Abstract
Posttranslational translocation of prepro-α-factor (ppαF) across the yeast endoplasmic reticulum membrane begins with the binding of the signal sequence to the Sec complex, a membrane component consisting of the trimeric Sec61p complex and the tetrameric Sec62p/63p complex. We show by photo-cross-linking that the signal sequence is bound directly to a site where it contacts simultaneously Sec61p and Sec62p, suggesting that there is a single signal sequence recognition step. We found no evidence for the simultaneous contact of the signal sequence with two Sec61p molecules. To identify transmembrane segments of Sec61p that line the actual translocation pore, a late translocation intermediate of ppαF was generated with photoreactive probes incorporated into the mature portion of the polypeptide. Cross-linking to multiple regions of Sec61p was observed. In contrast to the signal sequence, neighboring positions of the mature portion of ppαF had similar interactions with Sec61p. These data suggest that the channel pore is lined by several transmembrane segments, which have no significant affinity for the translocating polypeptide chain.
INTRODUCTION
In posttranslational protein translocation, polypeptides are first completely synthesized in the cytosol and released from the ribosome, before being transported across the endoplasmic reticulum (ER) membrane (for review, see Rapoport et al., 1999). Posttranslational translocation occurs in two distinct steps. In the first, the translocation substrate binds in an ATP-independent manner to a seven-component membrane protein complex, the Sec complex, by virtue of its signal sequence (Lyman and Schekman, 1997; Plath et al., 1998). In the second phase, the polypeptide chain is moved through the protein-conducting channel by a ratcheting mechanism involving the luminal ATPase BiP (called Kar2p in yeast) (Matlack et al., 1999). The Sec complex consists of the heterotrimeric Sec61p complex (containing Sec61p, Sbh1p, and Sss1p) and the tetrameric Sec62/63p complex (containing Sec62p, Sec63p, Sec71p, and Sec72p) (Deshaies et al., 1991; Panzner et al., 1995). The Sec61p complex also functions in cotranslational transport across the ER membrane when associated with the ribosome. In both translocation pathways, the Sec61p complex forms the protein-conducting channel, and the multispanning membrane protein Sec61p is likely its major component (for review, see Matlack et al., 1998; Johnson and van Waes, 1999).
In the past, we have used photo-cross-linking to analyze how the signal sequence of the secretory protein prepro-α-factor (ppαF) is recognized during the initial binding step in posttranslational translocation (Plath et al., 1998). With photoreactive probes positioned at single sites throughout the signal sequence, we found that the hydrophobic core of the signal sequence forms a helix that is contacted on opposite sides by transmembrane (TM) segments 2 and 7 of Sec61p. The signal sequence helix also interacts with Sec62p and Sec71p through a surface that partially overlaps with that contacting TM7 of Sec61p. Each residue of the signal sequence could be cross-linked to phospholipids, indicating that the signal sequence binding site is at the interface of the protein channel and the lipid phase (Martoglio et al., 1995; Plath et al., 1998). Residues immediately after the signal sequence were also in contact with Sec61p, notably with TM segment 8, but not with lipids, supporting a model in which the polypeptide chain inserts as a loop into the channel (Matlack et al., 1998; Mothes et al., 1994; Plath et al., 1998); the N terminus of the polypeptide chain would stay in the cytosol, the signal sequence would cross the membrane bound to TM2 and TM7, and the segment of the mature region immediately after the signal sequence would be located in the channel proper, leading the polypeptide chain back into the cytosol. Consistent with this model, residues of ppαF at the C terminus interact with cytosolic domains of Sec62p, Sec71p, and Sec72p and cannot be cross-linked to Sec61p (Lyman and Schekman, 1997; Plath et al., 1998).
The previous cross-linking experiments (Plath et al., 1998) left several important questions unanswered. One is whether signal sequence binding occurs in a single step. A two-step recognition by Sec62/63 complex components and Sec61p was proposed on the basis of experiments with a bifunctional amino group cross-linking reagent (Lyman and Schekman, 1997), but because lysines are lacking in the signal sequence of ppαF, the experiments did not actually monitor interactions of the signal sequence. Our previous experiments also did not address which TM segments of Sec61p form the actual pore through which polypeptides are transported, because the study was restricted to an analysis of the first step of translocation in which the substrate ppαF was only bound to the Sec complex, but not transported through it. Although it has been shown that substrates translocating through the ER membrane can be cross-linked to Sec61p (Sanders et al., 1992; Mothes et al., 1994; Musch et al., 1992; Lyman and Schekman, 1997; Matlack et al., 1997), there has been no attempt as yet to identify the segments of Sec61p that contact the polypeptide chain and thus line the channel pore. Here, we have addressed these questions by the use of site-specific photo-cross-linking in combination with a previously developed mapping technique that allows us to identify the cross-linking sites in Sec61p (Plath et al., 1998).
