent from ambient pressure, its validity has not been examined in humans.
The objectives of this study were twofold. First, we aimed to examine whether the Modelflow aortic age can quantify age-related increases in aortic stiffness without an influence of ambient blood pressure in humans. Second, we aimed to examine the effect of habitual vigorous exercise training on aortic stiffness in elderly subjects, as evidence that aortic age calculated in this fashion is sensitive to physiologically and clinically relevant stimuli to large vessel structure. We hypothesized that the Modelflow aortic age would reflect age-related intrinsic changes of the aortic wall, and that aortic stiffening with aging, as evidenced by an increased Modelflow aortic age, would be attenuated by life-long vigorous exercise training.
METHODS
Subjects
Twelve healthy sedentary young (age: 20–42 yr old) and 12 healthy sedentary elderly (age: 65–77 yr old) subjects were recruited to assess the effects of healthy sedentary aging on aortic stiffness. Eleven Masters athletes (fit elderly, age: 65–73 yr old) were also recruited to address whether their aortic ages are “younger” than their chronological age, since it has been reported that Masters athletes have more youthful-appearing cardiovascular structure and function (1, 47).
Subjects were excluded if one of following was present: 1) physiologically significant obstructive coronary artery disease, as determined by provocative ischemia during exercise electrocardiogram and echocardiogram; 2) significant valvular heart disease by echocardiogram; 3) renal dysfunction (serum creatinine > 2.0 mg/dl); 4) previous coronary artery bypass surgery; 5) arrhythmias, such as atrial fibrillation/flutter or left bundle branch block; 6) diabetes mellitus; 7) lung disease (pulmonary hypertension or chronic obstructive pulmonary disease); 8) untreated thyroid disorders; 9) hypertension (mean daytime blood pressure > 140/90 mmHg); 10) regular cigarette smoking within the previous 10 yr; 11) body mass index > 30 kg/m2; and 12) currently using cardiovascular medication.
Sedentary subjects were excluded if they engaged in endurance exercise for more than 30 min/session, three times per week. Masters athletes had participated in regular endurance competitions for 23 ± 8 yr (mean ± SD), with a weekly running mileage of 32 ± 10 miles or equivalent swimming or cycling training.
The experimental procedures were explained to all subjects, with informed consent obtained, as approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center at Dallas and Texas Health Presbyterian Hospital Dallas.
Measurements
All experiments were performed in the morning at least 2 h after a light breakfast in a quiet environmentally controlled laboratory, with an ambient temperature of 22–25°C. The subjects were asked to refrain from heavy exercise and caffeinated or alcoholic beverages for at least 24 h before the tests. All measurements were performed in the supine position.
Stroke volume.
A flow-directed, thermistor-tipped pulmonary artery catheter was inserted in the antecubital vein and advanced under fluoroscopic guidance into the main or proximal right pulmonary artery. Cardiac output determinations were based on the temperature changes recorded at the pulmonary artery after 10 ml of 0°C isotonic saline was injected into the right atrium (thermodilution method). Stroke volume was calculated from cardiac output, and each coincident heart rate from a three-lead ECG.
Stroke volumes were also measured noninvasively with the foreign gas (C2H2) rebreathing method (15, 23, 32, 42) to confirm the clinical utility of this index using available noninvasive methods.
Blood pressure.
Finger arterial pressure waveform was recorded by volume-clamp method with Photoplethysmography and Physiocal calibration (Portapres). Central arterial pressure waveform was mathematically reconstructed from the finger arterial pressure waveform (Beatscope 1.1a, FMS) (25). This reconstructed central blood pressure waveform was used for both the Modelflow aortic age and static systemic arterial stiffness calculation.
Lower body negative pressure.
To examine effects of blood pressure changes on the Modelflow aortic age, cardiac filling pressure was decreased in the sedentary elderly by applying lower body negative pressure (LBNP), as previously described (1, 35). We applied this physiological stimulus to alter stroke volume and blood pressure profiles rather than drug administration, since the drugs, which induce either vasodilatation or vasoconstriction, are likely to directly affect the target variable of the arterial pressure-area relationship, i.e., the Modelflow aortic age. Measurements of stroke volume and blood pressure were made 5 min after each −15- (LBNP 15) and −30-mmHg LBNP (LBNP 30) condition.
Data Analysis
The modelflow aortic age.
