Abstract
In ovarian cancer, the molecular targeted chemotherapeutics could increase the efficiency of low-dose radiotherapy while decreasing injury to adjusted organs. In irradiated A2780 human ovarian carcinoma cells, cytosolic phospholipase A2 (cPLA2) inhibitor AACOCF3 prevented activation of pro-survival Akt signaling and enhanced cell death. The potential molecular mechanisms of this effect could involve signaling through lysophosphatidic acid receptors. In the heterotopic A2780 tumor model using nude mice, cPLA2 inhibition significantly delayed tumor growth compared to treatment with radiation or vehicle alone. These results identify cPLA2 as a molecular target to enhance the therapeutic ratio of radiation in ovarian cancer.
Keywords: Cytosolic phospholipase A2, ovarian cancer, radiation therapy, lysophosphatidylcholine, lysophosphatidic acid
1. Introduction
Ovarian cancer is the fifth leading cause of cancer death among American women; it causes more mortality than any other gynecological cancer [1]. In 2009, twice as many women died of ovarian cancer compared to cancer of the uterine corpus, and more than three times as many compared to cervical cancer. Despite improvements in treatment over the past several decades, the mortality rates for ovarian cancer have remained relatively unchanged [1]. Following diagnosis, the primary form of treatment usually involves either a salpingooophorectomy or total hysterectomy [2]. The surgical removal of cancerous tissue is then succeeded by the administration of chemotherapeutic drugs such as cisplatin and other platinum-based chemotherapies [2]. These therapeutic agents have afforded modest increases in survival rates partially due to the acquired resistance [3]. Nonetheless, the combination of a platinum-based agent and a taxane such as paclitaxel is still considered the standard of care for the treatment of epithelial ovarian cancer [4; 5; 6].
Many women who have undergone surgery and chemotherapy regimens will eventually relapse due to microscopic residual disease. Such patients have an average survival of less than 3 years after chemotherapy [4]. Radiation therapy has been used to eliminate residual disease and thus reduce or delay recurrences [7]. However, the incorporation of radiation into the treatment protocols for ovarian cancer has been met with mixed results [8; 9]. The reluctance to target ovarian cancer cells with radiation partially results from injury to vital organs [10]. To avoid high levels of cytotoxicity to abdominal organs, radiation doses are currently limited to 30 Gy or less [10]. Despite these limitations, several studies have shown a survival advantage by using radiation therapy after surgery alone or surgery plus chemotherapy [9; 10; 11]. Furthermore, newer targeted therapies induce less toxicity as compared to traditional methods, and trials are underway to re-evaluate the role of radiotherapy in ovarian cancer [6].
Since cytotoxicity is a key limiting factor for the efficacy of radiotherapy, the use of agents that sensitize ovarian cancer to radiation-induced cell death could potentially improve the tumor response for ovarian cancer patients. We have previously shown that cytosolic phospholipase A2 (cPLA2) is a promising target for tumor sensitization to radiation therapy [12; 13]. This cytosolic enzyme is found in many different human and rodent tissues including ovarian tissue [14]. cPLA2 cleaves phosphatidylcholine (PC) at the sn-2 position, resulting in the formation of lysophosphatidylcholine (LPC) and arachidonic acid (AA) [15]. LPC has been implicated in cellular processes such as proliferation, migration, growth factor production, apoptosis, and adhesion molecule expression [15; 16; 17]. We have shown that in vascular endothelial cells, radiation-induced cPLA2-dependent accumulation of LPC leads to the phosphorylation/activation of the pro-survival kinases extra-cellular signal regulated protein kinase (ERK1/2) and Akt and results in radioresistance [12; 13]. LPC can also be converted to lysophosphatidic acid (LPA) via the actions of lysophospholipase D (LPLD), also known as autotaxin (ATX) [18; 19; 20; 21]. Among other functions, LPLD/ATX and LPA are important in angiogenesis, cell migration and metastasis of tumor cells, and tumor aggressiveness [19; 22; 23; 24; 25]. Ovarian cancers generate larger quantities of LPA than nonmalignant cells [26]. This phenomenon is attributed to the high activity of LPLD/ATX with the substrate specificity towards LPC that was found in peritoneal fluids from patients with ovarian cancer [27]. The prominent production of LPA in most ovarian cancers suggests that this is an autocrine system that promotes growth and invasion in such cancers. It has been demonstrated that specific G-protein coupled receptors (GPCRs) mediate the cellular effects of LPA [22]. Three of seven identified LPA-specific receptors, Edg-2/LPA1, Edg-4/LPA2, and Edg-7/LPA3, belong to the endothelial cell differentiation gene (EDG) family. Most ovarian cancer cells overexpress LPA receptors relative to normal ovarian cells, especially LPA2 and LPA3 [28; 29; 30]. Moreover, expression of LPA receptors was found to determine tumorigenicity and aggressiveness of ovarian cancer cells [23].
To investigate whether these cPLA2–dependent signaling events contribute to the ovarian cancer response to radiation, we assessed the effects of cPLA2 inhibition with arachidonyltrifluoromethyl ketone (AACOCF3) on irradiated ovarian carcinoma cells both in cell culture and in vivo. AACOCF3 is an analog of arachidonic acid that is able to permeate cell membranes and interact with cPLA2 active site [31; 32]. This inhibitor has been used for previous studies by our laboratory [12; 13] as well as by other groups [25; 33; 34]. In the present study, we found that inhibition of cPLA2 prior to irradiation decreased cell survival and tumor growth of cisplatin-sensitive ovarian cancer. These results suggest that cPLA2 is a potential molecular target for increasing the sensitivity of ovarian cancer cells to ionizing radiation.
2. Materials and methods
2.1. Cell cultures and treatment
The human ovarian epithelial adenocarcinoma cell lines A2780 and CP70 (obtained from Dr. Thomas C. Hamilton, Dept. of Medical Oncology, Fox Chase Cancer Center, Philadelphia, PA) were maintained in RPMI 1640 (Life Biosciences) plus 10% fetal bovine serum, 1% penicillin/streptomycin, 2 mM L-glutamine, and 0.25 units/ml insulin (Life Technologies, Grand Island, NY). The cells were kept in a 37°C and 5% CO2 environment. AACOCF3 was purchased from EMD Biosciences (San Diego, CA). Cells were pre-treated with either vehicle (70% EtOH) or 1 µmol/L AACOCF3 (in 70% EtOH) for 30 minutes prior to irradiation. A Mark I 137Cs irradiator (J.L. Shepherd and Associates) was used to deliver radiation to the cells.
2.2. Clonogenic survival
A2780 and CP70 cells were plated and allowed to attach for 3 hours. Cells were then treated with vehicle 70% EtOH or 1 µmol/L AACOCF3 for 30 minutes, followed by irradiation with 0, 2, 4, 6, or 8 Gy. The medium was changed after irradiation. After 10–14 days, plates were fixed with 70% EtOH and stained with 1% methylene blue. Colonies consisting of >50 cells were counted with a dissection microscope. The average surviving fraction was calculated as (number of colonies/number of cells plated)/(number of colonies for corresponding control/number of cells plated) with SEM from two experiments performed in triplicates.
