Abstract
Background
Guatemala is presently engaged in the Central America Initiative to interrupt Chagas disease transmission by reducing intradomiciliary prevalence of Triatoma dimidiata, using targeted cross-sectional surveys to direct control measures to villages exceeding the 5% control threshold. The use of targeted surveys to guide disease control programs has not been evaluated. Here, we compare the findings from the targeted surveys to concurrent random cross-sectional surveys in two primary foci of Chagas disease transmission in central and southeastern Guatemala.
Methodology/Principal Findings
Survey prevalences of T. dimidiata intradomiciliary infestation by village and region were compared. Univariate logistic regression was used to assess the use of risk factors to target surveys and to evaluate indicators associated with village level intradomiciliary prevalences >5% by survey and region. Multivariate logistic regression models were developed to assess the ability of random and targeted surveys to target villages with intradomiciliary prevalence exceeding the control threshold within each region. Regional prevalences did not vary by survey; however, village prevalences were significantly greater in random surveys in central (13.0% versus 8.7%) and southeastern (22.7% versus 6.9%) Guatemala. The number of significant risk factors detected did not vary by survey in central Guatemala but differed considerably in the southeast with a greater number of significant risk factors in the random survey (e.g. land surface temperature, relative humidity, cropland, grassland, tile flooring, and stick and mud and palm and straw walls). Differences in the direction of risk factor associations were observed between regions in both survey types. The overall discriminative capacity was significantly greater in the random surveys in central and southeastern Guatemala, with an area under the receiver-operator curve (AUC) of 0.84 in the random surveys and approximately 0.64 in the targeted surveys in both regions. Sensitivity did not differ between surveys, but the positive predictive value was significantly greater in the random surveys.
Conclusions/Significance
Surprisingly, targeted surveys were not more effective at determining T. dimidiata prevalence or at directing control to high risk villages in comparison to random surveys. We recommend that random surveys should be selected over targeted surveys whenever possible, particularly when the focus is on directing disease control and elimination and when risk factor association has not been evaluated for all regions under investigation.
Author Summary
Chagas disease is a vector-borne parasitic zoonosis endemic throughout South and Central America and Mexico. Guatemala is engaged in the Central America Initiative to interrupt Chagas disease transmission. A major strategy is the reduction of Triatoma dimidiata domiciliary infestations through indoor application of residual insecticides. Successful control of T. dimidiata will depend on accurate identification of areas at greatest risk for infestation. Initial efforts focused primarily on targeted surveys of presumed risk factors and suspected infestation to define intervention areas. This policy has not been evaluated and might not maximize the effectiveness of limited resources if high prevalence villages are missed or low prevalence villages are visited unnecessarily. We compare findings from the targeted surveys to concurrent random surveys in two primary foci of Chagas disease transmission in Guatemala to evaluate the performance of the targeted surveys. Our results indicate that random surveys performed better than targeted surveys and should be considered over targeted surveys when reliability of risk factors has not been evaluated, identify useful environmental factors to predict infestation, and indicate that infestation risk varies locally. These findings are useful for decision-makers at national Chagas Disease control programs in Central America, institutions supporting development efforts, and funding agencies.
Introduction
In Guatemala, nearly 4 million individuals are projected to be at risk for infection with Trypanosoma cruzi, the causative agent of Chagas disease, with approximately 30,000 new cases a year and a prevalence of 730,000 [1], [2]. The estimated prevalence and annual incidence is more than double any other country in Central America and is substantially greater than that observed in Mexico [1], [2]. Based on the results of the national survey of triatomine populations conducted from 1995–8, the principal focus of transmission is considered to be in the southeastern and central departments of the country where the prevalence of triatomine vectors [3], the estimated human population at risk for Trypanosoma cruzi infection [3], and the infection rate of triatomine vectors with T. cruzi [4] is greatest[1]. This is also the region where the vector Triatoma dimidiata (Latreille 1811) is most abundant [3], [4], [5].
The Guatemalan National Ministry of Health (GNMH) is engaged in the Central America Initiative to interrupt Chagas Disease transmission (IPCA) [6], [7], [8], and Guatemala is the country with the most progress to date [9]. All available information indicates that Rhodnius prolixus has been eliminated (GNMH communication) and populations of the indigenous T. dimidiata have been reduced in the domestic environment three to nine fold [10], [11]. However, since T. dimidiata is a native species also occurring in the peridomestic and sylvatic environments, elimination is virtually impossible [2], [12], [13], [14]. Therefore, the goal is to reduce and maintain T. dimidiata village level intradomiciliary prevalence and colonization (nymphal intradomiciliary prevalence) below 5% [1], [2], [6], [7], [8], [11], [15].
Vector control relies primarily on the intradomiciliary application of residual insecticides [16]. For the current control program, third-generation synthetic pyrethroids, including beta-cyfluthrin (12.5% suspension concentrate [s.c.], at 25% active ingredient [a.i.]/m2), cyfluthrin (10% wettable powder [w.p.], at 50 mg a.i./m2), delatamethrin (10% s.c. or 5% w.p. at 25 mg a.i./m2), and lambda-cyhalothrin (10% w.p. at 30 mg a.i./m2) (GNMH communication), were used based on market availability [17]. The current policy for selecting villages to spray entails a 5% intradomiciliary prevalence threshold but relies on targeted surveys of presumed risk factors and suspected infestation [11], [15], namely “villages suspected of being infested with R. prolixus or T. dimidiata, where infestation was reported or in rural areas where the majority of the houses are constructed with mud walls and/or thatched roofs” [15]. However, if villages with low prevalences are visited unnecessarily, or villages with high prevalences are missed, such a policy may not necessarily maximize the effectiveness of limited resources. In a resource limited setting, developing a rational control program to sustain T. dimidiata village intradomiciliary prevalence below 5%, will depend upon ensuring that control efforts are targeted to villages with the highest risk of infestation.
From 2000–3, GNMH, the Japanese International Cooperation Agency (JICA), and the Universidad del Valle de Guatemala (UVG) with other collaborating instituions undertook a series of targeted and random surveys to assess T. dimidiata prevalance prior to vector control [1], [11], [15], [18]. This study makes use of the data gathered in the central department of Baja Verapaz and southeastern department of Jutiapa to compare the effectiveness of random and targeted surveys in determining villages at high risk for T. dimidiata infestation in these two regions. Specifically, our objective was to evaluate the capability of the random and targeted survey methods in directing control to villages at greatest risk of infestation by comparing the ability of environmental and/or domiciliary risk factors to predict intradomestic prevalence >5% by survey and department.