The Modelflow aortic age was analyzed as described in the Supplemental Appendix. First, Modelflow stroke volumes were generated from a given central blood pressure waveform reconstructed from the finger blood pressure waveform (Beatscope 1.1a, FMS) by using input ages to the Modelflow system from 20 to 90 yr (49). Then the linear regression analysis between the input age and generated Modelflow stroke volumes was constructed. The Modelflow aortic age was then determined by an inverse function of this linear regression equation using stroke volume measured with thermodilution or the C2H2 rebreathing method. Thus, in essence, only the blood pressure waveform and an external measure of stroke volume are necessary to make a measurement of Modelflow aortic age.
Systemic arterial stiffness.
The static systemic arterial stiffness was calculated from central arterial pulse pressure reconstructed from the finger blood pressure waveform divided by stroke volume from thermodilution (pulse pressure/stroke volume).
Other indexes.
Central PWV and carotid β-stiffness index were measured by using Doppler and two-dimensional ultrasound (iE 33, Phillips) and applanation tonometry (SphygmoCor) using standard methods (11, 20, 34), since, in addition to systemic arterial stiffness, these two approaches have also been used in most large epidemiological and physiological studies. We measured these indexes only in the resting supine position, and not during LBNP, because the primary purpose of this study was the comparison between static systemic arterial stiffness and the Modelflow aortic age, as both of them are calculated from the same physiological variables: blood pressure and stroke volume. Moreover, it is technically challenging to obtain central PWV during LBNP, since the subject's lower body is sealed by the LBNP box.
CENTRAL PWV.
Central PWV was measured with Doppler ultrasound (iE 33, Phillips) and calculated as the distance between measurement sites divided by the time delay between the two waveforms (11, 34). Pulse transit time was calculated by subtracting the time between the peak of the R-wave and the foot of the carotid flow profile from the time between the peak of the R-wave and the foot of the femoral flow profile. Distance between arterial measurement sites was calculated by subtracting the distance between the carotid site and the sternal notch from the distance between the sternal notch and the femoral site.
CAROTID β-STIFFNESS INDEX.
β-Stiffness index of the common carotid artery was calculated from systolic and diastolic carotid dimensions and pressures (20, 34). Sequential measurement of right common carotid and brachial pressure waveforms with applanation tonometry (SphygmoCor) was immediately followed by brachial arm cuff blood pressure measurement (Suntech). Systemic diastolic and mean blood pressures were estimated from the brachial blood pressure waveform calibrated with arm-cuff systolic and diastolic blood pressures. These mean and diastolic blood pressures were used to calibrate a right common carotid blood pressure waveform to obtain carotid systolic (Ps) and diastolic blood pressures (Pd) (26, 34).
Cross-sectional area of the right common carotid artery was measured from the images derived from an ultrasound machine (iE33, Phillips) equipped with a high-resolution (Sono-CT) linear-array transducer (∼9 MHz). The measurements were made 1–2 cm proximal to the carotid bulb, with the transducer was placed at a 90° angle to the vessel so that near and far wall interfaces were clearly discernible. Acoustic quantification was applied for the edge detection of the internal arterial wall (Q-Lab, Phillips), and maximum and minimum areas were considered systolic (As) and diastolic (Ad) areas. β-Stiffness index of common carotid artery was calculated by the following equation: ln(Pd/Ps)/(As − Ad)/Ad.
Statistics
Numerical data are presented as means ± SD. Differences in variables among the sedentary elderly, fit elderly, and sedentary young individuals were compared by using one-way ANOVA. Differences in variables among different preload conditions of baseline, LBNP 15, and LBNP 30 were compared by using one-way repeated ANOVA. Student-Newman-Keuls corrected t-test was used for multiple comparisons during post hoc testing. Differences in variables between chronological age and the Modelflow aortic age were compared by using Student's paired t-tests. Statistics analysis was performed by computer software (SigmaStat 3.00, SPSS). P < 0.05 was considered to be statistically significant.
Age specificity was assessed as the coefficient of variation, which is calculated by group standard deviation divided by mean difference between groups. The reproducibility of indexes during LBNP was assessed as the typical error, which is calculated by the standard deviation of difference scores divided by √2 and expressed as a percentage of the grand mean (21).
RESULTS
Subject Characteristics
The sedentary elderly had higher body mass index and lower peak O2 uptake compared with the sedentary young and fit elderly; however, weight and height were not different between the groups (Table 1). Reconstructed central systolic and pulse pressures were higher, and stroke volume was smaller, in the sedentary elderly than in the sedentary young and fit elderly (Table 1).
