Abstract
Glutarimide-containing polyketides such as migrastatin (MGS) are well known for their ability to inhibit tumor cell migration. We have previously shown that MGS is derived from iso-migrastatin (iso-MGS) via a H2O-mediated ring-expansion rearrangement. A bacterial artificial chromosome (BAC) library of Streptomyces platensis NRRL18993, an iso-MGS producer, was constructed. From this library, pBS11001, a BAC clone harboring the intact iso-MGS biosynthetic gene cluster, was identified. Mobilization of pBS11001 into five heterologous Streptomyces hosts afforded recombinant strains, SB11001, SB11002, SB11003, SB11004, and SB11005, respectively. Under a standard set of media and fermentation conditions, the recombinant strains all produced the same profile of iso-MGS as that of S.platensis NRRL18993. These findings highlight the strength and flexibility of the BAC-based technology for natural product production and engineering in heterologous Streptomyces model hosts.
1. Introduction
“Combinatorial biosynthesis” – in its broadest sense, the generation of novel analogs of natural products by genetic engineering of biosynthetic pathways – complements traditional organic synthetic methods that often bear the burden of high production and environmental costs, particularly as the molecular complexity of target molecules increases. Development of expedient tools for recombinant DNA technologies in Streptomyces species and related microorganisms has made possible the application of genetic principles to meet the biotechnological challenge of drug discovery and development in these organisms. Specific, rather than random effects, can often be achieved by this approach, and target metabolites are produced by recombinant organisms amenable to large-scale fermentation.
Successful application of combinatorial biosynthesis requires (i) availability of the gene clusters encoding the synthesis of a given natural product or class thereof, (ii) genetic and biochemical characterization of the selected biosynthetic machinery to an extent sufficient for rational engineering, (iii) expedient genetic systems for in vivo manipulation of genes governing production of the target molecules in their native producers or heterologous hosts, and (iv) production of natural products or their engineered analogs in quantities sufficient for detection, isolation and characterization.1–4 In particular, successful application of combinatorial biosynthesis requires that the producing microorganism of choice be readily culturable and that the genetics of the host organism is compatible to those of the biosynthetic gene cluster of interest. This realization critically links the strength and flexibility of heterologous model hosts to the potential use of combinatorial biosynthesis strategies on novel natural products found in rare, slow-growing, or difficult to culture microrganisms.5–7 Notably, only ~1% of soil dwelling microorganisms have been cultivated in the lab and subsequently exploited for drug discovery and development purposes.8–10 This extraordinarily low, and discovery-limiting number is attributed to media, incubation times and inoculum sizes traditionally used to isolate and expediently culture rare soil bacteria. Advances in culturing techniques have broadened the repertoire of previously unculturable microbes examined for natural products. However, as surely as advances will continue to unveil new natural product scaffolds, the application of combinatorial biosynthesis methods to new biosynthetic gene clusters will require that target gene clusters reside and express in well characterized model organisms that are highly amenable to large scale fermentation, titer improvement and that do not call for highly specialized culturing methods or equipment.
Among the tools applicable to natural product production and engineering in heterologous hosts are bacterial artificial chromosomes (BACs). BAC vectors can harbor large DNA inserts (up to 600-kb), and BAC-based technology has been widely used in eukaryotic cell biology studies.11 However, there have been only a few reports of BAC-based strategies to clone large (>40-kb) natural product biosynthetic gene clusters for heterologous expression.12–16 Production of daptomycin, a lipopeptide antibiotic produced by Streptomyces roseosporus recently approved for the treatment of bacterial skin infections remains to our knowledge the only successful example. Daptomycin and a small library of engineered analogs were produced in Streptomyces lividans TK64 by expression of BAC clones harboring the entire 126-kb dpt biosynthetic gene cluster and its engineered progenies.16
There is a great need to investigate new hosts (preferably already optimized for high titer production of specific natural products) as producers of natural products for which combinatorial biosynthesis methods have been developed and for which there exists medical significance.5–7 Although a truly "universal" expression system suitable for all classes of natural products from all natural sources is unrealistic, the development of a suite of Streptomyces hosts enabling highly flexible and amplified production of a wide range of natural products from actinomycetes, represents a highly significant endeavor.
We are actively engaged in biosynthetic studies of the glutarimide-containing polyketides iso-migrastatin (iso-MGS), migrastatin (MGS), and the dorrigocins (DGNs) in Streptomyces platensis NRRL18993.17–20 Members of this class are potent inhibitors of human tumor cell migration21–25 and thus hold tremendous potential as inhibitors of tumor metastasis; metastasis being integrally dependent upon cell migration processes.26–28 Genome scanning of S. platensis NRRL18993 unveiled at least two glutarimide-containing polyketide biosynthetic loci, named the mgs (also known as DORR) and 088 clusters, respectively.29 However, inactivation of the mgs locus alone was sufficient to abolish the production of iso-MGS, MGS, and DGNs, suggesting that these metabolites share the same biosynthetic machinery.17 Since these early efforts, we have subsequently shown that neither MGS nor the DGNs are bona fide natural products.17 Rather, they are shunt metabolites of iso-MGS, derived from a H2O-mediated ring-expansion and ring-opeining rearrangement of iso-MGS (Figure 1).18,19 We also reported that iso-MGS can undergo a concerted [3,3]-sigmatropic rearrangement under neat heating conditions to afford MGS regio- and enantiospecifically.19
Figure 1.