Materials and Methods
Datasets
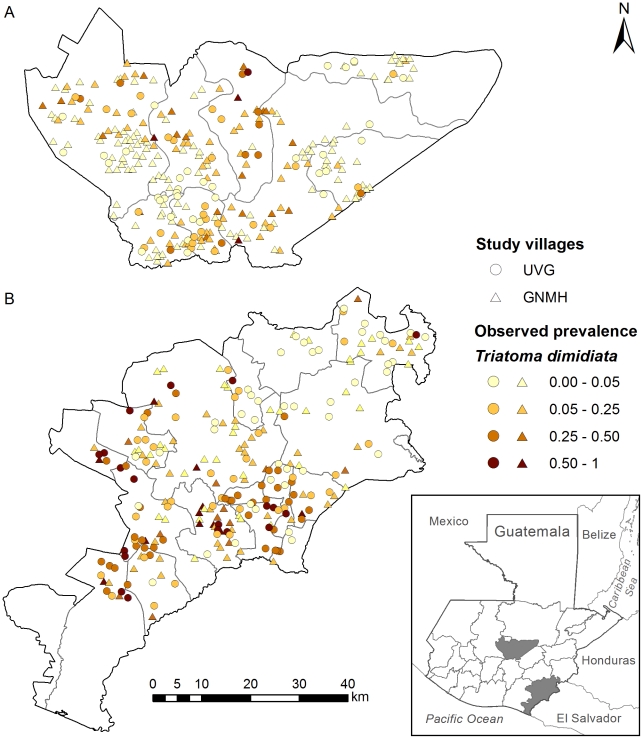
Triatoma dimidiata intradomiciliary prevalence data at the village level for the departments1 of Baja Verapaz and Jutiapa from 2000–3 were obtained from randomized cross-sectional pre-spray surveys implemented by UVG [10] and from targeted cross-sectional pre-spray surveys performed by GNMH [11], [18]. These departments are positioned within two principal regions of T. dimidiata infestation. Baja Verapaz is located in the temperate and subtropical dry forests [19] of central Guatemala, 89.93°–90.81°W and 13.74°–14.56°N, encompassing an area of 2864 km2. Jutiapa is positioned in the subtropical moist forest [19] in the southeast, 89.50°–90.30°W and 13.74°–14.56°N, covering an area of 3318 km2. The geographic distribution of villages surveyed by department and study is illustrated in Figure 1. Here, department prevalence refers to the proportion of villages within each department that are intradomiciliary infested with T. dimidiata, and village prevalence refers to the proportion of infested domiciles within each surveyed village.
Figure 1. Map of the geographic distribution and intradomiciliary prevalences of villages analyzed.
The location and intradomiciliary prevalences of villages analyzed in (A) Baja Verapaz and (B) Jutiapa. Each symbol represents a village, with circles symbolizing Universidad del Valle de Guatemala randomly sampled villages and triangles symbolizing Guatemala National Ministry of Health targeted villages. Shading indicates the level of intradomiciliary prevalence within each village. Inset: location of study departments within Guatemala and Central America. Note: Guatemala is divided into 22 departments and 331 municipalities [32] (www.state.gov/r/pa/ei/bgn/2045.htm). Health services, including vector control, are administered at the department level by each Health Area Authority [1].
Specific details of data collection and survey design have been previously published [1], . In brief, both surveys analyzed here are subsets of larger studies aimed at determining triatomine prevalence in central and southeastern Guatemala prior to a vector control campaign. Baja Verapaz and Jutiapa were selected here due to similarities in the broad geographic coverage of sampled villages, the presence of significant T. dimidiata infestation with limited R. prolixus infestation [3], [4], [18], [20], and the locations of the departments in two different regions, central and southeastern Guatemala. Moreover, the departments were analyzed separately as the vector surveys were administered at the department level [1] and due to the location of the departments in two different Holdridge Life zones. Baja Verapaz occurs in the subtropical and warm temperate dry forest and Jutiapa occurs in the subtropical moist forest [21].
The random data set was derived from a cross-sectional survey supported by the Tropical Disease Research and Training program (TDR), World Health Organization no. 990545 and the Centers for Disease Control and Prevention CoAg U50 CCU021236 by UVG in collaboration with GNMH from 2000–3 [10]. In each municipality, villages and domiciles were selected randomly [10]. All eight municipalities in Baja Verapaz and 16 of 18 municipalities in Jutiapa were surveyed. Within these municipalities, georeferenced data was obtained from 79 villages and 1021 domiciles in Baja Verapaz and 162 villages and 2215 domiciles in Jutiapa. Entomological evaluation was conducted using an abbreviated man-hour collection method [3]. For each domicile selected, the intradomestic and surrounding peridomestic environments were surveyed manually for triatomines by two entomology technicians for 15–30 minutes, as determined subjectively by the size of the house [10].
The targeted data set was derived from cross-sectional entomological surveys carried out by GNMH in collaboration with JICA from 2000–3 [1], [11], [18]. Domiciles were selected from a sampling frame that excluded villages sampled in the random survey. Within Baja Verapaz, all eight municipalities were surveyed while 14 of 18 were examined in Jutiapa. In contrast to the random survey, study villages were targeted in rural areas on the basis of anecdotal surveys, suspected infestation, previous infestation, or presumed risk factors, e.g., domiciles with walls made of mud and/or roofs constructed of thatch [11], [15]. Georeferenced data were obtained from 262 villages and 5306 domiciles in Baja Verapaz and 244 villages and 2954 domiciles in Jutiapa. Entomological evaluation was also conducted by an abbreviated man-hour collection method [3]. The intradomestic and peridomestic environments of selected domiciles were searched manually for triatomines for 30 minutes by one entomology technician and for 15 minutes by two technicians [11]. These findings were later used by GNMH to target pyrethroid spraying to domiciles and peridomestic annexes in villages with intradomiciliary prevalences >5% [1], [10], [11], [15], [18].
Environmental and socioeconomic data were obtained from multiple sources and are described in Table 1. Covariate and georeferenced infestation data were imported into the GIS TNTmips 2008:74 (Microimages, Lincoln, NE). Layers were processed and linked geographically. With the exception of land cover, environmental covariate values were defined using the geographic coordinates for each village. For land cover, the proportion of each land cover class (forest, grassland, cropland, wetland, and settlement) within a 2 km buffer of each village was determined. Domiciliary construction data were then summarized by calculating the proportion of each domicile construction material per village. All data were then extracted by village and exported for statistical analysis. Data were displayed and mapped using ArcView GIS v. 9.2 (Environmental Systems Research Institute, Inc., Redlands, CA).