MATERIALS AND METHODS
Sec61p Mutant Strains, Preparation of Membranes, Purification of Sec Complex and Kar2p, Reconstitution of Proteoliposomes, and Antibodies
Saccharomyces cerevisiae strains carrying Sec61p-Xa-mutations have been described previously [Plath et al., 1998; P105-GSIEGRGS-K108 (BWY25, N2/3C), L177-GSIEGRGS-G180 (BWY65, N4/5C), Y265-GSIEGRGS-P268 (BWY66, N6/7C), and S351-GSIEGRGS-E354 (BWY73, N7/8C); the sequence given between positions corresponds to the cleavage site for factor Xa]. Preparation of membranes from the different S. cerevisiae strains, purification of mutant Sec complexes from the membranes by affinity chromatography, purification of His-tagged Kar2p from Escherichia coli, and reconstitution of purified components into proteoliposomes were essentially done as described previously (Panzner et al., 1995). All antibodies used in this study are described in Plath et al. (1998).
Generation of ppαF Constructs
Generation of cDNA constructs coding for ppαF in which all wild-type lysine codons were altered to arginine codons, and single lysine codons were subsequently introduced at positions throughout the signal sequence or in the region immediately after the signal sequence, has been described previously (Plath et al., 1998). For this study, all additional ppαF-lysine mutants were generated by mutagenesis using the pAlter system (Promega, Madison, WI), by using appropriate oligonucleotides, and ppαF cDNA lacking all lysine codons as starting material.
Transcription and Translation
Transcription from ppαF-pAlter plasmids was carried out with T7 polymerase after linearization of the plasmids downstream of the ppαF cDNA to produce full-length mRNA (SalI cleavage), or within the ppαF cDNA to produce truncated mRNAs that lack the last five codons (Nci1 cleavage). ppαF mutants were synthesized in vitro in reticulocyte lysate in the presence of [35S]methionine and trifluoromethyl-diazirino-benzoic acid-lysyl-tRNA. Translation was stopped with 2 mM cycloheximide. When full-length ppαF molecules were generated, ribosomes were removed after translation by centrifugation for 10 min at 100,000 rpm in a Beckman TL 100 rotor.
Binding of Full-Length ppαF to Sec Complex, Photo-Cross-Linking, and Native Immunoprecipitation
For binding of ppαF to purified Sec complex, Sec complex containing proteoliposomes were incubated with the ppαF translation reaction for 20 min at 30°C in 150 mM potassium acetate and 20 mM HEPES, pH 7.5, and the samples were subsequently irradiated for 15 min on ice. Coimmunoprecipitation of ppαF bound and cross-linked to Sec complex was performed in digitonin with antibodies directed against Sec62p, as described previously (Matlack et al., 1997). For kinetic studies, the binding reaction was stopped by quick-freezing an aliquot of the sample on dry ice at different time points. In this case, irradiation with UV light was also done on the frozen samples.
Generation of Stable Transport Intermediates, and Cross-Linking
To generate stable transport intermediates, translation mixtures containing ribosome-tethered ppαF were incubated for 30 min with yeast membranes at 25°C or with proteoliposomes containing purified Sec complex and Kar2p at 30°C in the presence of 2 mM ATP, 20 mM creatine phosphate, 0.1 mg/ml creatine kinase, 150 mM potassium acetate, 5 mM magnesium acetate, and 20 mM HEPES, pH 7.5. The samples were subsequently irradiated for 15 min on ice.
Denaturing Immunoprecipitation and Factor Xa Cleavage
For denaturing immunoprecipitations of cross-linked products, the samples were incubated for 10 min at 50°C in 2% SDS. Subsequently, immunoprecipitation was performed with Sec61p or Sec62p antibodies in 50 mM Tris-HCl, pH 7.5, 150 mM sodium chloride, 1% Triton, and 0.1% SDS as described previously (Plath et al., 1998).
The mapping of the cross-linking sites of ppαF with the Sec61p-Xa-mutants was performed essentially as described previously (Plath et al., 1998). Briefly, denatured cross-linked products were incubated with antibodies directed against the C terminus of Sec61p and protein A-Sepharose beads. After incubation for 1 h at 4°C, the beads were isolated, washed, and half of the sample was incubated with the protease factor Xa for 90 min at 22°C. With the Sec61p-N6/7C mutant, factor Xa-cleavage was performed before immunoprecipitation. Two-thirds of the irradiated sample was incubated with factor Xa, divided into two parts, and immunoprecipitated under denaturing conditions with antibodies directed against either the N or C terminus of Sec61p. The untreated one-third of the sample was immunoprecipitated directly with antibodies directed against the C terminus of Sec61p.
Miscellaneous
Endoglycosidase H (EndoH) treatment of Sec61p-ppαF cross-linked product was performed on protein A-Sepharose beads after denaturing immunoprecipitation of cross-linked product with Sec61p antibodies. Washed beads were incubated in 50 mM sodium citrate, pH 5.5, 0.5% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, and 50 mU of endoH (Roche Diagnostics, Indianapolis, IN) for 1 h at 37°C. Analysis of all samples was performed by SDS-PAGE with 7.5–17.5% gels followed by autoradiography or quantification with a Fuji PhosphorImager BAS1000.