Table 1.
Subject characteristics
| Sedentary Elderly | Fit Elderly | Sedentary Young | |
|---|---|---|---|
| Male/female | 6/6 | 5/6 | 7/5 |
| Age, yr | 70 ± 3† | 68 ± 3† | 27 ± 7 |
| Height, cm | 168 ± 10 | 170 ± 12 | 171 ± 6 |
| Weight, kg | 73 ± 11 | 64 ± 14 | 69 ± 10 |
| BMI, kg/m2 | 25.8 ± 2.4*† | 22.0 ± 1.9 | 23.3 ± 2.5 |
| BSA, m2 | 1.85 ± 0.18 | 1.74 ± 0.25 | 1.80 ± 0.16 |
| SBP, mmHg | 138 ± 23† | 125 ± 23 | 105 ± 14 |
| DBP, mmHg | 71 ± 12 | 67 ± 11 | 72 ± 11 |
| PP, mmHg | 67 ± 14*† | 55 ± 17† | 37 ± 8 |
| SV, ml | 73 ± 17*† | 93 ± 22 | 89 ± 19 |
| V̇o2max, ml · min−1 · kg−1 | 21.9 ± 3.6*† | 38.2 ± 6.2 | 38.1 ± 7.8 |
Values are means ± SD. Subject characteristics are shown for sedentary elderly individuals (Sedentary Elderly), Masters athletes (Fit Elderly), and sedentary young individuals (Sedentary Young). BMI, body mass index; BSA, body surface area; SBP, DBP, and PP: reconstructed central systolic, diastolic, and pulse pressures, respectively; SV, stroke volume; V̇o2max, peak oxygen uptake.
P < 0.05, Sedentary Elderly vs. Fit Elderly.
P < 0.05, Sedentary Elderly vs. Sedentary Young.
Modelflow Aortic Age vs. Chronological Age
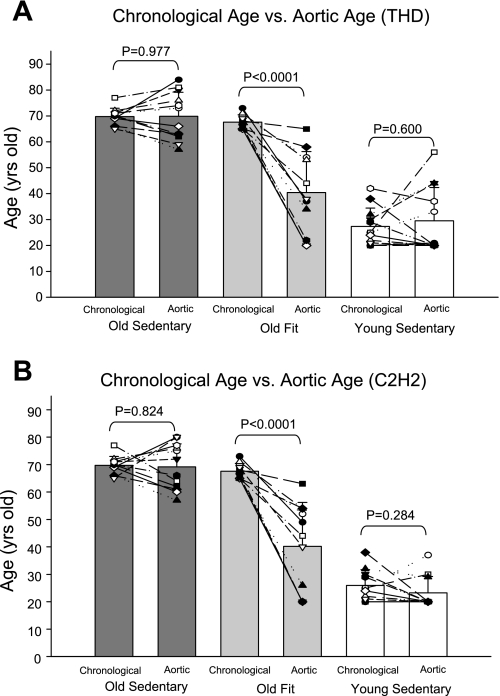
Modelflow aortic ages were comparable with chronological ages in both sedentary young and elderly, indicating that healthy sedentary subjects have aortas whose compliance is consistent with their actual age (Fig. 1A). Fit elderly had significantly younger Modelflow aortic ages than their chronological ages, indicating that the fit elderly have aortas that are more compliant than those of their age-matched peers (Fig. 1A). Similar results were obtained when stroke volume from the noninvasive method (C2H2 rebreathing) was used as the input stroke volume (Fig. 1B).
Fig. 1.
The comparisons between chronological age and the Modelflow aortic age in the sedentary elderly (Old Sedentary), Masters athletes (Old Fit), and young sedentary individuals (Young Sedentary). The Modelflow aortic age was estimated from stroke volume with thermodilution (THD; A) or C2H2 rebreathing (B) method.