Table 1. Summary of environmental and socioeconomic databases used in analyses.
| Resolution | ||||||
| Data type | Database | Source | Spatial | Temporal | Units | Citation |
| Environmental | Annual precipitation | WorldClim | 1 km | 1950–2000 | mm | www.worldclim.org [33], [34] |
| Digital elevation model | CGIAR-CSI | 90 m | 2004 | m | www.csi-cgiar.org [35] | |
| LST daytime and nighttime mean, max, min | MODIS | 1 km | 2001–3 | °C | lpdaac.usgs.gov [36] | |
| MIR mean, max, min | AVHRR/TFA | 1 km | 1992–6 | °C | Hay 2006 [37] | |
| NDVI mean, max, min | MODIS | 1 km | 2001–3 | lpdaac.usgs.gov [36] | ||
| RH mean, max, min | CRU/UEA | 10′ | 1961–90 | % | www.cru.uea.ac.uk [38] | |
| Land cover | SERVIR | 0.5 km | 2005 | www.servir.net [39] | ||
| Socioeconomic | House floor, wall and roof material | INE | Village | 2002 | www.ine.gob.gt/ [40] | |
Key to database abbreviations: LST, land surface temperature; MIR, middle infrared; NDVI, normalized difference vegetation index; RH, relative humidity; max, maximum average value; min, minimum average value. Key to database source abbreviations: CGIAR_CSI, Consultative Group for International Agriculture Research – Consortium for Spatial Information; MODIS, moderate resolution imaging spectroradiometer; AVHRR/TFA, advanced very high resolution radiometer transformed by temporal fourier analysis; CRU/UEA, Climate Research Unit,/University of East Anglia; INE, Instituto Nacional de Estadistica de Guatemala.
Statistical analysis
Analysis of T. dimidiata pre-spray prevalence data was limited to those villages where at least five domiciles were surveyed. Similarities in the geographic distribution of villages between the two studies were maximized by excluding villages from one study when their distance to the closest village in the opposing study exceeded five kilometers. For the remaining villages, descriptive statistics of T. dimidiata village and department level prevalences were summarized by study and department.
Analyses of risk factors associated with T. dimidiata intradomiciliary prevalence at the village level for each department and study were carried out using univariate and multivariate logistic regression. First, univariate logistic regression models for grouped data were fitted to each of the grouped climatic variables (land surface temperature, normalized difference vegetation index, middle infrared reflectance, and relative humidity) to identify covariates in each category that best discriminated village prevalence. For ease of interpretation and direct comparison of climate characteristics between studies, variables were selected from the analyses of the UVG random data set in each department. Variables with a Wald's P>0.05 were excluded from further analyses due to the large number of significant covariates. The best fit model for each category was then selected on the basis of its Akaike weight (wi). Although the number of parameters for each model was the same in this investigation, the statistic provided a simple and easily interpretable measure for model comparison [22], [23].
The environmental and domiciliary risk factors associated with T. dimidiata village prevalence >5% was investigated by univariate logistic regression for each study by department. The outcome variable was defined by village as T. dimidiata intradomiciliary prevalence ≤ or >5%. Explanatory variables included climate variables selected from the discriminative univariate analyses, the remaining environmental covariates (elevation, precipitation, and land cover), and all domiciliary construction covariates. A logistic regression model was fitted to each covariate to define the odds of infestation associated with each potential risk factor.
Predictions of the probability of village prevalence >5% were then made by fitting a series of multivariate logistic regression models using a jackknife procedure, whereby a single village was excluded and an estimate of its predictive probability was made using the remaining data [24], [25]. This method maximizes the data used to estimate a villages predictive probability and allows for model validation using independent data [24]. All significant covariates from the logistic regression models were used to fit multivariate models. Predictive models of environmental and domiciliary covariates for each study by department were generated individually and together. Diagnostic statistics were generated to compare model accuracy. The area under the receiver-operator curve (AUC) was calculated to compare overall model performance and kappa (κ), sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated across the range of predicted probability thresholds. All statistical analyses were conducted in Stata/IC version 10 (Stata Corporation, College Station, TX, USA).
Results
Prevalence and geographic distribution
The geographic distribution and intradomiciliary prevalences of villages selected for analysis are shown by department and study in Figure 1. In Baja Verapaz, villages in all eight municipalities were incorporated into the analysis of both surveys and included 894 domiciles in 72 villages from the random study and 4403 domiciles in 212 villages from the targeted study, representing 16.86% and 49.65% of villages, respectively, and 66.51% of villages overall (n = 427). Village prevalence of T. dimidiata was highest in the northwest and southern regions of the department. Department prevalence was highest in the random survey at 51.4% (95% CI 39.9–62.9), but not significantly different from the targeted survey with a prevalence of 39.2% (95% CI 32.6–45.7). In contrast, village prevalence was significantly higher in the random survey (13.0%, 95% CI 10.9–15.2) than in the targeted survey (8.7%, 95% CI 7.8–9.5).
T. dimidiata was distributed throughout Jutiapa with village prevalences highest in the central and southern regions. In the random study, 1919 domiciles in 138 villages and 16 municipalities were used for analyses, while in the targeted study, 2243 domiciles in 108 villages and 14 municipalities were used for analyses, representing 17.95% and 14.04% of villages, respectively, and 31.99% of villages overall (n = 769). Again, department prevalence was not significantly different between the random (68.8%, 95% CI 61.1–76.6) and targeted (62.0%, 95% CI 52.9–71.2) surveys, but village prevalences were significantly higher in the random (22.7%, 95% CI 20.9–24.6) than in the targeted (6.9%, 95% CI 5.9–8.0) surveys.
Environmental risk factors
The grouped climate variables that best explained T. dimidiata village prevalence are presented in Table 2. These covariates were used in all subsequent analyses.
Table 2. Significant grouped climate variables with highest Akaike weight (wi).
| Department | Covariate | Coefficient (95% CI) | P | AICc1 | wi |
| Baja Verapaz | LST daytime average (°C) | 0.29 (0.21,0.37) | 0.000 | 631.89 | 0.98 |
| NDVI minimum | −7.72 (−10.07,−5.38) | 0.000 | 643.45 | 0.71 | |
| MIR average (°C) | 0.30 (0.22,0.38) | 0.000 | 630.00 | 0.99 | |
| RH minimum | −0.11 (−0.19,−0.02) | 0.014 | 688.06 | 1.00 | |
| Jutiapa | LST daytime average (°C) | −0.38 (−0.46,−0.31) | 0.000 | 1957.28 | 1.00 |
| NDVI average | 8.05 (6.38,9.73) | 0.000 | 1967.49 | 1.00 | |
| MIR average (°C) | −0.44 (−0.51,−0.36) | 0.000 | 1909.13 | 1.00 | |
| RH maximum | 0.21 (0.12,0.30) | 0.000 | 2039.73 | 1.00 |
Key to covariate abbreviations: LST, land surface temperature; MIR, middle infrared; NDVI, normalized difference vegetation index; RH, relative humidity. Key to database statistical abbreviations: AICc: Akaike information criterion for small sample sizes; wi, Akaike weight.
