Abstract
We report a case of a male patient with autoimmune pancreatitis in whom biochemical examination revealed high plasma chromogranin A concentrations, histological demonstration of a small lymphocytic infiltrate and rapid decrease in size of the pancreatic mass following short-lasting therapy with methylprednisolone. To our knowledge, this is the first patient with autoimmune pancreatitis who had a simultaneous increase of serum chromogranin A levels, circulating and urinary serotonin concentrations and urine 5-hydroxyindoleacetic acid concentrations. This is one of the few cases of mass forming pancreatitis with small lymphocytic infiltrate found in a Caucasian patient and rapid decrease in size of the pancreatic mass following short-lasting therapy with methylprednisolone.
Key Words: Autoimmune diseases, Differential diagnosis, Pancreatitis, Therapeutics
Introduction
It is well known that the frequency of new diagnoses of autoimmune pancreatitis has increased in the past few years [1, 2]. Autoimmune pancreatitis is characterized by diffuse or focal pancreatic swelling with a narrowing of the pancreatic duct and/or common bile duct. Elevated circulating IgG or IgG4 concentrations seem to be the hallmark of sclerosing pancreatitis [3] as well as the histological findings of lymphoplasmacytic infiltration, especially concentrated on the pancreatic ducts [4]. From a therapeutic point of view, steroids are a well-known efficacious treatment of autoimmune pancreatitis; however we do not have sufficient information about the duration of this treatment [5].
We herein report the case of a patient with autoimmune pancreatitis in whom biochemical examination revealed high plasma chromogranin A concentration, histological demonstration of a small lymphocytic infiltrate and disappearance of the pancreatic mass following therapy with methylprednisolone.
Case Report
CA, a 71-year-old Caucasian male, was admitted in October 2006 to the emergency department of our hospital for the duration of one month for mild epigastric pain associated with 5 days of jaundice. The patient was a heavy smoker (20 cigarettes per day for 50 years) and slight drinker (40 g of alcohol per day for 50 years). His past history included vitiligo, diagnosed at 40 years of age, chronic gastritis (histological diagnosis), chronic duodenitis, duodenal ulcer and hypertension. He chronically consumed proton pump inhibitors and ACE inhibitors.
Biochemical examination revealed an increase in serum concentrations of bilirubin (total bilirubin 9.2 mg/dl), AST (174 U/l, upper reference value 37 U/l), ALT (359 U/l, upper reference value 40 U/l), plasma serotonin (2.63 μmol/l, upper reference value 1.14 μmol/l), urinary serotonin (862 μg/g creatinine, upper reference limit 110 μg/g creatinine), urinary 5-hydroxyindoleacetic acid (5-HIAA) (8.9 mg/24 h, upper reference value 8.0 mg/24 h), and chromogranin A (490 U/l, upper reference limit 17 U/l). Serum amylase, lipase, CEA, and CA 19-9 were within the normal reference values.
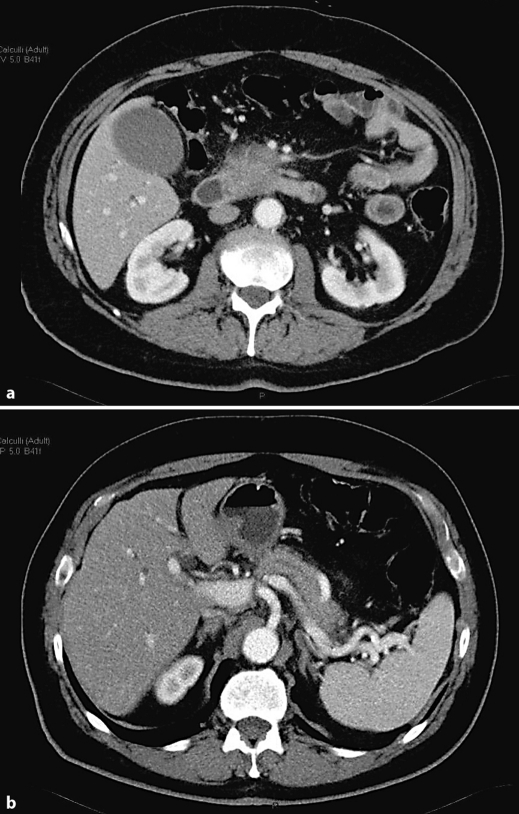
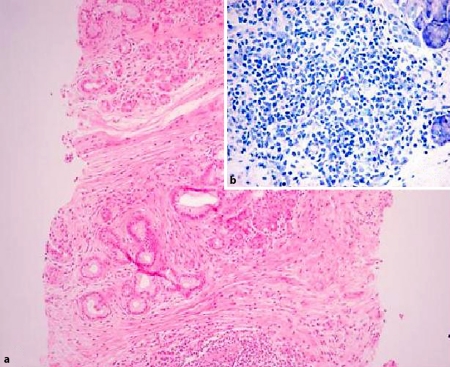
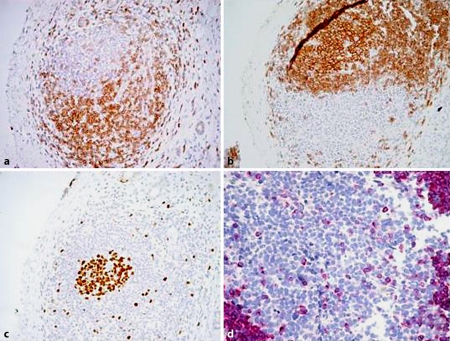