2.3. Western immunoblot analysis
A2780 or CP70 cells were plated and allowed to grow to near confluency and treated as required. Cells were harvested at 2, 3, 5, 10, 15, and 30 minutes and 2, 6, 18, and 24 hours after the beginning of irradiation. Total protein extraction was performed using an M-PER kit (Thermo Fisher Scientific, Rockford, IL). Protein concentration was quantified using BCA reagent (Thermo Fisher Scientific, Rockford, IL). Protein extracts (100 µg) were subjected to Western immunoblot analysis using antibodies for the detection of phospho-ERK1/2Thr202/Tyr204, total ERK1/2, phospho-AktThr308/Ser473 and total Akt (all from Cell Signaling Technologies, Danvers, MA). Antibody to β-actin (Sigma Aldrich, St. Louis, MO) was used to evaluate protein loading in each lane. Immunoblots were developed using Western Lightning Chemiluminescense Plus detection system (PerkinElmer, Wellesley, MA) according to the manufacturer’s protocol.
To assess for LPLD/ATX, LPA1, and LPA2, A2780 and CP70 cells were plated and allowed to grow to sub-confluency. Then conditioning media were collected, and cells were lysed. The conditioning medium was analyzed using an antibody to LPLD/ATX (Santa Cruz); the cell lysates were analyzed using antibodies to LPA1 (Novus Biologicals, Littleton, CO), LPA2 (Santa Cruz Biotechnology, Santa Cruz, CA), and β-actin (Sigma Aldrich, St. Louis, MO).
2.4. Mouse tumor model, treatment, and tumor growth delay study
Female athymic nude-Foxn1nu mice were purchased from Harlan Laboratories and maintained in sterile housing. Institutional Animal Care and Use Committee guidelines were followed when handling and treating all mice used in this study (IACUC animal protocol M/07-358). A2780 cells (106) were implanted into mouse hind limbs. Once tumors exceeded 200 mm3, the mice were stratified into four groups of 5 animals, each representing similar distributions of tumor sizes. A2780 tumor-bearing animals were administered intraperitoneally with vehicle (70% EtOH) or 10 mg/kg AACOCF3 30 minutes prior to irradiation with 3 Gy (Therapax DXT 300 X-ray machine; Pantak Inc.). The vehicle only and AACOCF3 only groups were not exposed to any radiation but were transported to the radiation facility along with the rest of the mice. The four treatment groups were EtOH only, EtOH plus irradiation, AACOCF3 only, and AACOCF3 plus irradiation. Treatment was repeated for 3 consecutive days. Tumor size was measured every other day by external digital caliper measurements.
2.5. Measurement of LPA levels in irradiated cells
A2780 cells were plated, allowed to grow to confluency, and irradiated with 3 Gy. LPA levels were measured in the conditioning medium up to 120 minutes after irradiation using a competitive ELISA kit according to manufacturer’s protocols (Echelon Biosciences, Inc., Salt Lake City, UT).
2.6 Statistical analysis
The mean and S.E.M. of each treatment group were calculated. Variance was analyzed by Student's t-test; P-value <0.05 was considered statistically significant.
3. Results
3.1. Inhibition of cPLA2 with AACOCF3 decreases clonogenic survival of irradiated A2780 cells
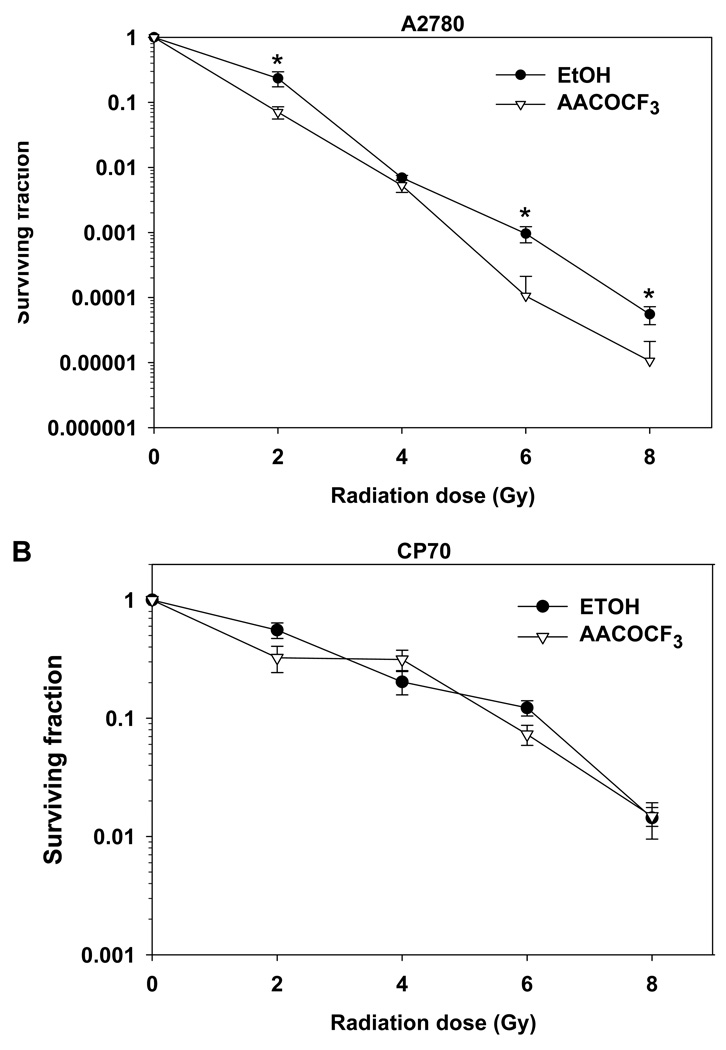
To determine whether cPLA2 inhibition affects cellular viability in irradiated tumor cells, we performed clonogenic survival assays for A2780 and CP70 cells (Fig. 1). Treatment with AACOCF3 decreased colony formation and enhanced cell death among irradiated A2780 cells. Statistically significant decreases in colony formation were observed at 2, 6, and 8 Gy among AACOCF3-treated cells, with the maximum effect occurring at 6 Gy (Fig. 1A). In contrast to A2780, CP70 cells displayed less radio-sensitivity overall and failed to exhibit decreased survival in response to cPLA2 inhibition and irradiation (Fig. 1B).
Fig. 1.
Inhibition of cPLA2 with AACOCF3 enhances cell death in irradiated ovarian carcinoma A2780 but not in CP70 cells. A2780 (A) and CP70 (B) cells were plated and treated with 1 µM AACOCF3 for 30 min prior to irradiation. After 1 week, colonies consisting of ≥ 50 cells were counted and normalized for plating efficiency. Shown are average surviving fractions and SEM from three experiments; * p < 0.05.