Glutarimide-containing polyketides isolated from S. platensis NRRL18993: iso-migrastatin and its H2O-mediated ring-expansion and ring-opening rearrangements to migrastatin and dorrigocins.
Predicated on the importance of iso-MGS to the glutarimide-containing polyketide class of natural products, enhanced production methods for iso-MGS are likely to make related compounds of medicinal interest more readily available. Moreover, we have also begun to develop combinatorial biosynthetic methods applicable to the glutarimide-containing polyketides. In developing a suite of heterologous hosts capable of producing iso-MGS and related analogs (engineered or naturally-derived) we report here: (i) strategy for, and construction of, BAC clones capable of harboring large biosynthetic gene clusters (up to 200-kb), (ii) isolation of BAC clones from S. platensis NRRL18993 that harbor the iso-MGS gene cluster, (iii) mobilization of the BAC clones into five heterologous Streptomyces hosts, and (iv) production of iso-MGS in the resultant recombinant strains. These results validate the BAC-based technology for expressing large biosynthetic gene clusters for natural product production and engineering in heterologous hosts.
2. Results
2.1. BAC vector design for cloning of large biosynthetic gene clusters for expression in Streptomyces hosts
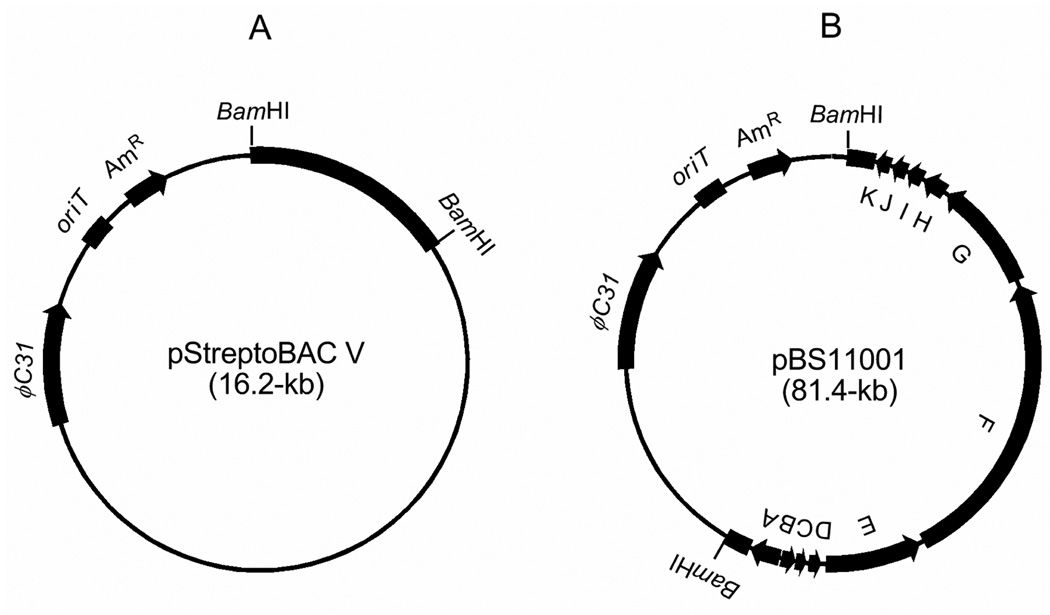
pStreptoBAC V, the BAC vector developed for cloning and expression of daptomycin in heterologous Streptomyces species,16 was adopted for this work. pStreptoBAC V features a set of carefully engineered characteristics, including (i) the origin of replication derived from pBACCe3.6 which allows maintenance of the BAC DNA in E. coli, (ii) the Streptomyces phage ΦC31 integrase responsible for site-specific integration into the att site of the chromosome, thereby stably maintaining the BAC in the heterologous Streptomyces host, (iii) the oriT element to facilitate efficient transfer between E. coli and Streptomyces by conjugation, (iv) the aac3(IV) apramycin resistance gene for use of apramycin as a selectable marker in both E. coli and Streptomyces, and (v) the BamHI sites for convenient cloning of the target natural product biosynthetic gene cluster in BAC library construction (Figure 2A).16
Figure 2.
BAC-based technology for natural product production and engineering in heterologous Streptomyces hosts: (A) the pStreptoBAC V vector, featuring two BamHI sites, the apramycin resistance marker (AmR), oriT, and ΦC31 and (B) the pBS11001 clone with 65-kb DNA insert from S. platensis NRRL18993 that harbors the intact mgs biosynthetic gene cluster.