RESULTS
Simultaneous Binding of the Signal Sequence to Sec61p and Sec62/71p
To examine interactions of the signal sequence of ppαF, we used a photo-cross-linking approach (Plath et al., 1998). Mutants of ppαF were generated each of which contained a single lysine codon at a different position of the signal sequence. Mutant polypeptides were synthesized in vitro in a reticulocyte lysate system in the presence of [35S]methionine and a modified lysyl-tRNA, carrying a carbene-generating probe in the side chain of the amino acid. A photoreactive probe is thus incorporated at the site of the lysine codon. After removal of the ribosomes by sedimentation, full-length ppαF chains were incubated with proteoliposomes containing purified Sec complex, but lacking BiP (Kar2p). Under these conditions, posttranslational transport arrests at the initial binding step, such that ppαF is stably associated with the Sec complex, but is not transported through the channel (Plath et al., 1998). The samples were then irradiated to induce cross-links of ppαF to neighboring proteins, and solubilized in digitonin, a detergent that leaves the Sec complex and its interaction with ppαF intact. The Sec complex was immunoprecipitated, and associated cross-linked and noncross-linked ppαF was analyzed by SDS-PAGE and autoradiography. To investigate whether the signal sequence contacts one component of the Sec complex before another, the incubation of ppαF with the proteoliposomes was performed for different time periods, and the binding reaction was stopped by placing the samples on dry ice before irradiation. Single photo-cross-linking probes at positions 10 through 14 of the signal sequence gave rise to different cross-linked products (Figure 1, A–E). Cross-links to different regions of Sec61p have different mobilities in SDS gels and are indicated by open and closed arrows (Plath et al., 1998). Cross-links to Sec62p and Sec71p are indicated by open squares; they run at the same position in SDS gels because the two proteins have approximately the same molecular weight. Each of the tested positions (10–14) gave different intensities of these cross-linked products, consistent with the idea that the signal sequence is bound in a specific manner. Within the error of the quantitations, the Sec61p and Sec62p/71p cross-links occurred with the same kinetics during the incubation of ppαF with the Sec complex (Figure 1F), indicating that the signal sequence does not contact one component earlier than the other. It should be noted that lipid cross-links also occurred with the same kinetics (Figure 1, A–E, open circles and our unpublished results). These data therefore suggest that there is a single binding step during which the signal sequence is brought into contact with Sec61p, Sec62/71p, and lipid.
Figure 1.
The signal sequence binds to Sec61p and Sec62p/71p with the same kinetics. (A) ppαF mutant containing a single photoreactive lysine derivative at position 10 (ppαF K10) was incubated with proteoliposomes containing purified Sec complex. At different time points, the binding reaction was stopped, and samples were irradiated with UV light as indicated. After solubilization with digitonin, the Sec complex was immunoprecipitated together with bound and cross-linked ppαF. The samples were analyzed by SDS-PAGE and autoradiography. Slow and fast mobility Sec61p cross-linked products are indicated by closed and open arrows, respectively; cross-linked products containing Sec62/71p by open squares; and lipid cross-links by open circles. (B) As in A, with a photoreactive lysine derivative at position 11 (ppαF K11). (C) As in A, with a photoreactive lysine derivative at position 12 (ppαF K12). (D) As in A, with a photoreactive lysine derivative at position 13 (ppαF K13). (E) As in A, with a photoreactive lysine derivative at position 14 (ppαF K14). (F) Quantification of the Sec61p cross-linked products (closed squares) and Sec62p/71p cross-linked products (stars) observed in (A-E) was performed with a PhosphorImager. Yields of cross-links are expressed relative to the amount of ppαF coimmunoprecipitated with the Sec complex. The Sec61p cross-links are the sum of all products containing Sec61p. Note that the outlier with position 11 at 0.5-min incubation is likely due to errors in the quantitation of the particularly weak Sec61p cross-links seen with this position.
To further test this idea, we investigated whether the signal sequence contacts Sec62p/71p and Sec61p at the same time. To this end, we used ppαF molecules that carry two photoreactive probes. We chose combinations of positions in the hydrophobic core of the signal sequence (positions 9, 10, 12, 14, and 15) and in the mature region of ppαF (position 28; the signal sequence is normally cleaved after position 22). Single probes at positions 10 or 14 gave strong cross-links to Sec62p/71p, and a prominent, slowly migrating Sec61p cross-linked band (Figures 1, A and E; and 2A, closed arrow), representing mainly cross-links of the signal sequence to TM segment 7 of Sec61p (Plath et al., 1998). Single probes at positions 9, 12, and 15, gave prominent, fast mobility Sec61p-cross-links, representing cross-links to TM2 of Sec61p (Figures 1C and 2A, open arrow; Plath et al., 1998), and a probe at position 28 gave fast mobility Sec61p cross-links that occur mostly to TM8 of Sec61p (Figure 2A; Plath et al., 1998). TM2 and TM7 of Sec61p are located on opposite sides of a helix formed by the hydrophobic core of the signal sequence (see scheme in Figure 2A; Plath et al., 1998). The side of the helix interacting with TM7 also contacts Sec62p/71p.
Figure 2.