Univariate logistic regression models were fitted to each of the grouped climate variables to determine the covariates that best discriminated intradomiciliary village prevalence. Model performance was evaluated by the selecting the covariate with the highest Akaike weight (wi).
Table 3 shows the significant results of the environmental risk factor analyses for village prevalence exceeding the 5% control threshold for each survey and department. For Baja Verapaz, the significant environmental risk factors were the same for both survey types and similarly describe the odds of infestation. The magnitude of the observed effect of each covariate with the exception of annual precipitation (equal impact) was greatest in the random study. An increase in the average daytime LST, average MIR, and proportion of cropland within a 2 km buffer of villages were associated with an increase in the risk of infestation. In contrast, minimum NDVI, minimum RH, and the proportion of evergreen forest within a 2 km buffer were associated with a decrease in the risk of infestation. Annual precipitation and elevation had weak negative effects.
Table 3. Estimates of effect of significant environmental risk factors on Triatoma dimidiata intradomiciliary prevalence >5%.
| Random survey | Targeted survey | ||||
| Department | Risk factor | OR (95%CI) | P | OR (95% CI) | P |
| Baja Verapaz | Annual precipitation (mm) | 0.999 (0.997,0.9999) | 0.040 | 0.999 (0.998,0.9998) | 0.011 |
| Elevation (m) | 0.996 (0.995,0.998) | 0.000 | 0.999 (0.998,0.9998) | 0.015 | |
| LST daytime average (°C) | 1.70 (1.34,2.16) | 0.000 | 1.24 (1.12,1.37) | 0.000 | |
| MIR average (°C) | 1.71 (1.33,2.20) | 0.000 | 1.20 (1.09,1.33) | 0.000 | |
| NDVI minimum | 7.34e-06 (1.40e-08,0.004) | 0.000 | 0.0005 (0.00002,0.02) | 0.000 | |
| RH minimum | 0.72 (0.57,0.92) | 0.008 | 0.85 (0.75,0.97) | 0.013 | |
| Cropland (%) | 292.52 (15.57,5496.13) | 0.000 | 10.66 (3.15,36.07) | 0.000 | |
| Evergreen forest (%) | 0.003 (0.0001,0.06) | 0.000 | 0.02 (0.004,0.16) | 0.000 | |
| Jutiapa | Annual precipitation (mm) | 1.006 (1.004,1.008) | 0.000 | 1.003 (1.002,1.005) | 0.000 |
| Elevation (m) | 1.003 (1.002,1.005) | 0.001 | |||
| LST daytime average (°C) | 0.57 (0.42,0.77) | 0.000 | |||
| MIR average (°C) | 0.40 (0.27,0.60) | 0.000 | 0.71 (0.54,0.94) | 0.015 | |
| NDVI average | 2.18e+5 (141.24,3.37e+08) | 0.001 | 8227.39 (4.33,1.56e+07) | 0.019 | |
| RH maximum | 1.70 (1.21,2.39) | 0.002 | |||
| Cropland (%) | 0.14 (0.02,0.81) | 0.028 | |||
| Grassland (%) | 12.36 (1.70,89.72) | 0.013 | |||
| Settlement (%) | 4.81e-07 (3.40e-11,0.007) | 0.003 | |||
Key to risk factor abbreviations: LST, land surface temperature; MIR, middle infrared; NDVI, normalized difference vegetation index; RH, relative humidity.
Univariate logistic regression models were developed to investigate the effect of each environmental covariate on Triatoma dimidiata intradomiciliary village prevalence >5% by survey and department. Odds ratios (OR) and 95% confidence intervals for significant risk factors are reported. Land cover classes represent the proportion of each land cover type within a 2 km buffer of analyzed villages.
In Jutiapa, fewer environmental risk factors were significant in the targeted study than in the random study. The direction of the relationships of similar significant risk factors in both studies was the same. As with the relationships of the covariates in the Baja Verapaz studies, the magnitude of the observed effects was greatest in the random study but not significantly different as the confidence intervals overlapped. For both studies, the average NDVI had a substantial positive effect on the risk of infestation, while the odds of infestation were negatively associated with the average MIR. In addition, the proportion of grassland with in a 2 km buffer of an infested village and the maximum RH were associated with an increased risk of infestation in the random study. Moreover, the average daytime temperature, and proportion of cropland and settlements within a 2 km buffer of infested villages were associated with a decreased risk of infestation.
Domiciliary risk factors
Significant domicile construction risk factors associated with village prevalence >5% are shown in Table 4. Fewer villages contained data on domicile construction materials than environmental covariates in each study and department. In Baja Verapaz, 64 of 72 villages in the random survey and 160 of 212 villages in the targeted survey had corresponding construction data, while in Jutiapa 123 of 138 villages in the random survey and 89 of 108 villages in the targeted survey had data on domicile construction covariates. The effect of similar domicile construction materials in both departments was consistent among studies. Risk was higher in adobe walled domiciles and lower in aluminum roofed domiciles in Baja Verapaz. In Jutiapa, risk was higher in domiciles with dirt floors and roofs of aluminum or tile and lower in domiciles with floors made of clay tile or cement.
Table 4. Estimates of effect of significant domicile construction materials on Triatoma dimidiata intradomiciliary prevalence >5%.