An abdominal ultrasonography showed a hypoechoic mass of about 3 cm in diameter of the head of the pancreas with a dilatation of both Wirsung duct and common bile duct. Computer tomography confirmed the echographic findings and also showed involvement of the duodenum, superior mesenteric artery, and splenic and portal veins; the lesion remained hypodense after contrast medium injection (fig. 1). In the following days there was an increase of jaundice and bilirubin reached 12.88 mg/dl and the patient underwent an ERCP, and an endoscopic sphincterotomy was performed and for the suspicion of a pancreatic tumour a wallstent was placed. To reach a diagnosis a core biopsy of the pancreas mass was performed. Sample tissue was fixed in 10% buffered formalin and embedded in paraffin. 4-µm-thin sections were cut and stained for haematoxylin-eosin and Giemsa. Immunohistochemistry was performed according to the APAAP and EnVision++ technique [6, 7] and the following molecules searched (source in brackets): CD3 (NeoMarkers), CD20 (Dako), CD10 (NovoCastra), Bcl-6 and IRTA-1 (kindly provided by Prof. B. Falini, University of Perugia), Bcl-2 (kindly provided by Prof. D. Mason, University of Oxford), and Ki-67/Mib1 (Dako). Histology showed fragments of pancreatic tissue whose structure was impaired by fibrotic bundles, replacing normal parenchyma with a moderate lymphoid infiltrate. The latter was organized in aggregates, mostly composed of small lymphocytes with regular nuclear profile and partially arranged in reactive looking follicles with prominent germinal centres; few mast cells and eosinophils were interspersed, while plasma cells were rare and located around the lymphoid foci (fig. 2). No lympho-epithelial lesions were seen. The residual acinar parenchyma was unremarkable. Immunohistochemistry proved the mixed reactive nature of the intra-pancreatic lymphoid infiltrate (CD3−/+, CD20+/−) with benign follicular structures that strongly expressed CD20, Bcl-6 and CD10, but not Bcl-2 (fig. 3) with high proliferation index in the germinal centres (Ki-67/Mib1), as expected. A diagnosis of autoimmune pancreatitis with fibrosis and benign follicular infiltrate consistent with an autoimmune pancreatitis was made. The clinical conditions of the patient progressively improved and the patient was discharged from the hospital on December 2006.
Fig. 1.
Baseline multidetector computer tomography. a Mass of the head of the pancreas. b Dilatation of the main pancreatic duct due to the head pancreatic mass.
Fig. 2.
a Pancreatic tissue with lymphoid aggregates and fibrotic bundles (haematoxylin-eosin, original magnification 100×). b Lymphoid nodules contain small lymphocytes with rare mast cells and plasma cells (Giemsa stain, original magnification 200x).
Fig. 3.
a CD3 staining in the follicle (EnVision++, original magnification 200×). b CD20 positivity in the same follicle (EnVision++, original magnification 200×). c Ki-67 staining: high proliferation index in the germinal centre (EnVision++, original magnification 200×). d Bcl-2 negativity in the germinal centre (APAAP, original magnification 400×).