3.2. Ionizing radiation causes early activation of Akt in A2780 cells and late activation of ERK1/2 in both A2780 and CP70 cells
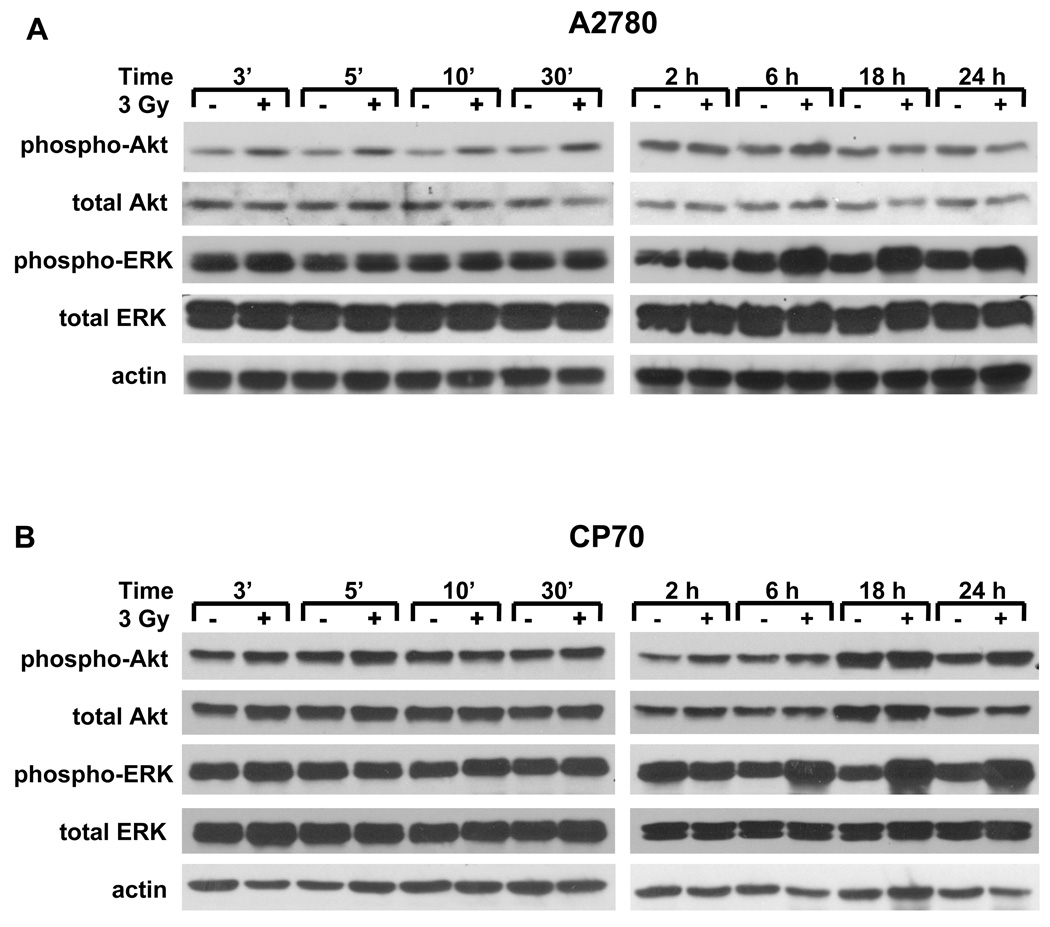
We have previously shown that activation of cPLA2 in response to irradiation of vascular endothelial cells resulted in phosphorylation of ERK1/2 and Akt within a few minutes [12; 13]. To determine if these kinases are activated in irradiated human ovarian carcinoma cells, we performed a time course study for both A2780 and CP70 cells (Fig. 2A). In the A2780 cells, considerably increased Akt phosphorylation was observed at 3, 5, 10, and 30 minutes after the beginning of irradiation with 3 Gy. An increase in early (≤30 minutes) ERK1/2 phosphorylation was not significant compared to Akt (Fig. 2A). In CP70 cells, we did not observe changes in early Akt and ERK1/2 phosphorylation (Fig. 2B).Since the pattern of ERK1/2 phosphorylation is often biphasic [35; 36] and late phosphorylation of ERK1/2 is known to be induced by cytotoxic insults in ovarian carcinoma cells [37; 38], we also assessed late activation of ERK1/2 in irradiated A2780 and CP70 cells. Western blot analysis revealed significant induction of ERK1/2 phosphorylation at 6, 18, and 24 hours following irradiation with 3 Gy in both A2780 and CP70 cell lines (Fig. 2).
Fig. 2.
Radiation induces distinct phosphorylation of pro-survival kinases Akt and ERK1/2 in A2780 and CP70 cells. A2780 (A) and CP70 (B) were treated with 3 Gy and lysed at 3 minutes-24 hours after the beginning of irradiation. Shown are the Western blot analyses with phospho-specific antibodies to AktThr308/Ser473 or ERK1/2Thr202/Tyr204, total Akt or ERK1/2, and actin.
3.3. Inhibition of cPLA2 with AACOCF3 prevents early activation of Akt in A2780 cells
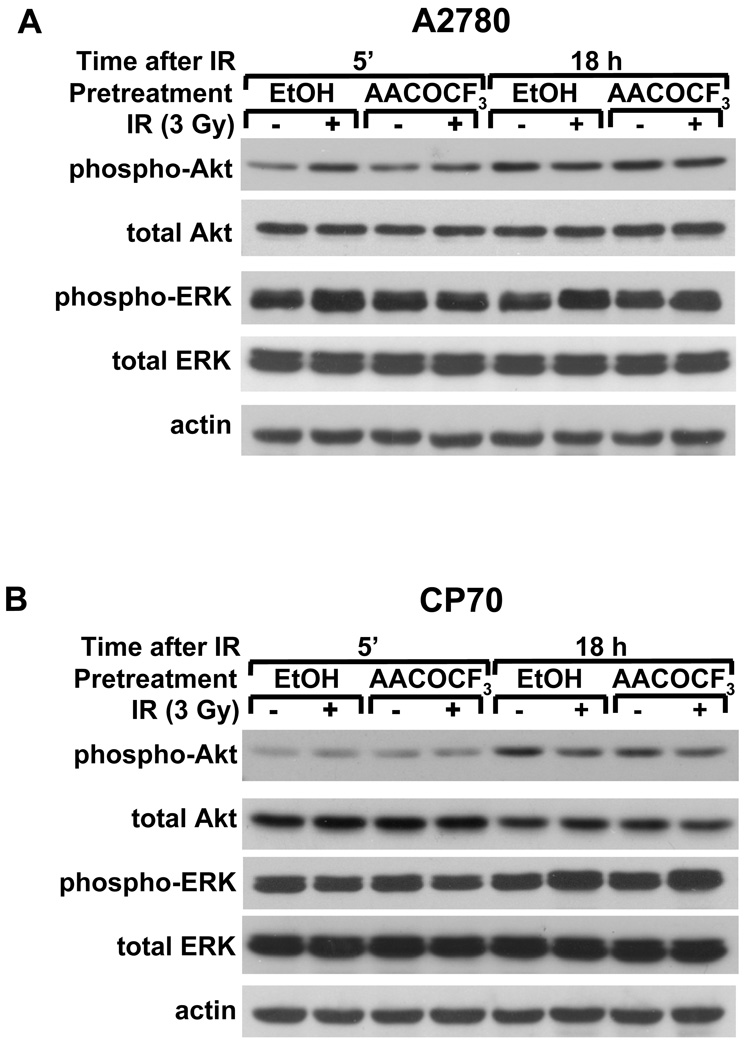
To determine whether radiation-induced early phosphorylation of Akt (5 min after IR) and late phosphorylation of ERK1/2 (18 h after IR) is cPLA2-dependent, we treated A2780 and CP70 ovarian carcinoma cells with AACOCF3 for 30 minutes prior to irradiation with 3 Gy (Figure 3). In A2780 cells, cPLA2 inhibition prevented early Akt activation (Fig. 3A, 5 min) but had no effect on ERK1/2 activation at 18 hours (Fig. 3A, 18 h). Inhibition of cPLA2 also had no effect on radiation-induced late ERK1/2 activation in CP70 cells (Fig. 3B).