| Random survey | Targeted survey | |||||
| Department | Location | Risk factor | OR (95% CI) | P | OR (95% CI) | P |
| Baja Verapaz | Wall | Adobe | 5.76 (1.08–30.60) | 0.004 | 13.10 (3.38,50.58) | 0.000 |
| Wood | 0.04 (0.004,0.46) | 0.009 | ||||
| Roof | Aluminum | 0.12 (0.01–0.94) | 0.044 | 0.11 (0.03,0.04) | 0.001 | |
| Tile | 8.04 (2.31,28.01) | 0.001 | ||||
| Jutiapa | Floor | Cement slab | 0.09 (0.01,0.87) | 0.037 | ||
| Cement tile | 0.05 (0.01–0.35) | 0.003 | ||||
| Ceramic | 7.60e-11 (9.27e-18-0.001) | 0.004 | ||||
| Clay tile | 3.66e-11 (1.25e-19-0.01) | 0.016 | 1.23e-12 (2.25e-24,0.67) | 0.047 | ||
| Earth | 26.84 (5.64–127.79) | 0.000 | 8.81 (1.69,46.04) | 0.010 | ||
| Wall | Brick | 0.001 (0.00001–0.37) | 0.015 | |||
| Block | 0.05 (0.004–0.56) | 0.016 | ||||
| Stick & mud | 11.97 (1.04–137.44) | 0.046 | ||||
| Palm & straw | 1.30e+16 (8.99–1.88e+31) | 0.037 | ||||
| Roof | Aluminum | 7.64 (1.66–35.14) | 0.009 | 9.96 (1.63,60.80) | 0.013 | |
| Concrete | 1.90e-26 (2.45e-41-1.47e-11) | 0.001 | ||||
| Tile | 0.15 (0.04–0.65) | 0.011 | 0.15 (0.03,0.79) | 0.026 |
Univariate logistic regression models were developed to investigate the effect of each domicile construction material on Triatoma dimidiata intradomiciliary village prevalence >5% by survey and department. Odds ratios (OR) and 95% confidence intervals for significant risk factors are reported. Domicile construction risk factors represent the proportion of domiciles per village constructed with each material as determined by the 2002 national census of the Guatemalan National Institute of Statistics [40].
In both departments, village prevalence in each study was often associated with different risk factors. For example, the targeted survey in Baja Verapaz found an increased risk associated with tile roofed domiciles that was not detected in the random study. Moreover, the random survey in Jutiapa detected a series of associations with wall materials not observed in the targeted survey. In particular, walls constructed of stick and mud or palm and straw were associated with considerable increases in the risk of infestation. Brick and block walls had marked protective effects. Interestingly, the direction of the effect of similarly significant materials such as aluminum and tile roofs contrasted between departments.
Predictive models
A summary of the performance of the multivariate logistic regression models ability to predict village prevalence >5% is presented in Table 5. Models were constructed using villages with data for both environmental and domicile construction covariates to allow for direct comparison. The area under receiver-operator curve (AUC) is the best measure of a model's overall discriminative ability [25], [26]. With the exception of the domicile construction material model in Baja Verapaz, the random models for both departments had reasonably good discriminative capacity and performed significantly better than the corresponding targeted models. All targeted models had poor discriminative capacity. Moreover, the environmental and combination models in the Baja Verapaz random surveys had similar predictive power and performed significantly better than the domicile construction material model. In the Jutiapa random surveys, no significant difference in predictive performance was detected between models.
Table 5. Diagnostic statistics for predictive models of Triatoma dimidiata intradomiciliary prevalence >5%.
| Accuracy measures | |||||||
| Dept/Study | Model | AUC (95% CI) | Max κ | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) | NPV % (95% CI) |
| BV/UVG | ENV | 0.84 (0.74,0.94) | 0.56 | 76.5 (58.4,88.6) | 80.0 (60.9,91.6) | 81.3 (63.0,92.1) | 75.0 (56.3,87.9) |
| DOM | 0.58 (0.44,0.72) | 0.16 | 82.4 (64.8,92.6) | 33.3 (17.9,52.9) | 58.3 (43.3,72.1) | 62.5 (35.9,83.7) | |
| ALL | 0.84 (0.74,0.93) | 0.51 | 64.7 (46.5,79.7) | 86.7 (68.4,95.6) | 84.6 (64.3,95.0) | 68.4 (51.2,82.0) | |
| BV/GNMH | ENV | 0.65 (0.56,0.73) | 0.24 | 80.3 (67.8,89.0) | 46.5 (36.5,56.7) | 48.0 (38.1,58.1) | 79.3 (66.3,88.4) |
| DOM | 0.65 (0.56,0.74) | 0.27 | 82.0 (69.6,90.2) | 48.5 (38.4,58.7) | 49.1 (39.5,59.6) | 81.4 (68.7,89.9) | |
| ALL | 0.65 (0.57,0.74) | 0.19 | 68.9 (55.6,79.8) | 59.6 (49.2,69.2) | 51.2 (40.0,62.3) | 75.6 (64.4,84.4) | |
| JU/UVG | ENV | 0.86 (0.78,0.93) | 0.57 | 82.9 (72.7,90.0) | 75.6 (59.4,87.1) | 87.2 (77.2,93.4) | 68.9 (53.2,81.4) |
| DOM | 0.77 (0.68,0.87) | 0.51 | 91.5 (82.7,96.2) | 56.1 (39.9,71.2) | 80.7 (70.9,87.8) | 76.7 (57.3,89.4) | |
| ALL | 0.84 (0.76,0.92) | 0.57 | 79.3 (68.6,87.1) | 80.5 (64.6,90.6) | 89.4 (79.2,94.8) | 66.0 (51.1,78.4) | |
| JU/GNMH | ENV | 0.67 (0.55,0.78) | 0.35 | 64.7 (50.0,77.2) | 71.1 (53.9,84.0) | 75.0 (59.4,86.3) | 60.0 (44.4,73.9) |
| DOM | 0.65 (0.53,0.77) | 0.30 | 66.7 (52.0,78.9) | 63.2 (46.0,77.7) | 70.8 (55.7,82.6) | 58.5 (42.2,73.3) | |
| ALL | 0.64 (0.52,0.76) | 0.30 | 54.9 (40.5,68.6) | 57.9 (40.9,73.3) | 63.6 (47.7,77.2) | 48.9 (33.9,64.0) | |
Key to department and study abbreviations: Dept, department; BV, Baja Verapaz; JU, Jutiapa; UVG, Universidad del Valle de Guatemala; GNMH; Guatemala National Ministry of Health. Key to model abbreviations: ENV, environmental model; DOM, domicile construction material model; ALL, combination of census and environmental models. Key to accuracy measure abbreviations: AUC, area under receiver-operator curve; Max κ, maximum kappa; PPV, positive predictive value; NPV, negative predictive value.
Multivariate logistic regression models were developed to estimate the predictive probability of Triatoma dimidiata intradomiciliary village prevalence >5%. For each department and study, predictive models of environmental and domicile construction risk factors were developed separately and together. Overall model accuracy was compared using the area under the receiver-operator curve (AUC). Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated using the probability threshold with maximum value of kappa (κ).
κ, sensitivity, specificity, PPV, and NPV all vary with the selection of the predicted probability threshold. The maximum κ obtained for each model is reported in Table 5 and the remaining accuracy measures are calculated using the corresponding threshold. All models from random surveys, with the exception of the domicile construction material model in Baja Verapaz, performed significantly better than chance alone. With regard to the random surveys, predictions based on environmental covariates had the greatest accuracy in Baja Verapaz, and environmental and combination models had similar and greater accuracy than domicile construction covariates in Jutiapa.