Modelflow Aortic Age vs. Other Indexes
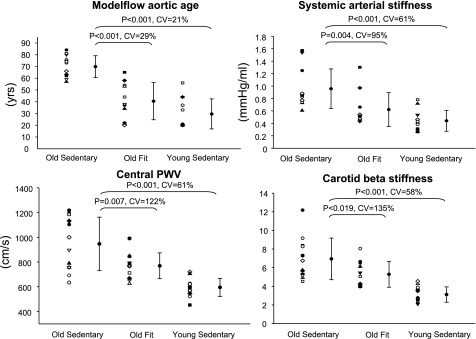
There were significant differences in both the Modelflow aortic age, static systemic arterial stiffness, central PWV, and carotid β-stiffness index between sedentary young vs. elderly, and between sedentary vs. fit elderly at baseline (Fig. 2), suggesting that all methods can similarly identify arterial stiffening with aging, as well as the beneficial effects of exercise training. However, the coefficient of variation between young vs. elderly (standard deviation divided by mean difference between sedentary young vs. elderly) was approximately three times smaller in the Modelflow aortic age than in the static systemic arterial stiffness, central PWV, or carotid β-stiffness index (Fig. 2), indicating that the Modelflow aortic age can more precisely identify arterial stiffening with aging compared with the static systemic arterial stiffness. Moreover, the coefficient of variation between sedentary vs. fit elderly was also more than three times smaller in the Modelflow aortic age than in the static systemic arterial stiffness, central PWV, or carotid beat stiffness index (Fig. 2).
Fig. 2.
The Modelflow aortic age, static systemic arterial stiffness, central pulse-wave velocity (PWV), and carotid β-stiffness index in Old Sedentary, Old Fit, and Young Sedentary. Coefficient of variation (CV) was calculated from the ratio of standard deviation to mean difference.
Modelflow Aortic Age vs. Systemic Arterial Stiffness During LBNP
As expected, reconstructed central blood pressures and stroke volume decreased with increasing levels of LBNP, confirming the predicted changes in hemodynamics during LBNP (Table 2). Typical error as a coefficient of variation between baseline vs. LBNP 15 and between LBNP 15 vs. LBNP 30 was very small (<7%) as a physiological index in the Modelflow aortic age and was approximately two times smaller than that observed for static systemic arterial stiffness (>12%) (Table 3).
Table 2.
Hemodynamic change during LBNP
| Condition | Baseline | LBNP 15 | LBNP 30 |
|---|---|---|---|
| SBP, mmHg | 139 ± 22 | 133 ± 15 | 127 ± 10 |
| DBP, mmHg | 72 ± 12 | 71 ± 10 | 73 ± 11 |
| PP, mmHg | 66 ± 13 | 62 ± 12 | 54 ± 9*† |
| SV, ml | 75 ± 17 | 62 ± 13* | 55 ± 16* |
Values are means ± SD. LBNP 15 and LBNP 30: −15- and −30-mmHg lower body negative pressure, respectively.
P < 0.05, LBNP 15 or LBNP 30 vs. Baseline.
P < 0.05, LBNP 30 vs. LBNP 15.
Table 3.
Reproducibility of stiffness indexes
| Condition | Baseline vs. LBNP 15, % | LBNP 15 vs. LBNP 30, % |
|---|---|---|
| Modelflow aortic age | ||
| Mean difference | 6.3 | 3.2 |
| Typical error as a CV | 6.5 | 6.8 |
| Systemic arterial stiffness | ||
| Mean difference | 9.8 | 0.7 |
| Typical error as a CV | 12.7 | 13.8 |
CV, coefficient of variation.
DISCUSSION
The primary findings of the present study were as follows: 1) the Modelflow aortic age, which conceptually reflects structural changes of the aortic wall with aging, indeed reflected aortic stiffening with healthy sedentary aging in humans; 2) the Modelflow aortic age could more precisely and specifically quantify aortic stiffening with aging compared with the static systemic arterial stiffness, central PWV, or β-stiffness index; and 3) Masters athletes exhibited a younger Modelflow aortic age compared with their chronological age, indicating that the Modelflow aortic age is sensitive to the beneficial effects of exercise training on aortic compliance.
Modelflow Aortic Age vs. Chronological Age
The finding that the Modelflow aortic ages were comparable with chronological ages in healthy sedentary subjects confirms our hypothesis that the Modelflow aortic age reflects aortic stiffening associated with age-related structural changes in the aortic wall. The Modelflow system for stroke volume estimation has been validated by several clinical or physiological studies in healthy individuals (5, 19, 45, 48). Since the Modelflow aortic age calculation is based on an inverse function of the Modelflow stroke volume calculation, this finding is consistent with these previous studies. Furthermore, the present finding proved the validity of the Modelflow system separately for young and elderly populations, which has not been done in previous studies. The fact that the Modelflow system works well, regardless of subject age, is essential for the concept of the Modelflow aortic age, since, if the Modelflow system is affected by age itself, it will serve as a confounding factor for the aortic age estimation.