Fig. 3.
cPLA2 is required for radiation-induced early Akt and ERK1/2 phosphorylation in A2780 but not in CP70 cells. A2780 (A) and CP70 (B) cells were treated with 1 µM AACOCF3 for 30 min prior to irradiation with 3 Gy and lysed 5 minutes or 18 hours after the beginning of irradiation. Shown are the Western blot analyses with phospho-specific antibodies to AktThr308/Ser473 or ERK1/2Thr202/Tyr204, total Akt or ERK1/2, and actin.
3.4. Inhibition of cPLA2 with AACOCF3 delays tumor growth in irradiated heterotopic mouse model of A2780 ovarian carcinoma
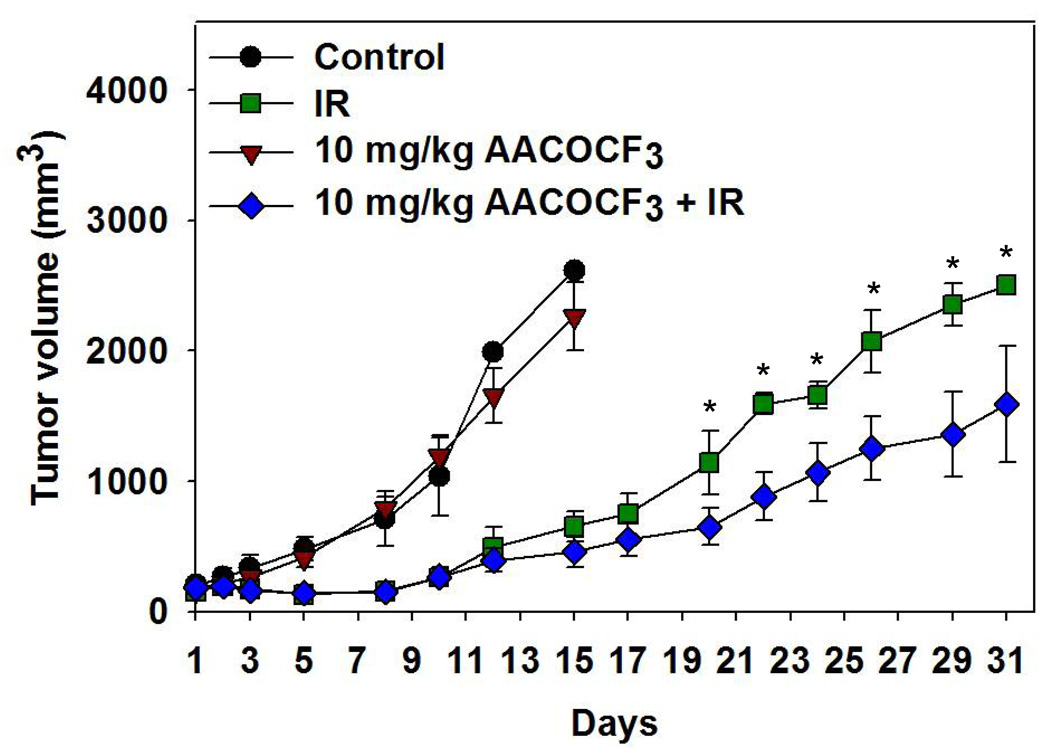
Previous studies by our laboratory have shown decreased growth of lung tumors in heterotopic mouse models treated with AACOCF3 and fractionated radiotherapy [13]. Using a similar model, we wanted to determine if cPLA2 inhibition could also increase the efficacy of radiation therapy and delay tumor growth in A2780 ovarian tumors. Tumor-bearing mice were given 3 consecutive daily treatments of vehicle control or AACOCF3 (10 mg/kg) in the presence or absence of radiation. Treatment response was monitored by caliper measurements at 48 h intervals. Radiation alone substantially inhibited growth of A2780 carcinoma cells and, for the first 10 days after treatment initiation, this treatment group demonstrated a nearly identical increase in tumor volume compared to mice that received a combined treatment of AACOCF3 and radiation (Fig. 4). After day 10, however, a visible distinction between these tumor growth rates could be detected. Subsequently, 31 days after the initiation of treatment, a statistically significant tumor growth delay was observed in mice that received a combination of AACOCF3 and radiation compared to mice treated with radiation (Fig. 4, delay of 10 days) or vehicle alone (Fig. 4, delay of 19 days).
Fig. 4.
Inhibition of cPLA2 with AACOCF3 decreases tumor size in irradiated mouse models. Using heterotopic tumor models of A2780 human ovarian carcinoma, nude mice were treated intraperitoneally with vehicle (control) or 10 mg/kg AACOCF3, and tumors were irradiated 30 minutes later with 3 Gy. Treatment was repeated for 5 consecutive days. Tumor volumes were calculated using external caliper measurements. Shown are the mean A2780 tumor volumes in each of the treatment groups (control, IR, AACOCF3, and AACOCF3 + IR) with SEM from group of 5 mice; * p < 0.05.