The signal sequence contacts Sec61p and Sec62p/71p simultaneously. (A) ppαF mutants, each containing a single photoreactive lysine derivative at the indicated position (ppαF K), were incubated with proteoliposomes containing purified Sec complex, and irradiated with UV light as indicated. After solubilization with digitonin, Sec complex-bound and -cross-linked ppαF was isolated and analyzed by SDS-PAGE. Slow and fast mobility Sec61p cross-linked products are indicated by closed and open arrows, respectively; cross-linked products to Sec62/71p by open squares; and lipid cross-links by open circles. Noncross-linked ppαF K28 runs somewhat differently than the other ppαF mutants, and often occurs in several bands. The scheme to the right shows a top view of the helix formed by the hydrophobic core of the ppαF signal sequence; the cross-linking partners of positions 9, 10, 12, 14, and 15 are indicated. (B) The experiment was performed as in A using ppαF mutants each carrying two photoreactive lysine derivatives at the indicated positions. Some combinations of photoreactive probes give a high-molecular-weight band. The mobility of this band is the same for all mutants tested, but its intensity is strong for some positions (*) and weak for others (**). (C) A cross-linking experiment similar to that described in A was performed with ppαF mutants carrying photoreactive groups at the indicated positions. After irradiation, the samples were denatured in SDS, and immunoprecipitated with antibodies directed against Sec61p or Sec62p as indicated.
The cross-linking results with various combinations of two photoreactive probes are shown in Figure 2B. In general, the cross-linking pattern was essentially the sum of the patterns seen with single probes. With most combinations, the yield of cross-linked products was even in quantitative agreement with the sum of the cross-linking yields of the two single lysine mutants (Table 1; our unpublished data). These results indicate that both probes in ppαF can simultaneously give cross-links. A high-molecular-weight band (star), indicative of a double-cross-link of ppαF to two membrane proteins, was readily seen with the probes at positions 10/14 and 10/28 (Figure 2B). This band represents a cross-link of ppαF to both Sec61p and Sec62p because it could be immunoprecipitated under denaturing conditions with antibodies directed against these proteins (Figure 2C). The prominence of a double cross-link of Sec61p and Sec62p with the combinations 10/14 and 10/28 is not surprising because ppαF with single probes at positions 10 and 14 gave particularly strong cross-links to Sec62p (Figures 1, A and E; and 2A). The high-molecular-weight band seen with the 10/14 and 10/28 combinations also contains a small amount of ppαF-Sec61p-Sec71p cross-links (our unpublished data), similarly to the results with single lysine mutants, for which the Sec62p/71p cross-linked band contained a small amount of cross-links to Sec71p in addition to much stronger cross-links to Sec62p (Plath et al., 1998). These data demonstrate that the signal sequence simultaneously contacts both Sec62p/71p and Sec61p, providing further evidence that it does not interact with one component before the other.
Table 1.
Estimating the expected intensity of a possible ppαF-Sec61p-Sec61p crosslink
| K9/14 | ΣK9 + K14 | K10/14 | ΣK10 + K14 | K9/15 | ΣK9 + K15 | K10/15 | ΣK10 + K15 | K10/28 | ΣK10 + K28 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sec61pS | 19.1 | 27.2 | 17.7 | 35.7 | 1.4 | 1.7 | 5.4 | 5.1 | 6.2 | 6.0 |
| Sec61pF | 2.7 | 4.5 | 1.2 | 1.4 | 4.6 | 10.1 | 3.6 | 7.5 | 11.1 | 14.8 |
| Sec62/71p | 1.5 | 1.9 | 2.5 | 4.2 | 0.8 | 0.6 | 1.6 | 2.9 | 3.0 | 2.6 |
| Sec61p × Sec62/71p (expected) | 0.13 | 0.85 | 0.03 | 0.21 | 0.37 | |||||
| HMW (*/**) (observed) | 0.02 | 0.42 | 0.03 | 0.15 | 0.42 | |||||
| Sec61p × Sec61p (expected) | 1.16 | 1.82 | 0.32 | 0.38 | 0.87 |
Quantification of the crosslinked products obtained with single- and double-lysine ppαF mutants shown in Figure 2 was performed with a PhosphorImager. The radioactivity contained in Sec61p crosslinks with fast or slow mobility in the SDS gel (Sec61pP or Sec61pS), in Sec62/71p cross-links, and in the HMW cross-link (* and **), is expressed as percentage of the radioactivity in ppαF coimmunoprecipitated with the Sec complex. The table gives the data for double-lysine ppαF mutants (ppαF Kx/x where x indicates the position of the photoreactive lysine derivative). In addition, for the same combination of lysines, the data of the single lysine ppαF mutants were added (e.g., ΣK9 + K14). The cross-linking yields of the single lysine ppαF mutants were used to estimate the theoretical yields of ppαF-Sec61p-Sec61p and ppαF-Sec61p-Sec62/71p double cross-links for the respective double lysine combinations. To this end, the percentage of the Sec61p cross-links from one position was multiplied with the percentage of the Sec61p crosslinks or Sec62/71p cross-links from the other position.
We could not detect double-cross-links to two Sec61p molecules, which are expected to run slightly above the ppαF-Sec61p-Sec62p cross-link in a SDS gel (Figure 2, B and C). An extremely weak high-molecular-weight cross-link was observed with probe combinations 9/14, 9/15, 10/15, 14/28, and 15/28 (Figure 2B, double-stars). The mobility of this cross-link is similar to that of the ppαF-Sec61p-Sec62p double cross-link obtained with the combinations 10/28 and 14/28, indicating that this weak cross-link also occurred between ppαF, Sec61p, and Sec62p, rather than between ppαF and two Sec61p molecules. The intensity of this band also corresponds to what would be expected from the intensity of the single Sec61p and Sec62p/71p cross-links (Table 1). It should be noted that our quantitative analysis predicts that the intensity of a ppαF-Sec61p-Sec61p-double cross-link, if it occurred, should have been significantly higher than that of the ppαF-Sec61p-Sec62p-double cross-link for each of the tested ppαF double lysine mutants (Table 1).