In contrast to healthy sedentary individuals, Masters athletes had younger Modelflow aortic ages than their chronological age, consistent with previous findings using central PWV, carotid β-stiffness index, or static systemic arterial compliance that exercise training ameliorates age-related changes in aortic compliance (8, 46, 47). This fact supports the notion that the Modelfow aortic age would be sensitive to physiologically relevant stimuli to aortic aging. Moreover, the present finding may provide evidence that life-long exercise training attenuates aortic stiffening with aging by changing structural or functional components of the aortic wall, which has theoretically never been proven by pressure-dependent indexes, since there remains the possibility that the beneficial effects of exercise training on arterial compliance are simply due to changes in blood pressure.
Modelflow Aortic Age vs. Other Indexes
Recent epidemiological studies have shown that pulse pressure is a stronger predictor of the risks of cardiovascular disease compared with diastolic or mean blood pressure in those over 50 yr of age (16–18). Pulse pressure arises as a consequence of blood flow ejected by the left ventricle and a buffering or cushioning function of the entire arterial tree, that is, systemic arterial compliance. Thus systemic arterial compliance or its reciprocal, arterial stiffness, is, in essence, characterized by the relationship between pulse pressure and stroke volume. In the present study, we used the ratio of pulse pressure to stroke volume as an index of static systemic arterial stiffness, according to previous reports (9, 12, 43). Since animal studies have shown that the proximal aorta accounts for 60–80% of static systemic arterial compliance (27, 43), this index is considered to reflect primarily central aortic stiffening. Indeed, several recent studies have shown that the ratio of pulse pressure to stroke volume or its reciprocal predicts cardiovascular risks better than pulse pressure (13, 14, 36). Overall, these findings collectively suggest that the static systemic arterial stiffness is a useful tool to estimate central arterial stiffening with aging compared with pulse pressure.
The Modelflow aortic age is determined by the aortic input impedance, a dynamic relationship between ascending aortic flow and thus stroke volume vs. central blood pressure via a three-element windkessel model (Supplemental Appendix). Consequently, the Modelflow aortic age is also estimated from stroke volume and blood pressure similar to the static systemic arterial stiffness. Nevertheless, Modelflow aortic age was more precise in identifying age-specific changes in aortic stiffening with sedentary aging than was static systemic arterial stiffness. There are several possible explanations for this age specificity of the Modelflow aortic age: 1) the Modelflow aortic age reflects dynamic aspects of arterial properties, while the static systemic arterial stiffness only reflects the static aspect of arterial compliance. Since the actual aortic pressure-flow relationship is dynamic, the Modelflow aortic age can more accurately estimate arterial properties than a static function of systemic arterial stiffness; 2) Modelflow aortic age is conceptually not influenced by the ambient blood pressure, while the static systemic arterial stiffness is confounded by the ambient blood pressure; and 3) Modelflow aortic age specifically (theoretically 100%) reflects only central aortic stiffening with aging, where aging effects on arteries are most manifest (and clinically relevant), while the static systemic arterial stiffness is a crude estimation for the entire arterial system, including branches out of the aorta, even though 60–80% changes in systemic arterial stiffness can be explained by the central aorta (27, 43). Our findings suggest that the Modelflow aortic age would be a better approach to accurately quantify aortic stiffening with human sedentary aging than the static systemic arterial stiffness. Whether this index for aortic stiffness would be a better predictor for the risk of cardiovascular diseases than the static systemic arterial stiffness or pulse pressure would need to be addressed in future studies.
We compared the Modelflow aortic age primarily with static systemic arterial stiffness, since both the Modelflow aortic age and static systemic arterial stiffness are generated similarly from blood pressure and stroke volume, which would allow the most direct comparison between two techniques to evaluate aortic structure. However, the age specificity of the Modelflow aortic age was equally superior to other indexes, such as central PWV and β-stiffness index, which have been widely used for recent epidemiological and physiological studies, with an approximately threefold reduction in coefficient of variation. To confirm that this comparison was not due to imprecise measurements of these indexes in our own laboratory, we also estimated the coefficient of variation of central PWV (82%) (47) and β-stiffness index of the common carotid artery (88%) (46) from previously published studies, which reported data from cohorts similar to those of the present study; both of these coefficients of variation are also substantially higher than that of the Modelflow aortic age by approximately fourfold. Collectively, these additional comparisons strongly support the utility of the Modelflow aortic age as an age-specific index compared with other indexes for assessing central arterial stiffness.