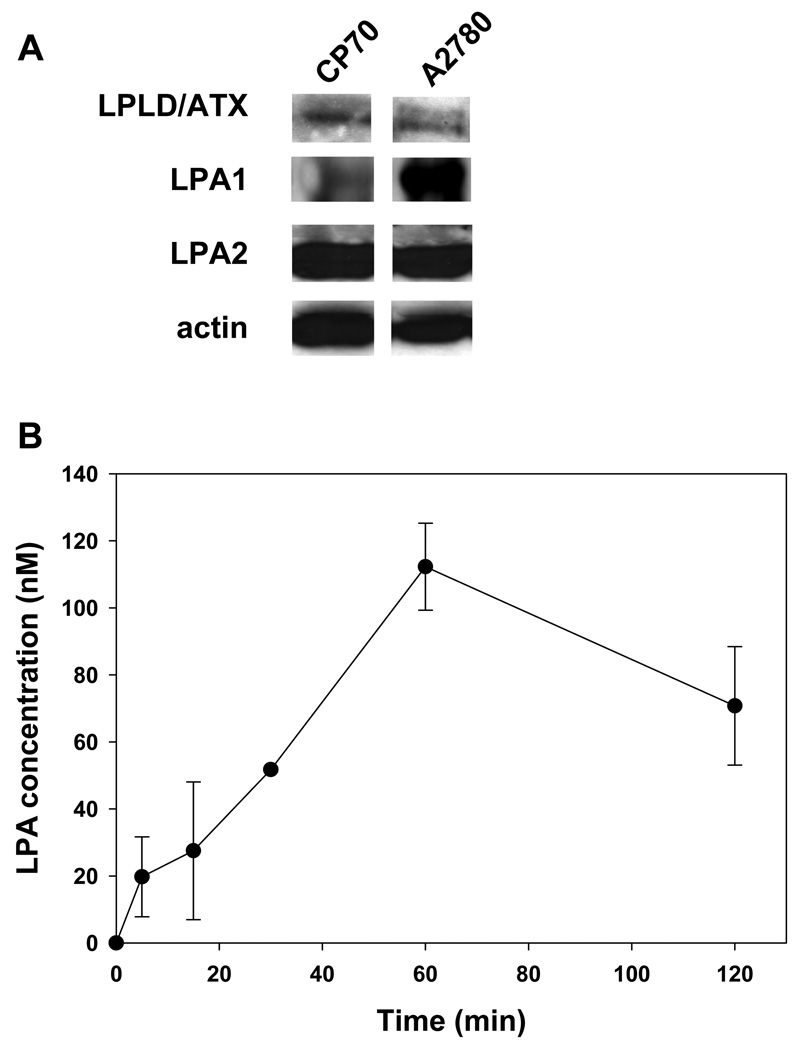
3.5. LPLD/ATX, LPA1, and LPA2 are expressed in A2780 and CP70 cells, and LPA levels in A2780 cells increase following irradiation
We have shown that the primary lipid second messenger that is produced by radiation-induced cPLA2 activation is LPC [12]. Since LPC is often converted to LPA by LPLD/ATX, and increased activity of LPLD/ATX and LPA receptors is associated with ovarian cancer progression [23; 24; 25], we assessed the protein levels of LPLD/ATX and LPA receptors 1 and 2 (LPA1 and LPA2) in non-irradiated A2780 and CP70 cells. The basal levels of LPLD/ATX and LPA2 were approximately the same in both cell lines, but A2780 cells displayed much higher levels of LPA1 (Fig. 5A). To determine whether LPA production is increased in response to radiation, LPA levels in A2780 cells were measured at 2–120 minutes after the beginning of irradiation with 3 Gy (Fig. 5). Elevated LPA production was detected within minutes after irradiation, but the most pronounced increase in LPA concentration occurred at 60 minutes after exposure (Fig. 5B).
Fig. 5.
LPLD/ATX, LPA and LPA 1 and 2 are expressed in ovarian cancer cells A2780 and CP70. (A). A2780 and CP70 ovarian carcinoma cells were grown to sub-confluency. Shown is Western blot analysis of total protein from conditioning medium using anti-LPLD/ATX antibody and from cell lysates using antibody to LRA1, LPA2 and actin. (B). A2780 cells were irradiated with 3 Gy. LPA levels were measured in conditioning medium from A2780 cells at 0–120 minutes after irradiation using ELISA. Shown are average LPA concentration and SEM from three experiments.
4. Discussion
Activation of pro-survival signaling cascades in response to ionizing radiation contributes to radioresistance and poor treatment outcomes. One important enzyme in this process is cPLA2 [39]. We have demonstrated that radiation-induced activation of cPLA2 leads to the production of LPC and subsequent activation of pro-survival kinases, such as Akt and ERK1/2, resulting in the promotion of cellular proliferation and tumor growth. Inhibition of cPLA2 prevents radiation-induced pro-survival signaling and, thus, improves tumor response to radiation therapy [12; 13].
We studied the effects of cPLA2 inhibition on the radiation response of two human ovarian carcinoma cell lines, A2780 and CP70. A2780 cells are known to be more sensitive to cisplatin while CP70 cells were derived by exposure of A2780 cells to cisplatin resulting in acquired cisplatin resistance [38; 40; 41]. Since radiation is a cytotoxic DNA-damaging insult similar to cisplatin, CP70 cells were also more resistant to radiation compared to A2780. With the acknowledged role of cPLA2 in radioresistance, we checked whether inhibition of this enzyme would affect survival of irradiated ovarian carcinoma cells. Contrary to our expectations, cPLA2 inhibition did not affect radiation response of radioresistant CP70 cells, but rather decreased survival of irradiated radiosensitive A2780 cells (Fig. 1). These data suggested that radiation induces cPLA2 activation in A2780 cells; however, the response of CP70 cells to radiation is cPLA2 independent. Indeed, we found that early activation of Akt occurs in A2780 but not in CP70 cells, and that it is abrogated with cPLA2 inhibition (Figs. 2, 3). Interestingly that radiation-induced late activation of ERK1/2 occurs in both cell lines, and it is cPLA2 independent (Figs 2, 3). Involvement of this late activation of ERK1/2 in survival of ovarian carcinoma cells treated with the DNA-damaging drug cisplatin has been reported by us previously [37; 38; 41; 42; 43; 44]. The cPLA2-dependent pro-survival signaling in irradiated ovarian carcinoma cells introduces new possibilities for pharmacologic interventions. In a tumor growth study, cPLA2 inhibition and irradiation resulted in a significant tumor growth delay of 10 days compared to mice that received radiation alone and 19 days compared to mice treated with vehicle alone (Fig. 4).