The Mature Region of Translocating ppαF Contacts Several TM Segments of Sec61p
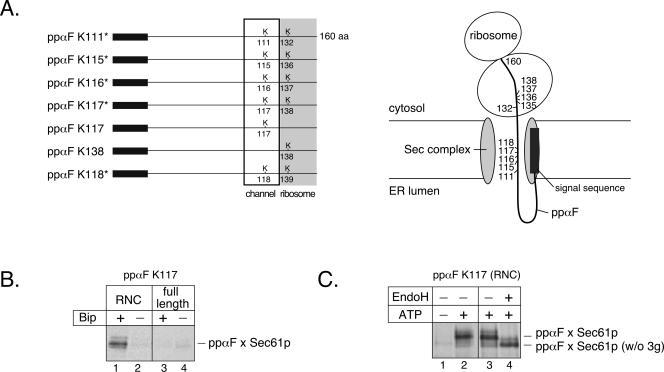
To determine which regions of Sec61p line the actual translocation pore, we performed photo-cross-linking experiments with ppαF chains that had been allowed to continue posttranslational translocation beyond the initial binding step analyzed before. To generate a late translocation intermediate, a truncated mRNA coding for a fragment of 160 amino acids of ppαF was translated in the reticulocyte lysate in vitro system (Musch et al., 1992). The nascent chain is only five amino acids shorter than full-length ppαF and remains associated with the ribosome because a stop-codon is lacking in the mRNA. After translation, ribosome-associated ppαF was incubated in the presence of ATP with yeast microsomes or with proteoliposomes containing purified Sec complex and luminal BiP (Kar2p). These conditions allow translocation to occur, but the ribosome at the C terminus prevents the polypeptide chain from moving completely into the lumen of the vesicles (Figure 3A, scheme). It should be noted that in this situation the ribosome simply functions as an obstacle but does not bind to the channel as in cotranslational translocation. With appropriately positioned photoreactive lysine derivatives, cross-linking of the mature region of ppαF to the surrounding Sec61p channel should be observed.
Figure 3.
Cross-linking of ribosome-tethered ppαF to Sec61p. (A) To probe the Sec61p environment of ppαF during translocation, the indicated lysine residues (K) were replaced by photoreactive lysine derivatives (left scheme). The signal sequence of ppαF is indicated by a filled box. Truncated mRNAs were translated to generate a ribosome nascent chain complex carrying the first 160 amino acids of ppαF. A translocation intermediate is produced upon transport into the ER, as indicated in the right scheme. Although most of the generated ppαF mutants carry two lysine residues, only one is located such that it can give cross-links to the Sec61p channel; the other is buried inside the ribosome. (B) Full-length ppαF or ribosome-nascent chain complexes (RNCs), containing a fragment of ppαF of 160 amino acids, both with a photoreactive probe at position 117, were incubated with proteoliposomes containing purified Sec complex and Kar2p, as indicated. Cross-links were induced by irradiation, and cross-linked products containing Sec61p were immunoprecipitated under denaturing conditions. (C) ppαF RNC, containing a photoreactive probe at position 117, were incubated with native yeast microsomes in the presence or absence of ATP, as indicated. Cross-links were induced by irradiation, and Sec61p cross-linked products were immunoprecipitated under denaturing conditions. Where indicated, the immunoprecipitated material was treated with EndoH, resulting in the removal of the three carbohydrate chains of ppαF (w/o 3g).
We generated mutants of ppαF that carry lysine codons in the region expected to be inside the channel. Introducing single lysine codons into the C-terminal part of ppαF turned out to be problematic because of the existence of four repeats in the ppαF gene with almost identical nucleotide sequence. We obtained two mutants with single lysine codons at positions 117 and 138 (ppαF K117 and ppαF K138). Five other mutants contained two lysine codons at positions 111 and 132, 115 and 136, 116 and 137, 117 and 138, and 118 and 139 (Figure 3A). Because ∼30–35 residues are buried inside the ribosome, probes at positions 132, 136, 137, 138, and 139 should not be able to cross-link to membrane proteins (Figure 3A, scheme). Indeed, with the mutant that contained a single probe at position 138 (ppαF K138), no cross-links to Sec61p were observed with native microsomes and only extremely weak ones with proteoliposomes containing purified components (our unpublished data). In contrast, ppαF K117 and the five ppαF mutants with two lysine codons in the C-terminal portion gave significantly stronger Sec61p cross-links (see below). The five mutants with two lysine codons therefore have a single probe available for cross-linking to Sec61p.