The pStreptoBAC V vector is also characterized by the replication region from the high-copy-number plasmid pUC19 which resides between the two cloning sites. This was designed to enable a high copy number of the pStreptoBAC V vector to be maintained thus facilitating its preparation. For BAC library construction the pUC19 replication region in pStreptoBAC V was removed via BamHI digestion and the remaining vector ligated with large DNA fragments from S. platensis. As a result, BAC clones composing the initial library remained single copy in E. coli.16
2.2 Construction of the S. platensis NRRL18993 BAC library and isolation of the BAC clone pBS11001 containing the intact iso-MGS gene cluster
To construct the BAC library, S. platensis NRRL18993 chromosomal DNA was prepared in agarose plugs and partially digested with BamHI. The resulting DNA fragments were size-fractionated by two rounds of pulse field gel electrophoresis (PFGE). DNA fragments ranging in size from 100 to 250 kb were ligated with BamHI-digested pStreptoBAC V vector. Ligation mixtures were then transformed into E. coli DH10B competent cells by electroporation. Approximately 1000 transformants were obtained per 20 ng of DNA. The average insert size was 75-kb as determined by PFGE analysis of HindIII digests from 18 randomly selected clones (Figure 3).
Figure 3.
Analysis of BAC clones of S. platensis NRRL18992 library. Eighteen randomly picked BAC clones were digested with HindIII and the digested DNAs were analyzed by pulsed field gel electrophoresis (PFGE). Lanes 1 to 18, HindIII-digested BAC DNAs; M, DNA marker, with relevant sizes noted on right.
Genome scanning of S. platensis NRRL18993 has previously unveiled at least two loci, mgs and 088, whose deduced biosynthetic machineries were consistent to glutarimide-containing polyketide biosynthesis,17,29 but gene inactivation suggested that the mgs locus was the one responsible for iso-MGS biosynthesis.17 To isolate BAC clones that harbor the intact mgs cluster, the S. platensis NRRL18993 library (2400 clones) was first screened by hybridization using an upstream gene (mgsA) of the sequenced mgs locus as a probe; 17 positive clones were identified in this manner. Positive clones were then screened by PCR using primers from a downstream gene (mgsK) of the sequenced mgs locus. From this screen was identified one positive clone, pBS11001, containing a 65-kb insert, end-sequencing of which confirmed that it contains the complete mgs gene cluster (Figure 2B).
2.3 Mobilization of pBS11001 into heterologous Streptomyces hosts
pBS11001 was mobilized into Streptomyces albus J1074, Streptomyces lividans K4–114, Streptomyces coelicolor M512, Streptomyces avermitilis SUKA4, and S. avermitilis SUKA5 via conjugation. Apramycin resistant exconjugants were selected, isolated, purified, and named SB11001 [S. albus J1074(pBS11001)], SB11002 [S. lividans K4–114(pBS11001)], SB11003 [S. coelicolor M512(pBS11001)], SB11004 [S. avermitilis SUKA4(pBS11001)], and SB11005 [S. avermitilis SUKA5(pBS11001)], respectively. Integrations of pBS11001 into the chromosomes of the heterologous Streptomyces hosts were verified by PCR using primers mgsK-F and mgsK-R, designed according to the mgsK sequence. The desired ~ 800-bp PCR products were obtained from all recombinant strains, indicating the correct integration of pBS11001 into the chromosome of each heterologous host.
2.4 Production of iso-MGS in heterologous Streptomyces hosts
Before exerting extensive efforts to optimize iso-MGS titers for each host, the five recombinant strains, with the S. platensis NRRL18993 as a control, were subjected to a standard set of media and fermentation conditions to examine if iso-MGS was produced in each of the heterologous Streptomyces hosts. Thus, each of the five recombinant strains SB11001, SB11002, SB11003, SB11004, or SB11005 was grown in B2 or R2YE medium, respectively. Incubations were conducted for 4–5 days in the presence of 5% amberlite XAD-16 resin, after which time secondary metabolites were collected from culture broth via resin isolation and extraction from resins with ethanol.17–20 Crude extracts were analyzed by HPLC, and iso-MGS was identified on the basis of retention time, UV-Vis spectroscopy, co-injections with authentic iso-MGS and LC-MS analysis. As shown in Figure 4, all recombinant strains carrying pBS11001 produced iso-MGS as the major product that possesses an identical retention time, displays an identical UV-Vis spectrum, and yielded identical [M+H]+ and [M+Na]+ ions upon LC-MS analysis to that of an authentic standard.17–19 Finally, to unambiguously confirm the identity of iso-MGS produced in the heterologous hosts, the major product was isolated from SB11001 for NMR analysis; both 1H and 13C NMR spectra were identical to those of authentic iso-MGS.17–19 These data, taken together, confirm the production of iso-MGS by expression of the mgs gene cluster in heterologous hosts.
Figure 4.
HPLC analysis of extracts from recombinant strains SB11001 (II), SB11002 (III), SB11003 (IV), SB11004 (V), SB11005 (VI), with S. platensis NRRL18993 as a control (I), cultured in (A) B2 or (B) R2YE medium. ▼, iso-MGS. Normalization of iso-MGS signal intensity between panel A and B analyses required that five times as much R2YE-derived extract be injected compared to the corresponding B2-derived extract (see experimental section 5.5).