To elucidate the molecular mechanisms of cPLA2-dependent radiation response in A2780 cells, we performed screening experiments for possible players in cPLA2/LPC pathway. Since LPC is frequently converted to LPA by LPLD/ATX, and LPA is associated with ovarian cancer progression, we investigated the effects of radiation on LPA production. Increased LPA concentration was detected within minutes of irradiation in A2780 cells and continued to rise until reaching a maximum level at 60 minutes (Fig. 5B). Interestingly, LPA has been reported to also activate cPLA2, thus possibly forming an autocrine loop in which it increases its own production [17; 23; 33; 34]. The enhanced levels of LPC [45; 46], LPA [17; 23; 25; 47; 48; 49], and LPLD/ATX [20; 24] have been found in the ascites of ovarian cancer patients. Furthermore, the previously-described “ovarian cancer activating factor” (OCAF) was later determined to be a combination of different forms of LPA [48]. Ascitic LPA levels in ovarian cancer were the highest among all cancers studied in one report [47]; this potent lipid mediator is also implicated in the generation of ascites, a major cause of morbidity in ovarian cancer [34]. Based on the higher than normal levels of LPC and LPA in the plasma of ovarian cancer patients, these bioactive lipids are being investigated as potential biomarkers of ovarian cancer.
In addition to LPA generation, we also observed that ATX/LPLD and LPA receptors 1 and 2 are present in both A2780 and CP70 cells (Figure 5A). LPA receptors are abnormally expressed in several different human cancers, and their activation correlates with tumorigenesis [19; 23; 47; 50]. Importantly, A2780 cells exhibited much higher expression of LPA1 compared to CP70 cells. This striking low level of LPA1 expression could be acquired by CP70 cells as a result of cispltin resistance and could be a key element in sensitivity of irradiated A2780 to cPLA2 inhibition compared to CP70 cells. Further investigation is needed, however, in order to determine the detailed molecular mechanisms of LPA receptors involvement in the differential response of ovarian cancer to radiation therapy.
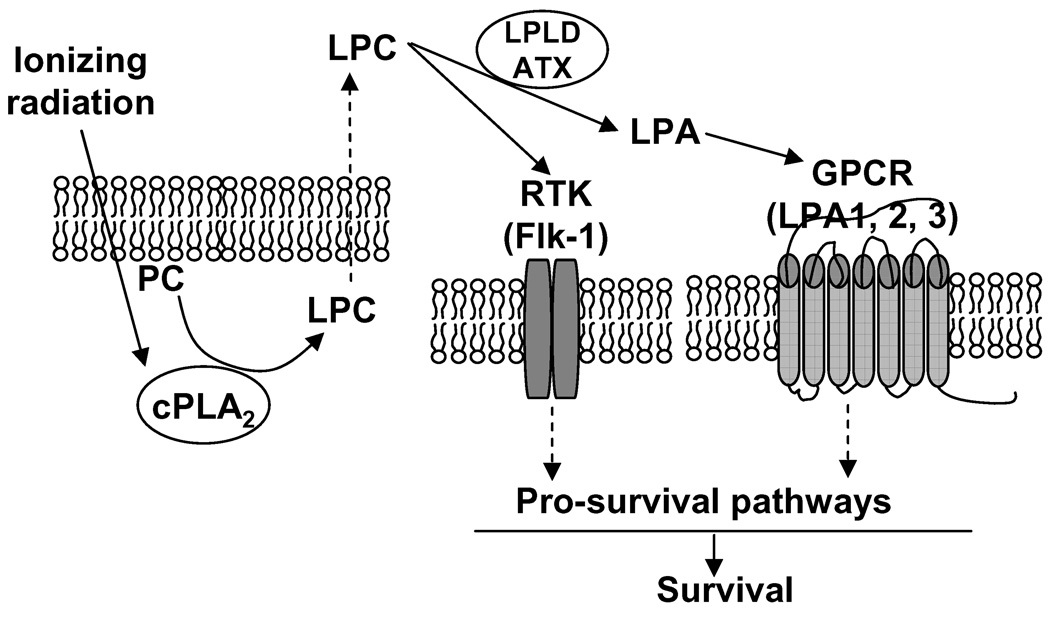
We summarize these findings in Figure 6. We propose that upon irradiation of ovarian carcinoma cells (A2780), the production of the second messenger LPC is increased as a result of radiation-induced cPLA2 enzymatic cleavage of PC. LPC can move through the cellular membrane and trigger activation of pro-survival signal transduction pathways through direct binding to RTKs (receptor tyrosine kinases) such as Flk-1 in endothelial cells as we reported previously [12]. Alternatively, LPC could be further metabolized to LPA, another biologically active lysophospholipid, by LPLD/ATX. LPA in turn activates LPA-specific GPCRs, LPA1 and LPA2. Activation of these receptors initiates pro-survival signal transduction pathways such as ERK1/2 and Akt. These data suggest that resistance to cPLA2 inhibition in irradiated CP70 cells is determined by lack of cPLA2 activation and dramatically low expression of LPA1 compared to A2780 cells (Fig. 5A). This could interrupt radiation-induced cPLA2-dependent pro-survival signaling which is intact in A2780 cells and, thus, desensitize CP70 cells to cPLA2 inhibition.
Fig. 6.
Proposed sequence of molecular events in irradiated ovarian carcinoma cells. Ionizing radiation triggers activation of cPLA2 followed by increased production of LPC and LPA, which activate pro-survival pathways by bind to specific receptors (Flk-1, LPA1 and LPA2).
A major cause of mortality in ovarian cancer patients is disease recurrence after initial treatment. Three-quarters of women who have a complete clinical response to chemotherapy will have a relapse. [8]. Radiation therapy could potentially reduce the rates of recurrence in ovarian cancer patients. The development of novel molecular targeted therapy that enhances radiation-induced cancer cell death while reducing normal tissue injury may improve the outcome of ovarian cancer. Taken together, these findings identify cPLA2 as one of key players in the response of ovarian carcinoma cells to ionizing radiation and a potential pharmaceutical target to lower radiation doses and to enhance radiotherapeutic ratio.