Discussion
Sustained control of T. dimidiata depends on the accurate identification of areas at greatest risk of infestation in order to efficiently target limited resources. In their efforts to eliminate Chagas disease from Guatemala, vector control initiatives have relied on targeted surveys of villages with presumed risk factors or suspected infestation [11], [15], [16], however, their performance has not been evaluated. The data sets analyzed here afforded a unique opportunity to compare the abilities of random and targeted baseline cross-sectional surveys of T. dimidiata village prevalence conducted concurrently in time and space and resulted in several important findings relevant to T. dimidiata vector control: 1) random surveys performed just as well if not better than targeted surveys at defining the risk of T. dimidiata infestation, 2) intradomiciliary and environmental risk factor associations with T. dimidiata prevalence >5% varied with geographic location, 3) environmental risk factors provide additional insight into the intradomiciliary risk of T. dimidiata prevalence exceeding the control threshold, and 4) predictive modeling has a role to play in directing T. dimidiata control in Guatemala if data sets are appropriately defined and expectations realistic. To our knowledge, this is the first study to compare targeted and random surveys for T. dimidiata and has implications for T. dimidiata control in Guatemala and Central America.
The failure of the targeted surveys to detect higher department and village prevalences than random surveys was surprising. These findings illustrate that the methods used to focus targeted surveys were not any better than random sampling at determining villages at greatest risk for T. dimidiata infestation. Therefore, presuming risk factors and infestation was inadequate and when initiating a program, efforts should favor risk factor evaluation and validation prior to targeting surveys or favor random sampling, as the results could reflect insufficiently defined risk factors and/or the assumption of geographic similarity in risk factor effect. Although, the findings could also be attributed to greater experience and expertise among UVG surveyors who conducted the random surveys [10], [18].
The analysis of the intradomiciliary and environmental risk factors further supports the notion that the poor performance of the targeted surveys resulted at least in part from insufficiently defined risk factors and geographic heterogeneity in their effect. The limited ability of the presumed risk factors is illustrated by our ability to detect further robust relationships with additional indicators in the analysis of the targeted survey data. Moreover, many of the risk factors contrasted in their significance and the direction of their effect between departments. Even the presumed risk factors contrasted in their significance between departments. Walls of adobe had strong positive association with T. dimidiata village prevalence exceeding the control threshold in Baja Verapaz only, while walls of stick and mud were significant in Jutiapa only. The lack of a significant association with thatch roofs in both surveys and departments likely reflects the inclusion of this risk factor to aide in the targeting of R. prolixus [2].
Particularly interesting was the contrasting relationship between tile roofs and infestation exceeding the control threshold in the departments. Tile roofs had a protective effect in Jutiapa but were associated with increased risk in infestation in Baja Verapaz. A similar increased risk was detected in Costa Rica where it was suggested that the presence of spare roofing tiles in the peridomestic environment provided suitable habitat for T. dimidiata [27]. Peridomestic surveys associated with the targeted study in Baja Verapaz found established T. dimidiata populations, although specific peridomestic environments were not reported [18]. These findings suggest the potential for roofing tiles to play a similar role in Baja Verapaz. Peridomestic populations are also present in Jutiapa [10] but were not reported here. The protective effect could indicate tile roofs in this region are associated with improved living conditions, thus, limiting intradomestic populations. In addition, previous studies in Jutiapa found no direct association between intradomestic and peridomestic infestation [10], indicating that spare roofing tiles in the peridomestic environment may be of little significance to intradomestic T. dimidiata populations in Jutiapa. More detailed studies are needed to clarify the variation of risk factors in different ecological settings.
Moreover, the analysis of the environmental covariates also illustrated the geographic heterogeneity in risk factor association with T. dimidiata infestation >5% and indicated their potential value as indicators of infestation exceeding the control threshold. For example, villages with higher temperatures, increasingly barren landscapes, and more cropland were associated with increases in prevalence above the threshold in Baja Verapaz, while in Jutiapa an increase in vegetated landscapes, the proportion of grassland, and maximum RH were associated with increased risk of infestation. Future surveys should evaluate the inclusion of environmental risk factors as an aide in focusing control efforts. Furthermore, the observed geographic heterogeneity of both domiciliary and environmental risk factors illustrates the need to evaluate risk factors prior to use in a particular geographic location and the risk in extrapolating findings beyond the geographic limits for which they were defined. This observed heterogeneity is even more important in light of recent molecular studies suggesting that T. dimidiata in Guatemala represents a geographically diverse species complex [28], [29] with one study elevating a member to specific status [28].
The findings from the predictive models indicate the potential for this type of analysis and risk mapping to aide in directing T. dimidiata control to regions at greatest risk as well as support the findings discussed above with regard to the abilities of the random surveys and potential value of environmental covariates. The reasonably high sensitivities and PPV's among the best performing models from the random surveys in both departments indicate marginal resource loss when applying control measures. Similarly, the respectable specificity and NPV's suggest that the number of positive villages missed would be moderately low. Moreover, the performance of the targeted surveys suggest that they might have a limited role to play in generating predictive models if risk factors are adequately defined first and sensitivity and PPV are reasonably good in targeting high risk villages. Although, one would have to accept a significant number of positive villages would be excluded from control due to the expected low specificity and NPV.
Also notable among the results were the performance of the environmental covariates in predicting risk of T. dimidiata prevalence >5%. The predictive performance of environmental models was just as good if not significantly better than domicile construction material models. As mentioned previously, this could relate to insufficiently defined risk factors and/or geographic heterogeneity in their effect. In addition, it could be that the association with environmental covariates is related to the peridomestic populations in these regions, implying that peridomestic populations give rise to intradomestic populations or are in constant movement from one environment to the other. However, it might also be that the environmental conditions that are present in a region dictate the domicile construction materials used and represent confounding relationships with existing covariates and subsequently the type of construction defines the temperature and relative humidity inside the domicile. This could explain why the predictive models combining both environmental and domicile construction risk factors failed to improve overall model performance. Future models might be improved by the inclusion of intradomiciliary physical variables such as temperature and relative humidity.
As with any study, it is important to point out the limitations that exist. First, the targeted sample is biased by the exclusion of villages sampled in the random survey. Differences in our results could reflect differences in the villages sampled, although, we tried to account for significant variation by comparing geographically similar villages. Secondly, the findings are relevant to surveys conducted by the man-hour collection method, which is labor intensive with small reward and likely varies with expertise and experience [30]. Other collection methods could be less biased by experience, more consistent and efficient, and better able to define risk factors. Thus, the lack of the results could reflect variation in the ability to adequately detect bugs and not the absence of bugs and their associations with the risk factors. In addition, neither study was designed with our analysis in mind and therefore doesn't allow for optimal comparison. Future studies could control for this by selecting villages from the same sample frame, choosing the same number of domiciles in each village to survey, and conducting surveys with similarly experienced technicians. In addition, a true comparison of survey effectiveness should balance scientific abilities against their cost, with decisions made accordingly.