The titers of iso-MGS from the recombinant strains were then determined by HPLC analysis with the S. platensis NRRL18993 as a control,17–19 and these data are shown in Table 1. The yields of iso-MGS from the heterologous hosts are lower than those noted for S. platensis NRRL18993 under the conditions examined, and yields in B2 medium universally surpass those obtained using R2YE medium. This is not surprising given the fact that the B2 medium and the fermentation conditions examined were optimized previously for iso-MGS production in S. platensis NRRL18993.17–19
Table 1.
iso-MGS production titters in wild-type and recombinant strains.
| Titer (mg/L) | ||
|---|---|---|
| Strains | B2 medium | R2YE medium |
| S. platensis NRRl18993 | 58 ± 8 | 17 ± 1 |
| S. albus J1074(pBS11001) (SB11001) | 46 ± 4 | 0.6 ± 0.3 |
| S. lividans K4–114(pBS11001) (SB11002) | 25 ± 2 | 15 ± 1 |
| S. coelicolor M512(pBS11001) (SB11003) | 23 ± 4 | 5.3 ± 1 |
| S. avermitilis SUKA4(pBS11001) (SB11004) | 2.6 ± 0.6 | 3.4 ± 0.8 |
| S. avermitilis SUKA5(pBS11001) (SB11005) | 4.2 ± 0.4 | 1.3 ± 0.4 |
3. Discussion
More than 70% of antibiotics and anticancer drugs in use today are natural products or bear structures inspired by natural products.30,31 Thus, despite a move away from natural products during the 1990s, these compounds remain the best source of leads for new drug discovery efforts. Most antibiotics are isolated from microorganisms and are the products of secondary metabolite machinery. Despite their wealth of structure diversity and creative occupancy of chemical space which has proven very important in the identification of new drug chemotypes, natural product titers are often limited by the poor growth of the original microbial producer. Thus, securing significant quantities of secondary metabolites from their native producers can often be challenging, if not rate-limiting, to the drug development process. Moreover, it has been estimated that greater than 99% of the environmental microbes responsible for natural product biosynthesis are not culturable in the laboratory setting.8–10 However, difficulties with microbial cultivation and natural product titers can be circumvented via the use of heterologous hosts.5–7 Such hosts are able to house a given biosynthetic gene cluster in a readily cultivatable system that often possesses a genome more amenable to improved natural product titers and is, ideally, permissive of a wider array of genetic modifications to the integrated biosynthetic gene cluster. The latter consideration is particularly important from a perspective of combinatorial biosynthesis.
It is not uncommon to use multiple-plasmids harboring different parts of one gene cluster32 or to incorporate multiple-steps to clone a gene cluster from different cosmid clones into one plasmid to achieve heterologous expression.33,34 Multi-step strategies were popularized following the development of Red/ET recombinant technologies but continue to involve multiple manipulations.35,36 Alternatively, BAC plasmids, with the ability to harbor large DNA, make possible one step cloning of large biosynthetic gene clusters.11–16 BACs capable of shuttling between E. coli and Streptomyces are particularly attractive, providing a means to conveniently transfer large natural product biosynthetic gene clusters from E. coli to Streptomyces.12,16 Despite the fact that such an attempt was first reported almost a decade ago,12–16 BAC-based production of natural products in heterologous Streptomyces hosts remain very limited. Underlying the significance of findings reported here, to our knowledge, only the daptomycin gene cluster has been successfully expressed in the heterologous Streptomyces host using the BAC-based technology.16
An ideal heterologous host should grow quickly, be genetically amenable to manipulation, provide all necessary precursors and have little or no significant capacity for competitive endogenous secondary metabolite production.5–7 Moreover, there must be in place a facile means by which to integrate and ensure functional expression of large biosynthetic clusters within the host. S. coelicolor and S. avermitilis are routinely used as heterologous Streptomyces hosts in large part because of the extent to which their genomes are understood including genome sequencing.37,38 Although the genomics of S. albus is not as well defined as for S. coelicolor or S. avermitilis, it also has been widely used because of its genetic amenability and rapid rate of growth.5–7 In this study, we used S. coelicolor M512 whose production of red pigment undecycloprodigiosin (Red) and actinorhodin (Act) has been abolished by inactivation of the afsR regulatory gene.39 S. lividans K4–114 whose entire act gene cluster has been deleted,40 and S. albus J1074, a fast-growing strain known as an empirically excellent heterologous hosts for natural product production,6 also were selected as hosts for iso-MGS production. The entire genome of S. avermitilis ATCC31267 has been sequenced,38 and two loci containing putative biosynthetic gene clusters were deleted from its chromosome to generate S. avermitilis SUKA4 and SUKA5, which are unable to produce the endogenous panel of metabolites including avermectin, oligomycin, polyenes, carotenoids, melanins, and two nonribosomal peptides. These S. avermitilis strains also were used as hosts allowing one to assess the impact of endogenous secondary metabolite pathways upon iso-MGS production. pBS11001 harboring the intact mgs cluster was integrated into the chromosome of all hosts. The resultant recombinant strains SB11001, SB11002, SB11003, SB11004, and SB11005 were all found to produce iso-MGS under the conditions originally optimized for the native producer S. platensis NRRL18993 (Figure 4 and Table 1).