Because our approach represents a new method to generate translocation intermediates in posttranslational translocation, we performed several control experiments to demonstrate its validity. One expectation of the experimental setup is that cross-linking to Sec61p should only occur when translocation across the membrane is permitted. Indeed, when ribosome-tethered ppαF containing a probe at position 117 was incubated with proteoliposomes containing Sec complex, irradiation generated cross-links to Sec61p only if the vesicles also contained BiP (Kar2p) (Figure 3B, lane 1), but not when they lacked BiP (lane 2). Thus, position 117 of ppαF stays outside the channel during the initial binding step and is only moved into the Sec61p channel when BiP-dependent translocation is allowed. With full-length ppαF K117, no Sec61p cross-links were obtained even when translocation was permitted, in agreement with the expectation that this polypeptide should move all the way into the lumen of the vesicles, and not be trapped inside the channel (Figure 3B, lanes 3 and 4). Similar control experiments were performed with native ER membranes (Figure 3C). Again, ribosome-tethered ppαF with a probe at position 117 only gave cross-links to Sec61p when translocation was initiated by addition of ATP (lanes 1and 2), whereas full-length ppαF did not give cross-links even when ATP was present (our unpublished data). Another test for our approach is based on the fact that ppαF, when transported into the ER lumen, becomes N-glycosylated at three sites in a region after the signal sequence. Our data demonstrate that the Sec61p cross-links observed with ribosome-associated ppαF K117 contained a glycosylated substrate, because they were sensitive to endoglycosidase H (Figure 3C, lanes 3 and 4). Thus, the N-terminal portion of the ppαF polypeptide chain, containing the three N-glycosylation sites, must have reached the ER lumen (Sec61p is not glycosylated). Together, these data provide strong evidence that position 117 of the translocation intermediate of ppαF is located inside the Sec61p channel.
To determine the approximate regions of Sec61p to which ppαF is cross-linked, we made use of a previously developed mapping technique (Plath et al., 1998). A set of Sec61p mutants was used, each of which has a single cleavage site for the protease factor Xa in one of the cytosolic or luminal loops between the TM segments of Sec61p (Wilkinson et al., 1996, 1997). The cross-links between ppαF and the Sec61p-Xa mutant molecules can be specifically cleaved with the protease factor Xa, allowing us to determine whether the ppαF chain has been cross-linked to the N- or C-terminal fragment of the Sec61p molecule. Combining the results obtained with different Sec61p-Xa-mutants, one can map the approximate site in Sec61p that interacts with ppαF. These experiments were performed with proteoliposomes containing purified mutant Sec complex and with yeast microsomes from mutant yeast strains. Translocation intermediates with ribosome-tethered ppαF chains carrying probes at different positions were generated (Figure 3A), cross-links were induced, and cross-linked products were immunoprecipitated with Sec61p antibodies under denaturing conditions. One-half of the samples was incubated with factor Xa protease, the other remained untreated. The samples were then separated by SDS-PAGE and analyzed by autoradiography. In case of the Sec61p mutant carrying a Xa site in the loop between TM domains 6 and 7, factor Xa cleavage was performed before immunoprecipitation. The data for ppαF with the photoreactive probe at position 117 are shown both for proteoliposomes containing purified Sec complex and Kar2p (Figure 4A) and for native microsomes (Figure 4B). The data for the other ppαF molecules tested are summarized with the quantification shown in Figure 4, C and D.
Figure 4.
Probing the Sec61p-environment of translocating ppαF. (A) The Sec complex was purified from yeast mutants each of which carries a single cleavage site for the protease factor Xa site in the loop between the indicated TM segments of Sec61p (for example, N7/8C contains a Xa site in the loop between TM segments 7 and 8 of Sec61p, see scheme for the relative positions of the Xa sites in the Sec61p molecule). Proteoliposomes containing the various mutant Sec complexes were incubated with ribosome-nascent chain complexes containing a 160-amino acid fragment of ppαF with a photoreactive group at position 117, and irradiated. For the Sec complexes N2/3C and N7/8C, the samples were denatured in SDS, immunoprecipitated with antibodies to the C terminus of Sec61p, and then analyzed with and without treatment with factor Xa, as indicated. For the N6/7C Sec complex, samples were split into three parts after irradiation. One part was directly immunoprecipitated with antibodies against the C terminus of Sec61p under denaturing conditions. The other two parts were first treated with factor Xa and subsequently immunoprecipitated under denaturing conditions with antibodies to the C or N terminus of Sec61p as indicated. The fragments of Sec61p containing cross-links are indicated in the autoradiograph (for example N2 indicates a cross-link to the Sec61p fragment comprising the N terminus to the loop between TM segments 2 and 3 of Sec61p). (B) An experiment similar to that described in A was performed with yeast microsomes purified from the indicated Sec61p-Xa mutant strains. (C) The experiment was performed as described in A with different ribosome-tethered ppαF mutants containing photoreactive groups at the indicated positions and quantitated with a phospholmager. The columns give the relative yields of radioactive label in the two Sec61p-fragments generated by factor Xa cleavage. For N6/7C, the calculation includes a correction for the different efficiencies of immunoprecipitation with antibodies against the N and C terminus of Sec61p. (D) As in C, but with native membranes. (E) Proteoliposomes containing purified Sec complex from the N7/8C Sec61p-Xa mutant were incubated with ribosome-tethered ppαF containing photoreactive groups at different positions as indicated, and irradiated. After denaturation in SDS, Sec61p cross-linked products were immunoprecipitated and separated by SDS-PAGE.