In January 2007 a new assessment of biochemical examinations was carried out. Total bilirubin was 0.99 mg/dl, and AST and ALT returned within the normal reference range. The IgGs were within the normal reference values (881 mg/dl, upper reference value 1,600 mg/dl), as well as both IgA (194 mg/dl, upper reference value 400 mg/dl) and IgM (32 mg/dl, upper reference value 230 mg/dl), and the autoantibodies were not detectable. It is worth noting that plasma concentrations of chromogranin A fell within the normal reference range (10 U/l) as well as those of plasma serotonin, urinary serotonin, and urinary 5-HIAA.
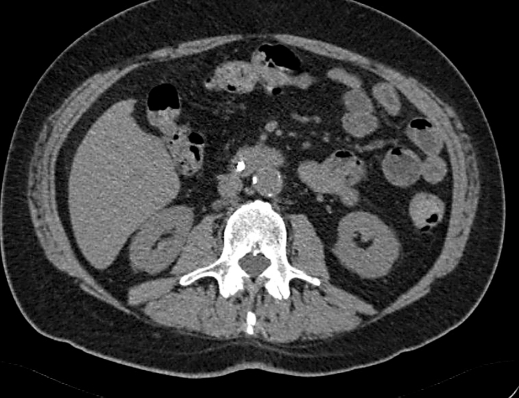
To better characterize the pancreatic mass, a contrast enhanced ultrasonography was carried out showing a diffuse hypoechoic pattern of the pancreas with late enhancement of the pancreatic lesion after contrast medium injection; an echoendoscopy confirmed the hypoechoic mass of 3 cm of diameter of the head of the pancreas. Because the size of the mass was unchanged from October 2006 and on the basis of a histological examination, a diagnosis of autoimmune pancreatitis was made and a treatment with methylprednisolone at a dosage of 1 mg/kg was started. After one month of this treatment, the patient underwent abdominal contrast enhanced CT which showed a clear decrease in size of the pancreatic mass. Six months after steroid treatment, the patient was in good general condition and the contrast enhanced CT showed a complete disappearance of the pancreatic mass (fig. 4).
Fig. 4.
Multidetector computer tomography carried out six months after steroid treatment showing the normalization of the pancreatic gland.
Discussion
The case reported presents some features that we consider worth reporting. To our knowledge, this is the first case of autoimmune pancreatitis with an increase in plasma and urinary serotonin, urinary 5-HIAA and chromogranin A. Why these biological parameters returned to normal levels before the steroid treatment is not clear, but the initial elevations cannot be related to the therapy, because the patient continued the PPI intake. In this case the firm diagnosis came from the histological examination of the pancreatic specimen obtained at core biopsy that showed pancreatic tissue whose structure was impaired by fibrotic bundles, replacing normal parenchyma with a moderate lymphoid infiltrate. The latter was organized in aggregates, mostly composed of small lymphocytes with regular nuclear profile and partially arranged in reactive looking follicles with prominent germinal centres; few mast cells and eosinophils were interspersed, while plasma cells were rare and located around the lymphoid foci. Immunohistochemistry proved the mixed reactive nature of the intra-pancreatic lymphoid infiltrate with benign follicular structures that strongly expressed CD20, Bcl-6 and CD10, but not Bcl-2 with high proliferation index in the germinal centres. In our experience of 15 cases of autoimmune pancreatitis [8, 9, 10, 11], this is the first case of autoimmune pancreatitis in whom a predominant T-lymphocyte expression was found. Japanese authors reported in the revised classification of 2006 that autoimmune chronic pancreatitis may be characterized, other than lymphoplasmacytic infiltrates, also by lymphocyte infiltration in which T cells are often predominant, and plasma cells are IgG4-positive [12]. Recently they also claimed that patients with neutrophilic infiltration in the epithelium of the pancreatic duct reported by American and Italian pathologists showed different clinicopathological features from autoimmune pancreatitis defined in Japan [13]. The third characteristic of this case is the spontaneous normalization of plasma and urinary serotonin, urinary 5-HIAA and chromogranin A. Finally, the inflammatory mass disappeared six months after steroid treatment, confirming that early introduction of steroid treatment is recommended especially for patients with obstructive jaundice associated with autoimmune pancreatitis histologically proven [14, 15] in order to avoid the mismanagement of a potentially resectable pancreatic malignancy [16].
Footnotes
Financial disclosure: The authors have not received any financial support, including pharmaceutical and industry support.