Pending fermentation optimization for each recombinant strain, the iso-MGS yield from heterologous hosts is, in some cases, on par with the native producer S. platensis NRRL18993. Conversely, production of iso-MGS in some host and medium combination is much lower than that with the native producer. While this may be attributed to a number of factors, including promoters, precursor availability, and competing biosynthetic pathways,5–7 it should be pointed out that the choice of media and fermentation conditions examined are biased towards the native producer S. platensis NRRL18993.17–19 Fermentation optimization would be the next logical step to improve iso-MGS titers in the recombinant strains.
Important to note is the impact of fermentation medium upon product profiles for all hosts evaluated. Both the native and recombinant strains for iso-MGS production produce what is clearly a more complex product profile following fermentation in R2YE than is the case for growth in B2 (Figure 4). This is most evident for iso-MGS production in SB11001 where the titer of 46 mg/L decreased to less than 1 mg/L in going from B2 to R2YE medium (Table 1). Any of the reasons noted above might explain this change although examination of Figure 4 makes clear that iso-MGS production in SB11001 in B2 is significantly cleaner than in R2YE. Within the margins of standard error, this is the case for all recombinant strains evaluated strongly suggesting that competing metabolic pathways detract from iso-MGS production in a manner partly driven by the R2YE medium. Nonetheless, we have demonstrated that BAC-based technology is highly practical and can be used as a general technology for producing natural products in high yields upon expression of the target gene cluster in selected model heterologous hosts. To achieve the highest titer of the target natural product in the recombinant strains, however, may require additional optimization of medium and fermentation conditions.
A practical technology for reliable production of natural products in user-friendly hosts provides tremendous opportunity to access natural products that cannot be accessed by other traditional means and allows the rapid correlation of genomics and bioinformatics information to specific natural products. Tools readily available for these model heterologous hosts can now be fully exploited for natural product production and engineering. The rewards of such efforts include a hastened ability to dissect biosynthetic pathways, as well as, the ability to enhance natural product titers and to engineer new analogs. Particularly appealing is the idea that a biosynthetic cluster (and associated secondary metabolite) whose importance might be marginalized by the genetics of its native producer can be made significantly more useful by simple transfer to a better host.
4. Conclusions
BAC-based technology was used to express the mgs gene cluster from S. platensis NRRL18993 in heterologous Streptomyces hosts. pBS11001, a BAC clone containing the intact mgs gene cluster, was obtained from the S. platensis NRRL18993 BAC library and transferred into five heterologous Streptomyces hosts. iso-MGS production was realized in all five resultant recombinant strains, highlighting BAC-based heterologous expression as a practical technology for natural product production and the broad exploitation of combinatorial biosynthesis methods for production of new natural product analogs not readily attained in their native producers or via conventional synthetic strategies.
5. Experimentals
5.1 Bacterial strains, plasmids and culture conditions
E. coli strains DH10B41 and ET12567/pUZ800242 were grown on solid or in liquid LB medium41 at 37 °C. S. platensis NRRL1899317–19 was cultured in TSB (Difco, Lawrence, KS) for 2 days at 28 °C for isolation of total DNA.42 S. platensis NRRL18993, S. albus J1074,6 S. lividans K4–114,40 and S. coelicolor M51239 were grown on ISP4 (Difco) medium for sporulation, while YMS medium43 was used for sporulation of S. avermitilis SUKA4 and SUKA5 strains (gift of Haruo Ikeda, Kitasato University, Japan). Antibiotics were added when necessary at the following concentrations: kanamycin, 50 µg/mL; apramycin, 25 µg/mL; ampicillin, 50 µg/mL.41,42
5.2 Genetic Manipulations
DNA manipulation was carried using standard methods.41,42 Genomic DNA of S. platensis NRRL18993 was isolated according to a general protocol.42 Unless specifically noted, restriction enzymes and other molecular biology reagents were purchased from Invitrogen and used as per manufacturer instructions. PCR amplification was carried out on a GeneAmp® PCR system 2400 (Applied Biosystems, Foster City, CA) with Takara LA Taq™ (Takara Bio USA, Madison, WI) with the supplied GC buffer II. Southern blotting was performed according to the standard protocols41 using the DIG-system (Roche, Palo Alto, CA). Appropriate DNA fragments were labeled with DIG-labelled dUTP and used as probes. BAC DNA was introduced into heterologous hosts by conjugation following literature methods.42
5.3 Construction of S. platensis NRRL18993 genomic BAC library and library screening