Nonetheless, the findings from our study lead us to several recommendations for T. dimidiata control in Guatemala and Central America. First, a priori knowledge, a prerequisite for targeted surveys, was not reliable for T. dimidiata surveys in Guatemala. Random surveys performed just as well if not better than targeted surveys, and have the additional benefit of risk factor detection, resulting from increased sample heterogeneity. Therefore, random surveys should be considered over targeted surveys if the reliability of the risk factors used to target surveys has not been evaluated. Secondly, risk factors for T. dimidiata infestation should be characterized for a particular geographic location through proper epidemiological investigation. One should keep in mind that the risk of extrapolation error increases as the distance from the source from which it was defined increases [31]. Furthermore, the role of environmental risk factors should be considered in addition to traditional intradomiciliary construction risk factors when investigating the risk of T. dimidiata infestation. Finally, our results indicate that predictive modeling has a role to play in targeting T. dimidiata control as long as the surveillance data is appropriately defined and/or model error is acceptable. It should be stressed that random surveys are not simply a luxury but an investment in programs. Future surveys should weigh their benefits as well cost when initiating a vector control program.
In conclusion, sustained control of T. dimidiata will depend on accurate and thorough epidemiological investigation. It is essential that the sample surveys on which decision making is based are evaluated to ensure that policy is not formed blindly and resources are not wasted. Here we show that a priori knowledge was not reliable in defining T. dimidiata risk in Guatemala. The random survey performed just as well if not better than the targeted survey. Moreover, our findings illustrate the blanket application of “presumed risk factors” should be applied with caution and based on initial scientific evaluation to ensure geographic extrapolation is appropriate. Future targeting of T. dimidiata surveys should also include environmental risk factors as they performed just as well if not better than domicile construction covariates at detecting infestation exceeding the control threshold. Random surveys were generally more successful at detecting risk factors and predicting infestation than targeted surveys and should be applied over targeted surveys when risk factor identification, predictive modeling and extrapolation to the general populations is the goal. These findings illustrate the need for studies that are well defined, geographically specific, and based on reliable epidemiological investigation.
Acknowledgments
We are grateful for the support provided by Dr. Robert Wirtz from the Division of Parasitic Diseases and Malaria, at the CDC, and to Dr. Simon Brooker at the London School of Hygiene and Tropical Medicine for comments on an earlier draft. We would also like to thank Jun Nakagawa and Ken Hashimoto from JICA for their advice and facilitating the database exchange. Technical assistance with the existing databases was kindly provided by Estuardo Barrios Girron, Gustavo Chajon, and Jose Roberto Ramirez at the Universidad del Valle de Guatemala, and Emil Cherrington at CATHALAC/SERVIR graciously provided support with the satellite imagery. This research could not have been conducted without the hard work of the UVG field technicians and the department vector control teams for which we are extremely grateful. Finally, we would like to acknowledge the late Dr. Hugo Alvarez, former Chief of the Guatemalan Chagas Program, for access to the national database for Guatemala.
Footnotes
The authors have declared that no competing interests exist.
This work was supported in part by UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) (grant no. A50685), the Overseas Research Studentship Award Scheme (ORSAS) (http://www.orsas.ac.uk), and Wilby and Gloria Coleman, United States of America. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.PAHO. Progress Report: Chagas Disease Vector Control Project, Republic of Guatemala (2000–2002) Pan American Health Organization; 2002. pp. 1–4. [Google Scholar]
- 2.Schofield CJ. Challenges of Chagas Disease Vector Control in Central America. World Health Organization; 2000. pp. 1–36. [Google Scholar]
- 3.Tabaru Y, Monroy C, Rodas A, Mejia M, Rosales R. The geographical distribution of vectors of Chagas' disease and populations at risk of infection in Guatemala. Medical Entomology & Zoology. 1999;50:9–17. [Google Scholar]
- 4.Monroy C, Rodas A, Mejia M, Rosales R, Tabaru Y. Epidemiology of Chagas disease in Guatemala: infection rate of Triatoma dimidiata, Triatoma nitida and Rhodnius prolixus (Hemiptera, Reduviidae) with Trypanosoma cruzi and Trypanosoma rangeli (Kinetoplastida, Trypanosomatidae). Mem Inst Oswaldo Cruz. 2003;98:305–310. doi: 10.1590/s0074-02762003000300003. [DOI] [PubMed] [Google Scholar]
- 5.Dorn PL, Monroy C, Curtis A. Triatoma dimidiata (Latreille, 1811): a review of its diversity across its geographic range and the relationship among populations. Infect Genet Evol. 2007;7:343–352. doi: 10.1016/j.meegid.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Ponce C. Current situation of Chagas disease in Central America. Mem Inst Oswaldo Cruz. 2007;102(Suppl 1):41–44. doi: 10.1590/s0074-02762007005000082. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Chagas Disease: Central American Initiative launched. TDR news. 1998;55:6. [PubMed] [Google Scholar]
- 8.WHA. Elimination of transmission of Chagas disease. World Health Assembly; 1998. pp. 10 10. 1–2. [Google Scholar]
- 9.PAHO. Agreements and recommendations from the IXth Annual IPCA meeting. Guatemala City: 2006. 5 [Google Scholar]
- 10.Hashimoto K, Cordon-Rosales C, Trampe R, Kawabata M. Impact of single and multiple residual sprayings of pyrethroid insecticides against Triatoma dimidiata (Reduviidae; Triatominae), the principal vector of Chagas disease in Jutiapa, Guatemala. Am J Trop Med Hyg. 2006;75:226–230. [PubMed] [Google Scholar]