References
- 1.Kim KP, Kim MH, Lee SS, Seo DW, Lee SK. Autoimmune pancreatitis: it may be a worldwide entity. Gastroenterology. 2004;126:1214. doi: 10.1053/j.gastro.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 2.Pearson RK, Longnecker DS, Chari ST, Smyrk TC, Okazaki K, Frulloni L, Cavallini G. Controversies in clinical pancreatology: Autoimmune pancreatitis: does it exist? Pancreas. 2003;27:1–13. doi: 10.1097/00006676-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, Kiyosawa K. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 4.Kawaguchi K, Koike M, Tsuruta K, Okamoto A, Tabata I, Fujita N. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: A variant of primary sclerosing cholangitis extensively involving pancreas. Hum Pathol. 1991;22:387–395. doi: 10.1016/0046-8177(91)90087-6. [DOI] [PubMed] [Google Scholar]
- 5.Song MH, Kim MH, Lee SK, Seo DW, Lee SS, Han J, Seo DW, Min YI, Song DE, Yu E. Regression of pancreatic fibrosis after steroid therapy in patients with autoimmune chronic pancreatitis. Pancreas. 2005;30:83–86. [PubMed] [Google Scholar]
- 6.Pileri SA, Roncador G, Ceccarelli C, Piccioli M, Briskomatis A, Sabattini E, Ascani S, Santini D, Piccaluga PP, Leone O, Damiani S, Ercolessi C, Sandri F, Pieri F, Leoncini L, Falini B. Antigen retrieval techniques in immunohistochemistry: comparison of different methods. J Pathol. 1997;183:116–123. doi: 10.1002/(SICI)1096-9896(199709)183:1<116::AID-PATH1087>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Sabattini E, Bisgaard K, Ascani S, Poggi S, Piccioli M, Ceccarelli C, Pieri F, Fraternali-Orcioni G, Pileri SA. The EnVision++ system: a new immunohistochemical method for diagnostics and research. Critical comparison with the APAAP, ChemMate, CSA, LABC, and SABC techniques. J Clin Pathol. 1998;51:506–511. doi: 10.1136/jcp.51.7.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pezzilli R, Broccoli PL, Melandri R, Vandelli A, Re G, Fontana G. Exocrine pancreatic involvement in Wegener's granulomatosis. A case report. Ital J Gastroenterol. 1991;23:258–260. [PubMed] [Google Scholar]
- 9.Pezzilli R, Casadei R, Calculli L, Santini D. Autoimmune pancreatitis. A case mimicking carcinoma. JOP. 2004;5:527–530. [PubMed] [Google Scholar]
- 10.Pezzilli R, Casadei R, Santini D. Autoimmune pancreatitis associated with anisakis infection. Dig Liver Dis. 2007;39:273. doi: 10.1016/j.dld.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Pezzilli R, Morselli Labate AM, Ceciliato R, Frulloni L, Cavestro GM, Comparato G, Ferri B, Corinaldesi R, Gullo L. Quality of life in patients with chronic pancreatitis. Dig Liver Dis. 2005;37:181–189. doi: 10.1016/j.dld.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Okazaki K, Kawa S, Kamisawa T, Naruse S, Tanaka S, Nishimori I, Ohara H, Ito T, Kiriyama S, Inui K, Shimosegawa T, Koizumi M, Suda K, Shiratori K, Yamaguchi K, Yamaguchi T, Sugiyama M, Otsuki M, Research Committee of Intractable Diseases of the Pancreas Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol. 2006;41:626–631. doi: 10.1007/s00535-006-1868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamisawa T. Recent topics in autoimmune pancreatitis. Pancreas. 2007;35:86. [Google Scholar]
- 14.Hirano K, Tada M, Isayama H, Yagioka H, Sasaki T, Kogure H, Nakai Y, Sasahira N, Tsujino T, Yoshida H, Kawabe T, Omata M. Long-term prognosis of autoimmune pancreatitis without and with corticosteroid treatment. Gut. 2007;56:1719–1724. doi: 10.1136/gut.2006.115246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkelberg DL, Sahani D, Deshpande V, Brugge WR. Autoimmune pancreatitis. N Engl J Med. 2006;355:2670–2676. doi: 10.1056/NEJMra061200. [DOI] [PubMed] [Google Scholar]
- 16.Kwon S, Kim MH, Choi EK. The diagnostic criteria for autoimmune chronic pancreatitis: It is time to make a consensus. Pancreas. 2007;34:279–286. doi: 10.1097/MPA.0b013e31802eff5f. [DOI] [PubMed] [Google Scholar]