S. platensis NRRL18993 was cultured in TSBY medium42 containing 0.5 % glycine for 2 days. The cells were washed twice with water and resuspended in 2 volumes of suspension buffer (10 mM Tris, 100 mM EDTA, pH 8.0). The above mixture was mixed 1:1 with 1.2 % certified™ Low Melt Agarose (Bio-Rad, Hercules, CA) and put into the plug module. After solidification, the plugs were immersed in lysozyme buffer (10 mM Tris, pH 8.0, 100 mM EDTA, 0.5 % sodium lauryl sarcosine, 1 mg/mL lysozyme) and placed at 37 °C for 12 h. The buffer was then replaced by proteinase buffer (10 mM tris, pH 8.0, 400 mM EDTA, 1 % sodium lauryl sarcosine, 0.1 %, 1 mg/mL proteinase K). The tube containing the plugs was placed at 50 °C for 48 h with one proteinase buffer change at 24 h. After being washed 5 times with TE buffer, the plugs were used for partial digestion. Partial digestion of genomic DNA in plugs (10) was performed in digestion buffer (20 unit BamHI, 50 mM Tris, 10 mM MgCl, 100 mM NaCl, 1 mM DTT, 0.01 % BSA) for 20 min at 37 °C on ice. The DNA fragments in the plug were separated by PFGE twice on 1 % certified™ Low Melt Agarose (Bio-Rad) as follows: 1st PFGE, 4 V/ cm with a pulse time of 1 to 50 s for 24 h; cutting out the agarose band containing DNA fragments ranging between 100–250 kb; subjecting the resultant DNA fragments to 2nd PFGE, 4 V/ cm with a pulse time of 3 to 8 s for 20 h. After PFGE, the low melt agarose containing DNA fragments between 100-kb to 250-kb were digested with GLase (Epicentre, Madison, WI) and the solution was ligated with prepared BAC vector directly by using Fast- Link DNA ligase (Epicentre, Madison, WI). Ligation was performed at 16 °C overnight. The ligation mixture was transformed into MegeX DH10B™ Electrocomp™ E. coli (Invitrogen, Madison, WI) by electroporation (1.5 kv/mm) using an E. coli Pulser (Bio-Rad). BAC DNA was extracted from randomly selected BAC clones and HindIII digested DNAs were run on PFGE (5 to 12 s in 14 h, 6v/cm) to check the insert size. The BAC clones were picked from the plate and placed into freezing medium in 96-well microplates, cultured overnight and then stored at −80 °C. The S. platensis NRRL18993 BAC library clones were transferred onto Hybond-N+ membranes (Amersham Pharmacia, Piscataway, NJ) and fixed for hybridization.41 The membranes were hybridized with the mgsA probe, which was amplified with primers mgsA-F (5'-AGCGAAGGAGCGGTCGGAGGG-3') and mgsA-R (5'-GTCAGGTGGCGGACGAGGAGA-3'). The positive clones were then screened by PCR with primers mgsK-F (5'-CTCGACCCGCATCTCAGCTCCGA-3') and mgsK-R (5'-CGTCCAGCTTTCCGGGGTTCTCG-3') using the following conditions: 94 °C for 2 min; 35 cycles each of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min; 72 °C for 7 min.
5.4 Fermentation of the recombinant strains and S. platensis NRRL18993
Fermentations of the recombinant strains, with S. platensis NRRL18993 as a control, were carried out by following the published procedures.17–19 Two different media were used for host fermentations. B2 medium has been previously used for iso-MGS and related glutarimide productions.17–20 R2YE medium42 also was evaluated as a fermentation medium; both B2 and R2YE media contained 5 % Amberlite® XAD-16 resin (Sigma) as a critical element permitting facile metabolite isolation following fermentation.17–19 Spore suspensions (50 µL) of S. platensis NRRL18993 and the recombinant strains SB11001, SB11002, SB11003, SB11004, and SB11005 were added to 50 mL of seed medium in 250-mL flasks, respectively. The seed cultures were incubated on a rotary shaker at 250 rpm and 28 °C for 30–48 h until the cells grew to log phase. The resulting seed culture (2.5 mL, i.e., 5% of the production medium) was then added to 50 mL of production media in 250-mL flasks, which were incubated on a rotary shaker at 250 rpm and 28 °C for 4–5 days for each strain, respectively.
5.5 Isolation and HPLC and LC-MS analysis of iso- MGS from the recombinant strains and S. platensis NRRL18993
Isolation of iso-MGS from the recombinant and S. platensis fermentations followed the methods previously described.17–19 The significantly more complex metabolite profiles of R2YE-derived extracts relative to their B2 counterparts required that more of each R2YE-derived extract be injected relative to the corresponding B2-derived extract. Resin-derived extracts from 50 mL of cultures in B2 or R2YE medium were dissolved in either 10 mL or 1 ml of EtOH, respectively. HPLC injection volumes of the B2- and R2YE-derived extracts were 20 µL and 10 µL, respectively. Analytical HPLC was carried out on a Varian system equipped with in-line Prostar 330 detector (Woburn, MA). The mobile phase was comprised of buffer A (15 % CH3CN in H2O containing 0.1 % HOAc) and buffer B (80 % CH3CN in H2O containing 0.1 % HOAc). LC-MS was carried out on an Agilent 1100 HPLC-MSD SL quadrupole mass spectrometer equipped with both orthogonal pneumatically assisted electrospray and atmospheric pressure chemical ionization sources (Santa Clara, CA). Analytical HPLC and LC-MS was conducted using a Microsorb-MV C18 column (250 × 4.6 mm, 5 µm) (Varian Inc. Palo Alto, CA) eluted with a linear gradient of 100 % buffer A and 0 % buffer B to 20 % buffer A and 80 % buffer B over 20 min, followed by 10 min at 20 % buffer A and 80 % buffer B at a flow rate of 1.0 mL/min with UV detection at 205 nm. LC-MS analysis of the major product in the recombinant strains, with retention time identical to iso-MGS, afforded the [M+H]+, [M+H2O]+, and [M+Na]+ ions at m/z of 490.4, 507.4, and 512.4, respectively. 1H and 13C NMR data were acquired on a VARIAN Inova-500 (500 MHz) spectrometer. The sample was dissolved in CDCl3 with TMS as an internal standard.