Acknowledgements
We would like to thank Ms. Allie Fu for help with animal studies. This work was supported by the Elizabeth H and James S. McDonnell Distinguished Professorship and grants from the National Institutes of Health grants to D.E.H. [Washington, DC; R01-140220; R01-CA112385 and R01-CA88076] and to E.M.Y from Elsa U. Pardee foundation (Midland, MI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
None declared.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet. 2009;374:1371–1382. doi: 10.1016/S0140-6736(09)61338-6. [DOI] [PubMed] [Google Scholar]
- 3.Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer. 2009;9:167–181. doi: 10.1038/nrc2583. [DOI] [PubMed] [Google Scholar]
- 4.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 5.Vasey PA. Ovarian cancer: front-line standard treatment in 2008. Ann Oncol. 2008;19 Suppl 7:vii61–vii66. doi: 10.1093/annonc/mdn479. [DOI] [PubMed] [Google Scholar]
- 6.Rochet N, Jensen AD, Sterzing F, Munter MW, Eichbaum MH, Schneeweiss A, Sohn C, Debus J, Harms W. Adjuvant whole abdominal intensity modulated radiotherapy (IMRT) for high risk stage FIGO III patients with ovarian cancer (OVAR-MRT-01) - Pilot trial of a phase I/II study: study protocol. BMC Cancer. 2007;7:227. doi: 10.1186/1471-2407-7-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosalaei A, Kazerooni T. Results of post-operative abdomino-pelvic radiotherapy in intermediate- and high-risk epithelial ovarian carcinoma. Eur J Cancer Care (Engl) 2008;17:371–376. doi: 10.1111/j.1365-2354.2007.00871.x. [DOI] [PubMed] [Google Scholar]
- 8.Gadducci A, Cosio S, Conte PF, Genazzani AR. Consolidation and maintenance treatments for patients with advanced epithelial ovarian cancer in complete response after first-line chemotherapy: a review of the literature. Crit Rev Oncol Hematol. 2005;55:153–166. doi: 10.1016/j.critrevonc.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Marsden DE, Friedlander M, Hacker NF. Current management of epithelial ovarian carcinoma: a review. Semin Surg Oncol. 2000;19:11–19. doi: 10.1002/1098-2388(200007/08)19:1<11::aid-ssu3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Firat S, Murray K, Erickson B. High-dose whole abdominal and pelvic irradiation for treatment of ovarian carcinoma: long-term toxicity and outcomes. Int J Radiat Oncol Biol Phys. 2003;57:201–207. doi: 10.1016/s0360-3016(03)00510-8. [DOI] [PubMed] [Google Scholar]
- 11.Sorbe B. Consolidation treatment of advanced (FIGO stage III) ovarian carcinoma in complete surgical remission after induction chemotherapy: a randomized, controlled, clinical trial comparing whole abdominal radiotherapy, chemotherapy, and no further treatment. Int J Gynecol Cancer. 2003;13:278–286. doi: 10.1046/j.1525-1438.2003.13193.x. [DOI] [PubMed] [Google Scholar]
- 12.Yazlovitskaya EM, Linkous AG, Thotala DK, Cuneo KC, Hallahan DE. Cytosolic phospholipase A2 regulates viability of irradiated vascular endothelium. Cell Death Differ. 2008;15:1641–1653. doi: 10.1038/cdd.2008.93. [DOI] [PubMed] [Google Scholar]
- 13.Linkous A, Geng L, Lyshchik A, Hallahan DE, Yazlovitskaya EM. Cytosolic phospholipase A2: targeting cancer through the tumor vasculature. Clin Cancer Res. 2009;15:1635–1644. doi: 10.1158/1078-0432.CCR-08-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonventre JV. The 85-kD cytosolic phospholipase A2 knockout mouse: a new tool for physiology and cell biology. J Am Soc Nephrol. 1999;10:404–412. doi: 10.1681/ASN.V102404. [DOI] [PubMed] [Google Scholar]
- 15.Chakraborti S. Phospholipase A(2) isoforms: a perspective. Cell Signal. 2003;15:637–665. doi: 10.1016/s0898-6568(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 16.Fujita Y, Yoshizumi M, Izawa Y, Ali N, Ohnishi H, Kanematsu Y, Ishizawa K, Tsuchiya K, Tamaki T. Transactivation of fetal liver kinase-1/kinase-insert domain-containing receptor by lysophosphatidylcholine induces vascular endothelial cell proliferation. Endocrinology. 2006;147:1377–1385. doi: 10.1210/en.2005-0644. [DOI] [PubMed] [Google Scholar]
- 17.Fang X, Gaudette D, Furui T, Mao M, Estrella V, Eder A, Pustilnik T, Sasagawa T, Lapushin R, Yu S, Jaffe RB, Wiener JR, Erickson JR, Mills GB. Lysophospholipid growth factors in the initiation, progression, metastases, and management of ovarian cancer. Ann N Y Acad Sci. 2000;905:188–208. doi: 10.1111/j.1749-6632.2000.tb06550.x. [DOI] [PubMed] [Google Scholar]
- 18.Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, Fukuzawa K. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem. 2002;277:39436–39442. doi: 10.1074/jbc.M205623200. [DOI] [PubMed] [Google Scholar]
- 19.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 20.Xie Y, Meier KE. Lysophospholipase D and its role in LPA production. Cell Signal. 2004;16:975–981. doi: 10.1016/j.cellsig.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Yuelling LM, Fuss B. Autotaxin (ATX): a multi-functional and multi-modular protein possessing enzymatic lysoPLD activity and matricellular properties. Biochim Biophys Acta. 2008;1781:525–530. doi: 10.1016/j.bbalip.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aoki J, Inoue A, Okudaira S. Two pathways for lysophosphatidic acid production. Biochim Biophys Acta. 2008;1781:513–518. doi: 10.1016/j.bbalip.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Yu S, Murph MM, Lu Y, Liu S, Hall HS, Liu J, Stephens C, Fang X, Mills GB. Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. J Natl Cancer Inst. 2008;100:1630–1642. doi: 10.1093/jnci/djn378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Fang XJ, Casey G, Mills GB. Lysophospholipids activate ovarian and breast cancer cells. Biochem J. 1995;309(Pt 3):933–940. doi: 10.1042/bj3090933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren J, Xiao YJ, Singh LS, Zhao X, Zhao Z, Feng L, Rose TM, Prestwich GD, Xu Y. Lysophosphatidic acid is constitutively produced by human peritoneal mesothelial cells and enhances adhesion, migration, and invasion of ovarian cancer cells. Cancer Res. 2006;66:3006–3014. doi: 10.1158/0008-5472.CAN-05-1292. [DOI] [PubMed] [Google Scholar]