Since the β-stiffness index also considers the curvilinear aortic compliance curve using an exponential correction (20), the age specificity of the Modelflow aortic age over the β-stiffness index may not be explained by the fact that the Modelflow aortic age is a pressure-independent index. However, pioneering work by Langewouters et al. (31) showed that an arctangent model with three free parameters explained >99% of the variance in area with pressure for each human aorta, providing strong evidence that the aortic compliance curve is best characterized by this sigmoid curve with the arctangent equation. Moreover, Wesseling et al. (49) showed that parameters of this arctangent model are linearly related with chronological age in human adults, on which the concept of the Modelflow aortic age is based. It is less likely that the simple exponential correction in the β-stiffness index reflects the full sigmoid curve of aortic pressure-area relationship, as well as age-related changes in the entire curve, particular when diastolic blood pressure is lower than the reflection points of the sigmoid curve. Therefore, the β-stiffness index may not be as blood pressure independent as the Modelflow aortic age.
Reproducibility of the Modelflow Aortic Age
Consistent with our hypotheses, the Modelflow aortic age showed a high reproducibility during blood pressure changes caused by cardiac unloading. Arterial stiffness indexes, such as PWV or static systemic arterial stiffness, are inherently confounded by the ambient blood pressures at which they are measured, since the arterial stress-strain relationship is not linear but curvilinear. Indeed, typical error was higher for systemic arterial stiffness than for the Modelflow aortic age in the present study. Although several methods of correcting for differences in distending pressure have been developed, including a statistical correction (40) or mathematical manipulation of the stiffness index (2, 20), until now there have been no established methods for evaluating arterial stiffness completely independent from ambient blood pressure. Therefore, the finding that the Modelflow aortic age is not substantially influenced by hemodynamic changes during LBNP strongly supports the clinical and/or practical utility of the Modelflow aortic age. The high reproducibility of the Modelflow aortic age suggests that this index is particularly useful to examine the effect of exercise training, anti-hypertensive drugs, or other interventions that involve blood pressure changes.
Clinical Implications
The present study primarily used stroke volume calculated from thermodilution, which required invasive right heart catheterization. However, noninvasive approaches to stroke volume estimation, such as foreign gas or CO2 rebreathing methods (32), ascending aortic flow measurement with Doppler (6), or B-mode echocardiography (10), etc., could be used for physiological or clinical noninvasive studies, since the concept of the Modelflow aortic age is not affected by the method of stroke volume estimation. Indeed, the Modelflow aortic ages calculated from the noninvasive method, C2H2 rebreathing, were comparable with those from thermodilution in the present study (Fig. 1B), confirming the reliability of the Modelflow aortic age with noninvasive methods, which are widely available (15, 42).
It should be emphasized that, because of its intuitive simplicity, the concept of aortic age might be much more meaningful for individual patients and has the potential to be a powerful motivating tool. For example, a 50-yr-old patient who is not very compliant with life-style interventions might be much more compelled by knowing that he had the aortic age of a 75 yr old, than he would be if he were told his β-stiffness index or PWV.
Study Limitations
Several limitations for the clinical utility of the Modelflow aortic age should be acknowledged, despite its conceptual advantages. First, the fundamental basis that supports the Modelflow aortic age was derived from the previous work characterizing age-related changes in aortic compliance parameters using human adults aged 20 up to 88 yr old (49). Thus this concept is most reliably utilized to quantify the aging process of aortic stiffening up to 88 yr old and possibly older, but not the growing process (children), limiting the lowest value of the Modelflow aortic age to 20 yr old. However, the clinical utility of the Modelflow aortic age would not be substantially attenuated, since aortic stiffness index is most relevant to the elderly population in clinical settings as a risk factor of cardiovascular diseases.
Second, we used commercially available software (BeatScope) to generate the central blood pressure waveforms from finger blood pressure waveforms, as well as the Modelflow system calculation. The detailed mathematical and engineering steps of this method are proprietary information; although the general physiological concepts underlying these mathematical models are published and accessible (25, 49), this fact may limit the further development and application of this index.