- 11.Nakagawa J, Hashimoto K, Cordon-Rosales C, Abraham Juarez J, Trampe R, et al. The impact of vector control on Triatoma dimidiata in the Guatemalan department of Jutiapa. Ann Trop Med Parasitol. 2003;97:288–297. doi: 10.1179/000349803235001895. [DOI] [PubMed] [Google Scholar]
- 12.Schofield CJ, Dujardin JP. Chagas disease vector control in Central America. Parasitol Today. 1997;13:141–144. doi: 10.1016/s0169-4758(97)89811-0. [DOI] [PubMed] [Google Scholar]
- 13.Zeledon R. El Triatoma dimidiata (Latreille, 1811) y su Relacion con la Enfermedad de Chagas; In: Distancia UEa., editor. San Jose: Universidad Estatal a Distancia; 1981. 146 [Google Scholar]
- 14.Acevedo F, Godoy E, Schofield CJ. Comparison of intervention strategies for control of Triatoma dimidiata in Nicaragua. Mem Inst Oswaldo Cruz. 2000;95:867–871. doi: 10.1590/s0074-02762000000600022. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa J, Cordon-Rosales C, Juarez J, Itzep C, Nonami T. Impact of residual spraying on Rhodnius prolixus and Triatoma dimidiata in the department of Zacapa in Guatemala. Mem Inst Oswaldo Cruz. 2003;98:277–281. doi: 10.1590/s0074-02762003000200019. [DOI] [PubMed] [Google Scholar]
- 16.Strosber AM, Barrio K, Stinger VH, Tashker J, Wilbur JC, et al. Chagas Disease: A Latin American Nemesis. Institute for OneWorld Health; 2007. pp. 1–105. [Google Scholar]
- 17.Yamagata Y, Nakagawa J. Control of Chagas disease. Adv Parasitol. 2006;61:129–165. doi: 10.1016/S0065-308X(05)61004-4. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa J, Juarez J, Nakatsuji K, Akiyama T, Hernandez G, et al. Geographical characterization of the triatomine infestations in north-central Guatemala. Ann Trop Med Parasitol. 2005;99:307–315. doi: 10.1179/136485905X29684. [DOI] [PubMed] [Google Scholar]
- 19.NGDC. Leemans Holdridge Life Zone Classifications. 1992 Available: http://www.fao.org/geonetwork/srv/en/main.home. Acessed 2011 Mar 9. [Google Scholar]
- 20.Bustamante DM, Monroy MC, Rodas AG, Juarez JA, Malone JB. Environmental determinants of the distribution of Chagas disease vectors in south-eastern Guatemala. Geospat Health. 2007;1:199–211. doi: 10.4081/gh.2007.268. [DOI] [PubMed] [Google Scholar]
- 21.Leemans R. Possible changes in natural vegetation patterns due to a global warming. Laxenburg: International Institute for Applied Systems Analysis; 1990. p. 108 108. 18. [Google Scholar]
- 22.Mazerolle MJ. Improving data analysis in herpetology: using Akaike's Information Criterion (AIC) to assess the strength of biological hypotheses. Amphibia-Reptilia. 2006;27:169–180. [Google Scholar]
- 23.Akaike H. A new look at the statistical model identification. IEEE Transaction on Automatic Control. 1974;19:716–723. [Google Scholar]
- 24.Olden JD, Jackson DA, Peres-Neto PR. Predictive models of fish species distributions: a note on proper validation and chance predictions. Transactions of the American Fisheries Society. 2002;131:329–336. [Google Scholar]
- 25.King RJ, Campbell-Lendrum DH, Davies CR. Predicting geographic variation in cutaneous leishmaniasis, Colombia. Emerg Infect Dis. 2004;10:598–607. doi: 10.3201/eid1004.030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 27.Starr MD, Rojas JC, Zeledon R, Hird DW, Carpenter TE. Chagas' disease: risk factors for house infestation by Triatoma dimidiata, the major vector of Trypanosoma cruzi in Costa Rica. Am J Epidemiol. 1991;133:740–747. doi: 10.1093/oxfordjournals.aje.a115949. [DOI] [PubMed] [Google Scholar]
- 28.Bargues MD, Klisiowicz DR, Gonzalez-Candelas F, Ramsey JM, Monroy C, et al. Phylogeography and Genetic Variation of Triatoma dimidiata, the Main Chagas Disease Vector in Central America, and Its Position within the Genus Triatoma. PLoS Negl Trop Dis. 2008;2:e233. doi: 10.1371/journal.pntd.0000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorn PL, Calderon C, Melgar S, Moguel B, Solorzano E, et al. Two Distinct Triatoma dimidiata (Latreille, 1811) Taxa Are Found in Sympatry in Guatemala and Mexico. PLoS Negl Trop Dis. 2009;3:e393. doi: 10.1371/journal.pntd.0000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schofield CJ. A comparison of sampling techniques for domestic populations of Triatominae. Trans R Soc Trop Med Hyg. 1978;72:449–455. doi: 10.1016/0035-9203(78)90160-8. [DOI] [PubMed] [Google Scholar]
- 31.Kitron U. Landscape ecology and epidemiology of vector-borne diseases: tools for spatial analysis. J Med Entomol. 1998;35:435–445. doi: 10.1093/jmedent/35.4.435. [DOI] [PubMed] [Google Scholar]
- 32.USDOS. Background Note: Guatemala. 2010. Available: http://www.state.gov/r/pa/ei/bgn/2045.htm. Accessed 2011 Mar 9.
- 33.Hijmans R, Cameron S, Parra J, Jones P, Jarvis A, et al. WorldClim. 2008. Available: http://www.worldclim.org/. Accessed 2011 Mar 9.
- 34.Hijmans R, Cameron S, Parra J, Jones P, Jarvis A. Very High Resolution Interpolated Climate Surfaces for Global Land Areas. Int J Climatol. 2005;25:1965–1978. [Google Scholar]
- 35.CGIAR-CSI. CGIAR Consortium for Spatial Information (CGIAR-CSI). 2008. Available: http://www.csi-cgiar.org/. Accessed 2011 Mar 9.
- 36.USGS-NASA. MODIS Data Products. 2006. Available: https://lpdaac.usgs.gov/lpdaac/products/modis_products_table. Accessed 2011 Mar 9.
- 37.Hay SI, Tatem AJ, Graham A, Goetz SJ, Rogers DJ. Global environmental data for mapping infectious disease distribution. In: Hay SI, Graham A, Rogers DJ, editors. Global Mapping of Infectious Diseases: Methods, Examples and Emerging Applications. London: Academic Press; 2006. pp. 38–71. [Google Scholar]
- 38.New M, Lister D, Hulme M, Makin I. A high-resolution data set of surface climate over global land areas. Clim Res. 2002;21:1–25. [Google Scholar]
- 39.Tullis JA, Cothren JD, Irwin DE, Yeager CP, Limp WF, et al. Yearly extraction of Central America's land cover for carbon flux monitoring. GIScience & Remote Sensing. 2007;44:334–355. [Google Scholar]
- 40.INE. Censos Nacionales XI de Población y VI Habatación. Ciudad de Guatemala. 2002. Available: http://www.ine.gob.gt/index.php/demografia-y-poblacion. Accessed 2011 Mar 9.