The results allow several conclusions. First, all positions of cross-linking probes gave similar Sec61p cross-linking patterns (Figure 4E), in contrast to the results obtained with probes in the signal sequence in which even neighboring positions gave very different Sec61p-cross-linking patterns (Figures 1 and 2). These data are consistent with a model in which the mature region of ppαF has no strong interactions when it is passing through the channel, whereas the signal sequence is specifically bound. Second, in contrast to the results with the signal sequence, each tested position of the mature region was cross-linked to about the same extent to several different regions of the Sec61p molecule. For example, with purified Sec complex and probes at different positions of ppαF, ∼30% of the label in the cross-linked product could be assigned to the Sec61p-fragment comprising the N terminus up to the loop between TM segments 2 and 3 (Figure 4C). About 20% of the label was present in the fragment comprising the loop between TM 7 and 8 up to the C terminus of Sec61p. With the factor Xa site in the loop between TM6 and 7, the C-terminal fragment contained ∼66% of the label. Assuming that the numbers are additive, ∼4% of the label is in the region between TM3 and 6, and ∼46% in TM7, or more precisely, in the region between the factor Xa cleavage site in the loop between TM segments 6 and 7 and that in the next loop. With native microsomes, ∼9% of the label was in the N-terminal fragment up to the loop between TM2 and 3, 35% in the region, including TM segments 3 and 4, 32% in the region comprising TM segments 5 through 7, and 24% in the C-terminal fragment, from the loop between TM segments 7 and 8 to the end (Figure 4D). Despite the quantitative differences between proteoliposomes and native membranes, it is clear that each position of the translocating polypeptide chain was cross-linked to several different regions of Sec61p, whereas in the case of the signal sequence each position was always in contact with primarily one TM segment. Thus, while the signal sequence binding site is formed by TM2 and TM7 (with some small contribution by TM1; Plath et al., 1998), several different regions line the pore through which the mature region of a polypeptide chain passes the membrane. It should be noted that, with probes in the mature region, each individual cross-link after factor Xa cleavage was much weaker than with probes in the signal sequence, making a more detailed mapping difficult.
DISCUSSION
Our results show that the signal sequence binds directly to a site at which it contacts both Sec61p and Sec62p/71p. Cross-links to these components occur with the same kinetics during the incubation of ppαF with the Sec complex, and the signal sequence contacts these components simultaneously. These results argue against the possibility that the signal sequence stably interacts with Sec62p/71p before being transferred into the channel. Rather, the signal sequence seems to bind in a single step to a site that is formed by both Sec61p and Sec62p/71p. However, we cannot exclude that there is a preceding, fast recognition step that went undetected in our kinetic experiments. Sec62/71p seems to be localized at a defined site relative to TM2 and TM7 of Sec61p, presumably the site where the signal sequence laterally exits into the lipid phase. It is unlikely that Sec62p/71p is directly involved in signal sequence recognition, simply because it is not required in the cotranslational mode of translocation in mammals or in the posttranslational mode in bacteria. Its role still remains to be clarified. Previously, a consecutive interaction of the signal sequence with Sec62/63p complex components and Sec61p was proposed on the basis of experiments with a bifunctional, amino group cross-linking reagent (Lyman and Schekman, 1997). An initial interaction of ppαF with Sec62p, Sec71p, and Sec72p was seen, whereas cross-links to Sec61p required a subsequent Kar2p- and ATP-dependent step. However, these experiments did not actually address interactions of the signal sequence because all cross-linkable lysines of ppαF are in the C-terminal portion of the polypeptide chain. A plausible alternative interpretation of these results is that in the initial binding stage, this portion is localized in the cytosol and cross-linkable to cytoplasmic domains of Sec62p, Sec71p, and Sec72p, whereas upon addition of Kar2p and ATP, it moves into the channel and is cross-linkable to Sec61p.
Our mapping experiments show that the translocation pore is lined by several regions of Sec61p. Single photoreactive probes in a translocating polypeptide chain that were located inside the channel gave cross-links to regions in the Sec61p molecule that are far apart in the primary sequence. For example, in experiments with proteoliposomes containing the purified Sec complex, ∼20–30% of all molecules cross-linked to the N-terminal two TM segments, about the same percentage to the C-terminal two TM segments, and a significant fraction cross-linked to internal regions. Surprisingly, proteoliposomes containing purified Sec complex and native microsomes gave reproducibly somewhat different results. One possibility is that the mutant Sec complexes containing factor Xa cleavage sites may be more stable in native membranes than after purification and reconstitution into membranes, and the results in particular for internal regions of Sec61p may therefore differ. Another possibility is that the cross-linking yields may not be completely additive when the results of different cleavage mutants are compared. Furthermore, we have used different sec61 mutants for the experiments with native microsomes and purified Sec complex. Despite the quantitative differences, the major conclusion, i.e., that different regions are involved in forming the translocation pore, is true for experiments with both types of membranes. The strong contribution by TM segment 7 suggests that it may play a dual role in forming both the signal sequence binding site and the translocation pore, and supports the idea that they are in proximity of one another.
In contrast to the results with probes in the signal sequence, different positions in a polypeptide segment located inside the actual channel pore gave identical cross-linking patterns. Obviously, the pore must be designed to allow the passage of polypeptides with widely different amino acid sequences, and our results indicate that there are indeed no specific contacts with the translocating polypeptide chain. The signal sequence, on the other hand, must be recognized by Sec61p and must therefore make specific interactions with certain TM segments.