Third, since the accuracy of the mathematically derived central blood pressure waveforms, as well as the Modelflow method, has not been well validated under physiological stress, such as LBNP, thermal stress, acute exercise, etc., the interpretation of the Modelflow aortic age measured under uncontrolled environmental and/or physical conditions needs to consider this limitation. However, the present finding that the Modelflow aortic age differed only slightly during LBNP supports the reliability of this index under moderate orthostatic stress. Moreover, the fact that the validity of the Modelflow method to estimate stroke volume has been confirmed by numerous studies (5, 19, 22, 24, 48, 49), including during open-heart cardiac surgery, which involves huge hemodynamic changes beyond normal physiological conditions, also supports the reliability of the Modelflow aortic age under various physiological conditions.
Conclusion
The Modelflow aortic age, which conceptually reflects the intrinsic structural changes associated with human aging, indeed accurately reflected aortic stiffening with healthy sedentary aging. Consistent with the concept, this index was not significantly influenced by hemodynamic changes, indicating that this index is reliable for quantifying intrinsic aortic stiffening with human aging. The fact that this index is sensitive to the effects of physiologically relevant stimuli, i.e., exercise training on aortic stiffening with aging, further supports the reliability and utility of this index, which provides an intuitive and easily understood context (biological age vs. chronological age) for measures of arterial stiffness.
GRANTS
This study was supported by National Institute on Aging Grant AG17479-05, and National Space Biomedical Research Institute Postdoctoral Fellowship Grant PR01101 and Career Development Award EO00007 through National Aeronautics and Space Administration NCC 9-58.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1. Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation 110: 1799–1805, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Armentano R, Megnien JL, Simon A, Bellenfant F, Barra J, Levenson J. Effects of hypertension on viscoelasticity of carotid and femoral arteries in humans. Hypertension 26: 48–54, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension 33: 1111–1117, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int 63: 1852–1860, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol 90: 437–446, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Bouchard A, Blumlein S, Schiller NB, Schlitt S, Byrd BF, 3rd, Ports T, Chatterjee K. Measurement of left ventricular stroke volume using continuous wave Doppler echocardiography of the ascending aorta and M-mode echocardiography of the aortic valve. J Am Coll Cardiol 9: 75–83, 1987 [DOI] [PubMed] [Google Scholar]
- 7. Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 39: 10–15, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Cameron JD, Rajkumar C, Kingwell BA, Jennings GL, Dart AM. Higher systemic arterial compliance is associated with greater exercise time and lower blood pressure in a young older population. J Am Geriatr Soc 47: 653–656, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Chemla D, Hebert JL, Coirault C, Zamani K, Suard I, Colin P, Lecarpentier Y. Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am J Physiol Heart Circ Physiol 274: H500–H505, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Christie J, Sheldahl LM, Tristani FE, Sagar KB, Ptacin MJ, Wann S. Determination of stroke volume and cardiac output during exercise: comparison of two-dimensional and Doppler echocardiography, Fick oximetry, and thermodilution. Circulation 76: 539–547, 1987 [DOI] [PubMed] [Google Scholar]
- 11. Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation 106: 2085–2090, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Dart AM, Kingwell BA. Pulse pressure–a review of mechanisms and clinical relevance. J Am Coll Cardiol 37: 975–984, 2001 [DOI] [PubMed] [Google Scholar]
- 13. de Simone G, Roman MJ, Koren MJ, Mensah GA, Ganau A, Devereux RB. Stroke volume/pulse pressure ratio and cardiovascular risk in arterial hypertension. Hypertension 33: 800–805, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Fagard RH, Pardaens K, Staessen JA, Thijs L. The pulse pressure-to-stroke index ratio predicts cardiovascular events and death in uncomplicated hypertension. J Am Coll Cardiol 38: 227–231, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Fontana P, Boutellier U, Toigo M. Reliability of measurements with Innocor during exercise. Int J Sports Med 30: 747–753, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham heart study. Circulation 100: 354–360, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, Levy D. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 103: 1245–1249, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Haider AW, Larson MG, Franklin SS, Levy D. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med 138: 10–16, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Harms MP, Wesseling KH, Pott F, Jenstrup M, Van Goudoever J, Secher NH, Van Lieshout JJ. Continuous stroke volume monitoring by modelling flow from non-invasive measurement of arterial pressure in humans under orthostatic stress. Clin Sci (Lond) 97: 291–301, 1999 [PubMed] [Google Scholar]