Acknowledgments
We thank the Analytical Instrumentation Center of the School of Pharmacy, University of Wisconsin-Madison for support in obtaining MS and NMR data, Prof. Jose A. Salas, University of Oviedo, Spain, for the S. albus J1074 strain, and Prof. Haruo Ikeda, Kitasato University, Japan, for the S. avermitilis SUKA4 and SUKA5 strains. This work was supported in part by NIH grants CA78747 and CA113297.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thomas MG, Bixby KA, Shen B. In: Antitumor Agents from Natural Products. Kingston DGI, Cragg GM, Newman DJ, editors. Boca Rato, FL: CRC Press; 2005. pp. 519–551. [Google Scholar]
- 2.Van Lanen S, Shen B. Drug Discov. Today: Technol. 2006;1:409–436. doi: 10.1016/j.ddtec.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Van Lanen S, Shen B. Curr. Opinion Microbiol. 2006;9:252–260. doi: 10.1016/j.mib.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Van Lanen S, Shen B. Curr. Opinion Drug Discov Devel. 2008;11:186–195. [PubMed] [Google Scholar]
- 5.Wenzel SC, Muller R. Curr. Opinion Biotechnol. 2005;16:594–606. doi: 10.1016/j.copbio.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Galm U, Shen B. Exp. Opin. Drug Discov. 2006;1:409–437. doi: 10.1517/17460441.1.5.409. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Wang Y, Pfeifer BA. Mol. Pharm. 2008;5:212–225. doi: 10.1021/mp7001329. [DOI] [PubMed] [Google Scholar]
- 8.Kaeberlein T, Lewis K, Epstein SS. Science. 2002;296:1127–1129. doi: 10.1126/science.1070633. [DOI] [PubMed] [Google Scholar]
- 9.Keller M, Zengler K. Nat. Rev. Microbiol. 2004;11:141–150. doi: 10.1038/nrmicro819. [DOI] [PubMed] [Google Scholar]
- 10.Handelsman J. Microbiol. Mol. Biol. Rev. 2004;68:669–685. doi: 10.1128/MMBR.68.4.669-685.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shizuya H, Birren B, Kim UJ, Mancino V, Slepak T, Tachiiri Y, Simon M. proc. Natl. Acad. Sci. USA. 1992;89:8794–8787. doi: 10.1073/pnas.89.18.8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sosio M, Giusino F, Cappellano C, Bossi E, Puglia AM, Donadio S. Nat. Biotechnol. 2000;18:343–345. doi: 10.1038/73810. [DOI] [PubMed] [Google Scholar]
- 13.Beja O, Suzuki MT, Koonin EV, Aravind L, Hadd A, Nguyen LP, Villacorta R, Amjadi M, Garrigues C, Jovanovich SB, Feldman RA, DeLong EF. Environ. Microbiol. 2000;2:516–529. doi: 10.1046/j.1462-2920.2000.00133.x. [DOI] [PubMed] [Google Scholar]
- 14.Martinez A, Kolvek SJ, Yip CLT, Hopke J, Brown KA, MacNeil IA, Osburne MS. Appl. Environ. Microb. 2004;70:2452–2463. doi: 10.1128/AEM.70.4.2452-2463.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Z, Qi J, Tsuge T, Oba Y, Kobayashi T, Suzuki Y, Sakagami Y, Ojika M. Biosci. Biotechnol. Biochem. 2005;69:1372–1380. doi: 10.1271/bbb.69.1372. [DOI] [PubMed] [Google Scholar]
- 16.Miao V, Coeffet-LeGal MF, Brian P, Brost R, Penn J, Whiting A, Martin S, Ford R, Parr I, Bouchard M, Silva CJ, Wrigley SK, Baltz RH. Microbiology. 2005;151:1507–1523. doi: 10.1099/mic.0.27757-0. [DOI] [PubMed] [Google Scholar]
- 17.Ju J, Lim S-K, Jiang H, Shen B. J. Am. Chem. Soc. 2005;127:1622–1623. doi: 10.1021/ja043808i. [DOI] [PubMed] [Google Scholar]
- 18.Ju J, Lim S-K, Jiang H, Seo J-W, Shen B. J. Am. Chem. Soc. 2005;127:11930–11931. doi: 10.1021/ja053118u. [DOI] [PubMed] [Google Scholar]
- 19.Ju J, Lim S-K, Jiang H, Seo J-W, Her Y, Shen B. Org. Lett. 2006;8:5865–5868. doi: 10.1021/ol062470p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ju J, Seo J-W, Her Y, Lim S-K, Shen B. Org. Lett. 2007;9:5183–5186. doi: 10.1021/ol702249g. [DOI] [PubMed] [Google Scholar]
- 21.Nakae K, Yoshimoto Y, Sawa T, Homma Y, Hamada M, Takeuchi T, Imoto M. J. Antibiot. 2000;53:1130–1136. doi: 10.7164/antibiotics.53.1130. [DOI] [PubMed] [Google Scholar]