Our experiments with two simultaneous photoreactive probes incorporated into ppαF did not give any evidence for ppαF-Sec61p-Sec61p double-cross-links. With cross-linking probes at opposite sides of a helix formed by the hydrophobic core of the signal sequence (e.g., positions 9 and 14 or 10 and 15; Figure 2A), one may have expected such a double-cross-link, if TM2 and TM7 belonged to different Sec61p molecules. Double-cross-links to Sec61p and Sec62p were observed with some combinations of photoreactive probes, and quantification indicates that the double-cross-links to two Sec61p molecules should have been even stronger if they occurred. Although of negative nature, these data therefore suggest that the signal sequence is not at the interface of two different Sec61p molecules, but rather intercalated into TM segments of a single molecule. Because the pore of the channel must be adjacent to the signal sequence binding site, it may also be generated by only one Sec61p molecule.
Acknowledgments
We are indebted to Kent Matlack for advice and critical reading of the manuscript. K.P. is an O'Donnell Fellow of the Life Sciences Research Foundation. The work was supported by a grant to T.A.R. from the National Institutes of Health (GM-54238). T.A.R. is an investigator of the Howard Hughes Medical Institute.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–06–0390. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-06-0390.
References
- Deshaies, R.J., Sanders, S.L., Feldheim, D.A., and Schekman, R. (1991). Assembly of yeast SEC proteins involved in translocation into the endoplasmic reticulum into a membrane-bound multisubunit complex. Nature 349, 806–808. [DOI] [PubMed] [Google Scholar]
- Johnson, A.E., and van Waes, M.A. (1999). The translocon: a dynamic gateway at the ER membrane. Annu. Rev. Cell. Dev. Biol. 15, 799–842. [DOI] [PubMed] [Google Scholar]
- Lyman, S.K., and Schekman, R. (1997). Binding of secretory precursor polypeptides to a translocon subcomplex is regulated by BiP. Cell 88, 85–96. [DOI] [PubMed] [Google Scholar]
- Martoglio, B., Hofmann, M.W., Brunner, J., and Dobberstein, B. (1995). The protein-conducting channel in the membrane of the endoplasmic reticulum is open laterally toward the lipid bilayer. Cell 81, 207–214. [DOI] [PubMed] [Google Scholar]
- Matlack, K.E., Misselwitz, B., Plath, K., and Rapoport, T.A. (1999). BiP acts as a molecular ratchet during posttranslational transport of prepro-alpha factor across the ER membrane. Cell 97, 553–564. [DOI] [PubMed] [Google Scholar]
- Matlack, K.E.S., Mothes, W., and Rapoport, T.A. (1998). Protein translocation - tunnel vision. Cell 92, 381–390. [DOI] [PubMed] [Google Scholar]
- Matlack, K.E.S., Plath, K., B., M. and Rapoport, T.A. (1997) Protein transport by purified yeast Sec complex and Kar2p without membranes. Science 277, 938–941. [DOI] [PubMed] [Google Scholar]
- Mothes, W., Prehn, S., and Rapoport, T.A. (1994). Systematic probing of the environment of a translocating secretory protein during translocation through the ER membrane. EMBO J. 13, 3937–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch, A., Wiedmann, M., and Rapoport, T.A. (1992). Yeast Sec proteins interact with polypeptides traversing the endoplasmic reticulum membrane. Cell 69, 343–352. [DOI] [PubMed] [Google Scholar]
- Panzner, S., Dreier, L., Hartmann, E., Kostka, S., and Rapoport, T.A. (1995). Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell 81, 561–570. [DOI] [PubMed] [Google Scholar]
- Plath, K., Mothes, W., Wilkinson, B.M., Stirling, C.J., and Rapoport, T.A. (1998). Signal sequence recognition in posttranslational protein transport across the yeast ER membrane. Cell 94, 795–807. [DOI] [PubMed] [Google Scholar]
- Rapoport, T.A., Matlack, K.E., Plath, K., Misselwitz, B., and Staeck, O. (1999). Posttranslational protein translocation across the membrane of the endoplasmic reticulum. Biol. Chem. 380, 1143–1150. [DOI] [PubMed] [Google Scholar]
- Sanders, S.L., Whitfield, K.M., Vogel, J.P., Rose, M.D., and Schekman, R.W. (1992). Sec61p and BiP Directly Facilitate Polypeptide Translocation into the ER. Cell 69, 353–365. [DOI] [PubMed] [Google Scholar]
- Shaw, A.S., Rottier, P.J., and Rose, J.K. (1988). Evidence for the loop model of signal-sequence insertion into the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 85, 7592–7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, B.M., Critchley, A.J., and Stirling, C.J. (1996). Determination of the transmembrane topology of yeast Sec61p, an essential component of the endoplasmic reticulum translocation complex. J. Biol. Chem. 271, 25590–25597. [DOI] [PubMed] [Google Scholar]
- Wilkinson, B.M., Esneult, Y., Craven, R.A., Skiba, F., Fieschi, J., Kepes, F., and Stirling, C.J. (1997). Molecular architecture of the ER translocase probes by chemical crosslinking of Sss1p to complementary fragments of Sec61p. EMBO J. 16, 4549–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]