- 26.Fang X, Schummer M, Mao M, Yu S, Tabassam FH, Swaby R, Hasegawa Y, Tanyi JL, LaPushin R, Eder A, Jaffe R, Erickson J, Mills GB. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim Biophys Acta. 2002;1582:257–264. doi: 10.1016/s1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- 27.Tokumura A, Kume T, Fukuzawa K, Tahara M, Tasaka K, Aoki J, Arai H, Yasuda K, Kanzaki H. Peritoneal fluids from patients with certain gynecologic tumor contain elevated levels of bioactive lysophospholipase D activity. Life Sci. 2007;80:1641–1649. doi: 10.1016/j.lfs.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 28.Goetzl EJ, Dolezalova H, Kong Y, Hu YL, Jaffe RB, Kalli KR, Conover CA. Distinctive expression and functions of the type 4 endothelial differentiation gene-encoded G protein-coupled receptor for lysophosphatidic acid in ovarian cancer. Cancer Res. 1999;59:5370–5375. [PubMed] [Google Scholar]
- 29.Fujita T, Miyamoto S, Onoyama I, Sonoda K, Mekada E, Nakano H. Expression of lysophosphatidic acid receptors and vascular endothelial growth factor mediating lysophosphatidic acid in the development of human ovarian cancer. Cancer Lett. 2003;192:161–169. doi: 10.1016/s0304-3835(02)00713-9. [DOI] [PubMed] [Google Scholar]
- 30.Nakamoto T, Yasuda K, Yasuhara M, Yoshimura T, Kinoshita T, Nakajima T, Okada H, Ikuta A, Kanzaki H. Expression of the endothelial cell differentiation gene 7 (EDG-7), a lysophosphatidic acid receptor, in ovarian tumor. J Obstet Gynaecol Res. 2005;31:344–351. doi: 10.1111/j.1447-0756.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 31.Farooqui AA, Ong WY, Horrocks LA. Inhibitors of brain phospholipase A2 activity: their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Pharmacol Rev. 2006;58:591–620. doi: 10.1124/pr.58.3.7. [DOI] [PubMed] [Google Scholar]
- 32.Trimble LA, Street IP, Perrier H, Tremblay NM, Weech PK, Bernstein MA. NMR structural studies of the tight complex between a trifluoromethyl ketone inhibitor and the 85-kDa human phospholipase A2. Biochemistry. 1993;32:12560–12565. doi: 10.1021/bi00210a002. [DOI] [PubMed] [Google Scholar]
- 33.Eder AM, Sasagawa T, Mao M, Aoki J, Mills GB. Constitutive and lysophosphatidic acid (LPA)-induced LPA production: role of phospholipase D and phospholipase A2. Clin Cancer Res. 2000;6:2482–2491. [PubMed] [Google Scholar]
- 34.Li H, Wang D, Zhang H, Kirmani K, Zhao Z, Steinmetz R, Xu Y. Lysophosphatidic acid stimulates cell migration, invasion, and colony formation as well as tumorigenesis/metastasis of mouse ovarian cancer in immunocompetent mice. Mol Cancer Ther. 2009;8:1692–1701. doi: 10.1158/1535-7163.MCT-08-1106. [DOI] [PubMed] [Google Scholar]
- 35.Karube H, Nishitai G, Inageda K, Kurosu H, Matsuoka M. NaF activates MAPKs and induces apoptosis in odontoblast-like cells. J Dent Res. 2009;88:461–465. doi: 10.1177/0022034509334771. [DOI] [PubMed] [Google Scholar]
- 36.Wadgaonkar R, Somnay K, Garcia JG. Thrombin induced secretion of macrophage migration inhibitory factor (MIF) and its effect on nuclear signaling in endothelium. J Cell Biochem. 2008;105:1279–1288. doi: 10.1002/jcb.21928. [DOI] [PubMed] [Google Scholar]
- 37.Yazlovitskaya EM, Pelling JC, Persons DL. Association of apoptosis with the inhibition of extracellular signal-regulated protein kinase activity in the tumor necrosis factor alpha-resistant ovarian carcinoma cell line UCI 101. Mol Carcinog. 1999;25:14–20. [PubMed] [Google Scholar]
- 38.Cui W, Yazlovitskaya EM, Mayo MS, Pelling JC, Persons DL. Cisplatin-induced response of c-jun N-terminal kinase 1 and extracellular signal--regulated protein kinases 1 and 2 in a series of cisplatin-resistant ovarian carcinoma cell lines. Mol Carcinog. 2000;29:219–228. [PubMed] [Google Scholar]
- 39.Linkous A, Yazlovitskaya E. Cytosolic phospholipase A2 as a mediator of disease pathogenesis. Cell Microbiol. 2010;12:1369–1377. doi: 10.1111/j.1462-5822.2010.01505.x. [DOI] [PubMed] [Google Scholar]
- 40.Godwin AK, Meister A, O'Dwyer PJ, Huang CS, Hamilton TC, Anderson ME. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci U S A. 1992;89:3070–3074. doi: 10.1073/pnas.89.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yazlovitskaya EM, DeHaan RD, Persons DL. Prolonged wild-type p53 protein accumulation and cisplatin resistance. Biochem Biophys Res Commun. 2001;283:732–737. doi: 10.1006/bbrc.2001.4849. [DOI] [PubMed] [Google Scholar]
- 42.DeHaan RD, Yazlovitskaya EM, Persons DL. Regulation of p53 target gene expression by cisplatin-induced extracellular signal-regulated kinase. Cancer Chemother Pharmacol. 2001;48:383–388. doi: 10.1007/s002800100318. [DOI] [PubMed] [Google Scholar]
- 43.Persons DL, Yazlovitskaya EM, Cui W, Pelling JC. Cisplatin-induced activation of mitogen-activated protein kinases in ovarian carcinoma cells: inhibition of extracellular signal-regulated kinase activity increases sensitivity to cisplatin. Clin Cancer Res. 1999;5:1007–1014. [PubMed] [Google Scholar]
- 44.Persons DL, Yazlovitskaya EM, Pelling JC. Effect of extracellular signal-regulated kinase on p53 accumulation in response to cisplatin. J Biol Chem. 2000;275:35778–35785. doi: 10.1074/jbc.M004267200. [DOI] [PubMed] [Google Scholar]
- 45.Xu Y. Sphingosylphosphorylcholine and lysophosphatidylcholine: G protein-coupled receptors and receptor-mediated signal transduction. Biochim Biophys Acta. 2002;1582:81–88. doi: 10.1016/s1388-1981(02)00140-3. [DOI] [PubMed] [Google Scholar]
- 46.Xiao YJ, Schwartz B, Washington M, Kennedy A, Webster K, Belinson J, Xu Y. Electrospray ionization mass spectrometry analysis of lysophospholipids in human ascitic fluids: comparison of the lysophospholipid contents in malignant vs nonmalignant ascitic fluids. Anal Biochem. 2001;290:302–313. doi: 10.1006/abio.2001.5000. [DOI] [PubMed] [Google Scholar]
- 47.Westermann AM, Havik E, Postma FR, Beijnen JH, Dalesio O, Moolenaar WH, Rodenhuis S. Malignant effusions contain lysophosphatidic acid (LPA)-like activity. Ann Oncol. 1998;9:437–442. doi: 10.1023/a:1008217129273. [DOI] [PubMed] [Google Scholar]
- 48.Xu Y, Gaudette DC, Boynton JD, Frankel A, Fang XJ, Sharma A, Hurteau J, Casey G, Goodbody A, Mellors A, et al. Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients. Clin Cancer Res. 1995;1:1223–1232. [PubMed] [Google Scholar]
- 49.Okita M, Gaudette DC, Mills GB, Holub BJ. Elevated levels and altered fatty acid composition of plasma lysophosphatidylcholine(lysoPC) in ovarian cancer patients. Int J Cancer. 1997;71:31–34. doi: 10.1002/(sici)1097-0215(19970328)71:1<31::aid-ijc7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 50.Wang P, Wu X, Chen W, Liu J, Wang X. The lysophosphatidic acid (LPA) receptors their expression and significance in epithelial ovarian neoplasms. Gynecol Oncol. 2007;104:714–720. doi: 10.1016/j.ygyno.2006.10.016. [DOI] [PubMed] [Google Scholar]