- 20. Hirai T, Sasayama S, Kawasaki T, Yagi S. Stiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosis. Circulation 80: 78–86, 1989 [DOI] [PubMed] [Google Scholar]
- 21. Hopkins WG. Measures of reliability in sports medicine and science. Sports Med 30: 1–15, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Jansen JR, Schreuder JJ, Mulier JP, Smith NT, Settels JJ, Wesseling KH. A comparison of cardiac output derived from the arterial pressure wave against thermodilution in cardiac surgery patients. Br J Anaesth 87: 212–222, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Jarvis SS, Levine BD, Prisk GK, Shykoff BE, Elliott AR, Rosow E, Blomqvist CG, Pawelczyk JA. Simultaneous determination of the accuracy and precision of closed-circuit cardiac output rebreathing techniques. J Appl Physiol 103: 867–874, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Jellema WT, Wesseling KH, Groeneveld AB, Stoutenbeek CP, Thijs LG, van Lieshout JJ. Continuous cardiac output in septic shock by simulating a model of the aortic input impedance: a comparison with bolus injection thermodilution. Anesthesiology 90: 1317–1328, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Karamanoglu M, Feneley MP. On-line synthesis of the human ascending aortic pressure pulse from the finger pulse. Hypertension 30: 1416–1424, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of a new technique. J Am Coll Cardiol 20: 952–963, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Kelly RP, Tunin R, Kass DA. Effect of reduced aortic compliance on cardiac efficiency and contractile function of in situ canine left ventricle. Circ Res 71: 490–502, 1992 [DOI] [PubMed] [Google Scholar]
- 28. Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. III. Cellular and molecular clues to heart and arterial aging. Circulation 107: 490–497, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. I. Aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Langewouters GJ, Wesseling KH, Goedhard WJ. The pressure dependent dynamic elasticity of 35 thoracic and 16 abdominal human aortas in vitro described by a five component model. J Biomech 18: 613–620, 1985 [DOI] [PubMed] [Google Scholar]
- 31. Langewouters GJ, Wesseling KH, Goedhard WJ. The static elastic properties of 45 human thoracic and 20 abdominal aortas in vitro and the parameters of a new model. J Biomech 17: 425–435, 1984 [DOI] [PubMed] [Google Scholar]
- 32. Laszlo G. Respiratory measurements of cardiac output: from elegant idea to useful test. J Appl Physiol 96: 428–437, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 37: 1236–1241, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27: 2588–2605, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Levine BD, Lane LD, Buckey JC, Friedman DB, Blomqvist CG. Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation 84: 1016–1023, 1991 [DOI] [PubMed] [Google Scholar]
- 36. Lind L, Andren B, Sundstrom J. The stroke volume/pulse pressure ratio predicts coronary heart disease mortality in a population of elderly men. J Hypertens 22: 899–905, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 113: 657–663, 2006 [DOI] [PubMed] [Google Scholar]
- 38. McEniery CM, Wilkinson IB, Avolio AP. Age, hypertension and arterial function. Clin Exp Pharmacol Physiol 34: 665–671, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol 21: 2046–2050, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Mitchell GF, Lacourciere Y, Ouellet JP, Izzo JL, Jr, Neutel J, Kerwin LJ, Block AJ, Pfeffer MA. Determinants of elevated pulse pressure in middle-aged and older subjects with uncomplicated systolic hypertension: the role of proximal aortic diameter and the aortic pressure-flow relationship. Circulation 108: 1592–1598, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Nichols W, O'Rourke M. McDonald's Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles (4th Ed.). London: Edward Arnold, 1998 [Google Scholar]
- 42. Peyton PJ, Bailey M, Thompson BR. Reproducibility of cardiac output measurement by the nitrous oxide rebreathing technique. J Clin Monit Comput 23: 233–236, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Stergiopulos N, Segers P, Westerhof N. Use of pulse pressure method for estimating total arterial compliance in vivo. Am J Physiol Heart Circ Physiol 276: H424–H428, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 111: 3384–3390, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Tam E, Azabji Kenfack M, Cautero M, Lador F, Antonutto G, di Prampero PE, Ferretti G, Capelli C. Correction of cardiac output obtained by Modelflow from finger pulse pressure profiles with a respiratory method in humans. Clin Sci (Lond) 106: 371–376, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 88: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 48. van Lieshout JJ, Toska K, van Lieshout EJ, Eriksen M, Walloe L, Wesseling KH. Beat-to-beat noninvasive stroke volume from arterial pressure and Doppler ultrasound. Eur J Appl Physiol 90: 131–137, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol 74: 2566–2573, 1993 [DOI] [PubMed] [Google Scholar]
- 50. Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 113: 664–670, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.