- 22.Woo EJ, Starks CM, Carney JR, Arslanian R, Cadapan L, Zavala S, Licari P. J. Antibiot. 2002;55:141–146. doi: 10.7164/antibiotics.55.141. [DOI] [PubMed] [Google Scholar]
- 23.Njardarson JT, Gaul C, Shan D, Huang X, Danishefsky SJ. J. Am. Chem. Soc. 2004;126:1038–1040. doi: 10.1021/ja039714a. [DOI] [PubMed] [Google Scholar]
- 24.Gaul C, Njardarson JT, Shan D, Dorn DC, Wu K, Tong W, Huang X, Moore MAS, Danishefsky SJ. J. Am. Chem. Soc. 2004;126:11326–11337. doi: 10.1021/ja048779q. [DOI] [PubMed] [Google Scholar]
- 25.Krauss IJ, Mandal M, Danishefsky SJ. Angew Chem. Int. Ed. 2007;46:5576–5579. doi: 10.1002/anie.200701837. [DOI] [PubMed] [Google Scholar]
- 26.Shan D, Chen L, Njardarson JT, Gaul C, Ma X, Danishefsky SJ, Huang X. Pro. Natl. Acad. Sci. USA. 2005;102:3772–3776. doi: 10.1073/pnas.0500658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodhouse EC, Chuaqui RF, Liotta LA. Cancer. 1997;80:1529–1537. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1529::aid-cncr2>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 28.Perez L, Danishefsky SJ. ACS Chem. Biol. 2007;2:159–162. doi: 10.1021/cb7000395. [DOI] [PubMed] [Google Scholar]
- 29.Farnet CM, Zazopoulos E, Staffo A, Yang X. WO 02/088176. International patent. 2002
- 30.Koehn FE, Carter GT. Nat. Rev. Drug Discov. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- 31.Newman DJ, Cragg GM. J. Nat. Prod. 2007;70:461–177. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 32.Xue Q, Ashley G, Hutchinson CR, Santi DV. Proc. Natl. Acad. Sci. USA. 1999;96:11740–11745. doi: 10.1073/pnas.96.21.11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDaniel R, Thamchaipenet A, Gustafsson C, Fu H, Betlach M, Betlach M, Ashley G. Proc. Natl. Acad. Sci. USA. 1999;96:1846–1854. doi: 10.1073/pnas.96.5.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang L, Shah S, Chung L, Carney J, Katz L, Khosla C, Julien B. Science. 2000;287:640–642. doi: 10.1126/science.287.5453.640. [DOI] [PubMed] [Google Scholar]
- 35.Gust B, Challis GL, Fowler K, Kieser T, Chater KF. Proc. Natl. Acad. Sci. USA. 2003;100:1541–1546. doi: 10.1073/pnas.0337542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wenzel SC, Gross F, Zhang Y, Fu J, Stewart AF, Muller R. Chem. Biol. 2005;12:349–356. doi: 10.1016/j.chembiol.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 37.Bentley SD, Chater KF, Cerdeno-Tarraga AM, Challis GL, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D, Bateman A, Brown S, Chandra G, Chen CW, Collins M, Cronin A, Fraser A, Goble A, Hidalgo J, Hornsby T, Howarth S, Huang CH, Kieser T, Larke L, Murphy L, Oliver K, O'Neil S, Rabbinowitsch E, Rajandream MA, Rutherford K, Rutter S, Seeger K, Saunders D, Sharp S, Squares R, Squares S, Taylor K, Warren T, Wietzorrek A, Woodward J, Barrell BG, Parkhill J, Hopwood DA. Nature. 2002;417:141–147. doi: 10.1038/417141a. [DOI] [PubMed] [Google Scholar]
- 38.Ikeda H, Ishikawa J, Hanamoto A, Shinose M, Kikuchi H, Shiba T, Sakaki Y, Hattori M, Ōmura S. Nat. Biotechnol. 2003;21:526–531. doi: 10.1038/nbt820. [DOI] [PubMed] [Google Scholar]
- 39.Floriano B, Bibb M. Mol. Microbiol. 1996;21:385–396. doi: 10.1046/j.1365-2958.1996.6491364.x. [DOI] [PubMed] [Google Scholar]
- 40.Ziermann R, Betlach MC. Biotechniques. 1999;26:106–110. doi: 10.2144/99261st05. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3rd ed. Plainview, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 42.Hopwood DA, Bibb MJ, Chater KF, Kieser T, Bruton CJ, Kieser HM, Lydiate DJ, Smith CP, Ward JM, Schrempf H. Genetic Manipulation of Streptomyces: A Laboratory Mannual. Norwich, UK: The John Innes Foundation; 2000. [Google Scholar]
- 43.Ikeda H, Kotaki H, Tanaka H, Omura SJ. Antimicrob. Agents Chemother. 1988;32:282–284. doi: 10.1128/aac.32.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]